Abstract
Objective
To validate scientifically the traditional use of Salacia leptoclada Tul. (Celastraceae) (S. leptoclada) and to isolate and elucidate the structure of the biologically active compound.
Methods
Bioassay-guided fractionation of the acetonic extract of the stem barks of S. leptoclada was carried out by a combination of chromatography technique and biological experiments in viro using Plasmodium falciparum and P388 leukemia cell lines as models. The structure of the biologically active pure compound was elucidated by 1D and 2D NMR spectroscopy and mass spectrometry.
Results
Biological screening of S. leptoclada extracts resulted in the isolation of a pentacyclic triterpenic quinone methide. The pure compound exhibited both in vitro a cytotoxic effect on murine P388 leukemia cells with IC50 value of (0.041±0.020) µg/mL and an antiplasmodial activity against the chloroquine-resistant strain FC29 of Plasmodium falciparum with an IC50 value of (0.052±0.030) µg/mL. Despite this interesting anti-malarial property of the lead compound, the therapeutic index was weak (0.788). In the best of our knowledge, the quinone methide pentacyclic triterpenoid derivative compound is reported for the first time in S. leptoclada.
Conclusions
The results suggest that furthers studies involving antineoplastic activity is needed for the development of this lead compound as anticancer drug.
Keywords: Salacia leptoclada, Quinone methide, Malaria, Therapeutic index, Cancer, Madagascar
1. Introduction
Parasitic infection causes, by Plasmodium species responsible of malaria which is a severe disease causing 300 to 500 millions of cases, and 1.1 million deaths per year in tropical regions worldwide, according to World Health Organization estimations[1],[2]. The most virulent protozoa, Plasmodium falciparum (P. falciparum), are the main cause of severe clinical malaria and death, and show an increasing prevalence of resistance to standard antimalarial drugs. The need of active antiplasmodial drugs with new mode of action becomes more and more urgent to replace ineffective drugs[3]–[5]. In many developing countries, medicinal plants were used by traditional healers to treat malaria for decade[6]–[11].
Madagascar is one of the countries in the world where plant based traditional medicine occupies an important place. Several plants of Malagasy flora are alleged to possess therapeutic values and are widely used by the local population. This is one of the reasons that people of this country are attached to the phytotherapy.
The genus Salacia (Celastraceae) is represented in the South of Madagascar by seven species[12],[13]. In Madagascar, Salacia leptoclada Tul. (Celastraceae) (S. leptoclada) is popularly known as Vandamena vahy or Vandamena tarike[14]. In Sakaraha, Manja and Toliara, the plant is traditionally used to treat malaria, diarrhea and asthma. Taking into account of these ethno-botanical data, we have investigated S. leptoclada for its antiplasmodial and cytotoxicity activities. The aim of the present study was to perform the bioassay-guided fractionation on the acetonic extract of the stem barks of S. leptoclada in order to validate scientifically the traditional use of this plant and to determine the nature of the biologically active compound. The chemical structure of the pure compound was elucidated using 1D, 2D NMR spectroscopy experiments and mass spectrometry by ESI.TOF.MS modes. This is the first report involving the chemical structure of a biologically active compound of S. leptoclada.
2. Materials and methods
2.1. Selection and collection of plant material
Ethnobotanical information about plant species selected for this study was obtained by interviewing traditional healers during field work which was conducted in the South of Madagascar. The stem barks of S. leptoclada were collected in the National Park Izombitse Sakaraha at nearly 165 km from Toliara town, in the south part of Madagascar. The plant was identified by comparison with reference specimens available at the Department of Botany; Parc Botanique et Zoologique de Tsimbazaza, Antananarivo, Madagascar. Voucher specimen with assigned sample number Rup-008 was deposited at the herbarium of the Laboratoire de Chimie Appliquée Rue Layflaylle, University of Toliara.
