Abstract
The peptide leptin conveys the availability of adipose energy stores to the brain. Increasing evidence implicates a significant role for extrahypothalamic sites of leptin action, including the dorsal vagal complex, a region critical for regulating visceral parasympathetic function. The hypothesis that leptin suppresses cellular activity in the dorsal motor nucleus of the vagus nerve (DMV) was tested using whole-cell patch-clamp recordings in brainstem slices. Leptin caused a rapid membrane hyperpolarization in 50% of rat DMV neurons. Leptin also hyperpolarized a subset of gastric-related neurons (62%), identified after gastric inoculation with a transneuronal retrograde viral tracer. The hyperpolarization was associated with a decrease in input resistance and cellular responsiveness and displayed characteristics consistent with an increased K+ conductance. Perfusion of tolbutamide (200 μM) reversed the leptin-induced hyperpolarization, and tolbutamide or wortmannin (10–100 nM) prevented the hyperpolarization, indicating that leptin activated an ATP-sensitive K+ channel via a phosphoinositide-3-kinase-dependent mechanism. Leptin reduced the frequency of spontaneous and miniature excitatory postsynaptic currents (EPSCs), whereas inhibitory postsynaptic currents (IPSCs) were largely unaffected. Electrical stimulation of the nucleus tractus solitarii (NTS) resulted in constant-latency EPSCs, which were decreased in amplitude by leptin. The paired-pulse ratio was increased, suggesting leptin effects involved activation of receptors presynaptic to the recorded neuron. A leptin-induced suppression of EPSCs, but not IPSCs, evoked by focal photolytic uncaging of glutamate within the NTS was also observed, supportive of leptin effects on the glutamatergic NTS projection to the DMV. Therefore, leptin directly hyperpolarized and indirectly suppressed excitatory synaptic activity to DMV neurons involved in visceral regulation, including gastric-related neurons.
THE ADIPOCYTE-DERIVED HORMONE leptin communicates changing energy needs to the brain, and evidence suggests that the leptin pathway plays a significant role in body weight control (1). Selective deletion of leptin receptors from proopiomelanocortin neurons in the arcuate nucleus or from the ventromedial hypothalamus results in obesity (2, 3). However, the degree of obesity is only a fraction of that observed with total leptin deficiency. These results suggest that the body weight and food intake effects of leptin are mediated by a distributed network of leptin-responsive cells that includes hypothalamic and brainstem autonomic control areas (4). Although leptin effects in these areas have been identified, they have not been widely studied (5-7).
The dorsal vagal complex (DVC) is an autonomic regulatory center located in the caudal medulla. Primary viscerosensory information is processed within the nucleus tractus solitarii (NTS) and subsequently relayed to the dorsal motor nucleus of the vagus nerve (DMV). Neurons of the DMV are the central origin of parasympathetic motor efferents that innervate corresponding postganglionic neurons in the viscera, especially the gastrointestinal tract. The NTS also contains fenestrated capillaries, potentially allowing circulating peptides, like leptin, access to the vagal complex (8, 9). The DMV is adjacent to the NTS, and most neurons have dendrites extending into the NTS. Also, unlike some peripherally produced peptides, leptin can be transported across the blood-brain barrier (10). Therefore, neurons in the DMV are receptive to changes in synaptic input from the NTS, and their activity may also be influenced directly by peripheral metabolic cues like leptin (11, 12).
Recent studies have reported leptin receptor gene expression in the dorsal vagal complex, including the DMV (5). In addition, fourth ventricle administration of leptin reduces food ingestion and weight gain within 24 h, and these effects are mimicked by microinjection of the peptide into the DVC (5). Several studies have shown heterogeneous responses to leptin in different central nervous system nuclei (13-15). Within the caudal NTS, leptin hyperpolarized most neurons via activation of an ATP-sensitive K+ (KATP) conductance and reduced excitatory, but not inhibitory, synaptic inputs, including in those premotor neurons related to gastric function (7). These data indicated an inhibition of putatively non-GABAergic NTS neurons, which may contribute to local circuit control within the vagal complex. As such, leptin would be expected to suppress viscerosensory and other excitatory activity related to visceral autonomic function, including gastric regulation, via actions directly at the level of the DVC. However, cellular correlates of leptin’s effects have not been defined in the DMV. In the present study, the hypothesis that leptin acts directly on neurons in the DMV via activation of an ATP-sensitive K+ conductance was tested using whole-cell recordings in medullary slices from rats. Acute effects of leptin were assessed on intrinsic membrane properties and synaptic responses in DMV neurons, including subsets of gastric-related motor neurons.
Materials and Methods
Animals
Male (21–60 d old) Sprague Dawley rats (Harlan, Indianapolis, IN) and lean and obese Zucker rats (a gift from Dr. J. Porter, Louisiana State University School of Dentistry, New Orleans, LA) were used for these experiments. Animals were housed in a vivarium under a 12-h light, 12-h dark cycle with food and water available ad libitum. The Tulane University Animal Care and Use Committee approved all animal procedures.
Retrograde transsynaptic neuronal tracing
Use of a transsynaptic retrograde label using pseudorabies virus (Bartha strain PRV-152; a gift from L. W. Enquist, Princeton University, Princeton, NJ) expressing enhanced green fluorescent protein (EGFP) has been described (7, 16-21). Under sodium pentobarbital anesthesia (50 mg/kg, ip), rats received injections of PRV-152 (2 × 108 pfu/ml) directed tangentially into the musculature along the greater curvature of the stomach using a Hamilton syringe fitted with a 26-gauge needle. Rats were maintained in a biosafety level 2 laboratory for up to 75 h post injection, at which time the incidence of transsynaptic labeling in the DMV has peaked (18). This type of injection results in a relatively selective, predictable, and sequential transsynaptic labeling of DMV neurons related to gastric motor control (18, 22, 23). It has previously been shown that injection into the lumen of the stomach, on the gastric surface, or ip injection (i.e. not im) does not result in specific or predictable labeling of gastric-related neurons in the DVC in this time frame. Recordings in this study were limited to infection times of less than 75 h, a time at which no apparent degradation of membrane or synaptic properties has been identified (7, 16-23).
Tissue preparation
Whole-cell patch-clamp recordings were made in transverse brain-stem slices (300–400 μm) containing the DMV from rats (21–60 d old). Under deep anesthesia (sodium pentobarbital; 100 mg/kg, ip) or halothane inhalation, animals were decapitated and the brainstem removed and immersed in ice-cold (0–4 C), oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mm) 124 NaCl, 3 KCl, 26 NaHCO3, 1.4 NaH2PO4, 11 glucose, 1.3 CaCl2, and 1.3 MgCl2 (pH 7.3–7.4), with an osmolality of 290–305 mOsm/kg. The brainstem then was mounted on a glass stage, and transverse slices were cut with a vibratome. The slices were then transferred to a submersion-type recording chamber mounted on a fixed-stage platform under an upright microscope (Olympus BX50WI, Melville, NY).
After an equilibration period of 1–2 h, whole-cell patch-clamp recordings were obtained from neurons in the DMV using patch pipettes with open tip resistance of 2–5 MΩ. Seal resistance was 1–5 GΩ, and series resistance was less than 24 MΩ, uncompensated. Patch pipettes were filled with (in mm) 130–140 K+ or Cs+ gluconate or KCl, 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 3 KOH or CsOH, 2–4 ATP, and 0.2% biocytin (pH 7.2). Added to the ACSF for specific experiments were tetrodotoxin (TTX; 2 μm; Sigma Chemical Co., St. Louis, MO, or Alomone Labs, Jerusalem, Israel), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μm; Sigma), bicuculline methiodide (30 μm; Sigma), picrotoxin (50–100 μm; Sigma), dl-2-amino-5-phosphono-valeric acid (AP-5; 50 μm; Sigma), diazoxide (200 μm; Sigma), tolbutamide (200 μm; Sigma), wortmannin (10–100 nm; Alomone Labs), and leptin (3 nm to 1 μm; Sigma or PeproTech, Rocky Hill, NJ). Wortmannin and diazoxide were dissolved in dimethylsulfoxide and added to ACSF to obtain a final dimethylsulfoxide concentration of less than 0.1%. Picrotoxin and tolbutamide were dissolved in 100% ethanol, with the final ethanol concentration in ACSF of less than 0.5%. Leptin (Sigma) was reconstituted in 15 mm HCl, pH normalized with NaOH. For all solvents, vehicle alone at the final concentration was without effect in separate recordings from DMV neurons. All other drugs were dissolved directly in the ACSF. Leptin was typically bath applied for 5–8 min and then removed from the perfusion system. A change in membrane potential was required to be at least 2 mV in amplitude, the onset was required to be associated temporally with the leptin application (i.e. usually beginning at about 2 min after changing solutions, the time it took for leptin to arrive at the recording chamber), and the response had to be saturated and stable within a few minutes (i.e. did not continually change) and had to be reversible or partially reversible upon washout of the drug. The value of the membrane potential was measured at a specific time after leptin application (i.e. 3-4 min after the drug arrived in the chamber). For some experiments, slices were perfused with ACSF containing 2.5 mm glucose by replacing glucose with equiosmolar amounts of sucrose.
