Abstract
Regulation of the platelet actin cytoskeleton by the Rho family of small GTPases is essential for the proper maintenance of hemostasis. However, little is known about how intracellular platelet activation from Rho GTPase family members, including Rac, Cdc42, and Rho, translate into changes in platelet actin structures. To better understand how Rho family GTPases coordinate platelet activation, we identified platelet proteins associated with Rac1, a Rho GTPase family member, and actin regulatory protein essential for platelet hemostatic function. Mass spectrometry analysis revealed that upon platelet activation with thrombin, Rac1 associates with a set of effectors of the p21-activated kinases (PAKs), including GIT1, βPIX, and guanine nucleotide exchange factor GEFH1. Platelet activation by thrombin triggered the PAK-dependent phosphorylation of GIT1, GEFH1, and other PAK effectors, including LIMK1 and Merlin. PAK was also required for the thrombin-mediated activation of the MEK/ERK pathway, Akt, calcium signaling, and phosphatidylserine (PS) exposure. Inhibition of PAK signaling prevented thrombin-induced platelet aggregation and blocked platelet focal adhesion and lamellipodia formation in response to thrombin. Together, these results demonstrate that the PAK signaling system is a key orchestrator of platelet actin dynamics, linking Rho GTPase activation downstream of thrombin stimulation to PAK effector function, MAP kinase activation, calcium signaling, and PS exposure in platelets.
Keywords: actin, PAK, platelets, Rac1, Rho GTPases
hemostasis is mediated at a cellular level by platelets (18, 23, 46). Platelets are molecularly tuned to detect disruptions of vascular integrity and to aggregate with one another at sites of vessel injury to form thrombi and effectively halt bleeding. Platelets are rapidly activated following binding to extracellular matrix proteins, such as collagen, and exposure to soluble agonists, such as the serine protease thrombin. During activation, platelets undergo a dramatic change in shape from discs to form finger-like filopodia and actin-rich sheets of lamellipodia on surfaces (32, 56, 57). At a cell physiological level, platelet cytoskeletal reorganization is mediated by a system of regulatory proteins, including the Rho GTPase family members Rho, Cdc42, and Rac, which have notable roles in regulating platelet function through the control of the actin cytoskeleton (1, 5, 32, 39, 40).
The Rho family of small GTP binding proteins, including members RhoA, Cdc42, and Rac1, are master organizers of the actin cytoskeleton and direct cell spreading, motility, and growth (47, 48). For the past two decades, these small GTPases have been investigated for separable as well as interdependent functions in filopodia and lamellipodia formation, membrane ruffling, and stress fiber formation (34). RhoA, Cdc42, and Rac1 help to orchestrate actin remodeling in a number of cell types, particularly platelets where these proteins converge to regulate platelet activation, secretion, aggregation, and thrombus stability (1, 32, 40, 48, 55). RhoA and Cdc42 have been shown to have roles in platelet contractility and secretion (1, 40, 44, 55). Platelet spreading is driven in part by Rac1, which is required for platelet lamellipodia formation and to maintain the stability of platelet aggregates (7, 32). While some of the tyrosine kinase signaling events upstream of Rac1 activation in platelet thrombotic functions have been defined, it is not yet well understood how signals downstream of Rac1 are translated into changes in platelet actin structures (57).
The Rho GTPase family members Cdc42 and Rac promote the autocatalytic activation of the p21-activated kinases, or PAKs, a family of serine/threonine protein kinases that represent the best characterized Rho GTPase effectors (2). PAKs have been shown to localize to actin-rich adhesions and to the leading edge of migrating cells to coordinate actin cytoskeletal dynamics and cell motility. Current models of PAK function describe a role for PAK in actin dynamics by way of a network of PAK substrates including filamin A, RhoGDI, Merlin, and LIMK1 (2). Other PAK effectors that also act as small GTPase-activating proteins (GAPs) and GEFs, such as GIT and PIX proteins, are also phosphorylated by PAK to subsequently drive the feed-forward activation of Rac1 in a cycle that regulates localized actin assembly and disassembly in processes of cell spreading and cell motility (2, 21, 47). Human platelets have been shown to express both group I and II PAKs [PAK1 and PAK2, and PAK3 and PAK4, respectively (4)], with PAK2 being first described as a kinase activated upon stimulation of platelets with the protease-activated receptor (PAR) agonist thrombin (2). Previous studies have shown that PAKs and PAK effectors such as LIMK1 are activated in platelets downstream of the collagen receptor GPVI (4); however, a role for PAK in platelet function in response to PAR signaling downstream of thrombin has not yet been defined (4, 13, 36, 50, 55).
In this study, a proteomic analysis of Rac1 binding partners reveals that Rac1 associates with a set of PAK effectors from thrombin-activated platelets, including GIT and PIX proteins and the Rho/Rac guanine nucleotide exchange factor GEFH1. Through the inhibition of platelet PAK, we show that these Rac-associated PAK effectors as well as LIMK1 and Merlin are phosphorylated upon platelet activation in a PAK-dependent manner and that PAK activity is required for the coordination of MAPK, Akt, and calcium signaling upstream of platelet lamellipodia formation, phosphatidylserine exposure, and aggregation by thrombin. These findings establish a model whereby PAK serves as a central organizer of thrombin signaling in platelet physiology.
MATERIALS AND METHODS
Reagents.
Bovine thrombin, EHT 1864, anti-vinculin (V9131), fatty-acid free BSA, and all other reagents were from Sigma-Aldrich except for as noted. For inhibitor studies, Y-27632, IPA-3, and PIR 3.5 were from Tocris. Human fibrinogen was from Enzyme Research. For Western blotting and immunocytochemistry experiments, anti-PAK2 pSer 20 (2607), PAK2 pSer192 (2605), PAK2 pThr 402 (2601), βPIX (4515), αPIX (4573), GEFH1 (4145), LIMK1 pThr 508 (3841), Merlin pSer518 (9163), FLNA pSer2152 (4761), MEK1/2 pSer217/221 (9154), ERK1/2 pThr202/Tyr204 (9106), Akt pSer473 (9271), Akt (9272), and GSK3βpSer9 (9336) were from Cell Signaling. GEFH1 pSer885 antibody was from Abcam. Rac1 (23A8) and FLNA antibodies were from Millipore. Total PAK (sc-881), LIMK1 (sc-5576), Merlin (sc-332), GSK3β (sc-881), and goat anti-rabbit and anti-mouse horseradish peroxidase secondary antibodies were from Santa Cruz Biotechnology. GIT1 and GIT2 antibodies were from Abgent and the University of California, Davis/National Institute of Neurological Disorders and Stroke/National Institute of Mental Health NeuroMab Facility. The anti-GIT1 pSer517 antibody was prepared as previously described (61). Alexa Fluor secondary antibodies, phalloidin, and Oregon Green BAPTA1-AM were from Life Technologies. pGEX-Rac1 plasmids were from Addgene. The d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (PPACK) anticoagulant was from Calbiochem. Glutathione-Sepharose 4B was from GE Healthcare Life Sciences.
Preparation of human washed platelets.
