Abstract
Introduction
Increasing evidence suggests the role of inflammation in enhancing neuronal excitability and contributing to epileptogenesis. Tetracycline-class antibiotics minocycline, doxycycline, and tetracycline have been shown to have anti-apoptotic and anti-inflammatory effects.
Methods
We investigated the anti-seizure effects of tetracycline-class antibiotics minocycline, doxycycline, and tetracycline in vivo by using the maximal electric shock (MES), 6Hz (minimal clonic seizure) test, and subcutaneous Metrazol (scMET) models of epilepsy.
Results
Minocycline, doxycycline, and tetracycline showed anticonvulsant effects in abolishing partial seizures in a mouse model of 6Hz seizure test. A dose-dependent effect was found, with ED50 of 170mg/kg for minocycline, 157 mg/kg for doxycycline, and 255 mg/kg for tetracycline with peak onset at 0.5 hours. At high doses, minocycline (250mg/kg) and doxycycline (150mg/kg) also have toxic effects, from motor impairments to respiratory failure and death. These drugs had no effects on the MES and scMET tests.
Conclusions
In three tests of anti-seizure activity, minocycline, doxycycline, and tetracycline were found to be protective in one: the 6Hz seizure model. Our data suggest that minocycline and other tetracycline-class drugs may offer some degree of anticonvulsant effect in the setting of CNS disease trials.
Keywords: minocycline, doxycycline, tetracycline, seizure, neuroprotection, antiepileptic drugs, anticonvulsant drugs, epilepsy
INTRODUCTION
Epilepsy is one of the most common neurological disorders with diverse etiologies. Aside from idiopathic or known genetic epilepsy syndromes, epilepsy can arise from central nervous system (CNS) infections, brain injuries, brain tumors, and cerebrovascular disorders including vascular malformations [1]. As many as one-third of the patients with epilepsy are refractory to pharmacological treatment [2], and mechanisms of treatment failure is still relatively poorly understood.
Recent data suggest that neuroinflammation may contribute to the pathogenesis of epilepsy. Insults to the central nervous system produce a neuroinflammatory response characterized by microglia and astrocyte activation, breakdown of the blood-brain-barrier (BBB), and acute up-regulation of pro-inflammatory cytokines such as interleukins and tumor necrosis factor [3-7]. In the case of epilepsy, the activation of these pro-inflammatory mediators can increase neuronal excitability, therefore enhancing seizure susceptibility [1, 8, 9].
Current anticonvulsant therapies are largely limited to those that interact with ion channels or synaptic vesicles [2]. However, attention has been turned toward anti-inflammatory and immunosuppressive drugs for the treatment or prevention of epilepsy. In particular, recent evidence shows that some anti-inflammatory drugs that inhibit metalloproteinase, microglia activation, and the inflammatory cascade such as the IL-1/Toll-like receptor (TLR) signaling cascade can have neuroprotective effects [1]. One report showed that doxycycline, a second-generation tetracycline, protects against seizures in the pilocarpine-induced seizure model in rats [10].
Minocycline, the most lipid-soluble of the tetracycline-class antibiotics, is of particular interest for its potential applicability as an antiepileptic drug. As an anti-inflammatory, minocycline has shown to inhibit apoptosis via attenuation of TNF-alpha and down-regulation of pro-inflammatory cytokine through caspase-dependent and caspase-independent pathways [5, 11]. Minocycline also has anti-inflammatory actions by inhibiting microglial activation and transmigration of T-lymphocytes. Patients taking minocycline shortly after acute ischemic stroke showed improved functional state and stroke severity over a 3-month period compared to those receiving placebo [12]. In the setting of brain arteriovenous malformations (AVM), preliminary evidence suggests that minocycline may reduce the risk for developing epilepsy and stabilize the rupture-prone, dysplastic vascular structures of the nidus [13].
