Abstract
AIM: To evaluate the clinical value of diffusion-weighted magnetic resonance imaging (DW-MRI) in predicting the response of rectal cancer to neoadjuvant chemoradiation.
METHODS: This prospective study was approved by our institutional review board, and informed consent was obtained from each patient. Fifteen patients (median age 56 years) with locally advanced rectal cancer were treated in our hospital from June 2006 to December 2007. All patients were stage IIIB-C according to the results of MRI and endorectal ultrasound examinations. All patients underwent pelvic irradiation with 45 Gy/25 fx per 35 days. The concurrent chemotherapy regimen consisted of capecitabine 625 mg/m2, bid (Monday-Friday), and oxaliplatin 50 mg/m2, weekly. The patients underwent surgery 5-8 wk after the completion of neoadjuvant therapy. T downstaging was defined as the downstaging of the tumor from cT3 to ypT0-2 or from cT4 to ypT0-3. Good regression was defined as TRG 3-4, and poor regression was defined as TRG 0-2. Diffusion-weighted magnetic resonance images were obtained prior to and weekly during the course of neoadjuvant chemoradiation, and the apparent diffusion coefficient (ADC) values were calculated from the acquired tumor images.
RESULTS: Comparison with the mean pretreatment tumor ADC revealed an increase in the mean tumor ADC during the course of neoadjuvant chemoradiation, especially at the 2nd week (P = 0.004). We found a strong negative correlation between the mean pretreatment tumor ADC and tumor regression after neoadjuvant chemoradiation (P = 0.021). In the T downstage and tumor regression groups, we found a significant increase in the mean ADC at the 2nd week of neoadjuvant therapy (P = 0.011; 0.004).
CONCLUSION: DW-MRI might be a valuable clinical tool to help predict or assess the response of rectal cancer to neoadjuvant chemoradiation at an early timepoint.
Keywords: Locally advanced rectal cancer, Neoadjuvant chemoradiation, Diffusion-weighted magnetic resonance imaging, Apparent diffusion coefficient
Core tip: This original study prospectively evaluated the clinical value of diffusion-weighted magnetic resonance imaging (DW-MRI) in predicting the response of rectal cancer to neoadjuvant chemoradiation. We found a strong negative correlation between the mean pretreatment tumor apparent diffusion coefficient (ADC) and tumor regression after neoadjuvant chemoradiation, as well as a significant increase in the mean ADC at the 2nd week in the T downstage and tumor regression groups. Therefore, DW-MRI might be a valuable clinical tool to help predict or assess the response of rectal cancer to neoadjuvant chemoradiation at an early timepoint.
INTRODUCTION
Neoadjuvant (chemo) radiation followed by total mesorectal excision has become the standard treatment for locally advanced rectal cancer (LARC)[1-3]. However, approximately 20%-30% of patients do not benefit from neoadjuvant treatment due to the radioresistance of the tumor[4], and ineffective neoadjuvant treatment may result in unnecessary toxicity and expense as well as delays in receiving the proper treatment. Meanwhile, 10%-30% of patients with a pathological complete response (pCR) have a favorable long-term outcome[5]. Recently, data have even suggested that surgery is unnecessary for clinical complete responders[6]. To effectively guide patient-tailored treatments, reliable and early assessment of the treatment response is important.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is a non-invasive functional MRI technique that is sensitive to the mobility of water protons in biological tissues, which is dependent on many factors, such as cell density, vascularity, the viscosity of the extracellular fluid, and cell membrane integrity[7-9]. The apparent diffusion coefficient (ADC) calculated from DW-MRI measurements can quantify and express these properties. However, published data on the value of DW-MRI as a predictive tool for anti-cancer treatment responses in patients with rectal cancer are scarce and conflicting. Most studies have found that the pretreatment ADC is negatively correlated with the response to treatment[10]. Furthermore, it is possible that necrotic areas with high pretreatment ADCs are less sensitive to radiation and chemotherapy, although several studies do not support this hypothesis, and others have obtained opposite results[11,12]. Therefore, we conducted this study to investigate the clinical value of DW-MRI as a predictor of the tumor response in patients receiving neoadjuvant chemoradiation therapy (CRT) for rectal cancer by measuring the tumor ADC.
