Abstract
Objective
To determined the antiparasitic activity of the isolated chitosan from Penicillium viridicatum, Penicillium aurantiogriseum and commercial chitosan against protoscolicidal of hydatid cysts were determined.
Methods
After isolating chitosan from fungal cell walls, four concentrations (50, 100, 200, 400 µg/mL) of each type of prepared chitosan and commercial chitosan were used for 10, 30, 60, and 180 min, respectively.
Results
Among different type of chitosan, commercial chitosan with the highest degree of deacetylation showed high scolicidal activity in vitro. Fungal chitosan could be recommended, as good as commercial chitosan, for hydatic cysts control.
Conclusions
It seems to be a good alternative to synthetic and chemical scolicidal.
Keywords: Chitosan, Protoccoleces, Echinococcus granulosus, Penicillium
1. Introduction
Echinococcosis is a zoonotic infection caused by adult or larval (metacestode) stages of cestodes belonging to the genus Echinococcus and the family Taeniidae[1]. Echinococcus granulosus (E. granulosus) produces unilocular cysts characterized by a thick, multilayered cyst wall with a thin, internal germinal membrane; protoscolices and daughter cysts, containing more protoscolices, may form internally; cysts are contained by a connective tissue capsule[2]. Hydatid cysts in humans can cause liver dysfunction or respiratory disease, and anaphylaxis occurs if the cysts rupture. For every hydatid cyst ingested by the definitive host, many worms can develop[3],[4]. Surgery is still the main treatment for hydatid disease[5]. But several groups of drugs including cytostatics, antibiotics, sulphonamides, antiprotozoal compounds and several antihelmintic drugs have been tested for their efficacy against the metacestode stage of Echinococcus[6]. Chitosan is a partially or fully deacetylated chitin, which exists in crustacean shells, cuticles of insects, and fungi cell wall[7].
Chitosan has been used in broad spectrum of agriculture, food, biotechnology, cosmeticology and pharmaceutical industries[8]. Chitosan is of unique biological characteristics such as biodegradability, biocompatibility and none-toxicity in mammalian cells[9]. Antifungal and antibacterial activity of fungal chitosan has also been observed against several fungi and bacteria, but a few researches have been carried out on fungal chitosan antiparasitic activity[10]–[13]. The aim of our investigation was to evaluate scolicidal activities of various concentrations of different types of fungal chitosan isolated from Penicillium viridicatum (P. viridicatum), Penicillium aurantiogriseum (P. aurantiogriseum) in comparison to commercial one against protoscolices of E. granulosus in vitro in different exposure time.
2. Materials and methods
2.1. Collection of protoscolices
Protoscolices were aspirated from the liver cysts of infected sheep slaughtered at Sari slaughterhouse, Mazandaran Province, in Northern Iran. The hydatid fluid was transferred into test tube under sterilized condition and set for 30 min. The sedimental protoscolices were washed with normal saline for three times. The viability of the protoscolices was assessed by eosin 0.1% under a light microscope. The protoscolices those did not absorb dye were considered potentially viable, otherwise recorded as dead.
2.2. Extraction of chitosan
Low molecular weight commercially chitosan (CC) was purchased from Fluka. Two Penicillium species, P. aurantiogriseum and P. viridicatum from the department of mycology at Mazandarane Agriculture University, were used as a source of fungal chitosan. The fungal chitin (FC) was deacetylated by a modified method of Rane and Hoover[14]. After cultivation of fungal mycelia, the dry biomass were suspended with NaOH solution (1 mol) and autoclaved at 121 °C for 20 min. The alkaline undissolved fractions were collected by centrifugation at 12 000 r/min for 20 min, washed with distilled water and recentrifuged to obtain pH=7. The precipitation was further extracted using 2% acetic acid at 95 °C for 8 h. The insoluble particles suspension was centrifuged, then the supernatant solution was neutralized with NaOH (2 mol/L), the solution centrifuged and the precipitated chitosan was washed with distilled water.
Degree of deacetylation (DD) of FC and CC was measured by FT-IR spectroscopy (Perkin Elmer Pergamon 781, from 4000 to 450) and the calculation proposed as DD=100-[(ACO/AOH)×100/1.33][15]. To prepare chitosan solutions, each prepared chitosan sample from Penicillium species and CC was dissolved in 1% (w/v) acetic acid solution and then the solution was neutralized by 1 mol NaOH (medium).
