Abstract
AIM: To elucidate the potential impact of intraoperative blood loss (IBL) on long-term survival of gastric cancer patients after curative surgery.
METHODS: A total of 845 stage I-III gastric cancer patients who underwent curative gastrectomy between January 2003 and December 2007 in our center were enrolled in this study. Patients were divided into 3 groups according to the amount of IBL: group 1 (< 200 mL), group 2 (200-400 mL) and group 3 (> 400 mL). Clinicopathological features were compared among the three groups and potential prognostic factors were analyzed. The Log-rank test was used to assess statistical differences between the groups. Independent prognostic factors were identified by the Cox proportional hazards regression model. Stratified analysis was used to investigate the impact of IBL on survival in each stage. Cancer-specific survival was also compared among the three groups by excluding deaths due to reasons other than gastric cancer. Finally, we explored the possible factors associated with IBL and identified the independent risk factors for IBL ≥ 200 mL.
RESULTS: Overall survival was significantly influenced by the amount of IBL. The 5-year overall survival rates were 51.2%, 39.4% and 23.4% for IBL less than 200 mL, 200 to 400 mL and more than 400 mL, respectively (< 200 mL vs 200-400 mL, P < 0.001; 200-400 mL vs > 400 mL, P = 0.003). Age, tumor size, Borrmann type, extranodal metastasis, tumour-node-metastasis (TNM) stage, chemotherapy, extent of lymphadenectomy, IBL and postoperative complications were found to be independent prognostic factors in multivariable analysis. Following stratified analysis, patients staged TNM I-II and those with IBL less than 200 mL tended to have better survival than those with IBL not less than 200 mL, while patients staged TNM III, whose IBL was less than 400 mL had better survival. Tumor location, tumor size, TNM stage, type of gastrectomy, combined organ resection, extent of lymphadenectomy and year of surgery were found to be factors associated with the amount of IBL, while tumor location, type of gastrectomy, combined organ resection and year of surgery were independently associated with IBL ≥ 200 mL.
CONCLUSION: IBL is an independent prognostic factor for gastric cancer after curative resection. Reducing IBL can improve the long-term outcome of gastric cancer patients following curative gastrectomy.
Keywords: Gastric carcinoma, Intraoperative blood loss, Blood transfusion, Postoperative complication, Prognosis
Core tip: Intraoperative blood loss (IBL) has been shown to be associated with poor outcome in various types of malignancy. In this study, we found that the overall survival of gastric cancer patients was significantly affected by the amount of IBL, and IBL was an independent prognostic factor in multivariate analysis. We suggest that meticulous surgery and new surgical methods such as the application of an ultrasonic scalpel in lymph node dissection should be used to decrease the amount of IBL and improve the long-term outcome of gastric cancer patients following curative gastrectomy.
INTRODUCTION
Radical gastrectomy with regional lymph node dissection is the only possible curative treatment for gastric cancer[1]. Even after R0 resection, a significant number of patients suffer from recurrence, especially those with advanced gastric cancer[2-4]. Tumor depth and lymph node status are well-known prognostic factors, and patient age and performance status have also been reported to have an impact on the long-term outcome of patients[5-7]. Besides these factors, a number of potential prognostic factors have been reported in recent years, such as perioperative blood transfusion and intraoperative blood loss (IBL)[8-11].
The impact of IBL on long-term outcome has previously been reported in patients with colorectal cancer, prostate cancer and pancreas cancer[12-14]. However, there are few reports assessing the relationship between IBL and long-term outcome in gastric cancer patients. Dhar et al[10] reported that more than 500 mL blood loss during surgery was an independent predictor of survival in gastric cancer patients with transmural depth invasion. Kamei et al[11] demonstrated that IBL was a crucial risk factor for peritoneal recurrence after curative resection for advanced gastric cancer. Unfortunately, the numbers of patients included in these aforementioned studies were small, and no further meticulous analysis was performed to explore the correlation between the prognosis of gastric cancer patients and the accurate amount of IBL.
The aim of the present study is to elucidate the potential impact of IBL on the long-term survival of gastric cancer patients after curative surgery in a single high-volume center in China.
MATERIALS AND METHODS
Patients
The surgical and pathological data of 845 patients with gastric cancer who had undergone curative gastrectomy (R0 resection) with lymph node dissection and had been followed up between January 2003 and December 2007 at Tianjin Medical University Cancer Institute and Hospital were reviewed in this study. All the patients had been histologically diagnosed with adenocarcinoma of the stomach. Patients who previously underwent gastric surgery or received preoperative chemotherapy were excluded. Patients with distant metastasis were also excluded. The study population consisted of 845 patients, 607 males (71.8%) and 238 females (28.2%) with a median age of 62 years (range, 23-89 years).
Surgical treatment and perioperative management
All the patients underwent gastrectomy with D1 or D2 lymph node dissection. The choice of surgical procedure for reconstruction was made by the surgeon. Resection margin was pathologically confirmed as negative. Postoperative adjuvant chemotherapy was administered according to tumor stage, physical condition and the patient’s willingness. Chemotherapeutics consisted of 5-fluorouracil, leucovorin and oxaliplatin. Radiotherapy was not administered in the present study.
IBL was visually estimated according to the weight or volume of blood absorbed by gauze and suction pump by anesthesiologists immediately after surgery. We obtained this information from anesthesia records. IBL ranged from 50 to 1500 mL and the median IBL was 200 mL for the whole group. The patients were divided into 3 groups according to the amount of IBL: group 1 (< 200 mL), group 2 (200-400 mL) and group 3 (> 400 mL). The entire transfusion history during hospital stay for surgery was recorded. Patients whose perioperative hemoglobin was less than 70 g/L or who lost a lot of blood during surgery were routinely given a red blood cell transfusion. Of the 845 patients, 211 had a perioperative red blood cell transfusion, and the remaining 634 did not receive a transfusion. Postoperative complications during hospitalization only included these directly associated with surgery, such as hemorrhage, wound dehiscence, anastomotic leak, pancreatic fistula, lymphatic fistula and abdominal or wound infection.