Recording pipettes were pulled from borosilicate glass capillaries of 1.65 mm outer diameter and 0.45 mm wall thickness (Garner Glass Co., Claremont, CA). Electrophysiological signals were recorded using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA), low-pass filtered at 5 kHz, digitized at 88 kHz (Neuro-corder; Cygnus Technology, Delaware Water Gap, PA), stored on videotape, and analyzed off-line on a PC using pCLAMP programs (Axon Instruments) or Mini-analysis (Synaptosoft, Decator, GA). Recordings were performed under visual control in a recording chamber on an upright, fixed-stage microscope equipped with infrared differential interference contrast and epifluorescence (Olympus BX50WI). Epifluorescence was briefly used to target fluorescent cells, at which time the light source was switched to infrared differential interference contrast to obtain the whole-cell recording. Once in the whole-cell configuration, cells were voltage clamped for 5 min near resting membrane potential (determined by temporarily removing the voltage clamp) to allow equilibration of recording-pipette contents with the intracellular milieu. Synaptic currents were examined at holding potentials positive and negative to resting membrane potential [−30 to 0 mV for inhibitory postsynaptic currents (IPSCs) and −60 to −80 mV for excitatory postsynaptic currents (EP-SCs)]. Rat DMV neurons received EPSCs and IPSCs, which could be entirely blocked by application of ionotropic glutamate receptor antagonists (i.e. CNQX and AP-5) or GABAA receptor antagonists (i.e. picrotoxin or bicuculline), respectively, as previously described (20, 21, 24, 25). Current-clamp (i.e. voltage) recordings were performed at resting membrane potential. Input resistance was assessed by measuring the voltage deflection at the end of the response to injected rectangular current pulses (100–500 ms of ±10–50 pA).
Stimulation and recording
Electrical stimulation of DMV afferents (putatively originating from the NTS) was performed using a concentric bipolar electrode (125 μm outer diameter, 12.5 μm inner diameter; FHC, Bowdoinham, ME) placed over the NTS. The stimulus intensity required for minimal response was determined, and then the intensity was increased until a constant-latency response was consistently obtained over 10 consecutive stimuli at 4- to 5-sec intervals (0.20-0.25 Hz). At these stimulation intervals, the PSC amplitude was relatively consistent from pulse to pulse and recovered fully before application of subsequent stimuli. Pairs or trains of stimuli (25-50 Hz interpulse frequency for EPSCs and 10-25 Hz interpulse frequency for IPSCs) were applied to establish constant-latency-evoked PSCs and paired-pulse ratios (20, 21, 25, 26).
Caged glutamate photolysis
Similar to previous descriptions of glutamate photolysis in the vagal complex (19, 20, 24), γ-(-carboxy-2-nitrobenzyl) ester, trifluoroacetic acid salt (i.e. CNB-caged glutamate, 250 μm; Molecular Probes, Eugene, OR) was added to recirculating ACSF and uncaged using brief pulses of UV light directed into the slice. Fluorescent light (UV filter; Chroma Technology, Rockingham, VT) was directed onto the slice through the ×40 objective used to obtain the recording. The objective was initially positioned directly over the recorded cell to observe a direct glutamate-induced inward current. It was then moved progressively further away from the recorded cell until a photolysis-mediated increase in synaptic events was found. Uncaging glutamate directly onto the recorded neuron (10- to 20-sec interval, 20- to 50-msec exposure) resulted in a fast inward current (50–200 pA at a holding potential of −60 mV). Exposure time was electronically controlled using a shutter (Vincent Associates, Rochester, NY). Opening the shutter with no UV filter or with other filters in place (e.g. fluorescein isothiocyanate) did not result in uncaging. The effective diameter of the uncaging (50–100 μm) was set by apertures in the light path and measured by moving the center of the illumination away from the cell and testing for a direct inward current after uncaging. The distance the objective was moved was analyzed post hoc by comparison with a scale micrometer (Microbrightfield, Williston, VT). No synaptic responses were observed when photolytic uncaging of glutamate occurred on the tractus solitarius (TS) or at sites just outside the NTS (which were used as negative controls).
Analysis
A value of twice the mean peak-to-peak noise level for a given recording in control solutions was used as the detection limit for minimal PSC amplitude (i.e. typically 5–10 pA). For spontaneous EPSCs and IPSCs (sEPSCs and sIPSCs), at least 2 min of activity was examined to identify leptin effects on amplitude and frequency distributions. Effects of leptin on spontaneous PSC frequency before, during, and after drug application were analyzed within a recording using the Kolmogorov-Smirnov (K-S) test (a nonparametric, distribution-free goodness-of-fit test for probability distributions). Typically, 100–300 events were compared for each condition. Pooled results from responding cells were analyzed using a paired t test; multiple groups of cells were compared using ANOVA. Proportions of responding cells from different groups were analyzed using a χ2 test of independence. Effects of leptin on spontaneous and evoked PSC amplitude were analyzed using a paired two-tailed t test. Measurements of five to 10 electrically evoked responses, not including failures, were used to obtain mean synaptic current amplitudes. Paired-pulse ratios were used as indirect measures of changes in the probability of release, in which a change in the amplitude ratio of the first to the second response to paired TS stimuli implicated a presynaptic site of action. Membrane potential values were compensated to account for junction potential (−8 mV). Results are reported as the mean ± sem unless indicated otherwise; significance was set at P < 0.05 for all statistical measures.
Histology
Recorded neurons were identified as EGFP labeled as described previously (7, 16, 18-21) via real-time visualization under fluorescent microscopy and/or via post hoc identification using the avidin-Texas Red reaction (Fig. 1). Subsequent to recording, slices were fixed in 4% para-formaldehyde in 0.15 m sodium phosphate buffer overnight. After rinsing three times with 0.01 m PBS, slices with EGFP-labeled cells (i.e. PRV-152 labeled) containing recorded, biocytin-filled neurons were immersed in Texas Red-conjugated avidin (1:400; Vector Laboratories, Burlingame, CA) to visualize the filled neurons. Slices were then washed in PBS, mounted on glass slides, and visualized using epifluorescence illumination (Leica DMLB) and a Spot RT CCD camera (Diagnostic Instruments, Sterling Heights, MI) or a confocal microscope (Zeiss LSM 510 META).
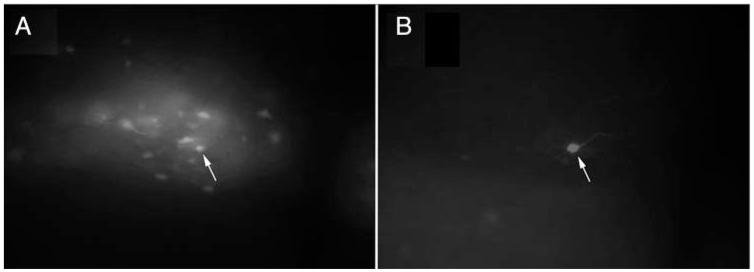
FIG. 1.
DMV neurons that expressed EGFP were visualized and targeted for whole-cell recordings. A, Fluorescent illumination of a gastric-related EGFP-labeled NTS neuron targeted for recording (×20); B, image of the same biocytin-filled neuron, reacted post hoc for Texas Red-Avidin (×20). Arrows point to the targeted neuron in both images.
Results
Effects of leptin on membrane potential
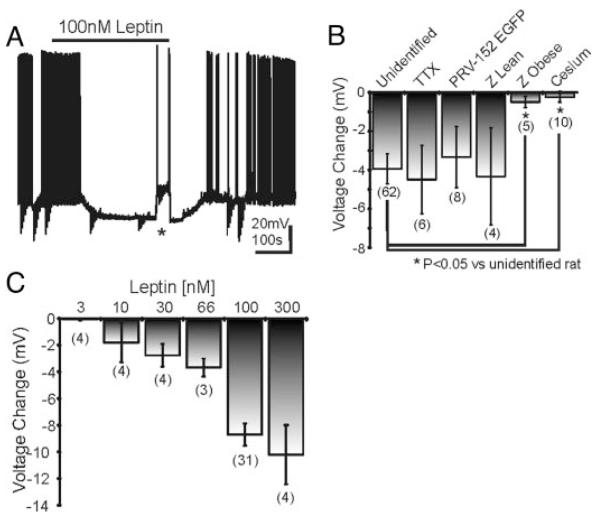
To analyze leptin effects on membrane potential, DMV neurons were recorded at rest in current-clamp mode. Alternatively, neurons recorded in voltage-clamp mode were monitored for changes in resting membrane potential by periodically removing voltage clamp (i.e. switch to I = 0). Bath application of leptin (100 nm) caused a rapid (range, 2-10 min) membrane hyperpolarization of −8.6 ± 0.7 mV from rest (−57.5 ± 1.3 mV) in 31 of 62 unidentified DMV neurons (Fig. 2, A and B). The membrane potential of the remaining neurons either depolarized (4.2 ± 0.6 mV; n = 9) or remained unchanged (−0.1 ± 0.2 mV; n = 22). Within a recording, the hyperpolarization was at least partially reversible within 15 min in 22 neurons. Application of different concentrations of leptin from 3-300 nm resulted in a concentration-dependent hyperpolarization of the membrane potential (Fig. 2C). In the presence of 2 μm TTX, a hyperpolarization was induced by leptin (100 nm) in four of six cells (−6.3 ± 2.1 mV; n = 4; Fig. 2B), indicative of a direct membrane hyperpolarization independent of action-potential-mediated synaptic transmission.
