Abstract
We used the perforated patch-clamp technique at 37°C to investigate the mechanisms underlying the activation of a transient large-conductance K+ (tBK) current in rabbit urethral smooth muscle cells. The tBK current required an elevation of intracellular Ca2+, resulting from ryanodine receptor (RyR) activation via Ca2+-induced Ca2+ release, triggered by Ca2+ influx through L-type Ca2+ (CaV) channels. Carbachol inhibited tBK current by reducing Ca2+ influx and Ca2+ release and altered the shape of spike complexes recorded under current-clamp conditions. The tBK currents were blocked by iberiotoxin and penitrem A (300 and 100 nM, respectively) and were also inhibited when external Ca2+ was removed or the CaV channel inhibitors nifedipine (10 μM) and Cd2+ (100 μM) were applied. The tBK current was inhibited by caffeine (10 mM), ryanodine (30 μM), and tetracaine (100 μM), suggesting that RyR-mediated Ca2+ release contributed to the activation of the tBK current. When IP3 receptors (IP3Rs) were blocked with 2-aminoethoxydiphenyl borate (2-APB, 100 μM), the amplitude of the tBK current was not reduced. However, when Ca2+ release via IP3Rs was evoked with phenylephrine (1 μM) or carbachol (1 μM), the tBK current was inhibited. The effect of carbachol was abolished when IP3Rs were blocked with 2-APB or by inhibition of muscarinic receptors with the M3 receptor antagonist 4-diphenylacetoxy-N-methylpiperidine methiodide (1 μM). Under current-clamp conditions, bursts of action potentials could be evoked with depolarizing current injection. Carbachol reduced the number and amplitude of spikes in each burst, and these effects were reduced in the presence of 2-APB. In the presence of ryanodine, the number and amplitude of spikes were also reduced, and carbachol was without further effect. These data suggest that IP3-generating agonists can modulate the electrical activity of rabbit urethral smooth muscle cells and may contribute to the effects of neurotransmitters on urethral tone.
Keywords: urethra, BK current, cholinergic, smooth muscle
although substantial evidence indicates that urethral smooth muscle (USM) plays an important role in the urinary continence mechanism of mammals (for review see Ref. 7), few studies have examined how the different ionic currents in USM contribute to the action potential (AP) or assessed if they are modified by excitatory neurotransmitters. We previously reported that freshly isolated USM cells generate APs in response to brief depolarizing current injection (20, 28) and demonstrated the presence of large-conductance, Ca2+-activated K+ (BKCa) currents and voltage-gated K+ (Kv2.1) currents in these cells. Kyle et al. (20) showed that most of the outward current in rabbit USM cells (RUSMCs) is due to the large-conductance K+ (BK) current and that the BK current appears to play the major role in limiting smooth muscle AP amplitude and duration.
Interestingly, this BK current has transient and sustained components and, thus, resembles the BK current in the bladder and USM of other species (17, 18), and we refer to the initial component as the transient BK (tBK) current. The first objective of this study was to examine the contribution of Ca2+ influx and Ca2+ release from internal stores via ryanodine receptors (RyRs) and inositol trisphosphate (IP3) receptors (IP3Rs) to activation of the tBK current. The second objective was to examine if IP3-generating agonists, such as phenylephrine and carbachol, altered tBK currents in USM cells, since little is known about the effects of excitatory neurotransmitters on isolated USM cells (1, 7, 31). Given that the tBK current contributes significantly to the shape of the USM AP (20), we also examined the effects of carbachol on bursts of APs recorded under current-clamp conditions to examine how IP3-generating agonists altered electrical activity in these cells.
MATERIALS AND METHODS
All procedures were carried out in accordance with European Union legislation and with approval of the Dundalk Institute of Technology Animal Care and Use Committee. Male and female New Zealand white rabbits (16–20 wk old) were humanely killed with a lethal injection of pentobarbitone (120 mg/kg iv).
Cell isolation.
The most proximal 1.5 cm of the urethra was removed and placed in Krebs solution. Strips (0.5 cm wide) of proximal urethra were cut into 1-mm3 pieces and stored in Hanks' Ca2+-free solution for 30 min and then incubated in dispersal medium containing (per 5 ml of Ca2+-free Hanks' solution; see Solutions) 15 mg of collagenase (type IA, Sigma), 1 mg of proteinase (type XXIV, Sigma), 10 mg of bovine serum albumin (Sigma), and 10 mg of trypsin inhibitor (Sigma) for 10–15 min at 37°C. Tissue was then transferred to Ca2+-free Hanks' solution and stirred for 10–15 min to release single smooth muscle cells. These cells were plated in petri dishes containing 100 μM Ca2+-Hanks' solution and stored at 4°C for use within 8 h.
Patch-clamp recordings.
Currents from RUSMCs were recorded with the perforated-patch configuration of the whole cell patch-clamp technique. The cell membrane was perforated using the antibiotic amphotericin B (600 μg/ml). Patch pipettes were initially front-filled by dipping into pipette solution and then back-filled with the amphotericin B-containing solution. Pipettes were pulled from borosilicate glass capillary tubing (1.5 mm OD, 1.17 mm ID; Clark Medical Instruments) to a tip diameter of ∼1–1.5 μm and resistance of 2–4 MΩ. Voltage-clamp commands were delivered via a patch-clamp amplifier (Axopatch 1D, Molecular Devices) connected to a Digidata 1322A AD/DA converter (Molecular Devices) interfaced to a computer running pClamp software (Molecular Devices).
Solutions.
The following solutions were used: Hanks' solution (in mM: 129.8 Na+, 5.8 K+, 135 Cl−, 4.17 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 1.8 Ca2+, 0.9 Mg2+, 0.4 SO42−, 10 glucose, 2.9 sucrose, and 10 HEPES, with pH adjusted to 7.4 with NaOH); Ca2+-free Hanks' perfusate solution (in mM: 129.8 Na+, 5.8 K+, 135 Cl−, 4.17 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 2.7 Mg2+, 0.4 SO42−, 10 glucose, 2.9 sucrose, 5 EGTA, and 10 HEPES, with pH adjusted to 7.4 with NaOH); Ca2+-free Hanks' cell dispersal solution (in mM: 141.2 Na+, 130.4 Cl−, 5.8 K+, 15.5 HCO3−, 0.44 H2PO4−, 0.33 HPO42−, 10 glucose, 2.9 sucrose, and 10 HEPES, with pH adjusted to 7.4 with NaOH); K+ pipette (perforated-patch) solution (in mM: 133 K+, 135 Cl−, 1 Mg2+, 0.5 EGTA, and 10 HEPES, with pH adjusted to 7.2 with KOH); and Cs+ pipette (perforated-patch) solution (in mM: 133 Cs+, 135 Cl−, 1.0 Mg2+, 0.5 EGTA, and 10 HEPES, with pH adjusted to 7.2 with CsOH).
During experiments, the dish containing the cells was superfused with Hanks' solution. In addition, the cell under study was continuously superfused with Hanks' solution by means of a close delivery system consisting of a pipette (200-μm tip diameter) placed ∼300 μm away. This could be switched, with a dead-space time of ∼5 s, to a solution containing a drug. All experiments were carried out at 36 ± 1°C.
Statistics.
Experiments on freshly dispersed RUSMCs were carried out on a minimum of three animals. In all experiments, n refers to the number of cells studied. Summary data are presented as means ± SE. Statistical comparisons were made on raw data using Student's paired t-test or ANOVA as appropriate; P < 0.05 was considered significant.
Drugs.
All drugs were made up in the appropriate stock solution and then diluted to their final concentrations in Hanks' solution. The drugs and their solvents (in parentheses) are as follows: amphotericin B (DMSO), penitrem A (DMSO), 4-diphenylacetoxy-N-methylpiperidine methiodide [4-DAMP (DMSO)], methoctramine (H2O), Cd2+ (H2O), carbachol (H2O), phenylephrine (H2O), prazosin (H2O), 2-aminoethoxydiphenyl borate [2-APB (ethanol)], and nifedipine (ethanol), obtained from Sigma; iberiotoxin (H2O), obtained from Tocris; and ryanodine (ethanol), obtained from Abcam and Tocris. Caffeine, obtained from Sigma, was added directly to Hanks' solution to give a 10 mM final concentration.
RESULTS
With use of our dispersal procedure, relaxed RUSMCs and interstitial cells of Cajal (ICCs) could be reliably isolated from the rabbit urethra. RUSMCs were easily distinguished from ICCs, as they were unbranched and spindle-shaped and contracted in response to depolarization or agonist application.
Identification of tBK currents in RUSMCs.
