Abstract
Besides its quintessential role in reproduction, 17β-estradiol (E2) is a potent anorexigenic hormone. E2 and the selective Gq-coupled membrane estrogen receptor (Gq-mER) ligand STX rapidly increase membrane excitability in proopiomelanocortin (POMC) neurons by desensitizing the coupling of GABAB receptors to G protein-coupled inwardly rectifying K+ channels (GIRKs), which upon activation elicit a hyperpolarizing outward current. However, it is unknown whether E2 and STX can modulate GABAB signaling in neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons. We used single-cell RT-PCR and whole cell patch clamping with selective pharmacological reagents to show that NPY/AgRP cells of mice express the GABAB-R1 and -R2 receptors and are hyperpolarized by the GABAB agonist baclofen in an E2-dependent manner. In males, E2 rapidly attenuated the coupling of GABAB receptors to GIRKs, which was blocked by the general PI3K inhibitors wortmannin and LY-294002 or the selective p110β subunit inhibitor TGX-221. The ERα-selective agonist propyl pyrazole triol mimicked the effects of E2. STX, in contrast, enhanced the GABAB response in males, which was abrogated by the estrogen receptor (ER) antagonist ICI 182,780. In gonadectomized mice of both sexes, E2 enhanced or attenuated the GABAB response in different NPY/AgRP cells. Coperfusing wortmannin with E2 or simply applying STX always enhanced the GABAB response. Thus, in NPY/AgRP neurons, activation of the Gq-mER by E2 or STX enhances the GABAergic postsynaptic response, whereas activation of ERα by E2 attenuates it. These findings demonstrate a clear functional dichotomy of rapid E2 membrane-initiated signaling via ERα vs. Gq-mER in a CNS neuron vital for regulating energy homeostasis.
Keywords: NPY, AgRP, estradiol, feeding
two cell populations residing within the arcuate nucleus (ARC) of the hypothalamus compose a critical circuit for regulating energy homeostasis: neuropeptide Y (NPY)/agouti-related peptide (AgRP) and proopiomelanocortin (POMC) neurons (12). Selective stimulation of NPY/AgRP neurons via optogenetics evokes intense feeding (3), and ablation of these neurons in adults causes starvation, evidently due to loss of inhibitory signaling to the brain stem (24, 52, 53). Similar and other approaches have revealed that POMC cells control the exact opposite actions in energy balance (3, 13, 32, 39, 50, 54). These two populations of neurons are thought to regulate feeding through sensing blood-borne metabolic factors and then synaptically releasing their expressed peptides or derivatives into various brain regions such as the paraventricular nucleus and lateral hypothalamus (12).
Estrogens, such as 17β-estradiol (E2), regulate energy balance in females and exert potent anorectic effects, at least partly through activating POMC neurons (34). E2 increases the expression of POMC and its derived anorectic peptides (30, 44), and deleting the estrogen receptor (ER)α from POMC cells results in hyperphagia and weight gain in females (54). We have shown that E2 rapidly increases the membrane excitability of POMC cells through a novel membrane-associated estrogen receptor coupled via Gqα (Gq-mER) to a phospholipase C-protein kinase C-protein kinase A signaling pathway (21, 31, 32). This mER signaling pathway rapidly attenuates the coupling of GABAB and μ-opioid receptors to G protein-coupled inwardly-rectifying K+ channels (GIRKs), which upon activation elicit a robust, hyperpolarizing outward current (21, 31).
Conversely, E2 attenuates the orexigenic actions of NPY and suppresses its expression (9, 30, 40). In addition, E2 reduces secretion of the peptide from immortalized hypothalamic neurons (11). We have shown that E2 decreases the membrane excitability of NPY/AgRP neurons, at least partially, through increasing the expression of KCNQ5 channels, which augments the M-current (36). However, whether this anorectic steroid also modulates synaptic GABAB currents, as in POMC cells, remains unknown. We hypothesized that, if baclofen, a GABAB agonist, inhibits NPY/AgRP cells, then E2, potentially via the Gq-mER, would enhance the coupling of the GABAB receptor to GIRKs, thereby reducing membrane excitability and curbing food consumption. Here, we used single-cell RT-PCR and visualized whole cell patch clamping with selective pharmacological reagents to investigate these possibilities.
MATERIALS AND METHODS
Animal care.
All animal treatments complied with guidelines from the National Institutes of Health and received approval from the Institutional Animal Care and Use Committee at Oregon Health & Science University or Western University of Health Sciences. NPY-GFP transgenic mice (from Dr. Brad Lowell, Harvard University) (47) of either sex lived under controlled temperature (25°C) and photoperiod (12:12-h light-dark) at Oregon Health & Science University and received food and water ad libitum. Following gonadectomy under 2% isoflurane, mice received a dose of 4 mg/kg carprofen (Rimadyl; Pfizer Animal Health, New York, NY) and then recovered for 1 wk before experimentation.
We have shown previously (32) that the membrane-initiated signaling of E2 attenuated the GABAB-GIRK channel coupling in POMC neurons similarly in intact male and ovariectomized female mice. Therefore, we used the same models to study the pharmacology of the rapid E2-induced actions in NPY/AgRP neurons. Due to the heterogeneous effects of E2 on the GABAB response in gonadectomized animals (see results), we decided to use ovariectomized females to study the expression of the PI3K p110β isoform in individual NPY/AgRP neurons and also used these cells to ascertain GABAB-R1 and -R2 expression.
In previous studies, the Gq-mER ligand STX showed a clear anorectic effect in female guinea pigs (37, 50). Since our in vitro experiments suggested that STX would also be anorectic in males, we wanted to compare food intake in both sexes. Topeka guinea pigs (Elm Hill Breeding Labs, Chelmsford, MA) of either sex (n = 10 males, n = 12 females) lived under controlled temperature (21–23°C) and photoperiod (12:12-h light-dark) at Western University of Health Sciences and received food and water ad libitum. Following gonadectomy under ketamine-xylazine (33 and 6 mg/kg sc, respectively) supplemented with 2% isoflurane, guinea pigs recovered for 4–8 days before initiation of the feeding studies (see below).
Drugs.