Human venous blood was drawn from healthy donors into sodium citrate and acid/citrate/dextrose as previously described (7, 32). Written informed consent was obtained from study participants, and the protocol was approved by the Oregon Health & Science University Institutional Review Board. Platelet-rich plasma was prepared by centrifugation of anti-coagulated blood at 200 g for 10 min. Platelets were further purified from platelet-rich plasma by centrifugation at 1,000 g in the presence of prostacyclin (0.1 μg/ml). Purified platelets were resuspended in modified HEPES/Tyrode buffer (129 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, and 1 mM MgCl2 pH 7.3) containing 0.1 μg/ml prostacyclin. Platelets were washed once by centrifugation and resuspended in modified HEPES/Tyrode buffer at indicated concentrations.
Protein capture and mass spectrometry.
Rac1-GST fusion proteins were prepared and conjugated to glutathione-Sepharose as previously described (6–8). Immobilized GST-Rac1 proteins were first loaded with 50 μM nucleotide (none, GDP, or GTPγS) in TENS buffer (10 mM Tris·HCl pH 7.5, 50 mM NaCl, 1 mM EDTA, and 1 mM dithiothreitol) for 1 h and then incubated on a rotator for 1 h at 4°C with precleared lysates from resting or thrombin-activated platelets. The immobilized Rac1 and platelet lysate mixtures were then washed in 10 volumes of buffer A (20 mM Tris·HCl pH 7.5, 1 mM DTT, 50 mM NaCl, 5% glycerol, 0.1% Triton X-100, and protease inhibitor cocktail). The GST-Rac1 affinity bound proteins from activated platelet lysates were separated by SDS-PAGE, and gel slabs corresponding to 75–250 kDa were cut out, digested with trypsin, and recovered peptides analyzed by liquid chromatography/tandem mass spectrometry as previously described (58) using an LTQ linear ion trap mass spectrometer (Thermo Scientific, San Jose, CA). Tandem mass spectral data were then interpreted using the program Sequest (Version 27, rev. 12; Thermo Scientific) and a human protein database containing 13,748 entries obtained from the Swiss Institute of Bioinformatics (Geneva, Switzerland). A static modification of 57 mass units was applied to cysteine residues, since proteins were alkylated with iodoacetamide before trypsinization, and a differential modification of 16 mass units to methionine residues to identify peptides undergoing oxidation during processing. Peptides and protein identifications were assembled and validated using the program Scaffold (Version 3.12; Proteome Software, Portland, OR), requiring two unique peptide identifications per protein, and minimum peptide and protein probabilities of 90 and 99%, respectively (25, 33).
Western blotting.
Western blot experiments were carried out as previously described (7). Briefly, platelet solutions were denatured in an equal volume of Laemmli sample buffer (Bio-Rad, Hercules, CA) with 0.5 M dithiothreitol (100°C, 5 min), separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and blotted with indicated antibodies and horseradish peroxidase-conjugated secondary antibodies as previously described (7). Protein was detected using ECL (Thermo Scientific).
Static adhesion assays.
For platelet spreading experiments, glass coverslips were coated with bovine thrombin (50 μg/ml) or human fibrinogen (50 μg/ml) followed by surface blocking with BSA (5 mg/ml). Inhibitors or vehicle were added to platelets in solution (2 × 107/ml) at the indicated concentrations for 10 min before exposure to immobilized surfaces at 37°C. After 45 min, nonadherent platelets were discarded and surface-bound platelets were washed three times with PBS. Coverslips were fixed in 4% paraformaldehyde and washed with PBS. To examine platelet morphology in solution, platelets (4 × 107/ml) we fixed with an equal volume of 8% paraformaldehyde before attachment to poly-l-lysine coated coverglass. Platelets were imaged using Kohler illuminated Nomarski differential interference contrast (DIC) optics with a Zeiss ×63 oil immersion 1.40-NA plan-apochromat lens on a Zeiss Axiovert 200M microscope as previously described (3, 7). For annexin V binding experiments, platelets were prepared as described above without fixation and incubated with annexin before visualization by fluorescence microscopy.
Calcium imaging.
For single-platelet calcium analyses, platelets were loaded with 15 μM Oregon Green BAPTA1-AM as previously described (22). Loaded platelets were allowed to settle onto fibrinogen-coated coverslips over a period of 30 min at 37°C in the presence or absence of 1 U/ml thrombin. The fluorescence intensities of single platelets were recorded and analyzed with a customized Matlab application as previously described (22). For comparison purposes, the average max-to-mean ratio ± SE of fluorescence intensities for at least five platelets from three fields of view was calculated for each treatment.
Fluorescence microscopy.
Purified human platelets (5 × 106/ml) were spread on coverglass coated with 50 μg/ml thrombin for 45 min at 37°C. Adherent platelets were washed three times with PBS before fixation with 4% paraformaldehyde and then washed in PBS and permeabilized with blocking buffer (PBS, 0.1% SDS + 1% BSA) for 1 h. Platelets were stained with anti-PAK (1:50), anti-Rac1 (1:100), or anti-vinculin (1:100) in blocking buffer overnight at 4°C. Invitrogen Alexa Fluor secondary antibodies (1:200) and Alexa Fluor 647-phalloidin or TRITC-phalloidin (1:100) were added in blocking buffer for 1 h. Coverslips were mounted with Fluoromount G (Southern Biotech) on glass slides and visualized with a Deltavision CoreDV Widefield Deconvolution System or a Zeiss Elyra SR-SIM Super-Resolution Structured Illumination Microscope (4).
Platelet aggregation.
For platelet aggregation studies, 300 μl of purified human platelets (2 × 108/ml) were pretreated with inhibitors for 10 min as indicated. Platelet aggregation was triggered by 0.07 U/ml thrombin and monitored under continuous stirring at 1,200 rpm at 37°C by measuring changes in light transmission using a PAP-4 aggregometer as previously described (7, 32).
RESULTS
Rac1 associates with a set of PAK effectors from thrombin-activated platelets.
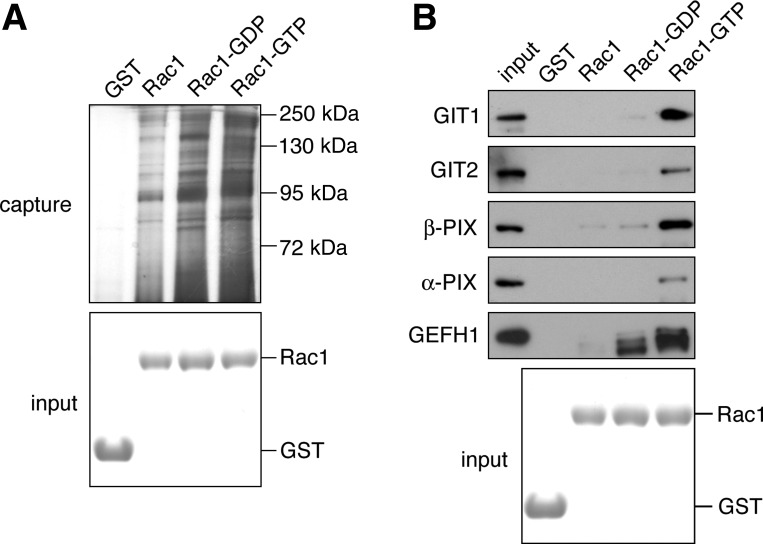
We previously determined that the Rho GTPase family member Rac1 is required for platelet lamellipodia formation and spreading as well as the maintenance of thrombus stability under physiological conditions of shear (7, 32). To better understand how signals from Rac1 lead to changes in platelet actin structures and morphology upon activation, we captured and identified Rac1-associated proteins from lysates of thrombin-activated platelets. Rac1-GST was conjugated to glutathione Sepharose, loaded with GDP, GTPγS, or no nucleotide and incubated with centrifugally cleared lysates of thrombin-activated platelets. As seen in Fig. 1A, immobilized Rac1 captured a number of platelet proteins as determined by the polyacrylamide gel electrophoresis and silver staining of Rac1 eluates. To identify these Rac-associated proteins, slabs of individual gel lanes in the range of 70–250 kDa from Rac1 elutes in the nucleotide free, GDP-loaded, and GTP-loaded states were digested with trypsin and the resulting peptide fragments were identified by mass spectrometry. As shown in Table 1, Rac1 captured proteins with putative roles in platelet function, including the Rac guanine nucleotide exchange factor Vav1 (6, 38, 41). Intriguingly, Rac1 captured a set GAPs and GEFs with known relationships to the PAK system, including GIT1, GIT2, βPIX, αPIX, and GEFH1 (21, 47, 60). Western blot analysis confirmed that GTPγS-loaded Rac1-GST captured GIT1, GIT2, βPIX, and αPIX (Fig. 1B) from thrombin-activated platelets, while minimal amounts of these proteins were associated with unloaded or GDP-loaded Rac1. Moreover, mass spectrometry and protein capture experiments using lysates of thrombin-stimulated platelets revealed that GDP-loaded as well as GTPγS-loaded Rac1 associated with the PAK-interacting Rho/Rac guanine nucleotide exchange factor GEFH1 (Fig. 1 and Table 1).
