Abstract
Platelet-derived growth factor (PDGF) stimulates vascular smooth muscle cell (VSMC) migration and neointimal formation in response to injury. We previously identified IQ-domain GTPase-activating protein 1 (IQGAP1) as a novel VEGF receptor 2 binding scaffold protein involved in endothelial migration. However, its role in VSMC migration and neointimal formation in vivo is unknown. Here we show that PDGF stimulation rapidly promotes IQGAP1 association with PDGF receptor-β (PDGFR) as well as IQGAP1 tyrosine phosphorylation in cultured VSMC. Overexpression or knockdown of IQGAP1 enhances or inhibits PDGFR autophosphorylation (p-PDGFR), respectively. Immunofluorescence and cell fractionation analysis reveals that PDGF-induced p-PDGFR localized in focal adhesions (FAs), but not caveolae/lipid rafts, is inhibited by IQGAP1 knockdown with siRNA. PDGF stimulation promotes IQGAP1 association with PDGFR/FA signaling protein complex. Functionally, IQGAP1 siRNA inhibits PDGF-induced FA formation as well as VSMC migration induced by PDGF. In vivo, IQGAP1 expression is markedly increased at neointimal VSMC in wire-injured femoral arteries. Mice lacking IQGAP1 exhibit impaired neointimal formation in response to vascular injury. In summary, IQGAP1, through interaction with PDGFR and FA signaling proteins, promotes activation of PDGFR in FAs as well as FA formation, which may contribute to VSMC migration and neointimal formation after injury. Our findings provide insight into IQGAP1 as a potential therapeutic target for vascular migration-related diseases.
Keywords: platelet-derived growth factor, IQGAP1, migration, vascular injury, vascular smooth muscle cell
vascular smooth muscle cell (VSMC) migration is a critical event for the development of atherosclerosis and restenosis after vascular injury (5, 20, 22). Platelet-derived growth factor (PDGF) is a key growth factor to promote VSMC migration and neointimal formation in response to injury primary through the receptor tyrosine kinase, PDGF receptor-β (PDGFR) (20). A fraction of PDGFR is localized and activated in caveolae/lipid raft signaling domains following PDGF stimulation (1, 13, 14). VSMC migration requires the laying down at the front of the cell and removal at the back of small punctate structures, focal adhesions (FAs), that link the cell to its basement membrane. Nascent FAs at the leading edge provide adhesion of the lamellipodia to the substrate and provide traction for propulsion of the cell over or through the extracellular matrix (5). Signaling from FAs influences cell adhesion, cell motility, gene expression, and the cell cycle. The major components of FAs include focal adhesion kinase (FAK), vinculin, and paxillin. The underlying molecular mechanisms by which PDGF promotes FA formation and migration in VSMCs are incompletely understood.
IQ-domain GTPase-activating protein 1 (IQGAP1) is a scaffold protein that plays a pivotal role in regulating actin cytoskeleton and cell migration by interacting directly with F-actin, active Rac1/Cdc42, cytoskeletal, adhesion, and signaling proteins (4, 15, 19, 32). In actively migrating cells, IQGAP1 accumulates at the leading edge and regulates actin assembly (4, 15, 32). We previously identified IQGAP1 as a novel vascular endothelial growth factor receptor 2 (VEGFR2)-binding scaffold protein involved in endothelial migration (33) and postischemic angiogenesis (27). IQGAP1 also binds to other receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) to regulate its phosphorylation (16), and fibroblast growth factor receptor 1 (FGFR1) to regulate actin assembly (2). Thus, IQGAP1 has been implicated to function to link growth factor receptor signaling to actin cytoskeleton and cell motility. However, the role of IQGAP1 in PDGF-stimulated FA formation and VSMC migration as well as neointimal formation after vascular injury in vivo is unknown.