2.3. Scolicidal assay
In our study, four concentrations (50, 100, 200, 400 µg/mL) of the chitosan were prepared. Then 10 000 washed protoscolices were added into 2 mL of each chitosan solutions and softly mixed. The mixed solution was incubated at 37 °C for 10, 30, 60, 180 min. Two milliliters of 0.1% eosin was then added to the sediment protoscolices. The pellet of protoscolices was examined under a light microscope. The percentages of dead cysts were determined by counting 500 cysts. No treated cysts were considered as a control group in each experiment. The experiments were performed in three times.
2.4. Statistical analysis
Triplicate trials were done for each experiments and statistical analysis for all the results was carried out using spss software. one sample t-test was performed to define significant variation between control and chitosan solutions at P<0.05.
3. Results
The scolicidal activity of FC and CC at different concentrations (50, 100, 200, 400 µg/mL) after 10, 30, 60 and 180 min of exposure time in comparison with control group are showed in Tables 1, 2 and 3.
Table 1. Scolicidal effect of different concentrations of prepared chitosan from P. viridicatum on the mortality rate of protoscolices of hydatid cyst at various exposure times.
| Exposure time (min) | Concentration (µg/mL) |
||||
| 50 | 100 | 200 | 400 | Control group | |
| 10 | 7 | 9 | 19 | 27 | 7 |
| 30 | 26 | 35 | 47 | 58 | 7 |
| 60 | 47 | 56 | 66 | 71 | 8 |
| 180 | 63 | 76 | 86 | 100 | 9 |
Table 2. Scolicidal effect of different concentrations of prepared chitosan from P. aurantiogeriseum on the mortality rate of protoscolices of hydatid cyst at various exposure times.
| Exposure time (min) | Concentration (µg/mL) |
||||
| 50 | 100 | 200 | 400 | Control group | |
| 10 | 5 | 7 | 19 | 28 | 7 |
| 30 | 15 | 25 | 41 | 46 | 7 |
| 60 | 49 | 53 | 62 | 70 | 8 |
| 180 | 79 | 76 | 83 | 100 | 9 |
Table 3. Scolicidal effect of different concentrations of commercially chitosanon the mortality rate of protoscolices of hydatid cyst at various exposure times.
| Exposure time (min) | Concentration (µg/mL) |
||||
| 50 | 100 | 200 | 400 | Control group | |
| 10 | 8 | 11 | 16 | 29 | 7 |
| 30 | 33 | 39 | 51 | 59 | 8 |
| 60 | 60 | 63 | 69 | 78 | 9 |
| 180 | 89 | 93 | 97 | 100 | 9 |
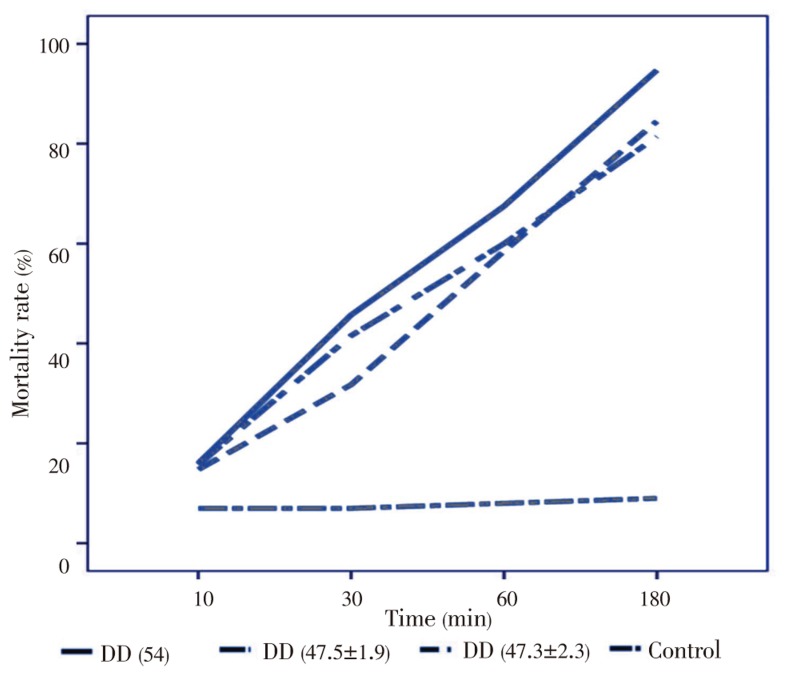
The maximum DD was obtained by CC (54%) and the minimum of this was 47.4% for P. viridicatum and P. aurantiogriseum respectively. No treated hydatic cyst is shown in Figure 1. Also, the dead protoscolex after treatment with chitosan is indicated in Figure 2. The efficacies of the deacetylations degree in toxicity of chitosan on hydatid cyst are shown in Figure 3. The hydatid cyst was susceptible after treatment in all of the concentrations. Protoscolex was more sensitive to CC than FC since CC at the lowest concentration indicated higher mortality rate. The result of our study demonstrated that the scolicidal effect at any concentrations of CC and FC shows significant difference compared with control groups (P=0.033, 0.018, 0.008, 0.006). Also, the scolicidal activity of CC and FC after 30, 60 and 180 min was significant compared with the control groups (P=0.016, 0.001, 0.001), but it was not significant after 10 min. It needs 100 µg/mL FC and CC after 60 min to reduce approximately 50% of the viable protosolexes. CC with higher DD (54%) showed higher mortality rate compared to FC ranging from 89% to 100% after 180 min of exposure time at all chitosan used dosages. The maximum mortality rate (100%) was achieved at 400 µg/mL concentration after 180 min exposure time.