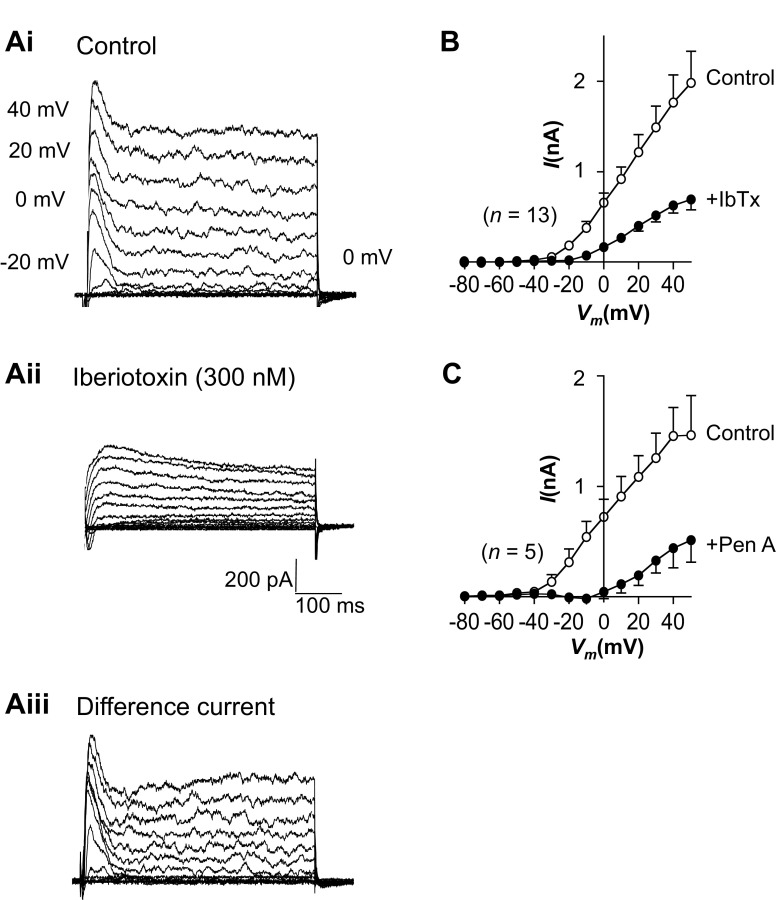
Currents were recorded under voltage-clamp conditions with use of the perforated-patch configuration of the patch-clamp technique. When held at −60 mV, the RUSMCs were electrically quiescent, as demonstrated previously (28). Cells were depolarized from −80 to +40 mV in 10-mV increments for 500 ms, and families of currents consisting of transient and sustained components were recorded at potentials positive to −30 mV, as shown in Fig. 1Ai. We first examined the effects of the selective BKCa channel toxin iberiotoxin (IbTx, 300 nM) to determine if BKCa channels contributed to these currents. As illustrated in Fig. 1Aii, IbTx reduced transient and sustained components of outward currents to leave a more slowly activating outward current. The biphasic nature of the BK currents can be clearly observed in Fig. 1Aiii, in which the IbTx-sensitive difference currents were obtained by digital subtraction of the recordings obtained in the presence of IbTx (Fig. 1Aii) from the control recordings (Fig. 1Ai). Figure 1B shows a summary from 13 similar experiments in which the peak current (obtained in the first 50 ms of the voltage step) was plotted against voltage in the absence and presence of IbTx. These data suggested that most of outward current in RUSMCs was BK current. Consistent with this, we found that another BK channel blocker, penitrem A (100 nM), produced effects very similar to those of IbTx in five cells, as shown in Fig. 1C.
Fig. 1.
Transient current in rabbit urethral smooth muscle cells is large-conductance K+ (BK) current. Ai and Aii: families of currents evoked from a holding potential of −80 mV to potentials up to +50 mV in the absence (control) and presence of 300 nM iberiotoxin (IbTx). Aiii: transient and sustained components of the IbTx-sensitive difference currents. B and C: summary current-voltage (I-Vm) relationships for the peak large-conductance K+ current in the absence (control) and presence of IbTx (n = 13) or penitrem A (PenA, n = 5).
These data confirm that 1) most of the outward current at physiological potentials is carried by BK channels and 2) transient and sustained components of outward current are carried through BK channels.
Contribution of external Ca2+ to activation of outward currents.
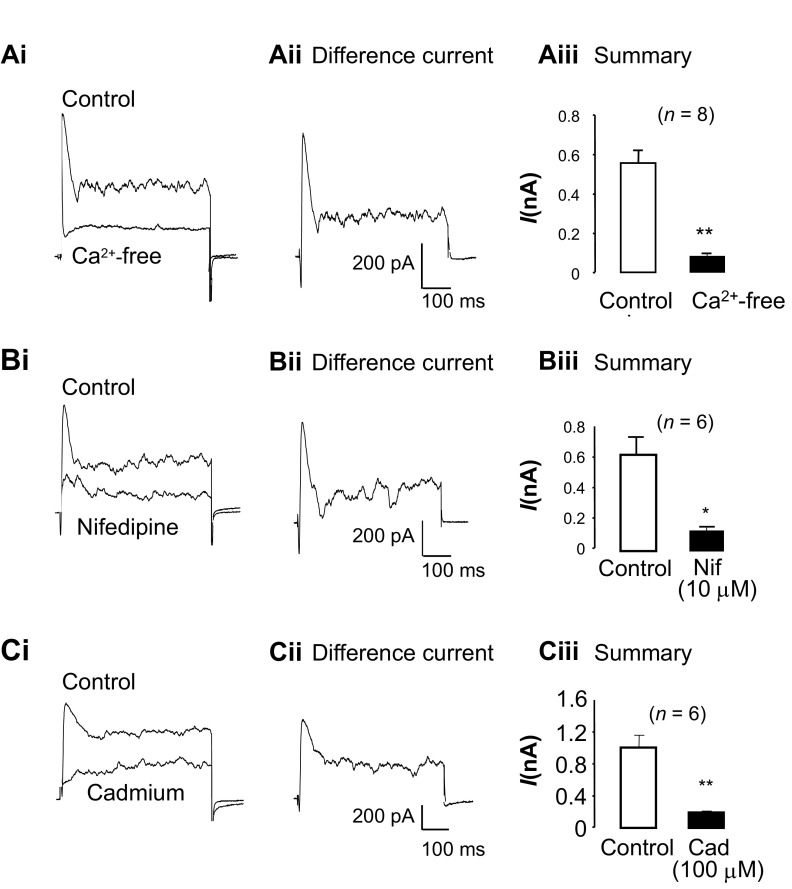
We first examined if Ca2+ influx was essential for activation of the tBK current by isosmotically substituting extracellular Ca2+ with Mg2+. As shown in Fig. 2Ai, Ca2+ removal abolished the tBK current and considerably reduced the sustained component of outward current. The difference current shown in Fig. 2Aii was obtained by digital subtraction of the current recorded in Ca2+-free solution from the control current recording and illustrates that transient inward and outward currents are sensitive to Ca2+ removal. Figure 2Aiii shows summary data from eight experiments in which the peak outward current was recorded before and after external Ca2+ was removed. The peak outward current was significantly reduced by ∼85%, from 558 ± 61 to 82 ± 14 pA (P < 0.001), when external Ca2+ was removed, demonstrating that external Ca2+ was essential for activation of the tBK current.
Fig. 2.
Transient BK (tBK) current depends on Ca2+ influx through L-type Cav channels. Ai: effect of Ca2+ removal after currents were evoked by steps from −60 to 0 mV. Aii: difference current obtained by digital subtraction of traces in Ai. Aiii: summary data for 8 experiments in which peak tBK current was measured before (control) and after Ca2+ removal. B and C: tBK current was also blocked in the presence of nifedipine and Cd2+. Bii and Cii: nifedipine- and Cd2+-sensitive difference currents. Biii and Ciii: summary data for nifedipine (Nif)- and Cd2+ (Cad)-sensitive curents. *P < 0.05, **P < 0.01 (by paired t-test).
To test if L-type CaV channel was the main influx pathway responsible for activating the tBK current, as demonstrated in bladder and vas deferens smooth muscle cells (18), we examined the effects of nifedipine on tBK currents. Figure 2Bi shows a typical example of tBK current in the absence and presence of nifedipine (10 μM) and illustrates that it abolished the current. The nifedipine-sensitive difference current is shown in Fig. 2Bii and, similar to Fig. 2Aii, consists of transient inward and outward currents, as well as sustained current. A summary of six similar experiments is presented in Fig. 2Biii, in which the peak outward current is shown in the absence and presence of nifedipine. L-type CaV channel blockade significantly reduced the tBK current by 84% from 559 ± 102 to 91 ± 14 pA (P < 0.05).
To confirm that these effects of nifedipine were due to inhibition of Ca2+ influx through L-type CaV channels, rather than an effect on dihydropyridine receptors [as in skeletal muscle (13, 22, 28)], we examined the effect of the inorganic blocker Cd2+ on tBK currents. As illustrated in Fig. 2, Ci and Cii, application of 100 μM Cd2+ produced effects very similar to the effect of external Ca2+ removal or nifedipine application. Figure 2Ciii demonstrates that the tBK current was significantly reduced from 981 ± 153 to 183 ± 29 pA by Cd2+ (P < 0.01, n = 6). These data are consistent with the idea that influx via L-type CaV channels is an essential step for activation of the tBK current.
Does the tBK current show voltage-dependent inactivation?
