Abstract
Forkhead box O 1 (Foxo1) controls the expression of proteins that carry out processes leading to skeletal muscle atrophy, making Foxo1 of therapeutic interest in conditions of muscle wasting. The transcription of Foxo1-regulated proteins is dependent on the translocation of Foxo1 to the nucleus, which can be repressed by insulin-like growth factor-1 (IGF-1) treatment. The role of Foxo1 in muscle atrophy has been explored at length, but whether Foxo1 nuclear activity affects skeletal muscle excitation-contraction (EC) coupling has not yet been examined. Here, we use cultured adult mouse skeletal muscle fibers to investigate the effects of Foxo1 overexpression on EC coupling. Fibers expressing Foxo1-green fluorescent protein (GFP) exhibit an inability to contract, impaired propagation of action potentials, and ablation of calcium transients in response to electrical stimulation compared with fibers expressing GFP alone. Evaluation of the transverse (T)-tubule system morphology, the membranous system involved in the radial propagation of the action potential, revealed an intact T-tubule network in fibers overexpressing Foxo1-GFP. Interestingly, long-term IGF-1 treatment of Foxo1-GFP fibers, which maintains Foxo1-GFP outside the nucleus, prevented the loss of normal calcium transients, indicating that Foxo1 translocation and the atrogenes it regulates affect the expression of proteins involved in the generation and/or propagation of action potentials. A reduction in the sodium channel Nav1.4 expression in fibers overexpressing Foxo1-GFP was also observed in the absence of IGF-1. We conclude that increased nuclear activity of Foxo1 prevents the normal muscle responses to electrical stimulation and that this indicates a novel capability of Foxo1 to disable the functional activity of skeletal muscle.
Keywords: Foxo1-GFP, skeletal muscle, excitability, calcium transients, voltage-gated sodium channel
the process by which electrical stimulation of a skeletal muscle fiber initiates muscle contraction is termed excitation-contraction (EC) coupling. This process begins physiologically with a local depolarization of the muscle fiber at the neuromuscular junction triggering an action potential, which is propagated both axially along the fiber and radially into the fiber (2, 4, 16, 18, 22). The action potential depolarization is generated primarily by current through Nav1.4 sodium channels, the skeletal muscle isoform of the voltage-gated sodium channel, occurring all along the sarcolemma and through the transverse (T)-tubule system of the fiber (1, 3, 23). Depolarization of the T-tubule membrane induces conformational changes in the voltage sensor for EC coupling, the voltage-gated calcium channel (Cav1.1), also known as the dihydropyridine receptor (DHPR), which is mechanically coupled to the skeletal muscle ryanodine receptor Ca2+ release channel (RyR1) in the sarcoplasmic reticulum (SR; Ref. 39). The RyR1 channels mediate rapid Ca2+ release from the SR into the cytosol in response to the muscle action potential in the T tubules, leading to Ca2+ binding to thin filament troponin C and activation for contraction (6, 39, 42).
The family of Forkhead box O (Foxo) transcription factors, of which Foxo1 is a member, is evolutionarily conserved and characterized by a 100-residue DNA-binding region called the Forkhead domain (11). In skeletal muscle, Foxo transcription factors control muscle atrophy/hypertrophy by promoting transcription of ubiquitin ligases. Upregulation of either of two isoforms of Foxo, Foxo1 and Foxo3A (also referred to as Foxo3), is independently sufficient to induce muscle atrophy (24, 40). Knockout of Foxo1 is embryonic lethal, and tissue-specific induced knockout of Foxo1 results in tumor growth (19, 44). Foxo3 has been shown to regulate both lysosomal and proteasomal degradation through transcriptional regulation of “atrogenes,” proteins that induce muscle atrophy, such as atrogin-1/MAFbx, MuRF-1, LC3, and Bnip3 (31, 48). Previous work from our laboratory used exogenously expressed Foxo1-green fluorescent protein (GFP) to explore the kinetics of nuclear-cytoplasmic localization of Foxo1 (41). We determined that localization and translocation of Foxo1-GFP were consistent with those of endogenous Foxo1 (41). These data indicate that Foxo1-GFP is regulated as is Foxo1, and that fibers expressing Foxo1-GFP undergo normal cellular signaling and intracellular Foxo1-GFP movements (41). However, the role of Foxo1 in the EC coupling process of muscle fibers has not yet been examined.
Here, we identify a novel role of Foxo1 in control of excitation, and thus EC coupling, of skeletal muscle. Using exogenously expressed Foxo1-GFP in cultured adult flexor digitorum brevis (FDB) muscle fibers, we have determined that increased nuclear Foxo1 in muscle fibers leads to prevention of SR calcium release and the subsequent muscle contraction by decreasing fiber excitability in response to electric field stimuli and that these effects are abolished in the presence of added IGF-1. However, the morphology of the T-tubule system is not altered and the overall health of the fibers does not appear compromised. A decrease in Nav1.4 expression in fibers overexpressing Foxo1 was also observed in the absence of IGF-1. We conclude that increased nuclear activity of Foxo1 leads to prevention of the normal muscle response to electrical stimulation and that this indicates a novel capability of Foxo1 to disable the functional activity of skeletal muscle.
MATERIAL AND METHODS
Muscle fiber culture and adenoviral infection.
Culture of FDB and infection were carried out as detailed previously (17, 41). Animals were euthanized by asphyxiation via CO2 followed by cervical dislocation according to protocols approved by the University of Maryland Institutional Animal Care and Use Committee. Briefly, the muscle was isolated from female adult CD-1 mice, enzymatically dissociated with collagenase type I (Sigma-Aldrich, St. Louis, MO) in MEM (Life Technologies, Carlsbad, CA) with 10% FBS and 50 μg/ml gentamicin for 2 h at 37°. Muscle was then gently triturated to separate fibers in MEM with FBS and gentamicin. Fibers were plated in MEM culture media and laminin-coated glass-bottomed dishes containing lysate with adenovirus coding for GFP or Foxo1-GFP (a gift from Dr. Joseph Hill, University of Texas Southwestern Medical Center; Ref. 35) or with no added virus. Fibers treated with IGF-1 (Sigma-Aldrich) were plated in a dish containing 100 ng/ml IGF-1 in addition to lysate, and this concentration of IGF-1 was maintained for the entirety of the experiments. Fibers were maintained in culture for 2 days at 37°C, 5% CO2 before the experiments.
Indo-1 ratiometric Ca2+ imaging.
Indo-1 acetoxymethyl (AM) ratiometric imaging and analysis were performed as previously described (17) but with some modifications for loading. Briefly, cultured FDB fibers were loaded with indo-1 AM (2 μM/l for 60 min at 22°C; Life Technologies) in L-15 media (ionic composition in mM: 137 NaCl, 5.7 KCl, 1.26 CaCl2, and 1.8 MgCl2, pH 7.4; Life Technologies). Then, the fibers were washed thoroughly with appropriate L-15 media to remove residual indo-1 AM. The culture dish was mounted on an Olympus IX71 inverted microscope and viewed with an Olympus ×60/1.20 NA water immersion objective. Fibers were illuminated at 360 nm, and the fluorescence emitted at 405/30 and 485/25 nm was detected simultaneously. The emission signals were digitized and sampled at 10 Hz using a built-in AD/DA converter of an EPC10 amplifier and the acquisition software Patchmaster (HEKA Instruments, Bellmore, NY). Field stimulation (1 ms, 10–25 V, alternating polarity) was provided by a custom pulse generator through a pair of platinum electrodes. The electrodes were closely spaced (0.5 mm) and positioned directly above the center of the objective lens to achieve semilocal stimulation.
