Abstract
Objectives
This study was performed to evaluate the effect of blood contamination on the compressive strength (CS) of Root MTA (RMTA) modified with Calcium chloride (CaCl2) and Disodium hydrogen phosphate (Na2HPO4) as setting accelerators over time.
Materials and Methods
A total of 110 cylindrical specimens of RMTA were divided into 6 experimental groups as follows: Group1, RMTA; Group 2, RMTA modified with CaCl2 (RMTA-C); Group 3, RMTA modified with Na2HPO4 (RMTA-N); Group 4, RMTA contaminated with blood; Group 5, RMTA-C contaminated with blood; Group 6, RMTA-N contaminated with blood. The CS of specimens in all groups was evaluated after 3 hr, 24 hr, and 1 wk. In the modified groups (groups 2, 3, 5, and 6) the CS of five specimens per group was also evaluated after 1 hr.
Results
Blood contamination significantly reduced the CS of all materials at all time intervals (p < 0.05). After 3 hr, the CS of specimens in the RMTA groups (with and without blood contamination) was significantly lower than those in the RMTA-C and RMTA-N groups (p < 0.05). The CS values were not significantly different at the other time intervals. In all groups, the CS of specimens significantly increased over time (p < 0.05).
Conclusions
Blood contamination decreased the CS of both original and accelerated RMTA.
Keywords: Blood contamination, Calcium chloride, Compressive strength, Disodium hydrogen phosphate, Mineral trioxide aggregate
Introduction
In most of its clinical applications, one or more surfaces of mineral trioxide aggregate (MTA) are exposed directly to blood which may also penetrate into body of the MTA slurry. Blood contamination of unset MTA adversely affects the setting reaction and mechanical strength of the material.1 Vanderweele et al. reported that following blood contamination of MTA in perforation sites, resistance to displacement decreased.2 In addition, Nekoofar et al. demonstrated that blood contamination influenced the development of MTA crystals and decreased its surface microhardness and compressive strength.3-5 It is well known that another disadvantage of MTA is its extended setting time.6-10
While MTA is a derivative of Type 1 Portland cement (PC), addition of setting accelerator admixtures such as calcium chloride (CaCl2) and disodium hydrogen phosphate (Na2HPO4) have been suggested to decrease the setting time of MTA.6,7,11-13 They also accelerate the early strength of PC.14 Therefore, they might be able to protect MTA against adverse effects of blood contamination by reducing the possibility of infusion of the blood into the material whilst at the same time improving its early strength. To date, no studies have reported the effects of blood contamination on rapid-setting MTA materials.
Root MTA (RMTA, Lotfi research group, Tabriz, Iran) is one of several commercially available types of MTA. Several cytological, animal and clinical studies have shown similar characteristics of RMTA and ProRoot White MTA (Dentsply Tulsa Dental, Tulsa, OK, USA).15-23 Scanning electron microscopic (SEM) analysis has shown that both ProRoot MTA and RMTA contained a complex mixture of mineral phases with highly visible randomly distributed particles of bismuth oxide.24 In these materials, the size of crystalline particles and bismuth oxide particles were the same.24 In addition, electron probe microanalysis has shown that their major components such as Lime (CaO), Silica (SiO2), Bismuth Oxide (Bi2O3), Aluminum Oxide (Al2O3) and Magnesium Oxide (MgO) are similar (Table 1).24
Table 1.
Comparison of the major components of Root MTA, ProRoot White MTA, and Original ProRoot MTA

RMTA, Root MTA; WMTA, White MTA; GMTA, Gray MTA.
Adapted with permission from Dr. Saeed Asgary.
The purpose of this study was to evaluate the effect of blood contamination on the compressive strength of RMTA, as a model of MTA-like materials, which was modified by adding CaCl2 and Na2HPO4, in order to investigate the ability of these admixtures to prevent adverse effects of blood contamination.
