Abstract
Vitamin B2 (riboflavin, RF) is essential for normal human health. Mammals obtain RF from exogenous sources via intestinal absorption and prevent its urinary loss by reabsorption in the kidneys. Both of these absorptive events are carrier-mediated and involve specific RF transporters (RFVTs). Chronic alcohol consumption in humans is associated with a high prevalence of RF deficiency and suboptimal levels, but little is known about the effect of chronic alcohol exposure on physiological and molecular parameters of the intestinal and renal RF transport events. We addressed these issues using rats chronically fed an alcohol liquid diet and pair-fed controls as a model. The results showed that chronic alcohol feeding significantly inhibits carrier-mediated RF transport across the intestinal brush-border and basolateral membrane domains of the polarized enterocytes. This inhibition was associated with a parallel reduction in the expression of the rat RFVT-1 and -3 at the protein, mRNA, and heterogeneous nuclear RNA (hnRNA) levels. Chronic alcohol feeding also caused a significant inhibition in RF uptake in the colon. Similarly, a significant inhibition in carrier-mediated RF transport across the renal brush-border and basolateral membrane domains was observed, which again was associated with a significant reduction in the level of expression of RFVT-1 and -3 at the protein, mRNA, and hnRNA levels. These findings demonstrate that chronic alcohol exposure impairs both intestinal absorption and renal reabsorption processes of RF and that these effects are, at least in part, mediated via transcriptional mechanism(s) involving the slc52a1 and slc52a3 genes.
Keywords: riboflavin transporter-1, riboflavin transporter-3, intestinal transport, renal transport
vitamin b2 (riboflavin, RF) is an indispensable micronutrient for normal cellular functions, growth, and development. In its coenzyme forms, i.e., riboflavin 5-phosphate and flavin adenosine dinucleotide, the vitamin plays a key metabolic role in the transfer of electrons in biological oxidation-reduction reactions involving carbohydrate, lipid, and amino acid metabolism, as well as in the conversion of vitamin B6 and folate to their active forms (5, 31). Recent studies have also demonstrated a role for RF in protein folding in the endoplasmic reticulum (40). RF deficiency leads to serious clinical abnormalities that include degenerative changes in the nervous system, anemia, growth retardation, skin lesions, and an increase in the susceptibility to carcinogens (5, 25, 31). In contrast to the negative effects of RF deficiency, RF supplementation/optimization appears to have the potential of protecting vital tissues from ischemia-induced oxidative injury (3), and is effective in the treatment of patients with RF-responsive multiple acyl-CoA dehydrogenase deficiency (16, 19).
Mammals obtain RF from exogenous sources, since they lack the ability for de novo synthesis of the vitamin. Two such sources are available to the intestine: a dietary source (absorbed in the small intestine) and a bacterial source where the vitamin is produced by the normal microflora of the large intestine (absorbed in the region of the gut) (11, 12, 34). Being the point of entry into the body compartment, the intestine therefore plays an important role in regulating normal RF body level. The other major player in regulating RF body homeostasis is the kidney, since it controls the exit point (urinary losses) of the vitamin from the body via its reabsorptive function of the filtered vitamin.
Previous studies from our laboratory and others have characterized both the intestinal (including colonic) and renal RF transport processes and found these events to be mediated via a regulated and specific carrier-mediated mechanism (15, 23, 28–30, 42, 43). These studies have shown that RF transport across both membrane domains of the polarized intestinal and renal epithelia cells, i.e., transport across the brush-border and the basolateral membrane domains (BBM and BLM, respectively), is carrier-mediated. Molecular identity of the systems involved in these transport events have also been clarified in recent years following the cloning of three RF transport systems from mammalian tissues, i.e., riboflavin transporter-1, -2, and -3 (RFVT-1, -2, and -3; products of the SLC52A1, SLC52A2, and SLC52A3 genes, respectively) (8, 41, 44, 45). Significant expression of RFVT-1 and -3 has been demonstrated in intestinal and renal epithelial cells, with expression of the RFVT-3 protein being exclusively localized at the apical BBM domain and that of RFVT-1 being predominantly localized at the BLM domain of these polarized epithelia (8, 35, 36).
Studies have reported high prevalence (ranges between 15 and 50%) of RF deficiency and suboptimal levels in patients with chronic alcoholism (4, 7, 9, 17, 22, 24, 27). Some of these cases were associated with clear clinical signs of deficiency that are manifested in the form of cheilosis and glossitis (7, 9). Because both the intestine and the kidney play key roles in maintaining and regulating RF body homeostasis, a negative effect of chronic exposure to alcohol on intestinal absorption and renal RF reabsorption may contribute toward the development of low RF levels. Very little, however, is known about the possible effect of chronic alcohol feeding on physiological and molecular parameters of intestinal and renal RF transport events. We addressed this issue using an established rat model chronically fed the Liber-Decarli alcohol-liquid diet. Our results show that chronic alcohol feeding leads to a significant inhibition in RF transport events across the jejunal and renal BBM and BLM; it is also associated with a significant inhibition in RF uptake in the colon. Furthermore, the data suggest that this inhibition is mediated, at least in part, at the level of transcription of the slc52a1 and slc52a3 genes.
MATERIALS AND METHODS
Materials
[3H]RF (specific activity 21.2 Ci/mmol; radiochemical purity >97%) was purchased from American Radiolabel (St. Louis, MO). Nitrocellulose filters (0.45 μm pore size) were purchased from Millipore (Fisher Scientific). Unlabeled RF and other chemicals, including molecular biology reagents, were from commercial sources. Oligonucleotide primers (Table 1) used in this study were synthesized by Sigma Genosys (Woodland, TX).
Table 1.
Combination of primers used to amplify the respective genes by real-time and semiquantitative PCR
| Gene Name | Forward and Reverse Primers (5′–3′) |
|---|---|
| Real-time PCR Primers | |
| rRFVT-1 | AGCTACCTGTAGTGGTGA; CTCAGCCCCTGAACCA |
| rRFVT-3 | TAAGGAAGATCACGGGCACCTC; GTCATCCAACTGGCCAACACAG |
| rβ-Actin | GTCAGGTCATCACTATCGGC; CATGGATGCCACAGGATTCC |
| hnRNA primers | |
| rRFVT-1 | CTTTAGGCCAGCTTCTTG; AAAATGCGAGTGGACAG |
| rRFVT-3 | TGGTGTTCTGCCCTCAG; GGGCCTGTGTTTGTAGC |
| rβ-Actin | CTGCTCTTTCCCAGATGAG; CTCATAGATGGGCACAGTG |
rRFVT, rat riboflavin transporter; hnRNA, heterogeneous nuclear RNA.
Animals and Alcohol Feeding
Male Wistar rats (Charles River, Wilmington, MA) were used in this study, as described by us previously (38). The experimental protocol was approved by the animal use committees of the Veterans Affairs Medical Center at Long Beach, CA, and the University of Southern California, Los Angeles, CA. Rats were fed Lieber-Decarli (20) ethanol-liquid diet (which provides 36% of total calories from ethanol) (Bio-Serv, Frenchtown, NJ); control rats were pair-fed the same liquid diet but without ethanol (maltose-dextrin replaced ethanol isocalorically) as described before (38). Rats were killed after 4 wk of chronic alcohol feeding, the jejunum and kidneys were removed, and brush-border membrane vesicles (BBMV) and basolateral membrane vesicles (BLMV) were isolated as described below (28, 29, 33, 42, 43). A portion of tissue from both the jejunum and kidney was stored in Trizol (Invitrogen, Carlsbad, CA) at −80°C for RFVT-1 and -3 mRNA and heterogeneous nuclear RNA (hnRNA) expression studies.