Rhod-2 x-y time lapse high-speed confocal Ca2+ imaging.
Rhod-2 measurements were carried out on a high-speed confocal system LSM 5 Live system (Carl Zeiss, Jena, Germany). Fiber culture chambers were loaded with 1 μM rhod-2 AM (Life Technologies, Carlsbad, CA) in L-15 media for 1 h at room temperature. Then, the fibers were washed thoroughly with L-15 media to remove residual rhod-2 AM. Individual fibers were imaged with a ×10/0.3 NA water-immersion objective lens. Excitation for rhod-2 was provided by the 532-nm line of a 100-mW diode laser, and emitted light was collected at >550 nm. One-millisecond electrical field stimuli were applied at a 1-Hz frequency for 6 s via two parallel platinum wires positioned at the bottom of the dish, ∼5 mm apart. Application of each stimulation protocol was synchronized relative to the start of confocal scan acquisition. Typically, the field stimulus was applied 300 ms after the start of the confocal scan sequence, thus providing control images before stimulation at the start of each sequence. These control images were used to determine the resting steady-state fluorescence level (F0). Average intensity of fluorescence within selected small (generally 300–450 μm2) regions of interest (ROIs) located at the ends of the fiber were measured with Zeiss LSM Image Examiner (Carl Zeiss, Jena, Germany). Images in x-y mode (frame size: 512 × 512 pixels; scan speed: 33.3 ms/frame for 10-s acquisition) were background corrected by subtracting an average value recorded outside the cell. The average F0 value in each ROI before electrical stimulation was used to scale Ca2+ signals in the same ROI as ΔF/F0. No attempts were made to estimate the actual cytosolic Ca2+ concentration.
Transverse tubular network imaging in living fibers.
Control, GFP, or Foxo1-GFP fibers were stained with the voltage-sensitive dye pyridinium,4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}-1-(3-sulfopropyl)-inner salt (di-8-ANEPPS; Life Technologies, Carlsbad, CA; 2.5 μM/l; in L-15 media for 1 h) and imaged on a Fluoview 500 confocal system (Olympus; ×60, 1.3 NA water-immersion objective; pixel dimensions 0.2 × 0.2 μm in x and y). Confocal images of the tubular network were obtained with 512 × 512 pixel x-y images (average of 8 images). Images were collected from randomly selected fibers using the same image acquisition settings and enhancing parameters. Images were background corrected, and a ROI of fixed dimensions was used to estimate average fluorescence profile within the ROI.
Action potential recordings.
Potentiometric dye action potential recordings and analysis were performed as previously described but with some modifications (37). FDB fibers were stained with 2.5 μM di-8-ANEPPS in the incubator for 3 h, followed by three washes in L-15 media. Fiber cultures were mounted on a Zeiss LSM 5 LIVE high-speed confocal system and stimulated with dual platinum field electrodes. Fiber fluorescence was excited with a 532-nm diode laser, and fluorescence emission >550 nm was sampled during repeated line scans through the interior of fibers (100 μs/line). The line scan was conducted at a depth of approximately 15–20 μm into the interior of the fiber. Signals were converted to −ΔF/F0 values, and four trials with the same electrode polarity were averaged to increase the signal-to-noise ratio. Action potentials were triggered using the same 1-ms electrical stimulus as in Ca2+ release assays. All single fiber recordings were performed at room temperature, 22°C. Where noted, tetrodotoxin (TTX; 1 μM, 5-min treatments; Sigma-Aldrich) were added to the recording solution to eliminate action potentials.
Western blotting.
Protein extraction and Western blotting techniques were performed as previously described (17, 41) with slight modifications. Briefly, dissociated FDB fibers were infected and cultured for 2 days and treated with IGF-1 when indicated. Culture plates containing fibers from FDB muscles provided a sufficient amount of cellular material for Western blot experiments. An independent experiment consisted of cultured isolated fibers from two whole FDB muscles, three to five mice per group. Cultured FDB fibers were homogenized with a mammalian protein extraction reagent (M-PER; Thermo Scientific, Rockford, IL) supplemented with protease inhibitor cocktail (Complete-Mini EDTA free; Roche Diagnostics) at 4°C for 60 min. This cell suspension was pipetted up and down to lyse fibers, The homogenates were subjected to centrifugation at 10,000 rpm for 10 min at 4°C. The supernatant was extracted and protein concentrations were measured using a Nanodrop-1000 spectrophotometer (Thermo Scientific). Then, 20 μg of protein samples were fractionated by 4–12% SDS-PAGE at 120 V at 4°C and transferred to PVDF membrane at 22 V overnight at 4°C. Blots were then processed and probed with antibodies against skeletal muscle voltage dependent sodium channel Nav1.4 (1:1,000; cat no. S9568; Sigma) and GAPDH (1:20,000; cat. no. G8795; Sigma). Blots were incubated with the appropriate horseradish peroxidase-labeled secondary antibodies (Cell Signaling Technology, Danvers, MA). Films were developed following the exposure to sensitive enhanced chemiluminescent substrate (Pierce ECL Western Blotting substrate; Thermo Scientific) to detect horseradish peroxidase on immunoblots. ImageJ (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/) was used to analyze data. The relative levels of Nav1.4 were calculated as a ratio against GAPDH and then normalized to those of controls run in the same gel. The normalized ratios of four Western blots were averaged and are presented as a bar graph ± SE.
Time-lapse imaging and fiber size analysis.
A long-term live cell incubation and automated microscope image acquisition system (Vivaview FL Incubator; Olympus) was used to monitor changes in single fiber size. Fibers were maintained in MEM without FBS and supplemented with 50 μg/ml gentamicin sulphate in 5% CO2 (37°C). Where indicated fibers were treated with 100 ng/ml IGF-1. Time-lapse imaging of several fibers was conducted for up to 72 h at 60-min acquisition intervals using a ×20 objective magnification and computer controlled microscope stage to return to the same fibers during each imaging sequence. Fibers in up to four culture dishes were monitored in parallel with the incubator microscope imaging system. For adenoviral mediated transfection, cultured fibers were incubated with the adenovirus for 1 h in MEM without antibiotics and no FBS added at 37°C, and then the medium was replaced and dishes were transferred to incubator equipped with an automated microscope imaging acquisition system. Fibers were imaged using differential interference contrast, and fiber width was measured at three different locations chosen randomly along the fiber length as indicated in results (see Fig. 8, A and B), using MetaMorph Premier (Molecular Devices Sunnyvale, CA). The average width per fiber was calculated as the mean of three measurements taken along the length of the fiber at the beginning (initial width: Iw) and at the end (final width: Fw) of the time-lapse experiment. The square of the ratio of the final width/initial width (final width/initial width)2 was used to quantify the change in muscle fiber volume, assuming that fiber size changed by the same fraction in the image plane (measured width in the xy image plane) and along the z-axis (not measured) and that the fiber length remained constant.