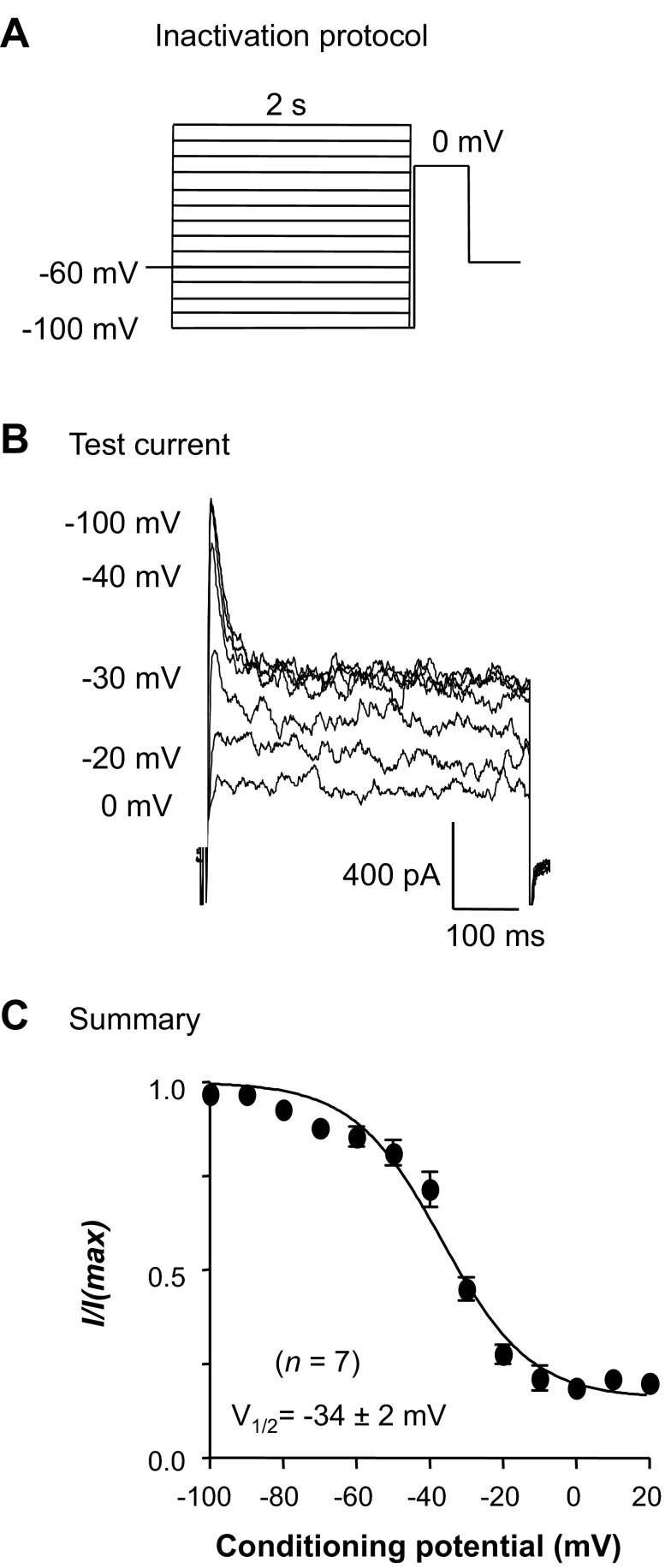
Given that the tBK current in RUSMCs was sensitive to Ca2+ removal and blockade of L-type CaV channels, we might expect that it would show an apparent voltage-dependent inactivation. This would presumably result from a reduction of Ca2+ influx caused by voltage-dependent inhibition of L-type CaV current. To test this idea, we used a double-pulse protocol (Fig. 3A) that comprised 2-s conditioning potentials from −100 to 20 mV in 10-mV increments prior to stepping to the test potential of 0 mV for 500 ms. As Fig. 3B suggests, the transient and sustained components of BK current appeared to exhibit voltage-dependent inactivation. The tBK current (measured within the first 50 ms of the depolarization) inactivated by <25% at conditioning potentials more negative than −40 mV. This was presumably due to the small contribution of T-type CaV currents to Ca2+ influx at these potentials (9). At more depolarized conditioning potentials, the current showed a steeper voltage dependence of inactivation, and the transient and sustained components of the BK current were maximally inactivated at potentials positive to −20 mV. When the peak current in the first 50 ms of the depolarization was plotted against the conditioning potential in seven cells, as shown in Fig. 3C, and fitted with a Boltzmann function, the voltage at which the current was half-maximally inactivated (inactivation V1/2) was −34 ± 2 mV and was similar to that of the L-type CaV current previously characterized in these cells (9).
Fig. 3.
Voltage-dependent inactivation of the underlying L-type CaV current reduced tBK current. A: standard double-pulse protocol. B: typical currents evoked by a test potential step to 0 mV. C: summary data for 7 experiments in which peak amplitude of the current at 0 mV was measured and normalized to the maximum (I/Imax). Data were plotted against conditioning potential and fitted with a Boltzmann function (solid line) to yield the voltage at which 50% of the channels were inactivated (V1/2).
Contribution of Ca2+ stores to tBK current.
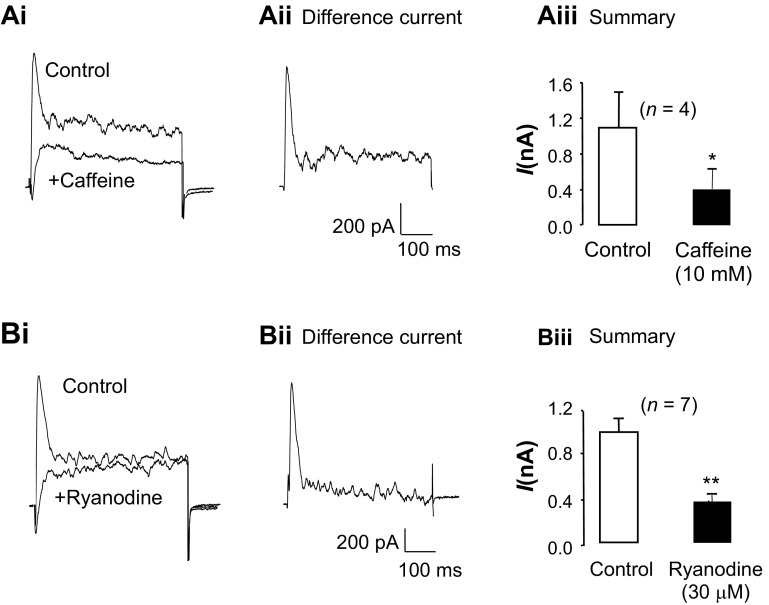
To assess if Ca2+ release via RyRs contributed to the tBK current, experiments were carried out with the RyR modulators caffeine and ryanodine. Caffeine (10 mM) was first used to deplete Ca2+ from the sarcoplasmic reticulum (SR) store and, as shown in Fig. 4, Ai and Aii, inhibited the tBK current. Figure 4Aiii shows the summary for four similar experiments in which the peak tBK current was significantly reduced by 63%, from 1,095 ± 387 to 406 ± 216 pA (P < 0.05), in the continued presence of caffeine, suggesting that Ca2+ release from the SR store via RyRs was an important step for tBK current activation.
Fig. 4.
tBK current depends on Ca2+ release via ryanodine receptors. Ai: effect of caffeine application after currents were evoked by steps from −60 to 0 mV. Aii: caffeine-sensitive difference current obtained by digital subtraction of traces in Ai. Aiii: summary data for 4 experiments in which peak tBK current was measured before (control) and after caffeine application. Bi: tBK current was also blocked in the presence of ryanodine. Bii: ryanodine-sensitive difference current. Biii: summary data for 7 experiments in which peak tBK current was measured before (control) and after ryanodine application. *P < 0.05, **P < 0.01 (by paired t-test).
As shown in Fig. 4, Bi and Bii, application of a high concentration of ryanodine (30 μM) also abolished the tBK current. In these experiments, cells were treated with ryanodine for ∼12 min, during which caffeine (10 mM) was applied repeatedly for 10 s at 80-s intervals to activate the RyRs and, thus, permit ryanodine to block the channels. Figure 4Biii shows a summary of seven similar experiments in which the peak tBK current was significantly reduced from 984 ± 110 to 362 ± 53 pA in the presence of ryanodine (P < 0.01). When the same protocol was repeated in the absence of ryanodine, the tBK current was unaffected, since its amplitude was 783 ± 162 pA under control conditions and 785 ± 225 pA following washout of the final application of caffeine. We also examined the effects of tetracaine (100 μM) on the tBK current in seven cells and found that it significantly reduced the peak current from 1,308 ± 225 to 412 ± 118 pA (P < 0.05).
Given that these RyR modulators may alter L-type Cav current, we examined the effects of each compound in RUSMCs recorded with Cs+-rich pipette solutions. Depolarizations from −60 to 0 mV evoked large inward currents with a mean amplitude of −323 ± 184 pA under control conditions, and this was not significantly affected in the presence of 30 μM ryanodine (−302 ± 89 pA, n = 4). Tetracaine (100 μM) reduced L-type current by ∼18%, from −559 ± 120 to −459 ± 136 pA (n = 8, P < 0.05). Similarly, caffeine significantly decreased the amplitude of the peak L-type Cav current from −412 ± 59 to −291 ± 44 pA (n = 10).
To assess if Ca2+ release from IP3Rs also contributed to the tBK current, we next examined if the IP3R inhibitor 2-APB (100 μM) altered the current. In seven experiments, the control tBK current was 943 ± 179 pA compared with 1,043 ± 177 pA in 2-APB, but the small increase in amplitude was not statistically significant. These data suggest that Ca2+ release via IP3Rs did not contribute to activation of the tBK current.
Inhibition of the BK current by IP3-generating agonists.
