Abstract
Recent advances in the treatment of achalasia include the use of high-resolution manometry to predict the outcome of patients and the introduction of peroral endoscopic myotomy (POEM). The first multicenter randomized, controlled, 2-year follow-up study conducted by the European Achalasia Trial group indicated that laparoscopic Heller myotomy (LHM) was not superior to pneumatic dilations (PD). Publications on the long-term success of laparoscopic surgery continue to emerge. In addition, laparoscopic single-site surgery is applicable to advanced laparoscopic operations such as LHM and anterior fundoplication. The optimal treatment option is an ongoing matter of debate. In this review, we provide an update of the current progress in the treatment of esophageal achalasia. Unless new conclusive data prove otherwise, LHM is considered the most durable treatment for achalasia at the expense of increased reflux-associated complications. However, PD is the first choice for non-surgical treatment and is more cost-effective. Repeated PD according to an “on-demand” strategy based on symptom recurrence can achieve long-term remission. Decision making should be based on clinical evidence that identifies a subcategory of patients who would benefit from specific treatment options. POEM has shown promise but its long-term efficacy and safety need to be assessed further.
Keywords: Esophageal achalasia, Endoscopic pneumatic dilations, Botulinum injection, Peroral endoscopic myotomy, Minimally invasive Heller myotomy
Core tip: Recent progress in esophageal achalasia includes the use of high-resolution manometry to predict the outcome, the introduction of peroral endoscopic myotomy (POEM). The best current treatment option is an ongoing matter of debate. Unless there are more new conclusive data to prove otherwise, laparoscopic Heller myotomy is the most durable treatment for achalasia at the expense of reflux complications. However, pneumatic dilation (PD) is the first choice for non-surgical treatment and is more cost-effective. Repeated PD according to an “on-demand” strategy based on symptom recurrence can achieve long-term remission. POEM is optimistic but needs more long-term efficacy and safety reports.
INTRODUCTION
Achalasia is one of the primary motility dysfunctions of the esophagus that affects both sexes and all races equally[1,2]. The selective loss of inhibitory neurons of the myenteric plexus, which produces vasoactive intestinal polypeptide, nitric oxide (NO), and inflammatory infiltrate, is responsible for abnormal lower esophageal sphincter (LES) dysfunction. This results in unopposed excitation of the LES, and dysfunction or failure of the LES to relax in response to each swallow[3]. Dysphagia for both liquid and solid foods is the most common symptom. Food regurgitation is one of the main associated problems, causing pulmonary complications such as chronic cough and aspiration pneumonia. Gradual weight loss usually occurs as a result.
Achalasia is diagnosed on the basis of tests such as barium esophagography, esophageal manometry, and endoscopy. Pseudoachalsia has to be ruled out by performing endoscopic ultrasound, or computed tomography[4]. A classic “bird-beak” of the gastroesophageal junction, with atonia and a dilated esophageal body detected by barium ingestion and fluoroscopy, are the typical radiological signs. Manometry is still the standard diagnostic test for achalasia. Conventional manometry must at least meet the criteria of absent or abnormal swallowing relaxation of the LES, and the absence of peristalsis in the esophageal body. However, the sensitivities of these traditional studies have been challenged by the recent emergence of advanced techniques for the diagnosis of esophageal achalasia such as the use of high-resolution manometry (HRM) and the addition of pressure topography plotting[5]. Together, these technologies are also called high-resolution esophageal pressure topography[6]. HRM with pressure topography plotting is capable of identifying impaired esophagogastric junction relaxation and subcategorize achalasia into three clinically relevant subtypes based on the contractile function of the esophageal body according to the Chicago classification[6]. Type I (classic achalasia) refers to patients with no significant pressurization within the esophageal body and impaired LES relaxation (Figure 1A). Water swallows cause rapid panesophageal pressurization, which may exceed LES pressure, causing the esophagus to empty for Type II disease (achalasia with compression) (Figure 1B). Type III achalasia, also known as spastic achalasia, is usually associated with rapidly propagated pressurization attributable to an abnormal lumen obliterating contraction (Figure 1C).
Figure 1.
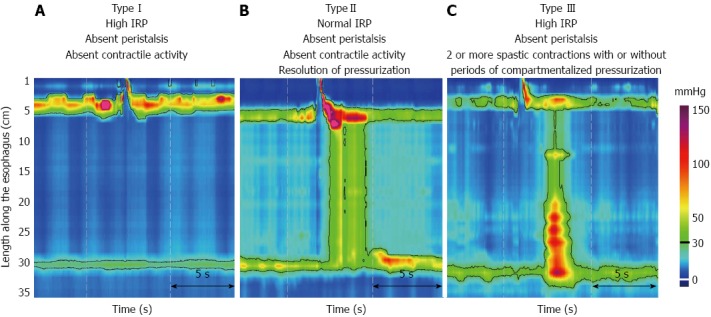
High-resolution manometry with pressure topography plotting classified achalasia into three subtypes. A: Type I (classic achalasia) refers to patients with absent of peristalsis, no pressurization within the esophageal body, high integrated relaxation pressure (IRP); B: Type II (achalasia with compression) refers to patients with absent of peristalsis, and contractile activity, panesophageal pressurization > 30 mmHg, and high IRP; C: Type III patient (spastic achalasia), associates with absent of peristalsis, and two or more spastic contractions with or without periods of compartmentalized pressurization and a high IRP.
Recent advances in the treatment of achalasia include the use of HRM to predict patient outcome, the introduction of peroral endoscopic myotomy (POEM), and laparoendoscopic single-site Heller myotomy with anterior fundoplication. Contributing to the ongoing debate on the superiority of pneumatic dilation (PD) vs laparoscopic Heller myotomy (LHM), the first multicenter, randomized, controlled, 2-year follow-up study conducted by the European Achalasia Trial group indicated that LHM was not superior to PD[7]. Nevertheless, publications on the long-term success of laparoscopic surgery continue to emerge. This review seeks to address this issue and provide an update on the current progress in the treatment of achalasia. Current available treatment modalities include relaxing the LES and relieving the esophageal obstruction[2]. The durability of a successful treatment, complication rates, and cost-benefit are the primary concerns.