In the present study, we demonstrate that IQGAP1 forms a complex with PDGFR and FA proteins such as vinculin, paxillin, and FAK in VSMCs in response to PDGF. Functionally, IQGAP1 is required for PDGF-induced phosphorylation of PDGFR localized in FAs, but not caveolae/lipid rafts, and formation of FAs as well as VSMC migration. Moreover, wire injury model with IQGAP1-deficient mice reveals that IQGAP1 plays an important role in neointimal formation in vivo.1
METHODS
Materials.
Antibodies to PDGFR-β, phospho-PDGFR (Tyr1021), IQGAP1, FAK, and phosphotyrosine (pTyr) were from Santa Cruz. Antibodies to caveolin-1 and paxillin were from BD Biosciences Pharmingen. Antibody against vinculin was from Sigma-Aldrich. Adenovirus expressing human IQGAP1 (Ad.IQGAP1) was generated by Vector Biolabs. Enhanced chemiluminescence (ECL) Western blotting detection reagents and nitrocellulose membranes (Hybond-ECL) were obtained from Amersham Biosciences Oligofectamine. Opti-MEM I Reduced-Serum Media were from Invitrogen. All other chemicals and reagents were from Sigma.
Cell culture.
Rat VSMCs were isolated from male Sprague-Dawley rat thoracic aortas by enzymatic digestion, as described previously (7). Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% bovine serum and used between passages 8 and 15. Cells were starved for 24 h with serum-free media before PDGF stimulation. For some experiments, VSMCs from other species, such as human and mouse aortic SMCs, were used (1).
siRNA transfection.
Control siRNA and IQGAP1 siRNA were obtained from Applied Biosystems. Sequence of IQGAP1 siRNA was as follows: sense, 5′-CAGCCGAUCUUUAUCAFAATT-3′; antisense, 5′-UUCUGAUAAAGAUCGGCUGCA-3′. VSMCs were seeded into culture dishes 1 day before transfection. Transfection of siRNA (30 nM) was performed using Oligofectamine (Invitrogen) according to the manufacturer's protocol. PDGF stimulation was performed 48 h after transfection.
Adenovirus transduction.
Nearly confluent VSMCs were infected with Ad.IQGAP1 or Ad.null (control) with 5 multiplicities of infection (MOIs) in serum-free medium for 24 h, followed by incubation in serum-free medium without virus for 24 h before PDGF stimulation, as reported previously (18).
Scratch wound assay.
VSMCs were cultured on six-well plates. Confluent cells were scraped using sterilized 10-μl pipette tips, washed with 0.1% serum media, and stimulated with 50 ng/ml PDGF for 10 h in mouse VSMCs or 24 h in rat VSMCs, as previously described (1, 33).
Cell proliferation assay.
VSMCs (105 cells) were seeded in six-well plates, and cell number with and without PDGF in 0.1% bovine serum containing culture medium was determined by counting with a hemocytometer, as described before (1, 33).
Confocal immunofluorescence microscopy in VSMCs.
VSMCs on glass coverslips were rinsed quickly in ice-cold PBS, fixed in freshly prepared 4% paraformaldehyde in PBS for 10 min at room temperature, permeabilized in 0.05% Triton X-100 in PBS for 5 min, and rinsed sequentially in PBS, 50 mmol/l NH4Cl, and PBS for 10 min each. After incubation for 1 h in blocking buffer (PBS + 3% BSA), cells were incubated with IQGAP1, paxillin, vinculin, or phospho-PDGFR antibodies for 18 h at 4°C, rinsed in PBS-BSA, and then incubated in either Alexa Fluor 488 or 546-conjugated goat anti-rabbit or mouse IgG for 1 h at room temperature. Cells on coverslips were mounted onto glass slides in Vectashield (Vector) and examined using the 488 and 543 nm lines of the argon ion and green HeNe lasers. Controls with no primary antibody showed no fluorescence labeling, and single-label controls were performed in double-labeling experiments. The individual areas of vinculin or paxillin staining were quantified in five different focal adhesions per cells (counted cells; 10 per each group).
Immunoprecipitation and immunoblotting.
