Abstract
Objective
To evaluate the antioxidant and radical scavenging activities of Solanum anguivi fruit (SAG) and its possible effect on mitochondrial permeability transition pore as well as mitochondrial membrane potential (ΔΨm) isolated from rat liver.
Methods
Antioxidant activity of SAG was assayed by using 2,2-diphenyl-1-picrylhydrazyl (DPPH), reducing power, iron chelation and ability to inhibit lipid peroxidation in both liver and brain homogenate of rats. Also, the effect of SAG on mitochondrial membrane potential and mitochondrial swelling were determined. Identification and quantification of bioactive polyphenolics was done by HPLC-DAD.
Results
SAG exhibited potent and concentration dependent free radical-scavenging activity (IC50/DPPH=275.03±7.8 µg/mL). Reductive and iron chelation abilities also increase with increase in SAG concentration. SAG also inhibited peroxidation of cerebral and hepatic lipids subjected to iron oxidative assault. SAG protected against Ca2+ (110 µmol/L)-induced mitochondrial swelling and maintained the ΔΨm. HPLC analysis revealed the presence of gallic acid [(17.54±0.04) mg/g], chlorogenic acid (21.90±0.02 mg/g), caffeic acid (16.64±0.01 mg/g), rutin [(14.71±0.03) mg/g] and quercetin [(7.39±0.05) mg/g].
Conclusions
These effects could be attributed to the bioactive polyphenolic compounds present in the extract. Our results suggest that SAG extract is a potential source of natural antioxidants that may be used not only in pharmaceutical and food industry but also in the treatment of diseases associated with oxidative stress.
Keywords: Solanum anguivi fruit, Antioxidant activity, Oxidative stress, Mitochondrial swelling, HPLC, Polyphenolic compounds, MPTP
1. Introduction
Mitochondria are the main site of oxygen metabolism in the cell[1]. They are unique organelles, accounting for about 85%-90% of oxygen consumed by the cell. The incomplete processing of oxygen and/or release of free electron in the mitochondria results in the production of reactive oxygen species (ROS)[2]. ROS released by the mitochondrial respiratory chain are a family of active molecules containing free radicals and are involved in the modulation of biological cell functions. However, excessive ROS bring about oxidative stress that cause injury to various cellular constituents such as lipid, protein and DNA, leading to the alteration in the integrity of cell membrane that consequently result in growth arrest, senescence or apoptosis[3]. They have been reported to play a major role in the pathogenesis of various human diseases including ischemia, carcinogenesis, inflammation/immune injury, arthritis, coronary diseases, hemorrhagic shock, cataract as well as age-related degenerative brain disorders[4],[5]. Oxidative stress is thought to affect many intracellular compartments in particular the mitochondria. Several studies have indicated that the mitochondrial permeability transition pore (MPTP) is involved in cellular responses to oxidative stress[6]–[8]. For instance, over accumulation of calcium, overproduction of ROS, high pH, low membrane potential and oxidized pyridine nucleotides which are associated with oxidative stress, can cause opening of the MPTP[9], resulting in a marked increase in inner membrane permeability as well as a decrease in membrane potential, leading to mitochondrial swelling, release of cytochrome c, cell damage and apoptosis[10]. Induction of MPTP has been reported to be prevented by antioxidants such as catalase and free radical scavengers[11]. Almost all organisms are equipped with antioxidant mechanisms to defend and repair oxidative damage. However, endogenous antioxidants may not be efficient in some cases; thus, exogenous antioxidant consumption can help the antioxidant mechanism of the organism to prevent against diseases associated with oxidative stress. Principal sources of exogeneous antioxidant include herbs, spices, and medicinal plants. Natural antioxidants from dietary plants are reported to prevent oxidative damage caused by free radical and active oxygen, and they also prevent the occurrence of disease, aging, and cancer[12].
Solanum anguivi Lam. (SAG) is a rare ethnomedicinal herb belonging to the family Solanaceae. The plant can be found in many places throughout non arid part of Africa. It is highly polymorphic and variable in its plant structure, fruits and leaf characters. The domesticated species are consumed as leafy and/or fruit vegetables that are rich in essential minerals and vitamins[13], and are recommended as a dietary staple or supplements for nursing mothers, the young, the aged, and anaemic patients[14]. The plant is used as therapeutic agent for various diseases. The roots are carminative and expectorant useful in coughs, cultarrhal affections, dysuria, colic, nasal ulcers, ingredient of dasamula, asthma, difficult parturition, tooth ache, cardiac disorder, worm complaints, spinal guard disorder, nervous disorder and fever. The leaves and fruits rubbed up with sugar are used as external application for itch[15]. The fruit of SAG is a ready sources of vegetable commonly consumed in Nigeria and other African countries because of the traditional believe that it reduces the risk of diabetes and artherosclerosis[16].
Recently, we have demonstrated the in vivo antioxidant activity of SAG in rat[17]; however, there is a lack of information in the literature regarding its in vitro antioxidant activity and its effect on mitochondrial swelling. Therefore, this study was undertaken to evaluate the antioxidant and radical scavenging activities of SAG by several in vitro test systems and its possible effect on MPTP as well as mitochondria membrane potential isolated from rat liver. Furthermore, we characterized qualitatively and quantitatively by HPLC-DAD some phenolics and flavonoids compounds present in SAG.
2. Materials and methods
2.1. Plant collection
The fruit of SAG was collected in October, 2011 from Ado Ekiti in Ekiti State, Nigeria. They were identified and authenticated at the herbarium of Plant Science and Forestry department, University of Ado Ekiti, Nigeria (voucher specimen number UHAE: 286). The fruits were air dried and grounded into a powdery fine texture and stored at room temperature in air tight polythene bag prior to use.
2.2. Preparation of extract
The powdered plant material (100 g) was extracted with 1 000 mL of methanol for 48 h on an orbit shaker. Extracts were filtered using a Buckner funnel and Whatman No. 1 filter paper. The filtrate was concentrated to dryness under reduced pressure to give a yield of (12.03 g) and later reconstituted in distilled water due to its solubility in water to give the required concentrations needed in this study.