MATERIALS AND METHODS
Our institutional review board approved this prospective study, and informed consent was obtained from each patient.
Patients and treatment
Fifteen patients (median age 56 years, range 32-69 years; 13 men and 2 women) with LARC were invited to participate in our study between June 2006 and December 2007. Each patient had histologically proven rectal adenocarcinoma of stage T3-T4 and was determined to be node-positive by endorectal ultrasound and pelvic MRI. Patients with a history of pelvic irradiation or chemotherapy, any other malignancy, or distant metastases were excluded (Table 1). The clinical and histopathological classification and stage according to the International Union Against Cancer TNM system[13] were recorded. Tumor regression grading was evaluated according to the criteria of Dworak et al[14] (grade 0, no regression; grade 1, minor regression, dominant tumor mass with obvious fibrosis in 25% or less of the tumor mass; grade 2, moderate regression, 26%-50% of the tumor mass; grade 3, good regression, more than 50% tumor regression; and grade 4, total regression, no viable tumor cells, only fibrotic mass). A pCR was defined as the absence of viable tumor cells in the primary tumor and lymph nodes (ypT0N0). T downstaging was defined as the downstaging of the tumor from cT3 to ypT0-2 or from cT4 to ypT0-3. Good regression was defined as TRG 3-4, and poor regression was defined as TRG 0-2.
Table 1.
Patients and treatment characteristics
| No. | Age (yr) | Preoperative stage | Surgical treatment | Postoperative stage |
| 1 | 56 | cT3N2M0 | LAR | ypT0N1M0 |
| 2 | 57 | cT4N2M0 | LAR | ypT3N0M0 |
| 3 | 46 | cT3N2M0 | LAR | ypT3N1M0 |
| 4 | 69 | cT4N1M0 | APR | ypT2N0M0 |
| 5 | 40 | cT3N2M0 | APR | ypT3N2M0 |
| 6 | 40 | cT3N1M0 | APR | ypT0N0M0 |
| 7 | 58 | cT4N1M0 | APR | ypT2N0M0 |
| 8 | 57 | cT3N2M0 | APR | ypT0N0M0 |
| 9 | 51 | cT4N2M0 | Exploratory laparotomy | ypT4N2M0 |
| 10 | 55 | cT3N1M0 | APR | ypT3N1M0 |
| 11 | 68 | cT3N2M0 | APR | ypT3N0M0 |
| 12 | 58 | cT3N2M0 | APR | ypT3N1M0 |
| 13 | 61 | cT3N2M0 | LAR | ypT1N1M0 |
| 14 | 32 | cT4N2M0 | APR | ypT3N1M0 |
| 15 | 55 | cT3N1M0 | APR | ypT3N1M0 |
LAR: Low anterior resection; APR: Abdominal perineal resection.
All patients received neoadjuvant concurrent CRT. Radiotherapy (RT) was delivered with a linear accelerator using 6- and 15-MV photons and a three-field technique (posterior-anterior and right and left laterals). Every patient underwent a planning computed tomography (CT) scan in the treatment position (prone position) using a belly board. Three-dimensional conformal RT was used for all patients based on the planning CT, with a total dose of 45 Gy at 1.8 Gy per fraction per day, Monday-Friday. Neoadjuvant chemotherapy was delivered concurrently with RT. Starting on day 1 of RT, patients received capecitabine 625 mg/m2 orally, bid (Monday-Friday), and oxaliplatin 50 mg/m2 weekly for five consecutive weeks. Surgical resection was scheduled for 5-8 wk after the completion of neoadjuvant treatment.
DW-MRI
Each enrolled patient was examined by DW-MRI at six scheduled times. The initial DW-MRI scan was performed 7 d prior to the start of RT. DW-MRI scans were then taken once weekly during the course of neoadjuvant treatment.