Isolation of BBMV and BLMVs from Rat Jejunal and Kidney Tissues and [3H]RF Uptake
Alcohol-fed rats and their pair-fed controls (two each) were killed with ketamine and xylazine, their jejunum and kidneys were removed, and BBMV and BLMV were isolated following well-established procedures from our laboratory and by others (28, 29, 33, 42, 43). These freshly isolated jejunal and kidney cortex BBMVs and BLMVs were preloaded with a buffer of 280 mM mannitol and 20 mM HEPES at pH 7.4. RF uptake studies were carried out by incubating vesicles (10 s; at 37°C; initial rate) in a buffer of 100 mM NaCl, 80 mM mannitol, and 20 mM HEPES, pH 7.4, and in the presence of labeled 0.5 μCi (240 nM) [3H]RF or in the presence of both unlabeled RF (1 mM) and labeled 0.5 μCi (240 nM) [3H]RF using a rapid-filtration method (10).
Rat Colonic Sheets Preparation and [3H]RF Uptake
Colons from chronically alcohol-fed rats and their pair-fed controls were removed and flushed with ice-cold saline. After being flushed, colons were cut open longitudinally, and two 1-cm-long sheets (pieces) were then immediately incubated in KR buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES, pH 7.4). The RF uptake was performed in the presence of labeled 0.5 μCi (24 nM) [3H]RF or in the presence of both unlabeled RF (1 mM) and labeled 0.5 μCi (24 nM) [3H]RF at 37°C for 5 min (initial rate, data not shown). Colonic sheets uptake was stopped, and radioactivity was determined.
Western Blotting
Western blotting was performed on purified BBMV and BLMV proteins. Eighty micrograms of either jejunal or renal BBMV and BLMV proteins isolated from the respective organs of alcohol-fed and control pair-fed rats were separated using 4–12% premade Bis-Tris minigel (Invitrogen). Gel-separated proteins were electroblotted onto polyvinylidene difluoride membrane (Bio-Rad) and then blocked with blocking solution (LI-COR Bioscience, Lincoln, NE) at room temperature for 1 h. The blocked membranes were then probed either with rat RFVT-3 (Santa Cruz) or RFVT-1 (Sigma) specific polyclonal antibodies (raised in rabbits) along with β-actin monoclonal antibody (raised in mouse)/GAPDH antibody (raised in rabbits) (Santa Cruz). The specific immunoreactive bands for rat (r) RFVT-3 and rRFVT-1 were determined as described recently (37, 38), and the respective bands were quantified using LI-COR Odyssey software.
cDNA Synthesis and Real-Time PCR
RNA isolated from the jejunum and kidney cortex of alcohol-fed rats and their pair-fed controls were used for real-time PCR analysis after DNase I (Invitrogen) treatment. cDNA was synthesized from total RNA by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and quantitative PCR was performed using rat RFVT-3 and -1 and β-actin primers (Table 1). The conditions for real-time RT-PCR are 95°C for 3 min, 95°C for 15 s, 58°C for 15 s, and 72°C for 15 s, 40 cycles. The data were normalized to rat β-actin and then quantified using a relative relationship method (Bio-Rad) (21).
hnRNA Analysis
Rate of hnRNA transcription (the first product of gene transcription) reflects the rate of gene transcription of many genes, including vitamin transporter genes (1, 6, 13, 37). We determined the level of rat RFVT-3 and -1 hnRNA expression in rat intestine and kidney cortex using cDNA synthesized from RNA, gene-specific primers that anneal to sequences in the intron/exon boundaries (Table 1), and semiquantitative PCR conditions as described before (6, 37) for the alcohol-fed rats and their pair-fed controls. The amplified PCR products were then run on agarose gel, and the specific bands were quantified using UN-SCAN-IT gel-digitizing software (Silk Scientific, Orem, UT).
Statistics
Transport data on carrier-mediated RF uptake in rat intestinal and renal BBMV and BLMV are means ± SE of multiple separate determinations from multiple rats and are expressed as the percentage relative to simultaneously performed controls. Carrier-mediated RF uptake was determined by subtracting uptake in the presence of 1 mM unlabeled RF from the uptake in its absence. Student's t-test was used for statistical analysis, and P < 0.05 was considered statistically significant. Protein, mRNA, and hnRNA experiments were measured on at least three separate occasions using different sample preparations.
RESULTS
Effect of Chronic Alcohol Feeding on Physiological and Molecular Parameters of Intestinal RF Absorption Process
Effect on physiological parameters of RF transport across jejunal BBM and BLM domains of the polarized rat intestinal epithelia.
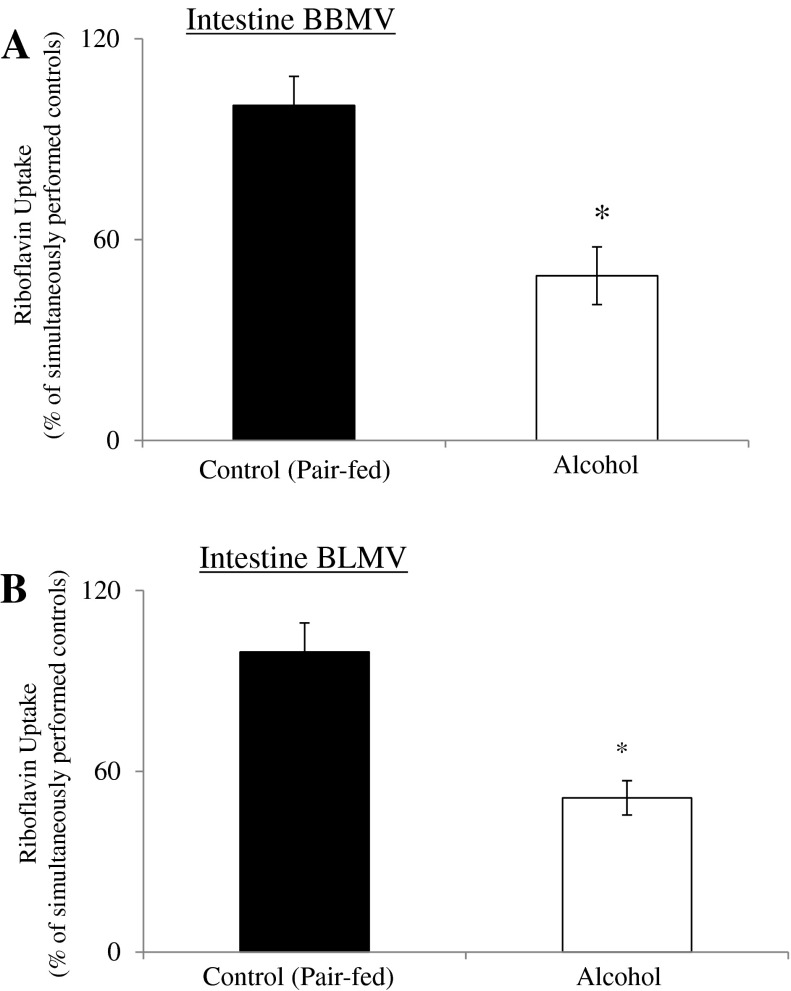
Figure 1A depicts the results of the effect of chronic alcohol feeding (4 wk) on carrier-mediated RF transport across the rat jejunal BBM. The study was done using purified BBMV isolated as described in materials and methods. The results showed a significant (P < 0.01) inhibition in the initial rate of carrier-mediated RF (240 nM) uptake by intestinal BBMV isolated from rats fed alcohol chronically compared with uptake by BBMV isolated from the jejunum of their pair-fed controls (Fig. 1A).
Fig. 1.