FIG. 2.
Leptin induced a membrane hyperpolarization in DMV neurons. A, Current-clamp recording of an unidentified DMV neuron depicting a characteristic leptin-induced hyperpolarization (100 nm; peak =−75 mV) from resting membrane potential (−57 mV). Downward deflections are responses to rectangular current steps; asterisk indicates where the membrane potential was transiently adjusted to preleptin levels to test for changes in whole-cell conductance and evoked action potential frequency. B, Histogram shows the leptin-induced hyperpolarization in normal Sprague Dawley rats, PRV-152-labeled neurons, and in the presence of TTX from Sprague Dawley rats and lean and obese Zucker rats. C, Histogram showing the concentration dependence of the response to leptin in unidentified NTS neurons. Number of replicates at each concentration is in parentheses below each bar.
The amplitude of the hyperpolarization was plotted as a function of the resting membrane potential of each cell at the time of leptin application. Extrapolation of linear regression plots revealed an estimated reversal potential of −90 mV for the hyperpolarization, which is near the calculated K+ equilibrium (calculated EK =−99.7 mV). Consistent with this analysis, the membrane potential of three DMV neurons was monitored after leptin application at three different membrane potentials, controlled with direct current injection through the recording pipette. The amplitude of the hyperpolarization was plotted as a function of the membrane potential during the 60 sec immediately preceding leptin application. Extrapolation of linear regression plots revealed an estimated reversal potential of −94.6 ± 2.9 mV for the hyperpolarization in these neurons (n = 3). This voltage was near the calculated K+ equilibrium potential of −99 mV and was more negative than the Cl- equilibrium potential of −83 mV. To further assess the involvement of Cl- in the leptin-induced hyperpolarization, eight neurons were recorded with KCl in the intracellular pipette, which reversed and increased the driving force for Cl- by setting the Cl- equilibrium potential near 0 mV. Three of these neurons were recorded in current-clamp mode to assess membrane potential deflections, and the other five cells were recorded in voltage-clamp mode to evaluate changes in whole-cell current. In current-clamp mode, leptin superfusion resulted in a hyperpolarization in one of three neurons (4 mV). The remaining two neurons were unchanged. In voltage-clamp mode, leptin superfusion resulted in an outward current in two of five neurons, with no change observed in the remaining three cells. A large depolarization or inward current was never apparent when KCl was used in the recording pipette. In a separate set of recordings, Cs+ was used as the primary cation in the recording pipette, which effectively blocked all outward K+ currents. No change in membrane potential was observed in any neurons in which Cs+ was used as the major cation in the recording pipette (−0.2 ± 0.1 mV; n = 10; Fig. 2B). Together, these data implicate K+ as the major cation responsible for the membrane hyperpolarization.
Obese Zucker rats (fa/fa) have a single point mutation in the long form of the leptin receptor (Ob-Rb), which suppresses activation of intracellular signaling cascades subsequent to leptin binding, whereas the homozygous (Fa/Fa) and heterozygous lean Zucker rats (Fa/fa) have functional Ob-Rb signaling. A hyperpolarization was observed after leptin application (100 nm) in three of four neurons from lean Zucker rats (−5.3 ± 2.8 mV; n = 3; Fig. 2B). However, no effect of leptin on membrane potential was detected in any neuron from obese Zucker rats (−0.5 ± 0.2 mV; n = 5; Fig. 2B), implicating the Ob-Rb in the response.
Leptin effects on neuronal excitability
In current-clamp configuration, rectangular current steps (400 msec; ±10-50 pA) were applied through the recording pipette to test the hypothesis that the leptin-induced hyperpolarization was associated with a modulation of neuronal excitability and/or input resistance. The hyperpolarization was accompanied by a 39% decrease in whole-cell input resistance, such that the input resistance was reduced from 614.5 ± 79.5 MΩ in control ACSF to 373.6 ± 48.0 MΩ in leptin (n = 20 from unidentified neurons; P < 0.05, paired t test; Fig. 3, A-C). Extrapolation of the slope conductance in control and leptin-containing ACSF revealed a reversal potential of −92.4 ± 4.7 mV (n = 20; Fig. 3B), which is close to the calculated reversal potential for K+. No change in input resistance was observed when Cs+ was included in the pipette (Fig. 3C). Overshooting action potentials were evoked by applying depolarizing current steps (10-20 pA) through the recording pipette. The leptin-induced hyperpolarization was accompanied by a decrease in action potential frequency in response to depolarizing current injection (Fig. 3D). Furthermore, in cells that hyperpolarized, the decrease in action potential frequency occurred when the membrane potential was adjusted to preleptin level with direct current injection. Leptin therefore activated a membrane conductance (i.e. a putative K+ current) and decreased the excitability of DMV neurons.
FIG. 3.
Leptin induced a decrease in whole-cell input resistance and responsiveness in DMV neurons. A, Current-clamp recording from the same unidentified DMV neuron as in Fig. 2A. Positive current injection to return the membrane potential to control level revealed a decrease in voltage deflection and decreased responsiveness to current injection after leptin (100 nm) application. B, Current vs. voltage (I-V) plot from the DMV neuron shown in A illustrates a characteristic reduction in input resistance subsequent to leptin application. Arrows indicate responses before, during, and after leptin application (reversal =−91.7 mV). The calculated reversal potentials for Cl- and K+ are indicated with a dashed line. C, Plot demonstrates decreases in whole-cell input resistance from the neuron groups examined. Asterisks indicate populations in which a significant reduction in input resistance was observed (P < 0.05). Number of replicates is in parentheses. D, Current vs. frequency (I-F) plot demonstrates the leptin-induced suppression of action potentials in DMV cells that were also hyperpolarized (n = 9). Asterisks indicate significant reduction in frequency vs. control (P < 0.05, paired t test).
Four neurons that were depolarized in response to leptin (100 nm) were subjected to a rectangular current step protocol (400 msec; ±10-50 pA) to obtain a current-voltage (I-V) plot. All four neurons exhibited an increase in the whole-cell input resistance suggestive of a decreased conductance (503.4 ± 78.3 MΩ in control ACSF; 644.4 ± 86.7 MΩ in leptin; n = 4; P < 0.05, paired t test). Extrapolation of the I-V plot revealed a membrane potential dependence of the leptin-induced depolarization that was near the reversal potential for K+ (−88.8 ± 6.4 mV; n = 4), suggesting leptin may have inhibited a tonically activated potassium conductance in this subset of DMV neurons.
Mechanisms of leptin-induced hyperpolarization
The data above suggested that leptin rapidly induced a K+-dependent membrane hyperpolarization in the majority of rat DMV neurons. Previous studies have shown that leptin altered the membrane potential via activation of an ATP-sensitive K+ conductance (7, 13, 14, 27), which involved a phosphoinositide-3-kinase (PI3K)-mediated mechanism in hypothalamic cell lines (28) and in neurons from a slice preparation containing either the hypothalamus or the NTS (7, 27). To investigate whether a similar conductance contributed to the leptin-induced hyperpolarization in DMV neurons, tolbutamide was used to block ATP-sensitive K+ channels, and the selective PI3K inhibitor wortmannin was used to test the contribution of PI3K to the leptin-induced hyperpolarization. In eight of 12 neurons tested, bath application of tolbutamide (200 μm) did not result in a change in the membrane potential (−0.1 ± 0.1 mV; n = 8; Fig. 4D). Superfusion of tolbutamide slightly depolarized the remaining subset of cells (2.8 ± 0.4 mV; n = 4). In current-clamp configuration, rectangular current steps (400 msec; ±50 pA) were applied to the membrane to obtain a current-voltage (I-V) plot. Extrapolation of a linear regression revealed a membrane potential dependence of the tolbutamide-induced depolarization that correlated with the reversal potential for K+ (−90.8 ± 4.9 mV; n = 4), suggesting tolbutamide decreased a tonically activated potassium conductance in this subset of DMV neurons.
FIG. 4.
