Summary
Cholera is an acute, secretory diarrhea caused by infection with Vibrio cholerae of the O1 and O139 serogroups. Cholera is endemic in over 50 countries and also causes large epidemics. Since 1817, seven cholera pandemics have spread from Asia to much of the world. The 7th pandemic began in 1961 and affects 3–5 million people each year, killing 120,000. Although mild cholera may be indistinguishable from other diarrheal illnesses, the presentation of severe cholera is distinct, with dramatic diarrheal purging. Management of patients with cholera involves aggressive fluid replacement; effective therapy can decrease mortality from over 50% to less than 0.2%. Antibiotics decrease volume and duration of diarrhea by 50% and are recommended for patients with moderate to severe dehydration. Prevention of cholera depends on access to safe water and sanitation. Two oral cholera vaccines are available and the most effective use of these in integrated prevention programs is being actively evaluated.
Cholera is an acute secretory diarrhea caused by the Gram-negative bacterium Vibrio cholerae (1–4). Cholera epidemics have been recently increasing in intensity, duration and frequency, highlighting the need for more effective approaches to prevention and control.
History
Descriptions of a disease thought to be cholera are found in Sanskrit back to the 5th century BC, and the disease has existed on the Indian subcontinent for centuries. In 1817, cholera spread beyond the Indian subcontinent and there were six world-wide cholera pandemics between 1817 and 1923. Between 1849 and 1854, London physician John Snow proposed that cholera was a communicable disease and that stool contained infectious material. He suggested that this infectious material could contaminate drinking water supplies, resulting in transmission of cholera. Filippo Pacini, working independently in Italy in 1854, first observed comma-shaped forms under a microscope in cholera stools. In 1884, Robert Koch first isolated V. cholerae in pure culture in work that began in Egypt and continued in Calcutta (Kolkata), India.
The ongoing seventh cholera pandemic began in Indonesia in 1961 and spread through Asia to Africa, Europe, and Latin America. This pandemic is caused by a new biotype of V. cholerae first isolated in 1905 in El Tor, Egypt (3). Although cholera is vastly under-reported, the WHO estimates that there are 3–5 million cases per year (5), predominantly in Asia and Africa, with periodic outbreaks such as recently in Haiti (6). Diarrheal diseases including cholera are the second leading cause of mortality worldwide among children under 5 years of age, and a principal cause of morbidity (7). Cholera is also a major cause of severe dehydrating diarrhea in adults.
Etiologic agent
V. cholerae is a member of the Vibrionaceae family of curved, Gram-negative rods that are found in coastal waters and estuaries (1;3). These organisms grow best in the presence of salt, although V. cholerae can grow in water of lower salinity when it is warmer and contains sufficient organic nutrients (8). V. cholerae is often associated with zooplankton and shellfish in water (8), and is capable of utilizing chitin as a carbon and nitrogen source (9). Chitin induces natural competence in V. cholerae, suggesting that lateral gene transfer may occur in water, particularly during zooplankton blooms (10). In water, V. cholerae enter a viable but non culturable form (11), also called active but non-culturable or conditionally viable environmental cells (4;12).
V. cholerae is classified into more than 200 serogroups based on the O antigen of the lipopolysaccharide (1); of these, only O1 and O139 serogroups cause epidemic cholera. V. cholerae O1 is further classified into two biotypes, classical and El Tor (3). There are two major serotypes, Ogawa and Inaba, which vary in prevalence over time (13). In 1992, V. cholerae O139 was first recognized in south Asia as a cause of epidemic cholera (14;15). This organism is derived from V. cholerae O1 El Tor by lateral transfer of a genomic island substituting the O139 for the O1 antigen, but is otherwise virtually identical to V. cholerae O1 El Tor (16;17). Although classical V. cholerae O1 caused the fifth and sixth pandemics (and presumably the earlier pandemics), the seventh pandemic is due to the El Tor biotype, which has now replaced the classical biotype.
Although earlier isolates of V. cholerae O1 were susceptible to most antibiotics, V. cholerae O139, as well as more recent isolates of V. cholerae O1 El Tor, have acquired an SXT element that mediates resistance to sulfamethoxazole-trimethoprim and streptomycin (18); this element is found in almost all strains isolated over the past decade (19). More recently, strains of V. cholerae O1 resistant to tetracycline, erythromycin, and/or ciprofloxacin have been recovered in Asia (19;20); some of these strains have acquired additional resistance genes in the SXT element. These multiresistant strains have not yet been recognized in other locations.
Pathogenesis and pathophysiology
After ingestion of V. cholerae, the majority are killed by gastric acid. Surviving organisms colonize the small intestine and elaborate cholera toxin, the major virulence factor for pathogenic strains of V. cholerae (3). Cholera toxin is a protein exotoxin that consists of a single A subunit associated with five B subunits (21). The B subunit pentamer binds to the ganglioside GM1 on eukaryotic cells, and the A subunit is translocated intracellularly, where it acts enzymatically to activate adenylate cyclase and elevate intracellular cAMP; this leads to chloride secretion through the apical chloride channel and secretory diarrhea (22–24). The second major virulence factor of pathogenic strains of V. cholerae is the toxin-co-regulated pilus, a colonization factor whose expression is regulated in parallel to cholera toxin (25;26).
The genes for cholera toxin are encoded within the genome of a filamentous bacteriophage, CTXφ (27). Classical and El Tor strains have different versions of this bacteriophage, which can insert at one or two attachment sites in the genome depending on the biotype. The bacterial cell surface receptor for CTXφ is the toxin-co-regulated pilus (27), which is itself encoded within a genomic island, vibrio pathogenicity island (VPI-1) (28;29). Evolution of virulence in V. cholerae involves sequential acquisition of VPI-1 followed by CTXφ.
All seventh pandemic strains of V. cholerae O1 El Tor contain VPI-1, as well as a second vibrio pathogenicity island VPI-2, and two genomic islands specific to the seventh pandemic strains, vibrio seventh pandemic islands 1 and 2 (30). Recent seventh pandemic strains have been described that have the classical form of CTXφ instead of El Tor CTXφ, or a variant of the El Tor CTXφ encoding the B subunit of cholera toxin (CtxB) found in classical V. cholerae O1 strains (31). The variant El Tor strains have largely replaced the earlier El Tor strains and may be associated with more severe diarrhea.
Epidemiology
Cholera occurs in both endemic and epidemic patterns. Cholera is endemic in many areas of Asia and Africa. In Asia, cholera occurs seasonally before and after the monsoon rains (3), and the incidence of disease peaks in children younger than five years, and may occur in neonates (32;33). Cholera epidemics occur in a longer cycle superimposed on existing endemic disease. This pattern relates to declining levels of population-level immunity from a previous outbreak, overlaid on cycles of climate variability (34). In recent years, devastating epidemics of cholera have occurred in Angola, Ethiopia, Zimbabwe, Pakistan, Somalia, Sudan, Vietnam, and Haiti (35). Among immunologically naïve populations, cholera affects all age-groups, and epidemics can be associated with high case fatality rates (35). This pattern was observed in Haiti, where cholera had been notably absent prior to 2010. Population density, lack of sanitation and health infrastructure, and logistical obstacles to appropriate case management also contribute to a high case fatality rate in epidemic settings.
Environmental factors are important in the epidemiology of cholera. Changes in surface water temperature and terrestrial nutrient discharge lead to a proliferation of phytoplankton and zooplankton and a consequent increase in V. cholerae (8;36;37). Cholera rates also increase dramatically during floods compared to non-flood periods (38). Natural disasters that disrupt public health facilities, such as cyclones and earthquakes, also contribute to cholera epidemics.
The infectious dose of V. cholerae O1 has been estimated to be 105 −108 in human volunteers, but may be as low as 103 in the presence of achlorhydria (39). The incubation period ranges between 12 hours and 5 days (1;40).
Molecular epidemiology
The genome of a V. cholerae O1 El Tor strain was sequenced in 2000 (41); as with all vibrios, this organism has a large and a small circular chromosome (42). All Vibrionaceae have a super-integron in the small chromosome that acts as a gene capture system (43;44). A comparison of genomic sequences of patient and environmental strains isolated over nearly 100 years demonstrated 12 distinct lineages of V. cholerae O1; the classical and El Tor O1 biotypes comprised one lineage in this phylogeny (31). All strains of V. cholerae O1 El Tor shared a highly conserved core genome, with variations due mainly to laterally transferred genetic elements and single nucleotide variation.
An analysis suggested that the 7th pandemic strains originated from a single source in the Bay of Bengal that has spread to distant locations in three independent but overlapping waves (45). The first wave, which spread from Asia into Africa and South America, lacked the SXT element. The second wave acquired the SXT element and replaced the isolate in the first wave; the third wave also contains the SXT element. Isolates in the Haiti outbreak are closely related to south Asian strains in the third pandemic wave (46).