Given the promising ability of minocycline to reduce inflammation in the CNS, we investigated whether minocycline and other tetracyclines have anti-seizure effects. By using in vivo models for partial, clonic, and tonic-clonic seizures, we describe for the first time, an anti-seizure property of minocycline, doxycycline, and tetracycline.
METHODS
Animals
Adult male CF no 1 albino mice (Charles River, Michigan) and Sprague-Dawley albino rats (Charles River, Michigan) were used for this study. All animals were maintained on adequate diet (Prolab RMH 3000) with free access to food and water. They are housed in plastic cages with controlled 12-hour alternating light and dark cycles. All animal experiments were performed in accordance with guidelines with NINDS Antiepileptic Drug Screening program.
6Hz (Minimal Clonic Seizure) Test
6Hz of alternating current (50 mA) was delivered for 3 s by corneal electrodes primed with an electrolyte solution containing an anesthetic agent (0.5% tetracaine HCl) to adult mice in order to assess a compound's efficacy against electrically induced seizures. Minocycline, doxycycline, tetracycline, or control 0.5% methylcellulose is pre-administered to CF mice (26-30 g, age 28-38 days) via i.p. injection at the following doses of 75, 100, 150, 250, and 500mg/kg. At varying times, individual mice (four per time point) are challenged with sufficient current delivered through corneal electrodes to elicit a psychomotor seizure in 97% of animals (32 mA for 3s) [14]. Untreated mice will display seizures characterized by a minimal clonic phase followed by stereotyped automatisms described originally as being similar to the aura of human patients with partial seizures. Animals not displaying this behavior are considered protected. The test may be evaluated quantitatively by measuring the responses at varying doses at a determined time of peak effect (TPE).
Maximal Electroshock Test (MES)
For all tests based on MES convulsions, 60Hz of alternating current (150mA) is delivered for 0.2s by corneal electrodes primed with an electrolyte solution containing an anesthetic agent (0.5% tetracaine HCl). Rats are tested at various intervals following doses of 30, 100 and 300 mg/kg minocycline, doxycycline, tetracycline, or control 0.5% methylcellulose given by i.p. injection of a volume of 0.01 mL/g. An animal is considered “protected” from MES-induced seizures upon abolition of the hindlimb tonic extensor component of the seizure (Swinyard et al., 1989; White et al., 1995a; White et al., 1995b).
Subcutaneous Metrazol Seizure Threshold Test (scMET)
Subcutaneous injection of the convulsant Metrazol produces clonic seizures in laboratory animals. The scMET test detects the ability of a test compound to raise the seizure threshold of an animal and thus protect it from exhibiting a clonic seizure. Mice are pretreated with 30, 100, and 300 mg/kg of minocycline, doxycycline, tetracycline, or control 0.5% methylcellulose given by i.p. injection. At the previously determined TPE of the test compound, the dose of Metrazol which will induce convulsions in 97% of animals (CD97: 85 mg/kg mice) is injected into a loose fold of skin in the midline of the neck. The animals are placed in isolation cages to minimize stress [15] and observed for the next 30 minutes for the presence or absence of a seizure. An episode of clonic spasms, approximately 3-5 seconds, of the fore and/or hindlimbs, jaws, or vibrissae is taken as the endpoint. Animals which do not meet this criterion are considered protected.
Minimal Motor Impairment
To assess a compound's side effects and toxicity, animals are monitored for overt signs of impaired neurological or muscular function. In mice, the rotarod [16] procedure is used to disclose minimal muscular or neurological impairment. When a mouse is placed on a rod that rotates at a speed of 6rpm, the animal can maintain its equilibrium for long periods of time. The animal is considered toxic if it falls off this rotating rod three times during a 1-min period. In rats, minimal motor deficit is indicated by ataxia, which is manifested by an abnormal, uncoordinated gait. In addition to MMI, animals may exhibit a circular or zigzag gait, abnormal body posture and spread of the legs, tremors, hyperactivity, lack of exploratory behavior, somnolence, stupor, catalepsy, loss of placing response and changes in muscle tone.