Materials and Methods
The materials and groups evaluated were Group1, RMTA (n = 15); Group 2, RMTA modified with CaCl2 (RMTA-C) (n = 20); Group 3, RMTA modified with Na2HPO4 (RMTA-N) (n = 20); Group 4, RMTA contaminated with blood (n = 15); Group 5, RMTA-C contaminated with blood (n = 20); Group 6, RMTA-N contaminated with blood (n = 20). The compressive strength of specimens was evaluated after 3 hours, 24 hours, and 1 week (5 specimens at each time interval). In addition, in the modified groups (2, 3, 5 and 6) the compressive strength of an additional 5 specimens in each group was evaluated after 1 hour to evaluate their initial strength.
Specimen preparation technique
Stainless steel cylindrical split molds with a height of 6 mm and an internal diameter of 4 mm were used according to ISO 9917-1.25
Group 1 (RMTA): RMTA powder was mixed with sterile water in a 3/1 ratio. The powder was weighed using a digital scale model PL303 with 0.001 g accuracy (Mettler Toledo Inc., Columbus, OH, USA). The volume of sterile water was determined using a transferpette with 0.001 mL accuracy (BRAND GMBH + Co KG, Wertheim, Germany). The powder and liquid were poured into plastic capsules and were mixed mechanically for 30 seconds using an amalgamator (Farazmehr Co., Esfahan, Iran) at 4,500 rpm. The mixed MTA slurries were then placed into the metallic moulds according to method described by Nekoofar et al.4
Group 2 (RMTA-C): The specimens were prepared in the same way as Group 1 but instead of sterile water, a 5% CaCl2 solution was used. To prepare this solution 5.0 g of CaCl2 (Merck, Darmstadt, Germany) was dissolved in 100 mL of sterile water.
Group 3 (RMTA-N): In this group the RMTA powder was mixed with 2.5% (weight) Na2HPO4 powder (Merck). The specimens were prepared in the same way as Group 1.
Groups 4, 5, and 6 (blood contaminated specimens): The specimens in groups 4, 5, and 6 were prepared in the same way as Groups 1, 2, and 3 respectively. The only difference being that before MTA placement the moulds were contaminated with blood by filling them with whole fresh human blood that was then removed by aspirating with a syringe to leave a coating of blood on the internal walls of the moulds. The fresh blood was obtained by a trained medical nurse from a volunteer member of the research group. The procedure was approved by the ethics committee of the Zahedan University of Medical Sciences.
All specimens were incubated at 37℃ in a fully saturated humidity. At each time interval the specimens were removed from the molds and assessed for the presence of voids or chipped edges and damaged specimens were excluded and replaced with new specimens.
The compressive strength of the specimens was then evaluated using a universal testing machine Model H5K5 (Haunsfield Test Equipment, Redhill, UK). The compressive load was recorded until the loading failure point was reached. Loading failure was used to calculate the compressive strength of the specimens in terms of megapascal (MPa) using the following equation: C = 4P/πD2 where P (N) is loading failure, and D (mm) is the diameter of the specimen.
The effects of blood contamination on compressive strength of the specimens at different time intervals were analyzed using T-tests using the Statistical Package for the Social Sciences version 16 (SPSS Inc., Chicago, IL, USA). Also the effects of setting accelerators themselves and time on compressive strength were analyzed with twoway analysis of variance (ANOVA) and post hoc Tamhane's. The level of significance for the data analysis was 95%. In order to assess the normality of the data, the error terms of all experimental groups calculated. Then the normal distribution of error terms were analyzed by one-sample kolmogorov-smirnov test which showed that the differences were not statistically significant (p < 0.05).
Results
A summary of the compressive strength (Mean ± SD) of experimental groups is shown in Table 2. The highest mean compressive strength value was recorded for the original RMTA group after 1 week (62.64 ± 3.2 MPa) and the lowest mean compressive strength value was recorded for the blood contaminated RMTA-C group after 1 hour (2.08 ± 0.23 MPa).
Table 2.