E2 (Steraloids, Wilton, NH), the ER antagonist ICI 182,780 (Fulvestrant; Tocris, Minneapolis, MN), and the selective ERα agonist PPT (propyl pyrazole triol, Tocris) were dissolved in 100% ethanol (1, 10, and 1 mM stock, respectively). Tetrodotoxin (TTX; Na+ channel blocker; Alomone Labs, Jerusalem, Israel) and baclofen (GABAB agonist; Sigma, St. Louis, MO) were dissolved in water (1 and 40 mM stocks, respectively). Wortmannin (PI3K inhibitor, Sigma), LY-294002 (PI3K inhibitor; EMD Millipore, Billerica, MA), and TGX-221 [P13K p110β subunit inhibitor; 7-methyl-2-morpholino-9-(1-(phenylamino)ethyl)-4H-pyrido[1,2-a]pyrimidin-4-one; synthesized by Dr. Kevan Shokat, UC San Francisco] were dissolved in DMSO (1, 40, and 20 mM stocks, respectively).
STX {2-(4-hydroxyphenyl)-3-phenylpent-2-enoic acid [4-(2-dimethylaminoethoxy)phenyl]amide, E-enantiomer}, a Gq-mER selective ligand, was produced by AAPharmaSyn (Ann Arbor, MI) under contract according to the published synthetic protocol (Tobias et al., 2006). STX was dissolved in 100% ethanol (1 mM stock) or polypropylene glycol (PPG, 20 mg/ml) for in vitro and in vivo experiments, respectively.
Feeding experiments.
We chose to use guinea pigs for the feeding experiments on the basis of our previous publications that the diphenylacrylamide compound STX decreases food intake and weight gain following ovariectomy in females (32, 37, 50) and the fact that STX is bioavailable (F = 22%) and centrally active in the female guinea pig (35). For the feeding studies, a Comprehensive Lab Animal Monitoring Systems (CLAMS; Columbus Instruments, Columbus, OH) recorded daily food intake in adult gonadectomized male (n = 10) and female guinea pigs (n = 12) as previously described (35). Animals were given 3 days to acclimate to their CLAMS chambers and daily handling/weighing procedures. After acclimation, analysis of food intake under ad libitum conditions took place for 7 days. Each morning (0800), animals were weighed, injected with STX (3 mg sc) or vehicle (150 μl sc), and then returned to their CLAMS chambers.
Preparation of hypothalamic slices.
Following rapid decapitation of 2- to 3-mo-old mice, brains were extricated and placed in ice-cold, carbogenated (95% O2, 5% CO2) cutting solution containing (in mM) 2 KCl, 2 MgCl2, 1.2 NaH2PO4-H2O, 10 HEPES, 208 sucrose, 10 dextrose, 26 NaHCO3, 2 MgSO4, and 1 CaCl2. Hypothalamic blocks were dissected and secured within the vibratome well. Five 250 μM coronal slices from the block were cut and then placed in an auxiliary chamber with carbogenated artificial cerebral spinal fluid (aCSF) containing (in mM) 124 NaCl, 5 KCl, 1.2 NaH2PO4-H2O, 5 HEPES, 10 dextrose, 26 NaHCO3, 2 MgSO4 (or 2 MgCl2 if BaCl2 was applied in experiment), and 2 CaCl2.
Electrophysiology.
After at least 1 h of recovery following collection, the slices were transferred to a recording chamber on an Olympus BX51WI with infrared differential interference contrast imaging and fluorescence. Slices received continuous perfusion of warmed (35°C), carbogenated aCSF at a rate of 2 ml/min by peristaltic pump. Drugs were diluted from stock solutions with aCSF in open-top 60-ml syringes and perfused throughout the recording chamber by the same pump. All cells were perfused with 0.5 μM TTX 5 min before and then during recording.
Whole cell voltage (−50 mV) and current-clamp recording were acquired using 3- to 4-mΩ borosilicate pipettes (1.5 mm OD; World Precision Instruments, Sarasota, FL) filled with internal solution containing (in mM) 128 potassium gluconate, 10 NaCl, 2 MgCl, 10 HEPES, 11 EGTA, 3 ATP, and 0.25 GTP (pH 7.26, 296 mOSM). pCLAMP 9 software was used to acquire and analyze data from an Axopatch 200A amplifier linked to Digidata 1322A and Minidigi 1A digitizers (Molecular Devices, Sunnyvale, CA). Only stable cells showing <20% change in access resistance throughout the experiment were analyzed. The −10-mV liquid junction potential was corrected offline. Current-voltage relationships were established for each cell by stepping the cell from −50 to −110 mV in 10-mV increments. The slope conductance was calculated between −60 and −80 mV. Under basal conditions, the NPY/AgRP cells in this study showed a mean RMP of −51.1 ± 0.6 mV (n = 113) and average input resistance of 2.3 ± 0.2 GΩ (n = 116).
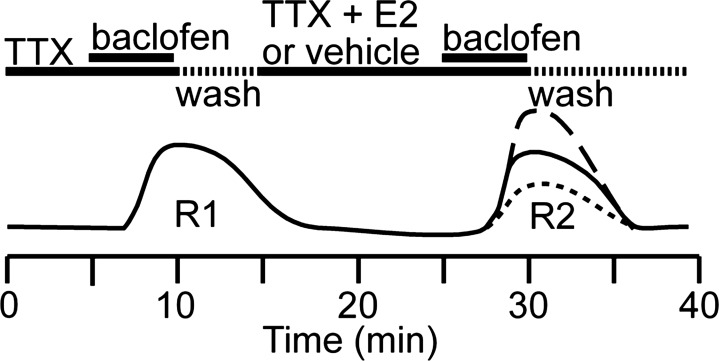
The protocol depicted in Fig. 1 was used to determine the effect of various pharmacological manipulations on coupling of GABAB receptors to GIRK channels: after obtaining a stable whole cell configuration, cells were perfused with 0.5 μM TTX for 5 min. Baclofen was then perfused at concentration of 10 μM to reach a steady-state outward current for the first response (R1). After washout of the drug, the current decreased to its resting level. Perfusion of vehicle or the selected treatment took place for 15 min, and then a second response to baclofen (10 μM) was elicited (R2). The magnitude (outward current) of each response was measured, and the effects were expressed as a percentage of R2/R1.
Fig. 1.
Depiction of the voltage-clamp protocol used to determine the effects of various pharmacological manipulations on the coupling of GABAB receptors to G protein-coupled inwardly rectifying K+ channels (GIRK) channels (see Electrophysiology in materials and methods).
NPY/AGRP cell harvesting and reverse transcription.
