Abstract
Growing concern about the influence of climate change on flowering plants, pollinators, and the mutualistic interactions between them has led to a recent surge in research. Much of this research has addressed the consequences of warming for phenological and distributional shifts. In contrast, relatively little is known about the physiological responses of plants and insect pollinators to climate warming and, in particular, how these responses might affect plant-pollinator interactions. Here, we summarize the direct physiological effects of temperature on flowering plants and pollinating insects to highlight ways in which plant and pollinator responses could affect floral resources for pollinators, and pollination success for plants, respectively. We also consider the overall effects of these responses on plant-pollinator interaction networks. Plant responses to warming, which include altered flower, nectar, and pollen production, could modify floral resource availability and reproductive output of pollinating insects. Similarly, pollinator responses, such as altered foraging activity, body size, and life span, could affect patterns of pollen flow and pollination success of flowering plants. As a result, network structure could be altered as interactions are gained and lost, weakened and strengthened, even without the gain or loss of species or temporal overlap. Future research that addresses not only how plant and pollinator physiology are affected by warming but also how responses scale up to affect interactions and networks should allow us to better understand and predict the effects of climate change on this important ecosystem service
Keywords: Mutualism, Networks, Plant-pollinator interactions, Pollination, Temperature, Thermoregulation
1 Introduction
Climate change is affecting a diversity of species in a variety of ways (Hughes, 2000; Parmesan, 2006; Walther et al., 2002). In particular, climate warming is causing shifts in the timing of life history events for many species (Parmesan and Yohe, 2003; Root et al., 2003). Insect larvae are maturing into adults sooner, some bird species are laying eggs earlier in the season, and many plants are blooming earlier (Hughes, 2000; Parmesan and Yohe, 2003). In addition to advancing many phenological events, climate warming is altering the distributions of both plant and animal species. For example, treelines are gradually increasing in elevation, and butterfly ranges are shifting northward (Hughes, 2000).
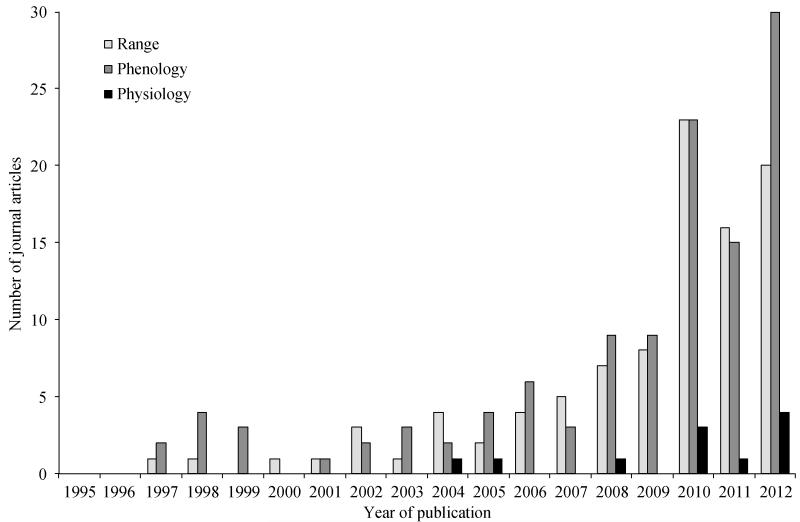
Whereas climate warming-induced shifts in species’ ranges and phenologies have received a great deal of recent study (e.g., Brooker et al., 2007; Cleland et al., 2012; Parmesan and Yohe, 2003; Pau et al., 2011), direct physiological effects of warming are less well-documented for some organisms or have yet to be mechanistically integrated with spatial and temporal shifts (Forrest and Miller-Rushing, 2010, but see, e.g., Kearney and Porter, 2009). This disparity is apparent in recent research on flowering plants and insect pollinators, where much work has been focused on shifts in flowering time and insect emergence and potential temporal mismatches between the two (e.g., Bartomeus et al., 2011; Forrest and Thomson, 2011; Hegland et al., 2009; Memmott et al., 2007; Rafferty and Ives, 2011). In contrast, relatively little research has addressed direct physiological effects (Fig. 1), yet these effects are likely to have important consequences for plant-pollinator interactions.
Fig. 1. Results of a search on the ISI Web of Science article database for journal articles published between 1995–2012 on climate change and pollination and either 1) ranges, 2) phenology, or 3) physiology.
To obtain a more inclusive sample, the topic search terms “range* and ‘climate change’ and pollinat*”, “phenolog* and ‘climate change’ and pollinat*”, and “physiolog* and ‘climate change’ and pollinat*” were used; thus, the results include articles on all aspects of climate change (not solely warming) and all taxonomic groups of pollinators (not solely insects).
Interactions among flowering plants and pollinators are ecologically important and economically valuable. Almost 88% of angiosperms rely on animals for pollination services (Ollerton et al., 2011), and the disruption of this interaction could cascade throughout ecological communities, affecting frugivores, seed dispersal, and plant recruitment (Kearns and Inouye, 1997). In economic terms, the ecosystem service that pollinators provide is worth an estimated $220 billion annually world-wide (Gallai et al., 2009). Some insect pollinators have declined globally, likely due to a suite of interacting factors (Potts et al., 2010), and parallel declines in insect-pollinated plants have been documented (Biesmeijer et al., 2006). Thus, understanding the physiological effects of climate warming on pollinators, their floral resources, and the mutualistic interactions between them is a pressing issue.