2.3. Chemicals
All chemicals used, including solvents, were of analytical grade. Folin Ciocalteu's phenol reagent, malonaldehyde bis-(dimethyl acetal) (MDA), thiobarbituric acid (TBA), sodium dodecyl sulfate, quercetin, rutin, chlorogenic acid and gallic acid, 1,1-Diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), were purchased from Sigma Chemical Co. (USA). Ferrous sulfate, FeCl3 were obtained from Vetec (Brazil). Methanol, acetic acid, gallic acid, chlorogenic acid and caffeic acid purchased from Merck (Darmstadt, Germany). High performance liquid chromatography (HPLC-DAD) was performed with a Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector and LC solution 1.22 SP1 software.
2.4. Animals
Male Wistar rats, weighing 270-320 g from our own breeding colony (Animal House-holding, UFSM, Brazil) were kept in cages with free access to food and water in a room with controlled temperature (22±3) °C and in 12 h light/dark cycle. The protocol of this study has been approved by the Brazilian Association for Laboratory animal Science (COBEA).
2.5. Determination of total flavonoid
The total flavonoid content of SAG was determined using quercetin as a reference compound[18]. Briefly, 50-500 mg/mL methanolic extract was mixed with 50 µL of aluminium trichloride and potassium acetate. The absorption at 415 nm was read after 30 min at room temperature. Standard quercetin solutions were prepared from 0.01 g quercetin dissolved in 20 mL of ethanol. All determinations were carried out in triplicate. The amount of flavonoids in both extracts was expressed as quercetin equivalent (QE).
2.6. Determination of total phenol
The determination of total phenolic content was carried out as described by Kamdem JP, et al[19]. Briefly, samples of SAG extract (50-500 mg/mL) were added to a test tube and the volume was adjusted to 1.4 mL with distilled water. Then, 0.2 mL of 10 % Folin-Ciocalteu reagent (diluted 1:1 with water) and 0.4 mL of sodium carbonate solution (7.5 %) were added sequentially to the test tube. The tubes were then incubated for 40 min at 45 °C and the absorbance was measured at 725 nm in a spectrophotometer (SP-2000UV, Biospectro, Brazil). The standard curve was prepared using 0, 1, 2.5, 5, 10 and 15 mg/mL solutions of gallic acid (0.1 mg/mL). Total phenol value was calculated and expressed as the microgram gallic acid equivalent (mg GAE) of dry extract.
2.7. DPPH Radical Scavenging activity of extract
DPPH radical-scavenging activity of SAG extract (50-500 mg/mL) and reference compound (Butylated hydroxyltoluene (BHT) (50-500 mg/mL) were determined as described by Shanab SM, et al[20]. The capacity of extracts scavenge the lipid-soluble 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical, which results in the bleaching of the purple color exhibited by the stable DPPH radical, is monitored at an absorbance of 517 nm.
2.8. Fe2+ chelation Assay
The ferrous ion chelating activity of extract was evaluated by a standard method[21] with minor changes. The reaction was carried out in Tris-HCL buffer (0.1 mol/L, pH 7.5). Briefly, various concentrations (50, 100, 200 and 500 mg/mL) SAG extract were added to 100 µmol/L ferrous sulfate solution. The reaction mixture was incubated for 30 seconds, before the addition of 1,10-phenanthroline (0.25% w/v). The absorbance was subsequently measured at 510 nm in a spectrophotometer. Buthylate hydroxytoluene (BHT), a standard antioxidant was used as a positive control.
2.9. Reducing power
The Fe3+ reducing power of the extract was determined as described by Kumar RS, et al.[22] with slight modification. Different concentrations (50, 100, 200 and 500 mg/mL) of the extract (0.5 mL) were mixed with 0.5 mL phosphate buffer (0.2 mol/L, pH 6.6) and 0.5 mL potassium ferrycyanate (0.1%), followed by incubation at 50 °C in a water bath for 20 min. After incubation, 0.5 mL of TCA (10%) was added to terminate the reaction. The upper portion of the solution (1 mL) was mixed with 1 mL distilled water, and 0.1 mL FeCl3 solution (1%) was added. The reaction mixture was left for 10 min at room temperature and the absorbance was measured at 700 nm against an appropriate blank solution. All tests were performed three times. A higher absorbance of the reaction mixture indicated greater reducing power. BHT at the same concentrations (50-500 mg/mL) used for the extract was a positive control.
2.10. Thiobarbituric acid reactive substances assay
Thiobarbituric acid reactive substances (TBARS) production was determined as described by Ohkawa H, et al[23]. Aliquots of the homogenate (100 µL) from brain and liver were incubated at 37 °C in a water bath for 1 h in the presence of different concentrations of SAG (200-500 µg/mL) and with Iron 10µmol/L. Color reaction was developed by adding 200 µL of 8.1% SDS (sodium dodecyl sulphate) to the reaction mixture. This was subsequently followed by the addition of 500 µL of acetic acid/ HCl buffer (pH 3.4) and 500 µL 0.6% thiobarbituric acid (TBA). This mixture was incubated at 97 °C for 1 h. TBARS produced were measured at 532 nm and the absorbance was compared with the standard curve using malondialdehyde (MDA).
2.11. Isolation of rat liver mitochondria
Rat liver mitochondria were isolated as previously described by Puntel RL, et al.[24] with some modifications. The livers were rapidly removed (within 1 min) and immersed in ice-cold isolation buffer I containing in mmol/L: 225 manitol, 75 sucrose, 1 K+-EGTA and 10 K+-HEPES, pH 7.2. The tissue was minced using surgical scissors and then extensively washed. The tissue was then homogenized in a power-driven, tight-fitting Potter-Elvehjem (Reviglass, Brazil) homogenizer with a teflon pestle. The resulting suspension was centrifuged for 7 min at 2 000'g in a Hitachi CR 21E centrifuge (Japan). The supernatant was centrifuged again for 10 min at 12 000'g. The pellet was re-suspended in isolation buffer II containing in mmol/L: 225 manitol, 75 sucrose, 1 K+-EGTA (ethyleneglycol tetraacetic acid) and 10 K+-HEPES [4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid], pH 7.2, and recentrifuged at 12 000'g for 10 min. The supernatant was decanted, and the final pellet was gently washed and resuspended in respiration buffer containing in mmol/L: 100 sucrose, 65 KCl, 10 K+ -HEPES and 0.05 EGTA, pH 7.2, to a protein concentration of 0.6 mg/mL.