Growth-arrested cells were stimulated with PDGF at 37°C, and cells were lysed with 500 μl of ice-cold lysis buffer, pH 7.4 (50 mM HEPES, 5 mM EDTA, 150 mM NaCl), 1% Triton X-100, 60 mM n-octyl-β-d-glucopyranoside, protease inhibitors (10 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin), and phosphatase inhibitors (50 mM sodium fluoride, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate). For immunoprecipitation, cell lysates (1,000 μg) were precipitated with antibody overnight at 4°C and then incubated with 20 μl of protein A/G-agarose beads for 1.5 h at 4°C. Cell lysates (25 μg) or immunoprecipitates were separated using SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes, blocked overnight in PBS containing 5% nonfat dry milk and 0.1% Tween 20, incubated overnight with primary antibodies, as described previously (1). After incubation with secondary antibodies, proteins were detected by ECL chemiluminescence.
Detergent-free purification of caveolin-rich membrane fractions.
Caveolae/lipid raft fractions were separated by the sodium carbonate-based detergent-free method (23). Briefly, VSMCs were scraped into 2 ml of 500 mM sodium carbonate containing protease inhibitors (pH 11). Homogenization was carried out sequentially in the following order using a loose-fitting Dounce homogenizer (10 strokes) and a sonicator (four 20-s bursts). The homogenates were adjusted to 45% sucrose by adding 90% sucrose in a buffer containing 25 mM Mes (pH 6.5) and 0.15 M NaCl and placed at the bottom of an ultracentrifugation tube. A 5–35% discontinuous sucrose gradient was formed above and centrifuged at 39,000 rpm at 4°C for 16–20 h in a Beckman SW-40Ti rotor. From the top of the tube, 13 fractions were collected. Caveolae/lipid raft fractions were accumulated in fractions 4–6. These fractions contained <5% of total tissue protein content. Proteins from each fraction were analyzed by Western blotting.
Vascular injury model.
Animal protocols were approved by the Animal Care and Use Committee of the University of Illinois at Chicago. IQGAP1−/− mice on mixed background (12) were backcrossed with C57BL/6 mice 10 generations, as reported (27). Age-matched C57BL/6 mice used for control [wild type (WT) mice] were from Jackson Laboratory. All mice were maintained at the University of Illinois at Chicago animal facilities. Transluminal arterial injury was performed in 12- to 16-wk-old male mice. Briefly, mice were anesthetized using an intraperitoneal injection of ketamine (100 mg/kg body wt) and xylazine (10 mg/kg). A straight spring wire (0.25 mm in diameter) was inserted into the left femoral artery and placed there for 1 min. This wire injury was reported to cause a complete removal of endothelium (21).
Histological analysis.
Three weeks after injury, mice were anesthetized and perfused in situ using 4% paraformaldehyde at 100 mmHg for tissue fixation. Injured arteries were excised and embedded in paraffin. Paraffin-embedded cross sections (5 μm thick) were stained with hematoxylin-eosin (HE) and elastica van Gieson (EVG). The cross-sectional area of the blood vessel layers, including the intima and medial areas, was measured in three sections from the middle segments of each artery at 150-μm intervals and analyzed using ImageJ 1.44p software (National Institutes of Health, Bethesda, MD). Tissue sections of blood vessels for immunohistochemical studies were incubated with primary antibody against IQGAP1 (Santa Cruz). This was followed by incubation with biotin-conjugated secondary antibody (Vector Laboratories). Next, we used R.T.U. Vectorstain Elite (Vector) followed by DAB visualization (Vector) as described previously (1). Immunofluorescence staining was performed with primary antibodies against IQGAP1 (Santa Cruz) and actin α-smooth muscle-Cy3 antibody (Sigma). Secondary antibodies were Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen).
Statistical analysis.
Results are expressed as means ± SE. Statistical significance was assessed by Student's paired two-tailed t-test or ANOVA on untransformed data, followed by comparison of group averages by contrast analysis using the Super ANOVA statistical program (Abacus Concepts, Berkeley, CA). P < 0.05 was considered to be statistically significant.