Elevated temperatures are known to affect the physiology of flowering plants in a number of ways, resulting in altered production of flowers, nectar, and pollen (e.g., Koti et al., 2005; Petanidou and Smets, 1996; Saavedra et al., 2003). With regard to insect pollinators, warming can influence foraging activity, body size at maturity, as well as individual life span (e.g., Bosch et al., 2000; Radmacher and Strohm, 2011; Willmer, 1983). The physiological impacts of climate warming may not have direct negative consequences for individual flowering plants or insect pollinators, and, in fact, some could even have direct positive effects. However, these physiological responses could, in turn, have opposing effects on the interactions between plants and pollinators. To date, there has been no synthesis of these physiological responses or their potential consequences for plant-pollinator interactions.
Here, we summarize what is currently known about the effects of elevated temperatures on the physiology of flowering plants and insect pollinators. Although other aspects of global climate change, such as elevated carbon dioxide levels and altered precipitation patterns, can also affect plant and insect physiology (e.g., Agrell et al., 2000; Erhardt and Rusterholz, 1997; Jablonski et al., 2002; Minckley et al., 2013; Reyer et al., 2013), either directly or interactively (e.g., Hoover et al., 2012), we restrict our synthesis to the effects of warming, in part because the relative wealth of studies makes possible the discussion of trends and mechanisms. Likewise, we focus on relatively immediate physiological responses to warming, though we recognize that evolutionary responses are likely (e.g., Gilman et al., 2012), as is interplay with behavioral responses. We explore first some ways in which plant physiological responses to warming might affect insect pollinators, followed by ways in which insect responses to warming might affect flowering plants, and finally ways in which these responses might affect plant-pollinator interaction networks. Our goal is both to clarify what we know about the physiological effects of warming on each mutualistic partner and to point to profitable directions for future research on this topic.
2 Physiological Effects on Flowering Plants and Potential Consequences for Pollinating Insects
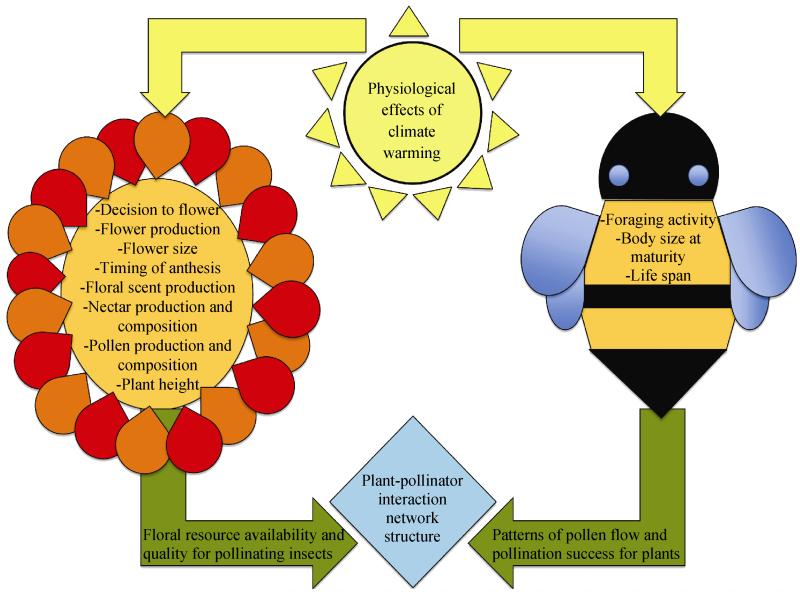
Increased temperatures can have a variety of effects on the physiology of flowering plants. Our focus here is on physiological effects that are likely to influence plant interactions with pollinating insects (Fig. 2). In addition to the many insect taxa that obtain nectar from flowers to fuel their flight and/or metabolic activity, many insects also rely on floral resources, especially pollen, to provision their eggs and developing offspring and, in some cases, to sustain themselves while overwintering (Kevan and Baker, 1983). Thus, the responses of flowering plants to warming are likely to affect floral visitors in a number of ways.
Fig. 2.
Framework showing the direct physiological responses (excluding those that result in phenological shifts) of flowering plants and insect pollinators to climate warming, which in turn can affect floral resources and pollination success for mutualists, and can shape plant-pollinator networks
2.1 Decision to flower and flower production
Elevated temperatures have been found to have varying effects on flower production. Some plants grown under higher temperatures may be less likely to flower or may produce fewer flowers. A study on Nuttall’s larkspur Delphinium nuttallianum found that plots undergoing experimental warming had fewer flowering plants than those in the control (unheated) plots. The same study also found that individual plants in heated plots on average had fewer flowers than plants in control plots (3.95 vs. 4.52 flowers/plant; Saavedra et al., 2003). Likewise, an experiment that simulated winter warming of plant species by 1.5 °C in the Tibetan plateau resulted in decreased flower production for several species (Liu et al., 2012). Lychee (Litchi chinensis) plants exposed to temperatures above 20 °C for 8 or more hours per day failed to flower (Menzel and Simpson, 1995). Conversely, studies performed on plants growing in arctic and alpine tundra found that after a few years of experimental warming, the plants’ flowering production increased (Arft et al., 1999). Mass flowering of several New Zealand species generally increased as a result of increasing temperatures, as well (Schauber et al., 2002). The mixed effects of warming on flower production suggest that particular species are stressed by higher temperatures while others are not. In many studies that involve experimental warming of plots, however, other variables such as soil moisture are affected by the treatment, making it difficult to isolate the effects of temperature. In general, species that rely on temperature cues to regulate flowering may be better able to respond to warmer conditions (Cleland et al., 2012; Willis et al., 2008), perhaps via a transcription factor that activates flowering at higher temperatures (Kumar et al., 2012), and those that are limited more by the availability of nitrogen than water may benefit from warming (de Valpine and Harte, 2001).