2.12. Standard incubation procedure
Measurements of mitochondrial trans-membrane electrical potential (ΔΨm) and mitochondrial swelling, determination were performed in a stirred cuvette mounted in a RF-5301 PC Shimadzu spectrofluorometer (Kyoto, Japan) at 30 °C. Mitochondria (0.6 mg protein) were added to 3 mL standard incubation buffer containing 100 mmol/L sucrose, 65 mmol/L KCl, 10 mmol/L K-HEPES, 50 µmol/L EGTA, (pH 7.2), 60 mmol/L ADP, 200 mmol/L MgCl2, 1 mmol/L Pi and 5 mmol/L succinate for mitochondrial swelling. The results shown are representative of a series of three to six independent experiments, using independently isolated mitochondrial preparations.
2.13. Measurement of mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential was estimated by fluorescence changes of safranine (5 µmol/L) recorded by a RF-5301 Shimadzu spectrofluorimeter operating at excitation and emission wavelengths of 495 and 586 nm, respectively, with slit widths of 3 nm. Values of mitochondrial membrane potential (ΔΨm) were expressed as the percent of control.
2.14. Measurement of mitochondrial swelling (MPTP)
Measurement of mitochondrial swelling was performed in a RF-5301 Shimadzu spectrofluorometer at 600 nm (slit 1.5 nm for excitation and emission[25]. Data are expressed as percentage of control.
2.15. Protein estimation
Protein concentration was measured by the method of Bradford M[26] using bovine serum albumin (BSA) as standard.
2.16. Quantification of compounds by HPLC-DAD
The phenolics and flavonoids in the extract were quantified by reverse phase chromatographic analysis by the method described by Sabir SM, et al.[27] with slight modifications. Reverse phase chromatographic analysis was carried out under gradient conditions using C 18 column (4.6 mm'250 mm) packed with 5-mm diameter particles. The mobile phase was water containing 2 % acetic acid (A) and methanol (B), and the composition gradient was: 5% (B) for 2 min; 25% (B) until 10 min; 40%, 50%, 60%, 70% and 80% (B) every 10 min. All samples and the mobile phase were filtered through a 0.45-mm membrane filter (Millipore, USA) and then degassed by ultrasonic bath prior to use. Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.031-0.250 mg/mL quercetin and rutin, and 0.006-0.250 mg/mL for gallic and chlorogenic acids. Quantification was carried out by integration of the peaks using the external standard method, at 257 nm for gallic acid, 325 nm for chlorogenic acid and 365 for quercetin and rutin. The flow rate was 0.8 mL/min and the injection volume was 40 mL. Chromatographic peaks were confirmed by comparing their retention time and diode-array-UV spectra with those of the reference standards. All chromatography operations were carried out at ambient temperature and in triplicate.
2.17. Statistical analysis
The experimental results were expressed as mean±standard error of mean (SEM) of three replicates and were subjected to one-way analysis of variance followed by Duncan's multiple range tests. Significant levels were tested at P<0.05.
3. Results
3.1. Phytochemical analysis
3.1.1. Total phenolic and flavonoid content in SAG
The methanolic extract of SAG fruit was evaluated for the phenolics and flavonoids content. In the present study, the total phenolic and flavonoid content were (11.13±0.73) mg GAE (Gallic acid equivalent)/g of dried weight and (9.53±0.49) mg QE (Quercertin equivalent)/g of dried weight (Table 1).
Table 1. Phenolics and flavonoids in SAG.
| Sample | Phenol mg/g GAE | Flavonoid mg/g QE |
| 17.13±0.73 | 9.53±0.49 |
3.1.2. HPLC analysis
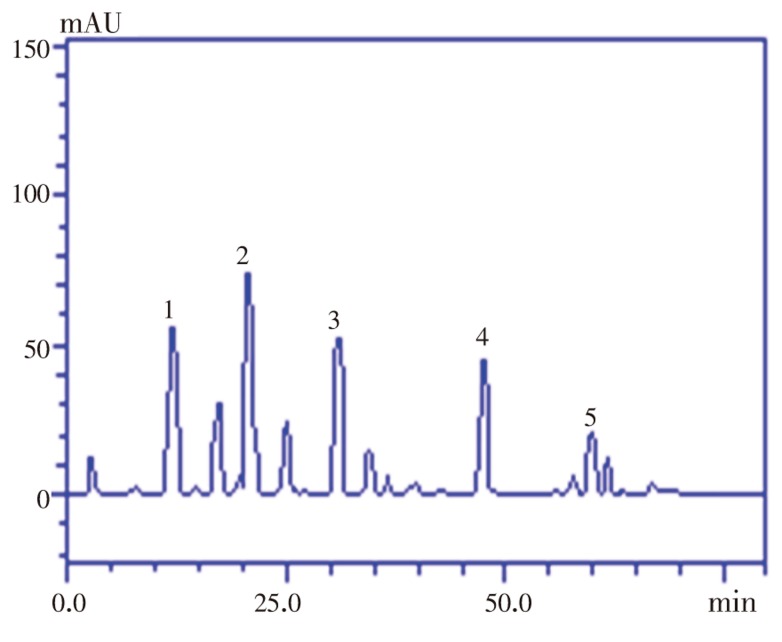
HPLC fingerprinting of extract revealed the presence of the phenolic acids such a gallic acid (tR=12.57 min), chlorogenic acid (tR=20.01 min), caffeic acid (tR=32.29 min), while the flavonoid contents included rutin (tR=47.58 min) and quercetin (tR=59.14 min) (Table 2 and Figure 1). The highest of the estimated phenolic acids was chlorogenic acid [(21.90±0.02) mg/g] while the least was caffeic acid [(16.64±0.01) mg/g]. The predominant of the estimated flavonoid contents is rutin [(14.71±0.03) mg/g]. The flavonoids and phenolics acids were identified by comparisons to the retention times and UV spectra of authentic standards analyzed under identical analytical conditions.
Table 2. HPLC fingerprinting of SAG.
| Compounds | Weight (mg/g) | (Percentage) % |
| Gallic acid | 17.54±0.04 | 1.75 |
| Chlorogenic acid | 21.90±0.02 | 2.19 |
| Caffeic acid | 16.64±0.01 | 1.64 |
| Rutin | 14.71±0.03 | 1.47 |
| Quercetin | 7.39±0.05 | 0.73 |
Results are expressed as mean±standard deviations (SD) of three determinations.
Figure 1. High performance liquid chromatographic profile of phenolics and flavonoids in methanolic extract of SAG fruit.