Figure 1. Viable protoscolices with no absorbed dye (control group).
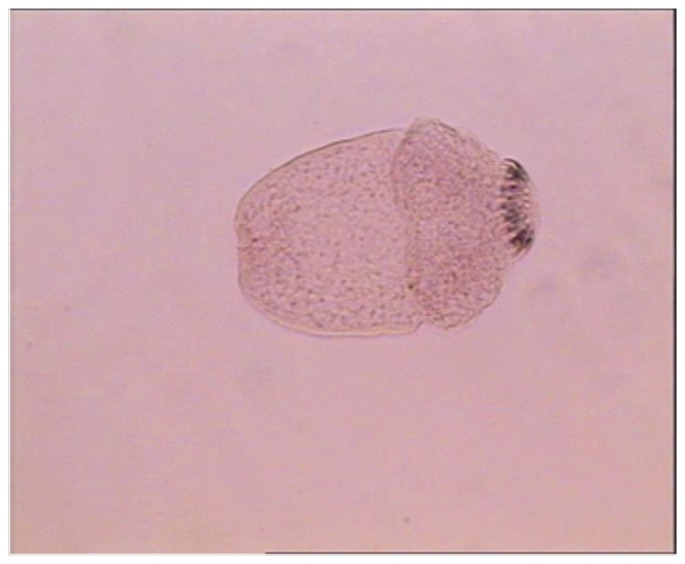
Figure 2. Dead protoscolices with absorbed dye (after treatment with chitosan).
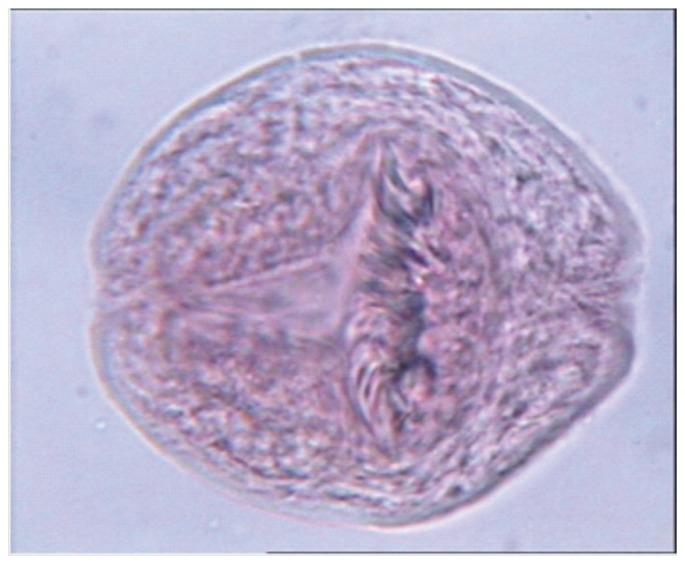
Figure 3. Effect of DD on the mortality rate of protoscolex.