RESULTS
IQGAP1 is expressed in various VSMCs and PDGF promotes the association of IQGAP1 with PDGFR.
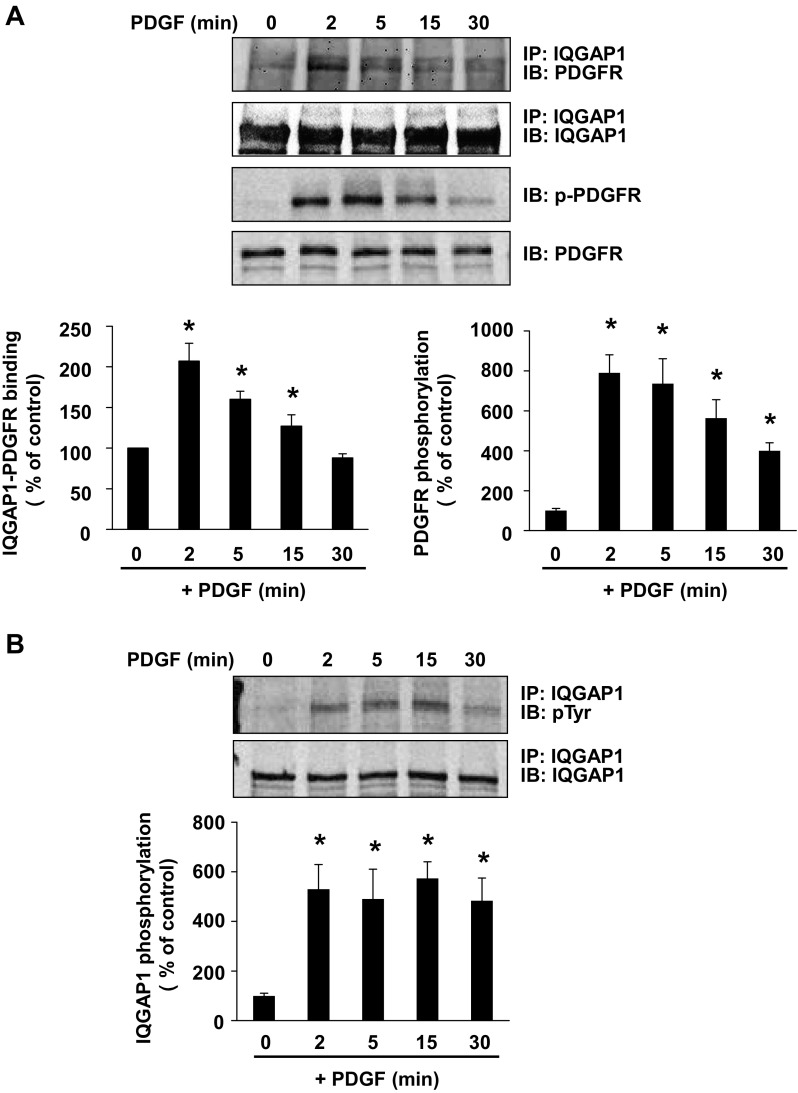
We first examined the expression of IQGAP1 in various VSMCs, and Western analysis with specific anti-IQGAP1 antibody showed the expression of IQGAP1 with a 190-kDa protein in whole cell lysates of rat, mouse, and human aortic quiescent VSMCs that were serum starved for 24 h (data not shown). To assess the relationship between IQGAP1 and PDGFR in PDGF-stimulated VSMCs, we examined whether IQGAP1 binds to PDGFR. Figure 1A using coimmunoprecipitation analysis shows that PDGF stimulation promoted IQGAP1 binding to the PDGFR with a peak at 2 min in VSMCs, which gradually decreased to the basal level within 30 min. The interaction of IQGAP1 to PDGFR was cotemporaneous with an increase in PDGFR phosphorylation (Fig. 1A). To investigate further the possibility that IQGAP1 is a component of PDGF signaling, we also examined whether IQGAP1 is tyrosine phosphorylated after PDGF stimulation in VSMCs. As shown in Fig. 1B, the level of tyrosine-phosphorylated IQGAP1 was markedly increased within 2 min, which gradually decreased and remained above baseline at least for 30 min after PDGF stimulation.