Gallic acid (peak 1), chlorogenic acid (peak 2), caffeic acid (peak 3), rutin (peak 4) and quercetin (peak 5).
3.2. Antioxidant assays
3.2.1. Scavenging activity on DPPH radical of SAG
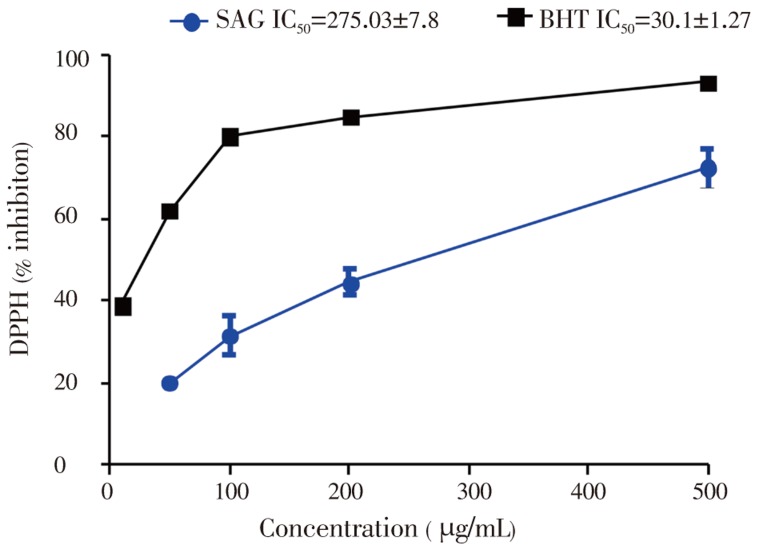
The results of DPPH radical scavenging activity of SAG and the standard antioxidant (BHT) are presented in Figure 3. The plant extract and the standard antioxidant (BHT) promoted an inhibition of DPPH radical with increasing concentrations. However, the percentage inhibition of the DPPH radical by the extract was lower than that of BHT. The IC50 (concentration that inhibits 50% of the DPPH radical) values of SAG and BHT were 275.03±7.8 and 30.1±1.27 µg/mL respectively (Figure 2).
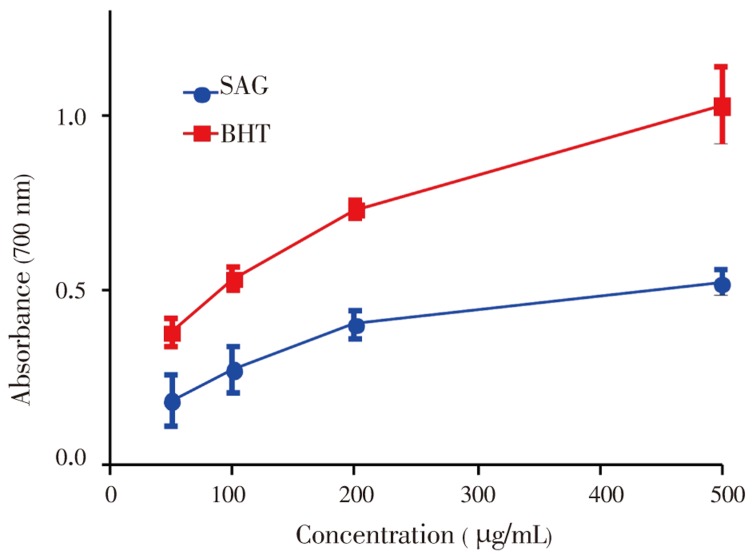
Figure 3. Reducing properties SAG and standard BHT.
Data show means±SEM values averages from 3 to 4 independent experiments performed in triplicate.
Figure 2. Quenching of DPPH color by SAG versus BHT.
Data represents means±SEM values averages from 3 to 4 independent experiments performed in triplicate.
3.2.2. Reducing power activity
The antioxidant activity of SAG was determined by measuring its ability to transform Fe3+ to Fe2+. As illustrated in Figure 3 the reducing power of SAG increased with an increase in concentration when compared to the standard BHT, suggesting the presence of reductants in the plant extract. However, the reducing power of BHT was relatively more pronounced than that of SAG.
3.2.3. Metal chelating activity
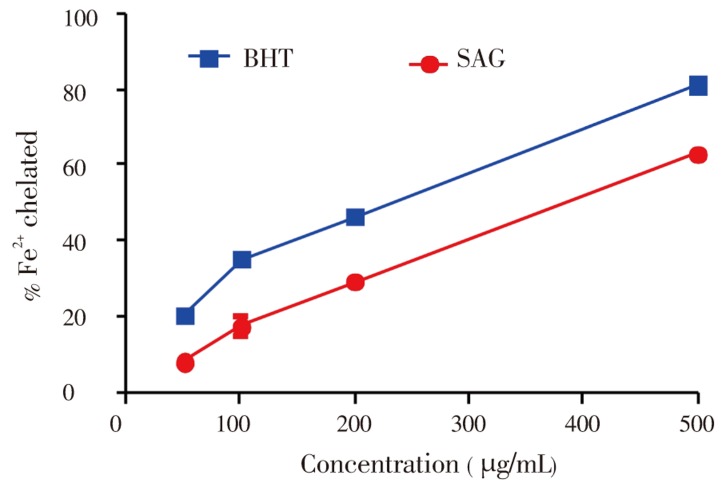
Figure 4 depicts the iron chelating ability of both SAG fruit and BHT, a known antioxidant. The ferrous ion-chelating effect of SAG and BHT correlated well with increasing concentrations. A maximum effect (50%) was evident at a concentration of 376 µg/mL, which was close to that of the chelating activity of BHT at concentration of 225 µg/mL.
Figure 4. Fe2+- chelating properties of SAG versuss BHT standard.
Data show means±SEM values averages from 3 to 4 independent experiments performed in triplicate.