Fig. 1.
Association of the Rac and p21-activated kinases (PAK) signaling systems in platelets. A: silver-stained polyacrylamide gel of Rac-associated proteins from thrombin-activated platelets identified by mass spectrometry (see Table 1). B: Western blot analysis of Rac1 captured GIT, PIX, and guanine nucleotide exchange factor GEFH1 proteins from thrombin-activated platelet lysates.
Table 1.
Mass spectrometry identification of selected Rac1 binding proteins from thrombin-stimulated platelets
| Protein Name | Accession No. | Unique Peptides Identified | Sequence Recovered | Probability of Identification |
|---|---|---|---|---|
| RAC1 | RAC1_Human | 12 | 55% | 100% |
| VAV1 | VAV_Human | 11 | 16% | 100% |
| GIT1 | GIT1_Human | 9 | 14% | 100% |
| GIT2 | GIT2_Human | 6 | 11% | 100% |
| βPIX | ARHG7_Human | 5 | 9.5% | 100% |
| αPIX | ARHG6_Human | 14 | 22% | 100% |
| GEFH1 | ARHG2_Human | 4 | 5.4% | 100% |
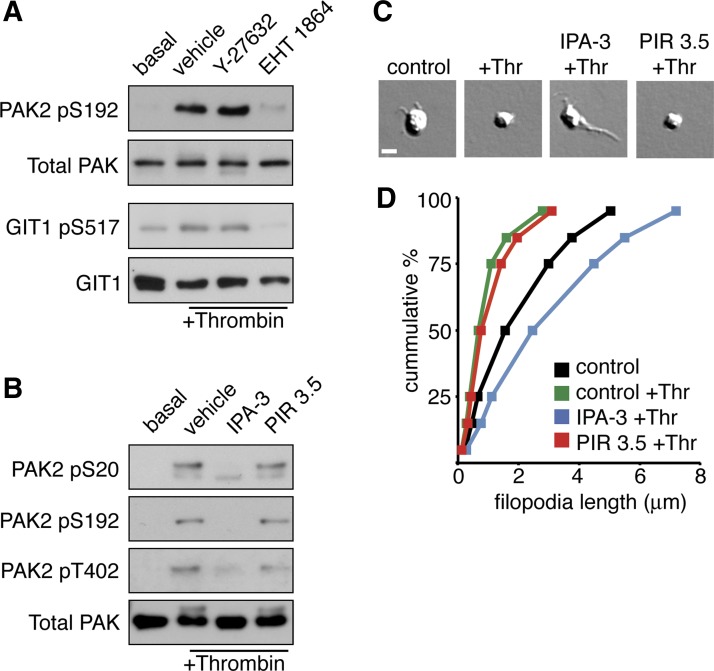
Previous studies have reported that Rac1 and PAK are activated in platelets upon stimulation with thrombin (1, 13, 50); however, it is not clear how Rac1 activation relates to PAK activation in thrombin-stimulated platelets. To determine the roles of Rho family GTPases in platelet PAK activation by thrombin, we examined the autocatalytic phosphorylation of PAK and the phosphorylation of the PAK substrate GIT1 in the absence and presence of Rho GTPase signaling pathway inhibitors. Western blot analysis of lysates from thrombin-stimulated platelets demonstrated that thrombin induces the autocatalytic activation and phosphorylation of PAK2 Ser192 well as the PAK-mediated phosphorylation of GIT1 Ser517 (Fig. 2A; Ref. 61). Treatment of platelets with the Rac-specific inhibitor EHT 1864 abrogated PAK activation and GIT1 phosphorylation elicited by thrombin. Inhibition of RhoA signaling with the Rho kinase inhibitor Y-27632 had no effect on thrombin-elicited PAK activation. These results support a role for Rac in thrombin-mediated PAK activation.
Fig. 2.
PAK activation in platelets upon stimulation with thrombin. A: human platelets treated with vehicle (DMSO), the Rho kinase (ROCK) inhibitor Y-27632 (10 μM), or the Rac inhibitor EHT 1864 (50 μM) before stimulation with 1 U/ml thrombin (Thr). Platelet PAK activation was determined by Western blot for phosphorylation of PAK2-Ser192 and phosphorylation of the PAK substrate GIT1-Ser517 under basal (lane 1) and thrombin-stimulated conditions (lanes 2–4). B: human platelets treated with vehicle (0.1%, DMSO), the inhibitor of PAK activation IPA-3 (10 μM), or the inactive PAK inhibitor relative PIR 3.5 (10 μM) before stimulation with 1 U/ml thrombin (Thr). PAK activation determined by Western blot for PAK2-pSer20, PAK2-pSer192 and PAK2-pThr402 under basal (lane 1) and thrombin-stimulated conditions (lanes 2-4). C: resting and thrombin-stimulated platelets treated with vehicle (0.1% DMSO), IPA-3 (10 μM), or PIR 3.5 (10 μM) were fixed in paraformaldehyde and examined for filopodia formation by differential interference contrast (DIC) microscopy. Scale bar = 1 μm. D: distribution analysis of measured platelet filopodia lengths from resting and thrombin-stimulated platelets treated with vehicle, IPA-3, or PIR 3.5 (n = 600 per condition).
PAK mediates platelet activation by thrombin.
Next, to determine the role of PAK in thrombin-stimulated platelets, we conducted a number of cell signaling studies using a specific inhibitor of PAK activation, IPA-3. This allosteric PAK inhibitor prevents activation of PAK by Rho GTPases Cdc42 and Rac (14, 54). IPA-3 covalently binds to PAK proteins to inhibit the activation of PAKs by the Rho family GTPases Rac and Cdc42 (14, 54). In studies of PAK function in contexts of molecular oncology and neuroscience, IPA-3 has been used in the range of 5 to 25 μM to inhibit PAK activity in epithelial cells and neurons (19, 24, 28, 35). In the current study, treatment of platelets with 1 U/ml thrombin readily activated PAK2, as evidenced by the autophosphorylation of PAK2 Ser20, Ser192, and Thr402 (Fig. 2B). As seen in Fig. 2B, PAK2 phosphorylation in response to thrombin was completely inhibited following a 10-min preincubation with 10 μM IPA-3. These experiments were complimented by the use of an inactive PAK inhibitor relative compound, PIR 3.5, which had no effect on thrombin-stimulated platelet PAK2 phosphorylation (Fig. 2B).