ED50 Calculation
Quantitative determinations of Median Effective (ED50) of candidate compounds are calculated in vivo. Studies are conducted utilizing an experimentally calculated time of peak effect. Time to peak effect was performed using four animals to select the time point at which the greatest treatment effect was seen. This time point (0.5 hours for all three drugs) was used to perform the quantitative study. Groups of at eight mice are tested with various doses of the candidate drug until at least two points can be clearly established between the limits of 100% protection and 0% protection. The dose of drug required to produce the desired endpoint in 50% of animals (ED50) in each test, the 95% confidence interval, the slope of the regression line, and the S.E.M. of the slope are calculated by fitting the data using a sigmoidal logistic function as previously described by Finney [17].
RESULTS
Minocycline, doxycycline, and tetracycline showed anticonvulsant effects in vivo
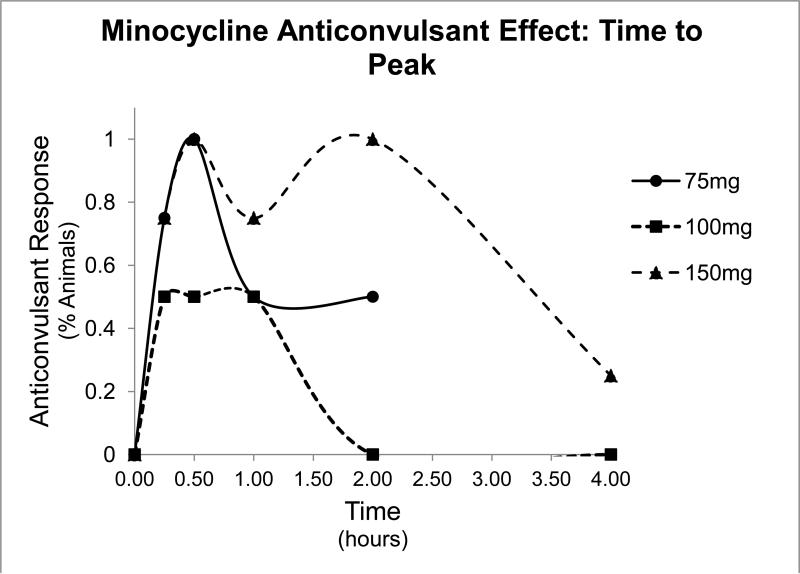
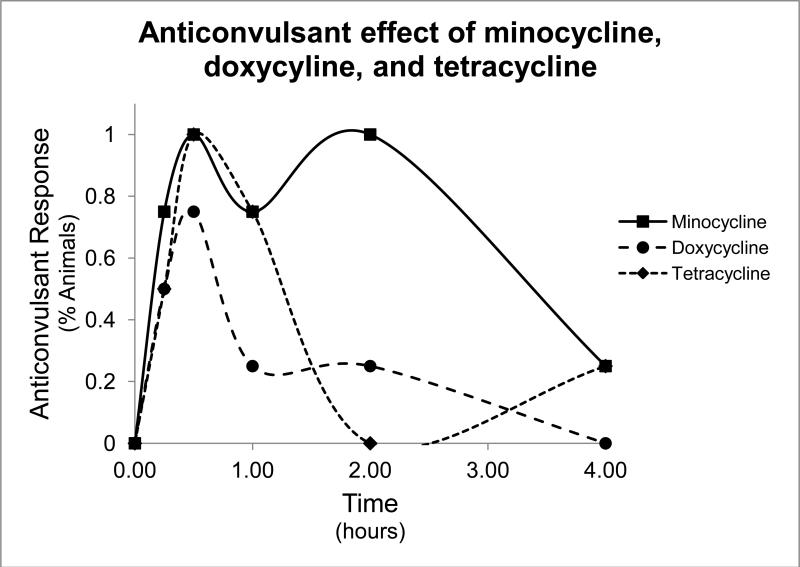
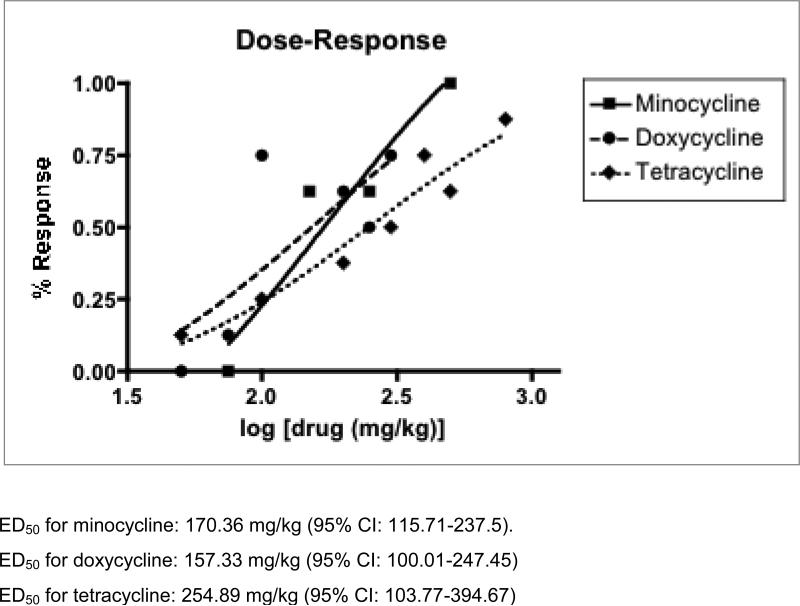
To test the anti-seizure effects of tetracycline-class antibiotics, we performed the 6Hz test. In this test, low frequency (6Hz) stimulations lasting 3 seconds are administered to the animals, which result in stereotyped automatisms similar to that of partial seizures in humans. Intraperitoneal injection of minocycline resulted in protection from clonic seizure in all doses tested (75mg/kg, 100mg/kg, and 