Effect of chronic alcohol feeding on carrier-mediated riboflavin (RF) uptake by rat jejunal brush-border membrane vesicles (BBMV) and basolateral membrane vesicles (BLMV). BBMV (A) and BLMV (B) were prepared from the jejunum of rats that were chronically fed alcohol liquid diet for 4 wk and their pair-fed controls and were briefly incubated at room temperature and then immediately used for uptake assay. [3H]RF (240 nM) was added to the vesicle-containing buffer at the time of the uptake experiment. Data are means ± SE of at least 5–10 independent uptake determinations from multiple sets of rats. *P < 0.01.
We also examined the effect of chronic alcohol feeding on carrier-mediated RF transport across rat jejunal BLM using purified jejunal BLMV. The results showed a significant (P < 0.01) inhibition on the initial rate of carrier-mediated RF (240 nM) uptake by jejunal BLMV of rats fed alcohol chronically compared with membrane vesicles from pair-fed controls (Fig. 1B).
These findings clearly show that both transmembrane components of the intestinal RF transepithelial absorptive events are negatively impacted by chronic alcohol exposure.
Effect on molecular parameters of RF transport in rat jejunum.
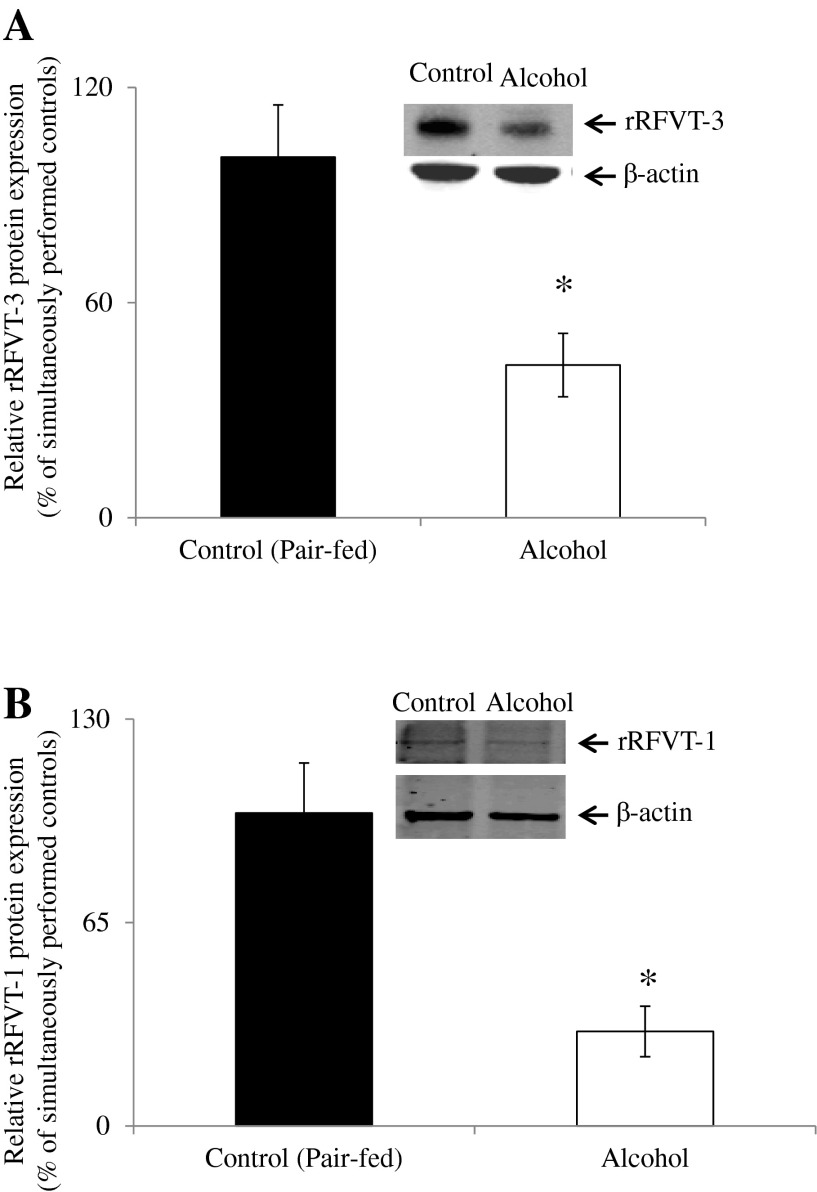
The RFVT-3 protein is expressed exclusively at the apical BBM of polarized intestinal (and renal) epithelial cells and plays a predominant role in RF transport across that membrane domain (8, 35, 36). On the other hand, RFVT-1 is the predominant RF transporter expressed at the BLM domain of intestinal (and renal) epithelial cells (35, 36). Thus, to examine the effect of chronic alcohol feeding on the level of expression of the RFVT-3 and RFVT-1 proteins, purified BBM and BLM preparations were analyzed by Western blotting. Rat specific anti-RFVT-3 and -RFVT-1 polyclonal antibodies were employed in these investigations. The results showed a significantly (P < 0.01 for both rRFVT-3 and rRFVT-1) lower level of expression of rRFVT-3 and rRFVT-1 proteins in jejunal BBM and BLM of rats chronically fed alcohol compared with their pair-fed controls (Fig. 2).
Fig. 2.
Effect of chronic alcohol feeding of rats on RF transporter (RFVT)-3 and RFVT-1 protein expression in intestinal brush-border membrane (BBM) and basolateral membrane (BLM) domains. Intestinal BBMV (A) and BLMV (B) proteins (80 μg) were isolated from chronic alcohol-fed rats (4 wk) and their pair-fed controls, and Western blotting was performed. BBMV and BLMV proteins were separated on NuPAGE 4–12% mini gel and electroblotted onto polyvinylidene difluoride (PVDF) membrane. Blots were incubated with rabbit polyclonal anti-RFVT-3 and -RFVT-1 primary antibodies and detected as described (37, 38). Samples were normalized relative to β-actin protein expression. Data are means ± SE of 3 separate samples from multiple sets of rats. *P < 0.01.
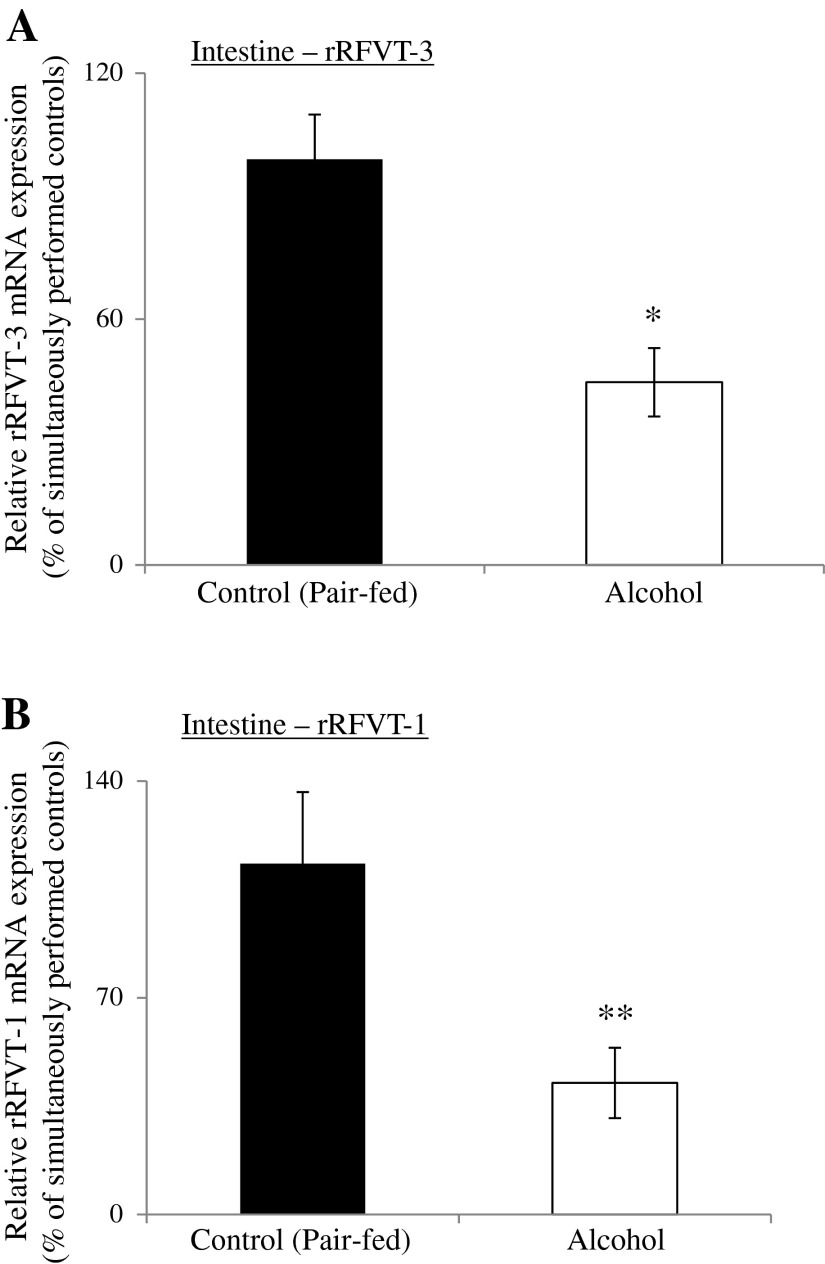
Next, we examined the effect of chronic alcohol feeding on the level of expression of rRFVT-3 and rRFVT-1 mRNA in rat jejunal mucosa; data were compared with the level of message expression of these transporters in pair-fed controls. The results showed a significant decrease in the level of expression of both the rRFVT-3 (P < 0.01) and rRFVT-1 (P < 0.05) mRNA in jejunal mucosa of alcohol-fed rats compared with controls (Fig. 3).