Single-cell harvesting took place as previously described (7, 55). Briefly, following microdissection from the slice, the ARC was incubated in aCSF containing protease (1 mg/ml) for 15–17 min at 37°C and then washed four times in low Ca2+ (1 mM) aCSF and two times in regular aCSF. Trituration of the tissue with flamed Pasteur pipettes of progressively smaller tip diameter allowed single cells to disperse onto a glass-bottomed 60-mm dish. Healthy cells adhered to glass after 12 min, and then unhealthy cells and debris were discarded with the continuous flow of carbogenated aCSF via peristaltic pump (2 ml/min).
Dispersed cells were patched with a Xenoworks microinjector system (Sutter Instruments, Navato, CA) using standard glass pipettes (1.5 mm OD/0.84 ID; World Precision Instruments, Sarasota, FL). Gentle negative pressure applied to the pipette allowed harvesting of cells with minimal collection of aCSF. The contents of the pipette were collected in 0.5-ml tubes containing 1× Invitrogen Superscript III Buffer, 15 U of RNasin (Promega), and 10 mM dithiothreitol (DTT) in 5 μl of diethylpyrocarbonate (DEPC)-treated water. Following collection, the cells were frozen on dry ice and later reverse-transcribed by adding dNTPs (0.5 mM, Promega), random primers (100 ng/tube, Promega), and anchored oligo(dT)20 primer (400 ng/tube, Invitrogen) to the tube. The cocktail was then heated to 65°C for 5 min and then cooled on ice for 5 min before addition of Superscript III reverse transcriptase (100 U/tube, Invitrogen), RNAsin (15 U), DTT (6 mM), and DEPC-treated water to a final volume of 20 μl. Reverse transcription (RT) followed accordingly: 25°C for 5 min, 50°C for 60 min, 70°C for 15 min, and 4°C for 5 min. aCSF collected near dispersed cells underwent RT as a negative control. Cells and tissue used for negative controls received the same processing as above, but without reverse transcriptase (−RT).
Primer design.
The NPY, GABAB-R1, and GABAB-R2 primer sequences were as previously published (36, 58). Mouse PI3K p110β primers (218 bp product; acc. no. NM_029094; forward primer, 812–833 nt; reverse primer 1010–1029 nt) were designed using the Clone Manager software (Sci Ed Software, Carry, NC). To avoid genomic DNA amplification, primer pairs crossed intron-exon boundaries.
Single-cell PCR.
Single cell PCR (SC-PCR) required 2–3 μl of cDNA template in a 30-μl PCR mix. Following established protocols (33, 56), 40 to 50 cycles of amplification took place with a C1000 Thermal Cycler (Bio-Rad, Hercules, CA). Ethidium bromide applied to a 2% agarose gel allowed visualization of PCR products, which were confirmed with sequencing.
Statistical analysis.
One-way ANOVA with Newman-Kuels post hoc test was used to compare groups for the electrophysiology experiments. A repeated-measures, multifactorial ANOVA followed by the least significant difference (LSD) test was used to compare groups for the in vivo feeding experiment. Results were considered significant if the probability of error was <5%. All data are reported as means ± SE.
RESULTS
NPY/AgRP cells respond to the GABAB agonist baclofen and express GABAB-R1 and -R2 mRNA.
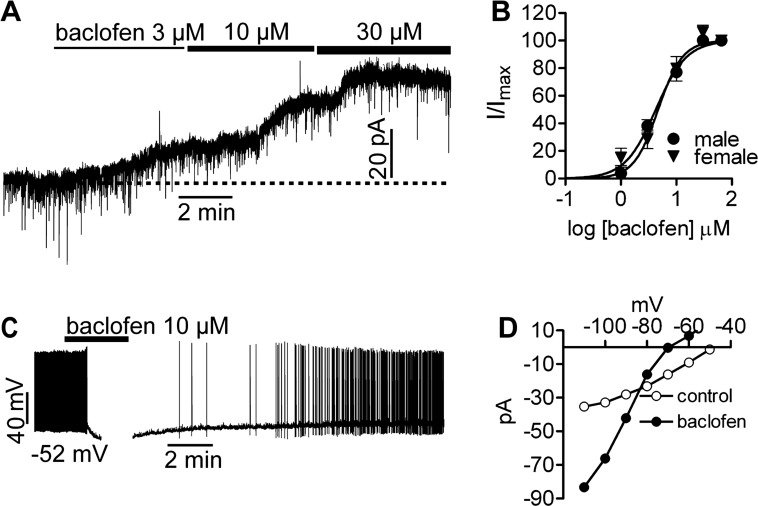
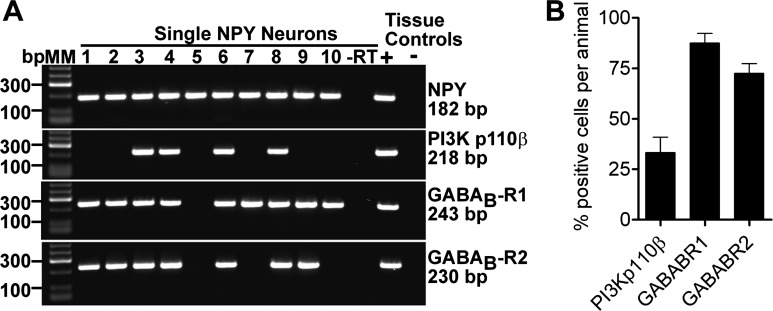
Since the effects of GABAB agonists such as baclofen have not been established in NPY/AgRP neurons, we measured the outward current (voltage clamp, −50 mV) in response to baclofen in the presence of TTX (0.5 μM; to block action potential-derived synaptic input). Virtually all NPY/AgRP neurons (n = 128) responded to baclofen. A cumulative dose response to the drug applied at 1, 3, 10, and 30 μM concentrations correspondingly generated a mean steady-state outward current of 4.0 ± 1.1 pA (n = 9), 8.4 ± 1.4 pA (n = 23), 14.6 ± 2.1 pA (n = 22), and 19.9 ± 3.5 pA (n = 11) in males and 5.8 ± 2.5 pA (n = 2), 7.7 ± 2.2 pA (n = 3), 21.6 ± 4.8 pA (n = 5), and 28.7 ± 5.9 pA (n = 7) in ovariectomized females (Fig. 2A). A few cells (n = 3) additionally received 65 μM baclofen to confirm that 30 μM elicited a maximum response. Normalizing these data to percent maximal outward current revealed that males and females shared a statistically equivalent EC50 of 4.3 and 4.7 μM, respectively (Fig. 2B). This is similar to the EC50 for baclofen-mediated inhibition of POMC neurons (31). In current clamp, 10 μM baclofen hyperpolarized NPY/AgRP cells (n = 11) by 16 ± 2 mV and inhibited firing (Fig. 2C). I-V relationships generated before and after application of the drug showed a reversal potential near EK+ (−83 mV) and a 54 ± 0.1% (n = 29) increase in slope conductance (Fig. 2D). BaCl2 (500 μM) abrogated the baclofen response (data not shown), which is consistent with the GABAB receptors activating GIRK channels (20, 28, 41). Single-cell RT-PCR on 40 neurons from four animals confirmed that 87.5 ± 4.8 and 72.5 ± 4.8% of NPY/AgRP neurons expressed GABAB-R1 and -R2, respectively (Fig. 3, A and B).