3.2.4. Lipid peroxidation inhibiting activity
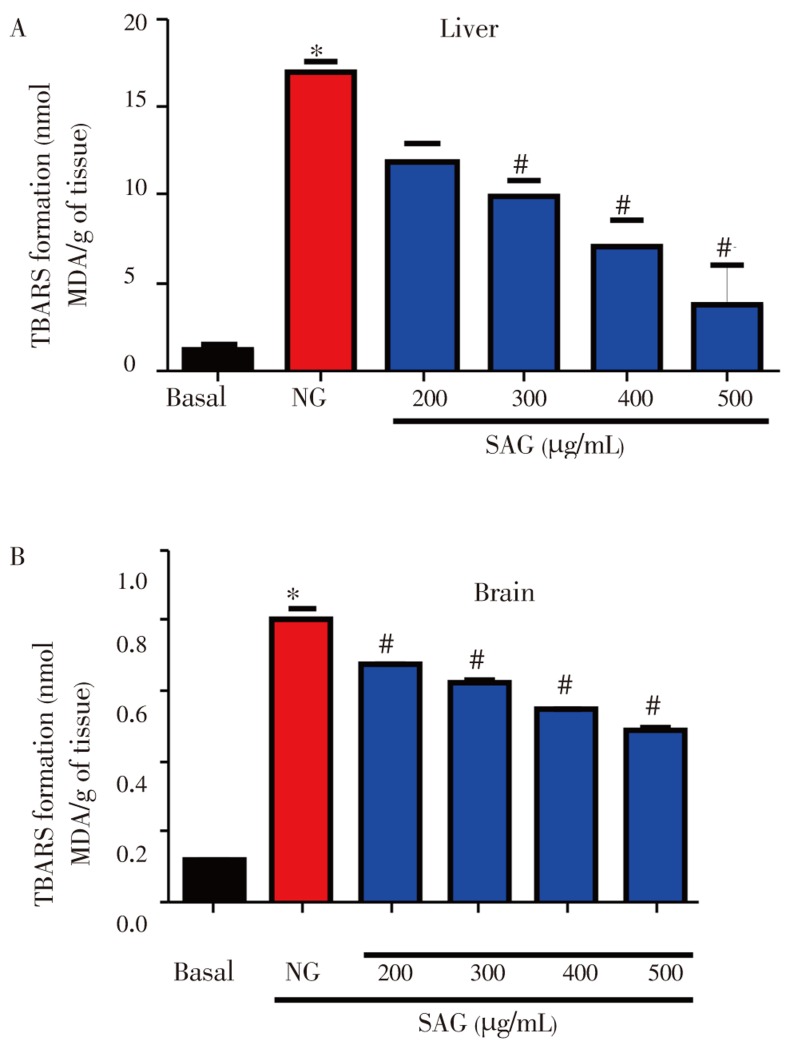
Fe (II) caused a significant increase in TBARS production in rat liver and brain homogenates when compared to their respective control (P<0.05, Figure 5A and B). SAG caused a significant decrease in Fe (II) -stimulated TBARS production in both rat liver and brain homogenates with increasing concentration (P<0.05, Figure 5A and B).
Figure 5. Effects of SAG on Fe2+ (10 µmol/L)-induced TBARS production in liver (A) and brain (B) homogenates.
The samples were incubated for 1 h with Fe2+ (10 µmol/L) in the presence or absence of plant extracts (basal). Mean±SEM, n=3-4 independent experiments. * represents significant difference from basal, # represents significant difference from NG at P<0.05. NG: negtive control.
3.3. Effect of methanolic extract of SAG on mitochondrial membrane potential
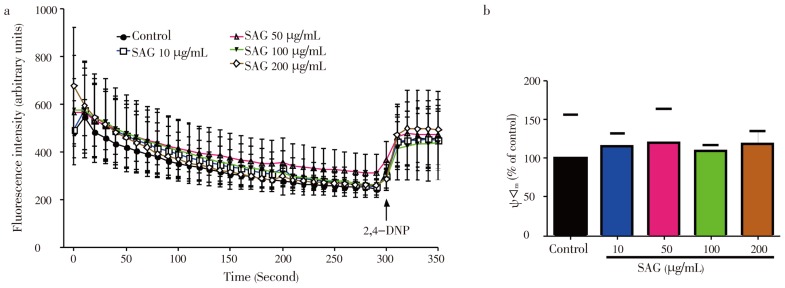
As observed in Figure 6A and B, SAG did not have any effect on membrane potential (ΔΨm) at all the concentrations tested when compared to the control (P>0.05).
Figure 6. Effect of SAG on mitochondrial membrane potential. Isolated rat liver mitochondria (0.6 mg/mL) were incubated in standard medium and the ΔΨm was monitored as described in experimental session.
a: Effect of SAG (10-200 mg/mL) on mitochondrial membrane potential; b: values of ΔΨm after adding the mitochondrial uncoupler 2,4-dinitro-phenol (2,4-DNP) point indicated by arrow in a). Experiments were performed three times using independent mitochondrial preparation. Mean±SEM, n=3. * Significant difference vs. control: P<0.05.
3.4. Effect of methanolic extract of SAG on mitochondrial permeability transition pore (MPTP)
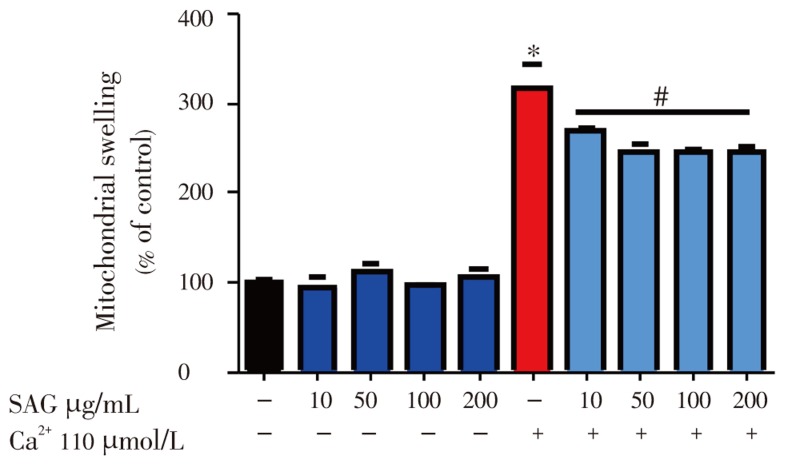
Figure 7 shows the effect of SAG on mitochondrial swelling in the presence or absence of calcium. Under basal conditions, SAG did not have any effect on mitochondrial swelling when compared to the control (P>0.05). However, Ca2+ (110 µmol/L) significantly and dramatically caused mitochondrial swelling when compared to the control (P<0.05) and this effect was significantly prevented by SAG [(10-200) µg/mL] in a dose dependent manner. This result indicates the prevention of MPTP opening by SAG.