150mg/kg, Table 1), and effects peaked at 30 minutes, as shown in the time to peak graph in Figure 1. Doxycycline and tetracycline also showed seizure protection at both doses tested (100mg/kg and 150 mg/kg, 200 mg/kg and 400 mg/kg, respectively, Tables 2 and 3). Control treatment with 0.5% methylcellulose demonstrated no seizure protection. Time to peak relationships for all test compounds at 150mg/kg concentration are shown in Figure 2. Dose-response relationships were found in all three compounds (Figure 3). The ED50 dose for anti-seizure effect during peak effect is 170.36 mg/kg for minocycline (95% CI: 115.7-237.5 mg, slope 4.49 ± 1.33), 157.33 mg/kg for doxycycline (95% CI: 100.01-247.45, slope 2.24 ± 0.69), and 254.89 mg/kg for tetracycline (95% CI 103.77-394.67, slope 2.07 ± 0.76).
Table 1.
Anticonvulsant Effect of Minocycline in 6Hz Test
| Time (Hours) | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 |
|---|---|---|---|---|---|
| Dose (mg/kg) | N/T | N/T | N/T | N/T | N/T |
| 75 | 3/4 | 4/4 | 2/4 | 2/4 | |
| 100 | 2/4 | 2/4 | 2/4 | 0/4 | 0/4 |
| 150 | 3/4 | 4/4 | 3/4 | 4/4 | 1/4 |
| Control | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
N/T: number of animals showing anticonvulsant effect / number of animals tested
Control: I.P injection of 0.5% methylcellulose
Figure 1.
Time to peak graph of minocycline. Percent of animals protected from seizures in the 6Hz test as a function of time at various doses of minocycline. The peak response occurred at 0.5 hour for all doses tested.
Table 2.