Evaluation of clinicopathological variables and survival
The clinicopathological features studied included gender, age, tumor location, tumor size, Borrmann type, histology, extranodal metastasis (EM), type of gastrectomy, combined organ resection, postoperative chemotherapy, tumour-node-metastasis (TNM) stage, extent of lymphadenectomy, postoperative complications, perioperative transfusion, and IBL. Clinicopathological features were first compared among the three groups and the impact of each factor on survival was evaluated to identify independent prognostic factors. We next determined whether IBL influenced cancer-specific survival by comparing overall survival among the three groups by excluding deaths due to reasons other than gastric cancer. Finally, we explored the possible factors associated with IBL and identified the independent risk factors for IBL ≥ 200 mL. The tumors were staged according to the 7th edition Union for International Cancer Control TNM classification system, whereas lymphadenectomy and lymph node stations were defined according to the 3rd English Edition of the Japanese Classification of Gastric Carcinoma. Tumors were classified into two groups based on histology: differentiated type including papillary, well or moderately differentiated adenocarcinoma; and undifferentiated type including poorly differentiated or undifferentiated adenocarcinoma, signet ring cell carcinoma and mucinous carcinoma.
Follow-up
The patients were followed up every 3 mo up to 2 years after surgery, then every 6 mo up to 5 years, and then every year or until death. Physical examination, laboratory tests, imaging and endoscopy were performed at each visit. The median follow-up was 39 mo (range 1-103 mo), and the last follow-up date was December 20, 2012. The overall survival rate was calculated from the day of surgical resection until time of death or final follow-up.
Statistical analysis
Categorical variables were analyzed by means of the χ2 or Fisher’s exact test. Overall survival curves were calculated using the Kaplan-Meier method based on the length of time between primary surgical treatment and final follow-up or death; the Log-rank test was used to assess statistical differences between the groups. Independent prognostic factors were identified by the Cox proportional hazards regression model. One-way analysis of variance (ANOVA) analysis or t test was used in univariate analysis to identify possible factors associated with IBL. Independent risk factors for IBL ≥ 200 mL were determined by logistic regression. P < 0.05 was considered statistically significant. The statistical analysis was performed using the statistical program SPSS 17.0 (SPSS, Chicago, IL, United States).
RESULTS
Clinicopathological features
Of the 845 patients, 397 (47.0%) patients underwent D2 or greater lymph node dissection, and the remaining 448 (53.0%) patients underwent D1 lymph node dissection. Sixty-seven patients underwent gastrectomy combined with other organ resections and 237 patients received postoperative adjuvant chemotherapy.
The patients were divided into three groups according to IBL (Table 1). The mean IBL was 99.4 mL in group 1, 223.2 mL in group 2 and 484.4 mL in group 3. There were no statistical differences in gender, age, Borrmann type, histology, EM and postoperative chemotherapy among the three groups. Tumors located in the upper one-third were more frequent in group 2 and group 3, while in group 1, 53.5% of tumors were located in the lower one-third. The incidence of postoperative complications and the ratios of tumors with a diameter ≥ 5 cm increased when the amount of IBL was high. Total gastrectomy and combined organ resection were more frequently performed in group 3 than in group 1 and group 2. Patients in group 2 and group 3 were more likely to have advanced tumor (T), node (N), and TNM stage than patients in group 1.
Table 1.
Case characteristics n (%)
| Characteristics |
IBL (mL) |
χ2 | P value | ||
| < 200 | 200-400 | > 400 | |||
| IBL (mean ± SD) | 99.3 ± 25.0 | 223.2 ± 41.6 | 484.4 ± 179.9 | ||
| Gender | 4.307 | 0.116 | |||
| Male | 269 (70.2) | 285 (71.6) | 53 (71.8) | ||
| Female | 114 (29.8) | 113 (28.4) | 11 (28.2) | ||
| Age (yr) | 2.488 | 0.288 | |||
| ≤ 65 | 230 (60.1) | 227 (57.0) | 32 (50.0) | ||
| > 65 | 153 (39.9) | 171 (43.0) | 32 (50.0) | ||
| Tumor location | 40.555 | < 0.001 | |||
| Lower 1/3 | 205 (53.5) | 148 (37.2) | 14 (21.9) | ||
| Middle 1/3 | 36 (9.4) | 41 (10.3) | 6 (9.4) | ||
| Upper 1/3 | 98 (25.6) | 164 (41.2) | 34 (53.1) | ||
| 2/3 or more | 44 (11.5) | 45 (11.3) | 10 (15.6) | ||
| Tumor size | 17.677 | < 0.001 | |||
| < 5 cm | 180 (47.0) | 155 (38.9) | 13 (20.3) | ||
| ≥ 5 cm | 203 (53.0) | 243 (61.1) | 51 (79.7) | ||
| Borrmann type | 5.180 | 0.075 | |||
| I/II | 169 (44.1) | 153 (38.4) | 33 (51.6) | ||
| III/IV | 214 (55.9) | 245 (61.6) | 31 (48.4) | ||
| Histology | 0.982 | 0.612 | |||
| Differentiated | 121 (31.6) | 139 (34.9) | 21 (32.8) | ||
| Undifferentiated | 262 (68.4) | 259 (65.1) | 43 (67.2) | ||
| Extranodal metastasis | 1.963 | 0.375 | |||
| Positive | 59 (15.4) | 71 (17.8) | 14 (21.9) | ||
| Negative | 324 (84.6) | 327 (82.2) | 50 (78.1) | ||
| Depth of invasion | 14.719 | 0.023 | |||
| pT1 | 14 (3.7) | 11 (2.8) | 0 (0.0) | ||
| pT2 | 53 (13.8) | 44 (11.1) | 0 (0.0) | ||
| pT3 | 21 (5.5) | 28 (7.0) | 6 (9.4) | ||
| pT4 | 295 (77.0) | 315 (79.1) | 58 (90.6) | ||
| Lymph node metastasis | 15.793 | 0.015 | |||
| pN0 | 173 (45.2) | 146 (36.7) | 19 (29.7) | ||
| pN1 | 56 (14.6) | 82 (20.6) | 9 (14.1) | ||
| pN2 | 85 (22.2) | 87 (21.9) | 15 (23.4) | ||
| pN3 | 69 (18.0) | 83 (20.9) | 21 (32.8) | ||
| TNM stage | 15.313 | 0.004 | |||
| I | 53 (13.8) | 43 (10.8) | 0 (0.0) | ||
| II | 132 (34.5) | 118 (29.6) | 19 (29.7) | ||
| III | 198 (51.7) | 237 (59.5) | 45 (70.3) | ||
| Chemotherapy | 2.036 | 0.361 | |||
| Yes | 104 (27.2) | 119 (29.9) | 14 (21.9) | ||
| No | 279 (72.8) | 279 (70.1) | 50 (78.1) | ||
| Type of gastrectomy | 37.357 | < 0.001 | |||
| Total | 51 (13.3) | 117 (29.4) | 24 (37.5) | ||
| Subtotal | 332 (86.7) | 281 (70.6) | 40 (62.5) | ||
| Combined organ resection | 22.256 | < 0.001 | |||
| Yes | 16 (4.2) | 38 (9.5) | 13 (20.3) | ||
| No | 367 (95.8) | 360 (90.5) | 51 (79.7) | ||
| Extent of lymphadenectomy | 7.230 | 0.027 | |||
| D2 and D2+ | 189 (49.3) | 188 (47.2) | 20 (31.3) | ||
| D1 | 194 (50.7) | 210 (52.8) | 44 (68.8) | ||
| Postoperative complications | 7.500 | 0.024 | |||
| Present | 20 (5.2) | 34 (8.5) | 9 (14.1) | ||
| Absent | 363 (94.8) | 364 (91.5) | 55 (85.9) | ||
IBL: Intraoperative blood loss; TNM: Tumour-node-metastasis.