Mean and standard deviation (SD) of compressive strength* of the groups over time
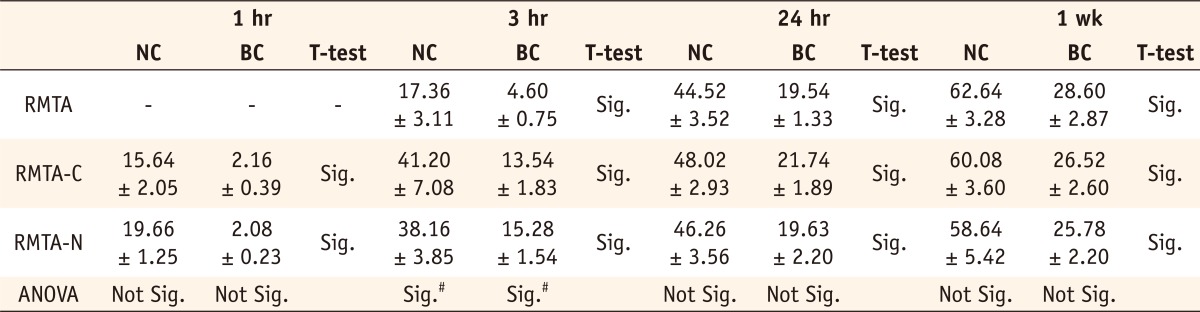
NC, No Contamination; BC, Blood Contamination; Sig., Statistically Significant (p < 0.05).
*All measurements are in MPa.
#The difference between modified groups themselves (RMTA-C & RMTA-N) was not statistically significant.
Effect of blood contamination
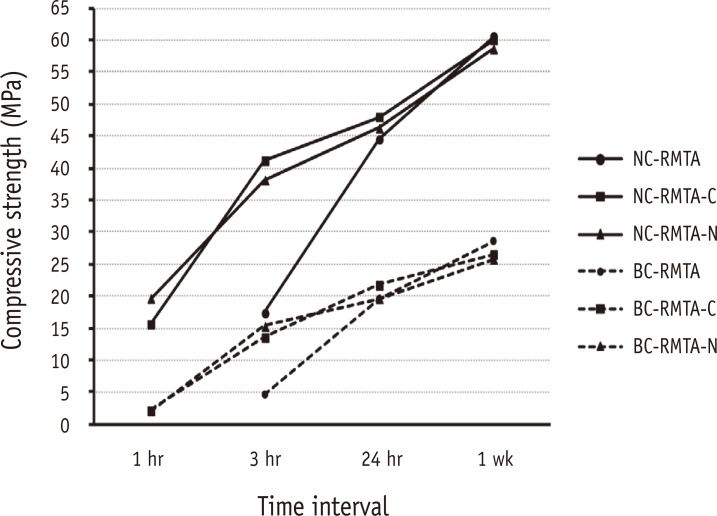
In blood contaminated specimens the compressive strength was significantly lower than that of specimens without blood contamination at all time intervals for all experimental materials (p < 0.05, Figure 1).
Figure 1.
Similar trends have been observed in both blood contaminated (dash lines) and uncontaminated (solid lines) groups but the former showed significantly lower compressive strengths in all time intervals.
RMTA, Root MTA; RMTA-C, RMTA modified with CaCl2; RMTA-N, RMTA modified with Na2HPO4; NC, No contamination; BC, Blood contamination.
Effect of accelerators
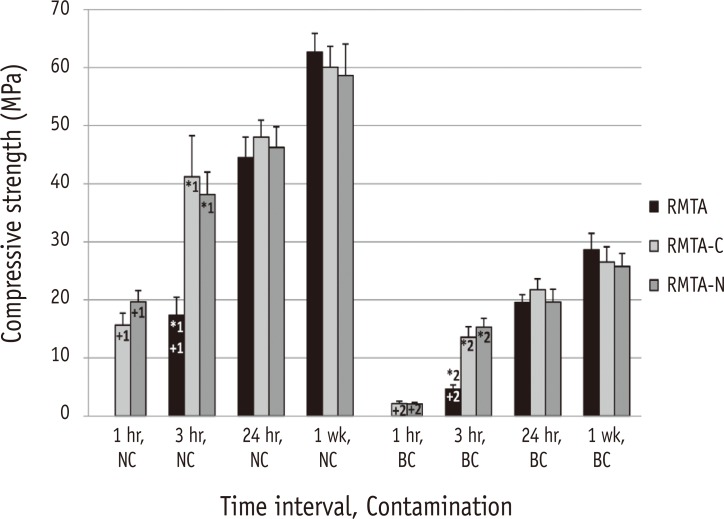
After 3 hours, the compressive strength of specimens in the RMTA groups (with or without blood contamination) was significantly lower than that of the same RMTA-C and RMTA-N groups (*1 and *2, p < 0.05, Figure 2). The difference was not significant at other time intervals (Figure 2). There was no significant difference between the compressive strength of the RMTA groups after 3 hours (with or without blood contamination) and that of the RMTA-C and RMTA-N groups after 1 hour (+1 and +2, Figure 2).