DWI was performed on a 1.5 T magnetic resonance machine (1.5 T Signa Twin Speeder with Excite, GE, United States) using a phased-array body coil. Before DW-MRI, standard T2-weighted fast spin echo sequence and T1-weighted spin echo sequence images were used for clinical staging. DWI echo planar images were acquired in the transverse plane using a GRE-EPI sequence (TR/TE 3000/min; field of view 22 cm2; matrix size 128× 128; slice thickness 4 mm; intersection gap 1 mm). DW-MR images and ADC maps were obtained using b values of 0 and 1000 s/mm2 applied in the x, y, and z directions. Patients did not undergo bowel preparation, receive anti-spasmodic medication, or undergo rectal distention before the MR examination. For the image analysis, the data were transferred to a Workstation (AW4.0, GE Medical Systems) and analyzed using the Functool dynamic analysis tool (GE Medical Systems). ADC values were calculated based on the ADC maps. The ADC map of the largest tumor extension in the transverse T2-weighted images was used for the analysis. Regions of interest (ROIs) were drawn manually along the edge of the tumor with a b value of 1000 s/mm2 on the selected ADC maps by an experienced radiologist (Zhang S, with 10 years of experience in clinical MRI), who did not participate in the treatment of the patients or the evaluation of the therapeutic effect.
Statistical analysis
Statistical analysis was performed using SPSS 12.0 statistical software. Paired comparisons were performed using the Wilcoxon test. Spearman’s correlation was used to assess the significance of differences between groups. A P value < 0.05 was considered statistically significant.
RESULTS
Treatment characteristics
After neoadjuvant treatment, pCR was observed in 2 patients. Downstaging of the tumor was observed in eight patients. The tumor regression grades after neoadjuvant treatment were grade 0-2 in 6 patients and grade 3-4 in 9 patients.
DW-MRI data
Diffusion data from 15 patients were obtained prior to and at constant intervals once weekly during the course of neoadjuvant treatment. The observed ADC values are shown in Table 2. A total of 88 ADC values were obtained in our study, and 2 ADC values were excluded due to measurement errors. Sample T2-weighted and diffusion-weighted images prior to treatment are shown in Figure 1. Sample ADC maps from the images taken weekly during the course of neoadjuvant treatment are shown in Figure 2.
Table 2.
Apparent diffusion coefficient values at six measurement times
| No. |
Apparent diffusion coefficient values (× 10-3 mm2/s) |
|||||
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
| 1 | 0.723 | 0.743 | 0.893 | 0.756 | 0.606 | 0.793 |
| 2 | 0.583 | 0.458 | 0.786 | 0.385 | 0.540 | 0.793 |
| 3 | 0.752 | 0.883 | 0.655 | 0.711 | 0.683 | 0.772 |
| 4 | 0.883 | 0.887 | 0.887 | 0.945 | 0.923 | 0.972 |
| 5 | 0.995 | 0.853 | 0.995 | 0.950 | 0.832 | 1.120 |
| 6 | 0.813 | 0.965 | 1.133 | 0.964 | 0.893 | 0.686 |
| 7 | 0.518 | 0.416 | 0.998 | 0.539 | 0.527 | 0.473 |
| 8 | 0.659 | 0.747 | 0.825 | 0.858 | 0.631 | 0.746 |
| 9 | 0.814 | 0.791 | 0.809 | 0.894 | 0.821 | 0.798 |
| 10 | 0.628 | 0.637 | 0.625 | 0.703 | 0.784 | 0.930 |
| 11 | 0.562 | 0.575 | 0.806 | 0.742 | 0.677 | 0.515 |
| 12 | 0.616 | 0.595 | 0.834 | 0.850 | 0.907 | 1.050 |
| 13 | 0.851 | 0.825 | 0.865 | -1 | 0.831 | 0.882 |
| 14 | 0.592 | -1 | 0.734 | 0.622 | 0.574 | 0.605 |
| 15 | 1.255 | 1.435 | 1.420 | 1.282 | 1.256 | 1.450 |
| 95%CI | 0.641-0.858 | 0.627-0.918 | 0.775-0.994 | 0.675-0.925 | 0.659-0.872 | 0.702-0.976 |
No data due to measurement error.
Figure 1.