. The sulfonylurea tolbutamide and the selective PI3K inhibitor wortmannin blocked the leptin-induced hyperpolarization in DMV neurons. A, Current-clamp recording at resting membrane potential showing application of leptin (100 nm) in the presence of tolbutamide (200μm). There was no effect of leptin in the presence of tolbutamide. Downward deflections indicate responses to negative current injection steps. B, Current-clamp recording at resting membrane potential showing a leptin-induced hyperpolarization (100 nm). Leptin superfusion (represented by horizontal bar) resulted in a 15-mV hyperpolarization (peak =−73 mV) from rest (−58 mV). Addition of tolbutamide (200 μm) depolarized the cell by 15 mV, returning the cell to preleptin conditions. C, Current-clamp recording at resting membrane potential showing application of leptin (100 nm) in the presence of wortmannin (10 nm). There was no effect of leptin on membrane potential in the presence of wortmannin. D, Plot shows the lack of effect of tolbutamide or wortmannin on membrane potential in DMV neurons and also depicts the lack of a leptin effect when applied in the presence of tolbutamide or wortmannin (replicate number in parentheses above bars). E, Plot represents changes in whole-cell resistance in unidentified DMV neurons before, in leptin, and in the presence of leptin and tolbutamide. Asterisk indicates significant changes from control (P < 0.05, paired t test).
Leptin failed to hyperpolarize DMV neurons in any cells preexposed to tolbutamide (n = 7; −0.2 ± 0.2 mV; Fig. 4, A and D), implicating an ATP-sensitive K+ channel in the response to leptin. Furthermore, tolbutamide was applied subsequent to leptin in four neurons to determine whether the putative K+ current activated by leptin would be affected. Tolbutamide reversed the leptin-induced hyperpolarization in each of the four neurons that had hyperpolarized in response to leptin, inducing a membrane depolarization (Fig. 4B). Tolbutamide application also returned the whole-cell input resistance to 99.9% of the preexposure value to leptin (778.8 ± 169.0 MΩ in control ACSF, 462.0 ± 114.9 MΩ in leptin, and 778.2 ± 149.9 MΩ in leptin plus tolbutamide; Fig. 4E). These data suggested that leptin hyperpolarized DMV neurons via activation of a KATP channel.
When bath-applied at resting membrane potential, wortmannin (10–100 nm) failed to influence the membrane potential of any neuron tested (0.3 ± 0.2 mV change for 100 nm wortmannin; n = 5; 0.5 ± 0.6 mV for 10 nm wortmannin; n = 7; Fig. 4D). Wortmannin prevented the leptin-induced hyperpolarization in all 15 neurons tested at either 100 nm (n = 8) or 10 nm (n = 7; Fig. 4, C and D), implicating involvement of PI3K in the leptin-induced membrane hyperpolarization. Together, these data suggested that the leptin-induced membrane hyperpolarization involved activation of an ATP-sensitive K+ channel via a PI3K-dependent mechanism in DMV neurons.
Leptin effects on membrane potential in gastric-related DMV neurons
Because leptin regulates feeding and ingestion in mammals, we tested the hypothesis that leptin would alter the membrane potential of central neurons most directly responsible for regulating gastric function. Gastric-related neurons were identified and targeted for recording after inoculation of the stomach wall with PRV-152 and subsequent retrograde labeling of DMV motor neurons, as previously described in detail (7, 18, 20, 21). Leptin application (100 nm) caused a membrane hyperpolarization in five of eight neurons expressing EGFP after PRV-152 inoculation of the stomach (n = 5; −7.0 ± 1.2 mV; Fig. 2B). Similar to unidentified DMV neurons, the membrane hyperpolarization was accompanied by a 45% decrease in whole-cell input resistance, such that the input resistance was reduced from 525.0 ± 89.0 MΩ in control ACSF to 287.6 ± 37.9 MΩ in leptin (Fig. 3B; n = 5; P < 0.05, paired t test). A decrease in evoked action potential frequency was also observed, indicating that leptin suppressed the cellular activity of gastric-related DMV neurons.
Leptin effects on spontaneous excitatory synaptic transmission
To describe the effects of leptin on excitatory synaptic activity, neurons were voltage-clamped at −65 mV, allowing for examination of spontaneous inward postsynaptic currents. As described previously (20, 21, 24, 25), these inward postsynaptic currents were distinguished from small outward currents by their polarity, were blocked by CNQX and AP-5, reversed polarity near 0 mV, and thus were considered to be glutamatergic sEPSCs. The frequency of sEPSCs under normal conditions was 3.7 ± 0.8 Hz (n = 12). With a similar time course to the leptin-induced membrane hyperpolarization (i.e. beginning <5 min after application), leptin (100 nm) decreased the frequency of sEPSCs in six of 12 DMV neurons in normal rats from 4.0 ± 1.4 Hz in normal ACSF to 1.9 ± 0.6 Hz in leptin (51% decrease; P < 0.05; paired t test; n = 6; Fig. 5). Effects on sEPSCs were reversible within a recording for five of these neurons such that after washing to control ACSF, the frequency of sEPSCs was 4.0 ± 2.2 Hz (n = 5). Of the remaining neurons tested, the K-S test showed five of the six neurons were unaffected by leptin (8% decrease; 2.5 ± 0.7 Hz in control ACSF to 2.3 ± 0.5 Hz in leptin; n = 5; P > 0.05). The remaining neuron exhibited a slight increase of sEPSC frequency in response to leptin (13% increase; n = 1). The overall frequency of sEPSCs in the population was significantly reduced in response to leptin (38% decrease; 3.7 ± 0.8 Hz in control ACSF to 2.3 ± 0.6 Hz in leptin; n = 12; P < 0.05; paired t test; Fig. 5C), whereas the amplitude of sEPSCs was unaffected (2.6% increase; −19.0 ± 1.1 pA in control ACSF to −19.5 ± 1.7 pA in leptin; n = 12; P > 0.05, paired t test; Fig. 5C).
FIG. 5.
Leptin suppressed excitatory synaptic frequency (sEPSCs and mEP-SCs) in the DMV. A, Voltage-clamp recording from an unidentified DMV neuron before, during, and after leptin (100 nm; Vm =−65 mV); A1, expanded portions of corresponding traces above in A. B, Cumulative frequency plot shows a significant decrease in the frequency of sEPSCs in this neuron (P < 0.05, K-S test). C, Histogram indicates changes in sEPSC frequency and amplitude in the entire population of DMV neurons (n = 12). Also depicted are changes in mEPSC frequency and amplitude from the entire population of DMV neurons (2 μm TTX; n = 8). Asterisks indicate significant changes (P < 0.05, paired t test).
Leptin effects on miniature excitatory synaptic transmission
To investigate the possible location of receptors responsible for the decrease in sEPSC frequency, we analyzed the effect of leptin on miniature EPSCs (mEPSCs) in unidentified DMV neurons. In the presence of TTX (2 μm), which blocked action-potential-dependent synaptic activity, mEPSC frequency was 2.7 ± 0.6 Hz (n = 8). In six of eight neurons, application of leptin (100 nm) significantly decreased the frequency of mEPSCs (38% decrease; 3.2 ± 0.6 Hz in control ACSF to 2.0 ± 0.5 Hz in leptin; n = 6; P < 0.05, paired t test). No leptin-induced change in frequency was observed in one of the remaining cells (9% decrease; P > 0.05, K-S test), whereas the other cell irreversibly increased in frequency by 38%. Overall, leptin (100 nm) perfusion resulted in a significant reduction in the frequency of mEPSCs in DMV neurons (33% decrease; 2.7 ± 0.6 Hz in normal ACSF to 1.8 ± 0.4 Hz in leptin; P < 0.05, paired t test; n = 8; Fig. 5C), without an accompanying change in mEPSC amplitude (16.6 ± 1.0 pA in normal ACSF to 16.4 ± 1.0 pA in 100 nm leptin; P > 0.05, paired t test; n = 5; Fig. 5C). This implied that leptin acted at receptors on terminals presynaptic to the recorded neuron.
Leptin effects on spontaneous inhibitory synaptic transmission
To describe the effects of leptin on inhibitory synaptic activity, neurons were voltage clamped at −10 mV, allowing for examination of spontaneous outward postsynaptic currents. As described previously (20, 21, 24, 25), these outward postsynaptic currents were distinguished from small inward currents by their polarity, were blocked by picrotoxin and bicuculline, reversed polarity near −70 mV, and thus were considered to be GABAergic sIPSCs. The frequency of sIPSCs under normal conditions was 2.4 ± 0.6 Hz (n = 12). Bath application of leptin (100 nm) resulted in a small decrease in the frequency of sIPSCs in only three of 12 DMV neurons in normal rats from 2.8 ± 1.0 Hz in normal ACSF to 2.4 ± 1.3 Hz in leptin (14% decrease; P < 0.05, K-S test; n = 3). Leptin perfusion also resulted in an increased frequency of sIPSCs in three of 12 DMV neurons from 1.9 ± 1.2 Hz in control ACSF to 2.8 ± 1.5 Hz in leptin (32% increase; P < 0.05, K-S test; n = 3), whereas the remaining six neurons were unaffected (6% decrease; 2.8 ± 0.9 Hz in control ACSF to 2.7 ± 0.8 Hz in leptin; n = 6; P > 0.05, paired t test). Overall, leptin (100 nm) failed to influence the frequency of sIPSCs in DMV neurons (2.4 ± 0.6 Hz in normal ACSF to 2.4 ± 0.5 Hz in leptin; P > 0.05, paired t test; n = 12; Fig. 6C). Leptin also failed to affect the amplitude of sIPSCs (4% from control; P > 0.05, paired t test; n = 12; Fig. 6C). These data indicated that leptin had no consistent effect on inhibitory synaptic input to DMV neurons.