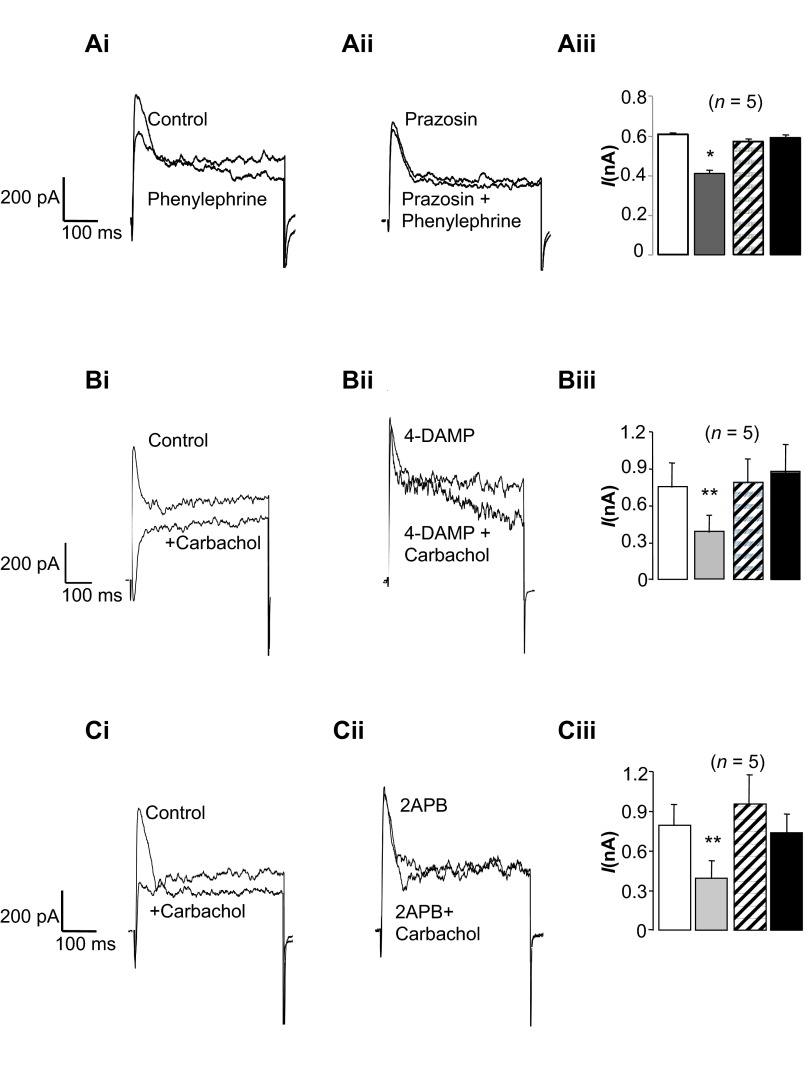
Although Ca2+ release via IP3Rs did not appear to contribute to tBK current activation in response to depolarizing steps, it is possible that agonist-induced Ca2+ release via IP3Rs could induce Ca2+-induced Ca2+ release (CICR) and, thus, inhibit the tBK current. Since the urethra is innervated by adrenergic and cholinergic nerves (14, 19, 33), we examined the effects of phenylephrine and the stable analog of acetylcholine, carbachol, on tBK currents and found that both inhibited tBK currents. As shown in Fig. 5A, application of phenylephrine (1 μM) decreased the amplitude of the tBK current evoked by a depolarizing step from −60 to 0 mV, and this effect was abolished in the presence of the α1-adrenoceptor antagonist prazosin (1 μM; Fig. 5Aii). Summary data from five similar experiments are shown in Fig. 5Aiii, in which phenylephrine reduced the peak tBK current from 607 ± 10 to 413 ± 15 pA (P < 0.05). Prazosin had no effect on the tBK current, and in its presence, phenylephrine failed to inhibit the current (571 ± 15 pA in prazosin compared with 590 ± 16 pA in prazosin + phenylephrine, P = not significant). These data are consistent with the idea that the inhibitory effects of phenylephrine on tBK current were mediated via activation of α1-adrenoceptors.
Fig. 5.
tBK current is inhibited when inositol trisphosphate (IP3)-generating agonists are applied. A–C: currents were evoked by steps from −60 to 0 mV, and effects of phenylephrine and carbachol were examined. Ai and Aii: phenylephrine reduced the amplitude of the tBK current, which was antagonized in the presence of prazosin (1 μM). Aiii: summary for 5 cells in which the peak current was measured in the absence of drugs (open bar) and in the presence of phenylephrine (gray bar), prazosin (hatched bar), and phenylephrine + prazosin (filled bar). Bi: carbachol application also inhibited the tBK current, which was abolished when M3 receptors were blocked with 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP, 1 μM). Biii: summary for 5 cells in which the peak current was measured in the absence of drugs (open bar) and in the presence of carbachol (gray bar), 4-DAMP (hatched bar), and carbachol + 4-DAMP (filled bar). Ci and Cii: inhibitory effects of carbachol were abolished when cells were preincubated with 2-aminoethoxydiphenyl borate (2-APB, 100 μM). Ciii: summary for 5 cells in which the peak current was measured in the absence of drugs (open bar) and in the presence of carbachol (gray bar), 2-APB (hatched bar), and carbachol + 2-APB (filled bar). *P < 0.05, **P < 0.01 (by ANOVA using Dunnett's post hoc test).
Carbachol (1 μM) appeared to be even more effective at inhibiting the tBK current, as shown in Fig. 5, B and C. To test if this effect was mediated via activation of M3 receptors, we examined the effects of the selective M3 receptor antagonist 4-DAMP on this response. As shown in Fig. 5Bii, 4-DAMP (1 μM) had no effect on the tBK current, and when carbachol was reapplied in its presence (Fig. 5Bii), 4-DAMP failed to inhibit the tBK current. In the five experiments summarized in Fig. 5Biii, application of carbachol reduced the peak tBK current by 48%, from 753 ± 199 to 387 ± 135 pA (P < 0.01). In the continued presence of 4-DAMP, the current was not significantly different from control (790 ± 196 pA) and was not significantly altered when carbachol was reapplied (876 ± 228 pA). In four experiments, we examined the effects of carbachol in the absence and presence of the selective M2 receptor antagonist methoctramine (1 μM). Under control conditions, carbachol reduced the tBK current from 502 ± 66 to 216 ± 52 pA, and in the presence of methoctramine and carbachol the current was still significantly decreased to 267 ± 58 pA compared with control (P < 0.05). Taken together, these data suggest that carbachol mediates its inhibitory effect on the tBK current via an effect on M3, rather than M2, receptors.
Since M3 receptor activation leads to generation of IP3, we next examined if the effects of carbachol were inhibited in the presence of the IP3R blocker 2-APB (100 μM). As shown in Fig. 5Ci, carbachol again inhibited the tBK current, and although 2-APB had little effect on the tBK current (Fig. 5Cii), reapplication of carbachol in the presence of 2-APB failed to inhibit the tBK current. These effects are summarized for five cells in Fig. 5Ciii. Under control conditions, tBK current amplitude was 790 ± 163 pA, and this was reduced by 45%, to 392 ± 136 pA (P < 0.01, by ANOVA), by carbachol. The tBK current amplitude was slightly increased to 952 ± 224 pA in the presence of 2-APB, but this was not significantly different from control. In the presence of 2-APB + carbachol, the mean tBK current amplitude was 735 ± 141 pA, which was not significantly different from the tBK current in 2-APB alone or the control tBK current.
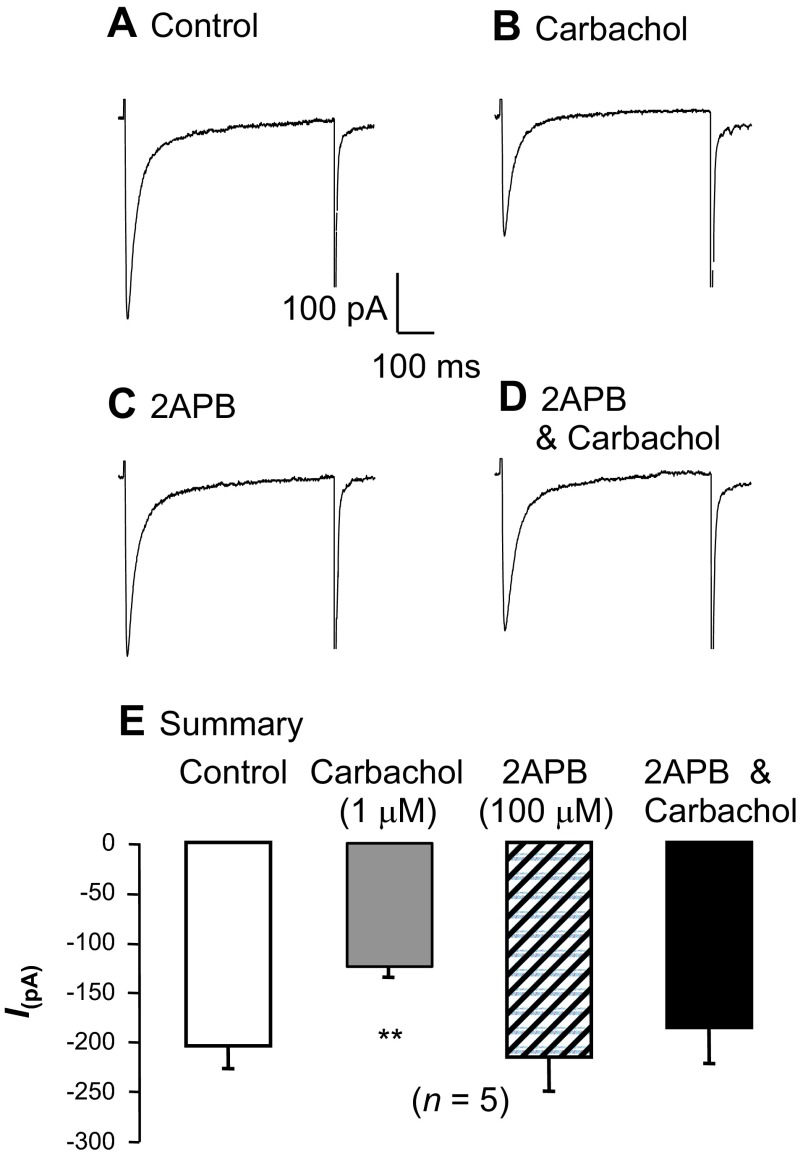
Inhibition of the L-type CaV current by carbachol.
Given that Ca2+ influx via L-type CaV channels and Ca2+ release via RyR contribute to activation of tBK current, we next tested the effects of carbachol on each component of the activation pathway. To record L-type CaV currents, contaminant K+ currents were blocked with intracellular Cs+, and cells were depolarized from −60 to 0 mV for 500 ms to elicit the peak L-type CaV currents, as shown in Fig. 6A. When carbachol (1 μM) was applied (Fig. 6B), the inward current amplitude was reduced by ∼50%. To test if this inhibitory effect was due to Ca2+-dependent inactivation of the L-type CaV current caused by IP3-mediated Ca2+ release from the SR, we next examined the effects of 2-APB on this response. Inhibition of the IP3R with 2-APB (100 μM) had little effect on the current itself (Fig. 6C), and the inhibitory effects of carbachol were abolished in the presence of 2-APB (Fig. 6D). These effects are reflected in the summary data for five similar experiments shown in Fig. 6E, where the mean peak L-type CaV current was reduced from −205 ± 23 to −124 ± 12 pA in the presence of 1 μM carbachol. Application of 100 μM 2-APB alone had little effect on the peak inward current (−216 ± 34 pA), and in the presence of 2-APB and carbachol, the current was not significantly reduced compared with control (−186 ± 36 pA). These data suggest that IP3-mediated Ca2+ release is responsible for inhibiting the L-type CaV current, and this could contribute to inhibition of tBK current.