2.2. Extraction and bioguided isolation
The air-dried and powdered stem barks of S. leptoclada (1.5 kg) were extracted (3×3 h) by maceration with acetone at room temperature on a shaker. The pooled organic solvents were dried over Na2SO4 and evaporated to dryness at 40 °C under reduced pressure to yield crude extract (22.5 g). Twenty gram of the acetonic crude extract was suspended in water and partitioned successively with cyclohexane, ethyl acetate and n-butanol to yield the corresponding soluble extracts. The activity was only found in the ethyl acetate extract (an inhibitory effect rate of 94.67% at 2 µg/mL).
Five gram of the ethyl acetate extract was first subjected to fractionation using silica gel column chromatography eluted with methylene chloride and a gradient of methanol which resulted into eight fractions (F1-F8). Two fractions, F2 and F3 showed strong cytotoxic activity with 98.12% of inhibition at 0.5 µg/mL. These fractions were selected for the following steps.
The fractions F2 and F3 were checked for their purity by analytical TLC, and the zones were detected both with a UV lamp at 254 and 365 nm and by spraying with sulfuric vanillin acid, followed by heating at 120 °C during 1-5 min. F2 and F3 were combined on the basis of TLC similarity and resubmitted to silica gel column chromatography. The elution was done using hexan and a gradient of acetone, which resulted into four fractions. The fraction F31 showed cytotoxic activity with 95.89% at 0.25 µg/mL. And 60 mg of F31 was subjected to further purification using a silica gel column chromatography, and using hexan and a gradient of ethyl acetate for elution. This furnished pure compound (8 mg).
The purity of the compound was then detected by analytical HLPC with the mixture of chloroform and methanol 1:1 (v/v) as mobile phase, and the chromatography was performed with isocratic regime during 25 min. The eluted compound was detected based on its UV absorption in the wavelength range of 190 nm to 315 nm. The product purity was 99.92% at λ=205 nm and the amorphous[α]D20=-18 °C [1:1 (v/v) chlorofor/methanol].
2.3. Antiplasmodial assay
The in vitro antiplasmodial test was based on the inhibition of [G-3H]-hypoxanthine uptake by P. falciparum cultured in human blood. Briefly, P. falciparum parasites were maintained in culture in a complete medium composed of RPMI-1640, 25 mmol/L HEPES, 25 mmol/L NaHCO3 and 10% pooled human serum, with uninfected human red blood cells at 2.5% haematocrit. According to IMRA (Institut Malgache de Recherches Appliquées) tests, 3 mg of each plant extract was accurately weighted and dissolved in a minimum quantity of methanol, and subsequent dilutions were made in distilled water. A single concentration of 10 µg/mL was used in the screening of the crude extract. The required quantity was introduced into flat-bottomed 96-well plates. The cell suspension (1% parasitaemia) was distributed at 0.2 mL per well containing the dried test extract in triplicate alongside untreated controls, and the plates were shaken vigorously using a microculture plate shaker. The culture was then incubated at 37 °C for 18 h under microaerophilic conditions obtained with the candle jar method[15]. Tritiated hypoxanthine with a specific activity of 14.1 Ci/mmol (DuPont NEN, Boston, USA) was then added to each well (0.5 µCi per well) and the incubation continued at 37 °C in the required atmosphere for a further 24 h. The contents of the well were then incubated at -30 °C and unfrozen at 50 °C to lyse the cells, harvested by filtration on glass filter papers using a Skatron apparatus and finally washed several times with water. Thereafter, the discs were dried and added to toluene scintillator in vials and the radioactivity incorporated into parasites was estimated in an LKB Wallac 1214 Reckbeta liquid scintillation counter. Using serial concentrations ranging to 0.01 to 5 µg/mL, the IC50 values were determined by linear regression method in n independent experiments.