Fig. 2.
NPY/AgRP neurons respond to baclofen, a GABAB agonist. A: baclofen elicits a concentration-dependent outward current in NPY/AgRP neurons, reaching its maximum effectiveness at ∼30 μM. B: intact male and hypoestrogenic female NPY/AgRP neurons show a statistically similar EC50 to baclofen of 4.3 and 4.7 μM, respectively. C: baclofen reversibly hyperpolarizes NPY/AgRP cells, inhibiting firing. D: I-V relationships before and after application of 10 μM baclofen shows a reversal near the calculated EK.
Fig. 3.
Most ARC NPY/AgRP neurons express GABAB-R1 and -R2, whereas only about one-third express PI3K p110β. A: gel representing mRNA expression of PI3K p110β, GABAB-R1, and GABAB-R2 in individual NPY/AGRP neurons from an ovariectomized female mouse. B: bar graph summarizing average transcript expression level across 4 ovariectomized females. Ten cells per animal were assayed for the GABAB receptors, while 12–29 (average of 19) cells per animal were examined for PI3K p110β.
E2 attenuates but STX enhances GABAB signaling in male NPY/AgRP cells.
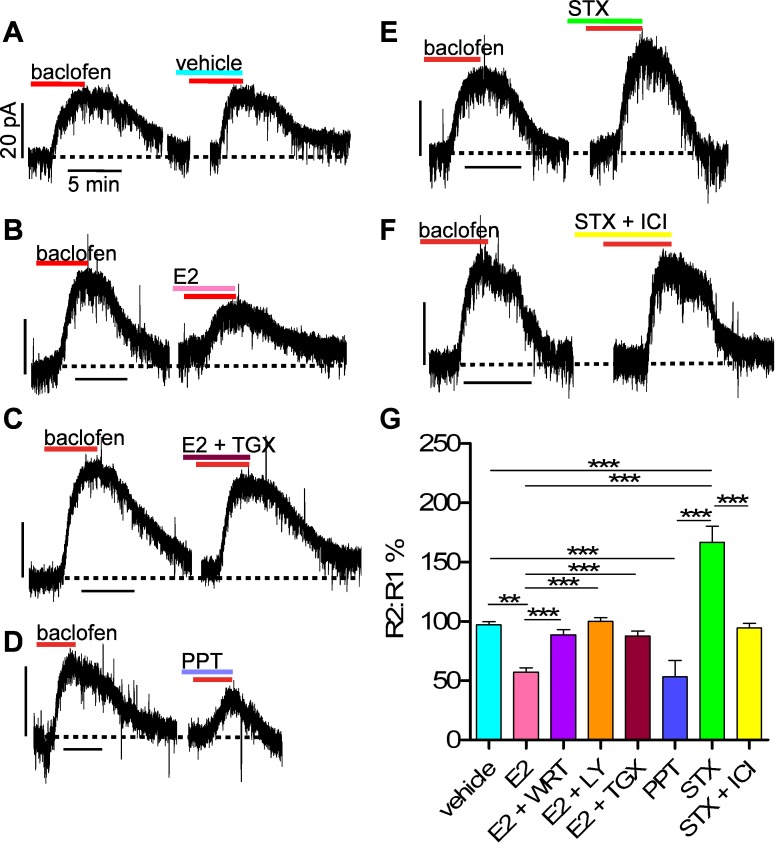
We used the whole cell voltage clamp protocol depicted in Fig. 1 to measure the rapid effects of E2 on GABAB coupling to GIRK channels in NPY/AgRP neurons, as previously described in POMC cells (31). We used a concentration of 10 μM baclofen to ensure a robust response. Under control conditions (no intervening drug) in intact males, 10 μM baclofen elicited a large response that subsided during a 20-min washout period, and a second, equal-amplitude response thereafter (n = 7; Fig. 4, A and G), suggesting that no rundown or desensitization occurred. Application of E2 (100 nM) during the washout period, which showed no effect on holding current, significantly attenuated the response (outward current) by 42.6 ± 3.5% (n = 8; P < 0.01; Fig. 4B). E2 did not alter reversal potential (EK+) for the GABAB-mediated effects (data not shown). We have previously shown that the EC50 for E2 in other ARC neurons is 7.5 nM, and the Ki for estrogen receptor antagonist ICI 182,780 is 0.3 nM (21). We opted to use a 100 nM concentration of E2 to promote more rapid pharmacokinetics in the slice (19, 27, 31). However, 10 nM E2 similarly attenuated the baclofen response by 52.0 ± 6.5% (n = 3).
Fig. 4.
The estrogen receptor (ER)α ligand propyl pyrazole triol (PPT) and the Gq-coupled membrane estrogen receptor (Gq-mER) ligand STX differentially modulate the GABAB response in NPY/AgRP cells from intact male mice. A–F: representative traces of GABAB responses before and after application of E2, PPT, or STX, with or without additional pharmacological manipulations (see below). Experiments were conducted as shown in Fig. 1. Dotted line represents baseline current. Vhold = −50 mV. Vertical scale bars represent 20 pA; all horizontal ones represent 5 min. For illustrative purposes, most of the 15-min vehicle or treatment period between GABAB responses (R1 and R2) is removed. Other small breaks in the recording signify removal of slightly prolonged return to baseline current following baclofen application. G: bar graphs summarizing effects of E2, STX, or PPT (all 100 nM) on the GABAB response (baclofen, 10 μM) in NPY/AgRP neurons from intact males. Baclofen elicited 2 equal-amplitude responses during perfusion of vehicle (n = 7), but E2 suppressed the response (n = 8). Coperfusing general PI3K inhibitors [wortmannin (WRT), 100 nM, n = 5; LY-294002 (LY), 10 μM, n = 4] or the p110β inhibitor TGX-221 (TGX, 11 nM, n = 6) with E2 reversed this effect. PPT mimicked the effects of E2 (n = 4). STX augmented the response (n = 5) but was rendered ineffective by coperfusing an ER antagonist [ICI 182,780 (ICI), 1 μM, n = 4]. **P < 0.01, ***P < 0.001, vs. vehicle control group.