Transmission of cholera
Patients infected with V. cholerae O1 or O139 who are asymptomatic generally shed the organism for only a few days; however, patients who are symptomatic shed the organism between two days and two weeks, and rarely longer (4;40). Transmission of cholera within households has been documented (40). V. cholerae are present in human stool both as individual planktonic cells as well as in biofilm-like aggregates (47;48). In environmental water, organisms convert to conditionally viable environmental cells (12) within 24 hours (49). These organisms are infectious upon reintroduction into humans, although the infectious dose in this form is not known. Filtration of water through sari cloth reduces cholera transmission by nearly 50%, consistent with removal of organisms attached to zooplankton (50).
The peak of a cholera epidemic is often preceded by increasing prevalence of the pathogenic strain in the environment (12). Bacteriophage that are lytic for V. cholerae O1 or O139 are also found in the stools of patients and in environmental water (12;51). Bacteriophage density increases as an outbreak proceeds, and these bacteriophages may modulate the severity and duration of an outbreak (12;51;52).
As V. cholerae leave the human, the organisms have a phenotype referred to as hyperinfectivity --that is, the infectious dose is 10 to 100 times lower compared to non-humanshed organisms (53). Hyperinfectivity of recently shed organisms persists in water for 5 to 24 hours, suggesting that organisms transmitted from person-to-person may be more infectious than those that have acclimated to the environment. When hyperinfectivity is incorporated into a mathematical model of a cholera outbreak, the characteristically explosive nature of a cholera outbreak is better reproduced than if hyperinfectivity is not included (54). Other key components of cholera transmission models (4;52;55;56) include the concentration of V. cholerae O1 or O139 in stool, the difference of infectivity between planktonic cells and stool aggregates, the rapidity of spread of the organism from human-to-human, the presence of lytic bacteriophage in stool and water, and the concentration in water of the conditionally viable environmental cells for environment-to-human transmission.
Host susceptibility
Concomitant infection with enteropathogenic bacteria or parasites exerts an immunomodulatory effect on V. cholerae-specific immunity (57;58), and a number of host factors contribute to susceptibility to cholera. In particular, retinol deficiency is associated with a higher risk of V. cholerae infection and with a higher risk of symptomatic disease (59;60). Blood group O has been associated with severe cholera in different populations (61–63). The prevalence of blood group O is lower in south Asia compared to other regions, perhaps related to evolutionary pressure from cholera (64). A family-based study from Bangladesh demonstrated that first-degree relatives of a cholera patient had greater odds of being infected compared to less closely related contacts in the same household independent of blood group (59), suggesting that additional genetic factors play a role in susceptibility. A variant in the promoter region of LPLUNC1, a component of the innate immune system, was associated with cholera in a candidate gene study (65;66). Additional studies of host genetic factors related to cholera may provide further insights into the interaction between V. cholerae and the host.
Diagnosis
According to the WHO (67), a case of cholera should be suspected when (1) a patient aged 5 years or more develops severe dehydration or dies from acute watery diarrhea, even in an area where cholera is not known to be present, or (2) a patient aged 2 years or more develops acute watery diarrhea in an area known to have cholera. Where microbiology facilities are available, V. cholerae infection can be confirmed by isolation of the organism from stool on selective media, followed by biochemical tests, as well as serogrouping and serotyping with specific antibodies (68). Enrichment of stool in alkaline peptone water can increase the sensitivity of culture (69). Cholera can be rapidly diagnosed by examining fresh human stool under 400 × darkfield microscopy for vibrio-shaped cells with darting motility that is abrogated with specific antibody (70); approximately half of culture-positive stools are positive on darkfield microscopy (47).
Immunoassays that detect cholera toxin (71;72) or V. cholerae O1 and O139 lipopolysaccharide (73–75) directly in stool have also been developed. Such assays can be used in settings with limited laboratory capacity and facilitate early detection of cases during an outbreak. One such commercially available dipstick for both O1 and O139-associated cholera has a 97% sensitivity and 71–76% specificity compared with PCR under field conditions (76). Dipstick assays may be more sensitive for detecting V. cholerae in patients previously treated with antibiotics.
Clinical manifestations
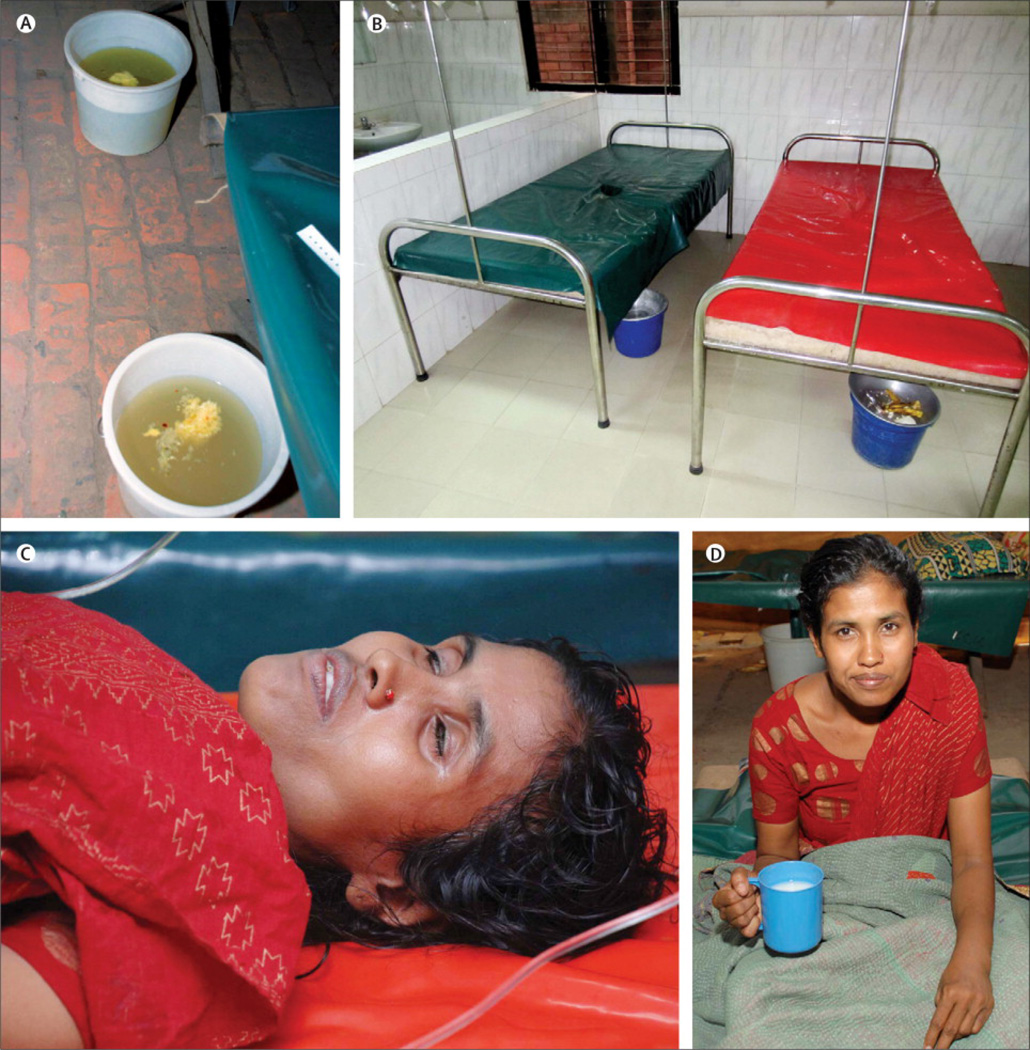
Few diseases give a clinical presentation as arresting as that of cholera. Massive watery diarrhea, up to 1 liter per hour, can lead to hypotensive shock and death within hours of the first symptom (“cholera gravis”). Death rates in untreated patients with severe cholera can exceed 70% (77). Although the stools of cholera patients may contain fecal matter or bile in the early phases, the characteristic “rice-water” stool of cholera develops with ongoing purging (Figure 1); this term refers to the similarity of the stool to water in which rice has been washed. Vomiting is a common feature, particularly early in illness. The diarrhea of cholera is typically painless and not accompanied by tenesmus; some patients may experience abdominal discomfort or cramping due to fluid distention of the bowel. Fever is rare and should raise suspicion for secondary infection.
Figure 1.
(A). “Rice water” stool in a patient with cholera. (B). Cholera cot used in management of patients with cholera to monitor ongoing volume losses in stool. (C). Patient with cholera before rehydration. (D). Patient with cholera eight hours after starting rehydration therapy. (A, C and D) reproduced from PLos Neglected Tropical Diseases (133) with permission.