TREATMENT OF ESOPHAGEAL ACHALASIA
Pharmacological management
Pharmacological management usually plays a minor role in the treatment of esophageal achalasia. Smooth muscle relaxation is partly effective for the reduction of LES pressure[8]. NO concentration in smooth muscle cells is increased by medication such as nitrates that increase cyclic GMP levels. Calcium antagonists block calcium entry and hence esophageal muscle contraction. When combined, these drugs can reduce LES pressure and ultimately relieve dysphagia but the efficacy is usually unsatisfactory and incomplete. Furthermore, side effects such as headache, dizziness, and pedal edema are important concerns, and they can be intolerable. These effects are similar to those with other drugs such as sildenafil[9].
Endoscopic treatment
Traditional endoscopic treatments for achalasia include injection of botulinum toxin and PD. Recently, a novel endoscopic technique, POEM, has been introduced and tested by gastroenterologists.
Botulinium toxin injection: Botulinum toxin is a biological toxin derived from Clostridium botulinum that causes paralysis of both voluntary and involuntary muscles[10]. It mainly acts at the terminal nerve endings of myoneural junctions by preventing the release of acetylcholine from vesicles, causing chemical denervation that can persist for several months. Botulinium toxin injection (BTI) is a treatment option for achalasia, and it is associated with a wide safety range and fewer complications[11]. Local injection of the toxin into the LES of patients with achalasia lowers sphincter tone, and the patient becomes asymptomatic. This treatment is reported to have excellent immediate responses (success rates > 90%)[11]. BTI is associated with a significant improvement in all objective tests of esophageal function, such as decreased LES pressure, increased esophageal diameter, and improvement of transit time by scintigraphy. Complications of BTI therapy for achalasia are minor, with approximately 25% of patients presenting with transient chest pain and < 5% complaining of reflux symptoms. The dosage used is too small to induce serious adverse effects such as generalized paralysis. The main drawback of BTI is its short duration of effect, which lasts only 6-9 mo in most patients. Based on the number of injections required, the treatment costs are 50% higher than those of PD. Success rates have been reported to be highest among elderly patients and in patients with an LES pressure not exceeding the upper normal level prior to treatment[12]. Therefore, it is currently recommended to treat only elderly and high-risk patients with concomitant comorbid diseases.
PD: In this simple forceful bougie dilation method, considerable stretching strength is required for the dilation to result in an effective mechanical tear in the muscle fibers of the LES. The most commonly used dilator is the Rigiflex dilator with a fully inflated diameter that is usually ≥ 3 cm to achieve a satisfactory result, and is able to achieve maximal pressure. This procedure can be guided using fluoroscopy[13-15] or endoscopy[2,16,17]. The number of dilation sessions and the inflation time needed for a successful dilation vary and are operator dependent. A single dilation session with a bigger dilator may be used in patients presenting with relapse based on symptom scores[18]. Progressive PD methods, such as a series of dilations on successive days using a larger dilator, have been proposed[19]. Immediate and short-term results have reportedly been good in most series[20-25]. However, the first 5-10 years of published follow-up studies have shown that 20%-75% of patients needed a second or even more dilatations[26,27]. Large-scale, long-term follow-up investigations reported unfavorable recurrence in fluoroscopy-guided PD patients[13,27,28]. Repeat PD according to an “on-demand” strategy based on symptom recurrence can achieve long-term remission[24]. Post-dilation radiographic findings in addition to the symptom-based scoring systems can reliably predict clinical remission and indicate the need for further treatment in patients with poor esophageal clearance after dilation to avoid progression to sigmoid type achalasia[29,30].
Complications caused by PD are uncommon. The most severe complication is perforation with an incidence of 1%-2% as shown in Table 1. These perforations are usually minor but can be hazardous if undetected after PD[31]. Reflux symptoms after PD are usually minor and transient, and can be easily controlled with proton-pump inhibitors.
Table 1.
Cumulative effectiveness of pneumatic dilations for treatment of achalasia by using low compliance “Rigiflex” dilators
| No. | Type of dilator (size, cm) | Improvement excellent/good | Mean follow-up (yr) | Complication perforation | Ref. |
| 125 | 3.0-4.0 | 50% | 12.00% | 0.01% | [27] |
| 54 | BMD | 36%-40% | 13.80% | 0.02% | [13] |
| 262 | 3.0-3.5 | 60% | 4.50% | 1.00% | [14] |
| 66 | 3.0-4.0 | 79% | 4.60% | 5.00% | [15] |
| 39 | 3.0-4.0 | 58.3%-78.0% | 9.30% | 5.40% | [24] |
| 50 | 3.0-4.0 | 67%-83% | 2.70% | 0.00% | [25] |
| 106 | 3.0-4.0 | 28%-62% | 3.20% | 2.80% | [28] |
| 209 | 3.0-4.0 | 72% | 5.80% | 0.00% | [23] |
| 55 | 3.0-3.5 | 74.50% | 2.30% | 0.00% | [22] |
| 43 | 3.0-3.5 | 54%-78% | 2.40% | 2.30% | [17] |
| 56 | 3.5 | 89.3%-92.9% | 0.50% | 0.00% | [21] |
| 32 | 3.0 | 69%-91% | 4.50% | 3.30% | [20] |
| 1097 | 3.0-4.0 | 28.0%-92.9% | 0.5%-15.0% | 1.0%-2.0% | Total |
BMD: Browne-McHardy dilator.
Self-expanding metalic stents: A study evaluating the utility of self-expanding, 30-mm metallic stents for achalasia at a single center over a 10-13-year period reported a long-term clinical success rate of > 80%[32,33]. No perforations or mortality associated with the treatment were reported, but stent migration occurred in 5% of patients, reflux in 20%, and chest pain in 38.7%. Overall, the authors claimed that self-expanding, 30-mm metallic stents were associated with a better long-term clinical efficacy in the treatment of patients with achalasia as compared with treatment with PD.
