vascular disease contributes to the mortality of multiple diseases including myocardial infarction, stroke, renal failure, and peripheral vascular disease. The etiology of many vascular diseases originates with endothelial and inflammatory cells that synthesize growth and chemotactic factors. As part of the response to injury hypothesis (9), vascular smooth muscle cells (VSMCs) respond to many of these factors by switching from a quiescent to an “activated” cell capable of migration and proliferation. VSMC migration from the media into the intima in response to these soluble factors leads to a loss of lumen diameter and has been suggested as the most critical cellular event in the development of multiple vascular diseases. Medial-to-luminal VSMC migration is also a characteristic maladaptive response to interventional procedures such as balloon angioplasty and stent placement (1, 10). The molecular mechanisms of VSMC migration are well described (for an excellent review see reference 2). In the injured artery, pro-migratory cytokines are varied and numerous (2, 4). Platelet-derived growth factor (PDGF) concentrations in particular are increased in vascular injury, and PDGF is an acknowledged potent proliferative and pro-migratory stimuli for VSMCs (3, 4). The coordinated activation of numerous proteins allows the smooth muscle cell to protrude lamellipodia, remodel actin, form new contacts with the substratum, and migrate toward the chemotactic stimulus. To put it into a pathophysiological perspective, VSMC cytoskeletal remodeling resulting in medial-to-intimal migration is requisite for loss of lumen diameter in response to injury, and molecules that regulate this process can be considered targets of rational drug therapy with which to intervene in many vascular pathological processes. Increasing our intelligence quotient (IQ) of VSMC migration by characterization of these proteins will lead to more effective treatment strategies.
IQGAP1: A Key Player in VSMC Signaling and Locomotion
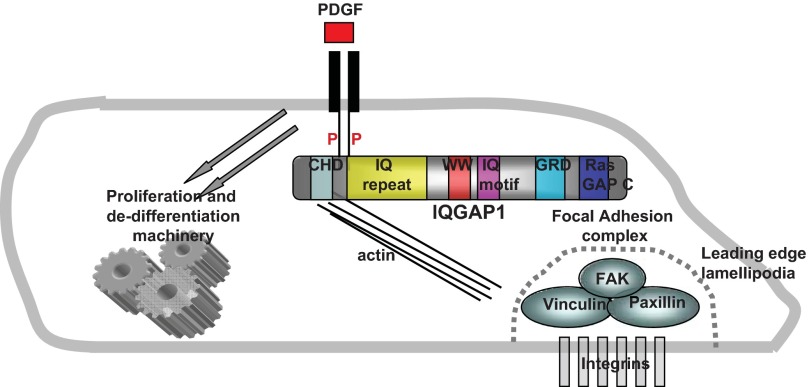
IQ motif containing GTPase-activating protein (IQGAP) is an ubiquitously expressed, highly conserved signaling protein. IQGAP1 possesses multiple conserved anchoring domains that mediate its interaction with many cytoplasmic and structural proteins including F-actin, ERK1/2, myosin light chain, and E-cadherin (for an excellent review see reference 7). IQGAP1 is central in regulation of several cellular processes including motility, adhesion, and cell-cell interactions. IQGAP1 is an effector for monomeric GTPases that regulate cell motility and also acts as a node for many key signaling pathways. With its multiple and varied protein interaction domains, it is not surprising this protein has been the subject of intense study in cell motility (Fig. 1).
Fig. 1.
IQGAP1 at the center of PDGF-initiated VSMC migration. Upon PDGF stimulation, IQGAP1 interacts with the phosphorylated PDGF receptor. Through its many protein-protein (P) interaction domains, IQGAP1 acts as a hub to congregate GTPases and other proteins that play a role in cell migration into reactive proximity with the focal adhesion complex. Domains in IQGAP1: CHD = calponin homology domain, interacts with F-actin; IQGAP repeat (IQ repeat) = IQGAP-specific repeat motif; WW = WW domain binds ERK1/2; IQ motif binds calmodulin and myosin essential light chain; GRD = Ras GAP-related domain, binds Rac and Cdc42; RasGAP COOH terminus (Ras Gap C), binds ERK1/2 and β-catenin.
In this issue of American Journal of Physiology-Cell Physiology, Kohno et al. (5) report that IQGAP1 may play an important role in regulation of VSMC migration, primarily by facilitating direct interaction between the platelet-derived growth factor receptor (PDGFR) and focal adhesion (FA) proteins. Considering that PDGF is such a potent proliferative and pro-migratory stimulus following vascular injury, this report has important ramifications for the treatment of vascular disease. Kohno and colleagues found that IQGAP1 interacts only with the activated form of PDGFR, and utilizing knockdown and overexpression strategies, propose IQGAP1 as necessary for PDGFR phosphorylation and thus its activation.