Anticonvulsant Effect of Doxycycline in 6Hz Test
| Time (Hours) | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 |
|---|---|---|---|---|---|
| Dose (mg/kg) | N/T | N/T | N/T | N/T | N/T |
| 100 | 2/4 | 3/4 | 1/4 | 1/4 | 0/4 |
| 150 | 2/4 | 2/3 | 4/4 | 2/4 | 3/3 |
| Control | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
N/T: number of animals showing anticonvulsant effect / number of animals tested
Control: I.P injection of 0.5% methylcellulose
Table 3.
Anticonvulsant Effect of Tetracycline in 6Hz Test
| Time (Hours) | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 |
|---|---|---|---|---|---|
| Dose (mg/kg) | N/T | N/T | N/T | N/T | N/T |
| 200 | 1/4 | 1/4 | 1/4 | 1/4 | 0/4 |
| 400 | 2/4 | 4/4 | 3/4 | 0/4 | 1/4 |
| Control | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
N/T: number of animals showing anticonvulsant effect / number of animals tested
Control: I.P injection of 0.5% methylcellulose
Figure 2.
Time to peak graph for minocycline, doxycycline, and tetracycline. Percent of animals protected from seizures in the 6Hz test as a function of time with 150mg/kg injection of minocycline (sqaures), 150mg/kg of doxycycline (circles), or 200mg/kg of tetracycline (diamonds).
Figure 3.
Dose-response graphs for minocycline, doxycycline, and tetracycline. This graph depicts the percentage of animals protected from the 6Hz test with various doses (in mg/kg) of minocycline (square), doxycycline (circles), or tetracycline (diamonds) with sigmoidal logistic functions for best-fit line.
Minocycline, doxycycline, and tetracycline did not show any protective effects in other seizure models
We also assessed tetraycycline-antibiotics’ anticonvulsant effects in other seizure models. In the maximal electrical shock (MES) test, 60Hz of alternating 50A current is delivered for 0.2s by corneal stimulation, causing tonic limb movements. Mice treated with IP minocycline, doxycycline, or tetracycline at all tested dose (30, 100, and 300mg/kg) did not abolish tonic hind limb extension. Subcutaneous injection of the convulsant Metrazol (scMET) produces clonic seizures, and the scMET test detects the ability of a test compound to raise the seizure threshold. Animals pretreated with various doses of minocycline, doxycycline, and tetracycline (30, 100, and 300mg/kg) as well as control injection with methylcellulose did not prevent the manifestation of clonic spasms in the fore- and hindlimbs, jaws, or vibrissae. These results are shown in Table 4
Table 4.
Minocycline, doxycycline, and tetracycline do not show anticonvulsant effects in MES and ScMET tests
| Minocycline | Doxycycline | Tetracycline | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (Hours) | 0.5 | 4.0 | 0.5 | 4.0 | 0.5 | 4.0 | 0.5 | 4.0 | |
| Dose (mg) | N/T | N/T | N/T | N/T | N/T | N/T | N/T | N/T | |
| 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| MES | 100 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/2 | 0/4 | 0/4 |
| 300 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| ScMET | 100 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/4 |
| 300 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
MES: Maximal Electrical Shock test
ScMET: Subcutaneous Metrazol test
Control: 0.5% methylcellulose
Minocycline, doxycycline, and tetracycline toxicity
To assess tetracycline-class antibiotic toxicity and other side effects, animals are evaluated for signs of motor and neurological impairment. Initial injection of minocycline resulted in vocalization and hyperactivity of all animals, likely related to the injection itself. With the peak of onset, injections of minocycline at 100mg and 150mg produced diarrhea in 5/8 mice, which resolved within 1 hour of minocycline injection (Table 5). All mice were also examined using the rotarod test, which is a sensitive test for revealing neuromuscular dysfunction. With 500mg of minocycline injection, half the animals exhibited motor impairments on the rotarod by falling off the rotating rod at least three times during a 1-min period. For high-dose minocycline treatments (250mg and 500mg), all animals tested became sluggish and cyanotic hours after injection (4-24 hours), and likely died from respiratory failure (Table 5). Control methylcellulose injection exhibited no toxicity.
Table 5.