Prognostic value of IBL in gastric cancer
Data from univariate and multivariate survival analyses are shown in Table 2. A total of 14 factors evaluated in the univariate analysis had a significant effect on survival: age (≤ 65 years vs > 65 years), tumor location, tumor size, Borrmann type (types I and II vs types III and IV), histology, EM, TNM stage, postoperative chemotherapy, type of gastrectomy, combined organ resection, extent of lymphadenectomy, IBL, perioperative transfusion and postoperative complications. Gender did not influence survival. In multivariate analysis, age, tumor size, Borrmann type, EM, TNM stage, postoperative chemotherapy, extent of lymphadenectomy, postoperative complications and IBL were found to be independent prognostic factors for overall survival (OS). The 5-year OS rates were 51.2%, 39.4% and 23.4% for IBL < 200, 200-400, and > 400 mL, respectively, (< 200 mL vs 200-400 mL, P < 0.001; 200-400 mL vs > 400 mL, P = 0.001) (Figure 1A). When deaths due to factors other than gastric cancer were excluded, cancer-specific survival was still significantly influenced by IBL (Figure 1B). The 5-year OS rates for patients with red blood cell transfusion vs those without were 37.0% and 45.7% (P = 0.013), respectively.
Table 2.
Survival analysis of all patients with gastric cancer
| Characteristics | n (%) | 5-yr OS |
Univariate analysis |
Multivariate analysis |
||
| χ2 | P value | HR (95%CI) | P value | |||
| Gender | 1.609 | 0.205 | ||||
| Male | 607 (71.8) | 42.20% | ||||
| Female | 238 (28.2) | 47.10% | ||||
| Age (yr) | 21.037 | < 0.001 | ||||
| ≤ 65 | 489 (57.9) | 50.10% | 1 (ref) | |||
| > 65 | 356 (42.1) | 34.60% | 1.372 (1.140-1.652) | 0.001 | ||
| Tumor location | 26.417 | < 0.001 | ||||
| Lower 1/3 | 367 (43.4) | 50.10% | 1 (ref) | |||
| Middle 1/3 | 83 (9.8) | 45.80% | 0.978 (0.680-1.407) | 0.905 | ||
| Upper 1/3 | 296 (35.0) | 39.50% | 0.931 (0.741-1.169) | 0.538 | ||
| 2/3 or more | 99 (11.7) | 29.30% | 1.149 (0.832-1.586) | 0.398 | ||
| Tumor size | 58.693 | < 0.001 | ||||
| < 5 cm | 348 (41.2) | 57.80% | 1 (ref) | |||
| ≥ 5 cm | 497 (58.8) | 33.60% | 1.411 (1.152-1.730) | 0.001 | ||
| Borrmann type | 13.517 | < 0.001 | ||||
| I/II | 355 (42.0) | 50.40% | 1 (ref) | |||
| III/IV | 490 (58.0) | 38.60% | 1.285 (1.062-1.556) | 0.010 | ||
| Histology | 6.783 | 0.009 | ||||
| Differentiated | 281 (33.3) | 49.80% | 1 (ref) | |||
| Undifferentiated | 564 (66.7) | 40.40% | 1.151 (0.939-1.412) | 0.176 | ||
| Extranodal metastasis | 52.773 | < 0.001 | ||||
| Negative | 701 (83.0) | 47.50% | 1 (ref) | |||
| Positive | 144 (17.0) | 24.30% | 1.543 (1.236-1.925) | < 0.001 | ||
| TNM stage | 147.103 | < 0.001 | ||||
| I | 96 (11.4) | 82.30% | 1 (ref) | |||
| II | 269 (31.8) | 58.40% | 2.253 (1.362-3.727) | 0.002 | ||
| III | 480 (56.8) | 27.50% | 4.736 (2.898-7.740) | < 0.001 | ||
| Chemotherapy | 10.999 | 0.001 | ||||
| Yes | 237 (28.0) | 50.60% | 1 (ref) | |||
| No | 608 (72.0) | 40.80% | 1.357 (1.093-1.684) | 0.006 | ||
| Extent of lymphadenectomy | 6.668 | 0.010 | ||||
| D2 and D2+ | 397 (47.0) | 48.40% | 1 (ref) | |||
| D1 | 448 (53.0) | 39.30% | 1.372 (1.126-1.671) | 0.002 | ||
| Type of gastrectomy | 21.400 | < 0.001 | ||||
| Subtotal | 653 (77.3) | 47.00% | 1 (ref) | |||
| Total | 192 (22.7) | 31.80% | 1.102 (0.849-1.430) | 0.466 | ||
| Combined organ resection | 10.310 | 0.001 | ||||
| No | 778 (92.1) | 44.60% | 1 (ref) | |||
| Yes | 67 (7.9) | 31.30% | 1.116 (0.811-1.536) | 0.501 | ||
| Intraoperative blood loss | 29.175 | < 0.001 | ||||
| < 200 mL | 383 (45.3) | 51.20% | 1 (ref) | |||
| 200-400 mL | 398 (47.1) | 39.40% | 1.242 (1.017-1.516) | 0.033 | ||
| > 400 mL | 64 (7.6) | 23.40% | 1.590 (1.140-2.217) | 0.006 | ||
| Perioperative transfusion | 6.145 | 0.013 | ||||
| No | 634 (75.0) | 45.70% | 1 (ref) | |||
| Yes | 211 (25.0) | 37.00% | 0.962 (0.748-1.180) | 0.708 | ||
| Postoperative complications | 28.320 | < 0.001 | ||||
| Absent | 782 (92.5) | 44.90% | 1 (ref) | |||
| Present | 63 (7.5) | 27.00% | 2.096 (1.525-2.881) | < 0.001 | ||
OS: Overall survival; TNM: Tumour-node-metastasis.