4. Discussion
Chemotherapy, surgery, and puncture with aspiration are the three traditional treatments for hepatic hydatid cysts. If patients for whom surgery is not suitable or they refuse surgery, chemotherapy and puncture are recommended as alternative medication[16]. Inactivation of the scolex with a protoscolicidal agent before surgery is intensely recommended, because the spillage of the cyst contents is a major cause of return[17]. During hydatid cyst surgery protoscolicidal agents used to reduce the risk of spillage of viable protoscolices for surgical success[16]. The benzimidazole derivatives and mebendazole are common drugs presently used for management of hydatids cysts. And these drugs have been successful in many cases[18]–[21]. However, like other chemical agents, mebendazole has some side effects including liver toxicity or poor absorption[15]. Our study aimed to decrease the high risk of problem and recurrence during the surgery. Chitin and chitosan have a wide range of uses. These properties, together with the very safe toxicity profile, make chitosan an exciting and promising excipient for the pharmaceutical industry for present and future applications. Biodegradability and biocompatibility as some of their specific properties make them suitable for medical application. Chitin and chitosan are basic polymers mostly used in the preparation of medical biomaterials[9]. Presence of more than 0.025% chitosan inhibited the growth of Escherichia coli, Fusarium, Alternaria and Helminth osporium[22]. The combination of chitosan with drug make it possible to provide suitable extended release in gastrointestinal tract for intestinal Cryptosporidium infections[22]. Therefore, in order to reduce the bad effects of using chitosan, toxicity testing is needed. Many researches have been done to find the toxicity of chitosan[9],[23],[24]. Chitosan is widely regarded as a non-toxic, biologically compatible polymer[25]. It is approved for dietary applications in Japan, Italy and Finland[26]. In this study, it was the first time to use fungal chitosan as an antiparasitic agent and our results at different concentrations (50, 100, 200, 400 µg/mL) after 10, 30, 60 and 180 min of exposure time showed high protoscolicidal effect of chitosan in comparison with control groups. The maximum mortality rate (100%) was achieved at 400 µg/mL concentration after 180 min exposure time. The parallel experiment showed that chitosan at the highest concentration (1 250 µg/mL) and exposure time (360 min) could stop the viability of Trichomonas gallinae completely[27]. Commonly, the highest degraded chitosan serves a better inhibitory effect on Escherichia coli, Staphylococcus aurous, Candida albicans, Saccharomyces unisporus, Saccharomyces bayanus and Porphyromonas gingivalis[28]–[30]. FC and CC seemed to be more effective against hydatid cyst contents rather than herbal component that discussed above, at the lowest concentration (0.02%) served a better killing effect on protoscolices (200 µg/mL after 180 min, 83%, 86% and 96%, respectively). The results have indicated that with the increase of chitosan concentration and exposure time, the viability of hydatic cysts decreased. DD as a main parameter could reduce the exposure time with the prolonged action. When DD of CC was 54%, the protoscolicidal activity of chitosan was at its highest point, while P. viridicatum and P. aurantiogriseum with 47.5% and 47.3%, DD indicated weaker protoscolicidal effect than commercial one. However, there is no significant difference between CC and FC. Uchida et al. found that chitosan with higher DD was more effective for inhibiting fungi and bacteria[31]. There is a correlation between antiparasitic effects and the content of protonated amino groups in chitosan structures. This may be attributed to the existence of nh3+ group which named DD. This parameter increased positive charge of chitosan molecule which interacts with the negative charge of parasite plasma membrane, upseting cell membrane and promoting the leakage of intracellular constituent. To administer of chitosan in combination to other agents, it might reduce the drug resistant, complications, and obtained much shorter periods of treatments. In conclusion, the data obtained clearly demonstrated that the chitosan derived from fungi and commercial one was protoscolicidal in vitro. Chitosan as a cationic natural polysaccharide and biopolymer was demonstrated to be safe for mammalian cells. Hence, chitosan was recognized as a good candidate among natural protoscolicidal agents. In addition, the chitosan has been commonly available, easily prepared, and inexpensive. Therefore, it may be promising for hydatid disease. However, in vivo efficacy of chitosan on hydatids and the possible side effects need further investigation.
Acknowledgments
This work was supported by a grant from Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran (Grant No. 91/150).
Comments
Background
Echinococcosis is a zoonotic infection caused by adult or larval (metacestode) stages of cestodes belonging to the genus Echinococcus and the family Taeniidae. Chitosan is a partially or fully deacetylated chitin, which exists in crustacean shells, insects' cuticles and fungi cell wall. chitosan has a wide range of uses. These properties, together with the very safe toxicity profile, make chitosan an exciting and promising excipient for the pharmaceutical industry for present and future applications.