POEM: This novel endoscopic esophagomyotomy method for the treatment of achalasia was first reported by Pasricha et al[34] in porcine models and then by Inoue et al[35] in humans. POEM is performed by dissection and division of the inner circular muscle layer of the esophagus through a submucosal tunnel created endoscopically by a small proximal opening of the esophageal mucosa. When compared with surgical myotomy, POEM can accomplish a longer myotomy. Extending the length of the myotomy to the thoracic esophagus is difficult for the surgeon, especially in patients with advanced disease and in those with severe fibrosis. Theoretically, the risk of injury to the vagus nerve should be lower with this approach.
Increasing numbers of reports on this technique have been published, and all of them showed good short-term results without serious complications; however, long term follow-up results are necessary[35-45]. A recently published prospective, international, multicenter study that aimed to determine the outcomes of 70 patients who underwent POEM at five centers in Europe and North America showed that the percentages of patients with symptom remission at 6 and 12 mo were 89% and 82%, respectively. Zhou et al[45] reported that POEM was a promising new treatment for failed Heller myotomy, resulting in short-term symptom relief in > 90% of cases. Nevertheless, POEM can be a challenging and demanding technique even for experienced endoscopists. Although air leak such as that caused by pneumomediastinum, pneumoperitoneum, and air embolism can be prevented by carbon dioxide insufflation, it can be hazardous in cases of purulent mediastinitis. If it occurs, extensive surgical procedures such as esophagectomy may be necessary instead of revisional surgery because of the inflamed and scarred tissue of the plane between the submucosal and muscular layers after the endoluminal approach[35-41].
Surgical treatment
Myotomy of the LES is the best treatment modality with satisfactory long-term results at the deleterious cost of a high incidence of postoperative reflux. Although controversy exists as to whether a concomitant antireflux procedure is necessary, minimally invasive LHM with a variety of fundoplication procedures has become the primary approach by many surgeons in the majority of patients with achalasia[46-49]. The overall success rates were between 77.0% and 97.2%[46-57] (Table 2). However, different surgeons have different opinions on the length of the myotomy. Generally, most surgeons choose a myotomy length of 4-5 cm onto the esophagus and 2-3 cm onto the stomach[58]. Another controversial issue among surgeons is whether a concomitant antireflux procedure is necessary. Currently, most surgeons perform minimally invasive LHM with a variety of fundoplication procedures in the majority of patients with achalasia, and partial fundoplications are preferred because 360° fundoplications cause more dysphagia[46,47,59,60]. Randomized controlled trials have shown that the addition of an antireflux procedure to a myotomy substantially reduces the postsurgical incidence and severity of pathological reflux[61,62]. Recently, laparoendoscopic single-site surgery has proven to be an archetypal shift to more minimally invasive surgery, and is applicable to advanced laparoscopic operations such as LHM and anterior fundoplication[63]. Esophagectomy may be needed in patients with recurrent disabling symptoms or severe complications.
Table 2.
Cumulative effectiveness of surgical myotomy for achalasia
| No. | Type of surgery | Improvement excellent/good | Mean follow-up (yr) | Complication acid reflux | Ref. |
| 52 | LHM-Dor operation | 92% | 4.3 | 11% | [51] |
| 53 | LHM-Dor operation | 92% | 3.0 | 9% | [61] |
| 75 | LHM-partial fundoplication | 84% | 5.6 | 15% | [48] |
| 71 | LHM-Dor | 85% | 6.0 | 12.70% | [52] |
| 248 | LHM+/-Dor | 88% | 3.4 | 3% | [55] |
| 211 | LHM-Dor | 89% | 5.3 | 34% | [56] |
| 161 | LHM-Dor | 97.20% | 4.6 | 15.70% | [47] |
| 200 | LHM-Dor | 85% | 3.5 | 28% | [57] |
| 46 | LHM-Toupet or Dor | 80% | 6.4 | 9% | [46] |
| 505 | LHM+/- fundoplication | 95% | 2.6 | 16% | [51] |
| 155 | LHM-Dor | 77% | 5.0 | 27% | [54] |
| 137 | LHM-Dor | 94.80% | 5.4 | 10.90% | [59] |
| 1860 | - | 77%-97.2% | 2.6-10.9 | 3%-34% | Total |
LHM: Laparoscopic Heller myotomy.
Overall, postsurgical complications are rare (< 4%)[64]. The major adverse event associated with surgery is severe reflux (3%-34%, Table 2). To minimize the reflux complications, it is generally accepted that a concomitant endoscopic examination during LHM to guide the myotomy and routine fundoplication is clinically necessary with either anterior fundoplication (Dor) or partial posterior fundoplication (Toupet)[65-67]. LMH is superior to thoracoscopic procedures because of the shorter operative time and hospital stay[68].
The reported incidence of esophageal perforation in LHM is 5%-10%. However, robotically assisted Heller myotomy (RAHM) is safer than LHM because it decreases the incidence of esophageal perforation to 0%, even in patients who had undergone previous treatment[69,70]. RAHM with partial fundoplication using a robotic platform appears to be a more precise and safer operation than laparoscopic myotomy, with improved postoperative quality of life. In addition, the outcomes of RAHM are slightly better than those of LHM, although the cost is higher[70].
DECISION MAKING: WHICH IS THE MOST APPROPRIATE TREATMENT
A proposed algorithm for the selection of the optimal treatment modality for esophageal achalasia is summarized in Figure 2. LHM and PD remain the key treatment options. The short-term efficacy of BTI therapy is similar to that of PD, although it is less effective for sustained symptomatic relief in patients with achalasia in comparison to PD and LHM. BTI is also effective in patients with tortuous megaesophagus and previous failed pneumatic dilatations; however, a high rate of relapse during the first year of follow-up has been reported[71]. The selection of BTI should be made with caution in certain patients because some surgeons reported that it increased the risk and difficulty of subsequent LHM[72]. Therefore, taking into account the lower durability of BTI therapy, it is a suitable alternative only in the minority of high-risk patients with comorbidity[2,8].
Figure 2.