FIG. 6.
Leptin failed to influence sIPSC within the DMV. A, Voltage-clamp recording from an unidentified DMV neuron before, during, and after leptin (100 nm; Vm =−10 mV); A1, Expanded portions of corresponding traces in A. B, Cumulative frequency plot indicates no change in the frequency of sIPSCs (P > 0.05, K-S test). C, Histogram depicts overall changes in sIPSC frequency and amplitude in the DMV (P > 0.05, paired t test).
Effects on synaptic transmission electrically evoked from the NTS
We previously showed a relatively selective inhibition of glutamatergic, but not GABAergic, synaptic activity within the NTS by leptin and a lack of leptin effects on identified GABAergic neurons in the nucleus (7). A concentric bipolar stimulating electrode (125 μm diameter) was placed over the NTS to test the hypothesis that leptin suppresses excitatory but not inhibitory synaptic input to DMV neurons. As previously described, excitatory currents (EPSCs) were evoked from the NTS when DMV neurons were recorded in voltage clamp at hyperpolarized holding potentials, whereas inhibitory currents (IPSCs) were evoked from the NTS when DMV neurons were recorded at depolarized holding potentials (20, 24). Subsequent to NTS stimulation (Vm =−65 mV), six neurons from unidentified rats received electrically evoked, constant-latency-evoked EPSCs. Leptin reversibly diminished the amplitude of constant-latency-evoked EPSCs in five of six neurons from −89.8 ± 8.2 pA in control ACSF to −67.8 ± 8.7 pA in leptin (25% decrease; n = 5; P < 0.05, paired t test; Fig. 7, A-C). All neurons were recorded using Cs+ as the primary intracellular cation, suggesting the effect of leptin on evoked EPSC amplitude was not due to a change in input resistance in the DMV neuron caused by postsynaptic leptin receptor activation. The paired-pulse ratio was examined to assess whether leptin may have acted presynaptically to suppress constant-latency EPSCs. Stimuli paired at 25-50 Hz revealed a paired-pulse depression in EPSC amplitude, where the amplitude of the second evoked EPSC was smaller than the first evoked EPSC. Bath application of leptin suppressed the amplitude of the first evoked EPSC to a larger degree than the second in all affected cells, revealing an increase in the paired-pulse ratio from 0.74 ± 0.02 in control ACSF to 0.96 ± 0.09 in leptin (n = 5; P < 0.05, paired t test; Fig. 7D). These data indicated that leptin decreased excitatory input within the DMV evoked by stimulation of the NTS.
FIG. 7.
Leptin effects on constant-latency-evoked PSCs after NTS stimulation. A, Traces showing the average response to NTS-evoked EPSCs before, during, and 10 min after perfusion of leptin (100 nm; Vm =−65 mV). Pairs of pulses were generated 30 msec (33 Hz) apart, resulting in EPSCs with paired-pulse depression of the second pulse. B, Currents normalized to amplitude of the first control EPSC in A indicate the first pulse was suppressed to a larger degree than the second in the presence of leptin. C, Percent suppression of evoked EPSC amplitude of the first response to NTS stimulation. Evoked EPSC amplitude was significantly reduced by leptin. D, Shown is a significant increase in the paired-pulse ratios (PPR) of NTS stimulation (numbers of cells are in parentheses). C and D, asterisks, P < 0.05, paired t test. E, Traces showing the average response to NTS-evoked IPSCs before, during, and 10 min after perfusion of leptin (100 nm; Vm =−10 mV). F, Percent suppression of evoked IPSC amplitude of the first response to TS stimulation. Evoked IPSC amplitude was not significantly affected by leptin.
The effects of leptin on evoked IPSCs presynaptic to the recorded neuron were examined in 10 neurons that received constant-latency IPSCs after stimulation of the NTS (Vm = −10 mV). The average amplitude of the evoked IPSCs was 94.8 ± 10.3 pA (Vm =−10 mV; n = 10). Leptin (100 nm) perfusion irreversibly enhanced the amplitude of evoked IPSCs in one neuron (19.7% from control; 131.8 pA in control ACSF to 167.3 pA in leptin-containing ACSF) but reversibly suppressed the amplitude in another two unidentified neurons (35 and 18% decrease; n = 2). Leptin (100 nm) failed to affect the amplitude of electrically stimulated IPSCs in the remaining eight neurons (7% decrease; 95.2 ± 13.0 pA to 94.5 ± 13.2 in leptin; n = 8; P > 0.05, paired t test). Cumulatively, leptin failed to influence the amplitude of NTS-evoked IPSCs within the entire population of DMV neurons recorded (2% decrease; 94.8 ± 10.3 pA in control ACSF to 92.9 ± 13.7 pA in leptin; n = 10; P > 0.05, paired t test; Fig. 7, E and F).
Effects on synaptic transmission evoked from the NTS using glutamate photolysis
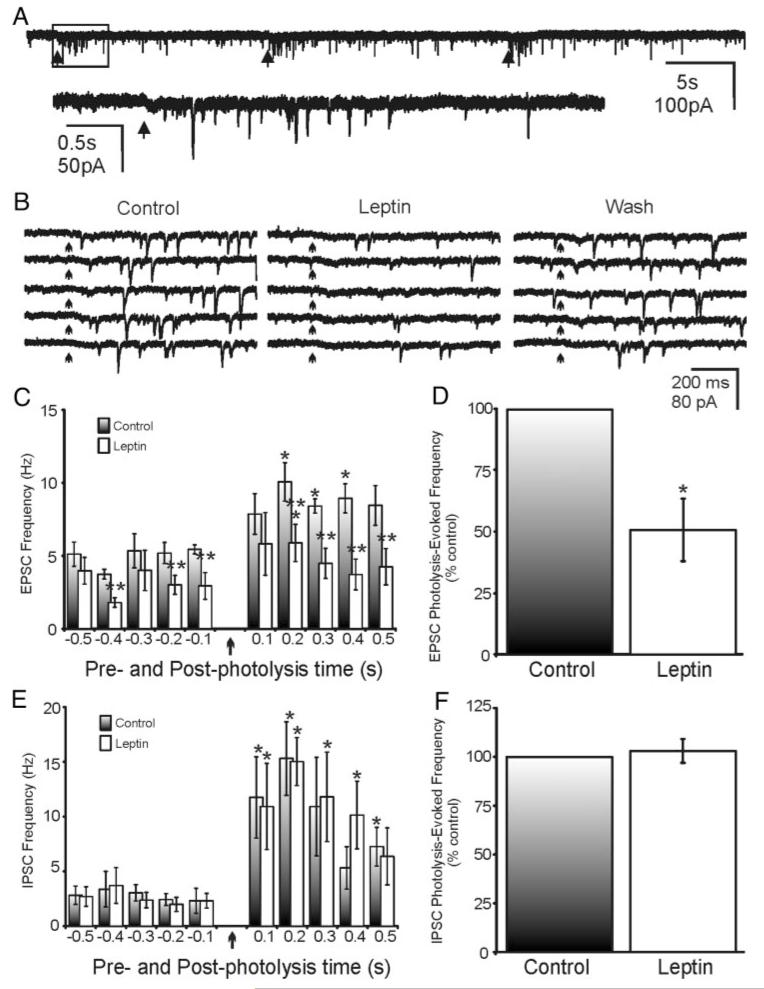
Electrical stimulation results in depolarization of cell somata within the NTS and could also activate fibers of passage traversing the NTS (20, 24). To test the effects of leptin more conclusively on the connections arising from intact neurons in the NTS that project to the DMV, we used glutamate photostimulation to focally release glutamate at discrete sites in the NTS while recording responses in the DMV. As previously described, focal uncaging of glutamate in the NTS results in increased EPSC frequency, which is easily observed at holding potentials slightly negative to rest, and focal uncaging of glutamate in the NTS results in increased IPSC frequency, which is readily observed at more depolarized holding potentials (20, 24). The glutamate photolysis-evoked increase in PSC frequency was abolished when the slice was bathed in TTX, suggesting that these observed responses were due to a glutamate-induced depolarization of NTS neurons, resulting in increased action potentials in intact projections to the DMV and not simply increased transmitter release caused by activation of receptors on terminals (20, 24). In the presence of picrotoxin, the frequency of EPSCs during the 500-msec interval before glutamate uncaging was 5.0 ± 0.5 Hz (Vm =−65 mV; n = 5). Leptin application resulted in a 38% decrease in the frequency of prestimulus EPSCs (5.0 ± 0.5 Hz in control ACSF to 3.1 ± 0.7 Hz in leptin; n = 5; P < 0.05, paired t test). Focal uncaging of glutamate within the NTS induced a transient increase in frequency of EPSCs within DMV neurons from 5.0 ± 0.5 Hz before glutamate photolysis to 8.8 ± 0.8 Hz after glutamate uncaging (n = 5), which typically lasted for 0.5-1.5 sec post stimulus (Fig. 8, A-C). Superfusion of leptin resulted in a 46% decrease in the glutamate photolysis-induced EPSC frequency (8.8 ± 0.8 Hz in control ACSF to 4.8 ± 1.0 Hz in leptin; n = 5; P < 0.05, paired t test). Correcting for sEPSC frequency before glutamate photolysis revealed a glutamate photolysis-evoked EPSC frequency within DMV neurons of 3.8 ± 0.4 Hz (n = 5). Leptin application resulted in a 51% reduction in the frequency of glutamate photolysis-evoked EPSCs from 3.8 ± 0.4 Hz in control ACSF to 1.9 ± 0.5 Hz in leptin (n = 5; P < 0.05, paired t test; Fig. 8D).