Figure 1.
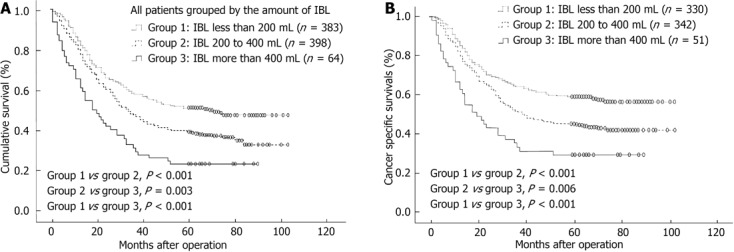
Overall survival and cancer-specific curves for all patients grouped by intraoperative blood loss. A: Overall survival curve; B: Cancer-specific survival curve. IBL: Intraoperative blood loss.
To assess the association between IBL and red blood cell transfusion, patients were categorized into 4 groups [IBL < 200 mL and transfusion (-); IBL < 200 mL, transfusion (+); IBL ≥ 200 mL, transfusion (-); IBL ≥ 200 mL, transfusion (+)], and OS was compared among these groups (Figure 2). As a blood loss of 200 mL was the median for the whole group, it was used for dichotomization in the statistical analysis. As a result, a IBL of 200 mL or more was a significant factor when excluding the influence of red blood cell transfusion (P = 0.001; P = 0.026). However, there was no significant difference in OS between patients with and without transfusion when the influence of IBL was excluded (P = 0.279; P = 0.078).
Figure 2.
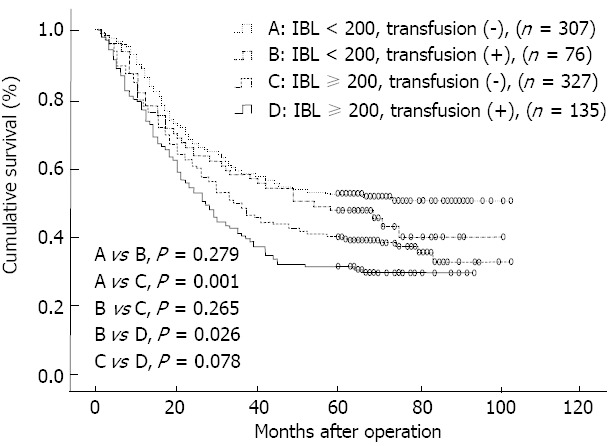
Overall survival curves for all patients classified according to intraoperative blood loss and red blood cell transfusion. IBL: Intraoperative blood loss.
The results of the stratified analysis are shown in Table 3. In patients with TNM stage I, those with IBL less than 200 mL had significantly better survival than those with IBL 200-400 mL (Figure 3A). In the patients staged with TNM II, those with IBL less than 200 mL had a significantly higher 5-year OS than those with IBL 200-400 mL or more than 400 mL, while there were no statistical differences in OS between those with IBL 200-400 mL and more than 400 mL (Figure 3B). For patients staged TNM III, OS did not differ significantly between those with IBL less than 200 mL and 200-400 mL, however, these patients had significantly higher 5-year OS than those with IBL more than 400 mL (Figure 3C).
Table 3.
Tumour-node-metastasis-stratified analysis of the overall survival
|
Group 11 |
Group 21 |
Group 31 |
χ2 | P value | ||||
| n | 5-yr OS | n | 5-yr OS | n | 5-yr OS | |||
| TNM | ||||||||
| I | 53 | 88.7 | 43 | 74.4 | 4.538 | 0.037 | ||
| II | 132 | 68.2 | 118 | 50.0 | 19 | 42.1 | 10.763 | 0.005 |
| III | 198 | 29.8 | 237 | 27.8 | 45 | 15.6 | 8.035 | 0.018 |
Group 1: IBL < 200 mL; Group 2: IBL 200-400 mL; Group 3: IBL > 400 mL. OS: Overall survival; TNM: Tumour-node-metastasis; IBL: Intraoperative blood loss.
Figure 3.