2.4. Cytotoxicity test
The acetonic crude extract, the fractions and the pure compound were systematically submitted to cytotoxicity test. To this end, murine P388 leukemia cells were grown in RPMI 1640 medium containing 0.01 nmol/L of β-mercaptoethanol, 10 mmol/L L-glutamine, 100 IU/mL G-penicillin, 100 µg/mL streptomycin, 50 µg/mL gentamycin, and 50 µg/mL nystatine, supplemented with 10% fetal calf serum. Cells were maintained at 37 °C in a humidified atmosphere with 5% of co2. The inoculums seeded at 104 cells/mL at an optimal volume of 0.1 mL per well, was introduced into flat-bottomed 95-well plates containing serial concentrations of compounds, alongside untreated controls.
Culture was then incubated at 37 °C for 71 h in a required atmosphere. Thereafter, cells were incubated at 37 °C with 0.02% neutral red dissolved in 1/9 methanol/water (0.1 mL per well) for 1 h and then washed with 1N PBS and finally lyzed with 1% sds. After a brief agitation on a microculture plate shaker, the plates were transferred to a Titertek Twinreader equipped with a 540 nm filter to measure the absorbance of the extracted dye. Cell viability was expressed as the percentage of cells incorporating dye relative to the untreated controls and IC50 values were determined by linear regression method.
2.5. Statistical analysis
All statistical calculations were carried out with GraphPadPrism4. The results are expressed as the mean±standard error of mean (SEM) of n independent experiments with individual values. Unpaired Student's t-test was used for statistical comparison; P values less than 0.01 were considered as significantly different from the control.
3. Results
3.1. Phytochemical studies
The bioassay-guided fractionation of the acetonic crude extract of the stem barks of S. leptoclada, owing to the use of repeated silica gel column resulted in the isolation of one triterpenic quinone, in pure form as evidenced by analytical TLC and by HPLC analysis.
The molecular formula of the pure compound was determined to be C30H40O4 by ESI.TOF.MS (m/z=487.2824 [M- (H-Na)+] calculated) and 1D, 2D NMR experiments.
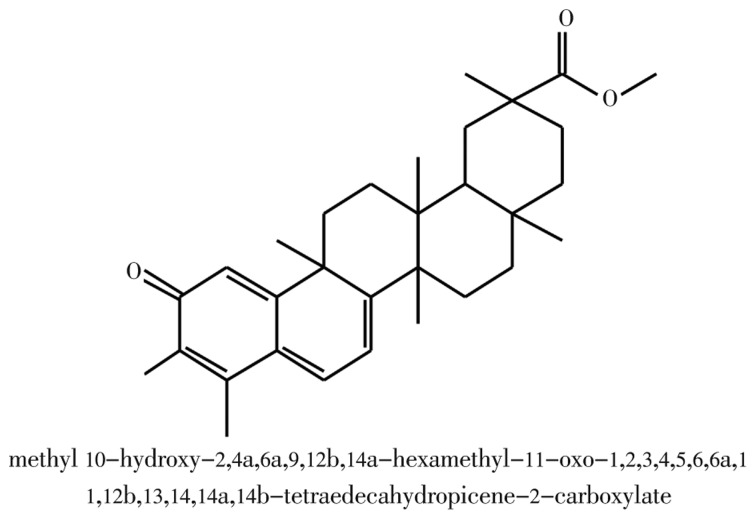
Examination of the 1D 1H, the 1D 13C (J-Module), and the 2D 1H-13C HSQC spectra data of the pure compound revealed the presence of two carbonyls carbons at δC 178.3 (C-29) and δC 178.7(C-2), eight alcens carbons of type (C=C) double bond at δC 117.1(C-4); δC 118.1(C-7); δC 119.6(C-1); δC 127.4(C-5); δC 134.0(C-6); δC 146.0(C-3); δC 164.7(C-10) and δC 170.0(C-8), ten quaternary C at: δC 30.5(C-17); δC 39.4(C-13); δC 40.4(C-20); δC 42.9(C-9); δC 45.0(C-14); δC 117.1(C-4); δC 127.4(C-5); δC 146.0(C-3); δC 164.7(C-10) and δC 170.0(C-8), four CH groups, seven CH2 groups, seven methyl groups and one hydroxyl (Table 1). The 1H and 13C chemical shift values of individual spin systems were determined by correlation in the 2D 1H-13C HSQC spectrum. The individual 1H and 13C chemical shift assigned by the 1H-1H COSY spectrum and 2D 1H-13C HSQC and HMBC correlation spectra, respectively (Table 1). The structures of the isolated phytochemical are given in Figure 1.