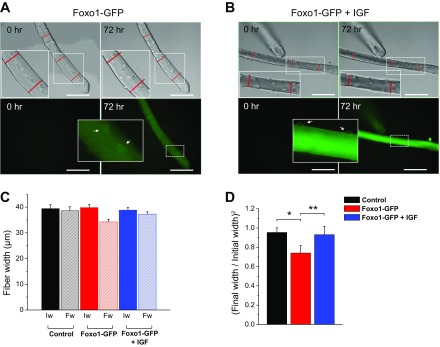
Fig. 8.
In vitro time-lapse study of atrophy in single FDB muscle fibers. Cultured FDB fibers were infected with a Foxo1-GFP adenoviral vector and incubated in the absence or presence of 100 ng/ml IGF-1 for 72 h. Foxo1-GFP signal was used to monitor Foxo1 expression and location. A: transmitted light images (top) and wide-field fluorescence images (bottom) of a representative muscle fiber after infection with Foxo1-GFP adenovirus showing the same fiber observed at the beginning of the time-lapse experiment (time 0; left) and after 72 h (right). B: transmitted light images (top) and wide-field fluorescence images (bottom) of another representative muscle fiber after infection with Foxo1-GFP adenovirus and treated with IGF-1 (100 ng/ml) showing the same fiber observed at the beginning of the time-lapse experiment (time 0; left) and after 72 h (right). Scale bars = 100 μm. Red lines in A and B indicate fiber width measurements from three different locations along the length of the fiber at time 0 and reused on panels from 72 h. Note the reduction on fiber width after 72 h in fibers expressing Foxo1-GFP. Insets are zoom-in versions of dashed regions. Arrows in A, bottom, show Foxo1-GFP nuclear localization, whereas arrows in B, bottom, show weaker Foxo1-GFP nuclear localization. C: summary of fiber width measurements for control fibers (n = 12, N = 3), fibers infected with Foxo1-GFP (n = 20, N = 3), and fibers expressing Foxo1-GFP and treated with IFG-1 (n = 24, N = 3). N indicates number of mice per condition, and n indicates number of fibers tested. The average width per fiber was calculated as the mean of 3 measurements taken along the length of the fiber at the beginning (initial width: Iw) and at the end (final width: Fw) of the time-lapse experiment. D: summary of fiber size measurements. The square of the ratio of the final width/initial width (final width/initial width)2 was used to quantify the change in muscle fiber diameter. Note the significant reduction of fiber size after 72 h in fibers expressing Foxo1-GFP. *P < 0.05, compared with control fibers. **P < 0.05, compared with fibers infected Foxo1-GFP and IGF-1 treated.
Data analysis and statistics methods.
To avoid one source of systematic bias, experimental and control measurements were alternated whenever possible and concurrent controls were always performed. All recordings were obtained at room temperature (19–22°C). Indo-1 and rhod-2 Ca2+ imaging as well as action potential waveform data was analyzed using OriginPro 8. T-tubule staining using di-8-ANEPPS was analyzed with ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/). Further data evaluation and statistical analysis were conducted using OriginPro 8 software (OriginLab, Northampton, MA). Summary data were reported as means ± SE when samples followed normal distributions and as medians when sample distributions were less well defined. Statistical significance was assessed using either parametric two sample t-test or with the nonparametric Mann-Whitney rank-sum test for unpaired data sets.
RESULTS
Fibers expressing Foxo1-GFP appeared healthy and responsive to chemical stimulation.
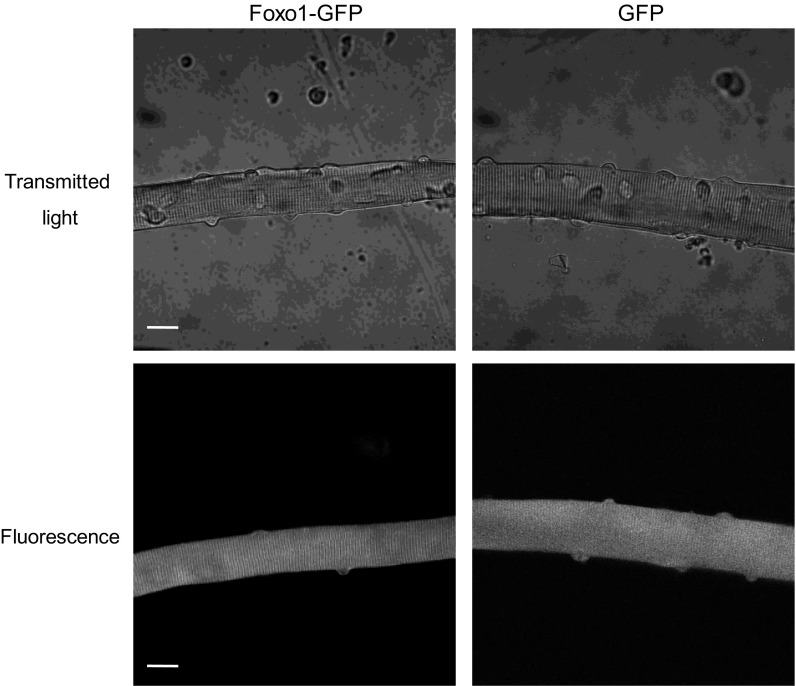
Fibers expressing Foxo1-GFP appeared normal with clearly visible striations that give rise to the skeletal and cardiac muscle alternative name: striated muscle (Fig. 1). These fibers also demonstrated functional Foxo1-GFP signaling in response to treatment with IGF and kinase inhibitors, with, respectively, increased or decreased Foxo1-GFP nuclear fluorescence as previously described by our laboratory (41). Furthermore, nuclear/cytoplasmic distribution of endogenous Foxo1 and exogenously expressed Foxo1-GFP was essentially the same, indicating that Foxo1 regulation is intact in fibers expressing Foxo1-GFP (41). Importantly, exogenous Foxo1-GFP expression was merely sevenfold that of endogenous Foxo1 expression (41). Overall, these fibers look healthy and typical for cultured fibers.
Fig. 1.
Fibers expressing Forkhead box O 1 (Foxo1)-green fluorescent protein (GFP) appear normal. Transmitted light images (top) and GFP fluorescence images (bottom) of Foxo1-GFP (left) or GFP (right) expressing flexor digitorum brevis (FDB) fibers. Signs of health are visible: striations and nuclei are predominantly peripheral, and fibers are smooth and straight. Scale bars = 20 μm.
Foxo1 suppresses stimulation-induced calcium transients.