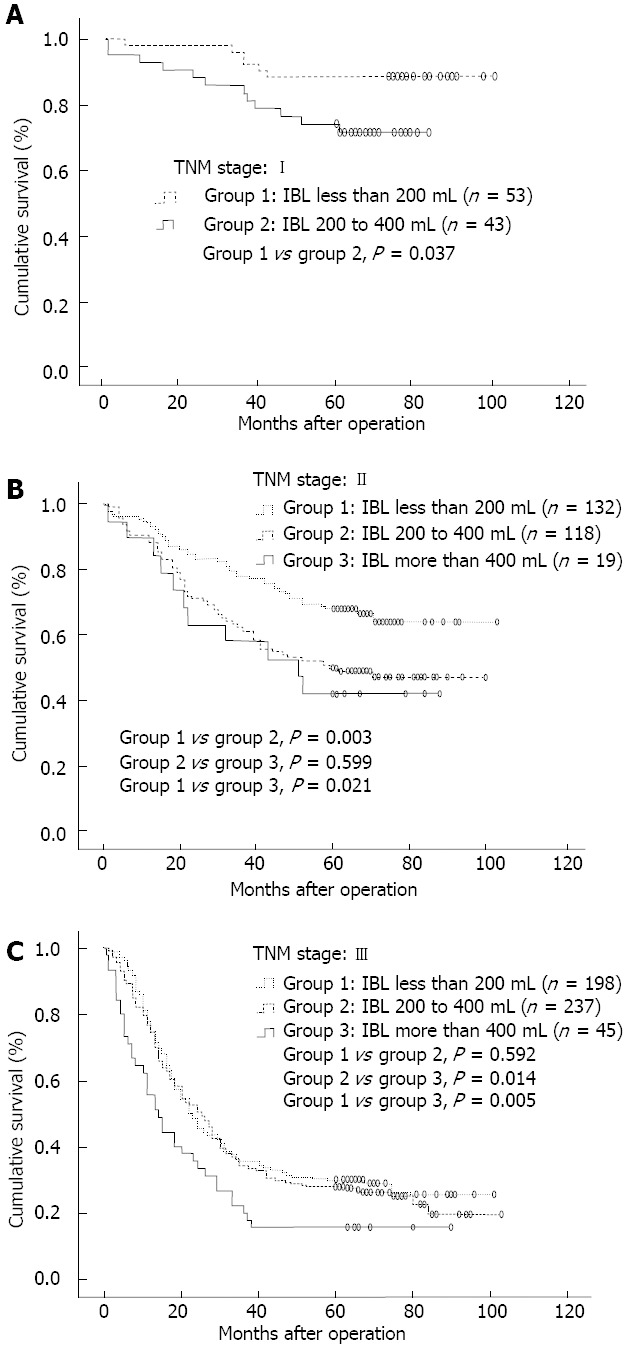
Overall survival curves. A: 96 patients staged tumour-node-metastasis (TNM) I; B: 269 patients staged TNM II; C: 480 patients staged TNM III. IBL: Intraoperative blood loss.
Risk factors associated with IBL
Univariate analysis of factors associated with the amount of IBL is shown in Table 4. Following one-way ANOVA analysis or t test, tumor location, tumor size, TNM stage, type of gastrectomy, combined organ resection, extent of lymphadenectomy and year of surgery were found to be significant factors associated with the amount of IBL. Factors which had no influence on IBL were gender, age, Borrmann type, histology, and EM. As patients with IBL less than 200 mL had the best survival, we further identified the independent risk factors for IBL ≥ 200 mL. Factors significant in the univariate analysis were included in the multivariate analysis. Tumor location, type of gastrectomy, combined organ resection and year of surgery were found to be independent risk factors for IBL ≥ 200 mL in the multivariate analysis (Table 5).
Table 4.
Association between clinicapothologic factors and the amount of intraoperative blood loss: univariate analysis
| Characteristics | n (%) | Amount of IBL (mL) (mean ± SD) | t/F | P value |
| Gender | 1.770 | 0.077 | ||
| Male | 607 (71.8) | 191.4 ± 128.6 | ||
| Female | 238 (28.2) | 175.2 ± 92.5 | ||
| Age (yr) | -1.128 | 0.260 | ||
| ≤ 65 | 489 (57.9) | 182.9 ± 121.8 | ||
| > 65 | 356 (42.1) | 192.3 ± 116.7 | ||
| Tumor location | 12.455 | < 0.001 | ||
| Lower 1/3 | 367 (43.4) | 160.9 ± 87.8 | ||
| Middle 1/3 | 83 (9.8) | 179.5 ± 103.0 | ||
| Upper 1/3 | 296 (35.0) | 213.2 ± 127.5 | ||
| 2/3 or more | 99 (11.7) | 210.6 ± 177.5 | ||
| Tumor size | -4.129 | < 0.001 | ||
| < 5 cm | 348 (41.2) | 166.7 ± 92.8 | ||
| ≥ 5 cm | 497 (58.8) | 200.9 ± 133.7 | ||
| Borrmann type | ||||
| I/II | 355 (42.0) | 187.5 ± 127.0 | 0.128 | 0.899 |
| III/IV | 490 (58.0) | 186.4 ± 114.3 | ||
| Histology | -0.160 | 0.873 | ||
| Differentiated | 281 (33.3) | 185.9 ± 107.7 | ||
| Undifferentiated | 564 (66.7) | 187.3 ± 125.3 | ||
| Extranodal metastasis | -1.040 | 0.299 | ||
| Negative | 701 (83.0) | 184.9 ± 119.7 | ||
| Positive | 144 (17.0) | 196.3 ± 119.6 | ||
| TNM stage | 4.974 | 0.007 | ||
| I | 96 (11.4) | 154.2 ± 67.1 | ||
| II | 269 (31.8) | 183.3 ± 135.9 | ||
| III | 480 (56.8) | 195.4 ± 117.1 | ||
| Type of gastrectomy | -5.963 | < 0.001 | ||
| Subtotal | 653 (77.3) | 173.8 ± 102.3 | ||
| Total | 192 (22.7) | 231.2 ± 158.1 | ||
| Combined organ resection | -5.329 | < 0.001 | ||
| Absent | 778 (92.1) | 180.5 ± 110.9 | ||
| Present | 67 (7.9) | 260.4 ± 180.0 | ||
| Extent of llymphadenectomy | -2.676 | 0.008 | ||
| D2 and D2+ | 397 (47.0) | 175.2 ± 95.4 | ||
| D1 | 448 (53.0) | 197.2 ± 136.9 | ||
| Year of surgery | -2.494 | 0.013 | ||
| 2003-2005 | 489 (57.9) | 195.1 ± 133.6 | ||
| 2006-2007 | 356 (42.1) | 174.3 ± 97.6 | ||
TNM: Tumour, node, metastasis; IBL: Intraoperative blood loss.