Table 1. 1H and 13C chemical shift, the 1H - 1H COSY, and important HMBC correlations.
| Position | δ 1H, multiplicity coupling constants | δ 13C | COSY | HMBC |
| 1 | 6.52, s | 119.6 | C3, C4, C9 | |
| 2 | 178.7 | |||
| 3 | 146.0 | |||
| 4 | 117.1 | |||
| 5 | 127.4 | |||
| 6 | 6.98, d | 134.0 | H7 | C4, C10, C8 |
| 7 | 6.34, d | 118.1 | H6 | C5, C9, C14 |
| 8 | 170.0 | |||
| 9 | 42.9 | |||
| 10 | 164.7 | |||
| 11a | 1.82, d | 33.6 | H25, H12b | C11,C10,C9,C12, C13 |
| 11b | 2.15, dd | H11a, H25 | C9, C12, C13 | |
| 12a | 1.78, d | 29.7 | H25 | C12, C13, C14 |
| 12b | 1.65, dd | H11a, H11b | C13, C14, C27 | |
| 13 | 39.4 | |||
| 14 | 45.0 | |||
| 15a | 1.65, dd | 28.7 | H15b, H16b | C14, C16, C17 |
| 15b | 1.55, dd | H25, H16a | C14, C17 | |
| 16a | 1.85, dd | 36.4 | H15b, H25 | C18, C22, C28 |
| 16b | 1.48, dd | H15b, H16a | C24 | |
| 17 | 30.5 | |||
| 18 | 1.56, dd | 44.3 | H19a, H27 | C27 |
| 19a | 2.41, m | 30.9 | H19b, H18, H30 | C18, C19, C20 |
| 19b | 1.68, dd | H27, H19a | C18, C20, C29 | |
| 20 | 40.4 | |||
| 21a | 2.18, m | 29.9 | H22a, H22b, H21b | C19 |
| 21b | 1.35, m | H22a, H22b, H21a | C21, C22 | |
| 22a | 0.98, m | 34.8 | H21a, H21b, H22b | C20,C18 |
| 22b | 2.10, dd | H28, H21a | C17 | |
| 23 | 2.21, s | 10.02 | C3, C4, C5 | |
| 24 | 10.68, s | |||
| 25 | 1.45, s | 38.2 | C8, C9, C10 | |
| 26 | 1.18, s | 21.6 | C14, C13, C15 | |
| 27 | 0.55, s | 18.3 | C12, C13, C18 | |
| 28 | 1.09, s | 31.6 | C16, C17, C18 | |
| 29 | 178.3 | |||
| 30 | 1.15, s | 32.7 | C19, C20, C29 | |
| 31 | 3.5, s | 51.5 | C29 |
Figure 1. Structure of pure compound.
3.2. Biological screening
The stem barks of S. leptoclada is used in the traditional medicine of Madagascar to treat malaria, asthma, and diarrhea and data were retrieved from a computerized compilation of medicinal plants of Madagascar[7]. To identify the bioactive fractions, we first submitted all the acetonic crude extract and four fractions (cyclohexane, ethyl acetate, n-butanol and aqueous extracts) from the stem barks of S. leptoclada to the in-vitro antiplasmodial test using serial concentrations. The growth inhibition of the parasite by the acetonic crude extract and four fractions was determined by comparison of radioactivity incorporated in the culture with the control culture and the IC50 values were determined by linear regression method. Results of evaluation of antiplasmodial activity of the acetonic crude extract and four fractions are outlined in Table 2.