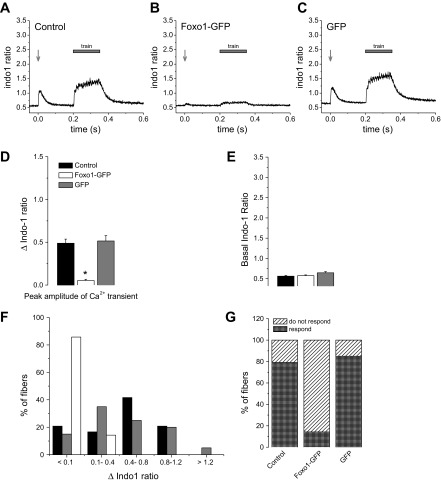
Although Foxo1-GFP fibers appeared healthy and biochemically functional, they responded to electrical stimulation in an abnormal manner. When control fibers, with or without GFP expression, were stimulated using either a single 1-ms, 25-V field stimulus or by a train of such pulses at 100 Hz, they exhibited robust calcium transients (Fig. 2, A and C) and contraction (Supplemental Video S1; Supplemental Material for this article is available online at the Am J Physiol Cell Physiol website). In stark contrast, fibers from the same muscle but expressing Foxo1-GFP generally respond to the same electric field stimuli with compromised calcium transients (Fig. 2B) or weak contraction (Supplemental Video S2). The average change in the indo-1 ratios (Δindo-1 ratio) of fibers in response to electrical simulation was significantly decreased in fibers expressing Foxo1-GFP (referred to here as Foxo1-GFP fibers) compared with control and GFP fibers (Fig. 2D; Δindo-1 ratio was 0.05 in fibers expressing Foxo1-GFP vs. 0.48 and 0.51 in control and GFP fibers, respectively; P < 0.05). The average change in indo-1 ratio in response to a single electrical stimulus of 25 V in Foxo1-GFP fibers was ∼10% that of control fibers or fibers expressing GFP (referred to here as GFP fibers; Fig. 2D). Interestingly, 86% of Foxo1-GFP fibers tested did not respond to electrical stimulation with a calcium transient, and the remaining small fraction (14%) of Foxo1-GFP fibers showed only a small increase in cytoplasmic calcium (Fig. 2F). In contrast, only 21% of control fibers and 15% of GFP fibers did not display a calcium transient upon electrical stimulation, and the other fibers displayed responses in a range of amplitudes (Fig. 2, F–G).
Fig. 2.
Calcium transients are ablated in fibers overexpressing Foxo1-GFP. A: control fibers (n = 24, N = 4) exhibit calcium transients in response to either a single 25-V field stimulation pulse (1-ms duration) or a 180-ms, 100-Hz train of such stimuli. B: fibers expressing Foxo1-GFP (n = 35, N = 5) show decreased calcium transients in response to the same pattern of electrical stimulation. C: fibers expressing GFP (n = 40, N = 4) alone respond to electrical stimulation with a calcium transient in a manner consistent with typical healthy FDB fibers. N indicates number of mice per condition, and n indicates number of fibers tested. D: average change in the indo-1 ratios of fibers in response to electrical simulation is dramatically decreased in fibers expressing Foxo1-GFP compared with control and GFP fibers. *P < 0.05, compared with control and GFP fibers. E: indo-1 resting ratios are not statistically different in fibers expressing Foxo1-GFP and fibers expressing GFP alone compared with control fibers. F: distribution of Δindo-1 amplitude in response to a single stimulus. The majority of Foxo1-GFP fibers have a range of no response to decreased intensity, whereas control and GFP fibers have varying degrees of strength. G: percentage of Foxo1-GFP fibers that exhibited a calcium transient, as defined by peak Δindo-1 ratio >0.1 in response to electrical stimulation is significantly lower than that of control fibers and GFP fibers.
Foxo1-GFP fibers do not exhibit altered resting calcium concentration.
Another factor that is related to the overall health of a fiber is the resting calcium concentration. Control, Foxo1-GFP, and GFP fibers all had resting indo-1 ratios that were not significantly different, indicating that resting calcium concentrations are not altered by Foxo1 (Fig. 2E; P > 0.05).
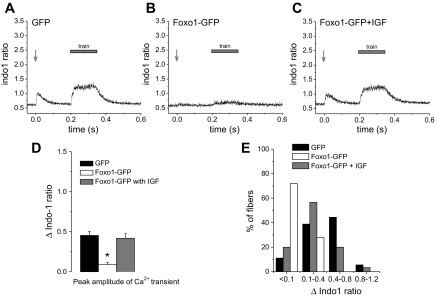
Foxo1-GFP fibers treated with IGF-1 responded to electrical stimulation.
Treatment with IGF-1 prevents nuclear targeting of Foxo1 (41, 47). To determine the role of nuclear Foxo1 in regulating the response to electrical stimulation, we treated Foxo1-GFP fibers with 100 ng/ml IGF-1 during the 2-day culture period and during the experiment to prevent functional activity of Foxo1 as a transcription factor by keeping it out of fiber nuclei. Remarkably, fibers expressing Foxo1-GFP but treated with IGF-1 (referred to here as Foxo1-GFP/IGF fibers) responded to electrical stimulation with appropriate calcium transients despite expression of Foxo1-GFP (Fig. 3, A–C). The difference between indo-1 fluorescence ratio signals in response to electrical stimulation in GFP fibers and Foxo1-GFP/IGF fibers was not significant (Fig. 3D, Δindo-1 ratio was 0.45 in GFP fibers vs. 0.41 in Foxo1-GFP/IGF fibers; P > 0.05), whereas Foxo1-GFP fibers exhibit significantly decreased indo-1 transients in response to electrical stimulation (Fig. 3D; Δindo-1 ratio was 0.08 in Foxo1-GFP fibers vs. 0.41 in Foxo1-GFP/IGF fibers; P < 0.05). Similar fractions of GFP fibers and of Foxo1-GFP/IGF fibers responded to electrical stimulation with strong, weak, or negligible Δindo-1 ratio signals, whereas the Foxo1-GFP fibers exhibited mostly weak or negligible calcium responses and did not contract or contracted in only a local fashion (Fig. 3E). These results indicate that Foxo1-GFP/IGF fibers consistently behave in the same manner as control fibers and that the presence of IGF during the culture period prevents the impairment in activation of calcium transients that develops in Foxo1-GFP fibers. These results provide evidence that the observed inability of Foxo1-GFP fibers to contract and release calcium results from functional, presumably transcriptional, activity of Foxo1 within the fiber nuclei.
Fig. 3.
Insulin-like growth factor-1 (IGF-1) treatment prevents Foxo1 effects on action potential induced Ca2+ transients. Cultured fibers expressing GFP (A; n = 36, N = 4), fibers expressing Foxo1-GFP (B; n = 25, N = 4), and fibers expressing Foxo1-GFP treated with IGF-1 from the time of infection (for 48 h; C; n = 30, N = 4) were stimulated using the same pattern of stimulation as detailed in Fig. 2. N indicates number of mice per condition, and n indicates number of fibers tested. The vast majority of the Foxo1-GFP fibers treated with IGF-1 did not have compromised calcium transients as those seen in untreated fibers expressing Foxo1-GFP. D: average change in indo-1 ratio in response to electrical stimulation is significantly reduced in fibers expressing Foxo1-GFP compared with fibers expressing GFP alone or fibers expressing Foxo1-GFP and treated with IGF-1. *P < 0.05, compared with fibers expressing Foxo1-GFP and treated with IGF-1. E: distribution of Δindo-1 amplitude in response to a single stimulus in fibers expressing GFP, fibers expressing Foxo1-GFP, and fibers expressing Foxo1-GFP and treated with IGF-1. Percentage of Foxo1-GFP fibers treated with IFG-1 that exhibited a calcium transient, as defined by peak Δindo-1 ratio >0.1 in response to electrical stimulation is higher than that of Foxo1-GFP fibers.