Minocycline Toxicity
| Time (Hours) | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 6.0 | 8.0 | 24.0 |
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | N/T | N/T | N/T | N/T | N/T | N/T | N/T | N/T |
| 150 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | |||
| 250 | 0/8 | 0/8 | 0/8 | 0/8 | 6/8 Death | 7/8 Death | 1/2 Motor Imp | 1/2 Death |
| 500 | 4/8 Motor Imp | 2/8 Motor Imp | 5/8 Motor Imp | 8/8 Death | ||||
N/T: number of animals showing toxic effect / number of animals tested
Motor Imp: motor impairment on rotarod test
Control injection of 0.5% methylcellulose showed no toxicity
Doxycycline showed the greatest toxicity profile, causing deaths with even low dose administration at 150mg/kg. At higher doses (300 and 500mg/kg), doxycycline caused severe motor impairments within first two hours of administration, and led to deaths of all animals tested by 6 hours (Table 6). Tetracycline had the most favorable toxicity profile, as no animals were noted to have neurological or motor deficits with up to 500mg/kg injection (Table 7).
Table 6.
Doxycycline Toxicity in mice
| Time (Hours) | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 6.0 | 8.0 | 24.0 |
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | N/T | N/T | N/T | N/T | N/T | N/T | N/T | N/T |
| 150 | 0/4 | 1/4 death | 0/4 | 0/4 | 1/4 death | |||
| 300 | 1/8 motor imp | 1/8 motor imp | 0/8 | 1/8 motor imp | 4/8 death | 7/7 death | 5/5 death | |
| 500 | 7/8 motor imp | 4/8 motor imp | 5/8 motor imp | 3/8 motor imp | 8/8 death | |||
N/T: number of animals showing toxic effect / number of animals tested
Motor Imp: motor impairment on rotarod test
Control injection of 0.5% methylcellulose showed no toxicity
Table 7.
Tetracycline Toxicity in Mice
| Time (Hours) | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 6.0 | 8.0 | 24.0 |
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | N/T | N/T | N/T | N/T | N/T | N/T | N/T | N/T |
| 150 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | |||
| 500 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | |||
N/T: number of animals showing toxic effect / number of animals tested
Control injection of 0.5% methylcellulose showed no toxicity
DISCUSSION
We provide the first evidence for anticonvulsant effect of minocycline and tetracycline-class antibiotics in abolishing in vivo focal seizures in mice. We show a dose-dependent effect with ED50 of 170mg/kg for minocycline, 157 mg/kg for doxycycline, and 255 mg/kg for tetracycline with peak onset at 0.5 hours. As expected, at very high doses, minocycline and doxycycline displayed toxic effects, ranging from motor impairment to respiratory failure and death.
Interestingly, tetracyclines were protective against seizures in the 6Hz model but not in other seizure models tested in this study. For decades, first-line anticonvulsant efficacy screening has been with MES and scMET but this practice is being re-evaluated in light of recent experience. Levetiracetam, a useful human anti-seizure medication, fails to demonstrate efficacy in MES and scMET but is effective in the 6Hz seizure model [18]. This alternative paradigm uses low frequency, long-duration corneal stimulation model to produce psychomotor seizures as a model for partial epilepsy [14,19]. Instead of the tonic extension seizures characteristic of the MES test, the 6Hz-induced seizure involve a minimal, clonic phased followed by stereotyped automatisms reminiscent of human auras in those with partial or limbic epilepsy. The potential benefits of using minocycline and other tetracycline-class antibiotics to protect against partial seizures make them exciting prospective agents for clinical use.