Fig. 6.
L-type CaV current is inhibited when IP3-generating agonists are applied. A and B: currents were evoked by steps from −60 to 0 mV under control conditions and were reduced in the presence of carbachol. C and D: although inhibition of Ca2+ release from IP3 receptors (IP3Rs) with 2-APB had little effect on the L-type CaV current, it blocked the inhibitory effect of carbachol. E: summary for 5 experiments in which the peak inward current was measured in the absence of drugs (control) and in the presence of carbachol, 2-APB, and carbachol + 2-APB. **P < 0.01 (by ANOVA using Dunnett's post hoc test).
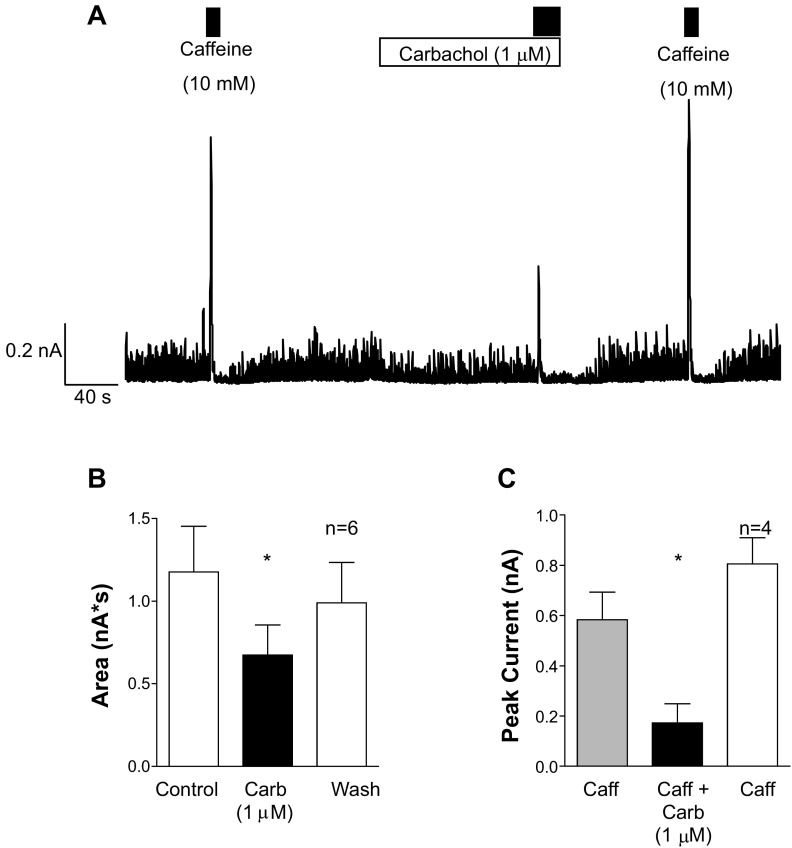
To determine whether the IP3-induced release of Ca2+ by carbachol affected the release of Ca2+ via the RyR, we next tested the effects of caffeine application (10 mM) in the absence and presence of carbachol (1 μM). The cells were voltage-clamped at 0 mV (with K+-rich pipette solutions), and at this potential they fired small spontaneous transient outward currents (STOCs), as shown in Fig. 7A. Application of caffeine evoked a large transient outward current (Fig. 7A) and transiently inhibited the STOCs for ∼10 s, presumably as a result of store depletion. Application of carbachol to the same cell (Fig. 7A) failed to evoke large-amplitude currents but consistently reduced the STOCs, suggesting that intracellular store content was reduced in its presence. To quantify the effect of carbachol on STOCs, we integrated the area under the current trace for 30 s in the absence, presence, and following washout of the agonist in six cells. As Fig. 7B suggests, the area was significantly reduced from 1.18 ± 0.28 to 0.67 ± 0.18 nA·s by carbachol (P < 0.05, ANOVA) and returned to 0.99 ± 0.25 nA·s following carbachol removal.
Fig. 7.
Carbachol application reduces the releasable pool of stored Ca2+. A: a cell was held at 0 mV and spontaneous transient outward currents (STOCs) were recorded. Application of caffeine (first black bar) elicited a large BK current and temporarily reduced the amplitude of STOCs. Application of carbachol (white bar) failed to elicit a large BK current but reduced the amplitude of the STOCs, and subsequent application of carbachol evoked a smaller current compared with control. Upon removal of carbachol, caffeine evoked currents of comparable amplitude to control. B: summary data for 6 cells in which the currents recorded over 30 s were integrated and plotted before (control), during (Carb), and after (Wash) application of carbachol. C: summary of 4 experiments in which the amplitude of the peak current evoked by caffeine was measured in the absence (gray bar) and presence (black bar) of carbachol and following the washout of carbachol (white bar). Caffeine responses were significantly reduced in the presence of carbachol, suggesting that the IP3-generating agonist reduced the releasable pool of Ca2+. *P < 0.05 (by ANOVA with Dunnett's post hoc test).
Caffeine responses were greatly reduced in the presence of carbachol, consistent with the idea that carbachol reduced the releasable pool of Ca2+ in this cell. When carbachol was removed, STOC amplitudes returned toward control levels, and reapplication of caffeine (Fig. 7A) evoked a large outward current, similar to that observed under control conditions. Figure 7C shows summary data from four similar experiments in which the amplitude of the caffeine-evoked current was measured under control conditions, in the presence of carbachol, and following 80 s of washout of carbachol. The amplitude of the caffeine-evoked current was 584 ± 110 pA, decreased to 173 ± 75 pA in the presence of carbachol (P < 0.05, by ANOVA), and then returned to 805 ± 105 pA following washout of carbachol. These data suggest that carbachol can also reduce Ca2+ release via the RyR, presumably by depleting the intracellular store Ca2+ concentration.
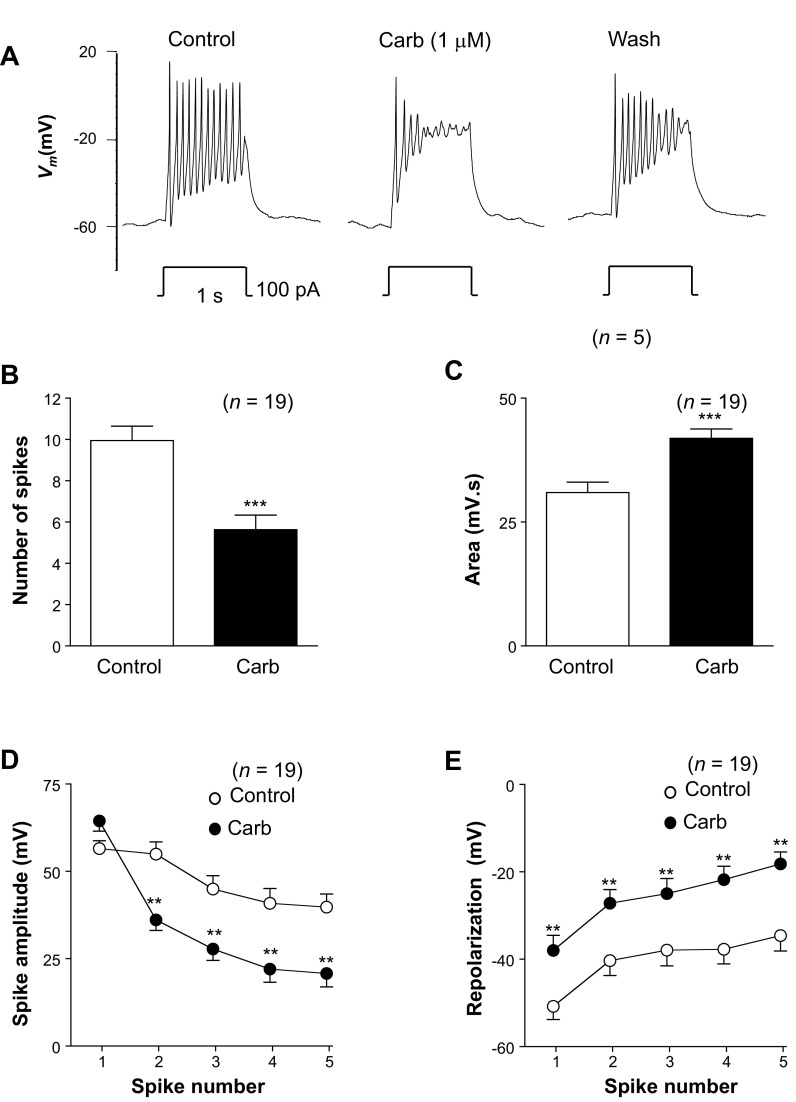
Modulation of spike complexes by carbachol.
The data presented above suggest that the tBK current in RUSMCs can be modulated by IP3-generating agonists, and these effects appear to be due to a reduction of L-type CaV current and decreased Ca2+ release from intracellular stores via the RyR. Given that the tBK current plays an important role in modulating the shape of the RUSMC AP (20), we next examined the effects of carbachol under current-clamp conditions. These recordings were made under current-clamp conditions with K+-rich patch pipettes using the perforated-patch configuration. Small hyperpolarizing current injections were used to reduce the resting membrane potential from about −40 to about −60 mV to remove any inactivation of L-type CaV currents. Figure 8A, left trace, illustrates a typical spike complex evoked in response to injection of a depolarizing current of 100 pA for 1 s. This complex comprises a series of 11 rapid spikes, which were superimposed on a depolarized plateau and showed a remarkable similarity to those recorded previously from the strips of rabbit urethra with intracellular microelectrodes (9). When current injections were repeated in the presence of 1 μM carbachol (Fig. 8A, middle trace), although the resting membrane potential was unaffected, the shape of the spike complex changed significantly. The most obvious changes were 1) decreased spike frequency, 2) reduced spike amplitude, 3) reduced amplitude of the repolarization phase of each spike, and 4) a more depolarized plateau potential. As shown in Fig. 8A, right trace, these effects began to reverse when carbachol was removed.