We recently demonstrated that E2 may use a PI3K signaling pathway to attenuate the GABAB response in POMC neurons (26) and hypothesized that E2 may utilize the same pathway in NPY/AgRP neurons. Coperfusion of the general PI3K blockers wortmannin (100 nM; n = 5) or LY-294002 (10 μM; n = 6) with E2 attenuated the suppression of the response (Fig. 4G). Furthermore, on the basis of the recent work highlighting the specific roles of PI3K isoforms in energy balance (2), we found that TGX-221 (11 nM), a selective inhibitor of PI3K p110β, potently blocked the suppression of the response by E2 (n = 4; Fig. 4C). ERα has been shown to complex with PI3K in hypothalamic and cortical neurons (11, 43), so we decided to test specifically if the attenuation of the GABAB response could be mimicked by the ERα-selective agonist PPT (15). Indeed, PPT (100 nM) replicated the effects of E2 to attenuate the GABAB response in NPY/AgRP neurons by 46.7 ± 13.8% (n = 4, P < 0.001; Fig. 4D).
In contrast to the ERα agonist PPT, the Gq-mER ligand STX (100 nM), which shows an ∼1 million-fold reduced binding affinity for the nuclear ERs (α/β) (31, 32), enhanced the GABAB response by 66.7 ± 13.5% (n = 5, P < 0.001; Fig. 4E). Coperfusion of the ER antagonist ICI 182,780 (1 μM) abrogated the STX-mediated augmentation of the GABAB response (n = 4; Fig. 4F). These data suggest that STX acts specifically on the membrane ER (Gq-mER) that we previously showed modulates POMC neuronal excitability and energy homeostasis (21, 31, 32).
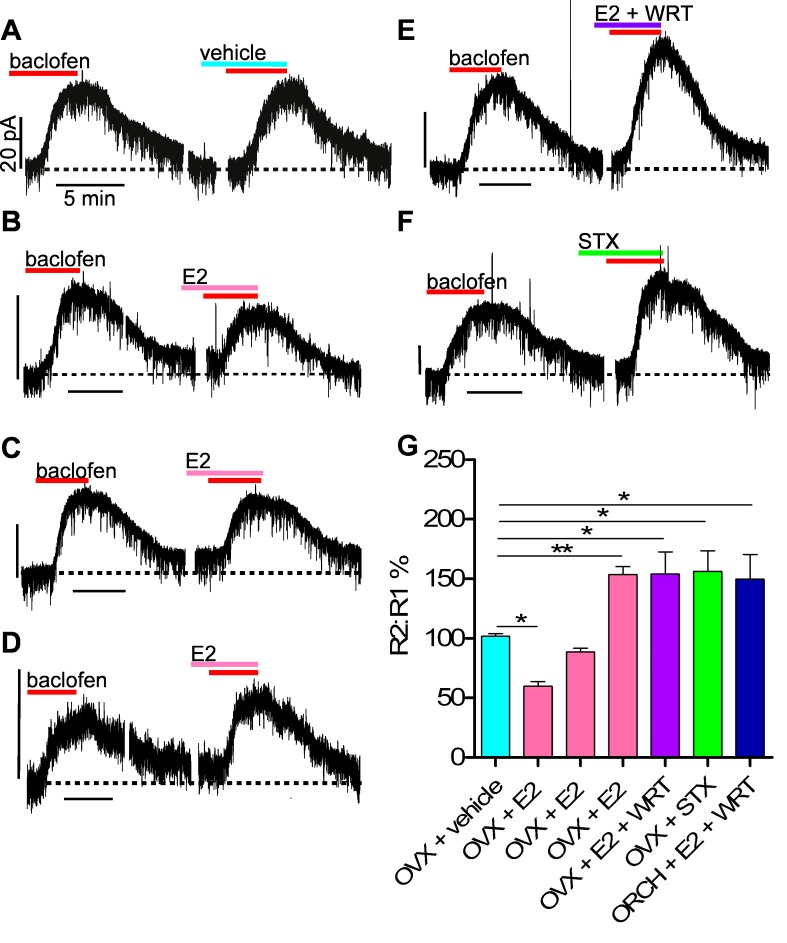
E2 and STX enhance GABAB signaling in NPY/AgRP cells from gonadectomized animals.
When we utilized our protocol (Fig. 1) in ovariectomized females, 10 μM baclofen elicited a robust response, which was consistently replicated following washout with vehicle (n = 4; Fig. 5, A and G). Application of E2 (100 nM) during the washout period, however, revealed three significantly different sets of responses compared with the control group. In 29% (7 of 24) of the neurons, E2 significantly reduced the amplitude of the response by 40.4 ± 3.7% (P < 0.05; Fig. 5B), whereas in 46% (11 of 24) of the neurons, E2 significantly enhanced the response by 53.5 ± 6.6% (P < 0.01; Fig. 5D). In the remaining 25% of the neurons (6 of 24), there was no net effect of E2 on the GABAB response (Fig. 5C). We believe the apparent lack of an effect in some NPY/AgRP neurons represents an equal balance between the ERα-mediated attenuation and the Gq-mER-mediated enhancement of the GABAB response (see discussion).
Fig. 5.