Fig. 3.
Effect of chronic alcohol feeding of rats on rat (r) RFVT-3 and rRFVT-1 mRNA expression in rat jejunal mucosa. Real-time PCR was performed using rRFVT-3 and rRFVT-1 gene-specific primers (Table 1) and normalized relative to rat β-actin. Data are means ± SE of 3–5 separate samples from 3–5 rats. *P < 0.01 and **P < 0.05.
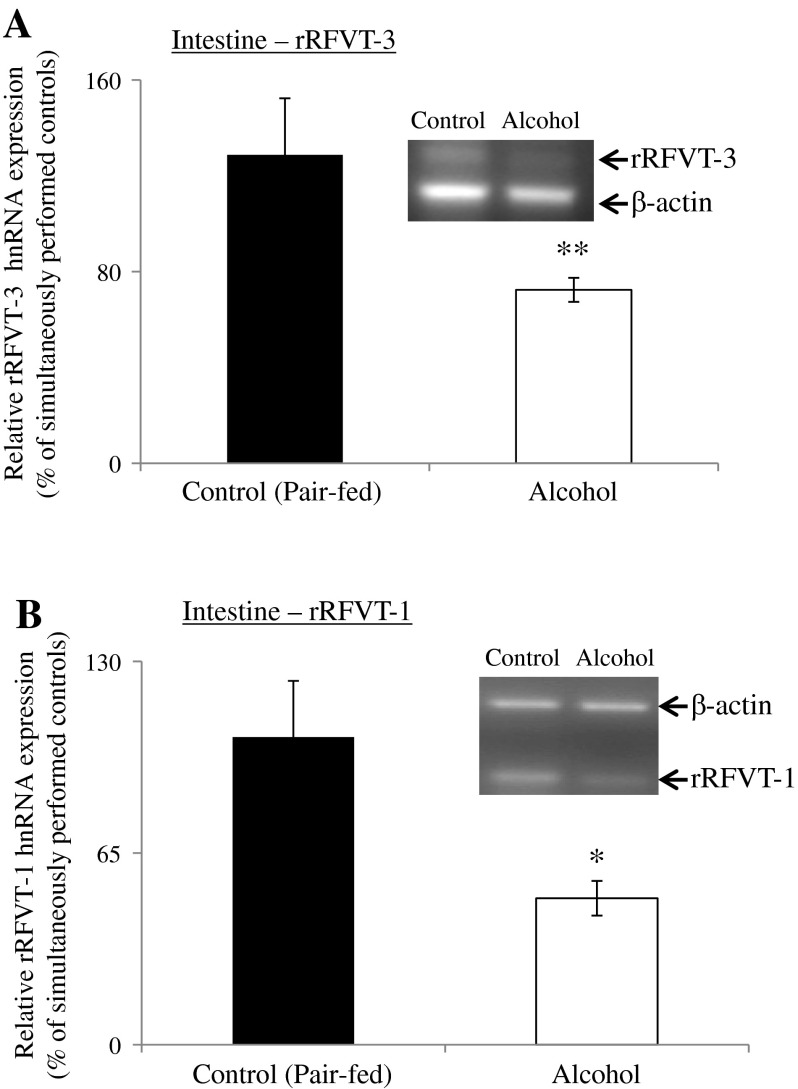
Because the level of hnRNA reflects the rate of transcription of the particular gene (a measure that has been used for such a purpose in a variety of investigations; see Refs. 1, 6, 13, and 37), we also examined the effect of chronic alcohol feeding on the level of expression of rRFVT-3 (Slc52a3) and rRFVT-1 (Slc52a1) hnRNA in rat jejunal mucosa. The results showed a significantly (P < 0.05 for RFVT-3 and P < 0.01 for RFVT-1) lower level of expression of the RFVT-3 and RFVT-1 hnRNA in alcohol-fed rats compared with their pair-fed controls (Fig. 4, A and B). These findings suggest that the inhibition in rat intestinal RF uptake by chronic alcohol feeding is, at least in part, being exerted at the level of transcription of the Slc52a3 and Slc52a1 genes.
Fig. 4.
Effect of chronic alcohol feeding of rats on RFVT-3 and RFVT-1 heterogeneous nuclear RNA (hnRNA) expression in rat jejunal mucosa. Semiquantitative RT-PCR was performed using rat RFVT-3, RFVT-1, and β-actin hnRNA gene-specific primers (Table 1). Data are means ± SE of 4–6 separate samples from 4–6 rats. *P < 0.01 and **P < 0.05.
Effect on RF uptake in rat colon (large intestine).
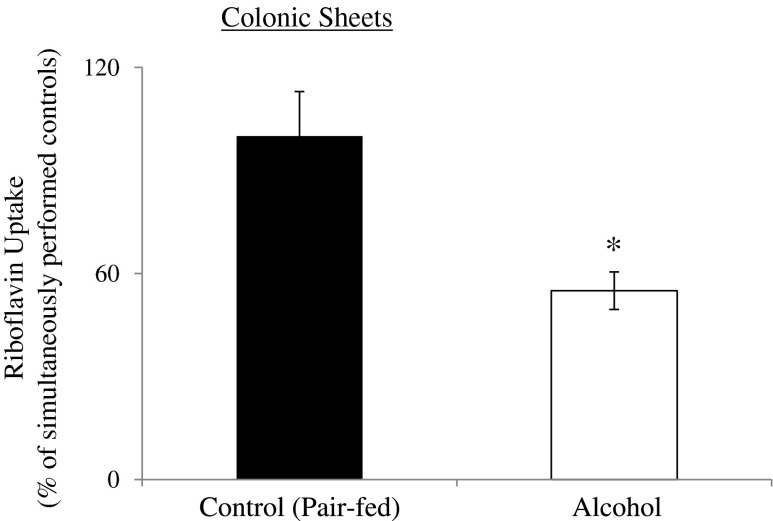
Previous studies have shown that the normal microflora of the large intestine synthesizes RF (11, 31) and that mammalian colonocytes have an efficient carrier-mediated mechanism for RF uptake via RF transporter (39). Therefore, we examined the effect of chronic alcohol feeding on the initial rate of carrier-mediated RF (24 nM) uptake by rat colon using colonic sheets. The results showed a significant (P < 0.01) inhibition in carrier-mediated RF uptake by colonic sheets from alcohol-fed rats compared with their pair-fed controls (Fig. 5). These results suggest that not only absorption of dietary RF in the small intestine is affected by chronic exposure to alcohol but also the absorption of the bacterially synthesized RF in the large intestine.