FIG. 8.
Leptin effects on PSCs within the DMV evoked by glutamate photolysis in the NTS. A, Repeated glutamate uncaging within the NTS resulted in evoked EPSCs in DMV neurons (Vm = −65 mV). Arrows indicate the time of uncaging. B, Effect of leptin (100 nm) on photolysis-evoked EPSCs in the same cell as in A (arrows indicate the time of uncaging). Shown are photolysis-evoked EPSCs in control, in leptin (100 nm), and after washout. Five separate photolysis-evoked responses are shown for each condition. C, Average frequency of EPSCs during 100-msec periods before and after glutamate uncaging (five neurons). Uncaging occurred at time 0 (arrow). Single asterisk indicates significance vs. prephotolysis values at P < 0.05 (paired t test); double asterisks indicate significant reduction of responses in the presence of leptin vs. control ACSF at P < 0.05 (paired t test). D, Effect of leptin on frequency (500 msec) of glutamate-evoked EPSCs across five neurons, corrected for spontaneous PSC frequency. Values for the leptin effect are normalized to control responses. Asterisk indicates significant reduction vs. control (P < 0.05, paired t test). E, Average frequency of IPSCs during 100-msec periods before and after glutamate uncaging (n = 5). Uncaging occurred at time zero (arrow). Single asterisk indicates significant change vs. prephotolysis values (P < 0.05). F, Effect of leptin on frequency (500 msec) of glutamate-evoked IPSCs across five neurons, corrected for spontaneous PSC frequency. Values for the leptin effect are normalized to control responses.
The frequency of IPSCs during the 500-msec interval before glutamate uncaging was 2.8 ± 0.8 Hz (Vm =−10 mV; n = 5). Leptin application did not affect the frequency of prestimulus IPSCs (2.8 ± 0.8 Hz in control ACSF to 2.6 ± 0.8 Hz in leptin; n = 5; P > 0.05, paired t test). Focal uncaging of glutamate within the NTS resulted in an increased frequency of IPSCs within DMV neurons from 2.8 ± 0.8 Hz before glutamate photolysis to 10.1 ± 2.2 Hz (n = 5), which typically lasted for 0.5-2.0 sec post stimulus (Fig. 8E). The frequency of glutamate photolysis-induced IPSCs remained unchanged in response to leptin (10.1 ± 2.2 Hz in control ACSF to 10.2 ± 2.6 Hz in leptin; n = 5; P < 0.05, paired t test). Correcting for sIPSC frequency before glutamate uncaging revealed a glutamate photolysis-evoked IPSC frequency within DMV neurons of 7.3 ± 2.3 Hz (n = 5). Leptin failed to influence the frequency of glutamate photolysis-evoked IPSCs from 7.3 ± 2.3 Hz in control ACSF to 7.6 ± 2.4 Hz in leptin (6% increase; n = 5; P > 0.05, paired t test; Fig. 8F). These data indicated that leptin potently suppressed EPSCs arising from intact neurons in the NTS to the DMV but had no effect on IPSCs originating from NTS neurons.
Mechanism of effects on synaptic transmission
DMV neurons were exposed to tolbutamide or wortmannin to determine whether leptin suppressed excitatory synaptic input via a mechanism similar to its effects on membrane potential. In these experiments, cesium was the primary intracellular cation to block K+ conductance changes, whereas TTX (2 μm) and picrotoxin (50 μm) were used to isolate effects on terminal release and occlude circuit-dependent changes in GABAA channel binding, respectively. In the presence of tolbutamide (200 μm), leptin (100 nm) failed to influence the frequency of mEPSCs (−4% from control; from 2.8 ± 0.5 Hz in ACSF containing tolbutamide to 2.7 ± 0.5 Hz in ACSF containing both leptin and tolbutamide; n = 5; P > 0.05, paired t test). The amplitude of mEPSCs was also unaffected when in the presence of tolbutamide (1% from control; from 23.0 ± 1.8 pA in ACSF containing tolbutamide to 23.5 ± 2.2 pA in ACSF containing both leptin and tolbutamide; n = 5; P > 0.05, paired t test). In the presence of wortmannin (10-100 nm; n = 10) or LY294002 (10 μm; n = 3), leptin (100 nm) failed to influence the frequency of mEPSCs in any cell tested (6% from control; from 1.6 ± 0.9 Hz in ACSF containing 100 nm wortmannin to 1.7 ± 0.9 Hz in ACSF containing both leptin and wortmannin; n = 5; P > 0.05, paired t test). Leptin also failed to influence the amplitude of mEPSCs in the presence of wortmannin (1% from control; from 20.1 ± 1.7 pA in ACSF containing wortmannin to 20.4 ± 2.0 pA in ACSF containing both leptin and wortmannin; n = 5; P > 0.05, paired t test). These data suggest that leptin suppressed excitatory synaptic activity via a mechanism similar to the leptin-induced membrane hyperpolarization.
Previous studies indicate that KATP channels may be responsible for suppression of synaptic activity at the terminals within several brain areas (7, 29-31). Slices were also perfused with the selective KATP channel agonist diazoxide to test whether activation of an ATP-sensitive K+ conductance on terminals presynaptic to the recorded neuron could suppress excitatory synaptic activity within the DMV. In the presence of TTX (2 μm), picrotoxin (50 μm), and internal Cs+, application of diazoxide (200 μm) alone resulted in a reduction of mEPSC frequency in each of three DMV neurons tested (25% decrease; from 3.2 ± 0.8 Hz in control ACSF to 2.4 ± 0.3 Hz in the presence of diazoxide; P < 0.05, paired t test). However, diazoxide failed to influence the overall amplitude of mEPSCs in affected cells (18.7 ± 1.5 pA in control to 17 ± 1.6 pA in diazoxide; P > 0.05, paired t test). These data support the finding that KATP channel opening suppressed glutamate release at the terminals presynaptic to the recorded DMV neuron.
Glucose sensitivity of the leptin effects
Insulin and leptin both inhibit hypothalamic neurons via a PI3K-mediated activation of KATP channels in 10 mm glucose (13, 14). However, recent evidence suggests that insulin (and possibly leptin) effects may be glucose sensitive (32). Therefore, we examined the effect of leptin on the membrane potential of DMV neurons in 2.5 mm glucose to determine whether glucose concentration qualitatively affected the leptin response. As previously noted (33), storage of slices for an extended period of time in 2.5 mm glucose was detrimental to cell viability. So, slices were stored in ACSF containing 11 mm glucose until they were transferred to the recording chamber, at which time the slices were superfused with ACSF containing 2.5 mm glucose with equiosmolar substitution of sucrose for 30 min. In 2.5 mm glucose, leptin hyperpolarized three of five DMV neurons by 5.1 ± 2.1 mV. The remaining two neurons were depolarized in response to leptin superfusion (4 mV each). We also assessed the ability of wortmannin (10 nm) to suppress the leptin effect in DMV neurons. Perfusion of leptin (100 nm) failed to influence the membrane potential of any DMV neuron in 2.5 mm glucose that was also preexposed to wortmannin (10 nm; n = 5). These data indicate that at 2.5 mm glucose, leptin results in a PI3K-mediated membrane hyperpolarization that mimics the effects observed at 11 mm glucose. The effects of leptin on sEPSC frequency were also examined in 2.5 mm glucose (n = 7). In these cells, leptin (100 nm) significantly decreased sEPSC frequency in four neurons (18 – 43% decrease in sEPSC frequency; P < 0.05, K-S test), increased frequency in one cell, and had no effect in the remaining two neurons.