Fig. 1.
Platelet-derived growth factor (PDGF) stimulation promotes IQ-domain GTPase-activating protein 1 (IQGAP1) association with PDGF receptor (PDGFR), and tyrosine phosphorylation of PDGFR and IQGAP1 in vascular smooth muscle cells (VSMCs). Rat aortic smooth muscle cells (RASMs) were stimulated with 50 ng/ml PDGF for indicated times (in minutes). A: lysates were immunoprecipitated (IP) with anti-IQGAP1 antibody, followed by immunoblotting (IB) with anti-PDGFR and anti-IQGAP1 antibodies. The same lysates were immunoblotted with anti-p-PDGFR (Tyr1021) or PDGFR antibodies. B: lysates were IP with anti-IQGAP1 antibody, followed by IB with anti-phosphotyrosine (pTyr) or anti-IQGAP1 antibodies. Values represent means ± SE of 3 independent experiments. *P < 0.05 vs. control cells.
IQGAP1 positively regulates PDGFR autophosphorylation in VSMCs.
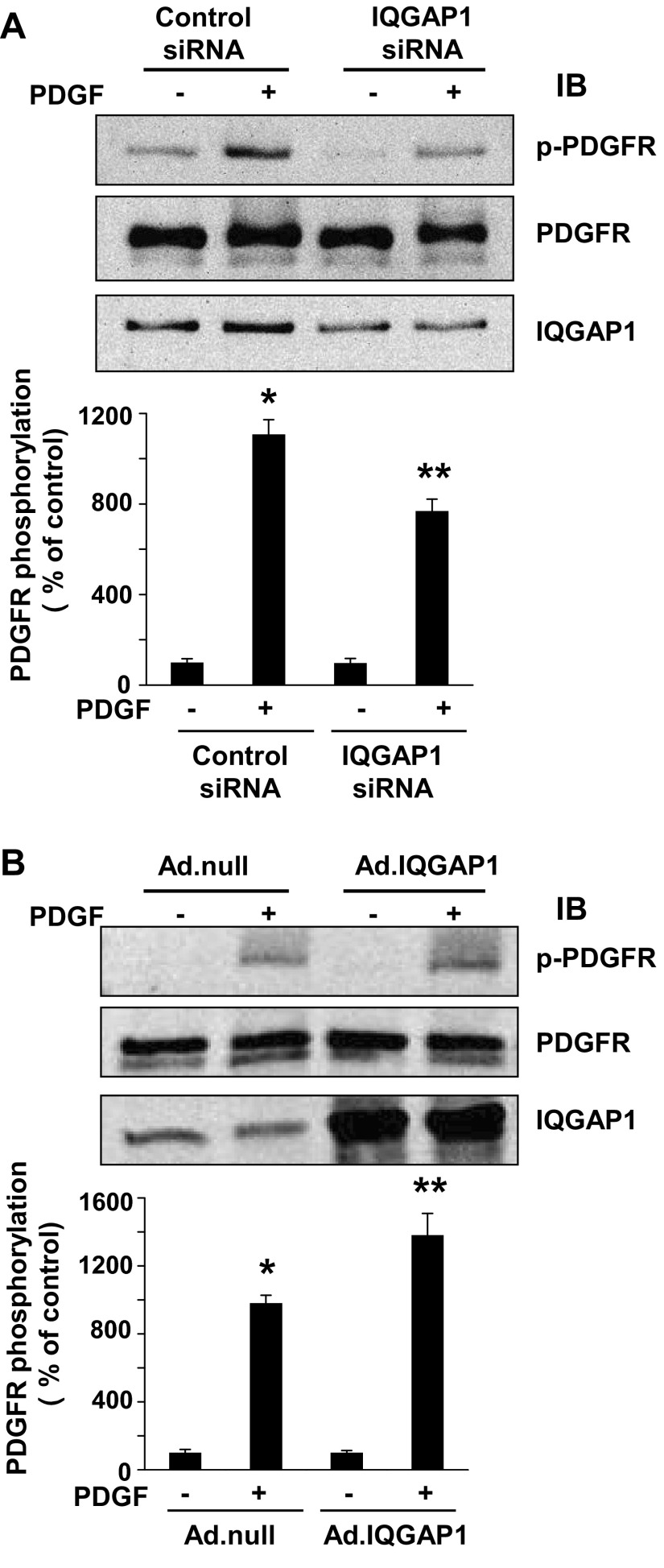
We next examined whether IQGAP1 regulates PDGFR autophosphorylation in VSMCs. Figure 2A shows that knockdown of endogenous IQGAP1 with specific siRNA significantly inhibited PDGF-induced PDGFR tyrosine phosphorylation (p-PDGFR) without affecting its protein expression (Fig. 2B). In contrast, overexpression of IQGAP1 using adenovirus significantly enhanced PDGF-induced p-PDGFR formation without affecting its basal phosphorylation or protein expression. These results suggest that IQGAP1 functions as a positive regulator for PDGFR autophosphorylation.