Dehydration and electrolyte abnormalities are the most important complications of cholera. Patients may be lethargic, and may have sunken eyes (Figure 1), dry mouth, cold clammy skin, decreased skin turgor, or wrinkled hands and feet. Kussmaul breathing may occur due to acidosis from stool bicarbonate losses and lactic acidosis associated with poor perfusion (78). The peripheral pulse is rapid and thready, and may become difficult to palpate as blood pressure drops; urine output decreases with time (79;80). Muscle cramping and weakness due to electrolyte losses and ion shifts (particularly potassium and calcium) are common. In children, depletion of glycogen stores and inadequate gluconeogenesis can lead to severe hypoglycemia, manifest by altered consciousness, seizures or even coma (81;82). “Cholera sicca” is an unusual form of the disease in which fluid accumulates in the intestinal lumen, and circulatory collapse and even death, can occur before the passage of the first loose stool (83).
The presentation of cholera differs between endemic and epidemic settings. In endemic settings, rates of asymptomatic V. cholerae infection range from 40 to 80% (4), and cholera may present as mild diarrhea indistinguishable from infection with other enteropathogens. The most severe cases of cholera in endemic settings are concentrated among young children and previously un-exposed individuals. In epidemic cholera in a previously un-exposed population, severe disease occurs in adults as frequently as in children and is associated with high case fatality rates (84). Laboratory studies are not required to care for the vast majority of patients with cholera although they may be useful in patients with ileus, confusion, coma, or seizures, or in those with no urine output in response to fluid replacement. Laboratory abnormalities include alterations in serum electrolytes (hypokalemia, hyponatremia, hypocalcemia), renal dysfunction, the effects of hemoconcentration, and in a small percentage of children, hypoglycemia. The clinical features of cholera due to V. cholerae O1 and O139 are similar (85;86). Complications from severe hypotension can include stroke (especially in elderly patients), renal compromise, and vomiting can lead to aspiration pneumonia (87), but cholera itself is an acute infection with no chronic manifestations.
Management
Rehydration is the cornerstone of management of patients with cholera. Early attempts at oral rehydration met with limited success because the physiologic requirements for sodium-glucose co-transport were not recognized. The introduction of oral rehydration solution (ORS) in the late 1960s, utilizing equimolar concentrations of sodium and glucose to maximize sodium uptake in the small intestine, and carefully replacing preceding and ongoing fluid losses, ushered in current cholera treatment (83;88).
Employing the current standard of care, the mortality of severe cholera can be reduced to less than 0.2%, even in resource-limited settings (3). However, there are obstacles to administering optimal rehydration, and mortality rates may still exceed 10% early in cholera epidemics before appropriate resources are available (89;90). In the epidemic in Haiti, the median time between onset of symptoms and death within the community was 12 hours (91). Decentralized treatment centers (such as oral rehydration points) improve access to therapy, reduce time to initial rehydration, and may be critical in managing outbreaks.
The approach to rehydration during severe cholera differs markedly from the approach to patients with gastroenteritis in developed countries because:
Patients with severe cholera present with a greater degree of initial dehydration.
Patients with severe cholera suffer more rapid ongoing losses once they come to medical attention.
Patients with severe cholera have proportionally greater electrolyte losses than seen in non-cholera gastroenteritis (Table 1).
Table 1.
Comparison of the composition of cholera stools and acceptable therapeutic fluids for cholera (67;134); (www.cotsprogram.com).
| Na+ | K+ | Cl- | HCO3- | Carbohydrate | Commentary | ||
|---|---|---|---|---|---|---|---|
| millimoles/liter | |||||||
| Electrolyte losses in stools* | Cholera stool, adult | 130 | 20 | 100 | 45 | -- | The mean maximal rate of purging in severe cholera exceeds 10 ml/kg/hour. Sodium losses in cholera stools exceed those seen in other causes of diarrheal illness. |
| Cholera stool, child | 100 | 30 | 90 | 30 | -- | ||
| Non-cholera stool child (ETEC) | 50 | 35 | 25 | 20 | -- | ||
| Intravenous Therapy | Lactated Ringer’s Solution | 130 | 4 | 109 | 28 | -- (278 if D5LR# available) | Lactated Ringer’s (LR) solution is usually readily available and preferred over normal saline because it contains potassium and bicarbonate. The optimal infusion, for cholera, such as ‘Dhaka solution’ contains more potassium and bicarbonate than LR and also contains dextrose; addressing complications of severe cholera including hypokaelemia hypoglycemia, and metabolic acidosis |
| Normal Saline | 154 | 0 | 154 | 0 | -- | ||
| Cholera Saline (Dhaka solution) | 133 | 13 | 154 | 48 | 140 | ||
| Oral Rehydration Therapy | ORS (WHO 2002)** | 75 | 20 | 65 | 10 (citrate) | 75 (glucose) | WHO ORS utilizes glucose as a carbohydrate source Rice based ORS formulation have been found in randomized trials to reduce the duration of diarrhea and stool losses in severe cholera (93) |
| Rice based ORS (eg. CeraORS 75®) | 75 | 20 | 65 | 10 (citrate) | 27 grams rice syrup solids | ||
| Home made ORS (135) | ~75 | 0 | ~75 | 0 | ~75 | ||
|
A home made preparation of ORS could be used in an emergency situation. | ||||||
Compositions are estimates of the mean electrolyte composition of stools and are shown to demonstrate the significant difference in the pathophysiology of pediatric and adult cholera and non-cholera childhood gastroenteritis.
Dextrose 5%-Lactated Ringer’s
In 2002, the WHO replaced its previous formulation of ORS with the current lower osmolarity formulation. Subclinical hyponatremia is common among cholera patients using the current WHO formulation of ORS (136;137), but the rates of symptomatic hyponatremia in cholera patients do not appear to be significantly increased (138).
For these reasons, the most common error in caring for cholera patients is to underestimate the speed and volume of fluids required. Patients with severe cholera typically require an average of 200 mL/kg of isotonic oral or intravenous fluids in the first 24 hours of therapy, and may require more than 350 mL/kg (67;92). Estimating and replacing ongoing losses, even after correcting the initial fluid deficit, is critical. The rate of ongoing fluid loss may exceed 20 mL/kg/hour; cholera cots are inexpensive and useful for estimating ongoing volume losses (Figure 1). In the absence of cholera cots, ongoing losses can be estimated as 10 to 20 mL/kg of body weight for each diarrheal stool or episode of vomiting.
In severe cholera, the initial fluid deficit should be replaced within 3–4 hours of presentation. The route of administration of fluids depends on the severity of dehydration (Table 2). Patients with severe (≥10%) dehydration are in hypovolemic shock and require immediate intravenous rehydration administered as rapidly as possible until circulation is restored. Oral rehydration should begin as soon as patients are capable of drinking (typically 3–4 hours), because more potassium, bicarbonate, and glucose are available in ORS than in standard intravenous fluids. In patients with some dehydration, the initial deficit should also be replaced rapidly, with ORS whenever possible, and patients should be monitored until signs of dehydration have resolved. Patients with some dehydration but with profound vomiting or ongoing stool losses, may rapidly progress to severe dehydration if only ORS is provided, and should receive concomitant intravenous and oral rehydration. In patients without dehydration, management consists of oral fluids to replace ongoing losses. WHO ORS utilizes glucose as a carbohydrate source. Rice-based ORS formulations, if available, have been found in randomized trials to reduce the duration of diarrhea and stool losses in severe cholera (93). Home made ORS can be used in an emergency situation (Table 1). In patients with symptomatic hypoglycemia, 0.25–0.5 g/kg of intravenous glucose can be administered and correction of the hypoglycemia monitored until fluid repletion and the ability to take ORS has occurred (82).
Table 2.
Approach to rehydration in the patient with suspected cholera (67)
| Degree of Dehydration | ||||
|---|---|---|---|---|
| None (< 5%) | Some (5–10%) | Severe (>10%) | ||
|
Clinical assessment for dehydration dehydration |
General appearance Eyes Thirst |
Well, alert Normal Drinks normally |
Restless, irritable Sunken Thirsty, drinks eagerly |
Lethargic or unconscious Sunken Drinks poorly or unable to drink |
|
Approach to rehydration# |
Skin turgor Pulse Requirement for fluid replacement |
Instantaneous Recoil Normal Ongoing losses only |
Non-instantaneous recoil Rapid, low volume 75 mL/kg in addition to ongoing losses |
Very slow recoil (>2 seconds) Weak or absent >100 mL/kg in addition to ongoing losses |
|
Preferred route of administration Timing |
Oral* Usually guided by thirst |
Oral or Intravenous Replace fluids over 3–4 hours |
Intravenous As rapidly as possible until circulation is restored, complete the remainder of fluids within 3 hours |
|
| Monitoring | Observe until it is determined that ongoing losses can be adequately replaced by ORS |
Observe every 1–2 hours until all signs of dehydration resolve and patient urinates |
Once circulation is established monitor every1–2 hours. |
Patients with co-morbid conditions including severe malnutrition, significant complications, infants and elderly patients may require adjustments from this standard which are detailed in the references.