Fig. 5.
Effect of chronic alcohol feeding of rats on carrier-mediated RF uptake by colonic sheets. Colonic sheets were prepared from chronic alcohol-fed and their pair-fed control rats. The initial rate of carrier-mediated [3H]RF (24 nM) uptake was determined. Data are means ± SE of multiple uptake determinations from 6 rats. *P < 0.01.
Effect of Chronic Alcohol Feeding on Physiological and Molecular Parameters of the Renal RF Reabsorption Process
Effect on physiological parameters of RF transport across the BBM and BLM domains of the polarized rat renal epithelia.
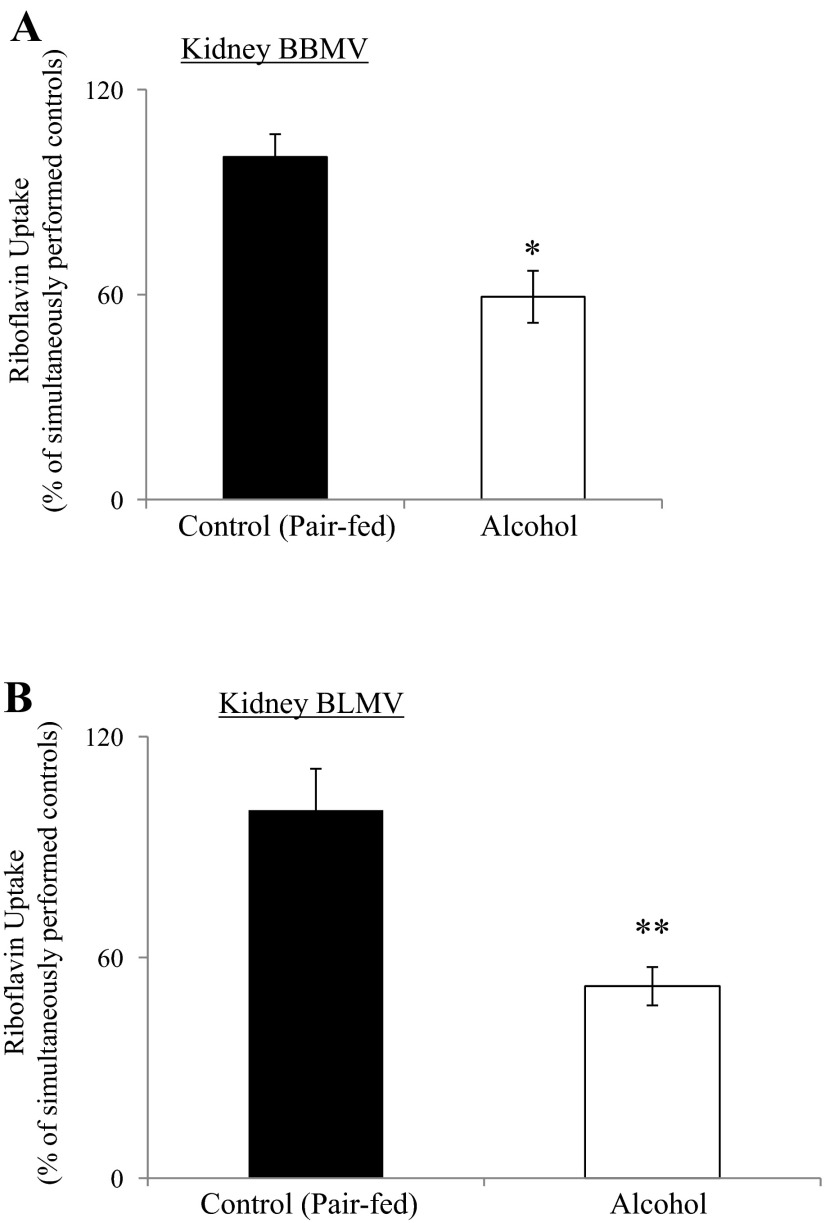
Figure 6A depicts the results on the effect of chronic alcohol feeding on carrier-mediated RF transport across rat renal BBM. The study was performed using purified renal BBMV. The results showed a significant (P < 0.01) inhibition of the initial rate of carrier-mediated RF (240 nM) uptake by vesicles isolated from rats fed alcohol chronically (4 wk) compared with those of pair-fed controls.
Fig. 6.
Effect of chronic alcohol feeding on carrier-mediated RF uptake by rat kidney BBMV and BLMV. BBMV (A) and BLMV (B) were isolated from the kidney cortex of rats chronically fed alcohol-liquid diet for 4 wk and their pair-fed controls. Vesicles were briefly incubated at room temperature and immediately used for uptake assay. [3H]RF (240 nM) was added to the vesicle-containing buffer at the time of the uptake experiment. Data are means ± SE of at least 8–11 separate uptake determinations from multiple sets of rats. *P < 0.01 and **P < 0.02.
We also examined the effect of chronic alcohol feeding on carrier-mediated RF transport across rat renal BLM using purified BLMV. The results again showed a significant (P < 0.02) inhibition of the initial rate of carrier-mediated RF (240 nM) uptake by jejunal BLMV of rats fed alcohol chronically for 4 wk compared with membrane vesicles isolated from pair-fed controls (Fig. 6B).
Effect on molecular parameters of renal RF transport.