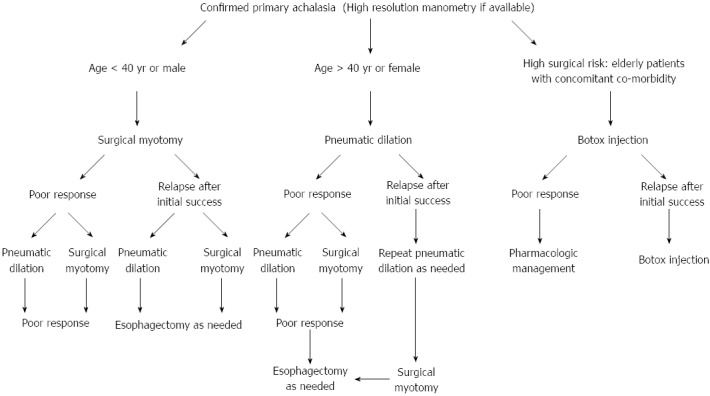
Proposed algorithm for the treatment of esophageal achalasia.
POEM can achieve favorable short-term results comparable to those of any of the above treatment modalities. Moreover, it enables the performance of a longer myotomy, especially in patients with advanced disease and those with severe fibrosis, with a lower risk of injury to the vagus nerve. Validation of the long-term durability and safety of this procedure could make POEM a breakthrough in the treatment of esophageal achalasia. Long-term follow-up of patients who undergo POEM is important to test the durability and safety of the procedure. After all, it is a very technically demanding procedure.
Surgery vs PD
The choice of LHM as the primary treatment for achalasia or as second-line treatment following the failure of nonsurgical intervention remains a topic of controversy after many decades in clinical practice. Several studies have shown that repeated PD according to an on-demand strategy based on symptom recurrence can increase success rates to levels comparable with LHM[26,73,74]. This was supported by the first multicenter, randomized controlled, 2-year follow-up study conducted by the European Achalasia Trial group, which indicated that LHM was not superior to PD[7], and it supports those who are in favor of PD, believing that PD and LHM are equally efficient. This is further supported by advantages of PD such as the fact that it is an outpatient procedure associated with minimal injury, and minimal reflux and bleeding. However, the follow-up duration of this study is not long enough to declare equality between the two procedures. In addition, another disadvantage of PD is that these patients usually require more than one treatment session. Nevertheless, publications on satisfactory long-term success of laparoscopic surgical outcome continue to emerge[75], and patients usually require only one treatment session. Moreover, some surgeons believe that LHM can be more difficult technically following PD but others claim that PD does not hinder future myotomy procedures[76].
Complications and cost-effectiveness, besides the durability of the procedure, are the main concerns for deciding on a treatment option. Perforation of the esophagus occurs in 1%-2% of patients during PD and can be hazardous if left undiscovered[31]. Mucosal tears occur in 12% of patients during LHM but can usually be repaired, and the patients recover. However, the main drawback of LHM is the incidence of acid reflux after surgery, which could be long lasting despite partial fundoplication. Reflux can usually be treated with proton-pump inhibitors; however, long term complications of reflux such as stricture, Barrett’s esophagus, and adenocarcinoma, although rare, must be kept in mind. By contrast, symptoms of reflux in post-PD patients are usually mild and transient and can be easily controlled by prescribing proton-pump inhibitors. When considering the cost-effectiveness of treatment strategies for achalasia, LHM has a higher initial cost and PD is the most cost-effective treatment option for adults with achalasia[77]. However, LHM can be cost-effective if the durability is > 10 years[78]. A recent meta-analysis conducted by Weber et al[79] showed that both PD and LHM are effective treatment options, but LHM might be more durable.
The experience of the surgeons and gastroenterologist is also an important factor for treatment success. More importantly, the decision should be based on clinical evidence that identifies a subcategory of patients who may benefit from a specific treatment option. In general, unless new conclusive data prove otherwise, LHM is the more durable treatment for achalasia, but PD is the first nonsurgical choice and is more cost-effective. Practically, the correction of failed operations for esophageal achalasia is challenging; however, those operations are also performed at high-volume centers using laparoscopic procedures, and many patients prefer to avoid esophagectomy. However, some researchers have reported adverse effects of repeated dilations, especially the risk of perforations, and this must be considered in the decision making process. LMH is recommended for younger patients (< 40 years), male sex, and those showing pulmonary symptoms and failed response to one or two initial dilations[2,8,80].
PREDICTORS OF RISK FACTORS FOR RELAPSE AFTER TREATMENT FOR ACHALASIA
To recognize the risk factors for relapse after treatment is an important issue. It is generally accepted that young age (< 40 years), male sex, a single dilation session with a 3.0-cm balloon, immediate or 3-mo post-treatment LES pressure > 15 mmHg, poor esophageal emptying on timed barium swallow, and classic achalasia are considered the predicting risk factors for relapse after PD[2,8,20,21]. Therefore, both timed barium esophagography and manometry, especially HRM, should be performed at baseline and post-PD, and compared to predict the outcome of patients. The possible impact of the results of HRM on treatment outcome was highlighted in Pandolfino’s landmark study, which showed that Type II achalasia patients were significantly more likely to respond to any therapy [BTI (71%), PD (91%), or LMH (100%)] compared with Type I (56% overall) or Type III (29% overall) patients. Type II achalasia was a predictor of positive treatment response, whereas Type III and pretreatment esophageal dilatation were predictive of a negative treatment response[81]. This was confirmed in another study by Pratap et al[82], which showed that patients with a Type II achalasia pattern (esophageal pressurization) on HRM were more likely to respond to all therapies such as PD, Heller myotomy, and BTI (70%-100% overall), as compared with Type I (≥ 63.3% overall) and Type III approximately 30% overall) patients. More evidence with larger prospective studies and long-term follow-up results are necessary in the new era of HRM (Table 3).
Table 3.
Summary of the cumulative efficacies and complications of current treatment options of achalasia
| PD | Surgical myotomy | BTI | POEM | |
| No. of studies | 12 | 12 | 9 | 11 |
| No. of patients | 1097 | 1860 | 315 | 210 |
| Excellent/good symptom response (range) | 28%-92.9% | 77%-97.2% | At 1 mo: 79% (64%-93%) At 1 yr: 41% (10%-55%) | 82%-100% |
| Follow up (yr) | 0.5-15 | 2.6-10.9 | 18 (6-30) | 0.1-1 |
| Major complications (range) | 1%-2% Perforation | 3%-34% Acid reflux | - | 0.03% Acid reflux |
PD: Pneumatic dilations; BTI: Botulinium toxin injection; POEM: Peroral endoscopic myotomy.