If losses are in excess of 10 ml/kg/hour per hour, it may not be possible to successfully employ oral therapy initially. An excellent resource is the Cholera Outbreak Training and Shigellosis (COTS) Program (www.cotsprogram.com) that provides free online information regarding the management of patients with cholera, based on WHO standards.
Antibiotics are adjunctive therapy in patients with moderate to severe dehydration from cholera (39). As in other infections, use of antibiotics in cholera may contribute to increasing antimicrobial resistance. However, effective antibiotics shorten the duration of diarrhea and reduce the volume of stool losses by up to 50%; they also reduce the duration of shedding of viable organisms in stool from several days to 1–2 days (77;94). Antibiotics can be administered once the initial fluid deficit is corrected and vomiting has resolved, ideally within 4 hours of initiating therapy. Antibiotic therapy should be based on prevailing local resistance patterns (Table 3).
Table 3.
Antibiotics for cholera.
| Class | Antibiotic | Pediatric Dose* | Adult Dose | Comment(s) |
|---|---|---|---|---|
| Tetracyclines | Tetracycline | 12.5 mg/kg/dose QID × 3 days |
500 mg QID × 3 days |
Antibiotic resistance to all tetracyclines is common (139) Empiric use is most appropriate in outbreaks caused by documented susceptible isolates. Tetracyclines are not recommended for pregnant women or children less than 8 years because of risk of irreversible discoloration of permanent teeth. |
| Doxycycline | 4–6 mg/kg × single dose |
300 mg × single dose |
||
| Fluoroquinolones | Ciprofloxacin | 15 mg/kg/dose BID × 3 days |
500 mg BID × 3 days |
In highly susceptible strains, single dose ciprofloxacin compares favorably against erythromycin (140) and doxycycline (141) in randomized trials. However, reduced susceptibility to fluoroquinolones has become common in endemic areas, and is associated with treatment failure (142;143). |
| Macrolides | Erythromycin | 12.5 mg/kg/dose QID × 3 days |
250 mg QID × 3 days |
Single dose azithromycin is the preferred therapy in children and has been shown to be more effective than ciprofloxacin in randomized trials in regions where reduced susceptibility to flouroquinolones are common (142;144). There are rare reports of macrolide resistance. |
| Azithromycin | 20 mg/kg × single dose |
1 gram × single dose |
Pediatric doses, based on weight, should not exceed maximum adult dose
QID: four times a day
BID, twice a day
Nutritional interventions include the resumption of a high energy diet immediately after the initial fluid deficit is corrected to prevent malnutrition as well as immediate complications including hypokalemia and hypoglycemia. For infants, breastfeeding should be encouraged in concert with ORS. In a randomized trial, zinc supplementation reduced the duration of diarrhea and volume of stool in children with cholera (95). Zinc supplementation after childhood diarrhea also reduced the incidence of subsequent episodes of diarrhea for several months (96;97); the WHO recommends zinc for children under 5 years of age with diarrhea (10 mg/day for children under 6 months and 20 mg/day for 10 days for children 6 months to 5 years) (67). Children with diarrhea in developing countries also benefit from supplementation with vitamin A (98). Antimotility agents and anti-emetics have no established benefit for treatment of cholera, and may prolong infection or have sedating effects that interfere with effective oral rehydration (67;99).
In an outbreak, clinicians and public health officials often need to manage many patients at the same time. Critical response features include establishing cholera treatment centers; training staff in case recognition and management; and providing safe water and sanitation. Depending on the local situation, radio ads, cell phone messaging, messages on billboards, community volunteers and other means may be important ways to educate the public on seeking medical care, oral rehydration use, sanitation, and other ways to prevent or minimize transmission. Other important components of the public health response include disinfectants, proper disposal of waste and cadavers, and coordinating the response with community, regional, national and international health authorities. An excellent resource that can assist in managing such features is available online at www.cotsprogram.com. Some countries are reluctant to declare a cholera epidemic because of concern over creating panic and the implications for tourism and exports; however, rapid reporting and a coordinated public health response should be encouraged to minimize the extent of the outbreak and prevent further spread.
Prevention of cholera
The response to the cholera pandemics of the 19th century led to the development of systems to provide safe water and adequate sanitation, but 1 billion people still lack access to safe water and remain at risk of cholera (100). Continued progress in providing safe water and adequate sanitation is a Millenium Development Goal but may take decades to achieve (101). During a cholera outbreak, the major response should focus on case detection, rehydration-based treatment and provision of safe water, in conjunction with adequate sanitation, hand-washing, and safe food preparation (102). These goals have been used for decades in areas that remain at risk for cholera, without reducing the ongoing impact of this disease, suggesting that consideration of additional control strategies, such as vaccination, is warranted (101;103;104).
Although safe and effective cholera vaccines exist, cholera vaccination is not yet part of cholera control programs outside of Vietnam; discussions are in progress regarding potential use in Haiti. The reasons for this are logistical, financial, and historical. Current cholera vaccines are given orally, have an excellent safety profile, and target induction of mucosal immunity. There are two oral killed vaccines that are licensed and commercially available. Dukoral (WCrBS, Crucell, Sweden) contains multiple biotypes/serotypes of V. cholerae O1 supplemented with 1 mg/dose of recombinant cholera toxin B subunit. Shanchol (Shantha Biotechnics-Sanofi Pasteur, India) and mORCVAX (VaBiotech, Vietnam) contain multiple biotypes/serotypes of V. cholerae O1 as well as V. cholerae O139 without supplemental cholera toxin B subunit. Shanchol is the bivalent vaccine internationally available (5).
Oral killed cholera vaccines have been administered to millions of recipients and are safe and immunogenic. The vaccines are administered as two or three doses depending on age and vaccine (Table 4). Overall, the vaccines provide 60–85% protective efficacy for 2–3 years, although protection among younger children is of shorter duration (105–115). Dukoral has been safely administered to individuals with HIV (111).
Table 4.
Internationally available oral killed cholera vaccines
| Vaccine | Doses* | Dosing interval* |
Dosing volume* |
Boosters* | Protective efficacy |
Comments | References |
|---|---|---|---|---|---|---|---|
| Dukoral** (Crucell) |
|
(106– 111;145;146) | |||||
| Children 2 – <6 years of age | 3 | 14 days (7– 42 permissible) | 3 ml Vaccine and 75 ml buffer | Every 6 months | 60–85% Protective Efficacy within 6 months of vaccination, | ||
| >6 years of age | 2 | 14 days (7– 42 permissible) | 3 ml Vaccine and 150 ml buffer | Every 2 years | decreasing to baseline over 24–36 months | ||
| Shanchol (Shantha Biotechnics-Sanofi Pasteur) | (105;112– 115;147; 148). | ||||||
| 60–70% Protective efficacy over 24–36 |
|
||||||
| >1 year of age | 2 | 14 days (window probably same as Dukoral) | 1.5ml | Every 2 years | months | ||
Per manufacturer
Listed field studies have involved both the current preparation of WC-rBS vaccine, supplemented with recombinant cholera toxin B subunit (rBS), and a previously available preparation of WC-BS containing non-recombinant B subunit (BS).
Re-analysis of original studies of oral cholera vaccine in Bangladesh in the 1980s disclosed a measurable herd effect (116), and modeling suggests that vaccinating 50% of a population could result in a greater than 90% reduction in cholera incidence in that population overall (117). A cost effectiveness model suggested that oral cholera vaccine could be cost effective in areas endemic for cholera (118).
A number of live attenuated oral cholera vaccines have also been developed, including CVD 103-HgR, Peru-15, and others (119). These genetically modified vaccine strains have in common the inability to express cholera toxin. These vaccines have been shown to be safe and immunogenic in volunteer studies (119–124); however, CVD 103-HgR failed to show protective efficacy when evaluated in a field study (125). Peru-15 has been shown to be safe and immunogenic in different age groups in Bangladesh (126), but has not yet been evaluated in field studies. A number of other cholera vaccines are in various stages of development, including subunit vaccines, live attenuated vaccines, and conjugate vaccines (127;128).
The WHO has endorsed the inclusion of oral vaccine in cholera control programs in endemic areas in conjunction with other preventive and control strategies (5). The WHO also recommends that oral vaccine be considered as part of an integrated control program in areas at risk for outbreaks (5). The use of vaccine in reactive situations (i.e. after an outbreak has occurred) is currently less certain. A case-control study in Vietnam suggested that such use could be of benefit (129), and modeling further supports such potential use (55;56;130–132). At present, the WHO suggests that oral cholera vaccine be considered as part of an integrated program in reactive situations in both epidemic and endemic settings in conjunction with provision of safe water, adequate sanitation, case detection and rehydration strategies, but that collection of additional data to support vaccination in such settings is warranted (5).