Fig. 2.
IQGAP1 is involved in PDGFR autophosphorylation in VSMCs. RASMs were transfected with IQGAP1 or control siRNAs (A) or infected with Ad.null or Ad.IQGAP1 (B) for 48 h. Growth-arrested RASMs were stimulated with 50 ng/ml PDGF for 5 min, and lysates were used for measurement of p-PDGFR (Tyr1021), total PDGFR, or IQGAP1. Values represent means ± SE of 3 independent experiments. *P < 0.05 or **P < 0.05 vs. control siRNA (A) or Ad.null (B) cells without or with PDGF treatment, respectively.
IQGAP1 regulates PDGFR autophosphorylation in non-caveolae/lipid rafts in VSMCs.
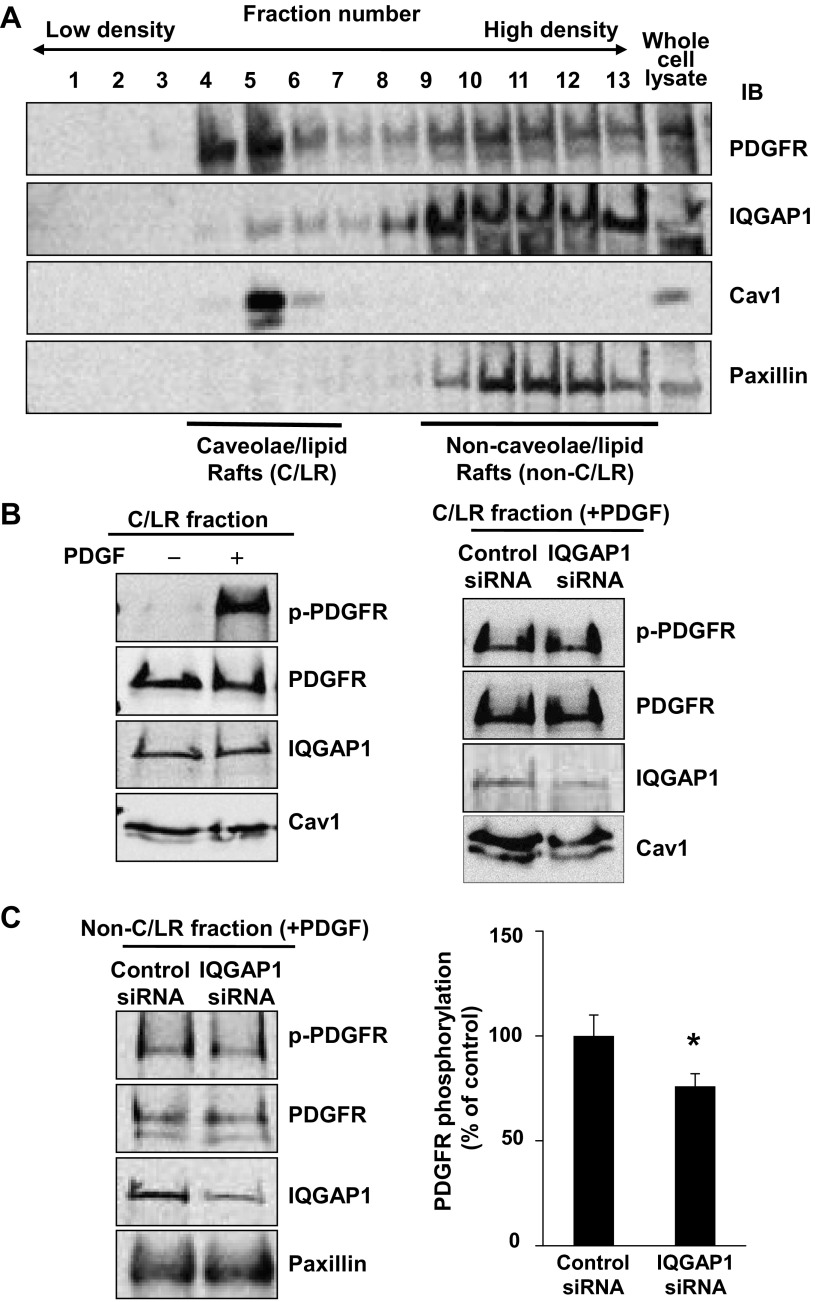
Since caveolae/lipid rafts are an important signaling domain in which a fraction of PDGFR is localized and activated following PDGF stimulation (1, 13, 14), we next examined whether IQGAP1 is localized in this compartment to regulate PDGFR activation. Detergent-free sucrose gradient fractionation revealed that IQGAP1 and PDGFR were localized in both caveolin-enriched caveolae/lipid rafts and non-caveolae/lipid raft fractions that contained paxillin, a marker protein for FAs (Fig. 3A). Of note, IQGAP1 was more predominantly localized in non-caveolae/lipid rafts as compared with PDGFR. Figure 3B shows that PDGF stimulation rapidly increased p-PDGFR at 5 min in caveolae/lipid raft fractions without affecting PDGFR and IQGAP1 