Whether species flower and how intensively they do so will shape floral resource availability for insect visitors, as well as the degree to which pollinators are attracted to those plants. Reductions in flower production under elevated temperatures would almost certainly mean reduced food availability, which could translate into reduced reproductive output (Boggs and Ross, 1993; Minckley et al., 1994) and population densities (Westphal et al., 2003) of insect pollinators. Alternatively, the pollinators of those plants that show increased flower production under warming could experience increased food availability and, if floral resources are limiting, population growth. At the community level, species-specific increases and decreases in flower production could balance out, such that there is no net change in floral resource abundance, at least for generalized pollinators. Irrespective of changes in absolute abundance, the diversity and quality of floral resources will likely change with the species composition of plants in flower, which could affect foraging distances (Jha and Kremen, 2013) and larval growth (Génissel et al., 2002).
2.2 Flower size and timing of anthesis
When plants do produce flowers under elevated temperatures, those flowers are likely to differ in several key traits that could affect how attractive, accessible, and profitable their rewards are for insect visitors. Together, the studies conducted to date point to a complex array of physiological effects of temperature on floral traits, the mechanisms for which likely depend in part on whether and at what developmental stages plants experience heat stress (Wahid et al., 2007). For instance, individual flower size can be affected by temperature. Experimentally warmed (23 °C) pumpkin plants Cucurbita maxima produced flowers of smaller diameter (Hoover et al., 2012). Corolla tube lengths of coastal morning glory Ipomoea trichocarpa flowers produced during a colder period were several mm shorter than flowers produced when temperatures were warmer. Temperature also dictated the timing of anthesis, with flowers opening 2-3 h earlier on warmer mornings (Murcia, 1990).
These temperature-induced changes in flower size and timing of anthesis could affect whether pollinators can obtain floral resources from a given species. In particular, floral dimensions are known to influence which pollinators are physically capable of accessing floral rewards, as documented by morphological relationships between the length of nectar spurs and proboscides (e.g., Nilsson, 1988). Even if rewards remain accessible, changes in floral dimensions could affect pollinator foraging efficiency, as flower size can, in part, determine how energetically costly it is to obtain those rewards (Harder, 1983). Likewise, if anthesis occurs earlier due to warmer morning temperatures, pollinator taxa active earlier may benefit from access to those rewards, but this could affect resource availability for pollinators active later in the day (Murcia, 1990). It is also worth noting that floral size can affect pollinator attraction (Totland, 2001).
2.3 Floral scent, nectar, and pollen production
The production of floral scent, nectar, and pollen can also be affected by temperature. Warmer temperatures might increase emissions and/or volatility of organic compounds produced by flowers (reviewed by Yuan et al., 2009), although some evidence suggests that endogenous floral scent production decreases with increasing temperature (Sagae et al., 2008). Nectar production, composition, and concentration have all been found to be influenced by temperature (reviewed by Pacini et al., 2003), though it appears that few studies have addressed these topics in the context of climate warming. Nectar volume and sugar concentration increased with temperature up to a point (38 °C) in a Mediterranean plant (Thymus capitatus; Petanidou and Smets, 1996), whereas temperature (23 °C vs. 19 °C) had a negative effect on the ratio of glucose to fructose in the nectar of pumpkin plants (Hoover et al., 2012). Alfalfa Medicago sativa plants subjected to fluctuating temperatures (18 to 32 °C) produced less nectar than those in a constant 25 °C temperature regime (57.1 vs. 68.4 μl/100 florets; Walker et al., 1974). Finally, temperature can also affect pollen performance and chemical composition (reviewed by Delph et al., 1997). The flowers of soybeans Glycine max grown under elevated temperatures (38 °C day, 30 °C night) produced 30–50% less pollen and pollen that was less likely to germinate (Koti et al., 2005). Similarly, peanut plants Arachis hypogaea exposed to high temperatures (up to 44 °C) produced less viable pollen (Prasad et al., 2003).
These modifications in floral odor and rewards could affect how likely insects are to visit certain flowers and the benefits they accrue. Altered floral scent emission or volatilization at higher temperatures could affect the detectability of flowers, particularly for pollinating insects, such as moths, that rely on long-distance cues to locate floral resources (Kevan and Baker, 1983; Yuan et al., 2009). Certainly, altered nectar production and composition could have both immediate effects on pollinator activity and energetics (Kudo and Harder, 2005) and longer-term consequences for pollinator fitness (Burkle and Irwin, 2009), perhaps especially for those insects, such as some lepidopterans and wasps, that rely on nectar for amino acids as well as for sugars (Kevan and Baker, 1983). Similarly, decreased pollen production is likely to affect the reproductive success of many bees, which may need to collect pollen from a large number of plants to successfully rear their offspring (Muller et al., 2006). While there has not been much conclusive research on whether less viable pollen is less appealing to pollinators, bumblebees favored potato Solanum tuberosum flowers with viable pollen grains as opposed to those with inviable or shrunken pollen grains, suggesting the former are more nutritious (Batra, 1993).