Areas of uncertainty in cholera
Cholera has had an immense impact on human history. Unfortunately, current control strategies have not proven highly effective in areas of the world bearing the global burden of cholera (101). Many areas of uncertainty remain. Will a new serogroup of V. cholerae arise, as O139 did? Why are altered variants of V. cholerae O1 El Tor developing? Will severe weather events such as regional flooding associated with global warming result in increased cholera? What role would surveillance, screening, vaccination or empiric treatment have in limiting the spread of cholera into immunological naïve populations? Would short course targeted chemotherapy with highly active antimicrobials among close community contacts of cholera patients limit transmission, or only lead to drug resistance? How can safe water and improved sanitation be attained in the many parts of the world lacking these? What are the obstacles to incorporation of current cholera vaccines into immunization programs in cholera endemic countries, and how can these be overcome? Who will support and pay for the manufacture, distribution and use of cholera vaccines? Will a vaccine stockpile be developed? And, if so, who will maintain, monitor and activate its use? Will the development of more effective or longer acting cholera vaccines simplify a number of these decisions?
We do not yet know the answers to these important questions, but the way forward will require scientific, medical, public health, environmental, financial, and political cooperation and action. As it has in the past, cholera remains largely a disease of impoverishment, social unrest and displacement, and continues to be a disease of major public health significance.
Search strategy
A search of Medline and Cochrane Library databases was performed using the terms “cholera”, “Vibrio cholerae”, and “randomized controlled trials” for the period January 1, 1966 through September 30, 2011 and in all languages.
Acknowledgments
This work was supported by the icddr,b and grants from the National Institutes of Health, AI058935 (SBC, ETR, FQ), U01 AI077883 (ETR, FQ) and an International Research Scientist Development Award, K01 TW07409 (JBH), a Physician-Scientist Early Career Award from the Howard Hughes Medical Institute (RCL), and a Charles H. Hood Foundation Child Health Research Award (JBH).
Conflict of interest statement. SBC, ETR, RCL, FQ and JBH report receiving support from grants from the National Institutes of Health on immunity to cholera. FQ reports receiving grants from the Gates Foundation and the Swedish International Development Agency (Sida) on vaccines and immunology in cholera. JBH reports receiving a grant from the Charles H. Hood Foundation on cholera immunology, and RCL reports receiving a grant from the Howard Hughes Medical Institute on human genetic susceptibility to cholera. SBC, ETR, and FQ are co-inventors on institutional patents related to cholera vaccine development. SBC and ETR are editors for the infectious diseases sections of UpToDate, a medical education program that includes topics related to cholera, and receive editorial payments from the publisher.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Morris JG., Jr. Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin Infect Dis. 2003 Jul 15;37(2):272–280. doi: 10.1086/375600. [DOI] [PubMed] [Google Scholar]
- 2.Greenough WB., III. The human, societal, and scientific legacy of cholera. J Clin Invest. 2004 Feb;113(3):334–339. doi: 10.1172/JCI20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004 Jan 17;363(9404):223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 4.Nelson EJ, Harris JB, Morris JG, Jr., Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009 Oct;7(10):693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholera vaccines: WHO position paper. Weekly Epidemiological Record. 2010;85(13):117–128. [PubMed] [Google Scholar]
- 6.Enserink MInfectious diseases. Haiti’s outbreak is latest in cholera’s new global assault. Science. 2010 Nov 5;330(6005):738–739. doi: 10.1126/science.330.6005.738. [DOI] [PubMed] [Google Scholar]
- 7.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005 Mar 26;365(9465):1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 8.Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996 Dec 20;274(5295):2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 9.Kirn TJ, Jude BA, Taylor RK. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature. 2005 Dec 8;438(7069):863–866. doi: 10.1038/nature04249. [DOI] [PubMed] [Google Scholar]
- 10.Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. Chitin induces natural competence in Vibrio cholerae . Science. 2005 Dec 16;310(5755):1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 11.Roszak DB, Colwell RR. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faruque SM, Islam MJ, Ahmad QS, Faruque AS, Sack DA, Nair GB, et al. Self-limiting nature of seasonal cholera epidemics: Role of host-mediated amplification of phage. Proc Natl Acad Sci U S A. 2005 Apr 26;102(17):6119–6124. doi: 10.1073/pnas.0502069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longini IM, Jr., Yunus M, Zaman K, Siddique AK, Sack RB, Nizam A. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J Infect Dis. 2002 Jul 15;186(2):246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- 14.Ramamurthy T, Garg S, Sharma R, Bhattacharya SK, Nair GB, Shimada T, et al. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993 Mar 13;341(8846):703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 15.Albert MJ, Siddique AK, Islam MS, Faruque AS, Ansaruzzaman M, Faruque SM, et al. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993 Mar 13;341(8846):704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 16.Comstock LE, Maneval D, Jr., Panigrahi P, Joseph A, Levine MM, Kaper JB, et al. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect Immun. 1995 Jan;63(1):317–323. doi: 10.1128/iai.63.1.317-323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bik EM, Bunschoten AE, Gouw RD, Mooi FR. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995 Jan 16;14(2):209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldor MK, Tschape H, Mekalanos JJ. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol. 1996 Jul;178(14):4157–4165. doi: 10.1128/jb.178.14.4157-4165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faruque SM, Islam MJ, Ahmad QS, Biswas K, Faruque AS, Nair GB, et al. An improved technique for isolation of environmental Vibrio cholerae with epidemic potential: monitoring the emergence of a multiple-antibiotic-resistant epidemic strain in Bangladesh. J Infect Dis. 2006 Apr 1;193(7):1029–1036. doi: 10.1086/500953. [DOI] [PubMed] [Google Scholar]
- 20.Kim HB, Wang M, Ahmed S, Park CH, Larocque RC, Faruque AS, et al. Transferable quinolone resistance in Vibrio cholerae . Antimicrob Agents Chemother. 2010 Feb;54(2):799–803. doi: 10.1128/AAC.01045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill DM. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- 22.Gill DM. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassel D, Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill DM, Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matson JS, Withey JH, DiRita VJ. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun. 2007 Dec;75(12):5542–5549. doi: 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996 Jun 28;272(5270):1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 28.Karaolis DK, Johnson JA, Bailey CC, Boedeker EC, Kaper JB, Reeves PR. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karaolis DK, Somara S, Maneval DR, Jr., Johnson JA, Kaper JB. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999 May 27;399(6734):375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 30.Dziejman M, Balon E, Boyd D, Fraser CM, Heidelberg JF, Mekalanos JJ. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1556–1561. doi: 10.1073/pnas.042667999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae . Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15442–15447. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deen JL, Von SL, Sur D, Agtini M, Lucas ME, Lopez AL, et al. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Negl Trop Dis. 2008;2(2):e173. doi: 10.1371/journal.pntd.0000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan AM, Hossain MS, Khan AI, Chisti MJ, Chowdhury F, Faruque AS, et al. Bacterial enteropathogens of neonates admitted to an urban diarrhoeal hospital in Bangladesh. J Trop Pediatr. 2009 Apr;55(2):122–124. doi: 10.1093/tropej/fmn090. [DOI] [PubMed] [Google Scholar]