Discussion
The predominant nature of the effect of leptin in the DMV was inhibitory, acting to hyperpolarize neurons, diminish their responsiveness, and decrease excitatory synaptic inputs. These effects are summarized in Fig. 9, which indicates the effects shown in the present study in combination with those from our previous work (7). These effects may be prominent during vagal reflex activation, when glutamatergic viscerosensory afferents and NTS-to-DMV synaptic connections are modulated. A minority of neurons did not respond to leptin, and an even smaller minority were depolarized by the peptide, which is consistent with the observation that leptin induced c-fos expression in only about 10% of neurons in the DVC (34). The effects of leptin occurred with an onset less than 5 min, similar to several of those effects observed in hypothalamic and brainstem neurons (7, 13, 15) but much shorter than the time frame used for most analyses of leptin effects on feeding in intact animals. Unlike some reports on inhibitory leptin responses (13), but similar to others (7, 35), these effects were concentration dependent and fully reversible, suggesting that leptin is a fully reversible agonist at its cognate receptor in the vagal complex. Similar to reported leptin effects within the NTS (7), leptin predominately induced a membrane hyperpolarization by activating a KATP conductance. Effects were evident in a subset of neurons identified as gastric related via the use of retrograde viral tracer PRV-152. We previously reported an absence of leptin effects in a subset of identified GABAergic neurons within the NTS (7). In agreement with this finding, leptin’s effects were not observed on inhibitory synaptic inputs to DMV neurons. This finding suggests a relatively selective action for leptin to inhibit excitatory, putatively glutamatergic neurons in central vagal circuits. The inhibition of KATP channels on glutamatergic neurons or terminals, in combination with inhibition of DMV neurons, suggests a role for leptin in potently inhibiting vagally mediated visceral motor activity.
FIG. 9.
Summary diagram indicating the location of leptin receptors and leptin effects in the DVC. Shown are four sites of action for leptin in the NTS and DMV: 1) suppression of viscerosensory glutamatergic input to NTS neurons (7); 2) hyperpolarization of glutamatergic NTS neurons (7), including those that project to DMV; 3) inhibition of glutamate release from NTS neurons onto DMV neurons; and 4) hyperpolarization of DMV neurons. No effect of leptin on the GABAergic neurons or on GABA release in NTS or DMV was observed.
Membrane effects
Previous studies of neurons in several brain regions have shown a leptin-induced membrane hyperpolarization due to activating a K+ conductance via a PI3K-dependent mechanism, including a leptin-induced activation of a KATP conductance in NTS neurons (7, 36, 37). Preapplication of tolbutamide prevented the leptin-induced hyperpolarization in DMV neurons. Furthermore, tolbutamide reversed the leptin-induced hyperpolarization, indicating that leptin enhanced the conductance of an ATP-sensitive K+ channel. Preapplication of wortmannin or LY294002 prevented the leptin-induced hyperpolarization, suggesting that PI3K activation was necessary in the intracellular cascade mediating the response within the DMV. This hyperpolarization was not observed in obese Zucker rats (fa/fa), which have a single point mutation in the long form of the leptin receptor (Ob-Rb), resulting in a reduced activation of intracellular signaling cascades subsequent to leptin binding (7, 38-43). Leptin therefore appears to hyperpolarize DMV neurons subsequent to binding the type I cytokine receptor, Ob-Rb, which then activates a KATP channel via a PI3K mechanism.
Most DMV neurons are continuously active at rest (i.e. they fire action potentials). Leptin application hyperpolarized most neurons in the DMV and decreased the action potential frequency. The hyperpolarization was accompanied by a decrease in whole-cell input resistance, which resulted in a decrease in cellular responsiveness. In agreement with a decreased cellular sensitivity, a reduction in action potential frequency was observed when DMV neurons were exposed to depolarizing current injection subsequent to leptin superfusion. Thus, elevated leptin levels within the DMV may result in a silencing of autonomic motor activity, and this effect includes inhibiting neuronal activity related to gastric control.
The effects of leptin were observed in solutions containing 2.5 or 11 mm glucose, indicating that glucose concentration in this range did not qualitatively alter the effect of leptin. This is important because a few recent studies have examined the sensitivity of neurons in the DVC to extracellular glucose concentrations (33, 44). However, direct effects of glucose on DMV vs. NTS neurons remains unclear. A small portion (~20%) of neurons in the vagal complex responded to reducing glucose levels either by a depolarization (~10%) or a hyperpolarization (~10%) of the membrane potential (33). Although the hyperpolarization induced by lowering glucose concentration was attributed to activation of a KATP conductance, low glucose (2 mm) was sufficient to maintain KATP channel closure and prevent fluctuations in the membrane potential in response to reducing glucose levels. A separate study reported that increasing glucose from 5 to 15 and/or 30 mm resulted in an outward current in DMV neurons (44). However, this outward current could be blocked with TTX, implicating modulation of afferent neurons, possibly GABAergic NTS neurons synaptically linked to the DMV, and not a direct membrane current in DMV neurons. Our data and previous literature suggest that glucose levels within the range of 2-11 mm fail to influence the leptin-induced hyperpolarization within the DMV. Additional studies will be required to determine whether extracellular glucose concentration quantitatively enhances or diminishes the amplitude of the response to leptin.
Synaptic effects
Glutamate is released within the DMV from terminals of NTS neurons (20, 24, 25), and probably also from terminals originating from neurons elsewhere in the brain or from TS (45). Bath application of leptin suppressed the frequency of spontaneous glutamatergic EPSCs in the DMV. Moreover, leptin suppressed the frequency, but not amplitude, of mEPSCs, implying a presynaptic action on terminals in apposition to DMV neurons. Previous studies have implicated KATP channels in modulating both action-potential-dependent and -independent synaptic transmission (7, 29-31). Preapplication of wortmannin and tolbutamide prevented the leptin-induced suppression of mEPSCs, implying a PI3K-activated KATP channel in the response. Leptin also suppressed the amplitude of constant-latency EPSCs evoked after stimulation of NTS, indicating that it suppressed glutamate-mediated input. There was a consistent increase in the paired-pulse ratio, which also argues for a presynaptic site of action at the level of terminals in the DMV. Notably, sIPSCs were largely unaffected by leptin. Superfusion of leptin resulted in a decrease in the frequency of glutamate photolysis-evoked EPSCs within the DMV, suggesting actions on circuits originating in the NTS. However, the frequency of glutamate photolysis-evoked IPSCs was not affected by leptin. These data are in agreement with the observed lack of membrane effects of leptin in GAD67/EGFP-expressing NTS neurons in mice (46), further indicative of a phenotypic effect of leptin on glutamatergic circuits in the vagal complex. The synaptic effects of leptin in the DMV appear to be due to hyperpolarization of glutamate neurons in the NTS and/or inhibition of glutamate release from synaptic terminals contacting DMV neurons.
The DVC and energy balance
Leptin receptors are expressed widely in autonomic centers implicated in regulating ingestive behaviors, including the vagal complex and several hypothalamic sites. Recent studies have implicated the body weight and food intake effects of leptin are resultant to leptin actions over a population of leptin-responsive cells, which may include neurons of the DVC in addition to hypothalamic neurons (2-4, 7). The DVC is a critical modulator of visceral function. As previously discussed, DMV motor neuron activity has been linked with behaviors related to feeding and satiety (7). Briefly, classical enhancement of feeding behavior is directly associated with increases in DMV motor activity, whereas satiety is directly correlated with decreases in DMV motor activity (47, 48). However, these responses may not be apparent due to the involvement of different pathways in gastric control at the level of the stomach and other viscera [e.g. the inhibitory non-adrenergic non-cholinergic (NANC) and excitatory cholinergic pathways]. Leptin induced a membrane hyperpolarization in a majority of DMV neurons and suppressed excitatory, but not inhibitory, synaptic activity within the DMV. This cellular inhibition resulted in a suppression of DMV neuron activity, including in gastric-related neurons, and classically would be expected to decrease gastric motility and/or reflex responses as has been previously observed in rats (49). Interestingly, peptides associated with orexigenic behavioral responses (e.g. hypocretin) often result in excitation of these same motor neurons (20), so cellular inhibition by leptin and anorexigenic peptides might be predicted. The ability of leptin to rapidly alter cellular activity within the DVC (i.e. NTS and DMV) probably reports a much faster feedback response to changing leptin levels than has been previously appreciated. These rapid effects can now be directly associated with neurons related to feeding and ingestion.
Similar to effects observed in diencephalic areas, alterations in insulin or glucose levels may also result in a modulation of an ATP-sensitive K+ conductance within the DVC (33, 50). In accordance with these observations, we found that application of tolbutamide, which mimics elevated glucose levels by closing ATP-sensitive K+ channels, failed to influence the membrane potential of most DMV neurons. This suggested that unlike in NTS neurons, ATP-sensitive K+ channels do not contribute to the resting membrane potential in most DMV cells (7). A leptin-induced activation of an ATP-sensitive K+ channel within the NTS and DMV is analogous to effects of lowering glucose in the vagal complex (44). Previous data show that glucose fails to excite most neurons within the DMV and that NTS neurons may be more sensitive to glucose-induced excitation (33, 44, 51). Although we did not detect a qualitative difference in leptin response in 2.5-11 mm glucose, it remains possible that the transient effects of leptin provide a mechanism for leptin regulation during changing energy needs. This may imply that leptin’s effects within the vagal complex are not all or none. Due to a possible common cellular target of these homeostatic regulators within the vagal complex (i.e. KATP channel), the leptin-activated KATP conductance observed here may instead be a substrate for insulin and glucose actions during changing energy needs. Correspondingly, the use of a common ionic mechanism for glucose- and leptin-sensing NTS neurons may be related to the proposed interactions between leptin and other regulators of energy, including glucose metabolism (52). The presence of leptin would thus prime DMV neurons, making them more responsive to changing glucose levels.