2.4 Plant height
Along with the effects of warming on floral traits, elevated temperatures can alter other plant characteristics in ways that could affect visitation by insect pollinators. For example, plant communities exposed to winter warming of 1.5 °C via open top chambers were several cm taller than communities in control chambers (Liu et al., 2012). A spring-flowering forest understorey plant (Anemone nemorosa) also displayed increased vegetative growth and height under warmer conditions (De Frenne et al., 2011). On the other hand, Silene noctiflora plants grown under elevated temperature (28 °C day, 24 °C night) were several cm shorter (Qaderi and Reid, 2008), as were Hypericum perforatum plants exposed to 3 °C winter warming (Fox et al., 1999), suggesting that the effects of temperature on plant height are species-specific and may depend on the availability of water and other resources.
Changes in the stature of plants could affect how likely insects are to encounter and visit flowers. Indeed, some pollinating insects are known to show height-specific foraging patterns (Levin and Kerster, 1973), and tall plants are generally expected to attract more pollinator visits (Aarssen, 1995). Therefore, reduced height could make it less likely that flowers will be detected by pollinators (Aspi et al., 2003; Donnelly et al., 1998), potentially affecting how much time and energy pollinators expend in locating these floral resources.
3 Physiological Effects on Pollinating Insects and Potential Consequences for Flowering Plants
Pollinators, too, are susceptible to many changes as a direct result of climate change. There has been relatively minimal research on the influence of warming temperatures on the physiology of many crucial pollinators. Because of this, we instead draw attention to what is known about the thermal ecology of insect pollinators, and how this may in turn be of significance for the pollination success of flowering plants (Fig. 2).
3.1 Foraging activity
At high temperatures, thermoregulatory limits dictate which pollinators can be active and when (reviewed by Willmer and Stone, 2004). Because body size is related to the ability to physiologically (vs. behaviorally) thermoregulate, pollinating insects of different sizes are likely to be affected differently by warming, with larger insects better able than smaller ones to regulate their temperatures (Bishop and Armbruster, 1999). However, larger-bodied insects may retain more heat and do not release this heat very quickly, thereby increasing the risk of overheating (Heinrich, 1993). Indeed, passive convective heat loss through the thorax was inversely correlated with overall body size of Asian honeybees (Apis spp.; Dyer and Seeley, 1987). Color and pile or fur thickness can also influence thermoregulatory ability in insects (Heinrich, 1974; Kingsolver and Watt, 1983; Willmer, 1983). The thermal limits of insect pollinators can directly translate into altered daily activity patterns and timing under elevated temperatures. For example, larger insects, including Bombus spp., tend to forage either earlier in the morning or later in the day, avoiding the hottest hours (Willmer, 1983), and honeybees A. mellifera in the Sonoran desert ceased foraging for pollen at temperatures above 40 °C (Cooper et al., 1985). However, with rising temperatures, insects incapable of physiological thermoregulation, such as Andrena bicolor, which is able to forage only on relatively warm, sunny days (Herrera, 1995), may reach minimum thoracic temperatures required for flight on a greater number of days, or perhaps earlier in the day.
If climate warming imposes new physiological constraints on the activity patterns of diurnal pollinating insects such that it alters the time of day at which they choose to visit flowers, then this will likely affect patterns of pollen flow, the likelihood of pollen receipt, and ultimately, pollination success. Plants with flowers that open later in the day, for example, could receive fewer visits if their pollinators restrict their visits to flowers earlier in the day, which would result in pollinator limitation and reduced fruit and seed set (Wilcock and Neiland, 2002). Because pollinators may have to restrict their foraging trips to shorter distances to avoid over heating during flight on very warm days, patterns of pollen flow might be altered. Shorter flight distances could result in plants receiving less outcross pollen from more-distant conspecifics (Herrera, 1987), affecting seed set and seedling survivorship (Price and Waser, 1979). Similarly, if daily temperature dictates the composition of active floral visitors based on intra- or inter-specific variation in body size (Herrera, 1997; Stone, 1994), plants could be more- or less-effectively pollinated (Sahli and Conner, 2007).
3.2 Body size at maturity
A common pattern among ectotherms, including insects, is that development at higher temperatures tends to produce adults that are smaller in size, possibly because development is accelerated (reviewed by King-solver and Huey, 2008). Several studies have determined that elevated temperatures (both constant and fluctuating) result in smaller weights of larvae or pupae in solitary bees, which would subsequently develop into smaller adults (e.g., Radmacher and Strohm, 2010; 2011). The size of tobacco hornworm Manduca sexta larvae also decreased with increasing temperatures of 20, 25, and 30 °C (Davidowitz et al., 2004), which would translate into smaller adult hawkmoths (Kingsolver et al., 2012).
Because pollinator effectiveness can vary with body size (e.g., Sahli and Conner, 2007), warming-induced changes in developmental physiology that lead to smaller adult pollinators could mean improved or reduced pollen delivery among plants and thus altered per-visit seed set, potentially shifting the costs and benefits of pollinator visits. Body size in bees has also been linked to for aging distance, with larger bees foraging over greater distances (Greenleaf et al., 2007). If the same relationship holds within species, then smaller pollinators might move pollen over smaller distances.