- 34.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005 Aug 4;436(7051):696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 35.Harris JB, Larocque RC, Charles RC, Mazumder RN, Khan AI, Bardhan PK. Cholera’s western front. Lancet. 2010 Dec 11;376(9757):1961–1965. doi: 10.1016/S0140-6736(10)62172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascual M, Rodo X, Ellner SP, Colwell R, Bouma MJ. Cholera dynamics and El Nino-Southern Oscillation. Science. 2000 Sep 8;289(5485):1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- 37.Jutla AS, Akanda AS, Griffiths JK, Colwell R, Islam S. Warming oceans, phytoplankton, and river discharge: implications for cholera outbreaks. Am J Trop Med Hyg. 2011 Aug;85(2):303–308. doi: 10.4269/ajtmh.2011.11-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack DA, Malek MA, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg. 2006 Jun;74(6):1067–1073. [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson EJ, Nelson DS, Salam MA, Sack DA. Antibiotics for both moderate and severe cholera. N Engl J Med. 2011 Jan 6;364(1):5–7. doi: 10.1056/NEJMp1013771. [DOI] [PubMed] [Google Scholar]
- 40.Weil AA, Khan AI, Chowdhury F, Larocque RC, Faruque AS, Ryan ET, et al. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis. 2009 Nov 15;49(10):1473–1479. doi: 10.1086/644779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae . Nature. 2000 Aug 3;406(6795):477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trucksis M, Michalski J, Deng YK, Kaper JB. The Vibrio cholerae genome contains two unique circular chromosomes. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14464–14469. doi: 10.1073/pnas.95.24.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe-Magnus DA, Guerout AM, Ploncard P, Dychinco B, Davies J, Mazel D. The evolutionary history of chromosomal super-integrons provides an ancestry for multiresistant integrons. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):652–657. doi: 10.1073/pnas.98.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazel D, Dychinco B, Webb VA, Davies J. A distinctive class of integron in the Vibrio cholerae genome. Science. 1998 Apr 24;280(5363):605–658. doi: 10.1126/science.280.5363.605. [DOI] [PubMed] [Google Scholar]
- 45.Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature. 2011 Sep 22;477(7365):462–465. doi: 10.1038/nature10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, et al. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011 Jan 6;364(1):33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson EJ, Chowdhury A, Harris JB, Begum YA, Chowdhury F, Khan AI, et al. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. Proc Natl Acad Sci U S A. 2007 Nov 27;104(48):19091–19096. doi: 10.1073/pnas.0706352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faruque SM, Biswas K, Udden SM, Ahmad QS, Sack DA, Nair GB, et al. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci U S A. 2006 Apr 18;103(16):6350–6355. doi: 10.1073/pnas.0601277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson EJ, Chowdhury A, Flynn J, Schild S, Bourassa L, Shao Y, et al. Transmission of Vibrio cholerae is antagonized by lytic phage and entry into the aquatic environment. PLoS Pathog. 2008 Oct;4(10):e1000187. doi: 10.1371/journal.ppat.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colwell RR, Huq A, Islam MS, Aziz KM, Yunus M, Khan NH, et al. Reduction of cholera in Bangladeshi villages by simple filtration. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1051–1055. doi: 10.1073/pnas.0237386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faruque SM, Naser IB, Islam MJ, Faruque AS, Ghosh AN, Nair GB, et al. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1702–1707. doi: 10.1073/pnas.0408992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen MA, Faruque SM, Mekalanos JJ, Levin BR. Modeling the role of bacteriophage in the control of cholera outbreaks. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4652–4657. doi: 10.1073/pnas.0600166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002 Jun 6;417(6889):642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartley DM, Morris JG, Jr., Smith DL. Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006 Jan;3(1):e7. doi: 10.1371/journal.pmed.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrews JR, Basu S. Transmission dynamics and control of cholera in Haiti: an epidemic model. Lancet. 2011 Apr 9;377(9773):1248–1255. doi: 10.1016/S0140-6736(11)60273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuite AR, Tien J, Eisenberg M, Earn DJ, Ma J, Fisman DN. Cholera epidemic in Haiti, 2010: using a transmission model to explain spatial spread of disease and identify optimal control interventions. Ann Intern Med. 2011 May 3;154(9):593–601. doi: 10.7326/0003-4819-154-9-201105030-00334. [DOI] [PubMed] [Google Scholar]
- 57.Chowdhury F, Begum YA, Alam MM, Khan AI, Ahmed T, Bhuiyan MS, et al. Concomitant enterotoxigenic Escherichia coli infection induces increased immune responses to Vibrio cholerae O1 antigens in patients with cholera in Bangladesh. Infect Immun. 2010 May;78(5):2117–2124. doi: 10.1128/IAI.01426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris JB, Podolsky MJ, Bhuiyan TR, Chowdhury F, Khan AI, Larocque RC, et al. Immunologic Responses to Vibrio cholerae in Patients Co-Infected with Intestinal Parasites in Bangladesh. PLoS Negl Trop Dis. 2009;3(3):e403. doi: 10.1371/journal.pntd.0000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris JB, Larocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, et al. Susceptibility to Vibrio cholerae Infection in a Cohort of Household Contacts of Patients with Cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2(4):e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chowdhury F, Khan AI, Harris JB, Larocque RC, Chowdhury MI, Ryan ET, et al. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr Infect Dis J. 2008 Nov;27(11):986–992. doi: 10.1097/INF.0b013e3181783adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barua D, Paguio AS. ABO blood groups and cholera. Ann Hum Biol. 1977 Sep;4(5):489–492. doi: 10.1080/03014467700002481. [DOI] [PubMed] [Google Scholar]
- 62.Harris JB, Khan AI, Larocque RC, Dorer DJ, Chowdhury F, Faruque AS, et al. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun. 2005 Nov;73(11):7422–7427. doi: 10.1128/IAI.73.11.7422-7427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaudhuri A, DasAdhikary CR. Possible role of blood-group secretory substances in the aetiology of cholera. Trans R Soc Trop Med Hyg. 1978;72(6):664–665. doi: 10.1016/0035-9203(78)90031-7. [DOI] [PubMed] [Google Scholar]
- 64.Glass RI, Holmgren J, Haley CE, Khan MR, Svennerholm AM, Stoll BJ, et al. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol. 1985 Jun;121(6):791–796. doi: 10.1093/oxfordjournals.aje.a114050. [DOI] [PubMed] [Google Scholar]
- 65.Larocque RC, Sabeti P, Duggal P, Chowdhury F, Khan AI, Lebrun LM, et al. A variant in long palate, lung and nasal epithelium clone 1 is associated with cholera in a Bangladeshi population. Genes Immun. 2009 Apr;10(3):267–272. doi: 10.1038/gene.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin OS, Uddin T, Citorik R, Wang JP, Della PP, Kradin RL, et al. LPLUNC1 Modulates Innate Immune Responses to Vibrio cholerae . J Infect Dis. 2011 Nov;204(9):1349–1357. doi: 10.1093/infdis/jir544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization. The treatment of diarrhoea, a manual for physicians and other senior health workers. (4th revision) 2005 [Google Scholar]
- 68.Qadri F, Wenneras C, Albert MJ, Hossain J, Mannoor K, Begum YA, et al. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997 Sep;65(9):3571–3576. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lesmana M, Rockhill RC, Sutanti D, Sutomo A. An evaluation of alkaline peptone water for enrichment of Vibrio cholerae in feces. Southeast Asian J Trop Med Public Health. 1985 Jun;16(2):265–267. [PubMed] [Google Scholar]
- 70.Benenson AS, Islam MR, Greenough WB., III. Rapid identification of Vibrio cholerae by darkfield microscopy. Bull World Health Organ. 1964;30:827–831. [PMC free article] [PubMed] [Google Scholar]
- 71.Yam WC, Lung ML, Ng MH. Evaluation and optimization of a latex agglutination assay for detection of cholera toxin and Escherichia coli heat-labile toxin. J Clin Microbiol. 1992 Sep;30(9):2518–2520. doi: 10.1128/jcm.30.9.2518-2520.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almeida RJ, Hickman-Brenner FW, Sowers EG, Puhr ND, Farmer JJ, III., Wachsmuth IK. Comparison of a latex agglutination assay and an enzyme-linked immunosorbent assay for detecting cholera toxin. J Clin Microbiol. 1990 Jan;28(1):128–130. doi: 10.1128/jcm.28.1.128-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colwell RR, Hasan JA, Huq A, Loomis L, Siebeling RJ, Torres M, et al. Development and evaluation of a rapid, simple, sensitive, monoclonal antibody-based coagglutination test for direct detection of Vibrio cholerae 01. FEMS Microbiol Lett. 1992 Oct 15;76(3):215–219. doi: 10.1016/0378-1097(92)90339-p. [DOI] [PubMed] [Google Scholar]