In all, leptin suppression of DMV motor activity may result in a decrease in gastric motor tone and subsequent satiety. Additionally, leptin suppression of activity within the DVC may be more closely related to autonomic effects of leptin on blood pressure, glucose production, and/or insulin sensitivity (44, 52, 53). Because of the increased incidence of various disease states such as obesity and diabetes, future studies will likely be focused on determining the effects of several metabolic cues and their integrative role within autonomic centers such as the DVC. Determining how metabolic cues affect neuronal activity and how these cues integrate activity within and across multiple central areas involved in energy homeostasis is critical for understanding autonomic and metabolic disorders such as diabetes, obesity, and hypertension that are increasingly affecting society.
Acknowledgments
We thank Dr. L. Enquist for supplying the PRV-152, Dr. T. Stuart for expanding the viral stock, and Dr. J. Porter for the Zucker rats. Thanks also to M. D. Bhaskaran and H. Gao for comments on the article. Deepest thanks to Dr. W. E. Armstrong for graciously lending his space, time, and effort during a critical and extremely difficult period for our lab and these studies.
This work was supported by the National Institutes of Health (DK56132), the National Science Foundation (IBN-0080322 and IOB-0518209), and the Louisiana Board of Regents.
Abbreviations
- ACSF
Artificial cerebrospinal fluid
- AP-5
dl-2-amino-5-phosphono-valeric acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DMV
dorsal motor nucleus of the vagus nerve
- DVC
dorsal vagal complex
- EGFP
enhanced green fluorescent protein
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- K-S
Kol-mogorov-Smirnov
- mEPSC
miniature EPSC
- NTS
nucleus tractus solitarii
- PI3K
phosphoinositide-3-kinase
- sEPSC
spontaneous EPSC
- TS
tractus solitarius
- TTX
tetrodotoxin
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 2.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 5.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GJ, Moran TH. Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779–3784. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- 7.Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol. 2006;573:395–412. doi: 10.1113/jphysiol.2006.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross PM, Wall KM, Wainman DS, Shaver SW. Subregional topography of capillaries in the dorsal vagal complex of rats. II. Physiological properties. J Comp Neurol. 1991;306:83–94. doi: 10.1002/cne.903060107. [DOI] [PubMed] [Google Scholar]
- 9.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- 10.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 11.Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 12.Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes. 1996;45:1511–1515. doi: 10.2337/diab.45.11.1511. [DOI] [PubMed] [Google Scholar]
- 13.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 14.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 15.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 16.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci USA. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irnaten M, Neff RA, Wang J, Loewy AD, Mettenleiter TC, Mendelowitz D. Activity of cardiorespiratory networks revealed by transsynaptic virus expressing GFP. J Neurophysiol. 2001;85:435–438. doi: 10.1152/jn.2001.85.1.435. [DOI] [PubMed] [Google Scholar]
- 18.Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol. 2003;464:525–539. doi: 10.1002/cne.10831. [DOI] [PubMed] [Google Scholar]
- 19.Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol. 2005;93:2530–2540. doi: 10.1152/jn.00429.2004. [DOI] [PubMed] [Google Scholar]
- 20.Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23:3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, Enquist LW. Pseudorabies virus infection of the rat central nervous system: ultra-structural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinaman L, Card JP, Enquist LW. Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci. 1993;13:685–702. doi: 10.1523/JNEUROSCI.13-02-00685.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017:208–217. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 26.Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J Neurophysiol. 1986;55:1076–1090. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- 27.Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey J, McKenna F, Herson PS, Spanswick D, Ashford ML. Leptin activates ATP-sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. J Physiol. 1997;504:527–535. doi: 10.1111/j.1469-7793.1997.527bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleidervish IA, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. J Neurosci. 2001;21:4600–4608. doi: 10.1523/JNEUROSCI.21-13-04600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto N, Komiyama S, Akaike N. Pre- and postsynaptic ATP-sensitive potassium channels during metabolic inhibition of rat hippocampal CA1 neurons. J Physiol. 2002;541:511–520. doi: 10.1113/jphysiol.2002.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa I, Ogawa Y, Noriyama Y, Nakase H, Yamashita M, Sakaki T. Chemical preconditioning prevents paradoxical increase in glutamate release during ischemia by activating ATP-dependent potassium channels in gerbil hippocampus. Exp Neurol. 2003;183:180–187. doi: 10.1016/s0014-4886(03)00158-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 33.Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 35.Glaum SR, Hara M, Bindokas VP, Lee CC, Polonsky KS, Bell GI, Miller RJ. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Mol Pharmacol. 1996;50:230–235. [PubMed] [Google Scholar]
- 36.Shanley LJ, Irving AJ, Rae MG, Ashford ML, Harvey J. Leptin inhibits rat hippocampal neurons via activation of large conductance calcium-activated K+ channels. Nat Neurosci. 2002;5:299–300. doi: 10.1038/nn824. [DOI] [PubMed] [Google Scholar]
- 37.Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545:933–944. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chua SC, Jr, White DW, Wu-Peng XS, Liu SM, Okada N, Kershaw EE, Chung WK, Power-Kehoe L, Chua M, Tartaglia LA, Leibel RL. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr) Diabetes. 1996;45:1141–1143. doi: 10.2337/diab.45.8.1141. [DOI] [PubMed] [Google Scholar]
- 39.da Silva BA, Bjorbaek C, Uotani S, Flier JS. Functional properties of leptin receptor isoforms containing the Gln→Pro extracellular domain mutation of the fatty rat. Endocrinology. 1998;139:3681–3690. doi: 10.1210/endo.139.9.6168. [DOI] [PubMed] [Google Scholar]
- 40.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita T, Murakami T, Iida M, Kuwajima M, Shima K. Leptin receptor of Zucker fatty rat performs reduced signal transduction. Diabetes. 1997;46:1077–1080. doi: 10.2337/diab.46.6.1077. [DOI] [PubMed] [Google Scholar]
- 42.Rosenblum CI, Tota M, Cully D, Smith T, Collum R, Qureshi S, Hess JF, Phillips MS, Hey PJ, Vongs A, Fong TM, Xu L, Chen HY, Smith RG, Schindler C, Van der Ploeg LH. Functional STAT 1 and 3 signaling by the leptinreceptor (OB-R); reduced expression of the rat fatty leptin receptor in transfected cells. Endocrinology. 1996;137:5178–5181. doi: 10.1210/endo.137.11.8895396. [DOI] [PubMed] [Google Scholar]
- 43.White DW, Wang DW, Chua SC, Jr, Morgenstern JP, Leibel RL, Baumann H, Tartaglia LA. Constitutive and impaired signaling of leptin receptors containing the Gln→Pro extracellular domain fatty mutation. Proc Natl Acad Sci USA. 1997;94:10657–10662. doi: 10.1073/pnas.94.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira M, Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol. 2001;536:141–152. doi: 10.1111/j.1469-7793.2001.t01-1-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang C, Fogel R, Zhang X. Lateral hypothalamus modulates gut-sensitive neurons in the dorsal vagal complex. Brain Res. 2003;980:31–47. doi: 10.1016/s0006-8993(03)02844-0. [DOI] [PubMed] [Google Scholar]
- 46.Williams KW, Smith BN. Leptin inhibits neurons in the rat dorsal motor nucleus of the vagus nerve. Society for Neuroscience; Washington, DC: 2005. [Google Scholar]
- 47.Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 48.Abrahamsson H, Jansson G. Elicitation of reflex vagal relaxation of the stomach from pharynx and esophagus in the cat. Acta Physiol Scand. 1969;77:172–178. doi: 10.1111/j.1748-1716.1969.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 49.Smedh U, Hakansson ML, Meister B, Uvnas-Moberg K. Leptin injected into the fourth ventricle inhibits gastric emptying. Neuroreport. 1998;9:297–301. doi: 10.1097/00001756-199801260-00022. [DOI] [PubMed] [Google Scholar]
- 50.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucosensing and the K(ATP) channel. Nat Neurosci. 2001;4:459–460. doi: 10.1038/87405. [DOI] [PubMed] [Google Scholar]
- 51.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 53.Bogacka I, Roane DS, Xi X, Zhou J, Li B, Ryan DH, Martin RJ. Expression levels of genes likely involved in glucose-sensing in the obese Zucker rat brain. Nutr Neurosci. 2004;7:67–74. doi: 10.1080/10284150410001710401. [DOI] [PubMed] [Google Scholar]