T-tubule system remained unaltered.
The T-tubule system is the membrane system along which depolarization spreads inward into the fiber (14, 15, 36). Breakdown of this system would disrupt propagation of the action potential and thus the contraction of the fiber. To examine the integrity of the T-tubule system we stained control fibers and Foxo1-GFP fibers with voltage-dependent membrane dye di-8-ANEPPS, which stains the T-tubule system and plasma membrane. Imaging these fibers using fluorescence confocal microscopy established that the morphology of the T-tubule system of Foxo1-GFP fibers (Fig. 4A) was unaltered compared with control fibers (Fig. 4B).
Fig. 4.
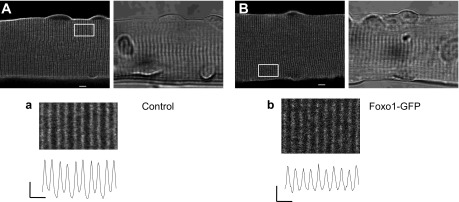
Transverse (T)-tubule arrangement and distribution as well as overall fiber morphology remain unaltered in fibers expressing Foxo1-GFP. Representative confocal (left) and transmitted light images (right) of a control fiber (A) or a fiber expressing Foxo1-GFP (B) cultured for 48 h and then stained with pyridinium,4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}-1-(3-sulfopropyl)-inner salt (di-8-ANEPPS). In control fibers (n = 14, N = 3), T-tubules are organized in a regular striated pattern, characterized by a ∼2-μm sarcomere length and ∼1 μm T-tubule “doublet” spacing. No changes in T-tubule morphology are seen in fibers expressing Foxo1-GFP (n = 18, N = 4). N indicates number of mice per condition, and n indicates number of fibers imaged. Scale bars in A and B = 5 μm. a and b, bottom: zoom-in versions of boxed regions indicated in A and B. Traces below zoom-in images are averaged fluorescence profiles across the box: vertical scale bar = 500 arbitrary units; horizontal scale bar = 2 μm.
Local calcium transients in fibers expressing Foxo1-GFP.
To further determine the effect of Foxo1 on muscle fiber excitability, we next used ultra-high-speed x-y imaging (33.3 ms per frame) to image the distribution of cytoplasmic Ca2+ along the entire length of the muscle fiber in contrast to the relatively small length of fiber monitored using the indo-1 spot illumination. Here we used the indicator rhod-2, which uses longer wavelengths than GFP and thus can be used in Foxo1-GFP or GFP expressing fibers. Monitoring the Ca2+ response along the entire length of FDB fibers in response to a 1-ms electric field stimuli repetitively applied at 1-s intervals, but with alternating polarity, we found that 82% of 17 control GFP fibers gave a synchronized Ca2+ transient over the entire fiber, independent of the alternating polarity of the stimulus (Fig. 5A). This is the typical response of a normal muscle fiber to a brief stimulus that activates a propagated action potential (38). In contrast, in response to the same alternating polarity electric field stimuli, 85% of 19 fibers expressing Foxo1-GFP gave only a reduced amplitude local Ca2+ response at either one end or the other end of the fiber (Fig. 5B). The fiber end exhibiting the local response alternated between ends when the polarity of the field stimulus was alternated, which is a characteristic sign of a local, nonpropagating membrane depolarization causing a local release of Ca2+, with the local depolarization alternating between ends when the direction of current flow is reversed. As quantified in Fig. 5C, Foxo1-GFP fibers demonstrate a 69.6% decrease in peak ΔF/F0 in the responding end of the fiber when compared with GFP controls following single field electrical stimulation (GFP: ΔF/F0 = 10.44 ± 0.43, n = 17; Foxo1-GFP: ΔF/F0 = 3.17 ± 0.21, n = 19; P < 0.05). Thus Foxo1-GFP-expressing fibers appear to have defective action potential initiation and/or propagation.
Fig. 5.
Fibers expressing Foxo1-GFP exhibit local Ca2+ responses. Control fibers expressing GFP (A, left) and fibers expressing Foxo1-GFP (B, left) were loaded with rhod-2 and their calcium responses to electrical stimulation were recorded. Time course of rhod-2 fluorescence in response to field stimulation (A and B, right). White boxes in A and B, left, show the regions of interest used to measure the calcium transients in response to electrical stimulation. GFP fibers responded to both polarities with calcium transients (A), whereas Foxo1-GFP fibers only responded locally to each polarity (B). C: bar plot summarizing differences in peak amplitude of rhod-2 ΔF/F0 transient. GFP fibers = 10.44 ± 0.43, n = 17, N = 3; Foxo1-GFP fibers = 3.17 ± 0.21, n = 19, N = 3. N indicates number of mice per condition, and n indicates number of fibers tested. *P < 0.05, compared with control GFP fibers.
Fibers expressing Foxo1-GFP failed to propagate action potentials.
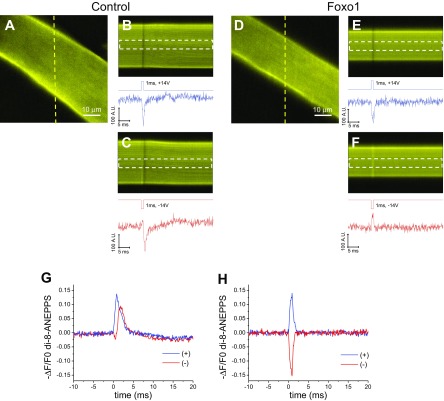
To further explore the cause of the failure of Foxo1 fibers to generate calcium transients and contract, we used di-8-ANEPPS to visualize the membrane potential response to electric stimulation. Both control fibers and Foxo1 fibers appeared the same when stained with this dye (Figs. 4 and 6, A and D). We determined the response of fibers to external electrical stimulation by taking a continuous line scan image of a line passing across the fiber before, during, and after stimulation with positive or negative electric field stimuli of 14-V, 1-ms duration, and then quantifying the fluorescence (Fig. 6, B–C and E–F). The fluorescence of di-8-ANEPPS decreases with the depolarization of the fiber (10). Control fibers responded to either positive or negative applied field stimuli with a decrease in di-8-ANEPPS fluorescence, indicating that applied field stimuli of either polarity generated a depolarizing action potential (Fig. 6, A–C and G; n = 10). In contrast, in Foxo1 fibers, a 1-ms, 14-V negative field stimulus caused an increase in di-8-ANEPPS signal, whereas a positive stimulus of 14 V caused a decrease in fluorescence (Fig. 6H; n = 10). Such reversal of membrane potential signal polarity with the alternation of the stimulus polarity is a characteristic of passive electrotonic polarization, which reverses with the reversal of polarity of the field stimulus. Measurement of membrane potential changes in control fibers treated with TTX to inactivate sodium channels showed a smaller and shorter increase or decrease in fluorescence, which alternated with the alternating polarity of the stimulus (data not shown); see Ref. 37, similar to the responses seen in Foxo1-GFP fibers untreated with TTX. These results demonstrate the inability of Foxo1-GFP fibers to respond to electrical stimulation by propagating action potentials.
Fig. 6.