Figure 2.
Mean Compressive strengths of uncontaminated and blood contaminated materials in each time interval.
RMTA, Root MTA; RMTA-C, RMTA modified with CaCl2; RMTA-N, RMTA modified with Na2HPO4; NC, No contamination; BC, Blood contamination.
*1 & *2, statistically significant; +1 & +2, statistically not significant.
Effect of time
In all groups, the compressive strength of specimens increased significantly over time (p < 0.05, Figure 1).
Discussion
MTA is a hydraulic cement that consists mainly of dicalcium silicate and tricalcium silicate that produces calcium silicate hydrate (CSH) gel and calcium hydroxide during the hydration process.26,27 The setting and strength of hydraulic cements depends on the formation of CSH gel and ettringate (hydrated calcium sulfoaluminate) on nucleation sites of calcium hydroxide crystals.5 Because of the various clinical applications of MTA, such as pulp capping, furcal perforation repair etc., sufficient strength is necessary for MTA to withstand compressive pressures. Also, compressive strength is an indicator of the setting and hydration processes.3,4,28-30 Therefore, in the present study the compressive strength was evaluated to assess the effects of blood contamination on features of accelerated RMTA.
In the present study, blood contamination resulted in lower compressive strength values for the original RMTA specimens at each time interval (Figure 1). This finding was similar to previous studies that concluded that blood contamination adversely affected the physical properties of MTA.2-4 Lack of acicular crystals that are indicative of ettringate formation have been reported in blood contaminated MTA by Nekoofar et al.3-5 In addition, it has been stated that the 'air entrainment' features of blood proteins affect the microstructure of cements and increases their porosity. An increase in the porosity of hydraulic cements such as MTA is associated with a decrease in compressive strength.31 However, in the present study the same phenomenon was also observed in the modified groups, which means that addition of CaCl2 and Na2HPO4 did not prevent the negative effects of blood on compressive strength.
Kogan et al. reported that 3% and 5% CaCl2 solutions reduced the compressive strength of MTA.6 Following incorporation of 10% CaCl2 to MTA powder, similar results were reported by Lee et al. during the initial phase of setting; however, the final compressive strength did not change.32 In the present study, the compressive strength of uncontaminated RMTA specimens modified with CaCl2 was higher than specimens in the original RMTA group after 3 hours (*1, Figure 2), which can be explained by a reduction in porosity following CaCl2 addition, as reported by Hong et al.8 However, such a difference was not observed at the later time intervals. In addition, the compressive strength of RMTA specimens modified with CaCl2 after 1 hour was comparable to the compressive strength of RMTA specimens after 3 hours (+1, Figure 2). Therefore, it can be assumed that the initial increase in the compressive strength of RMTA was related to the accelerated setting reaction induced by CaCl2. The same trend was observed in specimens modified by Na2HPO4 (*1 and +1, Figure 2). Therefore, it can be concluded that (as for CaCl2) Na2HPO4 only accelerated the setting reaction of RMTA and did not improve its hydration process and physical characteristics. Liu et al. reported a similar trend with an increase in initial compressive strength of tricalcium silicate (a major constituent of MTA) following incorporation of tricalcium aluminate.31 Comparison of blood contaminated specimens of all experimented materials also revealed the same pattern as uncontaminated ones (Figure 1; *2 and +2, Figure 2).