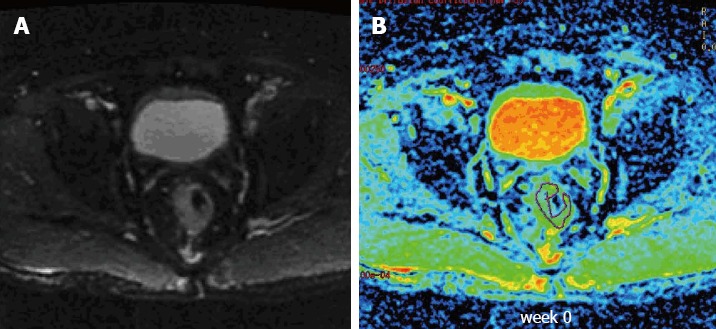
Sample T2-weighted and diffusion-weighted images from a patient with locally advanced rectal cancer prior to treatment. A: T2-weighted transaxial image through the pelvis prior to treatment; B: Corresponding apparent diffusion coefficient map of the patient obtained from the diffusion-weighted images. A region of interest was drawn around the tumor.
Figure 2.
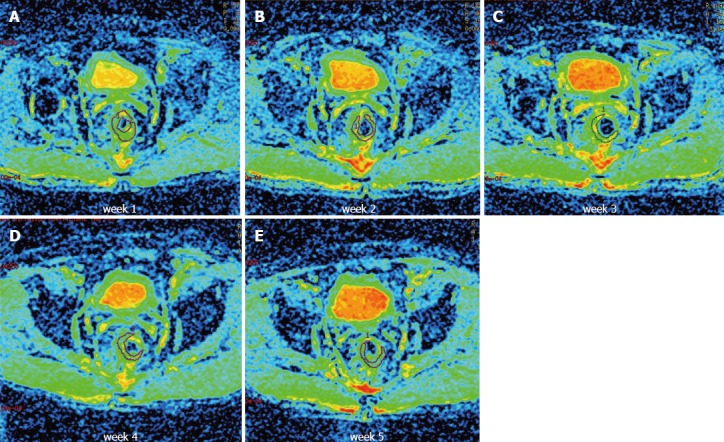
Apparent diffusion coefficient maps of one patient obtained at constant intervals once weekly during the course of neoadjuvant treatment from the diffusion-weighted images. A-E: Axial apparent diffusion coefficient maps obtained during treatment (weeks 1-5). A region of interest was drawn around the tumor.
The mean tumor ADC value slightly increased from 0.749 × 10-3 mm2/s (95%CI: 0.641 × 10-3-0.858 × 10-3 mm2/s) prior to treatment to 0.772 × 10-3 mm2/s (95%CI: 0.627 × 10-3-0.918 × 10-3 mm2/s) after the 1st week of treatment. There was also a significant increase at the 2nd week to 0.884 × 10-3 mm2/s 95%CI: 0.775 × 10-3-0.994 × 10-3 mm2/s). Subsequently, the mean ADC decreased to 0.800 × 10-3 mm2/s (95%CI: 0.675 × 10-3-0.925 × 10-3 mm2/s) at the 3rd week and 0.766 × 10-3 mm2/s (95%CI: 0.659 × 10-3-0.872 × 10-3 mm2/s) at the 4th week. ADC increased again at the 5th week to 0.839 × 10-3 mm2/s (95%CI: 0.702 × 10-3-0.976 × 10-3 mm2/s). We also observed a significant increase in the mean ADC value at the 2nd (P = 0.004) and 5th week (P = 0.033) during treatment relative to the values prior to treatment. The mean observed ADC values and P values are shown in Table 3.
Table 3.