Figure 7. Effect of SAG extract [(10-200) µg/mL] on mitochondrial swelling induced by 110 µmol/L calcium.
Isolated rat liver mitochondria (0.6 mg/mL) were incubated in standard medium and was monitored as described in experimental session. Experiments were performed three times using independent mitochondrial preparation. Mean±SEM, n=3. * Significant difference from control, # significant difference from Ca2+: P<0.05.
4. Discussion
There is increasing evidence that antioxidants derived from indigenous plant sources may be useful in preventing the deleterious consequences of oxidative stress and there is increasing interest in the protective biochemical functions of natural antioxidants contained in spices, herbs and medicinal plants[28].
Free radicals are major cause of the propagation stage of the oxidation process. The high potential for scavenging free radicals could inhibit the spread of oxidation. In this context, the free radical-scavenging activity of SAG extract was evaluated using DPPH. SAG demonstrated the capacity for scavenging free radicals by reducing the stable DPPH radical to the yellow coloured diphenylpicryl hydrazine and this capacity increased with increasing concentration. This result indicated the potential electron and/or hydrogen donating ability of SAG extract. However, the radical scavenging potential of the extract was lower than the standard antioxidant, BHT.
There are a number of assays designed to measure overall antioxidant activity, or reducing potential, as an indication of a host´s total capacity to withstand free radical stress[29]. Here we evaluated the reducing potential of SAG extract because the reducing power reflects the electron donating capacity of its bioactive compounds and may serve as a significant indicator of its antioxidant activity. Our results indicated that SAG extract like the standard antioxidant BHT, reduced Fe3+/ferricyanide complex to the ferrous form, indicating the presence of reductants in the solution. The existence of reductones from SAG are the keys to its reducing power, and these exhibit their antioxidant activities through the action of breaking the free radical chain by donating a hydrogen atom[30].
Iron is essential to life because of its requirements in various physiological and biochemical processes such as oxygen transport, respiration and its involvement in enzymatic activities. However, it has been implicated in the oxidative damages in lipids, proteins and other cellular components leading to occurrence of diseases such as cardiovascular and neurodegenerative diseases. The main strategy to avoid ROS generation which is associated with redox active metal catalysis involves chelating of the metal ions. In the present study, the antioxidative ability of SAG was estimated by assessing its iron chelating capacity and its ability to inhibit lipid peroxidation. Our results demonstrated that SAG interfered with the formation of 1,10-phenanthroline-Fe2+ complex, suggesting that it has chelating activity and captures ferrous ion before phenanthroline. Phenanthroline quantitatively form complexes with Fe2+ and the complexes formed can be disrupted in the presence of chelating agents, resulting in a decrease in the orange colour of the complex. The chelating ability (Figure 5) of SAG extract can be at least, in part, related to the presence of phenolic compounds found in this plant, especially caffeic acid. In agreement with this, phenolics have been shown to form complexes with iron, this is probably related to the strong nucleophilic character of the aromatic rings[31]. In addition, the potent chelating capacity of caffeic acid has been attributed to the presence of the catechol group[32].
Free Fe2+ can induce toxicity via stimulation of the Fenton reaction and its levels increased in some degenerative diseases. The ability of antioxidants to chelate and deactivate transition metals prevents such metal from participating in the initiation and progression of lipid peroxidation leading to oxidative stress through metal-catalyzed reaction[33]is considered as an antioxidant mechanism.
Here, we have observed that SAG significantly inhibited Fe2+-induced TBARS formation in rat liver and brain homogenates (Figure 6A and B). A plausible mechanism by which SAG confer protection against Fe2+-induced lipid peroxidation in these homogenates can be attributed to the presence of flavonoids found in this plant, which are well known to be chelator compounds. SAG contains flavonoids such as quercetin and rutin that may form redox inactive complexes with Fe2+, rendering this pro-oxidant unavailable for Fenton reaction. Earlier study has indicated that quercetin and its glycoside form, rutin, effectively block Fe2+-induced TBARS production in brain homogenates[34]. Flavonoids are potent antioxidants in lipid systems where they reduce oxidative modifications of membranes by restricting the access of oxidants to the bilayer and the propagation of lipid oxidation in the hydrophobic membrane matrix[35]. The free radical scavenging by flavonoids is highly dependent on the presence of a free 3-OH present, as well as the number of hydroxyl groups presented in the structure[36]. The multiple hydroxyl groups presented in the structure of quercetin and rutin found in SAG could have contributed to the antioxidant capacity of the plant especially the inhibition of lipid peroxide in brain and liver homogenates exposed to iron insult observed in this study.
Mitochondria plays a central role in energy metabolism and Ca2+ homeostasis in cells[37]–[39]. The mitochondrial permeability transition (MPT) is considered to contribute substantially to the regulation of normal mitochondrial metabolism and it is regarded as an important mediator of cell death through the opening of a membrane structure known as the mitochondrial permeability transition pore (MPTP)[40],[41]. The opening of this pore is accompanied by membrane potential (ΔΨ) collapse, calcium release, uptake of electrolytes and water, matrix swelling and ruptures of the mitochondrial outer membrane[42]. As a consequence, several factors are released into the cytosol including cytochrome c, apoptotic peptidase activating factor 1 (apaf-1), apoptosis-inducing factor (AIF) and caspase family members, which participate in apoptosis pathways[43]–[45]. Several agents, such as Ca2+, thiol oxidants, reactive oxygen species (ROS), and/or members of the Bcl−2 family of proteins can regulate cell death or survival by interference with MPTP opening[46]–[48]. In the present study, we used Ca2+ to induce MPTP opening. Recently, antioxidants from medicinal plants with calcium antagonization effect[49] and ROS scavengers have been proposed to be promising inhibitor of MPTP opening from a clinical perspective[50]. In the present study, SAG extract did not have any effect on mitochondrial membrane potential nor MPTP under basal condition. However, Ca2+-induced MPTP opening was significantly prevented by SAG extract, indicating a protection against MPTP opening, and the maintenance of the membrane potential of mitochondria. This effect can be due to the capacity of SAG extract counteract the effect of Ca2+ and/or ROS, related to its antioxidative activity.