These findings could explain the inability of accelerator admixtures to prevent adverse effects of blood contamination. While, blood penetrates into MTA slurries immediately after material placement, its adverse effects on compressive strength occur in the initial (early) phase of setting and subsequently are not prevented by setting accelerators. Protecting MTA from blood contamination may be the best strategy to preventing these adverse effects.
In the present study, the compressive strength of all specimens increased significantly over time (Figure 1), which has been also demonstrated in previous studies.2,3,30,31,33 The highest reported compressive strength of ProRoot MTA after 72 hours to 28 days was 71.36 - 86.02 MPa.4,28,29,33 In the present study the compressive strength of uncontaminated specimens of Root MTA after 7 days (58.64 - 62.64 MPa) was close to the reported range. However, despite improvement over time the compressive strengths of blood contaminated specimens did not reach the aforementioned level even after 1 week (Figure 1).
Conclusions
Blood contamination decreases the compressive strength of both original and accelerated RMTA, which means CaCl2 and Na2HPO4 as hydration accelerators could not prevent the adverse effects of blood contamination on the hydration processes of the material.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Chang SW. Chemical characteristics of mineral trioxide aggregate and its hydration reaction. Restor Dent Endod. 2012;37:188–193. doi: 10.5395/rde.2012.37.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanderweele RA, Schwartz SA, Beeson TJ. Effect of blood contamination on retention characteristics of MTA when mixed with different liquids. J Endod. 2006;32:421–424. doi: 10.1016/j.joen.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Nekoofar MH, Oloomi K, Sheykhrezae MS, Tabor R, Stone DF, Dummer PM. An evaluation of the effect of blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010;43:849–858. doi: 10.1111/j.1365-2591.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- 4.Nekoofar MH, Stone DF, Dummer PM. The effect of blood contamination on the compressive strength and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010;43:782–791. doi: 10.1111/j.1365-2591.2010.01745.x. [DOI] [PubMed] [Google Scholar]
- 5.Nekoofar MH, Davies TE, Stone D, Basturk FB, Dummer PM. Microstructure and chemical analysis of blood-contaminated mineral trioxide aggregate. Int Endod J. 2011;44:1011–1018. doi: 10.1111/j.1365-2591.2011.01909.x. [DOI] [PubMed] [Google Scholar]
- 6.Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32:569–572. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Ding SJ, Kao CT, Shie MY, Hung C, Jr, Huang TH. The physical and cytological properties of white MTA mixed with Na2HPO4 as an accelerant. J Endod. 2008;34:748–751. doi: 10.1016/j.joen.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Hong ST, Bae KS, Baek SH, Kum KY, Lee W. Microleakage of accelerated mineral trioxide aggregate and Portland cement in an in vitro apexification model. J Endod. 2008;34:56–58. doi: 10.1016/j.joen.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Lotfi M, Vosoughhosseini S, Saghiri MA, Mesgariabbasi M, Ranjkesh B. Effect of white mineral trioxide aggregate mixed with disodium hydrogen phosphate on inflammatory cells. J Endod. 2009;35:703–705. doi: 10.1016/j.joen.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Jeong YN, Yang SY, Park BJ, Park YJ, Hwang YC, Hwang IN, Oh WM. Physical and chemical properties of experimental mixture of mineral trioxide aggregate and glass ionomer cement. J Korean Acad Conserv Dent. 2010;35:344–352. [Google Scholar]
- 11.Torabinejad M, White DJ, inventors. Loma Linda University, assignee. Tooth filling material and method of use. 5415547. US Patent. 1995 May 16;
- 12.Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009;35:550–554. doi: 10.1016/j.joen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Huang TH, Shie MY, Kao CT, Ding SJ. The effect of setting accelerator on properties of mineral trioxide aggregate. J Endod. 2008;34:590–593. doi: 10.1016/j.joen.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Juenger MCG, Monteiro PJM, Gartner EM, Denbeaux GP. A soft X-ray microscope investigation into the effects of calcium chloride on tricalcium silicate hydration. Cement Concrete Res. 2005;35:19–25. [Google Scholar]