Upon treatment with thrombin, platelets undergo a characteristic change in morphology from discs to smaller spheres with filopodial extensions. To study the function of PAK in this shape change in solution, platelets were preincubated with IPA-3 for 10 min before the addition of thrombin for 5 min, fixed and bound to a surface of poly-l-lysine, and examined by Kohler-illuminated Nomarski DIC microscopy. As seen in Fig. 2C, treatment of platelets in solution with 1 U/ml thrombin led to a change in shape from a disc-like morphology to a small spherical shape indicative of platelet activation. Pretreatment of platelets with 10 μM IPA-3 altered this morphological change and led to the formation of extended filopodial structures. To quantify the effect of PAK inhibition on filopodia formation, we measured the filopodia length of 600 platelets per treatment condition. As plotted in Fig. 2D, the stimulation of platelets with thrombin reduced the mean filopodial length of platelets from 2.07 ± 1.92 to 0.96 ± 1.02 μm. The mean filopodial length of platelets treated with thrombin significantly increased to 3.11 ± 2.54 μm under conditions where PAK was inhibited with IPA-3. The ability of thrombin to cause a reduction in filopodial length was unaffected by the presence of PIR 3.5 (1.18 ± 1.36 μm).
Thrombin triggers PAK-dependent phosphorylation of PAK effectors in platelets.
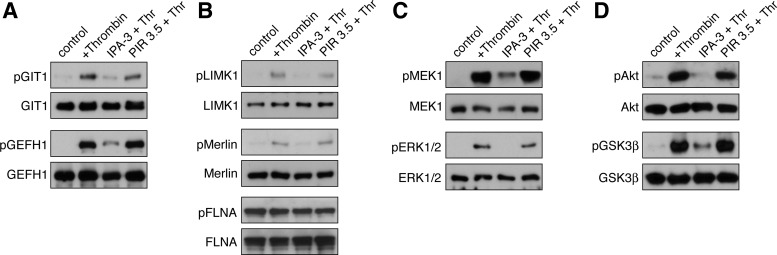
In nucleated cells, PAK mediates cell motility, migration, and functions associated with cell growth through the phosphoryation and activation of a complex set of PAK effector proteins, including GAP and GEF proteins captured in our proteomic screen from thrombin-stimulated platelets (Fig. 1). These effectors include GIT1, PIX, and GEFH1 (42, 60, 61), as well as classical effectors of PAK function such as LIMK1, Merlin, and filamin A (FLNA; Ref. 2). To first determine whether Rac-associated PAK effectors in thrombin-activated platelets are phosphorylated by PAK, platelets were preincubated with vehicle, IPA-3, or PIR 3.5. After a 5-min stimulation with 1 U/ml thrombin, platelets were lysed directly into Laemmli sample buffer and subject to SDS-PAGE and Western blot for GIT1 and GEFH1 phosphorylation. As seen in Fig. 3A, thrombin treatment resulted in the phosphorylation of GIT1 Ser517 and GEFH1 Ser885. The phosphorylation of GIT and GEFH1 upon platelet activation with thrombin was dependent on PAK kinase activity, as IPA-3 pretreatment prevented this thrombin-elicited phosphorylation. Thrombin treatment similarly resulted in a PAK-dependent phosphorylation of LIMK1 as well as Merlin. In nucleated cells, PAK phosphorylation of FLNA has been reported to control a key step in actin cytoskeletal reorganizations (53). Accordingly, we next examined the phosphorylation state of platelet FLNA in response to thrombin in PAK-inhibited conditions. As seen in Fig. 3B, platelet FLNA Ser2152 phosphorylation was not affected by thrombin stimulation or inhibition of PAK.
Fig. 3.
Thrombin-elicited PAK, MEK/ERK, and Akt signaling requires PAK. Purified human platelets were treated with vehicle (0.1% DMSO), 10 μM IPA-3, or 10 μM PIR 3.5 for 10 min before stimulation with thrombin (1 U/ml, 5 min). Western blot analysis of PAK-dependent phosphorylation of Rac-captured PAK effectors (A) and classical PAK effectors (B) as well as MEK and ERK (C) and Akt (D). Western blot results are representative of 4 separate experiments.
In concert with regulating actin cytoskeletal dynamics, PAKs are known to trigger the activation of the MAP kinase pathway through the phosphorylation of MEK proteins and the subsequent activation of ERK (15). As seen in Fig. 3C, PAK was found to be required for the complete activation of platelet MEK in response to thrombin stimulation, as IPA-3 pretreatment dramatically reduced MEK and abrogated ERK phosphorylation in response to thrombin (Fig. 3C). In addition to their roles in MAPK activation, the PAKs also support the activation of other key signaling systems, including the phosphatidylinositol 3-kinase/Akt pathway (20, 31). As seen in Fig. 3D, PAK activity was required for the complete activation of platelet Akt by thrombin, as IPA-3 pretreatment blocked Akt Ser473 phosphorylation and the downstream phosphorylation of the Akt substrate GSK3β Ser3. Pretreatment of platelets with the inactive IPA-3 relative molecule PIR 3.5 had no effect on thrombin-elicited platelet MEK, ERK, or Akt phosphorylation. These results suggest that in platelets PAK also has a central part in regulating platelet MAPK and Akt activation in response to thrombin stimulation.
A role for PAK in thrombin-stimulated platelet aggregation.
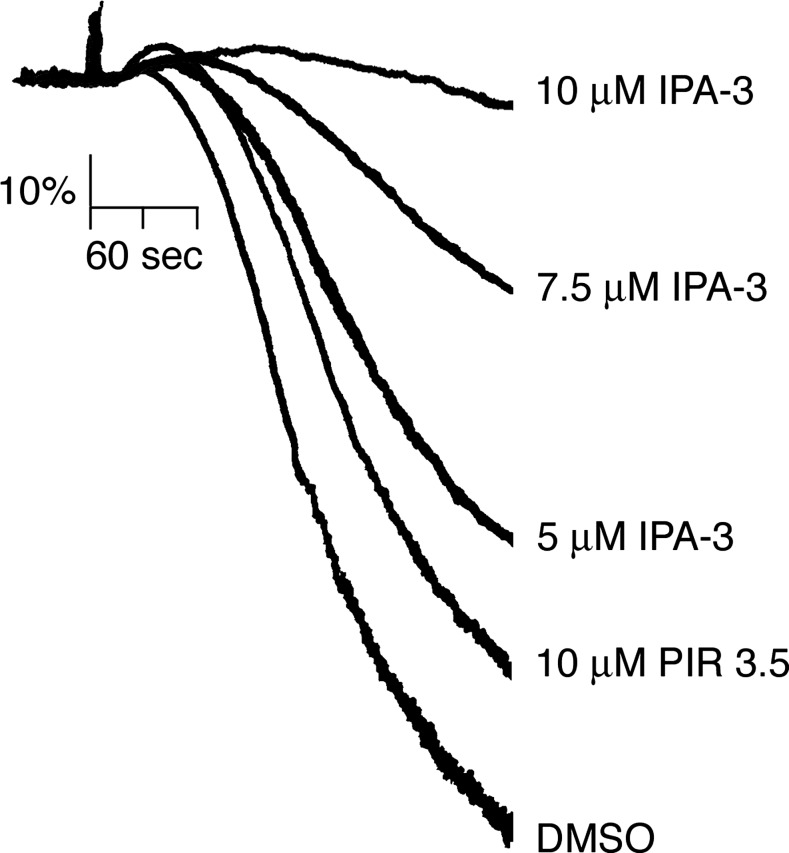
To determine if PAK has a role in platelet aggregation by thrombin, the effects of PAK inhibition on platelet aggregation in solution were assayed using a Born aggregometer. This apparatus measures light transmission through a suspension of platelets, with an increase in optical density correlating to platelet sphericity and shape change and a decrease indicative of aggregation. As shown in Fig. 4, purified human platelets readily aggregated in solution upon the addition of 0.07 U/ml thrombin. Pretreatment of platelets with IPA-3 dose dependently prevented the aggregation of platelets stimulated with thrombin compared with vehicle alone (0.1% DMSO; Fig. 4). Platelet aggregation in response to thrombin stimulation was minimally effected by 10 μM PIR 3.5 compared with equimolar concentrations of IPA-3 (Fig. 4).