Research frontiers
The aim of this investigation was to evaluate scolicidal activities of various concentrations of different types of FC isolated from P. viridicatum, P. aurantiogriseum in comparison to commercial one against protoscolices of E. granulosus in vitro in different exposure time.
Related reports
Hydatid cysts in humans can cause signs of liver dysfunction or respiratory disease, and anaphylaxis occurs if cyst ruptures. For every hydatid cyst ingested by the definitive host, many worms can develop. Chitosan has broad spectrum use as an antimicrobial agent.
Innovations and breakthroughs
Antifungal and antibacterial activity of FC has also been observed against several fungi and bacteria, but a few researches have been carried out on FC antiparasitic activity. Many of these scolicidal agent cause many original undesirable complications that limit their use. There is always a demand for finding natural materials with rapid and complete scolicidal effect with no local or systemic side effects as well as low cost. In this study authors demonstrated that FC has mentioned properties.
Applications
Chitin and chitosan have a wide range of uses. These properties, together with the very safe toxicity profile, make chitosan an exciting and promising excipient for the pharmaceutical industry for present and future applications. Biodegradability and biocompatibility as some of their specific properties make them suitable for medical application. Chitin and chitosan are basic polymers mostly used in the preparation of medical biomaterials.
Peer review
The data obtained clearly demonstrated that the chitosan derived from fungi and commercial one was protoscolicidal in vitro. Chitosan as a cationic natural polysaccharide and biopolymer was demonstrated to be safe for mammalian cells. Hence, chitosan was recognized as a good candidate among natural protoscolicidal agents. In addition, the chitosan has been commonly available, easily prepared, and inexpensive. Therefore, it may be promising for hydatid disease.
Footnotes
Foundation Project: This work was supported by a grant from Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran (Grant No. 91/150).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Cardona GA, Carmena D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet Parasitol. 2013;192(1–3):10–32. doi: 10.1016/j.vetpar.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Andresiuk MV, Gordo FP, Saarma M, Elissondo MC, Taraborelli A, Casalongue C, et al. et al. Echinococcus granulosus genotype G1 dominated in cattle and sheep during 2003-2006 in Buenos Aires province, an endemic area for cystic echinococcosis in Argentina. Acta Trop. 2013;127(2):136–142. doi: 10.1016/j.actatropica.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Pukar MM, Pukar SM. Giant solitary hydatid cyst of spleen—A case report. Int J Surg Case Rep. 2013;4(4):435–437. doi: 10.1016/j.ijscr.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teke Z, Yagci AB, Atalay AO, Kabay B. Splenic hydatid cyst perforating into the colon manifesting as massive lower gastrointestinal bleeding: an anusual presentation of disseminated abdominal echinococcosis. Singapore Med J. 2008;49(5):113–116. [PubMed] [Google Scholar]
- 5.Topcu O, Sumer Z, Tuncer E. Efficacy of chlorhexidine gluconate during surgery for hydatid cyst. World J Surg. 2009;33(6):1274–1280. doi: 10.1007/s00268-009-9971-z. [DOI] [PubMed] [Google Scholar]
- 6.Ceballos L, Virkel G, Elissondo C, Canton C, Canevari J, Murno G, et al. et al. A pharmacology-based comparison of the activity of albendazole and flubendazole against Echinococcus granulosus metacestode in sheep. Acta Trop. 2013;127(3):216–225. doi: 10.1016/j.actatropica.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Muzzarelli RA, Boudrant J, Meyer D, Manno N, DeMarchis M, Paoletti MG. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr Polym. 2012;87(2):995–1012. [Google Scholar]
- 8.Jae-Young J, Se-Kwon K. Chitosan as potential marine nutraceutical. Adv Food Nutr Res. 2012;65:121–135. doi: 10.1016/B978-0-12-416003-3.00007-X. [DOI] [PubMed] [Google Scholar]
- 9.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Li XF, Feng XQ, Yang S, Wang TP, Su ZX. Effects of molecular weight and concentration of chitosan on antifungal activity against Aspergillus niger. Iran Polym J. 2008;17(11):843–852. [Google Scholar]