- 74.Hasan JA, Huq A, Tamplin ML, Siebeling RJ, Colwell RR. A novel kit for rapid detection of Vibrio cholerae O1. J Clin Microbiol. 1994 Jan;32(1):249–252. doi: 10.1128/jcm.32.1.249-252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhuiyan NA, Qadri F, Faruque AS, Malek MA, Salam MA, Nato F, et al. Use of dipsticks for rapid diagnosis of cholera caused by Vibrio cholerae O1 and O139 from rectal swabs. J Clin Microbiol. 2003 Aug;41(8):3939–3941. doi: 10.1128/JCM.41.8.3939-3941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harris JR, Cavallaro EC, de Nobrega AA, Dos S Barrado JC, Bopp C, Parsons MB, et al. Field evaluation of crystal VC Rapid Dipstick test for cholera during a cholera outbreak in Guinea-Bissau. Trop Med Int Health. 2009 Sep;14(9):1117–1121. doi: 10.1111/j.1365-3156.2009.02335.x. [DOI] [PubMed] [Google Scholar]
- 77.Lindenbaum J, Greenough WB, Islam MR. Antibiotic therapy of cholera in children. Bull World Health Organ. 1967;37(4):529–538. [PMC free article] [PubMed] [Google Scholar]
- 78.Wang F, Butler T, Rabbani GH, Jones PK. The acidosis of cholera. Contributions of hyperproteinemia, lactic acidemia, and hyperphosphatemia to an increased serum anion gap. N Engl J Med. 1986 Dec 18;315(25):1591–1595. doi: 10.1056/NEJM198612183152506. [DOI] [PubMed] [Google Scholar]
- 79.Tariq M, Memon M, Jafferani A, Shoukat S, Gowani SA, Nusrat R, et al. Massive fluid requirements and an unusual BUN/creatinine ratio for pre-renal failure in patients with cholera. PLoS One. 2009;4(10):e7552. doi: 10.1371/journal.pone.0007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benyajati C, Keoplug M, Beisel WR, Gangarosa EJ, Sprinz H, Sitprija V. Acute renal failure in Asiatic cholera: clinicopathologic correlations with acute tubular necrosis and hypokalemic nephropathy. Ann Intern Med. 1960 May;52:960–975. doi: 10.7326/0003-4819-52-5-960. [DOI] [PubMed] [Google Scholar]
- 81.Lindenbaum J, Akbar R, Gordon RS, Jr., Greenough WB, III, Hirschorn N, Islam MR. Cholera in children. Lancet. 1966 May 14;1(7446):1066–1068. doi: 10.1016/s0140-6736(66)91011-7. [DOI] [PubMed] [Google Scholar]
- 82.Bennish ML, Azad AK, Rahman O, Phillips RE. Hypoglycemia during diarrhea in childhood. Prevalence, pathophysiology, and outcome. N Engl J Med. 1990 May 10;322(19):1357–1363. doi: 10.1056/NEJM199005103221905. [DOI] [PubMed] [Google Scholar]
- 83.Guerrant RL, Carneiro-Filho BA, Dillingham RA. Cholera, diarrhea, and oral rehydration therapy: triumph and indictment. Clin Infect Dis. 2003 Aug 1;37(3):398–405. doi: 10.1086/376619. [DOI] [PubMed] [Google Scholar]
- 84.Public health impact of Rwandan refugee crisis: what happened in Goma, Zaire, in July, 1994? Goma Epidemiology Group. Lancet. 1995 Feb 11;345(8946):339–344. [PubMed] [Google Scholar]
- 85.Dhar U, Bennish ML, Khan WA, Seas C, Huq KE, Albert MJ, et al. Clinical features, antimicrobial susceptibility and toxin production in Vibrio cholerae infection: comparison with V. cholerae O1 infection. Trans R Soc Trop Med Hyg. 1996 Jul;90(4):402–405. doi: 10.1016/s0035-9203(96)90522-2. [DOI] [PubMed] [Google Scholar]
- 86.Mahalanabis D, Faruque AS, Albert MJ, Salam MA, Hoque SS. An epidemic of cholera due to Vibrio cholerae O139 in Dhaka, Bangladesh: clinical and epidemiological features. Epidemiol Infect. 1994 Jun;112(3):463–471. doi: 10.1017/s0950268800051165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryan ET, Dhar U, Khan WA, Salam MA, Faruque AS, Fuchs GJ, et al. Mortality, morbidity, and microbiology of endemic cholera among hospitalized patients in Dhaka, Bangladesh. Am J Trop Med Hyg. 2000 Jul;63(1-2):12–20. doi: 10.4269/ajtmh.2000.63.12. [DOI] [PubMed] [Google Scholar]
- 88.Nalin DR, Cash RA, Islam R, Molla M, Phillips RA. Oral maintenance therapy for cholera in adults. Lancet. 1968 Aug 17;2(7564):370–373. doi: 10.1016/s0140-6736(68)90591-6. [DOI] [PubMed] [Google Scholar]
- 89.Walton DA, Ivers LC. Responding to cholera in post-earthquake Haiti. N Engl J Med. 2011 Jan 6;364(1):3–5. doi: 10.1056/NEJMp1012997. [DOI] [PubMed] [Google Scholar]
- 90.Mintz ED, Guerrant RL. A lion in our village--the unconscionable tragedy of cholera in Africa. N Engl J Med. 2009 Mar 12;360(11):1060–1063. doi: 10.1056/NEJMp0810559. [DOI] [PubMed] [Google Scholar]
- 91.Update: outbreak of cholera ---Haiti, 2010. MMWR Morb Mortal Wkly Rep. 2010 Dec 10;59(48):1586–1590. [PubMed] [Google Scholar]
- 92.Mahalanabis D, Wallace CK, Kallen RJ, Mondal A, Pierce NF. Water and electrolyte losses due to cholera in infants and small children: a recovery balance study. Pediatrics. 1970 Mar;45(3):374–385. [PubMed] [Google Scholar]
- 93.Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med. 2000 Feb 3;342(5):308–313. doi: 10.1056/NEJM200002033420502. [DOI] [PubMed] [Google Scholar]
- 94.Greenough IWB, Gordon RS, Rosenberg I, Davies BI, Benenson AS. Tetracycline in the treatment of cholera 1964. Lancet. 1964;1(7329):355–357. doi: 10.1016/s0140-6736(64)92099-9. [DOI] [PubMed] [Google Scholar]
- 95.Roy SK, Hossain MJ, Khatun W, Chakraborty B, Chowdhury S, Begum A, et al. Zinc supplementation in children with cholera in Bangladesh: randomised controlled trial. BMJ. 2008 Feb 2;336(7638):266–268. doi: 10.1136/bmj.39416.646250.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhandari N, Mazumder S, Taneja S, Dube B, Agarwal RC, Mahalanabis D, et al. Effectiveness of zinc supplementation plus oral rehydration salts compared with oral rehydration salts alone as a treatment for acute diarrhea in a primary care setting: a cluster randomized trial. Pediatrics. 2008 May;121(5):e1279–e1285. doi: 10.1542/peds.2007-1939. [DOI] [PubMed] [Google Scholar]
- 97.Lukacik M, Thomas RL, Aranda JV. A meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhea. Pediatrics. 2008 Feb;121(2):326–336. doi: 10.1542/peds.2007-0921. [DOI] [PubMed] [Google Scholar]
- 98.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li ST, Grossman DC, Cummings P. Loperamide therapyfor acute diarrhea in childre: systematic review and meta-analysis. PLoS.Med. 2007;4[(3]):e98. doi: 10.1371/journal.pmed.0040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.WHO. Fact and figures on water quality and health [Google Scholar]
- 101.Ryan ET. The cholera pandemic, still with us after half a century: time to rethink. PLoS Negl Trop Dis. 2011;5(1):e1003. doi: 10.1371/journal.pntd.0001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.World Health Organization Global Task Force on Cholera Control. Prevention. Prevention and control of cholera outbreaks: WHO policy and recommendations. 2011:2011. [Google Scholar]
- 103.Ivers LC, Farmer P, Almazor CP, Leandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010 Dec 18;376(9758):2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 104.Farmer P, Almazor CP, Bahnsen ET, Barry D, Bazile J, Bloom BR, et al. Meeting cholera’s challenge to Haiti and the world: a joint statement on cholera prevention and care. PLoS Negl Trop Dis. 2011;5(5):e1145. doi: 10.1371/journal.pntd.0001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009 Nov 14;374(9702):1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 106.Van Loon FP, Clemens JD, Chakraborty J, Rao MR, Kay BA, Sack DA, et al. Field trial of inactivated oral cholera vaccines in Bangladesh: results from 5 years of follow-up. Vaccine. 1996 Feb;14(2):162–166. doi: 10.1016/0264-410x(95)00122-h. [DOI] [PubMed] [Google Scholar]
- 107.Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Stanton BF, et al. Impact of B subunit killed whole-cell and killed whole-cell-only oral vaccines against cholera upon treated diarrhoeal illness and mortality in an area endemic for cholera. Lancet. 1988 Jun 18;1(8599):1375–1379. doi: 10.1016/s0140-6736(88)92189-7. [DOI] [PubMed] [Google Scholar]
- 108.Sanchez JL, Vasquez B, Begue RE, Meza R, Castellares G, Cabezas C, et al. Protective efficacy of oral whole-cell/recombinant-B-subunit cholera vaccine in Peruvian military recruits. Lancet. 1994 Nov 5;344(8932):1273–1276. doi: 10.1016/s0140-6736(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 109.Clemens JD, Sack DA, Ivanoff B. Misleading negative findings in a field trial of killed, oral cholera vaccine in Peru. J Infect Dis. 2001 Apr 15;183(8):1306–1309. doi: 10.1086/319673. [DOI] [PubMed] [Google Scholar]
- 110.Taylor DN, Cardenas V, Sanchez JL, Begue RE, Gilman R, Bautista C, et al. Two-year study of the protective efficacy of the oral whole cell plus recombinant B subunit cholera vaccine in Peru. J Infect Dis. 2000 May;181(5):1667–1673. doi: 10.1086/315462. [DOI] [PubMed] [Google Scholar]