Fig. 4.
Inhibition of PAK blocks platelet aggregation by thrombin. Replicate samples of washed human platelets (2 × 108/ml) were incubated with vehicle (DMSO) or increasing concentrations of IPA-3 or 10 μM PIR 3.5 before stimulation with thrombin (0.07 U/ml), and the change in optical density indicative of platelet aggregation was recorded. Results are representative of 4 separate experiments.
PAK supports platelet lamellipodia formation in response to thrombin.
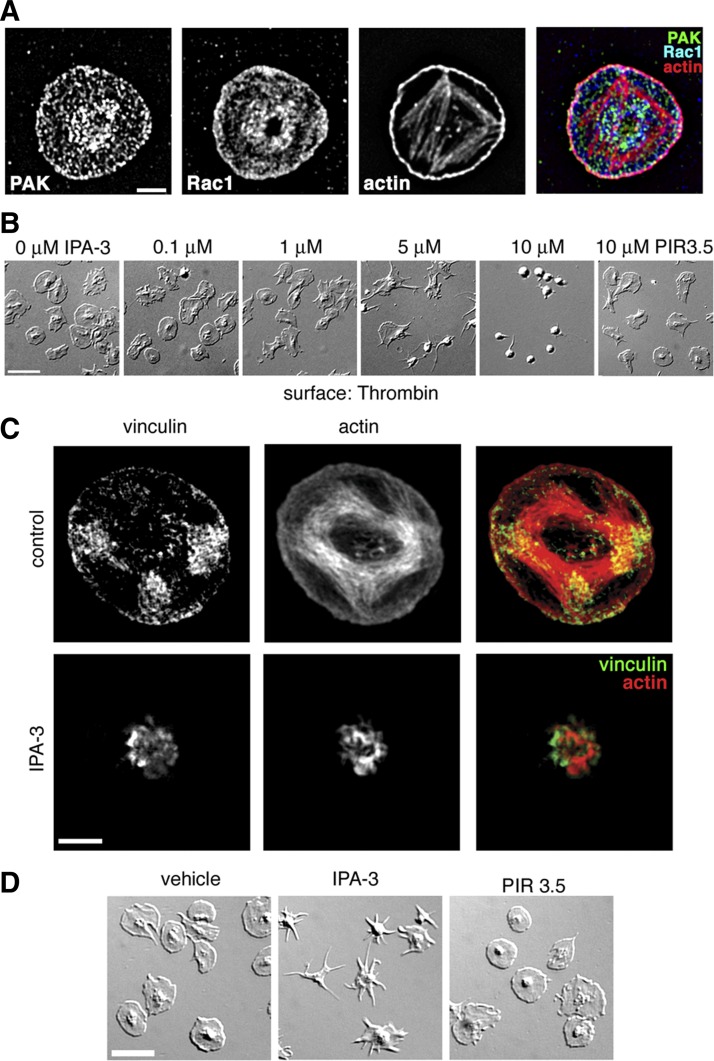
To examine the potential for an actin-localized coordination of PAK and to Rac signaling by thrombin, we next assayed by fluorescence deconvolution microscopy the colocalization of Rac1, PAK, and actin in platelets adherent to a surface coated with thrombin. Adherent platelets were fixed, permeabilized and stained for PAK, Rac1 and actin. As shown in Fig. 5A, PAK and Rac1 colocalized with lamellipodial actin in platelets spread on a surface of thrombin, with a high density of these proteins at focal adhesion regions, supporting a spatial role for organization of Rac and PAK pathways in the regulation of actin dynamics. Next, to examine the role of PAK inhibition on thrombin-induced lamellipodia formation as well as the formation of actin-rich adhesion structures, platelets (2 × 107/ml) pretreated with vehicle, IPA-3, or PIR 3.5 were incubated upon glass surfaces coated with thrombin and visualized by DIC microscopy. As seen in Fig. 5B, under control and PIR 3.5-treated conditions, platelets readily formed lamellipodia and spread out upon a surface of thrombin. Inhibition of PAK activation by IPA-3 dose dependently prevented lamellipodia formation and platelet spreading, suggesting that focal adhesion formation was similarly disrupted. To examine the architecture of platelet actin-rich adhesions, platelets spread on a surface of thrombin were fixed and stained for actin and as well as vinculin, a key component of focal adhesion structures (34). Super-resolution visualization by structured illumination microscopy revealed that platelets readily form actin-rich focal adhesions as they spread on thrombin-coated substrates. The pretreatment of platelets with IPA-3 before thrombin exposure prevented spreading, focal adhesion formation, and the efficiency of vinculin association with the actin cytoskeleton (Fig. 5C).
Fig. 5.
PAK activation is required for platelet lamellipodia and actin-rich adhesion formation in response to thrombin. A: purified human platelets spread on a surface of thrombin, stained for PAK, Rac, and actin and visualized by fluorescence deconvolution microscopy. Scale bar = 2 μm. B: representative DIC images of human platelets treated with vehicle (DMSO), increasing concentrations of IPA-3 or 10 μM PIR 3.5 on a surface of thrombin. Scale bar = 10 μm. C: super-resolution analysis of platelet focal adhesion formation of vehicle and 10 μM IPA-3 treated platelets on a surface of thrombin, visualized by SR-SIM of actin and vinculin staining. Scale bar = 2 μm. D: purified human platelets were spread on a surface of thrombin. After 30 min, unbound platelets were removed and activated platelets were treated with vehicle (0.1% DMSO), 10 μM IPA-3, or 10 μM PIR 3.5 for an additional 15 min. Platelet spreading and lamellipodial withdrawal were evaluated by DIC microscopy. Scale bar = 10 μm.