Propagation of an action potential is prevented by Foxo1 overexpression. A and D: representative confocal x-y images of a control fiber (A; n = 10, N = 3) and a fiber expressing Foxo1-GFP (D; n = 10, N = 3) stained with the voltage-sensitive dye di-8-ANEPPS. N indicates number of mice per condition, and n indicates number of fibers tested. Yellow lines in A and D indicate the location of the line scan used for B and C, and E and F, respectively. B, C, E, and F, bottom: graphs of the time course of di-8-ANEPPS fluorescence in response to field stimulation in the regions indicated by white dashed boxes. G and H: average change in di-8-ANEPPS fluorescence in control fibers (G) and fibers expressing Foxo1-GFP (H) in response to field stimulation of alternating polarity.
Expression of the sodium channel Nav1.4 protein was decreased by Foxo1-GFP.
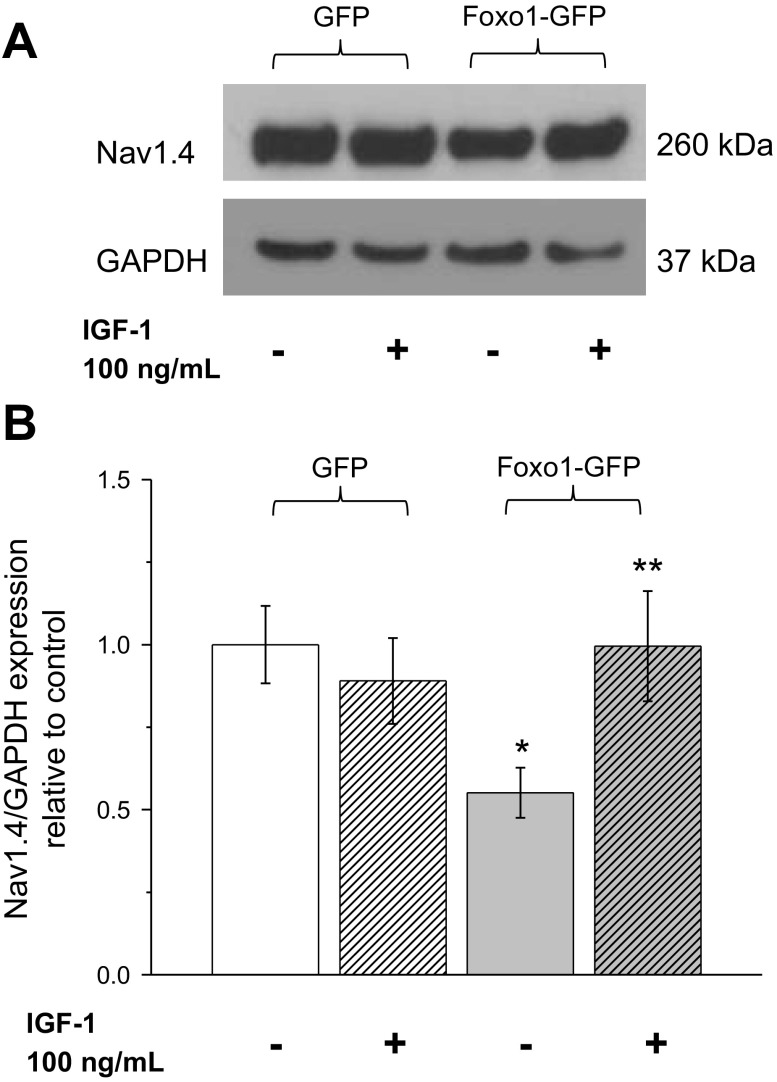
Due to the integral role of sodium channels in initiation and propagation of an action potential (3, 4, 23), we explored the possibility that overexpression of exogenous Foxo1-GFP negatively regulated expression of the skeletal muscle sodium channel Nav1.4. To this end, we used Western blot analysis of muscle fibers expressing GFP alone, expressing Foxo1-GFP, or expressing Foxo1-GFP but with IGF-1 treatment. Foxo1 fibers only expressed 55% of Nav1.4 compared with GFP fibers. Fibers expressing Foxo1-GFP and treated with IGF-1 had expression levels quite similar to that of GFP fibers (Fig. 7).
Fig. 7.
Foxo1 activity decreases the expression of the skeletal muscle sodium channel Nav1.4. A: Western blot analysis of whole cell homogenates prepared from FDB muscles using Nav1.4 and GAPDH antibodies. A: expression of Nav1.4 and GAPDH in fibers expressing GFP, fibers treated with IGF-1 expressing GFP fibers expressing Foxo1-GFP, and fibers treated with IGF-1 expressing Foxo1-GFP. Blot is representative of 4 independent experiments (4 mice per group). B: quantification of Western blots show that fibers expressing Foxo1-GFP have a decreased expression of Nav1.4 compared with fibers expressing GFP. *P < 0.05, compared with control GFP fibers. IGF-1 treatment of fibers expressing Foxo1-GFP recovers the loss of expression of Nav1.4. **P < 0.05 compared with Foxo1-GFP fibers.
We conclude that overexpression of Foxo1-GFP decreases the expression of the sodium channel Nav1.4 protein and thus prevents action potential initiation and/or propagation, thereby greatly decreasing the depolarization of the T-tubule membrane and the resulting calcium release and subsequent contraction of the muscle fiber. Furthermore, inhibition of nuclear influx of Foxo1-GFP by application of IGF-1 diminished this effect of Foxo1 on Nav1.4 expression, indicating that the nuclear activity of Foxo1, presumably as a transcription factor, regulates the level of Nav1.4 in skeletal muscle fibers.
Foxo1-GFP overexpression reduces muscle fiber size.
Previous work showed that Foxo transcription factors induce the expression of atrogenes (i.e., atrogin-1) and cause muscle atrophy (24, 40). We investigated whether overexpression of Foxo1-GFP in serum-free media caused muscle fiber atrophy in vitro, and this was indeed found to be the case over a period of 3 days in our adult muscle fiber culture system (Fig. 8). Muscle fibers were infected with Foxo1-GFP adenovirus, and automated microscope time-lapse imaging at 1-h intervals was used to characterize muscle fiber size changes over the course of 72 h in culture. Examination of the muscle fibers expressing Foxo1-GFP revealed a 25% reduction in average muscle fiber width in the image (xy) plane at the end of the 72-h time-lapse experiment compared with muscle width from the same fibers at the beginning of the experiment (Fig. 8, A and D). The addition of IGF-1 (100 ng/ml) to culture media prevented the loss in muscle size induced by Foxo1-GFP overexpression (Fig. 8, B and D). Serum deprivation resulted in a 5% reduction in average muscle size over the 72-h time lapse evaluated (Fig. 8, C and D). These findings indicate that Foxo1 overexpression leads to atrophy in muscle fibers cultured in serum-starved conditions and that IGF-1 supplementation prevents this effect.
DISCUSSION
The transcription factor Foxo1 controls muscle atrophy and regulates the expression of atrogenes such as atrogin-1 and MuRF1 (7, 33, 40). Here, we provide evidence for another role of Foxo1 in the degradation of skeletal muscle. The inability of fibers with increased nuclear levels of Foxo1 to propagate an action potential and activate calcium signaling or contract taken together demonstrates the capacity of Foxo1 to disable functional activity of skeletal muscle (Figs. 2, 3, and 6).