- 15.Zarabian M, Razmi H, Sharifian MR, Sharifi D, Sasani F, Mousavi A. An investigation on the histological responses of periapical tissues following retrofilling with Root MTA and Portland cement type I versus ProRoot MTA in the canine teeth of cats. J Dent (Tehran) 2004;1:31–38. [Google Scholar]
- 16.Razmi H, Zarabian M, Sharifian MR, Sharifi D, Sasani F, Ramezankhani N. A histologic evaluation on tissue reaction to three implanted materials (MTA, Root MTA and Portland cement type I) in the mandible of cats. J Dent (Tehran) 2004;1:62–69. [Google Scholar]
- 17.Razmi H, Sharifi D, Mottahari P, Khosravi MR. Pulp tissue reaction of dog canine to Root MTA and Portland cement compared to ProRoot MTA as pulp capping agents. J Dent (Tehran) 2006;3:3–8. [Google Scholar]
- 18.Moazami F, Shasiah S. The cellular behavior and SEM evaluation of ProRoot and Root MTAs on fibroblast L929. Iran Endod J. 2006;1:87–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Jahromi Z, Razavi SM, Esfahanian V, Feizi GH. Histological evaluation of inflammation after sealing furcating perforation in dog's teeth by four materials. Dent Res J (Isfahan) 2006;3:1–9. [Google Scholar]
- 20.Sharifian MR, Ghobadi M, Shokouhinejad N, Assadian H. Cytotoxicity evaluation of ProRoot MTA, Root MTA and Portland cement on human gingival fibroblasts. Iran Endod J. 2007;2:91–94. [PMC free article] [PubMed] [Google Scholar]
- 21.Kazem M, Eghbal MJ, Asgary S. Comparison of bacterial and dye microleakage of different root-end filling materials. Iran Endod J. 2010;5:17–22. [PMC free article] [PubMed] [Google Scholar]
- 22.Sheykhrezai MS, Aligholi M, Ghorbanzadeh R, Bahador A. A comparative study of antimicrobial activity of ProRoot MTA, Root MTA, and Portland cement on Actinobacillus actinomycetemcomitans. Iran Endod J. 2008;3:129–133. [PMC free article] [PubMed] [Google Scholar]
- 23.Haghgoo R, Abbasi F. Clinical and radiographic success of pulpotomy with MTA in primary molars: 30 months follow up. Iran Endod J. 2010;5:157–160. [PMC free article] [PubMed] [Google Scholar]
- 24.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate's composition with Portland cements and a new endodontic cement. J Endod. 2009;35:243–250. doi: 10.1016/j.joen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.ISO-Standards ISO 9917-1:2007. Dentistry-Water-based cements-Part 1: powder/liquid acid-base cements. Geneve: International Organization for Standardization; 2007. [Google Scholar]
- 26.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40:462–470. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41:408–417. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 28.Holt DM, Watts JD, Beeson TJ, Kirkpatrick TC, Rutledge RE. The anti-microbial effect against enterococcus faecalis and the compressive strength of two types of mineral trioxide aggregate mixed with sterile water or 2% chlorhexidine liquid. J Endod. 2007;33:844–847. doi: 10.1016/j.joen.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Nekoofar MH, Adusei G, Sheykhrezae MS, Hayes SJ, Bryant ST, Dummer PM. The effect of condensation pressure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2007;40:453–461. doi: 10.1111/j.1365-2591.2007.01236.x. [DOI] [PubMed] [Google Scholar]
- 30.Kayahan MB, Nekoofar MH, Kazandağ M, Canpolat C, Malkondu O, Kaptan F, Dummer PM. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2009;42:1004–1014. doi: 10.1111/j.1365-2591.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu WN, Chang J, Zhu YQ, Zhang M. Effect of tricalcium aluminate on the properties of tricalcium silicatetricalcium aluminate mixtures: setting time, mechanical strength and biocompatibility. Int Endod J. 2011;44:41–50. doi: 10.1111/j.1365-2591.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee BN, Hwang YC, Jang JH, Chang HS, Hwang IN, Yang SY, Park YJ, Son HH, Oh WM. Improvement of the properties of mineral trioxide aggregate by mixing with hydration accelerators. J Endod. 2011;37:1433–1436. doi: 10.1016/j.joen.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006;32:193–197. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]