- 11.Pires NR, Cunha PL, Maciel JS, Angelim AL, Melo VM, Paula RC, et al. et al. Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydr Polym. 2013;91(1):92–99. doi: 10.1016/j.carbpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Tayel AA, Moussa S, el-Tras WF, Knittel D, Opwis K, Schollmeyer E. Anticandidal action of fungal chitosan against Candida albicans. Int J Biol Macromol. 2010;47(4):454–457. doi: 10.1016/j.ijbiomac.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Zhao X, Han X, Du Y. Antifungal activity of oligochitosan against Phytophtora capsici and other pathogenic fungi in vitro. Pestic Biochem Phys. 2007;87(3):220–228. [Google Scholar]
- 14.Rane KD, Hoover DG. An evaluation of alkali and acid treatments for chitosan extraction from fungi. Process Biochem. 1993;28(2):115–118. [Google Scholar]
- 15.Manterola C, Mansilla JA, Fonseca F. Preoperative albendazole and scolices viability in patients with hepatic echinococcosis. World J Surg. 2005;29(6):750–753. doi: 10.1007/s00268-005-7691-6. [DOI] [PubMed] [Google Scholar]
- 16.Lv H, Jiang Y, Liao M, Sun H, Zhang S, Peng X. In vitro and in vivo treatments of Echinococcus granulosus with Huaier aqueous extract and albendazole liposome. Parasitol Res. 2013;112(1):193–198. doi: 10.1007/s00436-012-3125-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Ye B, Kong J, Cai H, Zhao Y, Han X, et al. et al. In vitro protoscolicidal effects of high-intensity focused ultrasound enhanced by a superabsorbent polymer. Parasitol Res. 2013;112(1):385–391. doi: 10.1007/s00436-012-3176-3. [DOI] [PubMed] [Google Scholar]
- 18.Mandal S, Mandal MD. Human cystic echinococcosis: epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asia Pac J Trop Med. 2012;5(4):253–260. doi: 10.1016/S1995-7645(12)60035-2. [DOI] [PubMed] [Google Scholar]
- 19.Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13(2):125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Falagas ME, Bliziotis IA. Albendazole for the treatment of human echinococcosis: a review of comparative clinical trials. Am J Med Sci. 2007;334(3):171–179. doi: 10.1097/MAJ.0b013e31814252f8. [DOI] [PubMed] [Google Scholar]
- 21.Polat C, Dervisoglu A, Hokelek M, Yetim I, Buyukkarabacak Y, Ozkutuk Y, et al. et al. Dual treatment of albendazole in hepatic hydatidosis: new therapeutic modality in 52 cases. J Gastroenterol Hepatol. 2005;20(3):421–425. doi: 10.1111/j.1440-1746.2004.03535.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar MN. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. [Google Scholar]
- 23.Ariani MD, Yuliati A, Adiarto T. Toxicity testing of chitosan from tiger prawn shell waste on cell culture. Dent J. 2009;42(1):15–20. [Google Scholar]
- 24.Lan HY, Qi W, Han F, Shao JZ, Gao JQ. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int J Nanomedicine. 2011;6:3351–3359. doi: 10.2147/IJN.S25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52(2):117–126. doi: 10.1016/s0169-409x(01)00231-9. [DOI] [PubMed] [Google Scholar]
- 26.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15(9):1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 27.Tavassoli M, Imani A, Tajik H, Moradi M, Pourseyed SH. Novel in vitro efficiency of chitosan biomolecule against Trichomonas gallinae. Iran J Parasitol. 2012;7(1):92–96. [PMC free article] [PubMed] [Google Scholar]
- 28.Tipparat H, Oraphan R. Effect of deacetylation conditions on antimicrobial activity of chitosans prepared from carapace of black tiger shrimp (Penaeus monodon) Songklanakarin J Sci Technol. 2008;30(Suppl 1):1–9. [Google Scholar]
- 29.Tayel AA, Moussa S, Opwis K, Knittel D, Schollmeyer E, Nickisch-Hartfiel A. Inhibition of microbial pathogens by fungal chitosan. Int J Biol Macromol. 2010;47(1):10–14. doi: 10.1016/j.ijbiomac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Ikinci G, Senel S, Akincibay H, Kaş S, Erciş S, Wilson CG, et al. et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm. 2002;235(1–2):121–127. doi: 10.1016/s0378-5173(01)00974-7. [DOI] [PubMed] [Google Scholar]
- 31.Uchida Y, Izume M, Ohtakara A. Skjak-Baek G, Anthonsen T, Sandford P. Chitin and chitosan: Sources, chemistry, biochemistry, physical properties and applications. London: Elsevier; 1989. Preparation of chitosan oligomers with purified chitosanase and its application; pp. 373–382. [Google Scholar]