Table 2. In vitro antiplasmodial and cytotoxic activities of the crude extract, differents fractions and the pure compound of stem barks of S. leptoclada.
| Antiplasmodial activity | Cytotoxicity activity | |
| Test | IC50 (µg/mL) | IC50 (µg/mL) |
| Acetonic extract | 0.85±0.05 | 0.72±0.02 |
| Cyclohexane | 23.17±2.03 | 35.61±1.01 |
| Ethyl acetate | 0.35±0.09 | 0.28±0.06 |
| n-Butanol | 34.68±1.01 | 43.59±0.82 |
| Aqueous | 41.09±1.71 | 51.32±2.01 |
| Fraction F2+3 | 0.175±0.05 | 0.15±0.04 |
| Fraction F31 | 0.076±0.02 | 0.069±0.03 |
| Pure compound | 0.052±0.03 | 0.041±0.02 |
As it can be deduced from the Table 2, the acetonic crude extract of the stem barks of Salacia leptoclada exhibited good antiplasmodial activity with the IC50=1 µg/mL and three fractions exhibited very weak antiplasmodial activity (Table 2). The antiplasmodial activity of the stem barks of S. leptaclada validates scientifically the use of this plant species in traditional medicine and may prove to be potentially useful as an antimalarial medicine. Aqueous, n-butanol and cyclohexane soluble fractions lack antiplasmodial activity. This could be due to the possible antagonist effects of the molecular structures mixture in the crude extract.
4. Discussion
The acetonic crude extract, four fractions (cyclohexane, ethyl acetate, n-butanol and aqueous extracts) and the pure compound from the stem barks of S. leptoclada were then submitted to the cytotoxicity test against P388 cell lines using the serial concentrations. The results of the cytotoxicity activity evaluation are outlined. The acetonic extract and ethyl acetate fraction exhibited good cytotoxicity activity with an IC50=1 µg/mL. To interpret these data, two cases must be considered: (1) the antiplasmodial activity of active constituents overlaps with the cytotoxicity activity of the unrelated compound; which may be the cases of the acetonic crude extract and ethyl acetate fraction; (2) the same constituents are responsible for both antiplasmodial and cytotoxic activities. One relevant example is the extract of Domohinea parrieri (Euphorbiaceae) which was found to contain phenantrenoids endowed with nicking DNA activities[16], and which exhibited good antiplasmodial activity. It is unlikely that the cytotoxic compound could be developed as antimalarial, but on the hand, screening of plant for antimalarial activity may lead to the discovery of useful anticancer drug.
The pure compound exhibited very good cytotoxicity activity with IC50 value of 0.035 µg/mL. Cytotoxicity activity of pure compound isolated from the stem barks of S. leptoclada was of great interest because it is closely related to chemical structure of the pure compound. Indeed, it was reported in the literature that the cytotoxicity activity of some pure compounds are linked to the presence of quinone methide in their chemical structure[17]. Indeed, recent findings based on the use of the computational analysis software especially the molecular orbital calculations indicate that quinone methide triterpenoids could induce cell apoptosis by quasi-intercalative interaction of these compounds with DNA followed by nucleophilic addition of the DNA base to carbon-6 of the triterpenoids[18]. In the best of our knowledge, the quinone methide triterpenoid derivative compound is reported for the first time in S. leptoclada Tul. and the result is in accordance with chemotaxonomic principle for related species containing such useful compounds and represent a powerful tool for therapeutic chemistry research field.
Triterpenic quinone was isolated from the stem barks of S. leptoclada, a medicinal plant species from Madagascar by the combination of bioassay-guided fractionation and gel chromatography. This lead compound was identified as the biologically active constituent against both the chloroquine-resistant strain FC29 of P. falciparum and against P388 leukemia cell lines. Because of its weak therapeutic index, furthers research involving in vivo anti-neoplastic activity is necessary for promoting these molecules as anticancer new lead compounds.