3.3 Life span
Warmer temperatures associated with climate change could also affect the life span of pollinating insects. For example, orange sulfur butterflies Colias eurytheme experiencing a 45 °C “warming” period in the middle of the “normal” temperature cycle of 32 to 20 °C had reduced life spans, with the average number of days males lived decreasing by nearly 40% (Kingsolver and Watt, 1983). Simulated longer summers and thus greater degree-day accumulations experienced by the solitary bee Osmia lignaria resulted in shorter life spans of bees that had overwintered (Sgolastra et al., 2011). Similarly, adult life span was reduced by up to several days in this species when exposed to sustained elevated pre-wintering temperatures (Bosch et al., 2000). Because these studies were all relatively short-term, it is possible that these responses are simply stress-related; additional data are needed to determine whether these species would exhibit shortened life spans under long-term warming.
For plants, reduced life span of certain pollinators essentially narrows the window of time in which pollen receipt and removal can be effected by those pollinators. Such an effect could be especially detrimental for plants that are both non-autogamous and rely on only a few species of pollinators during a brief flowering period. However, most plants have compensatory traits that can provide reproductive assurance in the absence of pollinator visitation and thus do not fall into this category (Bond, 1994). The consequences of a shortened period of pollinator availability could be similar to those of reduced phenological overlap, and individual plants that flower outside of the window of overlap with effective pollinators could have reduced reproductive output (Hegland et al., 2009; Rafferty and Ives, 2012).
4 Consequences for Plant-pollinator Networks
As the combined effects of warming on flowering plants and insect pollinators shape their pairwise interactions, they will also shape their overall interaction networks (Fig. 2). The structure and dynamics of plant-pollinator networks have received a great deal of study (e.g., Bascompte et al., 2003; Olesen et al., 2008), and some researchers have investigated how these networks might respond to perturbations, including climate change-induced phenological shifts (Memmott et al., 2007) and range shifts (Devoto et al., 2007). Recent empirical work makes it clear that, while buffered to some extent by generalization and the formation of new interactions, networks can be weakened over time by species loss and phenological mismatches (Burkle et al., 2013).
Physiological responses to warming may be capable of altering plant-pollinator networks even without changes in species composition or phenological overlap. The more subtle changes in interaction strength that could result from modified floral reward quality or reduced life span of pollinators, for example, could add up to significantly affect network structure and dynamics. Additionally, though we have discussed them separately, positive feedbacks between effects on pollinator and plant populations are probable, in that direct plant physiological responses that lead to reduced pollinator reproductive success could in turn lead to reduced pollination success. If, on the other hand, the responses of plants and pollinators are complementary, such that both flower size and body size are smaller or both anthesis and foraging occur earlier in the day, then there could be little net effect on interactions. Even if species’ responses are less directional and more variable, new interactions could be formed, buffering the overall network. In the end, however, physiological responses to climate warming could affect networks in many of the same ways that more obvious phenological shifts might, with some plant species visited by fewer pollinator species, and reduced diet breadths for some pollinators.
5 Conclusions
The maintenance of plant-pollinator interactions in the face of climate change is a complex and important conservation goal for the coming decades. Though researchers have made strides in documenting the physiological effects of warming for a number of plant and pollinator species, clearly there is much room for expansion in this field of research. As research on the consequences of climate change for plant and pollinator physiology moves forward, studies that more realistically incorporate the effects of warming should yield valuable insights. For example, simulations that account for thermal heterogeneity in landscapes and microclimatic variation at scales relevant for focal organisms are likely to more accurately predict the effects of climate warming (Sears et al., 2011). In addition, studies that integrate physiological, behavioral, and phenological responses and consider interactions among multiple drivers should advance our understanding of the overall effects of global change on plant-pollinator interactions (Hoover et al., 2012; Schweiger et al., 2010).
In some respects, studies at the network level automatically meet this objective, especially if networks are sampled over space and time. If, in fact, networks can be reshaped by physiological responses, even without the loss or gain of species or temporal overlap, then conserving species and their mutualists may not be enough to conserve their interactions (Kiers et al., 2010). To help answer this question, greater incorporation of species traits and abiotic factors in network studies would be particularly valuable. It is only by studying interactions that the individualistic physiological responses of each mutualistic partner can be put into a larger ecological context and the net effect on focal species can be understood.
Acknowledgements
We are grateful to three anonymous reviewers whose constructive comments greatly improved the manuscript. We thank G. Fitzpatrick for discussions that inspired some of the ideas presented here. NER was supported by Grant NIH K12 GM000708.