E2 and the Gq-mER ligand STX differentially modulate the GABAB response in NPY/AgRP cells from gonadectomized mice. A–F: representative traces of GABAB responses from ovariectomized (OVX) females before and after application of E2 or STX with or without additional pharmacological manipulations (see below). Experiments were conducted as shown in Fig. 1. Dotted line represents baseline current. Vhold = −50 mV. Vertical scale bars represent 20 pA; horizontal ones represent 5 min. For illustrative purposes, most of the 15-min treatment period between GABAB responses (R1 and R2) is removed. Other small breaks in the recording indicate removal of slightly prolonged return to baseline current levels following baclofen application. G: bar graphs summarizing effects of E2 and STX (both 100 nM) on the GABAB response (baclofen, 10 μM) in NPY/AgRP neurons. In OVX females, baclofen elicited 2 equal-amplitude responses during perfusion of vehicle (n = 4), but E2 suppressed (n = 7), enhanced (n = 11), or had no net effect (n = 6) on the GABAB response. Coperfusing a PI3K inhibitor (WRT, 100 nM, n = 6) with E2 or simply applying STX (n = 5) purely enhanced the response. E2 similarly affected the GABAB response in orchidectomized (ORCH) males (see results) and coperfusing wortmannin (n = 4) isolated the enhancement. *P < 0.05, **P < 0.01, vs. vehicle control group.
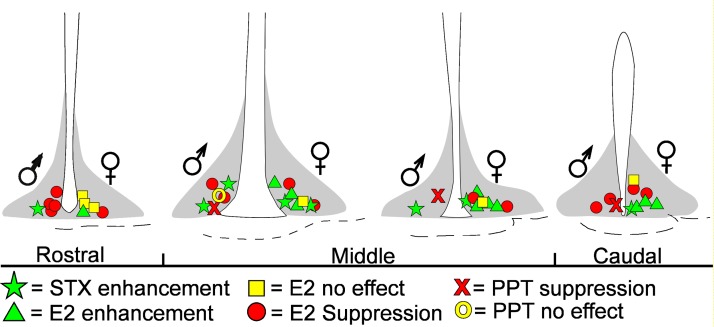
The different responses to E2 (enhancement, attenuation, no effect) showed no apparent anatomic segregation (Fig. 6), but coperfusing the PI3K blocker wortmannin (100 nM) with E2, which blocked the attenuation of the baclofen response by E2 in the male, isolated the enhancement of the GABAB response in female NPY/AgRP neurons (53.9 ± 18.7% increase; n = 4, P < 0.05; Fig. 5E). In support of the role of PI3K in mediating the attenuation of the GABAB-GIRK channel coupling, single-cell RT-PCR analysis revealed that 33.3 ± 7.6% of NPY/AgRP neurons (4 mice, 76 cells total) from ovariectomized females expressed PI3K p110β mRNA (Fig. 3, A and B).
Fig. 6.
Representative drawings of the 4 coronal slices through the mouse hypothalamus show the location of NPY/AgRP neurons that responded to E2, the ERα agonist PPT, or the Gq-mER ligand STX in intact males and OVX females. Left or right side of ventricle represents cell locations in males or females, respectively. Shaded area depicts ARC.
STX (100 nM), which selectively targets the Gq-mER, strictly enhanced the GABAB response in ovariectomized females by 59.7 ± 22.0% (n = 4, P < 0.05; Fig. 5F), similar to the males. A lower concentration of STX (10 nM) similarly enhanced the GABAB response (50.2 ± 4.7%, n = 3).
Interestingly, NPY/AgRP neurons from orchidectomized males responded to E2 in a similar manner to those from ovariectomized females (P = 0.76; Fisher exact probability test): in 42% (3 of 7) of the neurons, E2 attenuated the GABAB response by 35.5 ± 9.8%, whereas it enhanced the response by 84.0 ± 23.0% in 29% (2 of 7) of the neurons (data not shown). E2 showed no apparent effect in the remaining 29% (2 of 7) of cells. Coperfusing wortmannin with E2 isolated the enhancement of the baclofen response (49.7 ± 20.7% increase; n = 4, P < 0.05; Fig. 5G) in NPY/AgRP cells from orchidectomized males, similar to ovariectomized females.
STX application in vivo reduces food consumption.
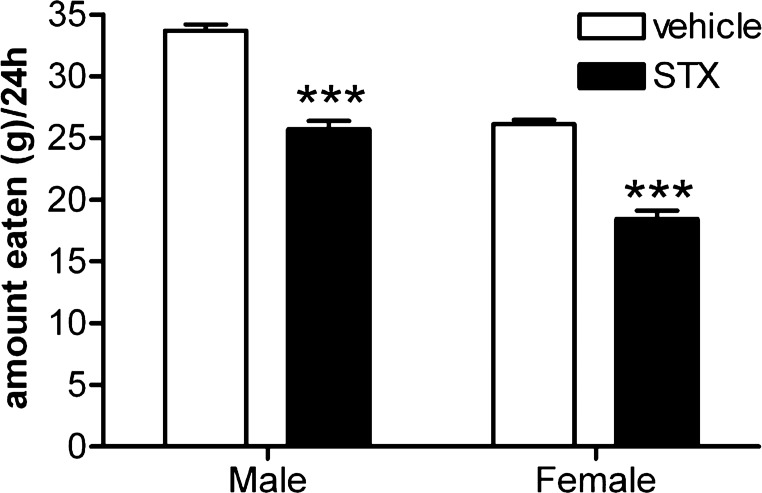
We have previously reported that STX reduces food consumption in female guinea pigs (35, 50). Here, we confirmed these findings and extended them to gonadectomized males. Daily subcutaneous injections of STX (at 0800) for 1 wk significantly reduced daily food consumption by greater than 20% in males and females (n = 5–7/group, P < 0.0001; F-value = 22.6; df = 1; Fig. 7). Although STX did not affect body weight over this short period of administration, our previous work showed that longer-term treatment significantly reduces post-ovariectomy weight gain in females (32, 37). These findings are consistent with the in vitro effects of STX on NPY/AgRP and POMC neurons (21, 31, 32).
Fig. 7.
STX treatment reduces food consumption in gonadectomized guinea pigs. Bar graph shows the average change in food consumption per day in male and female guinea pigs following daily injections with vehicle (n = 5 males, 5 females) or STX (n = 5 males, 7 females) for 1 wk. ***P < 0.0001 vs. vehicle control group. Partitioning these data into light and dark periods shows that STX-treated males consumed significantly less during the light period (P < 0.0001) but not the dark period; the STX-treated females, in contrast, consumed significantly less food during both periods (P < 0.0001).