Mean tumor apparent diffusion coefficient values and the P values for the comparisons with the pretreatment values
|
Apparent diffusion coefficient values (× 10-3 mm2/s) |
||||||
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
| All (n = 15) | 0.749 | 0.772 | 0.884 | 0.800 | 0.766 | 0.839 |
| P value | - | 0.672 | 0.004 | 0.077 | 0.586 | 0.033 |
| T downstage (n = 8) | 0.703 | 0.720 | 0.890 | 0.724 | 0.691 | 0.744 |
| P value | - | 0.964 | 0.011 | 0.406 | 0.578 | 0.284 |
| No T downstage (n = 7) | 0.803 | 0.824 | 0.878 | 0.876 | 0.851 | 0.948 |
| P value | - | 0.617 | 0.185 | 0.117 | 0.430 | 0.074 |
| Good regression (n = 9) | 0.659 | 0.671 | 0.852 | 0.696 | 0.674 | 0.714 |
| P value | - | 0.909 | 0.004 | 0.212 | 0.617 | 0.251 |
| Poor regression (n = 6) | 0.886 | 0.907 | 0.933 | 0.938 | 0.904 | 1.027 |
| P value | - | 0.669 | 0.372 | 0.264 | 0.785 | 0.086 |
Tumor ADC for the prediction of treatment response
We compared the tumor ADC values of the responder and non-responder groups to predict the treatment response based on T downstage and TRG criteria.
Downstaging of the tumor was observed for 8 of the 15 patients (53.3%). The ADC values at the 5th week during treatment increased for 6/8 patients with T downstaging and increased for 5/7 patients without T downstaging relative to the mean tumor ADC values before treatment. The mean observed ADC values for patients with and without T downstage are shown in Table 4. The difference between these two groups with respect to the mean ADC values measured at the six timepoints did not reach significance.
Table 4.
Mean tumor apparent diffusion coefficient values and P values for the comparisons between groups
| Group |
Apparent diffusion coefficient values (× 10-3 mm2/s) |
|||||
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
| T downstage (n = 8) | 0.702 | 0.720 | 0.890 | 0.724 | 0.690 | 0.743 |
| No T downstage (n = 7) | 0.803 | 0.824 | 0.877 | 0.875 | 0.851 | 0.947 |
| P value | 0.339 | 0.463 | 0.909 | 0.202 | 0.108 | 0.114 |
| Good regression (n = 9) | 0.658 | 0.670 | 0.851 | 0.618 | 0.673 | 0.713 |
| Poor regression (n = 6) | 0.885 | 0.907 | 0.933 | 0.938 | 0.903 | 1.027 |
| P value | 0.021 | 0.081 | 0.452 | 0.032 | 0.016 | 0.010 |
For the eight patients with tumor downstaging, there was a significant increase (P = 0.011) in the mean tumor ADC at the 2nd week of treatment relative to the ADC before treatment, whereas for the seven patients without tumor downstaging, there was no significant change in the ADC at any timepoint during treatment relative to the ADC values before treatment (Figure 3A, Table 3).
Figure 3.
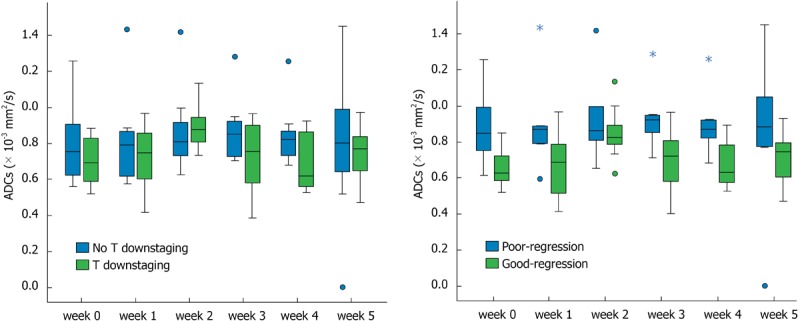
Mean tumor apparent diffusion coefficient values in patients at six measurement points. A: With and without T downstaging; B: With good regressin and poor regression. Cycle: Outlier; Star: Extreme value.
Good regression (TRG 3-4) was observed in 9 of the 15 patients (60%), and poor regression (TRG 0-2) was observed in 6 patients (40%). The ADC values at the 5th week during treatment were increased in 7/9 patients with good regression and 5/6 patients with poor regression relative to the mean tumor ADC values before treatment. The mean observed ADC values in patients with good regression and poor regression are shown in Table 4. Before treatment and at the 3rd, 4th, and 5th week during treatment, significant differences in the mean ADC values between the two groups were obtained.