IQGAP1: A Potential Target for Anti-Restenotic Therapy
Focal adhesions are the connection points through which the cytoskeleton connects to the substratum. The FA complex consists of varied proteins that transduce both regulatory signals and mechanical force (8). PDGF receptor binding activates GTPases that regulate actin polymerization in the lamellipodia at the leading edge and the formation of FA complexes. Continuous actin remodeling and FA formation at the leading edge are necessary for directional cellular locomotion (8, 11).
How does the pro-migratory signal get from the receptor to the focal adhesion? Kohno and colleagues show that PDGF stimulation induced colocalization of both IQGAP1 and phosphorylated PDGFR to focal adhesions. PDGF mediated IQGAP1 interaction with focal adhesion proteins vinculin, paxillin, and focal adhesion kinases. Depletion of IQGAP1 reduced the assembly of these proteins at the FA, pointing to IQGAP1 as a molecular link between chemotactic stimulation and locomotion. Functionally, absence of IQGAP1 significantly reduced PDGF-driven migration of VSMCs. Not surprisingly, in a murine carotid artery injury model, medial and neointimal area was reduced in IQGAP1−/− mice. As important as the in vivo experiment is, there are two important caveats to consider. The first is that, like most vascular injury models, immune cells certainly participate in restenosis formation (1, 9, 10). It is appropriately noted by the authors that the reduction in neointimal hyperplasia in the pan knockout mouse may be due to lack of IQGAP1 in other cell types, particularly endothelial and immune cells. Cell type specificity is an important consideration, as this manuscript suggests cell-specific effects on migration and proliferation. In fibroblasts, IQGAP1 is required for hyaluronan-induced, but not PDGF-BB-induced, fibroblast migration (6). This same report indicates that IQGAP1 appears to be required for PDGF-driven fibroblast proliferation, which is in contrast to Kohno et al., who report that IQGAP1 knockdown has no effect on PDGF-driven VSMC proliferation. Since the carotid artery wire injury model is proliferative as well as migratory, cell type-specific functional effects need to be characterized if IQGAP1 is to be considered a target of therapy. A second caveat is the observation that Kohno and colleagues show by immunohistochemistry that IQGAP1 expression is increased in injured arteries, suggesting regulated induction by proliferative, or inflammatory factors in vivo. This is an important point of specificity for a ubiquitously expressed protein such as IQGAP1.
Summary and Future Considerations
These and other investigators have previously shown that IQGAP1 interacts with other growth factor receptors and is involved in endothelial cell migration. The novelty of this report is that IQGAP1-mediated functional interactions regulating VSMC pathophysiological processes are unreported. Important future studies need to identify the mechanisms by which IQGAP1 regulates PDGFR activation, as well as assign cell type-specific functionality using conditional knockout or bone marrow transplantation approaches. Collectively, the present study and previously published work implicate IQGAP1 as an essential molecular link that integrates VSMC growth factors with migratory machinery. Raising our IQ of IQGAP1 function will raise our IQ about VSMC migration and treatment of vascular diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-090885, HL-115575, and HL-117724 (to M. V. Autieri).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.V.A. prepared figure, drafted, revised, and approved final version of manuscript.
REFERENCES
- 1. Epstein SE, Speir E, Unger EF, Guzman RJ, Finkel T. The basis of molecular strategies for treating coronary restenosis after angioplasty. J Am Coll Cardiol 23: 1278–1288, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res 100: 607–621, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb 13: 1218–1226, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest 89: 507–511, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kohno T, Urao N, Ashino T, Sudhahar V, Inomata H, Yamaoka-Tojo M, McKinney RD, Fukai T, Ushio-Fukai M. IQGAP1 links PDGF receptor-β signal to focal adhesions involved in vascular smooth muscle cell migration: role in neointimal formation after vascular injury. Am J Physiol Cell Physiol (May 8, 2013). 10.1152/ajpcell.00011.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kozlova I, Ruusala A, Voytyuk O, Skandalis SS, Heldin P. IQGAP1 regulates hyaluronan-mediated fibroblast motility and proliferation. Cell Signal 24: 1856–1862, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci 118: 2085–2092, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153: 1175–1186, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Schwartz RS, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR. Restenosis after balloon angioplasty: a practical proliferative model in porcine coronary arteries. Circulation 82: 2190–2200, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans 32: 416–420, 2004 [DOI] [PubMed] [Google Scholar]