Table 5.
Multivariate analysis of risk factors for intraoperative blood loss ≥ 200 mL
| Feature | HR | 95%CI | P value | |
| Tumor location | Upper 1/3 and 2/3 or more vs lower and middle 1/3 | 1.717 | 1.272-2.317 | < 0.001 |
| Tumor size | ≥ 5 cm vs < 5 cm | 1.129 | 0.833-1.513 | 0.434 |
| TNM stage | III vs I, II | 1.174 | 0.872-1.580 | 0.290 |
| Extent of gastrectomy | D1 vs D2 and D2+ | 1.161 | 0.860-1.566 | 0.330 |
| Type of gastrectomy | Total vs subtotal | 2.501 | 1.707-3.663 | < 0.001 |
| Combined organ resection | Present vs absent | 1.996 | 1.089-3.659 | 0.025 |
| Year of surgery | 2003-2005 vs 2006-2007 | 1.452 | 1.080-1.954 | 0.014 |
TNM: Tumour, node, metastasis.
DISCUSSION
The prognosis of gastric cancer is mainly associated with tumor depth and lymph node status[5,6]. To improve the outcome of gastric cancer, standard surgery with D2 lymph node dissection is recommended[15,16]. However, even after curative gastrectomy with D2 dissection, the prognosis remains poor. In the present study, we evaluated the potential prognostic factors and found that IBL was significantly associated with the survival of patients with gastric cancer after curative resection.
IBL has been reported to be associated with the prognosis of many malignant tumors[12-14]. Mörner et al[12] reported that the degree of IBL in colon cancer influenced long-term survival. In their study, blood loss of 250 mL or more during surgery was a risk factor for overall mortality in both univariate and multivariate analyses. Nagai et al[13] demonstrated that IBL greater than 2000 mL was related to poor prognosis in patients with pancreatic cancer. These authors suggested that successful curative resection with limited blood loss can contribute to improved survival. With regard to gastric cancer, few studies have focused on IBL. Dhar et al[10] reported that IBL more than 500 mL was an independent prognostic factor. Kamei et al[11] demonstrated that the cumulative survival rate was significantly lower in patients with IBL ≥ 475 mL than in patients with IBL < 475 mL (P = 0.0038), and IBL was a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. Our data are consistent with those results and strongly suggest that IBL, rather than transfusion, was an independent prognostic factor for gastric cancer after curative resection.
In previous studies, blood loss of 475 or 500 mL was proposed as a threshold for prognostic significance[10,11]. To date, no study has conducted a detailed statistical analysis by classifying patients into groups based on the level of IBL during resection for gastric cancer. When the thresholds were set at 200 and 400 mL, the OS was significantly affected based on a comparison between these 3 groups. The 5-year OS rates were 51.2%, 39.4% and 23.4% for IBL < 200 mL, 200-400 mL and > 400 mL, respectively (< 200 mL vs 200-400 mL, P < 0.001; 200-400 mL vs > 400 mL, P = 0.003; < 200 mL vs > 400 mL, P < 0.001). Even when deaths due to factors other than gastric cancer were excluded, the differences in cancer-specific survival among the three groups were still significant. This clearly demonstrated the negative influence of IBL on survival after curative gastrectomy. Pathological stage is assumed to be the most important prognostic factor for gastric cancer following curative gastrectomy. Therefore, we stratified patients by TNM stage. Even after stratification, the same trend, i.e., better outcomes in patients with a small amount of IBL, was still observed in each stage. Thus, reducing IBL in resectable gastric cancer may provide further improvements in survival. According to the results of the present study, for patients staged TNM I and II, IBL should be controlled within 200 mL to achieve a better outcome. In patients staged TNM III, IBL should be no more than 400 mL.
Blood transfusion is needed when performing complex surgery with a large amount of IBL. Although many studies[17-21] have confirmed that perioperative blood transfusion leads to poor outcome in gastric cancer, some studies[22-26] do not support this. In the present study, perioperative transfusion was a prognostic factor, but not an independent prognostic factor in the multivariate analysis. When the influence of IBL was excluded, OS did not differ significantly between patients with and without transfusion, although 5-year OS was higher in patients without transfusion than in patients with transfusion if the IBL was similar. However, when excluding the influence of transfusion, patients whose IBL was less than 200 mL had significantly better survival than those with IBL of 200 mL or more. The effect of IBL on survival was more pronounced than that of red blood transfusion.
It is still unclear why IBL affects the long-term outcome of patients. It is thought that excessive IBL reduces the body’s immunity and thus its ability to fight cancer cells[10]. In a study conducted by Bruns et al[27], IBL more than 700 mL following gastrointestinal surgery was associated with a significant decrease in natural killer cell activity, producing an unfavorable effect on patient survival. However, the degree of immune suppression was not assessed in this study. This should be examined in a future trial to clarify whether patients with excessive IBL have severe immune suppression resulting in a poor overall survival rate. Another possible explanation is that IBL is associated with peritoneal recurrence which leads to poor survival. It has been reported that operative blood loss is an independent risk factor for peritoneal recurrence of curatively resectable advanced gastric cancer[11]. In open abdominal surgery, most operative blood loss accumulates in the abdominal cavity, and thus, the peritoneal surface is considered to have direct contact with blood components. As extravascular blood cells, such as leukocytes and platelets, are activated, they may produce a number of soluble factors that may produce a favorable microenvironment for malignant cells. In fact, activated neutrophils, macrophages, and platelets are capable of producing a large amount of angiogenic factors, such as vascular endothelial growth factor, on the peritoneal surface, which is critical for the survival of isolated cancer cells[28,29]. Unfortunately, recurrence data was not obtained in our study.
IBL has been shown to be correlated with postoperative complications[30]. In the present study, the incidence of postoperative complications increased when the amount of IBL was high. Previous studies have affirmed the negative influence of postoperative complications on survival for many malignancies[31-35]. Sierzega et al[7] reported that anastomotic leakage was an independent prognostic factor for gastric adenocarcinoma following total gastrectomy. Tokunaga et al[35] found that postoperative intra-abdominal infectious complications had an adverse effect on 5-year OS and relapse-free survival rate. Our results were in accordance with those reports and showed that the presence of postoperative complications was an independent prognostic factor for OS. As a higher rate of complications was associated with a larger amount of IBL, we consider that the difference in the incidence of postoperative complications among the three groups was a possible contributing factor to the survival difference among the three groups.