Fig. 8.
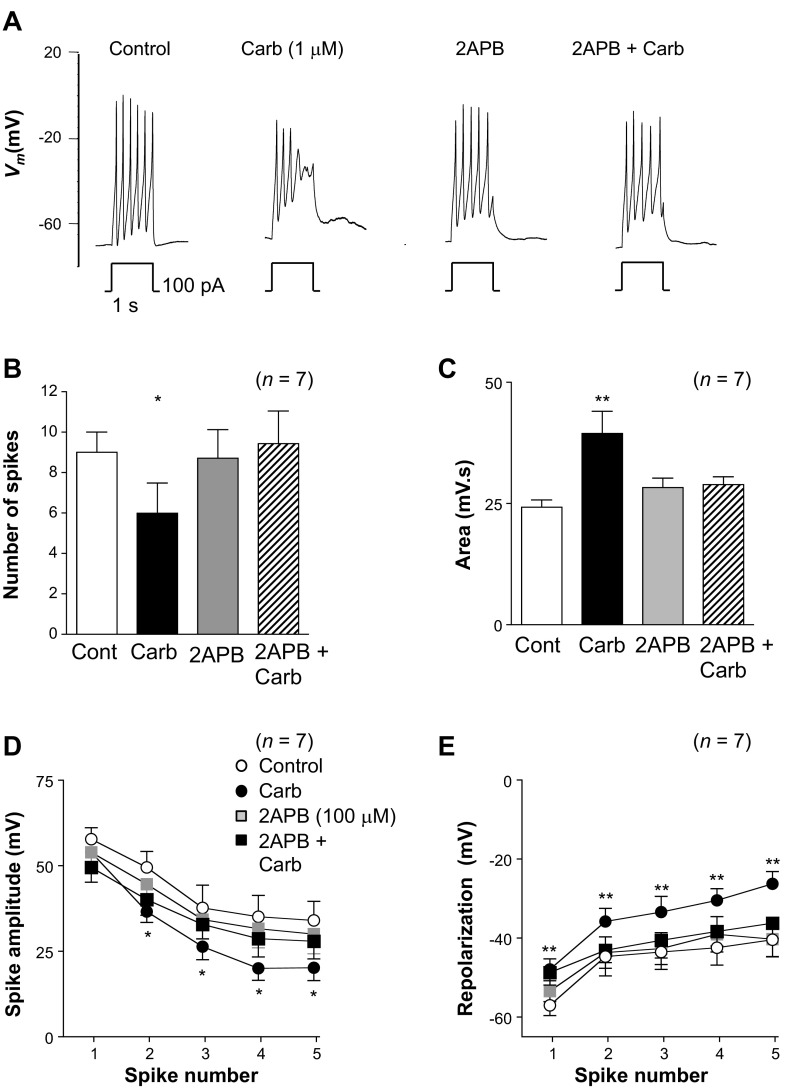
Carbachol application alters the shape of the spike complex. A: typical effect of injecting depolarizing current for 1 s in the absence (control) and presence of carbachol (Carb) and following washout of carbachol (Wash). B and C: application of carbachol reduced the number of spikes in the complexes but increased the area measured by integrating Vm. D: summary plot of the amplitude of the first 5 spikes in each complex in the absence (control) and presence of carbachol (n = 19). E: summary for the same 19 experiments in which the amplitude of repolarization for the first 5 spikes in a complex was measured in the absence (control) and presence of carbachol. **P < 0.01, ***P < 0.001 [by paired t-test (B and C) and ANOVA with Bonferroni's post hoc test (D and E)].
To quantify these effects on the spike complexes, we first measured the number of spikes evoked during the 1-s current injection in the absence and presence of carbachol in 19 experiments. The mean resting membrane potential was −56 ± 2 mV in control compared with −57 ± 2 mV in carbachol (n = 19). As Fig. 8B suggests, carbachol significantly reduced the number of spikes by 40%, from 10 ± 0.7 to 6 ± 0.7 (P < 0.001). We also measured the area under the curve during the current injection in the same 19 cells (Fig. 8C). The area under the curve was 31 ± 2 mV·s in control and significantly increased to 42 ± 2 mV·s in carbachol (P < 0.001), consistent with the idea that the cells were in a more depolarized state in the presence of carbachol than in the control condition during current injection.
We also compared the effects of carbachol on the individual spikes of each complex by measuring the absolute amplitude of each of the first five spikes in each complex in the absence and presence of carbachol (Fig. 8D). These were measured from the most hyperpolarized potential immediately preceding the upstroke to the peak of each spike. Under control conditions, the amplitude of the spikes ranged from 40 to 65 mV over the duration of the first five spikes in the complex (Fig. 8D). Although the amplitude of the first spike was 56 ± 2 mV under control conditions and 67 ± 4 mV in the presence of carbachol, this failed to reach statistical significance. However, the amplitudes of all subsequent spikes were significantly smaller in carbachol than in control (P < 0.01).
Since carbachol inhibited the main current responsible for repolarization of RUSMCs (the tBK current), we also measured the potential to which the membrane returned following each spike. Under control conditions, the first spike usually returned the membrane potential to −51 ± 3 mV, and the four subsequent spikes repolarized the cells to between −40 and −35 mV (Fig. 8E). However, in the presence of carbachol, there was significantly less repolarization following each spike than in control conditions. The first spike resulted in repolarization to only −37 ± 3 mV, and for each subsequent spike, repolarization varied between −30 and −18 mV, which was significantly different from control. Taken together, these data suggest that carbachol application changed the shape of the evoked spike complexes significantly, and this may result in the cells spending more time in a depolarized state during each spike complex.
2-APB reduced the effects of carbachol on spike complexes.
To test if the effects of carbachol on the spike complexes were mediated exclusively by elevating levels of IP3, rather than PKC or diacylglycerol, we next examined the effects of 2-APB on the carbachol responses. Figure 9A shows the effect of carbachol on the spike complexes in the absence and presence of 2-APB (100 μM). Although 2-APB produced a slight decrease in the amplitude of the spikes and the associated repolarizations, it abolished the effect of carbachol (Fig. 9A, right trace). Summary data from seven similar experiments are shown in Fig. 9, B–E. Carbachol reduced the number of spikes per complex significantly from 9 ± 1 to 6 ± 1.5 (P < 0.05), and although 2-APB did not change the number of spikes per complex, reapplication of carbachol in the presence of 2-APB failed to significantly alter the number of spikes compared with control (Fig. 9B). As Fig. 9C suggests, carbachol increased the area under the spike complex from 24.3 ± 1.3 to 38.1 ± 4.1 mV·s (P < 0.01). Application of 2-APB alone did not alter the area under the spike complex (27.9 ± 1.7 mV·s), and reapplication of carbachol in the presence of 2-APB failed to significantly alter the area (28.43 ± 1.5 mV·s) compared with control. A similar pattern was observed when the effect of 2-APB was examined on the carbachol effects on spike complex amplitude (Fig. 9D) and the amplitude of repolarization (Fig. 9E). Although carbachol did not significantly change the amplitude of the first spike, it reduced the amplitude of spikes 2–5 in each complex by ∼15 mV compared with control (Fig. 9D). These effects were abolished when carbachol was applied in the presence of 2-APB (Fig. 9D). Although 2-APB alone caused a small reduction in the amplitude of the spikes compared with control (Fig. 9D), this effect was not significant.
Fig. 9.
Inhibition of IP3-mediated Ca2+ release reduces the response to carbachol. A: typical effect of injecting depolarizing current for 1 s in the absence of carbachol (control) and in the presence of carbachol, 2-APB, and 2-APB + carbachol. B: carbachol significantly reduced the number of spikes in the complexes, and this effect was blocked when carbachol was reapplied in the presence of 2-APB (2-APB + carb). C: area measured by integrating Vm was increased in the presence of carbachol, and this effect was abolished in the presence of 2-APB (2-APB + carb). D: summary plot of the amplitude of the first 5 spikes in each complex in the absence (control) and presence of carbachol, 2-APB, and 2-APB + carbachol. E: summary for 7 experiments in which the amplitude of repolarization for the first 5 spikes in each complex was measured under control conditions and in the presence of carbachol, 2-APB, and 2-APB + carbachol. *P < 0.05, **P < 0.01 (by ANOVA using Dunnett's post hoc test).
Ryanodine mimics the effect of carbachol on spike complexes.