FUTURE PERSPECTIVES
Most existing studies point toward autoimmune mechanisms affecting neurons possibly after an infectious event and an association with certain genetic factors as the possible etiology[83]. The identification of an immunomodulatory drug for the treatment of achalasia is a target to achieve in the future. Evidence indicates that transplanting neuronal stem cells could be “a dream come true” achievement in the future[84]. Theoretically, if this works, both LES function and peristalsis should recover.
CONCLUSION
The debate on PD and LHM is on-going. Unless new conclusive data prove otherwise, LHM is a more durable treatment option for achalasia at the expense of increased reflux complications. However, PD is the first nonsurgical choice and is more cost-effective. Repeated PD according to an on-demand strategy based on symptom recurrence can achieve long-term remission. It is recommended that the decision making should be based on clinical evidence that identifies a subcategory of patients who may benefit from a specific treatment option. POEM is a promising strategy, but more long-term efficacy and safety studies are necessary.
ACKNOWLEDGMENTS
The authors thank Dr. John Pandolfino for providing the figures of HMR and the interpretation.
Footnotes
P- Reviewers Garrigues V, Holscher AH, Tsuboi K S- Editor Gou SX L- Editor Kerr C E- Editor Ma S
References
- 1.Chuah SK, Hsu PI, Wu KL, Wu DC, Tai WC, Changchien CS. 2011 update on esophageal achalasia. World J Gastroenterol. 2012;18:1573–1578. doi: 10.3748/wjg.v18.i14.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuah SK, Wu KL, Hu TH, Tai WC, Changchien CS. Endoscope-guided pneumatic dilation for treatment of esophageal achalasia. World J Gastroenterol. 2010;16:411–417. doi: 10.3748/wjg.v16.i4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champion JK, Delise N, Hunt T. Myenteric plexus in spastic motility disorders. J Gastrointest Surg. 2001;5:514–516. doi: 10.1016/s1091-255x(01)80089-5. [DOI] [PubMed] [Google Scholar]
- 4.Chuah SK, Kuo CM, Wu KL, Changchien CS, Hu TH, Wang CC, Chiu YC, Chou YP, Hsu PI, Chiu KW, et al. Pseudoachalasia in a patient after truncal vagotomy surgery successfully treated by subsequent pneumatic dilations. World J Gastroenterol. 2006;12:5087–5090. doi: 10.3748/wjg.v12.i31.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahrilas PJ. Esophageal motor disorders in terms of high-resolution esophageal pressure topography: what has changed. Am J Gastroenterol. 2010;105:981–987. doi: 10.1038/ajg.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 8.Wen ZH, Gardener E, Wang YP. Nitrates for achalasia. Cochrane Database Syst Rev. 2004;(1):CD002299. doi: 10.1002/14651858.CD002299.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortolotti M, Mari C, Lopilato C, Porrazzo G, Miglioli M. Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology. 2000;118:253–257. doi: 10.1016/s0016-5085(00)70206-x. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh B, Das SK. Botulinum toxin: a dreaded toxin for use in human being. J Indian Med Assoc. 2002;100:607–608, 610-612, 614. [PubMed] [Google Scholar]
- 11.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774–778. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 12.Neubrand M, Scheurlen C, Schepke M, Sauerbruch T. Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy. 2002;34:519–523. doi: 10.1055/s-2002-33225. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt VF, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut. 2004;53:629–633. doi: 10.1136/gut.2003.029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikaeli J, Bishehsari F, Montazeri G, Yaghoobi M, Malekzadeh R. Pneumatic balloon dilatation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther. 2004;20:431–436. doi: 10.1111/j.1365-2036.2004.02080.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan KC, Wong SK, Lee DW, Mui WL, Chan AC, Ng EK, Wu JC, Sung JJ, Chung SC. Short-term and long-term results of endoscopic balloon dilation for achalasia: 12 years’ experience. Endoscopy. 2004;36:690–694. doi: 10.1055/s-2004-825659. [DOI] [PubMed] [Google Scholar]
- 16.Chuah SK, Hu TH, Wu KL, Kuo CM, Fong TV, Lee CM, Changchien CS. Endoscope-guided pneumatic dilatation of esophageal achalasia without fluoroscopy is another safe and effective treatment option: a report of Taiwan. Surg Laparosc Endosc Percutan Tech. 2008;18:8–12. doi: 10.1097/SLE.0b013e31815c1ba2. [DOI] [PubMed] [Google Scholar]
- 17.Dobrucali A, Erzin Y, Tuncer M, Dirican A. Long-term results of graded pneumatic dilatation under endoscopic guidance in patients with primary esophageal achalasia. World J Gastroenterol. 2004;10:3322–3327. doi: 10.3748/wjg.v10.i22.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabharwal T, Cowling M, Dussek J, Owen W, Adam A. Balloon dilation for achalasia of the cardia: experience in 76 patients. Radiology. 2002;224:719–724. doi: 10.1148/radiol.2243011049. [DOI] [PubMed] [Google Scholar]
- 19.Vantrappen G, Hellemans J. Treatment of achalasia and related motor disorders. Gastroenterology. 1980;79:144–154. [PubMed] [Google Scholar]
- 20.Chuah SK, Hu TH, Wu KL, Hsu PI, Tai WC, Chiu YC, Lee CM, Changchien CS. Clinical remission in endoscope-guided pneumatic dilation for the treatment of esophageal achalasia: 7-year follow-up results of a prospective investigation. J Gastrointest Surg. 2009;13:862–867. doi: 10.1007/s11605-009-0804-z. [DOI] [PubMed] [Google Scholar]