References
- Aarssen LW. Hypotheses for the evolution of apical dominance in plants: implications for the interpretation of over-compensation. Oikos. 1995;74:149–156. [Google Scholar]
- Agrell J, McDonald EP, Lindroth RL. Effects of CO2 and light on tree phytochemistry and insect performance. Oikos. 2000;88:259–272. [Google Scholar]
- Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, et al. Responses of tundra plants to experimental warming: Meta-analysis of the international tundra experiment. Ecol. Monogr. 1999;69:491–511. [Google Scholar]
- Aspi J, Jäkäläniemi A, Tuomi J, Siikamäki P. Multilevel phenotypic selection on morphological characters in a meta-population of Silene tatarica. Evolution. 2003;57:509–517. doi: 10.1111/j.0014-3820.2003.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, et al. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20645–20649. doi: 10.1073/pnas.1115559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J, Jordano P, Melián CJ, Olesen JM. The nested assembly of plant-animal mutualistic networks. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra SWT. Male-fertile potato flowers are selectively buzz-pollinated only by Bombus terricola Kirby in upstate New York. J. Kansas Entomol. Soc. 1993;66:252–254. [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, Ohlemuller R, Edwards M, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Bishop JA, Armbruster WS. Thermoregulatory abilities of Alaskan bees: effects of size, phylogeny and ecology. Funct. Ecol. 1999;13:711–724. [Google Scholar]
- Boggs CL, Ross CL. The effect of adult food limitation on life history traits in Speyeria mormonia (Lepidoptera: Nymphalidae) Ecology. 1993;74:433–441. [Google Scholar]
- Bond WJ. Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Phil. Trans. R. Soc. B. 1994;344:83–90. [Google Scholar]
- Bosch J, Kemp WP, Peterson SS. Management of Osmia lignaria (Hymenoptera: Megachilidae) populations for almond pollination: methods to advance bee emergence. Environ. Entomol. 2000;29:874–883. [Google Scholar]
- Brooker RW, Travis JMJ, Clark EJ, Dytham C. Modelling species’ range shifts in a changing climate: The impacts of biotic interactions, dispersal distance and the rate of climate change. J. Theor. Biol. 2007;245:59–65. doi: 10.1016/j.jtbi.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Burkle L, Irwin R. Nectar sugar limits larval growth of solitary bees (Hymenoptera: Megachilidae) Environ. Entomol. 2009;38:1293–1300. doi: 10.1603/022.038.0441. [DOI] [PubMed] [Google Scholar]
- Burkle LA, Marlin JC, Knight TM. Plant-pollinator interactions over 120 years: Loss of species, co-occurrences, and function. Science. doi: 10.1126/science.1232728. In press. (published online February 28, 2013) [DOI] [PubMed] [Google Scholar]
- Cleland EE, Allen JM, Crimmins TM, Dunne JA, Pau S, et al. Phenological tracking enables positive species responses to climate change. Ecology. 2012;93:1765–1771. doi: 10.1890/11-1912.1. [DOI] [PubMed] [Google Scholar]
- Cooper PD, Schaffer WM, Buchmann SL. Temperature regulation of honey bees Apis mellifera foraging in the Sonoran desert. J. Exp. Biol. 1985;114:1–15. [Google Scholar]
- Davidowitz G, D’Amico LJ, Nijhout HF. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Research. 2004;6:49–62. [Google Scholar]
- De Frenne P, Brunet J, Shevtsova A, Kolb A, Graae BJ, et al. Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Glob. Change Biol. 2011;17:3240–3253. [Google Scholar]
- Delph LF, Johannsson MH, Stephenson AG. How environmental factors affect pollen performance: Ecological and evolutionary perspectives. Ecology. 1997;78:1632–1639. [Google Scholar]
- Devoto M, Zimmermann M, Medan D. Robustness of plant-flower visitor webs to simulated climate change. Ecol. Austral. 2007;17:37–50. [Google Scholar]
- Donnelly SE, Lortie CJ, Aarssen LW. Pollination in Verbascum thapsus (Scrophulariaceae): The advantage of being tall. Am. J. Bot. 1998;85:1618–1625. [PubMed] [Google Scholar]
- Dyer FC, Seeley TD. Interspecific comparisons of endothermy in honey-bees (Apis): Deviations from the expected size-related patterns. J. Exp. Biol. 1987;127:1–26. [Google Scholar]
- Forrest J, Miller-Rushing AJ. Toward a synthetic understanding of the role of phenology in ecology and evolution. Phil. Trans. R. Soc. B. 2010;365:3101–3112. doi: 10.1098/rstb.2010.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest JRK, Thomson JD. An examination of synchrony between insect emergence and flowering in Rocky Mountain meadows. Ecol. Monogr. 2011;81:469–491. [Google Scholar]
- Fox LR, Ribeiro SP, Brown VK, Masters GJ, Clarke IP. Direct and indirect effects of climate change on St John’s wort Hypericum perforatum L. (Hypericaceae) Oecologia. 1999;120:113–122. doi: 10.1007/s004420050839. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Rusterholz H-P. Effects of elevated CO2 on flowering phenology and nectar production. Acta Oecologica. 1997;18:249–253. doi: 10.1007/s004420050385. [DOI] [PubMed] [Google Scholar]
- Gallai N, Salles J-M, Settele J, Vaissiere BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009;68:810–821. [Google Scholar]
- Génissel A, Aupinel P, Bressac C, Tasei J-N, Chevrier C. Influence of pollen origin on performance of Bombus terrestris micro-colonies. Entomol. Exp. Appl. 2002;104:329–336. [Google Scholar]