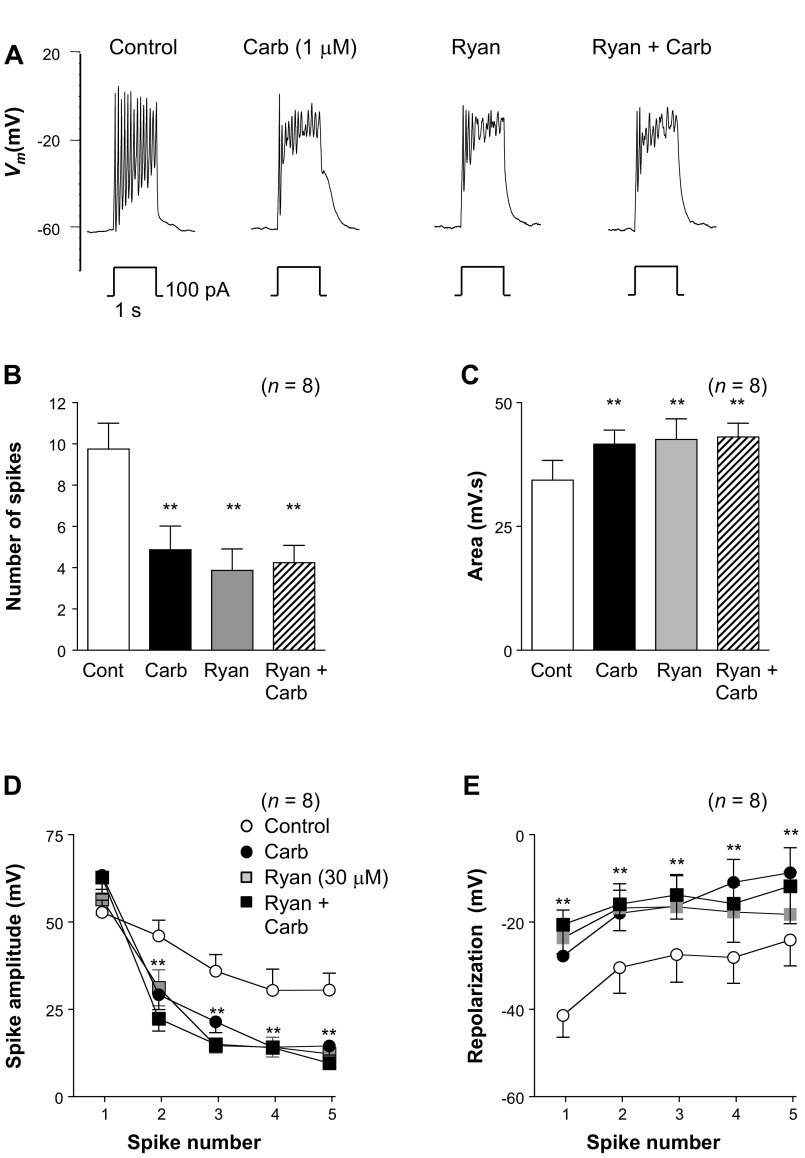
The above data are consistent with the idea that carbachol mediates its effects predominantly through an IP3-mediated mechanism that results in store release of Ca2+. If this is the case, we might expect that application of ryanodine would also empty the stores and, thus, mimic the effect of carbachol. Figure 10A shows a typical example of an experiment in which the effects of ryanodine (30 μM) were examined in the absence and presence of carbachol. Under control conditions (Fig. 10A, left trace) current injection evoked a series of oscillatory spike complexes. In this example, application of carbachol markedly reduced spike complex amplitude and modestly reduced spike complex frequency. Application of 30 μM ryanodine produced effects very similar to effects of carbachol, and when carbachol was reapplied in the continued presence of ryanodine, it had no further effect (Fig. 10A, right trace). Summary data from eight similar experiments are shown in Fig. 10, B–E. Carbachol reduced the number of spikes per complex significantly from 9.8 ± 1.3 to 4.9 ± 1.1 (Fig. 10B; P < 0.01). Ryanodine also significantly decreased the number of spikes per complex to 3.9 ± 1 (Fig. 10B), but this was not reduced further upon reapplication of carbachol. As Fig. 10C suggests, carbachol increased the area under the spike complex from 34.3 ± 4 to 41.6 ± 2.8 mV·s (P < 0.01). Application of ryanodine alone also significantly increased the spike complex area (42.6 ± 4 mV·s) compared with control, and reapplication of carbachol in the presence of ryanodine failed to further increase the area significantly (43.1 ± 2.8 mV·s). Ryanodine also mimicked the effects of carbachol on spike amplitude and repolarization amplitude, as illustrated in Fig. 10, D and E. Although carbachol appeared to increase the amplitude of the first spike (Fig. 10D), this was not significant. However, the amplitude of the remaining spikes was significantly reduced by ∼15 mV (P < 0.01) in the presence of carbachol. Application of ryanodine also produced a significant reduction in spike amplitude of ∼15 mV, and reapplication of carbachol failed to alter this further (Fig. 10D). As shown in Fig. 10E, the amplitudes of the repolarizations were also significantly reduced in the presence of carbachol or ryanodine. For example, under control conditions (Fig. 10E), the first spike repolarized to −41.4 ± 5 mV, and this was reduced to −27.8 ± 6 mV in the presence of carbachol (Fig. 10E; P < 0.01), −23.6 ± 6.4 mV in the presence of ryanodine (Fig. 10E; P < 0.01), and −20.6 ± 6.7 when carbachol was reapplied in the presence of ryanodine (Fig. 10E; P < 0.01). A similar effect was seen in the remaining four spikes shown in Fig. 10E, suggesting that ryanodine mimicked the effect of carbachol.
Fig. 10.
Ryanodine application mimics the effect of carbachol. A: typical effect of injecting depolarizing current for 1 s in the absence and presence of carbachol, ryanodine (Ryan), and ryanodine + carbachol. B: carbachol significantly reduced the number of spikes in the complexes, and this effect was mimicked by ryanodine; carbachol had no additional effect when reapplied in the presence of ryanodine (Ryan + Carb). C: area measured by integrating Vm was increased in the presence of carbachol, and this effect was mimicked in the presence of ryanodine; carbachol had no additional effect in the presence of ryanodine (Ryan + Carb). D: summary plot of the amplitude of the first 5 spikes in each complex under control conditions and in the presence of carbachol, ryanodine, and ryanodine + carbachol. E: summary for 7 experiments in which the amplitude of repolarization for the first 5 spikes in each complex was measured under control conditions and in the presence of carbachol, ryanodine, and ryanodine + carbachol. **P < 0.01 (by ANOVA using Dunnett's post hoc test).
DISCUSSION
The major objective of this study was to examine the mechanisms responsible for activating the tBK current in RUSMCs and determine how this current was modulated by the IP3-generating agonists phenylephrine and carbachol. We previously demonstrated that most of the K+ current evoked in sheep and rabbit USM cells by membrane depolarization was a tBK current (17, 20). While this tBK current appears to play an important role in shaping the APs evoked in RUSMCs (20), the mechanism(s) responsible for activating the tBK current has not been previously investigated.
Furthermore, although a number of studies have demonstrated an adrenergic and cholinergic innervation of the urethra (2, 14, 16, 19, 22, 24, 33), few studies have examined how RUSMC currents are modulated by excitatory IP3-generating neurotransmitters. In the present study we demonstrated that Ca2+ influx through L-type CaV channels and Ca2+ release via RyRs were essential for tBK current activation. In this study, we have shown that agonist-induced IP3R activation can result in inhibition of the tBK and, consequently, alter the shape of AP complexes recorded under current-clamp conditions.
Blockade of Ca2+ influx through L-type CaV channels with nifedipine or Cd2+ abolished the tBK current in these cells. In addition, the voltage-dependent “inactivation” of the tBK current was very similar to that of L-type CaV channels characterized previously in these cells (9). Therefore, it is tempting to speculate that the tBK current was activated exclusively by Ca2+ influx and that the transient nature of the current simply reflects the Ca2+ concentration at the cytosolic surface of the BK channels. Indeed, the transient component of the BK current appears to follow a time course similar to that of the decay of the L-type CaV channels. When one examines the reduction in tBK current resulting from voltage-dependent L-type CaV channel inactivation (Fig. 3), it is apparent that the tBK current showed a graded response to Ca2+ levels, rather than an all-or-nothing, threshold response. However, our data obtained with the RyR modulators caffeine, ryanodine, and tetracaine suggest that Ca2+ release from intracellular stores is also essential for activation of the tBK current in a manner similar to that described previously by Imaizumi et al. (18) in bladder and vas deferens smooth muscle. Imaizumi et al. proposed that Ca2+ influx via L-type CaV current activated RyR-mediated Ca2+ release (CICR) and that this was primarily responsible for activating the tBK current in the cells they studied. Here, we confirm that a similar mechanism appears responsible for activating the tBK current in RUSMCs. The small reduction of L-type CaV current with caffeine and tetracaine (but not ryanodine) may have contributed to the effects of these drugs on tBK current amplitude. However, this is unlikely to account for the entire effect of the RyR modulators on the tBK current, since a similar reduction (∼25%) can be achieved during voltage-dependent inactivation with a conditioning potential of −50 mV (9), which only reduces the tBK current slightly (Fig. 3A). Furthermore, ryanodine had no significant effect on L-type Cav current, yet it significantly reduced the amplitude of the tBK current, consistent with the idea that Ca2+ release and Ca2+ influx contribute to the tBK current.
Our data suggest that Ca2+ release via IP3Rs does not contribute to activation of the tBK current in RUSMCs, since 2-APB failed to alter this current. However, in the presence of IP3-generating agonists, such as phenylephrine and carbachol, the tBK current was inhibited. Since M2 and M3 receptors are present in USM (24, 25, 35), experiments were carried out to examine which muscarinic receptor subtype contributed to the effects of carbachol. The sensitivity of the response to 4-DAMP (4), but not the M2 receptor antagonist methoctramine (23), suggests that the main effect of carbachol was mediated via M3 receptors.
Although 2-APB effectively abolished the response to carbachol, a number of studies have suggested that this blocker has nonspecific effects, including inhibition of store-operated Ca2+ entry (6); therefore, its effects should be interpreted with caution. We find that it does not alter L-type CaV current or the tBK current. The finding that the tBK current is dependent on store release suggests that 2-APB has little effect on store filling in these cells.
Since the tBK current in RUSMCs relies on Ca2+ influx and Ca2+ release via CICR, carbachol could modulate either of these pathways to inhibit the tBK current. Indeed, carbachol is known to inhibit L-type CaV channels in a variety of smooth muscles by Ca2+-dependent inhibition of the L-type CaV channels (34) or activation of pertussis toxin-insensitive G proteins (26). In RUSMCs, carbachol inhibited the L-type CaV current, an effect that was blocked in the presence of 2-APB. Thus it appears that the decrease in L-type CaV current was mediated via an elevation of intracellular Ca2+ concentration as a result of Ca2+ release following IP3R activation. This inhibition of L-type CaV current would reduce Ca2+ influx considerably and would presumably decrease activation of the tBK current.