As IBL is an independent prognostic factor and patients with IBL less than 200 mL had the best outcome, it is necessary to explore the potential factors influencing IBL and to develop new surgical methods to reduce IBL. It is obvious that IBL could be affected by the type of gastrectomy and combined organ resection. Patients with tumors located in the upper 1/3 or more than 2/3 the area usually undergo a total gastrectomy or combined spleen resection, which may result in a larger amount of IBL. Lymph node dissection is considered to be a complex procedure and can easily lead to bleeding, especially dissection of the lymph nodes around the celiac trunk. We have used an ultrasonic scalpel for lymph node dissection of gastric cancer since 2006. Ultrasonic surgical devices have been reported to provide advantages in terms of operative time and blood loss[36,37]. A study conducted by Inoue K and colleagues showed that blood loss was significantly lower in patients using ultrasonic scalpel than in those not using the ultrasonic scalpel (median 351.0 mL vs 569.5 mL; P = 0.016)[38]. From this point of view, it is actually the application of the ultrasonic scalpel that leads to reduced IBL rather than the year, although year of surgery was found to be an independent risk factor for IBL in the present study.
In conclusion, IBL was found to be an independent prognostic factor for gastric cancer after curative resection. It can be used to stratify the risk for gastric cancer prognosis. Meticulous surgery is needed and new methods should be considered to decrease the amount of IBL and improve the long-term outcome of patients following curative gastrectomy.
COMMENTS
Background
Intraoperative blood loss (IBL) has been shown to be associated with poor outcome in various types of malignancy. However, the relationship between the amount of IBL and outcome of gastric cancer is still unclear.
Research frontiers
IBL can not be avoided in surgery. Excessive blood loss may result in more postoperative complications and poorer prognosis. Research has shown the negative association between IBL and prognosis of many malignancies. Few researchers have focused on IBL during resection of gastric cancer. In this study, the authors demonstrated that IBL was an independent prognostic factor for gastric cancer after curative resection.
Innovations and breakthroughs
Many studies have affirmed that perioperative blood transfusion leads to poor outcome in gastric cancer. However, when performing complex surgery, blood transfusion is required due to a large amount of IBL, which was also reported to have an adverse effect on survival. The impact of IBL on survival may be confused by blood transfusion. This study evaluated the prognostic value of both factors on survival in gastric cancer patients after curative resection and found that IBL influenced the prognosis of gastric cancer rather than blood transfusion.
Applications
By understanding the negative association between the amount of IBL and prognosis of gastric cancer, this study may stimulate surgeons to pay attention to decreasing the amount of IBL during curative gastrectomy.
Terminology
IBL is the amount of blood loss during surgery which is visually estimated by anesthesiologists immediately after surgery. Extranodal metastasis was defined as the presence of tumor cells in extramural soft tissue that was discontinuous with either the primary lesion or locoregional lymph nodes.
Peer review
The IBL and perioperative transfusion have been the topics concerned by surgeons. And IBL has been shown to be associated with poor outcome in various types of malignancy. This study shows that IBL is an independent prognostic factor for gastric cancer patients after curative resection. This conclusion has some significance for guiding clinical work.
Footnotes
P- Reviewers Hajifathalian K, Ji JF, Mann O S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–357. doi: 10.1046/j.1365-2168.2000.01358.x. [DOI] [PubMed] [Google Scholar]
- 3.Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89:255–261. doi: 10.1002/1097-0142(20000715)89:2<255::aid-cncr8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Prediction of early and late recurrence after curative resection for gastric carcinoma. Cancer. 1996;77:2445–2448. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2445::AID-CNCR5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, Arai K, Kodera Y, Nashimoto A. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 6.Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–316. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierzega M, Kolodziejczyk P, Kulig J. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg. 2010;97:1035–1042. doi: 10.1002/bjs.7038. [DOI] [PubMed] [Google Scholar]
- 8.Hyung WJ, Noh SH, Shin DW, Huh J, Huh BJ, Choi SH, Min JS. Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol. 2002;9:5–12. doi: 10.1245/aso.2002.9.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Ojima T, Iwahashi M, Nakamori M, Nakamura M, Naka T, Katsuda M, Iida T, Hayata K, Yamaue H. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J Gastrointest Surg. 2009;13:1821–1830. doi: 10.1007/s11605-009-0973-9. [DOI] [PubMed] [Google Scholar]
- 10.Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Watanabe R, Kohno H, Nagasue N. Long-term survival of transmural advanced gastric carcinoma following curative resection: multivariate analysis of prognostic factors. World J Surg. 2000;24:588–593; discussion 593-594. doi: 10.1007/s002689910099. [DOI] [PubMed] [Google Scholar]
- 11.Kamei T, Kitayama J, Yamashita H, Nagawa H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg. 2009;33:1240–1246. doi: 10.1007/s00268-009-9979-4. [DOI] [PubMed] [Google Scholar]
- 12.Mörner ME, Gunnarsson U, Jestin P, Svanfeldt M. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg. 2012;255:1126–1128. doi: 10.1097/SLA.0b013e3182512df0. [DOI] [PubMed] [Google Scholar]