DISCUSSION
We have shown for the first time that NPY/AgRP cells respond to the GABAB agonist baclofen and that E2 modulates this robust inhibitory response. Low concentrations of E2 (10 nM) suppressed GABAB signaling in all NPY/AgRP cells from intact male mice, whereas E2 suppressed the signaling in only about one-third of NPY/AgRP neurons from ovariectomized females and orchidectomized males. In contrast, the Gq-mER-selective ligand STX (10 nM) augmented GABAB signaling in both sexes by greater than 50% and curbed food consumption in guinea pigs. Based on our current findings in NPY/AgRP neurons and previous work in POMC neurons (21, 31, 32, 50), the Gq-mER regulates NPY/AgRP and POMC neurons in a reciprocal manner by enhancing or attenuating GABAB receptor coupling to GIRK channels, respectively.
GABAergic neurotransmission accounts for the vast majority of inhibitory synaptic activity in the hypothalamus (17, 46), and it is essential for some of the functions of NPY/AgRP neurons (45, 52). Deletion of GABA from NPY/AgRP neurons render the mice lean and resistant to obesity (45), and chronic infusion of a GABAA partial agonist into the parabrachial nucleus prevents starvation following NPY/AgRP cell ablation (52). Furthermore, important metabolic signals such as ghrelin and leptin require GABAergic transmission within the ARC for full efficacy (45, 48).
Although GABAergic synaptic activity and its role in the control of energy homeostasis have been extensively studied in POMC neurons (18, 21, 31, 32, 48), very little is known about this signaling in NPY/AgRP neurons. Here, we show for the first time that the GABAB agonist baclofen robustly hyperpolarizes NPY/AgRP cells, which corroborates the finding that most of these neurons express GABA-R1 and -R2 (Fig. 2). The reversal potential near EK, curvilinear I/V plot, and sensitivity to barium blockade suggest that the GABAB receptor is coupled to GIRK channels, as we have previously demonstrated for POMC and other hypothalamic neurons (22, 23, 49, 57).
Besides its critical role in reproduction, it has long been recognized that E2 is an anorexigenic hormone, based on the findings that E2 replacement attenuates post-ovariectomy weight gain in rodents through decreasing food intake and increasing energy expenditure (4, 10, 12, 29, 35, 54). These actions of E2 are thought to be mediated in large part by the transcriptional activity of ERα, since a loss-of-function mutation in the receptor is associated with hyperinsulinemia and obesity in humans (42), and globally deleting it causes obesity in mice (14, 16). Furthermore, conditional deletion of ERα in POMC neurons of female mice results in hyperphagia and weight gain (54), which is not surprising given that E2 regulates the expression of a plethora of genes associated with the control of energy homeostasis (25, 37).
We have identified a novel Gq-mER signaling pathway that is also important for the anorexigenic actions of E2 on POMC neurons (21, 31, 32). We have designed a Gq-mER-selective ligand, STX, that potently increases the membrane excitability in POMC cells through attenuating GABAB-GIRK coupling via a PLC-PKC-PKA signaling pathway (31) In addition, STX attenuates the presynaptic, CB1 receptor-mediated decrease in glutamate release, further enhancing the excitability of these neurons (50). STX predictably mimics the anorexigenic effects of E2 to reduce food intake and weight gain following ovariectomy (32, 37).
In contrast to its excitatory actions in POMC neurons, the “nonselective” E2 both enhanced and attenuated the GABAB receptor-GIRK channel coupling in NPY/AgRP cells of gonadectomized mice, whereas the selective Gq-mER ligand STX always enhanced the coupling. Moreover, in intact males, E2 and the selective ERα agonist PPT attenuated GABAB receptor-GIRK channel coupling, whereas STX enhanced the coupling in an ICI 182,780-sensitive manner. These data collectively suggest that E2 suppresses or augments GABAB-mediated currents in these orexigenic neurons through binding ERα or a putative Gq-mER, respectively. We previously have shown that NPY/AgRP neurons express ERα transcript and protein (36), and the cloning of the Gq-mER is a work in progress. Since the NPY/AgRP neurons that differentially responded to E2 showed no physiological (i.e., resting membrane potential or input resistance) or anatomic (Fig. 6) differences, we believe the direction of the response depends on the expression level of ERα vs. Gq-mER. Indeed, the Gq-mER ligand STX always enhanced the response to baclofen in both males and females. The present in vitro findings support our previous in vivo findings that STX, but not E2, treatment significantly decreases NPY mRNA expression in the ARC of ovariectomized female guinea pigs (37). Overall, E2 is clearly anorexigenic in females because of its pronounced excitatory effects on POMC neurons (13, 31, 32, 50).
The pathway by which E2-ERα suppresses GABAB signaling in NPY/AgRP neurons appears to require PI3K, specifically the catalytic p110β subunit. We previously reported that E2 also suppresses GABAB signaling in POMC neurons, in part via a PI3K signaling pathway (26). Interestingly, deleting p110β, but not p110α, in POMC cells results in diet-induced obesity, whereas mice with the same deletion in NPY/AgRP neurons resist obesity (2). These findings would support the argument that the p110β subunit plays a vital role in regulating energy homeostasis. Single-cell PCR verified that NPY/AgRP neurons from ovariectomized females expressed p110β mRNA (Fig. 3), and blockade of PI3K or selective blockade of the p110β catalytic subunit abrogated the inhibitory effects of E2 on GABAB receptor-GIRK channel coupling. Since the selective ERα agonist PPT mimicked the inhibitory effects of E2 on the coupling, presumably increasing membrane excitability, the PI3K signaling pathway may underlie the stimulatory effects of NPY on GnRH and LH secretion in females (1, 5). Indeed, NPY mRNA expression increases in the ARC at the time of the preovulatory LH surge in female rats (6). The Gq-mER signaling pathway in NPY/AgRP and POMC neurons may, therefore, be specific for the control of energy homeostasis, whereas the ERα-PI3K pathway in NPY/AgRP neurons may be exclusive for the reproductive pathway.
The anorexigenic actions of E2 are critical throughout the lifespan of women, who show increased risk for insulin resistance, central adiposity, and cardiovascular disease during menopause (8). Although E2 replacement (i.e., hormone replacement therapy, HRT) can help reverse these effects, HRT also increases the risk for cancer and stroke (38, 51). Selective activation of Gq-mER, on the other hand, elicits robust anorexigenic effects without the systemic risks associated with activating transcription factors ERα and ERβ (35).