For the nine patients with good regression, there was a significant increase (P = 0.004) in the mean tumor ADC value at the 2nd week of treatment relative to the ADC values before treatment, whereas for the six patients with poor regression, no significant change in ADC was observed at any timepoint during treatment (Figure 3B, Table 3). The two patients with pCR demonstrated lower ADC (0.659 × 10-3 mm2/s and 0.813 × 10-3 mm2/s) before treatment but significantly increased tumor ADC (0.825 × 10-3 mm2/s and 1.133 × 10-3 mm2/s) at the 2nd week of treatment.
DISCUSSION
The recent trend toward patient-tailored treatment for LARC has highlighted the need for a reliable method for the early assessment of treatment response. DWI-MRI may be a promising functional imaging tool for the prediction of treatment response. In our study, DW-MRI was investigated as a potential clinical tool to predict or assess the response of rectal tumors to neoadjuvant concurrent CRT at an early timepoint.
Our results show that CRT induced a significant increase in mean tumor ADC in LARC. Because the ADC values obtained from DWI measurements reflect tumor cellularity and anti-tumor treatment decreases tumor cellularity, CRT should increase the ADC value. The administration of CRT results in cell swelling, necrosis, and apoptotic cell death. When CRT is initiated, the ADC may rapidly decrease over several hours due to cell swelling, followed by an increase over several days concurrent with cell death. Increased ADC values have also been correlated with tumor necrosis and reduced cell density[15], and most studies have found an increase in ADC after CRT[12,16,17]. For example, Kim et al[12] recently showed that neoadjuvant CRT caused a significant increase in the ADC values of 76 rectal cancer patients. In contrast, Hein et al[18] reported a decrease in the ADC after CRT in all nine of their patients, and they attributed this result to intratumoral radiation-induced fibrosis and cytotoxic edema as well as to the method employed (ROI excluding apparent necrotic areas).
Our results indicate that the mean pre-CRT ADC was negatively correlated with tumor regression (P = 0.021) but not with T downstaging (P = 0.339). T downstaging and TRG criteria were used because these are common factors used for the evaluation of treatment responses[19,20]. The TRG was not completely concordant with T downstaging, and some studies have shown that the pretreatment ADC value is negatively correlated with treatment response in rectal cancer and other tumors[10,21-25]. Dzik-Jurasz et al[10] found a strong negative correlation between the mean pretreatment tumor water ADC and the percent change in the size of the tumor after chemotherapy and chemoradiation in 2002. ADC values are generally higher for necrotic tumors than for solid or viable tumors[26]. Because necrotic areas in tumors are resistant to radiation, it may be hypothesized that tumors with necrotic areas, and thus high pretreatment ADC values, would have less favorable treatment responses. However, other studies have obtained different results; for example, several studies of rectal and other tumors found no correlation between the pretreatment ADC value and treatment response[11,27,28], whereas another study found a positive correlation[12]. Several factors may explain these different correlations, such as small sample sizes, the use of different methods for calculating the ADC, and the use of different indicators for the evaluation of treatment response.
A substantial change in the mean ADC value at the 2nd week of CRT predicted the tumor response of LARC in our study. Most studies have assumed that CRT decreases tumor cellularity and results in a substantial change in the ADC value[18,26]. Although decreasing tumor cellularity will lead to a reduction in tumor size, this reduction is typically observed 3 wk or more after the start of CRT[29,30]. Thus, a more rapid evaluation or prediction of treatment response would be clinically useful. We found a significant increase in the mean ADC at the 2nd week in the T downstage (P = 0.011) and good regression (P = 0.004) groups but not in the groups of patients without T downstaging and with poor regression. We believe that the significant increase in the mean ADC at the 2nd week of treatment was correlated with tumor necrosis and apoptosis, which reduce cell density, after the start of therapy. Similar results have been obtained in several other studies. For example, one study examined the ADC data of nine patients with LARC, and a significant change in the mean ADC starting at week 2 of CRT was observed[18]. In another study focused on the early detection of responses to CRT in cervical cancer, the changes in the ADC value after 2 wk of therapy were also significantly correlated with the treatment response[27].