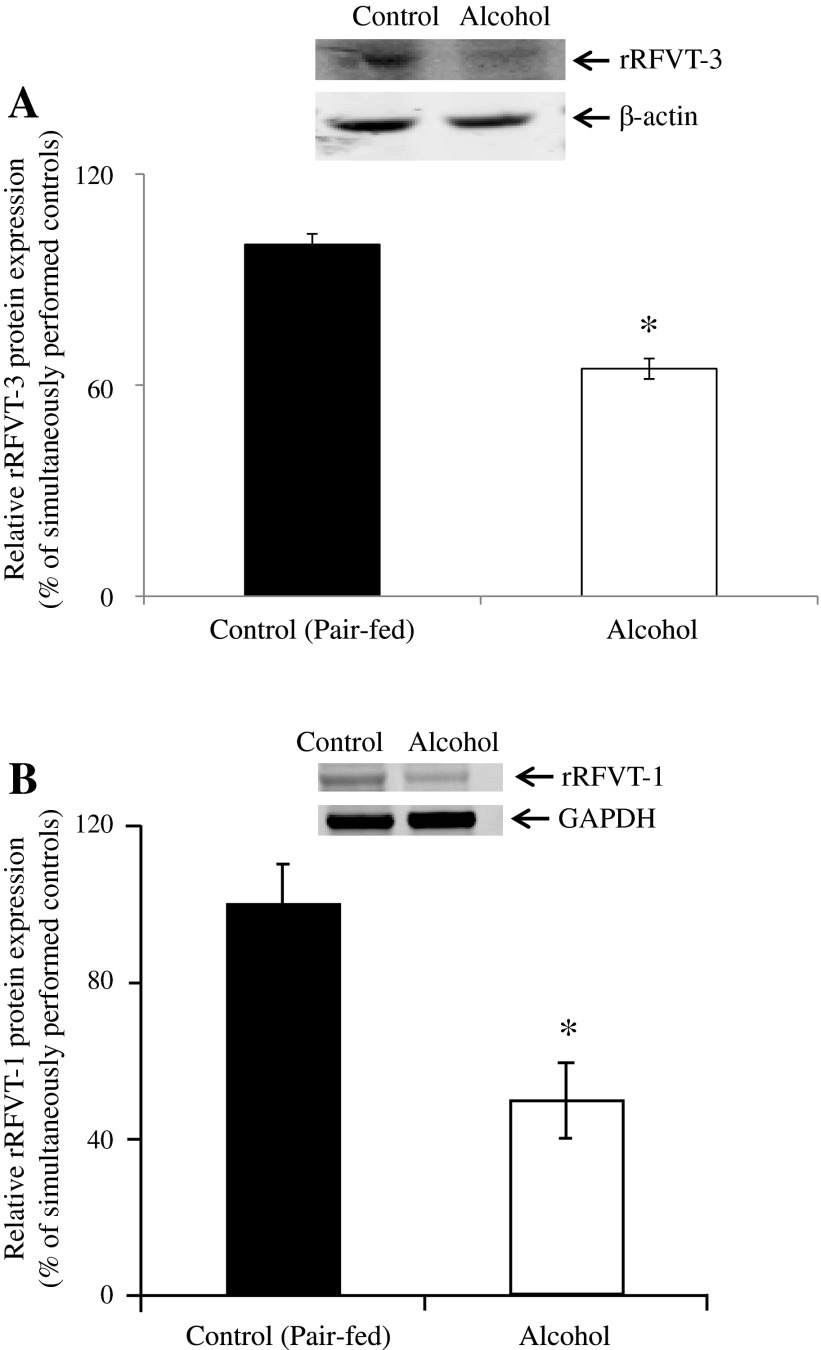
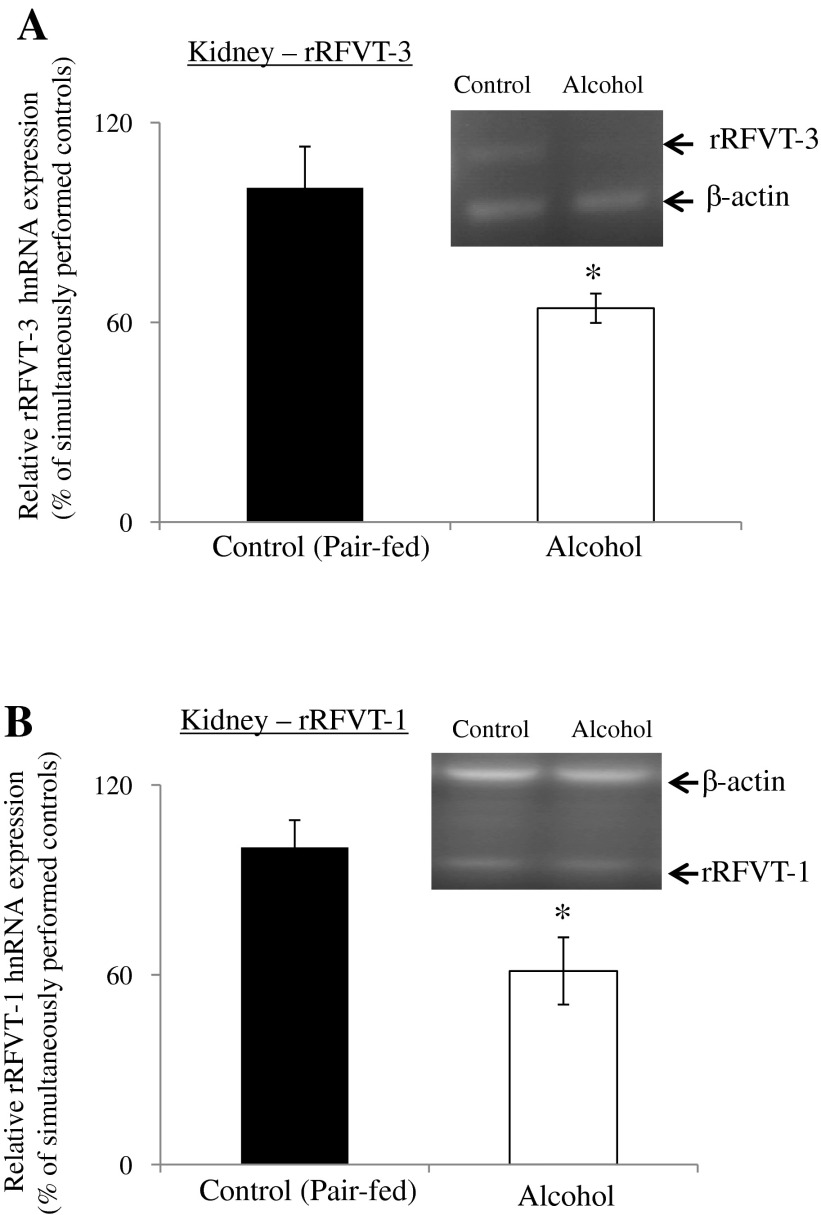
The effect of chronic alcohol feeding on the level of expression of the RFVT-3 and RFVT-1 proteins was examined by means of Western blotting using purified renal BBM and BLM preparations, respectively. The results showed a significantly (P < 0.01 for both) lower level of expression of rRFVT-3 and rRFVT-1 proteins in rats chronically fed alcohol compared with their pair-fed controls (Fig. 7).
Fig. 7.
Effect of chronic alcohol feeding of rats on RFVT-3 and RFVT-1 protein expression in kidney BBM and BLM domains. Kidney BBMV (A) and BLMV (B) proteins (80 μg) were isolated from chronic alcohol-fed rats (4 wk) and their pair-fed controls, and Western blotting was performed. BBMV and BLMV proteins were separated on NuPAGE 4–12% mini gel and electro blotted onto PVDF membrane. Blots were incubated with rabbit polyclonal anti-RFVT-3 and -RFVT-1 primary antibodies. Samples were normalized relative to β-actin or GAPDH protein expression. Data are means ± SE of 3 separate samples from multiple sets of rats. *P < 0.01.