- 21.Rai RR, Shende A, Joshi A, Mathur A, Nijhawan S. Rigiflex pneumatic dilation of achalasia without fluoroscopy: a novel office procedure. Gastrointest Endosc. 2005;62:427–431. doi: 10.1016/j.gie.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Iwakiri K, Kawami N, Sano H, Umezawa M, Kotoyori M, Hoshihara Y, Nomura T, Miyashita M, Sakamoto C. Predictors of a better outcome of pneumatic dilatation in patients with primary achalasia. J Gastroenterol. 2010;45:153–158. doi: 10.1007/s00535-009-0145-4. [DOI] [PubMed] [Google Scholar]
- 23.Hulselmans M, Vanuytsel T, Degreef T, Sifrim D, Coosemans W, Lerut T, Tack J. Long-term outcome of pneumatic dilation in the treatment of achalasia. Clin Gastroenterol Hepatol. 2010;8:30–35. doi: 10.1016/j.cgh.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Katsinelos P, Kountouras J, Paroutoglou G, Beltsis A, Zavos C, Papaziogas B, Mimidis K. Long-term results of pneumatic dilation for achalasia: a 15 years’ experience. World J Gastroenterol. 2005;11:5701–5705. doi: 10.3748/wjg.v11.i36.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boztas G, Mungan Z, Ozdil S, Akyüz F, Karaca C, Demir K, Kaymakoglu S, Besisik F, Cakaloglu Y, Okten A. Pneumatic balloon dilatation in primary achalasia: the long-term follow-up results. Hepatogastroenterology. 2005;52:475–480. [PubMed] [Google Scholar]
- 26.Zerbib F, Thétiot V, Richy F, Benajah DA, Message L, Lamouliatte H. Repeated pneumatic dilations as long-term maintenance therapy for esophageal achalasia. Am J Gastroenterol. 2006;101:692–697. doi: 10.1111/j.1572-0241.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 27.West RL, Hirsch DP, Bartelsman JF, de Borst J, Ferwerda G, Tytgat GN, Boeckxstaens GE. Long term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol. 2002;97:1346–1351. doi: 10.1111/j.1572-0241.2002.05771.x. [DOI] [PubMed] [Google Scholar]
- 28.Vela MF, Richter JE, Khandwala F, Blackstone EH, Wachsberger D, Baker ME, Rice TW. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol. 2006;4:580–587. doi: 10.1016/s1542-3565(05)00986-9. [DOI] [PubMed] [Google Scholar]
- 29.Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50:765–770. doi: 10.1136/gut.50.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuah SK, Hu TH, Wu KL, Chen TY, Changchien CS, Lee CM. The role of barium esophagogram measurements in assessing achalasia patients after endoscope-guided pneumatic dilation. Dis Esophagus. 2009;22:163–168. doi: 10.1111/j.1442-2050.2008.00888.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin MT, Tai WC, Chiu KW, Chou YP, Tsai MC, Hu TH, Lee CM, Changchien CS, Chuah SK. Delayed presentation of intrathoracic esophageal perforation after pneumatic dilation for achalasia. World J Gastroenterol. 2009;15:4461–4463. doi: 10.3748/wjg.15.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao JG, Li YD, Cheng YS, Li MH, Chen NW, Chen WX, Shang KZ. Long-term safety and outcome of a temporary self-expanding metallic stent for achalasia: a prospective study with a 13-year single-center experience. Eur Radiol. 2009;19:1973–1980. doi: 10.1007/s00330-009-1373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li YD, Cheng YS, Li MH, Chen NW, Chen WX, Zhao JG. Temporary self-expanding metallic stents and pneumatic dilation for the treatment of achalasia: a prospective study with a long-term follow-up. Dis Esophagus. 2010;23:361–367. doi: 10.1111/j.1442-2050.2010.01048.x. [DOI] [PubMed] [Google Scholar]
- 34.Pasricha PJ, Hawari R, Ahmed I, Chen J, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Gostout CJ. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761–764. doi: 10.1055/s-2007-966764. [DOI] [PubMed] [Google Scholar]
- 35.Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 36.Stavropoulos SN, Harris MD, Hida S, Brathwaite C, Demetriou C, Grendell J. Endoscopic submucosal myotomy for the treatment of achalasia (with video) Gastrointest Endosc. 2010;72:1309–1311. doi: 10.1016/j.gie.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Swanström LL, Rieder E, Dunst CM. A stepwise approach and early clinical experience in peroral endoscopic myotomy for the treatment of achalasia and esophageal motility disorders. J Am Coll Surg. 2011;213:751–756. doi: 10.1016/j.jamcollsurg.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhou PH, Cai MY, Yao LQ, Zhong YS, Ren Z, Xu MD, Chen WF, Qin XY. [Peroral endoscopic myotomy for esophageal achalasia: report of 42 cases] Zhonghua Weichang Waike Zazhi. 2011;14:705–708. [PubMed] [Google Scholar]
- 39.von Renteln D, Inoue H, Minami H, Werner YB, Pace A, Kersten JF, Much CC, Schachschal G, Mann O, Keller J, et al. Peroral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol. 2012;107:411–417. doi: 10.1038/ajg.2011.388. [DOI] [PubMed] [Google Scholar]
- 40.Costamagna G, Marchese M, Familiari P, Tringali A, Inoue H, Perri V. Peroral endoscopic myotomy (POEM) for oesophageal achalasia: preliminary results in humans. Dig Liver Dis. 2012;44:827–832. doi: 10.1016/j.dld.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Gutschow CA, Hölscher AH. Myotomy for esophageal achalasia - laparoscopic versus peroral endoscopic approach. Endoscopy. 2010;42:318–319. doi: 10.1055/s-0029-1244071. [DOI] [PubMed] [Google Scholar]