Signaling networks such as the mammalian target of rapamycin (7), small GTPase (32), and phosphatidylinositol 3-kinase/Akt (11) systems are required to maintain platelet spreading. Given the role of PAK in platelet lamellipodia formation, we hypothesized that PAK would similarly be required for platelet lamellipodia maintenance. To examine if continuous PAK activation is required to maintain platelet lamellipodial structures, purified human platelets were first spread on a surface of thrombin. After 30 min, nonadherent platelets were washed away and platelets were exposed to the PAK inhibitor IPA-3 or vehicle alone for an additional 15 min. DIC microscopy reveals that DMSO or PIR 3.5 treatment did not reverse the spreading of platelets on a surface of thrombin (Fig. 5D). However, treatment with IPA-3 led to the withdrawal of platelet lamellipodia (Fig. 5D).
Role of PAK in platelet calcium signaling and procoagulant activity.
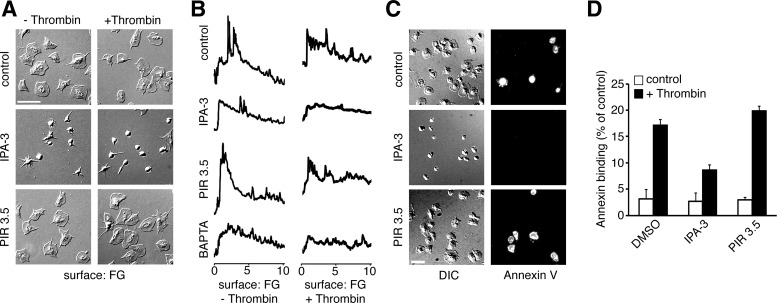
To examine the roles of PAK in functional events downstream of platelet activation, we next assayed calcium mobilization in single thrombin-stimulated platelets adherent to a surface of fibrinogen. As seen in Fig. 6A, purified human platelets readily adhered to and spread on a surface of fibrinogen. Pretreatment of platelets with IPA-3 prevented platelet spreading on fibrinogen in both the presence and absence of 1 U/ml thrombin in solution. To assay calcium dynamics, platelets were loaded with the calcium reporter dye Oregon Green BAPTA 1-AM prior to exposure to thrombin and/or fibrinogen. As shown in Fig. 6B, platelets adherent to fibrinogen, in both the absence and presence of thrombin in solution, elicited a sustained, oscillatory calcium response that coincided with lamellipodia formation and platelet spreading. Calcium mobilization in platelets on fibrinogen in both the absence and presence of thrombin was inhibited by pretreatment of platelets with the PAK inhibitor IPA-3. The max-to-mean ratio of calcium fluorescence intensities of platelets on fibrinogen significantly decreased from 4.0 ± 0.21 to 2.6 ± 0.20 in the presence of IPA-3. A similar decrease in the calcium fluorescence intensity max-to-mean ratio was observed in thrombin-stimulated platelets on fibrinogen in the presence of IPA-3 (3.8 ± 0.18 vs. 2.7 ± 0.13 in the presence of vehicle vs. IPA-3, respectively; means ± SE; n = 3). A similar level of inhibition was observed in the presence of the calcium chelator BAPTA-AM, with a max-to-mean ratio of 2.5 ± 0.24 and 2.4 ± 0.15 in the absence and presence of thrombin, respectively.
Fig. 6.
PAK is required for platelet calcium signaling. A: platelets treated with vehicle, 10 μM IPA-3 or 10 μM PIR 3.5 on a surface of fibrinogen in the absence or presence of thrombin (1 U/ml) in solution. B: replicate samples of platelets on a surface of fibrinogen, loaded with Oregon Green BAPTA1-AM analyzed for calcium spiking in the absence or presence of thrombin (1 U/ml) in solution. C and D: visualization and quantification of PS exposure of platelets pretreated with vehicle, 10 μM IPA, or 10 μM IPR 3.5 on a surface of fibrinogen with thrombin in solution visualized by annexin V-FITC staining. Results are representative of 4 separate experiments.
Platelet calcium signaling is intimately linked to the membrane translocation and externalization of phosphatidylserine (PS), a classical marker of apoptosis induction that also serves a procoagulant role in platelet function (27, 45). As single-platelet calcium mobilization in response to thrombin was abrogated in the presence of IPA-3, we hypothesized that inhibition of PAK would also block platelet PS exposure in response to thrombin. To test this, thrombin-stimulated platelets were exposed to a surface of immobilized fibrinogen 30 min before staining with the PS marker annexin V-FITC. As shown in Fig. 6C, a fraction of fibrinogen-bound platelets stained positive with annexin V in the absence of thrombin. Inhibition of PAK with IPA-3 prevented spreading on fibrinogen as well as PS exposure as determined by the absence of annexin V binding. Quantification of annexin V-positive platelets shows that under basal conditions on fibrinogen, 3.13 ± 1.83% of platelets bound annexin V on their surface in the presence of vehicle (0.1% DMSO). Similar basal levels of annexin V binding were seen for platelets pretreated with IPA-3 or PIR 3.5 (2.67 ± 1.63 and 2.94 ± 0.55%, respectively). Upon stimulation with thrombin, 17.2 ± 0.97 and 19.8 ± 0.85% of platelets bound annexin V in the presence of vehicle and PIR 3.5, respectively. Pretreatment with IPA-3 significantly reduced thrombin-stimulated platelet annexin V binding levels to 8.70 ± 0.93%. Together, these results show that the PAK system is involved in the signaling cascade to drive PS exposure in platelets in response to thrombin stimulation (Fig. 7).
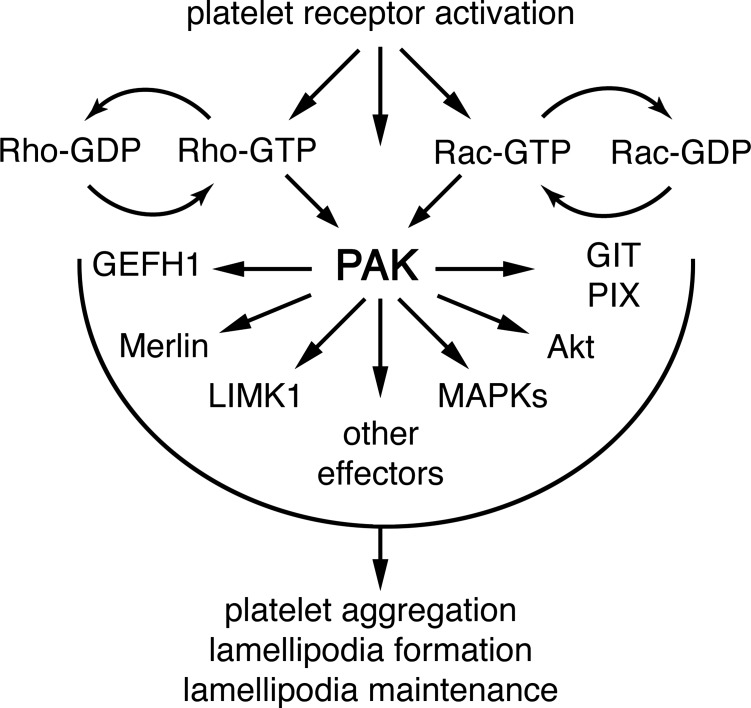
Fig. 7.
A PAK-centric model of platelet activation. PAK links Rho GTPase family signaling to MAP kinase, Akt, and calcium systems to drive platelet lamellipodia formation and aggregation.
DISCUSSION
The p21-activated kinases, or PAKs, are well known for connecting the 21-kDa Rho GTPase family members Cdc42 and Rac to a number of actin-associated cellular functions (2). In this study, through proteomics, signaling, and cell physiological studies of platelet biology, we find that following stimulation of platelets with thrombin, the PAK signaling system serves roles downstream of Rac1 activation to support activation of MEK/ERK, Akt, and calcium signaling as well as platelet spreading and aggregation.