While the anti-seizure effects of minocycline, doxycycline, and tetracycline is promising as a new class of antiepileptic therapy, the toxic effects of these drugs need to be considered. Minocycline has been known to cause common gastrointestinal side effects, as seen in a randomized placebo-control study of minocycline use in Huntington's disease [20-22]. The same study showed nervous system side effects of minocycline including dizziness, vertigo, and intracranial hypertension, though the mechanisms for these effects are unknown. Minocycline has also been linked to respiratory hypersensitivity reactions and lupus-like syndromes leading to respiratory failure, which may explain the respiratory distressed seen in animals in our study. However, the dose of minocycline does used in this study far exceeds that of typical use in clinical trials. Rodents require an approximately 10-fold higher dose of tetracyclines to achieve comparable plasma levels of drug because of differing pharmacokinetics from humans [23, 24]. Therefore, minimal dose tested in this study, 75 mg/kg that achieved anticonvulsant activity in mice was about 2.5 fold higher than the 30 mg/kg mouse analogue of the commonly used clinical dose of 200mg per day in humans. Several published randomized, placebo controlled clinical trials using an equivalent dose of minocycline for relatively long treatment durations (6 months and greater) showed that it was well-tolerated with few adverse effects [20-22, 25]. Future studies addressing the mechanisms for minocycline and doxycycline-induced toxicity may facilitate development of approaches to further reduce risk in the clinical setting.
The mechanism for minocycline and other tetracycline-class antibiotics’ anticonvulsant effects remains to be elucidated. Minocycline has multiple effects on the nervous system. First, minocycline is a potent matrix metalloproteinase-9 inhibitor (MMP-9), which plays important role in mediating tissue injury during human ischemic stroke [26]. Second, it is a potent inhibitor of microglial activation and other inflammatory responses [27]. Third, it is a potent inhibitor of poly-ADP-ribose polymerase 1 (PARP-1), which causes energy depletion and cell necrosis [7, 28]. Therefore, it is possible that minocycline and other tetracyclines can suppress seizures by their anti-inflammatory effects. The short time course of peak effect suggests that minocycline and other tetracyclines have other immediate effects on suppressing neuronal excitability besides its anti-inflammatory roles.
There are several limitations to our current study. First, we did not demonstrate the mechanism(s) by which the tested tetracycline-class agents protect against seizures. Secondly, we had a relatively small sample size for the tests performed. Our study serves primarily as an initial screen for the potential anticonvulsant effects of tetracyclines, and future studies using molecular and electrophysiological tools can elucidate the mechanism of these protective effects and also assess their role in preventing the development of epilepsy in genetic models of epilepsy and brain injury models. Although it is currently unknown how the findings of this study will translate to human clinical trials, an important finding of this study is that tetracycline-class drugs showed at least partial anti-convulsant activity, and seizure severity and duration did not worsen with tetracyclines in any of these acute seizure models.
In conclusion, our study shows for the first time, a possible role for minocycline and tetracycline-derivatives in preventing and stopping partial seizures in vivo. This new therapeutic approach may lead to promising new AED in the treatment of epilepsy with special relevance to cerebrovascular malformations.
Highlights.
Minocycline shows anticonvulsant effects against minimal clonic seizure.
Anticonvulsant effects were seen with doxycline and tetracycline.
These drugs showed dose-dependent effect and peak onset at 0.5 hours.
Toxic effects were seen with very high doses, from motor impairments to death.
Acknowledgements
This work was supported in part by NIH R01 NS34949 and the NINDS Anticonvulsant Screening Program (ASP) and we gratefully acknowledge the technical assistance of Tracy Chen, Ph.D. for experiments performed through the ASP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures and Support
All authors report no financial conflicts of interest.