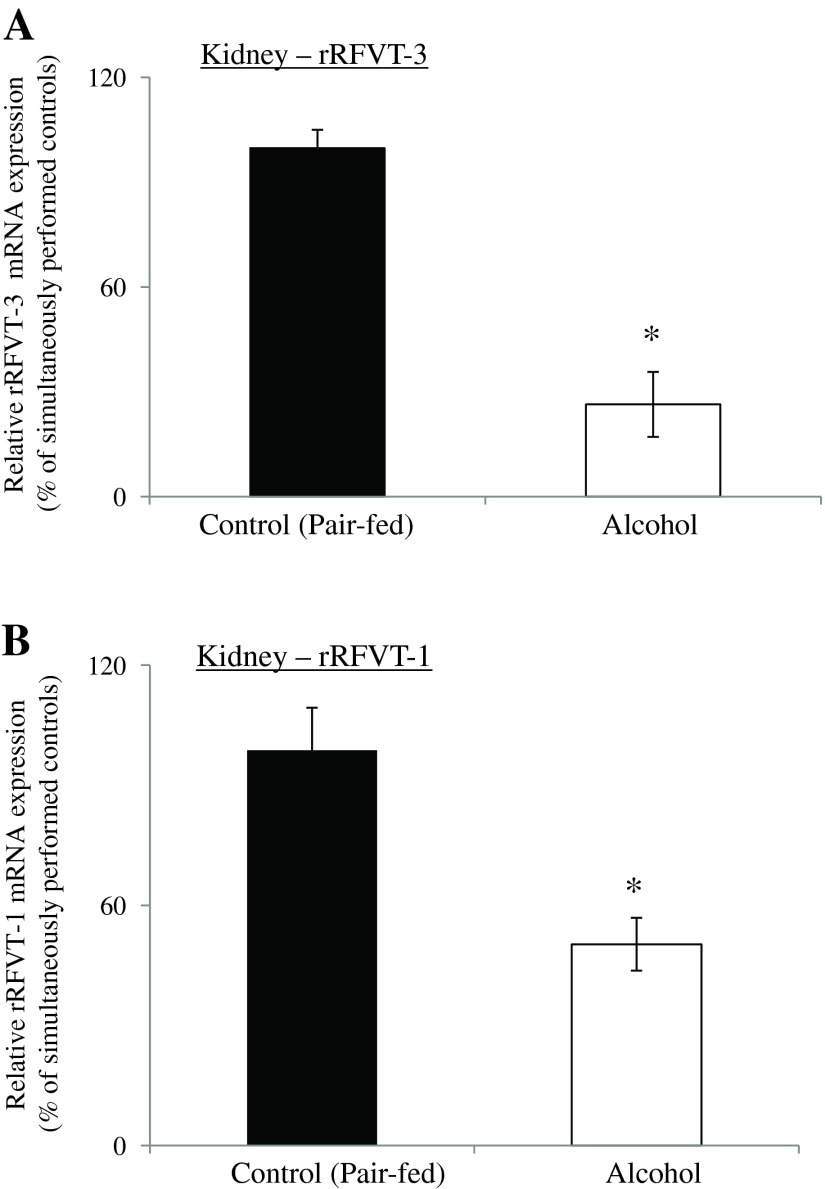
Figure 8 shows the effect of chronic alcohol feeding on the level of expression of rRFVT-3 and rRFVT-1 mRNA in rat kidney cortex. As can be seen, chronic alcohol feeding led to a significant (P < 0.01 for both) reduction in the level of expression of rRFVT-3 and rRFVT-1 mRNA in the kidney of alcohol-fed rats compared with their pair-fed controls (Fig. 8).
Fig. 8.
Effect of chronic alcohol feeding of rats on RFVT-3 and RFVT-1 mRNA expression in rat kidney cortex. Real-time PCR was performed using rRFVT-3 and rRFVT-1 gene-specific primers (Table 1) and normalized relative to rat β-actin. Data are means ± SE of 4 separate samples from 4 rats. *P < 0.01.
In other studies, we examined the effect of chronic alcohol feeding on the level of expression of rRFVT-3 (Slc52a3) and rRFVT-1 (Slc52a1) hnRNA in rat kidney cortex to determine if such exposure to ethanol affects the transcriptional level of the relevant genes. The results showed a significant (P < 0.03 for both) decrease in the level of expression of rRFVT-3 and rRFVT-1 hnRNA in the kidneys of alcohol-fed rats compared with pair-fed controls (Fig. 9, A and B, respectively). These results suggest that the inhibition in renal RF uptake by chronic alcohol feeding is (at least in part) being exerted at the level of transcription of the Slc52a3 and Slc52a1 genes.
Fig. 9.
Effect of chronic alcohol feeding of rats on RFVT-3 and RFVT-1 hnRNA expression in rat kidney cortex. Semiquantitative RT-PCR was performed using rRFVT-3, rRFVT-1, and rβ-actin hnRNA gene-specific primers (Table 1). Data are means ± SE of 4 separate samples from 4 rats. *P < 0.03.
DISCUSSION
Chronic alcohol consumption in humans is associated with a high prevalence of RF deficiency and suboptimal levels (4, 7, 9, 17, 22, 24, 27). Little is known on whether impairment in intestinal absorption and renal reabsorption processes of the vitamin contributes to the development of these abnormalities. Because the intestine is the site that controls the entry of RF into the body compartment, and the kidney is the site that controls the degree of its loss, a negative effect of chronic alcohol exposure on these two important steps may contribute to the development of RF deficiency and suboptimal levels in chronic alcoholism. We investigated this issue in the current study using a well-established alcohol-fed rat model by examining the effect of chronic alcohol feeding on physiological and molecular parameters of intestinal and renal RF transport events. Our results showed that chronic exposure to alcohol leads to impairments in physiological and molecular parameters of intestinal and colonic RF absorption processes. Similarly, RF transport across the renal BBM and BLM was also compromised.
Our findings show that chronic exposure to alcohol leads to significant inhibition in carrier-mediated RF uptake across both the intestinal BBM and the BLM domains. This inhibition was associated with a significant reduction in the level of expression of the predominant BBM RF transporter, i.e., rRFVT-3, and the BLM RF transporter, i.e., rRFVT-1, at the protein and mRNA levels in alcohol-fed rats compared with their pair-fed controls. The latter finding suggests possible involvement of transcriptional mechanism in mediating, at least part of, the inhibitory effect. In support of the latter suggestion was the finding that the level of expression of hnRNA (an indicator for gene transcription rate) Scl52a3 (the gene that encodes RFVT-3) and Slc52a1 (the gene that encodes RFVT-1) is also significantly inhibited upon chronic exposure of rats to alcohol.
Not only RF transport in the small intestine (site of absorption of dietary RF) is inhibited by chronic exposure to ethanol, but also transport of the vitamin in the colon (the site of absorption of the bacterially synthesized RF) (12, 34, 39) is similarly affected. These findings suggest that chronic alcohol exposure can compromise the bioavailability of RF from both of its exogenous sources (i.e., the dietary and the bacterial sources). It is relevant to mention here that, while the majority of ingested alcohol is absorbed in the proximal part of the gastrointestinal tract, alcohol could also reach the colon from the blood side (26). Also, colonic bacteria convert ethanol to its more toxic metabolite, acetaldehyde (26), and colonic mucosa has low levels of alcohol dehydrogenase (32). Thus, the observed inhibition in colonic RF uptake on chronic alcohol exposure could be due to the combined effects of alcohol and its metabolite, acetaldehyde.
As to RF transport across the renal BBM and BLM, chronic alcohol exposure was also found to lead to a significant inhibition in both of these transport events. This inhibition was again associated with a parallel reduction in expression of RFVT-1 and RFVT-3 at the protein, mRNA, and hnRNA levels, which again suggests that at least part of the inhibitory effect is mediated at the level of transcription of the Scl52a3 and Slc52a1 genes.
While our data suggest the involvement of transcriptional mechanism(s) in the inhibitory effect of chronic alcohol feeding on intestinal and renal RF transport, they do not exclude the possibility that part of the effect is also mediated via changes in RNA stability of the involved transporters; further studies are required to address the latter issue. Also, our findings on the inhibition in RF intestinal and renal transport events by chronic alcohol exposure are in contrast to the induction in the activity/expression of other transporters and channels (2, 14, 18, 46). In summary, our data in the current study show that chronic alcohol exposure leads to impairment in the two important steps involved in the regulation of normal RF body homeostasis, i.e., the entry step at the level of the intestine and the exit step at the level of the kidneys. Furthermore, this inhibition appears to be, at least in part, mediated via transcriptional mechanisms involving the Scl52a3 and Slc52a1 genes.