Two-day cultured muscle fibers overexpressing Foxo1-GFP appeared structurally normal and demonstrated normal cellular Foxo1 signaling (Figs. 1 and 4; see also Ref. 41). However, these fibers did not respond properly to electrical stimulation as do similar fibers not expressing Foxo1-GFP: they did not have normal calcium transients or propagate action potentials (Figs. 2, 3, and 6) as do fibers not expressing Foxo1-GFP. Expressing GFP alone or GFP fusion constructs of a variety other proteins does not cause similar suppression of fiber excitability (29, 30). The basal levels of cytoplasmic calcium of fibers expressing Foxo1-GFP were not changed from those of control fibers (Fig. 2E), indicating that the homeostatic mechanisms of calcium regulation were conserved, that sarco(endo)plasmic reticulum Ca2+-ATPase was functional, and that the RyR1 was not leaking in fibers expressing Foxo1-GFP. Foxo1 overexpression also did not alter the morphology of the T-tubule system, eliminating the possibility that a cause for the failure of fibers expressing Foxo1 to contract was the inability of current to flow through the T-tubule system (Fig. 4). Fibers overexpressing Foxo1-GFP showed signs of accentuated atrophy compared with control counterparts, and this effect was attenuated in the presence of IGF-1 (Fig. 8).
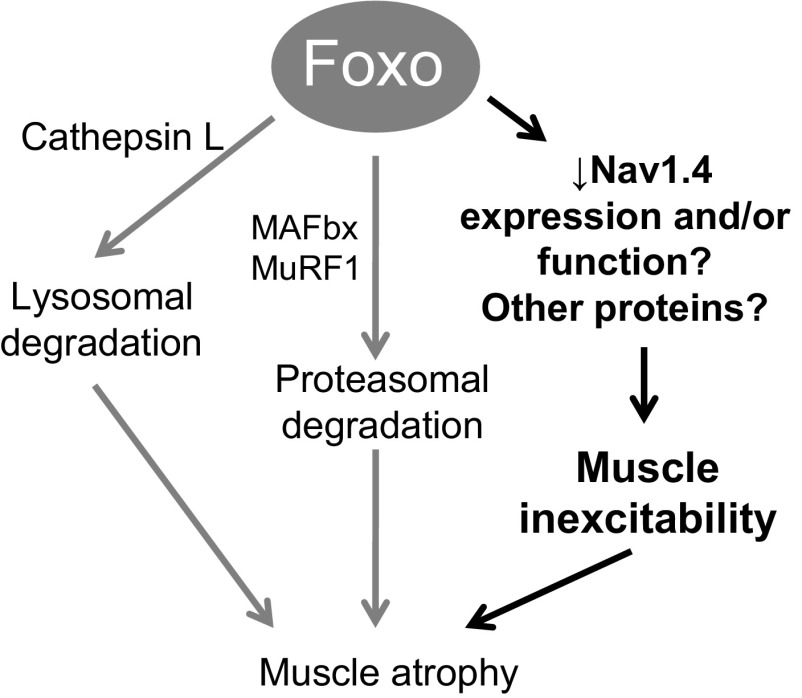
Nav1.4 is an essential part of excitation of skeletal muscle fibers (3, 23). As such, its regulation is of importance to muscle excitability. The ability of Foxo1 to diminish the excitability of a fiber, by regulating the expression of Nav1.4 (Fig. 7), and possibly its function, indicates the likely involvement of Foxo1 in another aspect of regulation of skeletal muscle health (Fig. 9). This is supported by a recent finding that in a cardiomyocyte cell line sodium channel Nav1.5 expression is negatively regulated by Foxo1 (32). Mao et al. (32) demonstrated that Foxo1 directly binds to the insulin responsive element, a conserved DNA sequence 5′-CAAAACA-3′ in the promoter region of SCN5A, the cardiac isoform of the sodium channel α1-subunit (Nav1.5), and inhibits its promoter activity. This inhibition downregulates Nav1.5 expression and sodium channel activity in cardiac myocytes. Using an in silico search of the upstream promoter region (bp: −5,000) in the Ensembl data base for the mouse SCN4A gene for the skeletal muscle sodium channel (Nav 1.4), we have identified three potential Foxo binding elements. The molecular mechanisms underlying the regulation of Nav1.4 expression are largely unknown and whether Foxo1 binds to SCN4a promoter and inhibits its activity remains undetermined. Intriguingly, this aspect of Foxo1 regulation suggests a positive feedback loop in which once Foxo1 is activated and begins to cause muscle atrophy, the muscle also loses its ability to respond to stimulation, thus increasing its atrophy as seen in models of disuse and denervation (12, 13, 25, 34, 45).
Fig. 9.
A working model that can account for the effects of Foxo1 on muscle excitability. Foxo1 regulates expression of proteins that carry out lysosomal degradation and proteasomal degradation leading to muscle atrophy. We here propose a novel mechanism in which Foxo1 transcriptional activity results in the inability of muscle fibers to respond to electrical stimulation. Foxo1 activity decreases the expression and/or function of sodium channel Nav1.4 and possibly other proteins critical for maintenance of excitability. Then, the lack of response to electrical stimulation and inability to contract, as seen in denervation and disuse, will eventually contribute to muscle atrophy.
Previous reports have shown that phosphatidylinositol 3-kinase (PI3K)-AKT pathway mediates the efflux of nuclear Foxo1 induced by IGF-1 (5, 8, 41). The IGF-1-induced increase in Nav1.4 expression in Foxo1-GFP fibers is likely mediated through activation of the PI3K-AKT pathway via phosphorylation of Foxo1, resulting in a predominant Foxo1 cytoplasmic localization. Whether PI3K-AKT pathway directly contributes to the increase on Nav1.4 expression induced by IGF-1 in muscle fibers overexpressing Foxo1 requires further work. Our data allow us to propose the hypothesis that IGF-1, either systemically supplied or locally expressed by muscle cells (27, 46), regulates the transcription and function of the main skeletal muscle fiber sodium channel Nav1.4. Our results also provide new mechanistic insight into the regulation and maintenance of the electrical properties of skeletal muscle fibers and its association with IGF-1 signaling, ultimately to Nav1.4 expression level.
Other mechanisms may also be involved in the regulation of NaV1.4 expression at the transcriptional level. In addition to the IGF1-PI3K-AKT-Foxo1 pathway, other growth factors, hormones, cytokines and myokines and their corresponding signaling pathways, transcription factors, and transcriptional regulators could potentially regulate Nav1.4 expression. For instance, it has been shown that transforming growth factor-β1, a proinflammatory cytokine released by myofibroblasts, regulates transcription and function of Nav1.5 in ventricular myocytes via a transforming growth factor-β1-PI3K-AKT-Foxo1 pathway (26). There is evidence of an NF-κB-dependent transcriptional regulation of the SCN5A sodium channel by angiotensin II through the production of reactive oxygen species resulting in NF-κB binding to the sodium channel promoter (43). Finally, in addition to AKT, other kinases such as serum and glucocorticoid-induced kinase 1 (9), cyclin-dependent kinase 2 (21), and IκB kinase (20) phosphorylate Foxo proteins, resulting in their net cytoplasmic translocation. Whether other extracellular messengers (i.e., proinflammatory cytokines) or intracellular signaling molecules (i.e., serum and glucocorticoid-induced kinase 1) or different transcription factors (i.e., NF-κB) suppress Nav1.4 expression in skeletal muscle fibers remains to be evaluated.