There are several limitations of our study. First, the study sample size was small. Second, the sample slice with the largest tumor extension was selected to determine the ADC value, and the use of this slice may not have adequately captured the heterogeneity of the tumor. Third, the ROIs were drawn manually, and this process may have influenced the ADC value and introduced subjectivity. The reason the ROIs were drawn manually by a single experienced radiologist was to obtain more uniform and stable ADC values.
Our study and several previous studies highlight the value of DW-MRI as a predictive tool for the response of rectal cancer to chemoradiation. However, there are some difficulties associated with incorporating DW-MRI into routine clinical practice. The reproducibility of DWI has been insufficiently investigated, and the cut-off values used to determine treatment response vary between treatments and ADC measurement techniques. Thus, a standardized guideline to predict or assess treatment response is needed before DWI can be implemented in clinical practice.
In this study, the tumor ADC values changed during the course of neoadjuvant chemoradiation. The pretreatment tumor ADC value was negatively correlated with tumor regression after chemoradiation for the treatment of LARC, and the ADC value at the 2nd week of therapy was significantly correlated with the tumor response. Our results indicate that DW-MRI may be a valuable clinical tool to help predict or assess the responses of rectal tumors to neoadjuvant concurrent chemoradiation at an early timepoint.
COMMENTS
Background
Neoadjuvant (chemo)radiation followed by surgery has become the standard treatment for locally advanced rectal cancer (LARC). However, approximately 20%-30% of patients do not benefit from this neoadjuvant treatment due to radioresistance of the tumor. Functional non-invasive diffusion-weighted magnetic resonance imaging (DW-MRI) studies are increasingly used to predict response to cancer therapy, but definitive evidence is limited, especially for patients with rectal cancer treated with neoadjuvant chemoradiation therapy (CRT).
Research frontiers
DW-MRI is a non-invasive functional MRI technique. To date, published data on the value of DW-MRI as a predictive tool for assessing responses to anti-cancer treatment in patients with rectal cancer are scarce and conflicting.
Innovations and breakthroughs
The authors found that CRT induced a significant increase in the mean apparent diffusion coefficient (ADC) value of LARC. The pretreatment tumor ADC was negatively correlated with tumor regression after CRT for the treatment of LARC, and the ADC value at the 2nd week of therapy was significantly correlated with the tumor response.
Applications
The results of this study suggest that DW-MRI may be a valuable clinical tool to help predict or assess the responses of rectal tumors to neoadjuvant concurrent CRT at an early timepoint.
Terminology
DW-MRI is a non-invasive functional MRI technique that provides information by measuring water proton mobility in tissues. ADC values can be calculated from DWI measurements according to the impediment to free diffusion of water molecules in a single voxel due to restricting barriers such as membranes, macromolecules, and fibers inside different tissue compartments.
Peer review
This is an interesting study that investigates the use of DW-MRI as a predictor of the tumor response in 15 patients with rectal cancer undergoing CRT therapy by measuring the tumor ADC. This is an emerging field in which new knowledge is needed, and this study, despite its limits, provides novel information that may help to settle the current debate about the utility of DW-MRI as a predictive tool for the response to anti-cancer treatment.