Here, we have shown that E2 via a putative Gq-mER rapidly enhances the coupling of GABAB receptors to GIRK channels in NPY/AgRP neurons, thereby increasing the inhibitory tone in these orexigenic cells. Our previous work has shown that STX exerts the exact opposite effect on POMC neurons (31, 32), which serve an opposing role in controlling energy homeostasis. Thus, STX has the potential to significantly decrease the risk for insulin resistance and obesity in menopausal women without the peripheral side effects associated with traditional HRT.
GRANTS
The research reported in this publication was supported by National Institute of Health R01 grants NS-38809 (M. J. Kelly), NS-43330 (O. K. Rønnekleiv), DK-68098 (M. J. Kelly and O. K. Rønnekleiv), and DA-024314 (E. J. Wagner). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.W.S., E.J.W., O.K.R., and M.J.K. conception and design of research; A.W.S., M.A.B., and E.J.W. performed experiments; A.W.S., M.A.B., and E.J.W. analyzed data; A.W.S., E.J.W., O.K.R., and M.J.K. interpreted results of experiments; A.W.S. and M.A.B. prepared figures; A.W.S. drafted manuscript; A.W.S., E.J.W., O.K.R., and M.J.K. edited and revised manuscript; A.W.S., E.J.W., O.K.R., and M.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kevan M. Shokat (UC San Francisco) for supplying TGX-221. We also thank Drs. Casey Nestor and Mark Bailey for comments on the manuscript and acknowledge the technical support of Marina V. Rulevskaya and M. Rick Rollins.
REFERENCES
- 1.Acosta-Martinez M, Horton T, Levine JE. Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol Metab 18: 48–50, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJH, Batterham RL, Ashford MLJ, Vanhaesebroeck B, Withers DJ. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab 10: 343–354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42: 461–471, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bauer-Dantoin AC, McDonald JK, Levine JE. Neuropeptide Y potentiates luteinizing hormone (LH)-releasing hormone-stimulated LH surges in pentobarbital-blocked proestrous rats. Endocrinology 129: 402–408, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Bauer-Dantoin AC, Urban JH, Levine JE. Neuropeptide Y gene expression in the arcuate nucleus is increased during preovulatory luteinizing hormone surges. Endocrinology 131: 2953–2958, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Bosh M, Tonsfeldt K, Rønnekleiv O. mRNA expression of ion channels in GnRH neurons: Subtype-specific regulation by 17β-estradiol. Mol Cell Endocrinol 367: 85–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Crowley WR, Tessel RE, O'Donohue TL, Adler BA, Kalra SP. Effects of ovarian hormones on the concentrations of immunoreactive neuropeptide Y in discrete brain regions of the female rat: correlation with serum luteinizing hormone (LH) and median eminence LH-releasing hormone. Endocrinology 117: 1151–1155, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Czaja JA. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol Behav 33: 553–558, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-α in clonal, immortalized hypothalamic neurons. Int J Obes 35: 198–207, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 30: 367–398, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13: 89–94, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Geary N. Estradiol, CCK and satiation. Peptides 22: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha-and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 206: 13–22, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20: 68–100, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kelly MJ, Loose MD, Rønnekleiv OK. Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci 12: 2745–2750, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly M, Rønnekleiv O. Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature. Front Neuroendocrinol 33: 376–387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol (Lond) 401: 437–453, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol 51: 605–612, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology 64: 114–123, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Loose MD, Rønnekleiv OK, Kelly MJ. Neurons in the rat arcuate nucleus are hyperpolarized by GABAB and μ-opioid receptor agonists: Evidence for convergence at a ligand-gated potassium conductance. Neuroendocrinology 54: 537–544, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids 70: 397–406, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol 506: 895–911, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Micevych P, Kelly M. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology 96: 103–110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen JM, Quinn CC, Leach R, Findlay JBC, Boyett MR. Effect of extracellular cations on the inward rectifying K+ channels Kir2.1 and Kir.31/Kir.34. Exp Physiol 84: 471–488, 1999 [PubMed] [Google Scholar]
- 29.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J Clin Invest 121: 604–612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol 19: 426–431, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23: 9529–9540, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26: 5649–5655, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 152: 1503–1514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roepke TA. Oestrogen modulates hypothalmic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol 21: 141–150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlová-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 151: 4926–4937, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roepke TA, Smith AW, Ronnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci 31: 11825–11835, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology 149: 6113–6124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA 288: 321–333, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Rother E, Belgardt BF, Tsaousidou E, Hampel B, Waisman A, Myers MG, Jr, Bruning JC. Acute selective ablation of rat insulin promoter-expressing (RIPHER) neurons defines their orexigenic nature. Proc Natl Acad Sci USA 109: 18132–18137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res 191: 173–177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slugg RM, Zheng SX, Fang Y, Kelly MJ, Rønnekleiv OK. Baclofen inhibits guinea pig magnocellular neurones via activation of an inwardly-rectifying K+ conductance. J Physiol (Lond) 551: 295–308, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331, No.16: 1056–1061, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Spampinato S, Merlo S, Molinaro G, Battaglia G, Bruno V, Nicoletti F, Sortino M. Dual effect of 17β-estradiol on NMDA-induced neuronal death: involvement of metabotropic glutamate receptor 1. Endocrinology 153: 5940–5948, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol 341: 68–77, 1994 [DOI] [PubMed] [Google Scholar]
- 44a.Tobias SC, Qiu J, Kelly MJ, Scanlan TS. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. Chem Med Chem 1: 565–571, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11: 998–1000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van den Pol AN. Weighing the role of hypothalamic feeding neurotransmitters. Neuron 40: 1059–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing stong Renilla green fluorescent protein in NPY neurons. J Neurosci 29: 4622–4639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71: 142–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner EJ, Bosch MA, Kelly MJ, Rønnekleiv OK. A powerful GABAB receptor-mediated inhibition of GABAergic neurons in the arcuate nucleus. NeuroReport 10: 2681–2687, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Washburn N, Borqquist A, Wang K, Jeffery G, Kelly M, Wagner EJ. Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology 97: 160–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA 289: 2673–2684, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137: 1225–1234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci USA 105: 2687–2692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Y, Nedugadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14: 453–465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27: 10153–10164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29: 10552–10562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. GABAB receptor mediated inhibition of GnRH neurons is suppressed by kisspeptin-GPR54 signaling. Endocrinology 150: 2388–2394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci 29: 13823–13836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]