- 42.von Renteln D, Fuchs KH, Fockens P, Bauerfeind P, Vassiliou MC, Werner YB, Fried G, Breithaupt W, Heinrich H, Bredenoord AJ, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145:309–311.e3. doi: 10.1053/j.gastro.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 43.Lee BH, Shim KY, Hong SJ, Bok GH, Cho JH, Lee TH, Cho JY. Peroral endoscopic myotomy for treatment of achalasia: initial results of a korean study. Clin Endosc. 2013;46:161–167. doi: 10.5946/ce.2013.46.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minami H, Isomoto H, Yamaguchi N, Matsushima K, Akazawa Y, Ohnita K, Takeshima F, Inoue H, Nakao K. Peroral endoscopic myotomy for esophageal achalasia: Clinical impact of 28 cases. Dig Endosc. 2013:Apr 14; Epub ahead of print. doi: 10.1111/den.12086. [DOI] [PubMed] [Google Scholar]
- 45.Zhou PH, Li QL, Yao LQ, Xu MD, Chen WF, Cai MY, Hu JW, Li L, Zhang YQ, Zhong YS, et al. Peroral endoscopic remyotomy for failed Heller myotomy: a prospective single-center study. Endoscopy. 2013;45:161–166. doi: 10.1055/s-0032-1326203. [DOI] [PubMed] [Google Scholar]
- 46.Kilic A, Schuchert MJ, Pennathur A, Gilbert S, Landreneau RJ, Luketich JD. Long-term outcomes of laparoscopic Heller myotomy for achalasia. Surgery. 2009;146:826–831; discussion 831-833. doi: 10.1016/j.surg.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 47.Gockel I, Junginger T, Eckardt VF. Long-term results of conventional myotomy in patients with achalasia: a prospective 20-year analysis. J Gastrointest Surg. 2006;10:1400–1408. doi: 10.1016/j.gassur.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Li YM, Li L. Meta-analysis of randomized and controlled treatment trials for achalasia. Dig Dis Sci. 2009;54:2303–2311. doi: 10.1007/s10620-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 50.Bonatti H, Hinder RA, Klocker J, Neuhauser B, Klaus A, Achem SR, de Vault K. Long-term results of laparoscopic Heller myotomy with partial fundoplication for the treatment of achalasia. Am J Surg. 2005;190:874–878. doi: 10.1016/j.amjsurg.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Douard R, Gaudric M, Chaussade S, Couturier D, Houssin D, Dousset B. Functional results after laparoscopic Heller myotomy for achalasia: A comparative study to open surgery. Surgery. 2004;136:16–24. doi: 10.1016/j.surg.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Costantini M, Zaninotto G, Guirroli E, Rizzetto C, Portale G, Ruol A, Nicoletti L, Ancona E. The laparoscopic Heller-Dor operation remains an effective treatment for esophageal achalasia at a minimum 6-year follow-up. Surg Endosc. 2005;19:345–351. doi: 10.1007/s00464-004-8941-7. [DOI] [PubMed] [Google Scholar]
- 53.Rosemurgy AS, Morton CA, Rosas M, Albrink M, Ross SB. A single institution’s experience with more than 500 laparoscopic Heller myotomies for achalasia. J Am Coll Surg. 2010;210:637–645, 645-647. doi: 10.1016/j.jamcollsurg.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z, Bessell JR, Chew A, Watson DI. Laparoscopic cardiomyotomy for achalasia: clinical outcomes beyond 5 years. J Gastrointest Surg. 2010;14:594–600. doi: 10.1007/s11605-010-1158-2. [DOI] [PubMed] [Google Scholar]
- 55.Portale G, Costantini M, Rizzetto C, Guirroli E, Ceolin M, Salvador R, Ancona E, Zaninotto G. Long-term outcome of laparoscopic Heller-Dor surgery for esophageal achalasia: possible detrimental role of previous endoscopic treatment. J Gastrointest Surg. 2005;9:1332–1339. doi: 10.1016/j.gassur.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Deb S, Deschamps C, Allen MS, Nichols FC, Cassivi SD, Crownhart BS, Pairolero PC. Laparoscopic esophageal myotomy for achalasia: factors affecting functional results. Ann Thorac Surg. 2005;80:1191–1194; discussion 1194-1195. doi: 10.1016/j.athoracsur.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Torquati A, Richards WO, Holzman MD, Sharp KW. Laparoscopic myotomy for achalasia: predictors of successful outcome after 200 cases. Ann Surg. 2006;243:587–591; discussion 591-593. doi: 10.1097/01.sla.0000216782.10502.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oelschlager BK, Chang L, Pellegrini CA. Improved outcome after extended gastric myotomy for achalasia. Arch Surg. 2003;138:490–495; discussion 495-497. doi: 10.1001/archsurg.138.5.490. [DOI] [PubMed] [Google Scholar]
- 59.Rebecchi F, Giaccone C, Farinella E, Campaci R, Morino M. Randomized controlled trial of laparoscopic Heller myotomy plus Dor fundoplication versus Nissen fundoplication for achalasia: long-term results. Ann Surg. 2008;248:1023–1030. doi: 10.1097/SLA.0b013e318190a776. [DOI] [PubMed] [Google Scholar]
- 60.Patti MG, Herbella FA. Fundoplication after laparoscopic Heller myotomy for esophageal achalasia: what type. J Gastrointest Surg. 2010;14:1453–1458. doi: 10.1007/s11605-010-1188-9. [DOI] [PubMed] [Google Scholar]
- 61.Richards WO, Torquati A, Holzman MD, Khaitan L, Byrne D, Lutfi R, Sharp KW. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405–412; discussion 412-415. doi: 10.1097/01.sla.0000136940.32255.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falkenback D, Johansson J, Oberg S, Kjellin A, Wenner J, Zilling T, Johnsson F, Von Holstein CS, Walther B. Heller’s esophagomyotomy with or without a 360 degrees floppy Nissen fundoplication for achalasia. Long-term results from a prospective randomized study. Dis Esophagus. 2003;16:284–290. doi: 10.1111/j.1442-2050.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 63.Yano F, Omura N, Tsuboi K, Hoshino M, Yamamoto SR, Kashiwagi H, Yanaga K. Single-incision laparoscopic Heller myotomy and Dor fundoplication for achalasia: report of a case. Surg Today. 2012;42:299–302. doi: 10.1007/s00595-011-0089-1. [DOI] [PubMed] [Google Scholar]