Early experiments of Rho GTPase family signaling in platelets suggested that PAK functions primarily downstream of Rac and Cdc42 platelets (55). More recently, studies of platelets from Cdc42 knockout mice have suggested that Cdc42 plays a role in PAK activation in platelets as well as filopodia formation, platelet aggregation, and Akt activation (1, 5, 39). As the roles of Rac and Cdc42 in platelet function become further defined, these Rho family GTPases may similarly converge or separately route through PAK pathways and specific PAK effectors (2, 5). Future studies that take advantage of knockout mouse models of Rac and Cdc42 will aid in clarifying whether Cdc42 and Rac play synergistic roles in mediating mouse platelet activation by thrombin. Notably, such studies will require careful interpretation as mouse platelets have a distinct set of receptors for thrombin (mouse platelets express PAR3 and PAR4; human platelets express PAR1 and PAR4) that are cleaved with differing efficacy by thrombin to drive platelet activation (12). In this study, we show that Rac and PAK colocalize at focal adhesion and lamellipodial regions in human platelets (Fig. 5A) and that specific inhibition of Rac blocks the thrombin-induced activation of PAK in human platelets (Fig. 2A). Using a PAK isoform specific inhibitor, we show that inhibition of PAK1/2/3 activity alters platelet filopodia and lamellipodia dynamics and prevents platelet spreading on a surface of thrombin (Figs. 2 and 5). PAK inhibition also prevented platelets from establishing focal adhesions on an immobilized surface of thrombin, as determined by super-resolution fluorescence microscopy characterization of the platelet actin cytoskeleton (Fig. 5C).
Our proteomic analysis of Rac1 binding partners from thrombin-activated platelets revealed that Rac1 associates with a set of PAK effectors linked to focal adhesion regulation from thrombin-activated platelets, including GIT and PIX proteins as well as GEFH1 (Fig. 1 and Table 1). Intriguingly, PAK2 (also referred to as γ-PAK; Ref. 2), was originally characterized as a thrombin-activated kinase associated with a thrombin-responsive signaling complex consisting of small GTPases and GTPase activating proteins, or GAPs (52). Together with our work herein, this study suggests that a multimember signaling complex consisting of Rac and PAK and regulatory proteins such as GIT, PIX, and GEFH1 may work in concert to mediate thrombin-triggered platelet response. GEFH1 phosphorylation by PAK is hypothesized to coordinate RhoA, Cdc42, and Rac1 pathways (60). In nucleated cells, PAK phosphorylation of GEFH1 helps to drive the localized activation of Rho during cytokinesis (10); however, GEFH1 function has not yet been examined in platelets. GITs are multifunctional scaffolding proteins that serve to target PAKs as well as MAPKs to regions of dynamic actin signaling (29, 59, 61). GITs are closely associated with the PIX proteins, a set of PAK-interacting GEFs that recruit PAK to Rac1- and Cdc42-containing focal adhesion complexes and activate Rac1 locally at focal adhesions in a feedback mechanism that negatively regulates focal adhesion complex maturation and promotes cell migration (26, 30, 51). GIT1 associates with PIX and is tyrosine phosphorylated in thrombin-activated platelets (43); however, the functions of GIT and PIX in platelet biology remain to be defined. In addition to recruiting PAK to focal adhesions, GIT1 and βPIX are also PAK substrates; however, a functional role for GIT/PIX phosphorylation by PAK remains to be examined. Here we show that GIT1 is phosphorylated at a previously characterized PAK phosphorylation site in platelets following activation with thrombin in a Rac- and PAK-dependent manner upstream of platelet lamellipodia formation. These results suggest that GIT as well as GEFH1 phosphorylation may link PAK activity to platelet focal adhesion regulation.
In addition to GIT1 and GEFH1, we also examined the phosphorylation of other PAK target proteins with more established roles in regulation of the actin cytoskeleton. In cancer cells, the NF2 gene product and PAK effector Merlin acts to translate receptor signals into cytoskeletal changes through interactions with a number of signaling systems, including small GTPases, PAKs and MAPKs (49). Here we show that platelets express Merlin and that Merlin is phosphorylated in platelets in a PAK-dependent manner upon stimulation with thrombin. While stimulation of platelets with thrombin sets forth the activation of a number of PAK effectors with roles in actin dynamics, not all PAK effectors have apparent roles, as PAK-mediated FLNA phosphorylation remains unaffected by thrombin stimulation under control and PAK-inhibited conditions. These results suggest that PAK does not regulate platelet actin dynamics through FLNA phosphorylation under static conditions but do not rule out a role for PAK and FLNA under conditions of physiological shear (17). Along these lines, we have recently shown that PAK plays a key role in maintaining platelet aggregate stability on collagen under shear flow (4). While PAK phosphorylates an expansive set of targets to mediate cell spreading (2), LIMK1 may be the best characterized PAK substrate in connecting Rho family GTPases and PAK to actin regulation (9). Following its phosphorylation by PAK as well as ROCK, LIMK1 phosphorylates and inactivates cofilin to increase actin polymerization (9). Accordingly, PAK may regulate platelet actin dynamics through the LIMK1-mediated phosphorylation of cofilin. Intriguingly, LIMK1 also contributes to platelet function through glycoprotein Ib-IX-mediated TxA2 synthesis; however, the roles of PAK phosphorylation actin-independent processes such as platelet TxA2 synthesis remain to be examined (16, 36, 37). Together with data demonstrating a role for PAK in thrombin-mediated platelet activation, these findings connect platelet Rho GTPase signaling to a system of PAK effectors with central roles in platelet physiological functions, including lamellipodia formation, aggregation, and calcium-mediated phosphatidylserine exposure.
GRANTS
The M. J. Murdock Charitable Trust supported super-resolution microscopy studies. This work was supported by National Institutes of Health Grants R01-CA-142928 (to J. Chernoff) and R01-HL-101972 (to O. J. T. McCarty) and American Heart Association Grants 13POST13730003 (to J. E. Aslan) and 13EIA12630000 (to O. J. T. McCarty.). J. E. Aslan is a Fulbright Scholar. K. M. Haley is an MRF Early Clinical Investigator. C. P. Loren is a FV Leiden Scholar. A. Itakura is a Vertex Scholar. O. J. T. McCarty is an American Heart Association Established Investigator.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.E.A. conception and design of research; J.E.A., S.M.B., C.P.L., K.M.H., A.I., J.P., D.L.G., and L.L.D. performed experiments; J.E.A., S.M.B., C.P.L., K.M.H., A.I., and L.L.D. analyzed data; J.E.A., D.L.G., L.L.D., J.C., E.M., and O.J.T.M. interpreted results of experiments; J.E.A., S.M.B., C.P.L., K.M.H., and L.L.D. prepared figures; J.E.A. and L.L.D. drafted manuscript; J.E.A., J.C., E.M., and O.J.T.M. edited and revised manuscript; J.E.A., S.M.B., C.P.L., K.M.H., A.I., J.P., D.L.G., L.L.D., J.C., E.M., and O.J.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Kaech Petrie and A. Snyder (Oregon Health & Science University Advanced Light Microscopy Core) for imaging assistance. We thank G. Tormoen, A. Spencer, and L. Replogle for technical assistance.