Our data also suggest that carbachol reduced the releasable pool of Ca2+ in the SR, since it decreased STOCs in RUSMCs and reduced the amplitude of caffeine-evoked currents. Interestingly, carbachol did not appear to be as effective as caffeine at evoking large outward BK currents. It is possible that the concentration of carbachol was too low to raise Ca2+ sufficiently to stimulate the BK channels at this potential (0 mV). However, this concentration of carbachol appeared to release sufficient Ca2+ to apparently reduce store content (as evidenced by decreased STOCs and reduced caffeine responses) and cause Ca2+-dependent inactivation of L-type CaV channels. It is tempting to speculate that the Ca2+ released following stimulation of IP3Rs may not be in the vicinity of the BK channels and is therefore unable to stimulate them, but this will clearly require experimental validation.
Although numerous studies have demonstrated that the urethra receives an adrenergic and cholinergic innervation, few studies have examined how USM cell currents are modulated by muscarinic or adrenergic receptor agonists. The majority of data obtained in the urethra have used tension recording to demonstrate the contribution of adrenergic and cholinergic nerves (3, 8, 33). Less frequently, intracellular recording with sharp microelectrodes from strips of urethra (15, 16, 19) was used to demonstrate that urethral tone is frequency-modulated (14, 15, 19), since excitatory agonists increase the frequency of slow waves (15) and spike complexes (10, 11). The present study sheds some light on how urethral smooth muscle currents are altered in the presence of excitatory agonists. In contrast to the effect of adrenergic and cholinergic receptor agonists on urethral ICCs (28, 29, 32), we found no evidence that oscillatory inward currents or depolarizations were evoked by these agonists in RUSMCs, consistent with the idea that RUSMCs do not possess significant Cl− current (28). However, in the present study, we have demonstrated that L-type CaV channels and tBK currents are modulated in the presence of carbachol. The decrease in both currents in response to muscarinic stimulation results from elevated intracellular Ca2+ concentration caused by Ca2+ release from the intracellular stores, rather than direct modulation of the ion channels by the agonist, as evidenced by blockade of the effect of carbachol with 2-APB and ryanodine. Although inhibition of the L-type CaV channels occurred in the presence of carbachol, this was also associated with a reduction in the amplitude of the repolarizing BK current. Consequently, the balance between inward and outward currents was altered significantly in the presence of excitatory agonists, with the result that the spike complex shape was changed. Thus the number of spikes per complex was reduced and the amplitude of the plateau (see Fig. 8, middle) was more depolarized in carbachol. It is possible that the latter change could result in less Ca2+ influx, if more voltage-dependent inactivation of the Ca2+ current occurred. Alternatively, if the amplitude of this plateau was about −20 mV, it would be right at the peak of the L-type CaV window current in these cells (9) and, thus, may actually enhance influx. These effects were abolished in the presence of 2-APB, consistent with the idea that the release of Ca2+ from IP3-sensitive stores was responsible. The inability of carbachol to further alter the shape of the spike complexes in the presence of ryanodine also supports the idea that carbachol mediated its effects predominantly through the release of store Ca2+. It appears from our data that the net effect of carbachol was to permit smooth muscle cells to spend significantly more time at more depolarized potentials, even though the depolarizing L-type CaV current was reduced in amplitude. Similar effects on APs have been observed in guinea pig ileal smooth muscle cells, where prolonged APs are observed in response to brief applications of carbachol (13). In RUSMCs, the effect of carbachol on the spike complexes, combined with the increased frequency of firing from pacemaking ICCs in response to excitatory nerve stimulation, would lead to an increased concentration of Ca2+ in the bulk smooth muscle and a significant increase in contraction when excitatory nerves are stimulated.
GRANTS
This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-68565. B. D. Kyle was in receipt of a Ph.D. studentship from the Council of Directors, Ireland.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.D.K., E.B., and R.L. performed the experiments; B.D.K., E.B., and R.L. analyzed the data; B.D.K. prepared the figures; B.D.K. and M.A.H. drafted the manuscript; B.D.K., E.B., G.P.S., N.G.M., K.D.T., and M.A.H. approved the final version of the manuscript; R.L., G.P.S., N.G.M., K.D.T., and M.A.H. interpreted the results of the experiments; G.P.S., N.G.M., K.D.T., and M.A.H. are responsible for conception and design of the research; G.P.S., K.D.T., and M.A.H. edited and revised the manuscript.
REFERENCES
- 1. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Andersson KE. New roles for muscarinic receptors in the pathophysiology of lower urinary tract symptoms. BJU Int 86: 36–42, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev 45: 253–307, 1993 [PubMed] [Google Scholar]
- 4. Barlow RB, Shepherd MK. A further search for selective antagonists at M2-muscarinic receptors. Br J Pharmacol 89: 837, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barlow RB, Berry KJ, Glenton PA, Nilolaou NM, Soh KS. A comparison of affinity constants for muscarine-sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29°C and in ileum at 29°C and 37°C. Br J Pharmacol 58: 613–620, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bridgewater M, Macneil HF, Brading AF. Regulation of tone in pig urethral smooth muscle. J Urol 150: 223–228, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Bradley JE, Anderson UA, Woolsey SM, Thornbury KD, McHale NG, Hollywood MA. Characterization of T-type calcium current and its contribution to electrical activity in rabbit urethra. Am J Physiol Cell Physiol 286: C1078–C1088, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Callahan SM, Creed KE. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol 74: 353–358, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Faoite A, Thornbury KD, McHale NG, Sergeant GP, Hollywood MA. Muscarinic stimulation enhances spontaneous electrical activity in the rabbit urethra through activation of M3 receptors (Abstract). Proc Physiol Soc 15: PC37, 2009 [Google Scholar]
- 12. Dulhunty AF. Excitation-contraction coupling from the 1950s into the new millennium. Clin Exp Pharmacol Physiol 33: 763–772, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gordienko DV, Harhun MI, Kustov MV, Pucovský V, Bolton TB. Sub-plasmalemmal [Ca2+]i upstroke in myocytes of the guinea-pig small intestine evoked by muscarinic stimulation: IP3R-mediated Ca2+ release induced by voltage-gated Ca2+ entry. Cell Calcium 43: 122–141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hashimoto S, Kigoshi S, Muramatsu I. Neurogenic responses of urethra isolated from the dog. Eur J Pharmacol 213: 117–123, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Hashitani H, Edwards FR. Spontaneous and neurally activated depolarization in smooth muscle cells of the guinea pig urethra. J Physiol 514: 459–470, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol 118: 1627–1632, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Characterization of outward K+ currents in isolated smooth muscle cells from sheep urethra. Am J Physiol Cell Physiol 279: C420–C428, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Imaizumi Y, Torii Y, Ohi Y, Nagano N, Atsuki K, Yamamura H, Muraki K, Watanabe M, Bolton TB. Ca2+ images and K+ current during depolarization in smooth muscle of the guinea-pig vas deferens and urinary bladder. J Physiol 510: 705–719, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ito Y, Kimoto Y. The neural and non-neural mechanisms involved in urethral activity in rabbits. J Physiol 367: 57–72, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kyle B, Bradley E, Ohya S, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. Contribution of Kv2.1 channels to the delayed rectifier current in freshly dispersed smooth muscle cells from rabbit urethra. Am J Physiol Cell Physiol 301: C1186–C1200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamb GD. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil 23: 81–91, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Mattiasson A, Andersson KE, Andersson PO, Larsson B, Sjögren C, Uvelius B. Nerve-mediated functions in the circular and longitudinal muscle layers of the proximal female rabbit urethra. J Urol 143: 155–160, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Melchiorre C, Angeli P, Lambrecht G, Mutschler E, Picchio MT, Wess J. Antimuscarinic action of methoctramine, a new cardioselective M-2 muscarinic receptor antagonist, alone and in combination with atropine and gallamine. Eur J Pharmacol 144: 117–124, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Mutoh S, Latifpour J, Saito M, Weiss RM. Evidence for the presence of regional differences in the subtype specificity of muscarinic receptors in rabbit lower urinary tract. J Urol 157: 717–721, 1997 [PubMed] [Google Scholar]
- 25. Nagahama K, Tsujii T, Morita T, Azuma H, Oshima H. Differences between proximal and distal portions of the male rabbit posterior urethra in the physiological role of muscarinic cholinergic receptors. Br J Pharmacol 124: 1175–1180, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pucovský V, Zholos AV, Bolton TB. Muscarinic cation current and suppression of Ca2+ current in guinea pig ileal smooth muscle cells. Eur J Pharmacol 346: 323–330, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Rios E, Stern MD. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct 26: 47–82, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol 526: 359–366, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol 283: C885–C894, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Interstitial cells of Cajal in the urethra. J Cell Mol Med 10: 280–291, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thor KB. Targeting serotonin and norepinephrine receptors in stress urinary incontinence. Int J Gynaecol Obstet 86 Suppl 1: S38–S52, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Thornbury KD, Hollywood MA, McHale NG, Sergeant GP. Cajal beyond the gut: interstitial cells in the urinary system—towards general regulatory mechanisms of smooth muscle contractility? Acta Gastroenterol Belg 74: 536–542, 2011 [PubMed] [Google Scholar]
- 33. Thornbury KD, Hollywood MA, McHale NG. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol 451: 133–144, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Unno T, Komori S, Ohashi H. Inhibitory effect of muscarinic receptor activation on Ca2+ channel current in smooth muscle cells of guinea-pig ileum. J Physiol 484: 567–581, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamanishi T, Chapple CR, Yasuda K, Yoshida K, Chess-Williams R. The role of M2 muscarinic receptor subtypes mediating contraction of the circular and longitudinal smooth muscle of the pig proximal urethra. J Urol 168: 308–314, 2002 [PubMed] [Google Scholar]