Acknowledgments
This research was founded by the Third World Academy of Science (TWAS Fellowship for Research and Advanced Training FR number: Grant No. 3240224121 and the International Foundation for Science (IFS, Stockholm, Sweden) and the Organization for the Prohibition of Chemical Weapons (OPCW) (IFS Research Grant No. F/4921-2).
Comments
Background
The P. falciparum, is the main cause of severe clinical malaria and death, and shows an increasing prevalence of resistance to standard antimalarial drugs. The need of active antiplasmodial drugs with new mode of action and low toxicity becomes more and more urgent to replace ineffective drugs.
Research frontiers
The authors have selected S. leptoclada through ethno-botanical survey. Bio-guided chromatographic fractionation of the acetonic extract of the stem bark of this plant using P. falciparum and P388 cell lines as in vitro models has conducted to the isolation of bioactive compound. The structure of the biologically active lead compound was elucidated by nmr spectroscopy (1D; 2D) and mass spectrometry.
Related reports
Recent findings have indicated that quinone methides from Celastraceae family are cytotoxic (induction of cell death). The authors of the present work have reported for the first time the antiplasmodial and cytotoxic activities of the root bark of S. leptoclada and it bioactive compound.
Innovations and breakthroughs
The authors have demonstrated that during biological screening of plant extracts for antimalarial activity, bioactive pure compound may become anticancer new lead compound if it therapeutic index is less than 1 as reported for the quinone methide pentacyclic triterpenoid derivative isolated from medicinal plant species S. leptoclada.
Applications
The present study has indicated that S. leptoclada could serve as source of anticancer drugs. The research describes how indigenous knowledge can be integrated in the process of discovery of new drugs from plants for commercial purpose as well as the search for the biologically active compounds in Salacia relative genus that have never been used in folk medicine.
Peer review
This is a valuable research work in which the authors have demonstrated that ethno-botanical data from Malagasy traditional healers on S. leptoclada have lead into the isolation and structure characterization of quinone methide using 1D, 2D NMR spectroscopy experiments and mass spectrometry (ESI-TOF-MS). This is the first report involving the chemical structure of a biologically active compound of S. leptoclada.
Footnotes
Foundation Project: Supported by the Third World Academy of Science (TWAS Fellowship for Research and Advanced Training FR number: Grant No. 3240224121 and the International Foundation for Science (IFS, Stockholm, Sweden) and the Organization for the Prohibition of Chemical Weapons (OPCW) (IFS Research Grant No. F/4921-2).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Aydin MF, Sahin A. Malaria epidemiology in Mersin province, Turkey from 2002 to 2011. Iran J Parasitol. 2013;8(2):296–301. [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain K, Shafee M, Khan N, Jan S, Tareen A, Khan M. Sero-prevalence of pediatric malaria in quetta, balachistan, Pakistan. Iran J Parasitol. 2013;8(2):342–347. [PMC free article] [PubMed] [Google Scholar]
- 3.Franco J, Blanckie MA, Toth D, Smith PJ, Capuano J, Fastnacht K, et al. et al. A structural comparative approach to identifying novel antimalarial inhibitors. Comput Biol Chem. 2013;45:42–47. doi: 10.1016/j.compbiolchem.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Domenech-Carbo'A, Maciuk A, Figadère B, Poupon E, Cebrian-Torrejon G. Solid-state electro chemical assay of heme-binding molecules for screening of drugs with antimalarial potential. An Chem. 2013;85(8):4014–4021. doi: 10.1021/ac303746k. [DOI] [PubMed] [Google Scholar]