- 13.Nagai S, Fujii T, Kodera Y, Kanda M, Sahin TT, Kanzaki A, Yamada S, Sugimoto H, Nomoto S, Takeda S, et al. Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas. 2011;40:3–9. doi: 10.1097/MPA.0b013e3181f7147a. [DOI] [PubMed] [Google Scholar]
- 14.Oefelein MG, Colangelo LA, Rademaker AW, McVary KT. Intraoperative blood loss and prognosis in prostate cancer patients undergoing radical retropubic prostatectomy. J Urol. 1995;154:442–447. doi: 10.1097/00005392-199508000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 16.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 17.Dhar DK, Kubota H, Tachibana M, Kotoh T, Kinugasa S, Shibakita M, Kohno H, Nagasue N. A tailored perioperative blood transfusion might avoid undue recurrences in gastric carcinoma patients. Dig Dis Sci. 2000;45:1737–1742. doi: 10.1023/a:1005538429420. [DOI] [PubMed] [Google Scholar]
- 18.Kaneda M, Horimi T, Ninomiya M, Nagae S, Mukai K, Takeda I, Shimoyama H, Chohno S, Okabayashi T, Kagawa S. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion. 1987;27:375–377. doi: 10.1046/j.1537-2995.1987.27587320526.x. [DOI] [PubMed] [Google Scholar]
- 19.Fong Y, Karpeh M, Mayer K, Brennan MF. Association of perioperative transfusions with poor outcome in resection of gastric adenocarcinoma. Am J Surg. 1994;167:256–260. doi: 10.1016/0002-9610(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 20.Maeta M, Shimizu N, Oka A, Kondo A, Yamashiro H, Tsujitani S, Ikegchi M, Kaibara N. Perioperative allogeneic blood transfusion exacerbates surgical stress-induced postoperative immunosuppression and has a negative effect on prognosis in patients with gastric cancer. J Surg Oncol. 1994;55:149–153. doi: 10.1002/jso.2930550304. [DOI] [PubMed] [Google Scholar]
- 21.Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol) 2006;18:60–66. doi: 10.1016/j.clon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Kampschöer GH, Maruyama K, Sasako M, Kinoshita T, van de Velde CJ. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. 1989;13:637–643. doi: 10.1007/BF01658891. [DOI] [PubMed] [Google Scholar]
- 23.Moriguchi S, Maehara Y, Akazawa K, Sugimachi K, Nose Y. Lack of relationship between perioperative blood transfusion and survival time after curative resection for gastric cancer. Cancer. 1990;66:2331–2335. doi: 10.1002/1097-0142(19901201)66:11<2331::aid-cncr2820661113>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Choi JH, Chung HC, Yoo NC, Lee HR, Lee KH, Kim JH, Roh JK, Min JS, Lee KS, Kim BS. Perioperative blood transfusions and prognosis in patients with curatively resected locally advanced gastric cancer. Oncology. 1995;52:170–175. doi: 10.1159/000227452. [DOI] [PubMed] [Google Scholar]
- 25.Bortul M, Calligaris L, Roseano M, Leggeri A. Blood transfusions and results after curative resection for gastric cancer. Suppl Tumori. 2003;2:S27–S30. [PubMed] [Google Scholar]
- 26.Sánchez-Bueno F, García-Marcilla JA, Pérez-Abad JM, Vicente R, Aranda F, Lujan JA, Parrilla P. Does perioperative blood transfusion influence long-term prognosis of gastric cancer. Dig Dis Sci. 1997;42:2072–2076. doi: 10.1023/a:1018818517811. [DOI] [PubMed] [Google Scholar]
- 27.Bruns CJ, Schäfer H, Wolfgarten B, Engert A. Effect of intraoperative blood loss on the function of natural killer cells in tumors of the upper gastrointestinal tract. Langenbecks Arch Chir Suppl Kongressbd. 1996;113:146–149. [PubMed] [Google Scholar]
- 28.McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–1331; discussion 1331-1332. doi: 10.1001/archsurg.134.12.1325. [DOI] [PubMed] [Google Scholar]
- 29.Seo KH, Ko HM, Choi JH, Jung HH, Chun YH, Choi IW, Lee HK, Im SY. Essential role for platelet-activating factor-induced NF-kappaB activation in macrophage-derived angiogenesis. Eur J Immunol. 2004;34:2129–2137. doi: 10.1002/eji.200424957. [DOI] [PubMed] [Google Scholar]
- 30.Sah BK, Zhu ZG, Chen MM, Xiang M, Chen J, Yan M, Lin YZ. Effect of surgical work volume on postoperative complication: superiority of specialized center in gastric cancer treatment. Langenbecks Arch Surg. 2009;394:41–47. doi: 10.1007/s00423-008-0358-7. [DOI] [PubMed] [Google Scholar]
- 31.Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. 2005;48:1021–1026. doi: 10.1007/s10350-004-0869-4. [DOI] [PubMed] [Google Scholar]
- 32.Bell SW, Walker KG, Rickard MJ, Sinclair G, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg. 2003;90:1261–1266. doi: 10.1002/bjs.4219. [DOI] [PubMed] [Google Scholar]
- 33.Hirai T, Yamashita Y, Mukaida H, Kuwahara M, Inoue H, Toge T. Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today. 1998;28:576–579. doi: 10.1007/s005950050187. [DOI] [PubMed] [Google Scholar]
- 34.Rizk NP, Bach PB, Schrag D, Bains MS, Turnbull AD, Karpeh M, Brennan MF, Rusch VW. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198:42–50. doi: 10.1016/j.jamcollsurg.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–1583. doi: 10.1245/s10434-012-2720-9. [DOI] [PubMed] [Google Scholar]
- 36.Litta P, Fantinato S, Calonaci F, Cosmi E, Filippeschi M, Zerbetto I, Petraglia F, Florio P. A randomized controlled study comparing harmonic versus electrosurgery in laparoscopic myomectomy. Fertil Steril. 2010;94:1882–1886. doi: 10.1016/j.fertnstert.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 37.Targarona EM, Balague C, Marin J, Neto RB, Martinez C, Garriga J, Trias M. Energy sources for laparoscopic colectomy: a prospective randomized comparison of conventional electrosurgery, bipolar computer-controlled electrosurgery and ultrasonic dissection. Operative outcome and costs analysis. Surg Innov. 2005;12:339–344. doi: 10.1177/155335060501200409. [DOI] [PubMed] [Google Scholar]
- 38.Bornstein RF, Greenberg RP, Leone DR, Galley DJ. Defense mechanism correlates of orality. J Am Acad Psychoanal. 1990;18:654–666. doi: 10.1521/jaap.1.1990.18.4.654. [DOI] [PubMed] [Google Scholar]