- Gilman RT, Fabina NS, Abbott KC, Rafferty NE. Evolution of plant-pollinator mutualisms in response to climate change. Evol. Appl. 2012;5:2–16. doi: 10.1111/j.1752-4571.2011.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf SS, Williams NM, Winfree R, Kremen C. Bee foraging ranges and their relationship to body size. Oecologia. 2007;153:589–596. doi: 10.1007/s00442-007-0752-9. [DOI] [PubMed] [Google Scholar]
- Harder LD. Flower handling efficiency of bumblebees: Morphological aspects of probing time. Oecologia. 1983;57:274–280. doi: 10.1007/BF00379591. [DOI] [PubMed] [Google Scholar]
- Hegland SJ, Nielsen A, Lazaro A, Bjerknes A-L, Totland Ø . How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009;12:184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- Heinrich B. The Hot-blooded Insects: Strategies and Mechanisms of Thermoregulation. Harvard Univ. Press; Cambridge, MA: 1993. [Google Scholar]
- Heinrich B. Thermoregulation in endothermic insects. Science. 1974;185:747–756. doi: 10.1126/science.185.4153.747. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Components of pollinator “quality”: Comparative analysis of a diverse insect assemblage. Oikos. 1987;50:79–90. [Google Scholar]
- Herrera CM. Floral biology, microclimate, and pollination by ectothermic bees in an early-blooming herb. Ecology. 1995;76:218–228. [Google Scholar]
- Herrera CM. Thermal biology and foraging responses of insect pollinators to the forest floor irradiance mosaic. Oikos. 1997;78:601–611. [Google Scholar]
- Hoover SER, Ladley JJ, Shchepetkina AA, Tisch M, Gieseg SP, et al. Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecol. Lett. 2012;15:227–234. doi: 10.1111/j.1461-0248.2011.01729.x. [DOI] [PubMed] [Google Scholar]
- Hughes L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000;15:56–61. doi: 10.1016/s0169-5347(99)01764-4. [DOI] [PubMed] [Google Scholar]
- Jablonski LM, Wang X, Curtis PS. Plant reproduction under elevated CO2 conditions: A meta-analysis of reports on 79 crop and wild species. New Phytol. 2002;156:9–26. [Google Scholar]
- Jha S, Kremen C. Resource diversity and landscape-level homogeneity drive native bee foraging. Proc. Natl. Acad. Sci. U.S.A. 2013;110:555–558. doi: 10.1073/pnas.1208682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Porter W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- Kearns CA, Inouye DW. Pollinators, flowering plants, and conservation biology. Bioscience. 1997;47:297–307. [Google Scholar]
- Kevan PG, Baker HG. Insects as flower visitors and pollinators. Annu. Rev. Entomol. 1983;28:407–453. [Google Scholar]
- Kiers ET, Palmer TM, Ives AR, Bruno JF, Bronstein JL. Mutualisms in a changing world: An evolutionary perspective. Ecol. Lett. 2010;13:1459–1474. doi: 10.1111/j.1461-0248.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Watt WB. Thermoregulatory strategies in Colias butterflies: Thermal stress and the limits to adaptation in temporally varying environments. Am. Nat. 1983;121:32–55. [Google Scholar]
- Kingsolver JG, Huey RB. Size, temperature, and fitness: Three rules. Evol. Ecol. Research. 2008;10:251–268. [Google Scholar]
- Kingsolver JG, Diamond SE, Seiter SA, Higgins JK. Direct and indirect phenotypic selection on developmental trajectories in Manduca sexta. Funct. Ecol. 2012;26:598–607. [Google Scholar]
- Koti S, Reddy KR, Reddy VR, Kakani VG, Zhao D. Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination, and tube lengths. J. Exp. Botany. 2005;56:725–736. doi: 10.1093/jxb/eri044. [DOI] [PubMed] [Google Scholar]
- Kudo G, Harder LD. Floral and inflorescence effects on variation in pollen removal and seed production among six legume species. Funct. Ecol. 2005;19:245–254. [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, et al. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature. 2012;484:242–245. doi: 10.1038/nature10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA, Kerster HW. Assortative pollination for stature in Lythrum salicaria. Evolution. 1973;27:144–152. doi: 10.1111/j.1558-5646.1973.tb05926.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Mu J, Niklas KJ, Li G, Sun S. Global warming reduces plant reproductive output for temperate multi-inflorescence species on the Tibetan plateau. New Phytol. 2012;195:427–436. doi: 10.1111/j.1469-8137.2012.04178.x. [DOI] [PubMed] [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Menzel CM, Simpson DR. Temperatures above 20 °C reduce flowering in lychee (Litchi chinensis Sonn.) J. Hortic. Sci. 1995;70:981–987. [Google Scholar]
- Minckley RL, Wcislo WT, Yanega D, Buchmann SL. Behavior and phenology of a specialist bee (Dieunomia) and sunflower (Helianthus) pollen availability. Ecology. 1994;75:1406–1419. [Google Scholar]
- Minckley RL, Roulston TH, Williams NM. Resource assurance predicts specialist and generalist bee activity in drought. Proc. R. Soc. B. 2013;280:20122703. doi: 10.1098/rspb.2012.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Diener S, Schnyder S, Stutz K, Sedivy C, et al. Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee-flower relationships. Biol. Conserv. 2006;130:604–615. [Google Scholar]
- Murcia C. Effect of floral morphology and temperature on pollen receipt and removal in Ipomoea trichocarpa. Ecology. 1990;71:1098–1109. [Google Scholar]
- Nilsson LA. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. [Google Scholar]
- Olesen JM, Bascompte J, Elberling H, Jordano P. Temporal dynamics in a pollination network. Ecology. 2008;89:1573–1582. doi: 10.1890/07-0451.1. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals. Oikos. 2011;120:321–326. [Google Scholar]