It has been postulated that there is a relation between MPTP and ΔΨm. A report by Madesh M, et al.[51] showed that for some apoptotical stimuli, MPTP formation is dependent of mitochondrial transmembrane depolarization. Despite the fact that we have not tested the effect of Ca2+ on ΔΨm, we can presume that the MPTP observed in this study would have been a consequence of mitochondrial transmembrane depolarization induced by Ca2+.
Overall, the results obtained in the present study clearly demonstrated the efficacy of SAG extract in free radical scavenging, ferric reducing power, metal iron chelating that was associated with inhibition of lipid peroxidation in rat liver and brain homogenates. Also, SAG extract inhibited Ca2+-induced mitochondrial swelling and did not have any effect on mitochondrial membrane potential nor mitochondrial permeability transition pore (MPTP). Although we have not isolated the compounds responsible for the observed activity, we speculate that it may be related to the bioactive polyphenolic compounds found in the composition of this plant. SAG is a functional food that can be very effective in the treatment of various disease and this is consistent with the traditional use of this plant in folk medicine.
Acknowledgments
The authors acknowledge the financial support of Educational Trust Fund, Nigeria and Doctoral fellowship in Professor Joao B.T. Rocha's Laboratory, Federal University of Santa Maria, Brazil. This research was funded by Educational Trust Fund, Nigeria with grant number ETF/ES/AST&D/AAU/AKUNGBA/1:2011.
Comments
Background
Mitochondrial permeability transition pore (MPTP) is activated due to oxidative stress. Antioxidants have been shown to prevent MPTP. The authors tried to established that polyphenolic compounds contained in fruit of SAG have antioxidant properties and are responsible for prevention of mitochondrial swelling which occur as a result of MPTP.
Research frontiers
The authors established that SAG fruit protected mitochondria membrane potential and prevented mitochondrial swelling and that the antioxidant capacity of SAG is responsible for this action. The HPLC analysis of SAG revealed the possible bioactive compounds responsible for these effects.
Related reports
This appears to be the first study on HPLC fingerprint, comprehensive antioxidants studies and effect of SAG fruit on mitochondrial membrane potential and mitochondrial swelling.
Innovations and breakthroughs
The antioxidant activity and HPLC fingerprint reveal the polyphenols from SAG fruit, prevention of mitochondria membrane permeability pore, and no effect on mitochondria membrane potential by fruit of SAG.
Applications
The study showed that the fruit of SAG is rich in antioxidants that can be used as food additives to prevent diseases associated with oxidative stress.
Peer review
This is a good study in which the authors established that SAG fruit has antioxidant activity, preventing mitochondria swelling and maintained mitochondria membrane potential. The results are very relevant because mitochondria swelling and a decrease of membrane potential has been implicated in many diseases associated with oxidative stress.
Footnotes
Foundation Project: Educational Trust Fund, Nigeria with grant number ETF/ES/AST&D/AAU/AKUNGBA/1:2011.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Bolanos JP, Moro MA, Lizasoain I, Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications. Adv Drug Deliv Rev. 2009;61(14):1299–1315. doi: 10.1016/j.addr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Selivanov VA, Votyakova TV, Pivtoraiko VN, Zeak J, Sukhomlin T, Trucco M, et al. et al. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. Plos Comput Biol. 2011;7(3):1001115. doi: 10.1371/journal.pcbi.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Monco J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Georgakilas AG. Oxidative stress, DNA damage and repair in carcinogenesis: Have we established a connection? Cancer Lett. 2012;327(1–2):3–4. doi: 10.1016/j.canlet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 6.Khan MU, Cheema Y, Shahbaz AU, Ahokas RA, Sun Y, Gerling IC, et al. et al. Mitochondria play a central role in nonischemic cardiomyocyte necrosis: common to acute and chronic stressor states. Pflugers Arch. 2012;464(1):123–131. doi: 10.1007/s00424-012-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindeque JZ, Levanets O, Louw R, van der Westhuizen FH. The involvement of metallothioneins in mitochondrial function and disease. Curr Protein Pept Sci. 2010;11(4):292–309. doi: 10.2174/138920310791233378. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Alvarez S, Solesio ME, Manzanares J, Jordanand J, Galindo MF. Lactacystin requires reactive oxygen species and bax redistribution to induce mitochondria-mediated cell death. Br J Pharmacol. 2009;158(4):1121–1130. doi: 10.1111/j.1476-5381.2009.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puntel RL, Roos DH, Folmer V, Nogueira CW, Galina A, Aschner M, et al. et al. Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening. Toxicol Sci. 2010;117(1):133–143. doi: 10.1093/toxsci/kfq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12(4):537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corte CLD, Soares FAA, Aschner M, Rocha JBT. Diphenyl diselenide prevents methylmercury-induced mitochondrial dysfunction in rat liver slices. Tetrahedron. 2012;68(5):10437–10443. [Google Scholar]
- 12.Hung JC, Jong TK, En-Shyh L. Comparative antioxidant properties of water extracts from different parts of beefsteak plant (Perilla frutescens) J Food Drug Anal. 2009;17(6):489–496. [Google Scholar]
- 13.Denton OA, Nwangburuka CC. Genetic variability in eighteen cultivars of Solanum anguivi Lam. using principal component analysis (PCA) and single linkage cluster analysis (SLCA) Ann Biologic Res. 2011;2(4):62–67. [Google Scholar]
- 14.Elekofehinti OO, Adanlawo IG, Saliu JA, Sodehinde SA. Saponins from Solanum anguivi fruits exhibit hypolipidemic potential in Rattus novergicus. Der Pharmacia Lett. 2012;4(3):811–814. [Google Scholar]
- 15.Johnson M, Wesely EG, Selvan N, Chalini K. Comparative phytochemical and isoperoxidase studies on leaf and leaves derived callus of Solanum anguivi Lam. J Chem Pharm Res. 2010;2(4):899–906. [Google Scholar]
- 16.Elekofehinti OO, Adanlawo IG, Fakoya A, Saliu JA, Sodehinde SA. Effect of saponin from Solanum anguivi Lam. fruit on heart and kidney superoxide dismutase, catalase and malondialdehyde in rat. Curr Res J Biol Sci. 2012;4(4):530–533. [Google Scholar]
- 17.Elekofehinti OO, Adanlawo IG, Komolafe K, Ejelonu OC. Saponins from Solanum anguivi fruits exhibit antioxidant potential in Wistar rats. Ann Biol Res. 2012;3(7):3212–3217. [Google Scholar]