GRANTS
The study was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK-58057, DK-56061, and AA-018071).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.S.S. and H.M.S. conception and design of research; V.S.S., S.B.S., and A.G. performed experiments; V.S.S., S.B.S., A.G., and H.M.S. analyzed data; V.S.S., S.B.S., and H.M.S. interpreted results of experiments; V.S.S. and A.G. prepared figures; V.S.S. and H.M.S. drafted manuscript; V.S.S., S.B.S., A.G., and H.M.S. edited and revised manuscript; V.S.S., S.B.S., A.G., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increase ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139: 434–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao HF, Song JZ, Duke BJ, Ma HP, Denson DD. Ethanol stimulates epithelial sodium channels by elevating reactive oxygen species. Am J Physiol Cell Physiol 303: C1129–C1138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betz AL, Ren XD, Ennis SR, Hultquist DE. Riboflavin reduces edema in focal cerebral ischemia. Acta Neurochir Suppl (Wien) 60: 314–317, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bonjour JP. Vitamins and alcoholism. V Riboflavin, VI Niacin, VII Pantothenic acid, and VIII Biotin. Int J Vitam Nutr Res 50: 425–440, 1980 [PubMed] [Google Scholar]
- 5.Cooperman JM, Lopez R. Riboflavin. In: Handbook of Vitamins: Nutritional, Biochemical and Clinical Aspects, edited by Machlin LJ. New York, NY: Dekker, 1984, p. 299–327 [Google Scholar]
- 6.Elferink CJ, Reiners JJ., Jr Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcript run-on assay. Biotechniques 20: 47–477, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholic. I. Aetiological role of aneurin and other B-complex vitamins. Br Med J 2: 1290–1292, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimura M, Yamamoto S, Murata T, Yasujima T, Inoue K, Ohta KY, Yuasa H. Functional characteristics of the human ortholog of riboflavin transporter 2 and riboflavin-responsive expression of its rat ortholog in the small intestine indicate its involvement in riboflavin absorption. J Nutr 140: 1722–1727, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Hell D, Six P. Thiamin, riboflavin-und pyridoxine-versorgung bie chronicschem alkoholismus. Dtsch Med Wochenschr 102: 962–966, 1977 [DOI] [PubMed] [Google Scholar]
- 10.Hopfer U, Nelson K, Perrotto J, Isselbacher KJ. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem 248: 25–32, 1973 [PubMed] [Google Scholar]
- 11.Iinuma S. Synthesis of riboflavin by intestinal bacteria. J Vitaminol (Kyoto) 1: 6–13, 1955 [DOI] [PubMed] [Google Scholar]
- 12.Kasper H. Vitamin absorption in the colon. Am J Proctol 21: 341–345, 1970 [PubMed] [Google Scholar]
- 13.Kohler CU, Roos PH. Focus on the intermediate state: immature mRNA of cytochrome P450-methods and insights. Anal Bioanal Chem 392: 1109–1122, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kubisch CH, Gukovsky I, Lugea A, Pandol SJ, Kuick R, Misek DE, Hanash SM, Logsdon CD. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas 33: 68–76, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kumar CK, Yanagawa N, Ortiz A, Said HM. Mechanism and regulation of riboflavin uptake by human renal proximal tubule epithelial cell line HK-2. Am J Physiol Renal Physiol 274: F104–F110, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Law LK, Tang NL, Hui J, Fung SL, Ruiter J, Wanders RJ, Fok TF, Lam CW. Novel mutations in ETFDH gene in Chinese patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin Chim Acta 404: 95–99, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Leevy CM, Baker H. Vitamins and alcoholism. Am J Clin Nutr 21: 1325–1328, 1968 [DOI] [PubMed] [Google Scholar]
- 18.Li HS, Zhang JY, Thompson BS, Deng XY, Ford ME, Wood PG, Stolz DB, Eagon PK, Whitcomb DC. Rat mitochondrial ATP synthase ATP5G3:cloning and upregulation in pancreas after chronic ethanol feeding. Physiol Genomics 6: 91–98, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Liang WC, Ohkuma A, Hayashi YK, Lopez LC, Hirano M, Nonaka I, Noguchi S, Chen LH, Jong YJ, Nishino I. ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord 19: 212–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 21.Livek KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta DeltaC(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Majumdar SK, Shaw GK, Thomson AD. Vitamin utilization status in chronic alcoholics. Internat J Vit Nutr Res 51: 54–58, 1981 [PubMed] [Google Scholar]
- 23.Middleton HM. Uptake of riboflavin by rat intestinal mucosa in vitro. J Nutr 120: 588–593, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Neville JN, Eagles JA, Samson G, Olson RE. Nutritional status of alcoholics. Am J Clin Nutr 21: 1329–1340, 1968 [DOI] [PubMed] [Google Scholar]
- 25.Pangrekar J, Krishnaswamy K, Jagadeesan V. Effects of riboflavin deficiency and riboflavin administration on carcinogen-DNA binding. Food Chem Toxicol 31: 745–750, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Rajendram R, Preedy VR. Effect of alcohol consumption on the gut. Dig Dis 23: 214–221, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal WS, Adham NF, Lopez R, Cooperman JM. Riboflavin deficiency in complicated chronic alcoholism. Am J Clin Nutr 26: 858–860, 1973 [DOI] [PubMed] [Google Scholar]
- 28.Said HM, Arianas P. Transport of riboflavin in human intestinal brush border membrane vesicles. Gastroenterology 100: 82–88, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Said HM, Hollander D, Mohammadkhani R. Uptake of riboflavin by intestinal basolateral membrane vesicles: a specialized carrier-mediated process. Biochim Biophys Acta 1148: 263–268, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Said HM, Ma TY. Mechanism of riboflavine uptake by Caco-2 human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 266: G15–G21, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Said HM, Ross C. Riboflavin. In: Modern Nutrition in Health and Disease Modern Nutrition in Health and Disease (11th ed.), edited by Ross C, Caballero B, Cousins RJ, Tucker KL, Ziegler TR. 2011, p. 325–330 [Google Scholar]
- 32.Salaspuro M. Bacterioclonic pathway for ethanol oxidation: characteristics and implications. Ann Med 28: 195–200, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Scalera V, Storelli C, Storelli-Joss C, Haase W, Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J 186: 177–181, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorrell MF, Frank O, Thompson AD, Aquino A, Baker H. Absorption of vitamins from the large intestine. Nutr Res Int 3: 143–148, 1971 [Google Scholar]
- 35.Subramanian VS, Rapp L, Marchant JS, Said HM. Role of cysteine residues in cell surface expression of the human riboflavin transporter-2 (hRFT2) in intestinal epithelial cells,. Am J Physiol Gastrointest Liver Physiol 301: G100–G109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian VS, Subramanya SB, Rapp L, Marchant JS, Ma TY, Said HM. Differential expression of human riboflavin transporters-1, -2, and -3 in polarized epithelia: a key role for hRFT-2 in intestinal riboflavin uptake. Biochim Biophys Acta 1808: 3016–3021, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian VS, Ghosal A, Subramanya SB, Lytle C, Said HM. Differentiation-dependent regulation of intestinal vitamin B2 uptake: Studies utilizing human derived intestinal epithelial Caco-2 cells and native rat intestine. Am J Physiol Gastrointest Liver Physiol 304: G741–G748, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanya SB, Subramanian VS, Sekar VT, Said HM. Thiamin uptake by pancreatic cells: effect of chronic alcohol feeding/exposure. Am J Physiol Gastrointest Liver Physiol 301: G896–G904, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomie S, Yuasa H, Inoue K, Watanabe J. Transport functions of riboflavin carriers in the rat small intestine and colon: site difference and effects of tricyclic-type drugs. Drug Deliv 8: 119–124, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 290: 1571–1574, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem 145: 437–443, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Yanagawa N, Jo OD, Said HM. Riboflavin transport by rabbit renal brush border membrane vesicles. Biochim Biophys Acta 1330: 172–178, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Yanagawa N, Jo OD, Said HM. Riboflavin transport by rabbit renal basolateral membrane vesicles. Biochim Biophys Acta 1415: 56–62, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr 140: 1220–1226, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol 295: C632–C641, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Zinchuk V, Zinchuk O, Akimaru K, Moriya F, Okada T. Ethanol consumption alters expression and colocalization of bile salt export pump and multidrug resistance protein 2 in the rat. Histochem Cell Biol 127: 503–512, 2007 [DOI] [PubMed] [Google Scholar]