Footnotes
P- Reviewer Trastulli S S- Editor Zhai HH L- Editor A E- Editor Ma S
References
- 1.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Popek S, Tsikitis VL. Neoadjuvant vs adjuvant pelvic radiotherapy for locally advanced rectal cancer: Which is superior. World J Gastroenterol. 2011;17:848–854. doi: 10.3748/wjg.v17.i7.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onaitis MW, Noone RB, Hartwig M, Hurwitz H, Morse M, Jowell P, McGrath K, Lee C, Anscher MS, Clary B, et al. Neoadjuvant chemoradiation for rectal cancer: analysis of clinical outcomes from a 13-year institutional experience. Ann Surg. 2001;233:778–785. doi: 10.1097/00000658-200106000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U, Silva E Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90–99; discussion 99-101. doi: 10.1016/j.gassur.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717; discussion 717-718. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 8.Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007;25:4104–4109. doi: 10.1200/JCO.2007.11.9610. [DOI] [PubMed] [Google Scholar]
- 9.deSouza NM, Riches SF, Vanas NJ, Morgan VA, Ashley SA, Fisher C, Payne GS, Parker C. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol. 2008;63:774–782. doi: 10.1016/j.crad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 11.DeVries AF, Kremser C, Hein PA, Griebel J, Krezcy A, Ofner D, Pfeiffer KP, Lukas P, Judmaier W. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:958–965. doi: 10.1016/s0360-3016(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, Choi BI. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116–125. doi: 10.1148/radiol.2532090027. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL. Current TNM staging of colorectal cancer. Lancet Oncol. 2007;8:572–573. doi: 10.1016/S1470-2045(07)70185-7. [DOI] [PubMed] [Google Scholar]
- 14.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 15.Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol. 2003;45:208–213. doi: 10.1016/s0720-048x(02)00310-8. [DOI] [PubMed] [Google Scholar]
- 16.Mardor Y, Pfeffer R, Spiegelmann R, Roth Y, Maier SE, Nissim O, Berger R, Glicksman A, Baram J, Orenstein A, et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21:1094–1100. doi: 10.1200/JCO.2003.05.069. [DOI] [PubMed] [Google Scholar]
- 17.Song I, Kim CK, Park BK, Park W. Assessment of response to radiotherapy for prostate cancer: value of diffusion-weighted MRI at 3 T. AJR Am J Roentgenol. 2010;194:W477–W482. doi: 10.2214/AJR.09.3557. [DOI] [PubMed] [Google Scholar]
- 18.Hein PA, Kremser C, Judmaier W, Griebel J, Pfeiffer KP, Kreczy A, Hug EB, Lukas P, DeVries AF. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol. 2003;45:214–222. doi: 10.1016/s0720-048x(02)00231-0. [DOI] [PubMed] [Google Scholar]
- 19.Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 20.Chapet O, Romestaing P, Mornex F, Souquet JC, Favrel V, Ardiet JM, d’Hombres A, Gerard JP. Preoperative radiotherapy for rectal adenocarcinoma: Which are strong prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61:1371–1377. doi: 10.1016/j.ijrobp.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, Husband JE. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001–1008. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 23.Mardor Y, Roth Y, Ochershvilli A, Spiegelmann R, Tichler T, Daniels D, Maier SE, Nissim O, Ram Z, Baram J, et al. Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia. 2004;6:136–142. doi: 10.1593/neo.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E, Filip C, Haustermans K. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int J Radiat Oncol Biol Phys. 2012;82:863–870. doi: 10.1016/j.ijrobp.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 25.Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y, Zhang XY. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254:170–178. doi: 10.1148/radiol.2541082230. [DOI] [PubMed] [Google Scholar]
- 26.Uhl M, Saueressig U, van Buiren M, Kontny U, Niemeyer C, Köhler G, Ilyasov K, Langer M. Osteosarcoma: preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusion-weighted magnetic resonance imaging. Invest Radiol. 2006;41:618–623. doi: 10.1097/01.rli.0000225398.17315.68. [DOI] [PubMed] [Google Scholar]
- 27.Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213–220. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Lee JY, Lee JM, Han JK, Choi BI. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol. 2011;21:987–995. doi: 10.1007/s00330-010-1989-y. [DOI] [PubMed] [Google Scholar]
- 29.Kim YC, Lim JS, Keum KC, Kim KA, Myoung S, Shin SJ, Kim MJ, Kim NK, Suh J, Kim KW. Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging. 2011;34:570–576. doi: 10.1002/jmri.22696. [DOI] [PubMed] [Google Scholar]
- 30.Ross BD, Moffat BA, Lawrence TS, Mukherji SK, Gebarski SS, Quint DJ, Johnson TD, Junck L, Robertson PL, Muraszko KM, et al. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther. 2003;2:581–587. [PubMed] [Google Scholar]