- 18.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and praline contents in Burkina fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- 19.Kamdem JP, Stefanello ST, Boligon AA, Wagner C, Kade IJ, Pereira RP, et al. et al. In vitro antioxidant activity of stem bark of Trichilia catigua Adr. Juss (Meliaceae) Acta Pharm. 2012;62:371–382. doi: 10.2478/v10007-012-0026-x. [DOI] [PubMed] [Google Scholar]
- 20.Shanab SM, Mostafa SS, Shalaby EA, Mahmoud GI. Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. Asian Pac J Trop Biomed. 2012;2(8):608–615. doi: 10.1016/S2221-1691(12)60106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puntel RL, Nogueira CW, Rocha JB. Krebs cycle intermediates modulate thiobarbituric reactive species (TBARS) production in rat brain in vitro. Neurochem Res. 2005;30(2):225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 22.Kumar RS, Rajkapoor B, Perumal P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac J Trop Biomed. 2012;2(4):256–261. doi: 10.1016/S2221-1691(12)60019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Puntel RL, Roos DH, Folmer V, Nogueira CW, Galina A, Aschner M, et al. et al. Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent on the classical mitochondrial permeability transition pore opening. Toxicol Sci. 2010;117(1):133–143. doi: 10.1093/toxsci/kfq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Votyakova TV, Reynolds IJ. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J Neurochem. 2005;93(3):526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1979;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Sabir SM, Ahmad SD, Hamid A, Khan MQ, Athayde ML, Santos DB, et al. et al. Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem. 2012;131(3):741–747. [Google Scholar]
- 28.Zahin M, Aqil F, Ahmad I. The in vitro antioxidant activity and total phenolic content of four indian medicinal plants. Int J Pharm Pharm Sci. 2009;1:88–95. [Google Scholar]
- 29.Gulcin I, Bursal E, Sehitoglu MH, Bilsel M, Goren AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chemi Toxicol. 2010;48(8–9):2227–2238. doi: 10.1016/j.fct.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 30.Thenmozhi A, Nagalakshmi K, Rao U. Qualitative analysis of phytochemicals, and comparative superoxide radical scavenging along with reducing potency of Solanum nigrum using various solvent extracts. Int J Green Pharm. 2011;5(4):318–324. [Google Scholar]
- 31.Costa P, Goncalves S, Valentao P, Andrade PB, Coelho N, Romano A. Thymus lotocephalus wild plants and in vitro cultures produce different profiles of phenolic compounds with antioxidant activity. Food Chem. 2012;135:1253–1260. doi: 10.1016/j.foodchem.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda H, Kimura Y, Masaki M, Iwahashi H. Caffeic acid inhibit the formation of 1-hydroxyethyl radical in the reaction mixture of rat liver microsomes with ethanol partly through its metal chelating activity. J Cli Biochem Nutr. 2011;48(3):187–193. doi: 10.3164/jcbn.10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elekofehinti OO, Kade IJ. Aqueous extract of African egg plant inhibit Fe2+ and SNP induced lipid peroxidation in rat's brain- In vitro. Der Pharma Lett. 2012;4(5):1352–1359. [Google Scholar]
- 34.Omololu PA, Rocha JB, Kade IJ. Attachment of rhamnosyl glucoside on quercetin confers potent iron-chelating ability on its antioxidant properties. Exp Toxicol Pathol. 2011;63(3):249–255. doi: 10.1016/j.etp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Kaurinovic B, Popovic M, Vlaisavljevic S, Schwartsova H, Vojinovic-Miloradov M. Antioxidant profile of Trifolium pratense L. Molecules. 2012;17(9):11156–11172. doi: 10.3390/molecules170911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair SS, Nithyakala CM, Rozario RV, Jennifer J, Somashekharaiah BV. Biochemical characterization of selected plant species and investigation of phytochemicals for in-vitro antioxidant activity. Int J Pharmacogn Phytochem Res. 2012;4(3):127–133. [Google Scholar]
- 37.Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013;698(1–3):6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Costa RO, Ferreiro E, Oliveira CR, Pereira CMF. Inhibition of mitochondrial cytochrome c oxidase potentiates Aβ-induced ER stress and cell death in cortical neurons. Mol Cellular Neurosci. 2013;52:1–8. doi: 10.1016/j.mcn.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Eckmann J, Eckert SH, Leuner K, Muller WE, Eckert GP. Mitochondria: mitochondrial membranes in brain ageing and neurodegeneration. Int J Biochem Cell Biol. 2013;45(1):76–80. doi: 10.1016/j.biocel.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Javadov S, Karmazyn M, Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther. 2007;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- 41.Norenberg MD, Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50(7–8):983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarba C. Relationship between membrane potential, calcium fluxes and matrix swelling in rat liver mitochondria; effect of ethanol feeding. Ann Romanian Soc Cell Biol. 2009;14:9–27. [Google Scholar]
- 43.Ge R, Ma WH, Li YL, Li QS. Apoptosis induced neurotoxicity of Di-n-butyl-di-(4-chlorobenzohydroxamato) Tin (IV) via mitochondria-mediated pathway in PC12 cells. Toxicol In Vitro. 2013;27(1):92–102. doi: 10.1016/j.tiv.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Selimovic D, Porzig BB, El-Khattouti A, Badura HE, Ahmad M, Ghanjati F, et al. et al. Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal. 2013;25(1):308–318. doi: 10.1016/j.cellsig.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Greetham D, Kritsiligkou P, Watkins RH, Carter Z, Parkin J, Grant CM. Oxidation of the yeast mitochondrial thioredoxin promotes cell death. Antioxid Redox Signal. 2013;18:375–385. doi: 10.1089/ars.2012.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, et al. et al. Mitochondrial permeability transition in the diabetic heart: Contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol. 2012;52(5):1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Petronilli V, Sileikyte J, Zulian A, Dabbeni-Sala F, Jori G, Gobbo S, et al. et al. Switch from inhibition to activation of the mitochondrial permeability transition during hematoporphyrin-mediated photooxidative stress. Unmasking pore-regulating external thiols. Biochim et Biophys Acta. 2009;1787:897–904. doi: 10.1016/j.bbabio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004;61(3):372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 49.Wu PF, Zhang Z, Wang F, Chen JG. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol Sin. 2010;31(12):1523–1531. doi: 10.1038/aps.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20(1–4):1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 51.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155(6):1003–1016. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]