REFERENCES
- 1.Vezzani A, Bartfai T, Bianchi M, Rossetti C, French J. Therapeutic potential of new antiinflammatory drugs. Epilepsia. 52(Suppl 8):67–9. doi: 10.1111/j.1528-1167.2011.03242.x. [DOI] [PubMed] [Google Scholar]
- 2.Hitiris N, Brodie MJ. Modern antiepileptic drugs: guidelines and beyond. Curr Opin Neurol. 2006;19:175–80. doi: 10.1097/01.wco.0000218235.67840.82. [DOI] [PubMed] [Google Scholar]
- 3.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galic MA, Riazi K, Henderson AK, Tsutsui S, Pittman QJ. Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol Dis. 2009;36:343–51. doi: 10.1016/j.nbd.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heo K, Cho YJ, Cho KJ, Kim HW, Kim HJ, Shin HY, Lee BI, Kim GW. Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci Lett. 2006;398:195–200. doi: 10.1016/j.neulet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Liu ZR, Chen J, Zhang SJ, Quan QY, Huang YG, Jiang W. Roles of astrocytes and microglia in seizure-induced aberrant neurogenesis in the hippocampus of adult rats. J Neurosci Res. 88:519–29. doi: 10.1002/jnr.22224. [DOI] [PubMed] [Google Scholar]
- 7.Yin P, Yang L, Zhou HY, Sun RP. Matrix metalloproteinase-9 may be a potential therapeutic target in epilepsy. Med Hypotheses. 76:184–6. doi: 10.1016/j.mehy.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 52(Suppl 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- 9.Rao RS, Medhi B, Saikia UN, Arora SK, Toor JS, Khanduja KL, Pandhi P. Experimentally induced various inflammatory models and seizure: understanding the role of cytokine in rat. Eur Neuropsychopharmacol. 2008;18:760–7. doi: 10.1016/j.euroneuro.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira CR, Damasceno FM, de Aquino-Neto MR, de Andrade GM, Fontenele JB, de Medeiros TA, Viana GS. Doxycycline protects against pilocarpine-induced convulsions in rats, through its antioxidant effect and modulation of brain amino acids. Pharmacol Biochem Behav. 98:525–32. doi: 10.1016/j.pbb.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–79. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–52. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 13.Frenzel T, Lee CZ, Kim H, Quinnine NJ, Hashimoto T, Lawton MT, Guglielmo BJ, McCulloch CE, Young WL. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis. 2008;25:157–63. doi: 10.1159/000113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toman JE, Everett GM, Richards RK. The search for new drugs against epilepsy. Tex Rep Biol Med. 1952;10:96–104. [PubMed] [Google Scholar]
- 15.Swinyard EA, Clark LD, Miyahara JT, Wolf HH. Studies on the mechanism of amphetamine toxicity in aggregated mice. J Pharmacol Exp Ther. 1961;132:97–102. [PubMed] [Google Scholar]
- 16.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:208–9. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 17.Finney DJ. Probit analysis. 3d ed. University Press; Cambridge [Eng.]: 1971. [Google Scholar]
- 18.Rowley NM, White HS. Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: Correlation with other seizure and epilepsy models. Epilepsy Res. 92:163–9. doi: 10.1016/j.eplepsyres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Brown WC, Schiffman DO, Swinyard EA, Goodman LS. Comparative assay of an antiepileptic drugs by psychomotor seizure test and minimal electroshock threshold test. J Pharmacol Exp Ther. 1953;107:273–83. [PubMed] [Google Scholar]
- 20.NINDS I. A futility study of minocycline in Huntington's disease. Mov Disord. 2010;25:2219–24. doi: 10.1002/mds.23236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NINDS I. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–71. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 22.NINDS I. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31:141–50. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CZ, Yao JS, Huang Y, Zhai W, Liu W, Guglielmo BJ, Lin E, Yang GY, Young WL. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J Cereb Blood Flow Metab. 2006;26:1157–64. doi: 10.1038/sj.jcbfm.9600268. [DOI] [PubMed] [Google Scholar]
- 24.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–9. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- 25.Metz LM, Li D, Traboulsee A, Myles ML, Duquette P, Godin J, Constantin M, Yong VW. Glatiramer acetate in combination with minocycline in patients with relapsing--remitting multiple sclerosis: results of a Canadian, multicenter, double- blind, placebo-controlled trial. Mult Scler. 2009;15:1183–94. doi: 10.1177/1352458509106779. [DOI] [PubMed] [Google Scholar]
- 26.Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–93. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 28.Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–90. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]