- 111.Lucas ME, Deen JL, Von SL, Wang XY, Ampuero J, Puri M, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005 Feb 24;352(8):757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 112.Anh DD, Canh dG, Lopez AL, Thiem VD, Long PT, Son NH, et al. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine. 2007 Jan 22;25(6):1149–1155. doi: 10.1016/j.vaccine.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 113.Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, et al. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS One. 2008;3(6):e2323. doi: 10.1371/journal.pone.0002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trach DD, Clemens JD, Ke NT, Thuy HT, Son ND, Canh DG, et al. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet. 1997 Jan 25;349(9047):231–235. doi: 10.1016/s0140-6736(96)06107-7. [DOI] [PubMed] [Google Scholar]
- 115.Thiem VD, Deen JL, Von SL, Canh dG, Anh DD, Park JK, et al. Longterm effectiveness against cholera of oral killed whole-cell vaccine produced in Vietnam. Vaccine. 2006 May 15;24(20):4297–4303. doi: 10.1016/j.vaccine.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Ali M, Emch M, Von SL, Yunus M, Sack DA, Rao M, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005 Jul 2;366(9479):44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 117.Longini IM, Jr., Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS Med. 2007 Nov 27;4(11):e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeuland M, Cook J, Poulos C, Clemens J, Whittington D. Costeffectiveness of new-generation oral cholera vaccines: a multisite analysis. Value Health. 2009 Sep;12(6):899–908. doi: 10.1111/j.1524-4733.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 119.Ryan ET, Calderwood SB, Qadri F. Live attenuated oral cholera vaccines. Expert Rev Vaccines. 2006 Aug;5(4):483–494. doi: 10.1586/14760584.5.4.483. [DOI] [PubMed] [Google Scholar]
- 120.Cohen MB, Giannella RA, Bean J, Taylor DN, Parker S, Hoeper A, et al. Randomized, controlled human challenge study of the safety, immunogenicity, and protective efficacy of a single dose of Peru-15, a live attenuated oral cholera vaccine. Infect Immun. 2002 Apr;70(4):1965–1970. doi: 10.1128/IAI.70.4.1965-1970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levine MM, Kaper JB, Herrington D, Ketley J, Losonsky G, Tacket CO, et al. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 122.Kotloff KL, Wasserman SS, O’Donnell S, Losonsky GA, Cryz SJ, Levine MM. Safety and immunogenicity in North Americans of a single dose of live oral cholera vaccine CVD 103-HgR: results of a randomized, placebo-controlled, double-blind crossover trial. Infect Immun. 1992 Oct;60(10):4430–4432. doi: 10.1128/iai.60.10.4430-4432.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kenner JR, Coster TS, Taylor DN, Trofa AF, Barrera-Oro M, Hyman T, et al. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995 Oct;172(4):1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 124.Sack DA, Sack RB, Shimko J, Gomes G, O’Sullivan D, Metcalfe K, et al. Evaluation of Peru-15, a new live oral vaccine for cholera, in volunteers. J Infect Dis. 1997 Jul;176(1):201–205. doi: 10.1086/514025. [DOI] [PubMed] [Google Scholar]
- 125.Richie EE, Punjabi NH, Sidharta YY, Peetosutan KK, Sukandar MM, Wasserman SS, et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000 May 8;18(22):2399–2410. doi: 10.1016/s0264-410x(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 126.Qadri F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, et al. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine. 2007 Jan 4;25(2):231–238. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 127.Garcia L, Jidy MD, Garcia H, Rodriguez BL, Fernandez R, Ano G, et al. The vaccine candidate Vibrio cholerae 638 is protective against cholera in healthy volunteers. Infect Immun. 2005 May;73(5):3018–3024. doi: 10.1128/IAI.73.5.3018-3024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liang W, Wang S, Yu F, Zhang L, Qi G, Liu Y, et al. Construction and evaluation of a safe, live, oral Vibrio cholerae vaccine candidate, IEM108. Infect Immun. 2003 Oct;71(10):5498–5504. doi: 10.1128/IAI.71.10.5498-5504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Anh DD, Lopez AL, Thiem VD, Grahek SL, Duong TN, Park JK, et al. Use of oral cholera vaccines in an outbreak in Vietnam: a case control study. PLoS Negl Trop Dis. 2011;5(1):e1006. doi: 10.1371/journal.pntd.0001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reyburn R, Deen JL, Grais RF, Bhattacharya SK, Sur D, Lopez AL, et al. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis. 2011;5(1):e952. doi: 10.1371/journal.pntd.0000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miller Neilan RL, Schaefer E, Gaff H, Fister KR, Lenhart S. Modeling optimal intervention strategies for cholera. Bull Math Biol. 2010 Nov;72(8):2004–2018. doi: 10.1007/s11538-010-9521-8. [DOI] [PubMed] [Google Scholar]
- 132.Chao DL, Halloran ME, Longini IM., Jr. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7081–7085. doi: 10.1073/pnas.1102149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chowdhury F, Khan AI, Faruque AS, Ryan ET. Severe, acute watery diarrhea in an adult. PLoS Negl Trop Dis. 2010;4(11):e898. doi: 10.1371/journal.pntd.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Molla AM, Rahman M, Sarker SA, Sack DA, Molla A. Stool electrolyte content and purging rates in diarrhea caused by rotavirus, enterotoxigenic E. coli, and V. cholerae in children. J Pediatr. 1981 May;98(5):835–838. doi: 10.1016/s0022-3476(81)80863-3. [DOI] [PubMed] [Google Scholar]
- 135.WHO. Global Task Force on Cholera Control, First steps for managing an outbreak of acute diarrhoea. :2010. [Google Scholar]
- 136.Alam NH, Majumder RN, Fuchs GJ. Efficacy and safety of oral rehydration solution with reduced osmolarity in adults with cholera: a randomised double-blind clinical trial. CHOICE study group. Lancet. 1999 Jul 24;354(9175):296–299. doi: 10.1016/s0140-6736(98)09332-5. [DOI] [PubMed] [Google Scholar]
- 137.Murphy C, Hahn S, Volmink J. Reduced osmolarity oral rehydration solution for treating cholera. Cochrane Database Syst Rev. 2004;(4):CD003754. doi: 10.1002/14651858.CD003754.pub2. [DOI] [PubMed] [Google Scholar]
- 138.Alam NH, Yunus M, Faruque AS, Gyr N, Sattar S, Parvin S, et al. Symptomatic hyponatremia during treatment of dehydrating diarrheal disease with reduced osmolarity oral rehydration solution. JAMA. 2006 Aug 2;296(5):567–573. doi: 10.1001/jama.296.5.567. [DOI] [PubMed] [Google Scholar]
- 139.Yamamoto T, Nair GB, Albert MJ, Parodi CC, Takeda Y. Survey of in vitro susceptibilities of Vibrio cholerae O1 and O139 to antimicrobial agents. Antimicrob Agents Chemother. 1995 Jan;39(1):241–244. doi: 10.1128/aac.39.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saha D, Khan WA, Karim MM, Chowdhury HR, Salam MA, Bennish ML. Single-dose ciprofloxacin versus 12-dose erythromycin for childhood cholera: a randomised controlled trial. Lancet. 2005 Sep 24;366(9491):1085–1093. doi: 10.1016/S0140-6736(05)67290-X. [DOI] [PubMed] [Google Scholar]
- 141.Khan WA, Bennish ML, Seas C, Khan EH, Ronan A, Dhar U, et al. Randomised controlled comparison of single-dose ciprofloxacin and doxycycline for cholera caused by Vibrio cholerae 01 or 0139. Lancet. 1996 Aug 3;348(9023):296–300. doi: 10.1016/s0140-6736(96)01180-4. [DOI] [PubMed] [Google Scholar]
- 142.Saha D, Karim MM, Khan WA, Ahmed S, Salam MA, Bennish ML. Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med. 2006 Jun 8;354(23):2452–2462. doi: 10.1056/NEJMoa054493. [DOI] [PubMed] [Google Scholar]
- 143.Islam MS, Midzi SM, Charimari L, Cravioto A, Endtz HP. Susceptibility to fluoroquinolones of Vibrio cholerae O1 isolated from diarrheal patients in Zimbabwe. JAMA. 2009 Dec 2;302(21):2321–2322. doi: 10.1001/jama.2009.1750. [DOI] [PubMed] [Google Scholar]
- 144.Kaushik JS, Gupta P, Faridi MM, Das S. Single dose azithromycin versus ciprofloxacin for cholera in children: a randomized controlled trial. Indian Pediatr. 2010 Apr;47(4):309–315. doi: 10.1007/s13312-010-0059-5. [DOI] [PubMed] [Google Scholar]
- 145.Peltola H, Siitonen A, Kyronseppa H, Simula I, Mattila L, Oksanen P, et al. Prevention of travellers’ diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet. 1991 Nov 23;338(8778):1285–1289. doi: 10.1016/0140-6736(91)92590-x. [DOI] [PubMed] [Google Scholar]
- 146.Clemens JD, Sack DA, Harris JR, van LF, Chakraborty J, Ahmed F, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 147.Saha A, Chowdhury MI, Khanam F, Bhuiyan MS, Chowdhury F, Khan AI, et al. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine. 2011 Sep 9; doi: 10.1016/j.vaccine.2011.08.108. [DOI] [PubMed] [Google Scholar]
- 148.Kanungo S, Paisley A, Lopez AL, Bhattacharya M, Manna B, Kim DR, et al. Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: a randomized, placebo-controlled trial. Vaccine. 2009 Nov 16;27(49):6887–6893. doi: 10.1016/j.vaccine.2009.09.008. [DOI] [PubMed] [Google Scholar]