- 64.Parise P, Santi S, Solito B, Pallabazzer G, Rossi M. Laparoscopic Heller myotomy plus Dor fundoplication in 137 achalasic patients: results on symptoms relief and successful outcome predictors. Updates Surg. 2011;63:11–15. doi: 10.1007/s13304-011-0050-2. [DOI] [PubMed] [Google Scholar]
- 65.Bloomston M, Brady P, Rosemurgy AS. Videoscopic Heller myotomy with intraoperative endoscopy promotes optimal outcomes. JSLS. 2002;6:133–138. [PMC free article] [PubMed] [Google Scholar]
- 66.Frantzides CT, Moore RE, Carlson MA, Madan AK, Zografakis JG, Keshavarzian A, Smith C. Minimally invasive surgery for achalasia: a 10-year experience. J Gastrointest Surg. 2004;8:18–23. doi: 10.1016/j.gassur.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 67.Rawlings A, Soper NJ, Oelschlager B, Swanstrom L, Matthews BD, Pellegrini C, Pierce RA, Pryor A, Martin V, Frisella MM, et al. Laparoscopic Dor versus Toupet fundoplication following Heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trial. Surg Endosc. 2012;26:18–26. doi: 10.1007/s00464-011-1822-y. [DOI] [PubMed] [Google Scholar]
- 68.Ramacciato G, Mercantini P, Amodio PM, Corigliano N, Barreca M, Stipa F, Ziparo V. The laparoscopic approach with antireflux surgery is superior to the thoracoscopic approach for the treatment of esophageal achalasia. Experience of a single surgical unit. Surg Endosc. 2002;16:1431–1437. doi: 10.1007/s00464-001-9215-2. [DOI] [PubMed] [Google Scholar]
- 69.Galvani C, Gorodner MV, Moser F, Baptista M, Donahue P, Horgan S. Laparoscopic Heller myotomy for achalasia facilitated by robotic assistance. Surg Endosc. 2006;20:1105–1112. doi: 10.1007/s00464-005-0272-9. [DOI] [PubMed] [Google Scholar]
- 70.Shaligram A, Unnirevi J, Simorov A, Kothari VM, Oleynikov D. How does the robot affect outcomes A retrospective review of open, laparoscopic, and robotic Heller myotomy for achalasia. Surg Endosc. 2012;26:1047–1050. doi: 10.1007/s00464-011-1994-5. [DOI] [PubMed] [Google Scholar]
- 71.Ghoshal UC, Chaudhuri S, Pal BB, Dhar K, Ray G, Banerjee PK. Randomized controlled trial of intrasphincteric botulinum toxin A injection versus balloon dilatation in treatment of achalasia cardia. Dis Esophagus. 2001;14:227–231. doi: 10.1046/j.1442-2050.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 72.Bonavina L, Incarbone R, Antoniazzi L, Reitano M, Peracchia A. Previous endoscopic treatment does not affect complication rate and outcome of laparoscopic Heller myotomy and anterior fundoplication for oesophageal achalasia. Ital J Gastroenterol Hepatol. 1999;31:827–830. [PubMed] [Google Scholar]
- 73.Bravi I, Nicita MT, Duca P, Grigolon A, Cantù P, Caparello C, Penagini R. A pneumatic dilation strategy in achalasia: prospective outcome and effects on oesophageal motor function in the long term. Aliment Pharmacol Ther. 2010;31:658–665. doi: 10.1111/j.1365-2036.2009.04217.x. [DOI] [PubMed] [Google Scholar]
- 74.Allescher HD, Storr M, Seige M, Gonzales-Donoso R, Ott R, Born P, Frimberger E, Weigert N, Stier A, Kurjak M, et al. Treatment of achalasia: botulinum toxin injection vs. pneumatic balloon dilation. A prospective study with long-term follow-Up. Endoscopy. 2001;33:1007–1017. doi: 10.1055/s-2001-18935. [DOI] [PubMed] [Google Scholar]
- 75.Richter JE, Boeckxstaens GE. Management of achalasia: surgery or pneumatic dilation. Gut. 2011;60:869–876. doi: 10.1136/gut.2010.212423. [DOI] [PubMed] [Google Scholar]
- 76.Popoff AM, Myers JA, Zelhart M, Maroulis B, Mesleh M, Millikan K, Luu MB. Long-term symptom relief and patient satisfaction after Heller myotomy and Toupet fundoplication for achalasia. Am J Surg. 2012;203:339–342; discussion 342. doi: 10.1016/j.amjsurg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 77.O’Connor JB, Singer ME, Imperiale TF, Vaezi MF, Richter JE. The cost-effectiveness of treatment strategies for achalasia. Dig Dis Sci. 2002;47:1516–1525. doi: 10.1023/a:1015811001267. [DOI] [PubMed] [Google Scholar]
- 78.Karanicolas PJ, Smith SE, Inculet RI, Malthaner RA, Reynolds RP, Goeree R, Gafni A. The cost of laparoscopic myotomy versus pneumatic dilatation for esophageal achalasia. Surg Endosc. 2007;21:1198–1206. doi: 10.1007/s00464-007-9364-z. [DOI] [PubMed] [Google Scholar]
- 79.Weber CE, Davis CS, Kramer HJ, Gibbs JT, Robles L, Fisichella PM. Medium and long-term outcomes after pneumatic dilation or laparoscopic Heller myotomy for achalasia: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:289–296. doi: 10.1097/SLE.0b013e31825a2478. [DOI] [PubMed] [Google Scholar]
- 80.Alderliesten J, Conchillo JM, Leeuwenburgh I, Steyerberg EW, Kuipers EJ. Predictors for outcome of failure of balloon dilatation in patients with achalasia. Gut. 2011;60:10–16. doi: 10.1136/gut.2010.211409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pratap N, Kalapala R, Darisetty S, Joshi N, Ramchandani M, Banerjee R, Lakhtakia S, Gupta R, Tandan M, Rao GV, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil. 2011;17:48–53. doi: 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruiz-de-León A, Mendoza J, Sevilla-Mantilla C, Fernández AM, Pérez-de-la-Serna J, Gónzalez VA, Rey E, Figueredo A, Díaz-Rubio M, De-la-Concha EG. Myenteric antiplexus antibodies and class II HLA in achalasia. Dig Dis Sci. 2002;47:15–19. doi: 10.1023/a:1013242831900. [DOI] [PubMed] [Google Scholar]
- 84.Micci MA, Learish RD, Li H, Abraham BP, Pasricha PJ. Neural stem cells express RET, produce nitric oxide, and survive transplantation in the gastrointestinal tract. Gastroenterology. 2001;121:757–766. doi: 10.1053/gast.2001.28633. [DOI] [PubMed] [Google Scholar]