- 5.Xu YP, Pieters L. Recent developments in antimalarial natural products isolated from medicinal plants. Mini Rev Med Chem. 2013;13(7):1056–1072. doi: 10.2174/1389557511313070009. [DOI] [PubMed] [Google Scholar]
- 6.Randrianarivelojosia M, Rasidimanana VT, Rabarison H, Cheplogoi PK, Ratsimboson M, Mulholland DA. Plant traditionally prescribed to treat tazo (malaria) in the eastern region of Madagascar. Malar J. 2003;2:25. doi: 10.1186/1475-2875-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyang NJ, Verpoorte R. A rewiew of the medicinal potentials of plants of the genus Vernonia (Asteraceae) J Ethnopharmacol. 2013;146(3):681–723. doi: 10.1016/j.jep.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Ngbolua KN, Rakotoarimanana H, Rafatro H, Ratsimamanga US, Mudogo V, Mpiana PT, et al. et al. Comparative anti-malarial and cytotoxic activities of two Vernonia species: V. amygdalina from the Democratic Republic of Congo and V. cinerea subsp vialis endemic to Madagascar. Int J Biol Chem Sci. 2011;5(1):345–353. [Google Scholar]
- 9.Challand S, Willcox M. A clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated malaria. J Altern Complement Med. 2009;15(11):1231–1237. doi: 10.1089/acm.2009.0098. [DOI] [PubMed] [Google Scholar]
- 10.Musila MF, Dossaji DF, Nguta JM, Lukhoba CW, Munya JM. In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalaria plants. J Ethnopharmacol. 2013;146(2):557–561. doi: 10.1016/j.jep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Zofou D, Tene M, Tane P, Titanji VP. Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug resistant W2 mef Plasmodium falciparum strain. Parasitol Res. 2012;110(2):539–544. doi: 10.1007/s00436-011-2519-9. [DOI] [PubMed] [Google Scholar]
- 12.Missouri Botanical Gardens and the Royal Botanic Gardens . Missouri: Missouri Botanical Gardens and the Royal Botanic Gardens; 2012. The Plant List (Version 1) [Online] Available from: http://www.theplantlist.org/. [Accessed on 30 June 2013]. [Google Scholar]
- 13.Tropicos® . Missouri: Tropicos®; 2012. Catalogue of the vascular plants of Madagascar. [Online] Available from: http://www.tropicos.org/Name/15600013. [Accessed on 30 June 2013]. [Google Scholar]
- 14.Beaujard P. Plants and traditional medicine in the South-East region of Madagascar. J Ethnopharmacol. 1988;23:165. doi: 10.1016/0378-8741(88)90004-9. [DOI] [PubMed] [Google Scholar]
- 15.Ngbolua KN, Herintsoa R, Hajatiana R, Suzanne RU, Mudogo V, Mpiana PT, et al. et al. Pharmacological screening of some traditionally-used anti-malarial plants from the Democratic Republic of Congo compared to its ecological taxonomic equivalence in Madagascar. Int J Biol Chem Sci. 2011;5(5):1797–1804. [Google Scholar]
- 16.Cui BL, Chai HY, Santisuk T, Reutrakul V, Farnsworth NR, Cordell GA, et al. et al. Novel cytotoxic 1H-cyclopenta[b]benzofuran lignans from Aglaia elliptica. Tetrahedron. 1997;53(52):17625–17632. [Google Scholar]
- 17.Satish K, Vishnuvardhan MV, Nayak VL, Srihari G, Subrahamanyam M, Rao TP, et al. et al. Cytotoxic diterpenoid quinonemethides from the roots of Pygmacopremna herbacea. Bioorg Med Chem Lett. 2011;21(15):4582–4584. doi: 10.1016/j.bmcl.2011.05.109. [DOI] [PubMed] [Google Scholar]
- 18.Ishii Y, Okamura T, Inoue T, Fukuhara K, Umemura T, Nishikawa A. Chemical structure determination of DNA bases modified by active metabolites of lucidin-3-O-primeveroside. Chem Res Toxicol. 2010;23(1):134–141. doi: 10.1021/tx900314c. [DOI] [PubMed] [Google Scholar]