- Pacini E, Nepi M, Vesprini JL. Nectar biodiversity: A short review. Plant Syst. Evol. 2003;238:7–21. [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006;37:637–69. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Pau S, Wolkovich EM, Cook BI, Davies TJ, Kraft NJB, et al. Predicting phenology by integrating ecology, evolution and climate science. Glob. Change Biol. 2011;17:3633–3643. [Google Scholar]
- Petanidou T, Smets E. Does temperature stress induce nectar secretion in Mediterranean plants? New Phytol. 1996;133:513–518. [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Price MV, Waser NM. Pollen dispersal and optimal out-crossing in Delphinium nelson. Nature. 1979;277:294–297. [Google Scholar]
- Qaderi MM, Reid DM. Combined effects of temperature and carbon dioxide on plant growth and subsequent seed germinability of Silene noctiflora. Int. J. Plant Sci. 2008;169:1200–1209. [Google Scholar]
- Radmacher S, Strohm E. Factors affecting offspring body size in the solitary bee Osmia bicornis (Hymenoptera, Megachilidae) Apidologie. 2010;41:169–177. [Google Scholar]
- Radmacher S, Strohm E. Effects of constant and fluctuating temperatures on the the development of the solitary bee Osmia bicornis (Hymenoptera: Megachilidae) Apidologie. 2011;42:711–720. [Google Scholar]
- Rafferty NE, Ives AR. Effects of experimental shifts in flowering phenology on plant-pollinator interactions. Ecol. Lett. 2011;14:69–74. doi: 10.1111/j.1461-0248.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- Rafferty NE, Ives AR. Pollinator effectiveness varies with experimental shifts in flowering time. Ecology. 2012;93:803–814. doi: 10.1890/11-0967.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer CPO, Leuzinger S, Rammig A, Wolf A, Bartholomeus RP, et al. A plant’s perspective of extremes: Terrestrial plant responses to changing climatic variability. Glob. Change Biol. 2013;19:75–89. doi: 10.1111/gcb.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Saavedra F, Inouye DW, Price MV, Harte J. Changes in flowering and abundance of Delphinium nuttallianum (Ranunculaceae) in response to a subalpine climate warming experiment. Glob. Change Biol. 2003;9:885–894. [Google Scholar]
- Sagae M, Oyama-Okubo N, Ando T, Marchesi E, Nakayama M. Effect of temperature on the scent emission and endogenous volatile profile of Petunia axillaris. Biosci. Biotechnol. Biochem. 2008;72:110–115. doi: 10.1271/bbb.70490. [DOI] [PubMed] [Google Scholar]
- Sahli HF, Conner JK. Visitation, effectiveness, and efficiency of 15 genera of visitors to wild radish Raphanus raphanistrum (Brassicaceae) Am. J. Bot. 2007;94:203–209. doi: 10.3732/ajb.94.2.203. [DOI] [PubMed] [Google Scholar]
- Schauber EM, Kelly D, Turchin P, Simon C, Lee WG, et al. Masting by eighteen New Zealand plant species: The role of temperature as a synchronizing cue. Ecology. 2002;83:1214–1225. [Google Scholar]
- Schweiger O, Biesmeijer JC, Bommarco R, Hickler T, Hulme PE, et al. Multiple stressors on biotic interactions: How climate change and alien species interact to affect pollination. Biol. Rev. 2010;85:777–795. doi: 10.1111/j.1469-185X.2010.00125.x. [DOI] [PubMed] [Google Scholar]
- Sears MW, Raskin E, Angilletta MJ. The world is not flat: Defining relevant thermal landscapes in the context of climate change. Integr. Comp. Biol. 2011;51:666–675. doi: 10.1093/icb/icr111. [DOI] [PubMed] [Google Scholar]
- Sgolastra F, Kemp WP, Buckner JS, Pitts-Singer TL, Maini S, et al. The long summer: Pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. J. Insect Physiol. 2011;57:1651–1659. doi: 10.1016/j.jinsphys.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Stone GN. Activity patterns of females of the solitary bee Anthophora plumipes in relation to temperature, nectar supplies and body size. Ecol. Entomol. 1994;19:177–189. [Google Scholar]
- Totland O. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology. 2001;82:2233–2244. [Google Scholar]
- de Valpine P, Harte J. Plant responses to experimental warming in an montane meadow. Ecology. 2001;82:637–648. [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007;61:199–223. [Google Scholar]
- Walker AK, Barnes DK, Furgala B. Genetic and environmental effects on quantity and quality of alfalfa nectar. Crop Sci. 1974;14:235–238. [Google Scholar]
- Walther G-R, Post E, Convey P, Menzel A, Parmesan C, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Westphal C, Steffan-Dewenter I, Tscharntke T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003;6:961–965. [Google Scholar]
- Wilcock C, Neiland R. Pollination failure in plants: Why it happens and when it matters. Trends Plant Sci. 2002;7:270–277. doi: 10.1016/s1360-1385(02)02258-6. [DOI] [PubMed] [Google Scholar]
- Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Davis CC. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17029–17033. doi: 10.1073/pnas.0806446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer PG. Thermal constraints on activity patterns in nectar-feeding insects. Ecol. Entomol. 1983;8:455–469. [Google Scholar]
- Willmer PG, Stone GN. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv. Stud. Behav. 2004;34:347–466. [Google Scholar]
- Yuan JS, Himanen SJ, Holopainen JK, Chen F, Stewart CN. Smelling global climate change: Mitigation of function for plant volatile organic compounds. Trends Ecol. Evol. 2009;24:323–331. doi: 10.1016/j.tree.2009.01.012. [DOI] [PubMed] [Google Scholar]