REFERENCES
- 1.Akbar H, Shang X, Perveen R, Berryman M, Funk K, Johnson JF, Tandon NN, Zheng Y. Gene targeting implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet filopodia formation, secretion and aggregation. PLoS One 6: e22117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell 100: 97–108, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Aslan JE, Itakura A, Gertz JM, McCarty OJ. Platelet shape change and spreading. Methods Mol Biol 788: 91–100, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Aslan JE, Itakura A, Haley KM, Tormoen GW, Loren CP, Baker SM, Pang J, Chernoff J, McCarty OJ. p21 activated kinase signaling coordinates glycoprotein receptor vi-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear. Arterioscler Thromb Vasc Biol 33: 1544–1551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslan JE, McCarty OJ. Rho GTPases in platelet function. J Thromb Haemost 11: 35–46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslan JE, Spencer AM, Loren CP, Pang J, Welch HC, Greenberg DL, McCarty OJ. Characterization of the Rac guanine nucleotide exchange factor P-Rex1 in platelets. J Mol Signal 6: 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslan JE, Tormoen GW, Loren CP, Pang J, McCarty OJ. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood 118: 3129–3136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslan JE, You H, Williamson DM, Endig J, Youker RT, Thomas L, Shu H, Du Y, Milewski RL, Brush MH, Possemato A, Sprott K, Fu H, Greis KD, Runckel DN, Vogel A, Thomas G. Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol Cell 34: 497–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol 39: 1071–1076, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell 12: 699–712, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood 108: 3045–3052, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 407: 258–264, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Crespin M, Vidal C, Picard F, Lacombe C, Fontenay M. Activation of PAK1/2 during the shedding of platelet microvesicles. Blood Coagul Fibrinolysis 20: 63–70, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol 15: 322–331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol 22: 6023–6033, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estevez B, Stojanovic-Terpo A, Delaney MK, O'Brien KA, Berndt MC, Ruan C, Du X. LIM Kinase-1 selectively promotes glycoprotein Ib-IX-mediated TXA2 synthesis, platelet activation, and thrombosis. Blood 121: 4586–4594, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falet H, Pollitt AY, Begonja AJ, Weber SE, Duerschmied D, Wagner DD, Watson SP, Hartwig JH. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med 207: 1967–1979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest 115: 3355–3362, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci 29: 15859–15869, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol 10: 1356–1364, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci 119: 1469–1475, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Itakura A, Aslan JE, Sinha S, White-Adams TC, Patel IA, Meza-Romero R, Vandenbark AA, Burrows GG, Offner H, McCarty OJ. Characterization of human platelet binding of recombinant T cell receptor ligand. J Neuroinflammation 7: 75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the “magic bullet”. Nat Rev Drug Discov 2: 775–789, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kalin S, Amstutz B, Gastaldelli M, Wolfrum N, Boucke K, Havenga M, DiGennaro F, Liska N, Hemmi S, Greber UF. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J Virol 84: 5336–5350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 13: 383–393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leytin V. Apoptosis in the anucleate platelet. Blood Rev 26: 51–63, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Li A, Ma Y, Yu X, Mort RL, Lindsay CR, Stevenson D, Strathdee D, Insall RH, Chernoff J, Snapper SB, Jackson IJ, Larue L, Sansom OJ, Machesky LM. Rac1 drives melanoblast organization during mouse development by orchestrating pseudopod- driven motility and cell-cycle progression. Dev Cell 21: 722–734, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci 115: 1497–1510, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Mao K, Kobayashi S, Jaffer ZM, Huang Y, Volden P, Chernoff J, Liang Q. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J Mol Cell Cardiol 44: 429–434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem 280: 39474–39484, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81: 53–62, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O'Brien T, Vucic D, Harris AL, Belvin M, Friedman LS, Blackwood EM, Koeppen H, Hoeflich KP. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci USA 108: 7177–7182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey D, Goyal P, Bamburg JR, Siess W. Regulation of LIM-kinase 1 and cofilin in thrombin-stimulated platelets. Blood 107: 575–583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey D, Goyal P, Siess W. Lysophosphatidic acid stimulation of platelets rapidly induces Ca2+-dependent dephosphorylation of cofilin that is independent of dense granule secretion and aggregation. Blood Cells Mol Dis 38: 269–279, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Pearce AC, Wilde JI, Doody GM, Best D, Inoue O, Vigorito E, Tybulewicz VL, Turner M, Watson SP. Vav1, but not Vav2, contributes to platelet aggregation by CRP and thrombin, but neither is required for regulation of phospholipase C. Blood 100: 3561–3569, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Pleines I, Eckly A, Elvers M, Hagedorn I, Eliautou S, Bender M, Wu X, Lanza F, Gachet C, Brakebusch C, Nieswandt B. Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood 115: 3364–3373, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 119: 1054–1063, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Qian F, Le Breton GC, Chen J, Deng J, Christman JW, Wu D, Ye RD. Role for the Guanine nucleotide exchange factor phosphatidylinositol-3,4,5-trisphosphate-dependent rac exchanger 1 in platelet secretion and aggregation. Arterioscler Thromb Vasc Biol 32: 768–777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem 282: 15667–15678, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Sato H, Suzuki-Inoue K, Inoue O, Ozaki Y. Regulation of adaptor protein GIT1 in platelets, leading to the interaction between GIT1 and integrin alpha(IIb)beta3. Biochem Biophys Res Commun 368: 157–161, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Schoenwaelder SM, Hughan SC, Boniface K, Fernando S, Holdsworth M, Thompson PE, Salem HH, Jackson SP. RhoA sustains integrin alpha IIbbeta 3 adhesion contacts under high shear. J Biol Chem 277: 14738–14746, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, O'Reilly LA, Henley KJ, Ono A, Hsiao S, Willcox A, Roberts AW, Huang DC, Salem HH, Kile BT, Jackson SP. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 114: 663–666, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 11: 264–274, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci 124: 679–683, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr 5: 170–180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci 11: 471–484, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki-Inoue K, Yatomi Y, Asazuma N, Kainoh M, Tanaka T, Satoh K, Ozaki Y. Rac, a small guanosine triphosphate-binding protein, and p21-activated kinase are activated during platelet spreading on collagen-coated surfaces: roles of integrin alpha(2)beta(1). Blood 98: 3708–3716, 2001 [DOI] [PubMed] [Google Scholar]
- 51.ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol 172: 759–769, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teo M, Manser E, Lim L. Identification and molecular cloning of a p21cdc42/rac1-activated serine/threonine kinase that is rapidly activated by thrombin in platelets. J Biol Chem 270: 26690–26697, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol 4: 681–690, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther 8: 2559–2565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vidal C, Geny B, Melle J, Jandrot-Perrus M, Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood 100: 4462–4469, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des 15: 1358–1372, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost 3: 1752–1762, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Yan FF, Pratt EB, Chen PC, Wang F, Skach WR, David LL, Shyng SL. Role of Hsp90 in biogenesis of the beta-cell ATP-sensitive potassium channel complex. Mol Biol Cell 21: 1945–1954, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin G, Zheng Q, Yan C, Berk BC. GIT1 is a scaffold for ERK1/2 activation in focal adhesions. J Biol Chem 280: 27705–27712, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem 279: 18392–18400, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell 20: 237–249, 2005 [DOI] [PubMed] [Google Scholar]