In this experimental setting, Foxo1 overexpression and its effects on EC coupling were evaluated in muscle fibers from FDB muscle, a muscle composed primarily of fast-twitch muscle fibers, and changes in excitability and fibers size were evident. However, the mechanistic details of this Foxo1-dependent regulation of fiber excitability at the subcellular level remain unsettled. In addition, it will be interesting to explore whether the effects of Foxo1 overexpression on excitability also apply to slow-twitch muscle fibers.
The present system of overexpression of Foxo1 can also be used as a model for overactivity or dysregulation of Foxo1 and the resulting effects on skeletal muscle, because overexpression effectively mimics activation by increasing the nuclear concentration of Foxo1 as occurs during dysregulation of Foxo1. Although the role of Foxo1 in skeletal muscle is established as a positive regulator of both lysosomal and proteasomal degradation leading to muscle atrophy (7, 28, 48), we reveal that dysregulation of Foxo1 can also cause impairment of muscle excitation and its ability to contract. With identification of this novel aspect of Foxo signaling, further understanding of the role of Foxo1 in muscle is now an even more attractive therapeutic avenue in the treatment and prevention of muscle atrophy.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01-AR056477 and R37-AR055099. T. N. Schachter was partially supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Training Grant T32-AR-007592 to the Interdisciplinary Program in Muscle Biology and National Heart, Lung, and Blood Institute Training Grant T32-HL-072751 to the Program in Cardiac and Vascular Cell Biology, University of Maryland School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.O.H.-O., T.N.S., and M.F.S. conception and design of research; E.O.H.-O. and T.N.S. performed experiments; E.O.H.-O. and T.N.S. analyzed data; E.O.H.-O., T.N.S., and M.F.S. interpreted results of experiments; E.O.H.-O. and T.N.S. prepared figures; E.O.H.-O., T.N.S., and M.F.S. drafted manuscript; E.O.H.-O., T.N.S., and M.F.S. edited and revised manuscript; E.O.H.-O., T.N.S., and M.F.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Joseph Hill, University of Texas Southwestern Medical Center, for kindly providing the adenovirus coding for Foxo1-GFP.
REFERENCES
- 1. Adrian RH, Chandler WK, Hodgkin AL. Voltage clamp experiments in striated muscle fibres. J Physiol 208: 607–644, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adrian RH, Costantin LL, Peachey LD. Radial spread of contraction in frog muscle fibres. J Physiol 204: 231–257, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adrian RH, Marshall MW. Sodium currents in mammalian muscle. J Physiol 268: 223–250, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adrian RH, Peachey LD. Reconstruction of the action potential of frog sartorius muscle. J Physiol 235: 103–131, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biggs WH, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96: 7421–7426, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107: 2587–2600, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21: 952–965, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiFranco M, Capote J, Vergara JL. Optical imaging and functional characterization of the transverse tubular system of mammalian muscle fibers using the potentiometric indicator di-8-ANEPPS. J Membr Biol 208: 141–153, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 14: 83–97, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89: 823–839, 2000. [DOI] [PubMed] [Google Scholar]
- 13. Fitts RH, Romatowski JG, Peters JR, Paddon-Jones D, Wolfe RR, Ferrando AA. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercortisolemia and ameliorated by dietary supplementation. Am J Physiol Cell Physiol 293: C313–C320, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annu Rev Physiol 56: 509–534, 1994. [DOI] [PubMed] [Google Scholar]
- 15. Franzini-Armstrong C, Porter KR. Sarcolemmal invaginations constituting the T system in fish muscle fibers. J Cell Biol 22: 675–696, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gage PW, Eisenberg RS. Action potentials, afterpotentials, and excitation-contraction coupling in frog sartorius fibers without transverse tubules. J Gen Physiol 53: 298–310, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernández-Ochoa EO, Robison P, Contreras M, Shen T, Zhao Z, Schneider MF. Elevated extracellular glucose and uncontrolled type 1 diabetes enhance NFAT5 signaling and disrupt the transverse tubular network in mouse skeletal muscle. Exp Biol Med (Maywood) 237: 1068–1083, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodgkin AL, Horowicz P. Potassium contractures in single muscle fibres. J Physiol 153: 386–403, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosaka T, Biggs WH, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA 101: 2975–2980, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117: 225–237, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science 314: 294–297, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Huxley AF, Taylor RE. Local activation of striated muscle fibres. J Physiol 144: 426–441, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jurkat-Rott K, Fauler M, Lehmann-Horn F. Ion channels and ion transporters of the transverse tubular system of skeletal muscle. J Muscle Res Cell Motil 27: 275–290, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279: 41114–41123, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33: 155–165, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Kaur K, Zarzoso M, Ponce-Balbuena D, Guerrero-Serna G, Hou L, Musa H, Jalife J. TGF-β1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PLoS One 8: e55391, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des 13: 663–669, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129: 227S-–237S., 1999. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol 155: 27–39, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol 168: 887–897, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007. [DOI] [PubMed] [Google Scholar]
- 32. Mao W, You T, Ye B, Li X, Dong HH, Hill JA, Li F, Xu H. Reactive oxygen species suppress cardiac NaV1.5 expression through Foxo1. PLoS One 7: e32738, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am J Physiol Cell Physiol 297: C548–C555, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Midrio M. The denervated muscle: facts and hypotheses. A historical review. Eur J Appl Physiol 98: 1–21, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation 114: 1159–1168, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peachey LD. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol 25, Suppl: 209–231, 1965. [DOI] [PubMed] [Google Scholar]
- 37. Prosser BL, Hernandez-Ochoa EO, Lovering RM, Andronache Z, Zimmer DB, Melzer W, Schneider MF. S100A1 promotes action potential-initiated calcium release flux and force production in skeletal muscle. Am J Physiol Cell Physiol 299: C891–C902, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prosser BL, Hernandez-Ochoa EO, Zimmer DB, Schneider MF. Simultaneous recording of intramembrane charge movement components and calcium release in wild-type and S100A1−/− muscle fibres. J Physiol 587: 4543–4559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 325: 717–720, 1987. [DOI] [PubMed] [Google Scholar]
- 40. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schachter TN, Shen T, Liu Y, Schneider MF. Kinetics of nuclear-cytoplasmic translocation of Foxo1 and Foxo3A in adult skeletal muscle fibers. Am J Physiol Cell Physiol 303: C977–C990, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature 242: 244–246, 1973. [DOI] [PubMed] [Google Scholar]
- 43. Shang LL, Sanyal S, Pfahnl AE, Jiao Z, Allen J, Liu H, Dudley SC. NF-κB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol 294: C372–C379, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegué E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339, 2007. [DOI] [PubMed] [Google Scholar]
- 45. Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil 17: 487–495, 1996. [DOI] [PubMed] [Google Scholar]
- 47. Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem 277: 45276–45284, 2002. [DOI] [PubMed] [Google Scholar]
- 48. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.