localization. Depletion of IQGAP1 with siRNA did not affect PDGFR localization and phosphorylation in the caveolae/lipid rafts while it significantly inhibited p-PDGFR in non-caveolae/lipid raft fractions (Fig. 3C). These results suggest that IQGAP1 positively regulates PDGFR activation in non-caveolae/lipid rafts in VSMCs.
Fig. 3.
IQGAP1 regulates PDGFR autophosphorylation in non-caveolae/lipid rafts in VSMCs. A: PDGFR and IQGAP1 are localized at caveolae/lipid rafts (C/LR) and non-C/LR. Growth-arrested RASMs were fractionated by sucrose gradient centrifugation. Fractions from the top (fraction 1) to the bottom (fraction 13) were immunoblotted with ant-PDGFR, anti-IQGAP1, or anti-caveolin-1 (Cav1) antibodies. C/LR and non-C/LR fractions were accumulated in fractions 4–6 and 9–13, respectively. B, left: RASMs were stimulated with or without PDGF (50 ng/ml) for 5 min, and followed by C/LR fractionation. Equal amounts of protein from C/LR fractions were IB with antibodies as indicated. B, right, and C: effect of IQGAP1 siRNA on PDGFR autophosphorylation in C/LR (B) and non-C/LR (C). RASMs transfected with IQGAP1 or control siRNA for 48 h were stimulated with 50 ng/ml PDGF for 5 min. Equal amounts of protein from C/LR and non-C/LR fractions were IB with antibodies, as indicated. *P < 0.05 vs. control cells.
Activated PDGFR and IQGAP1 localize at FAs in PDGF-stimulated VSMCs.
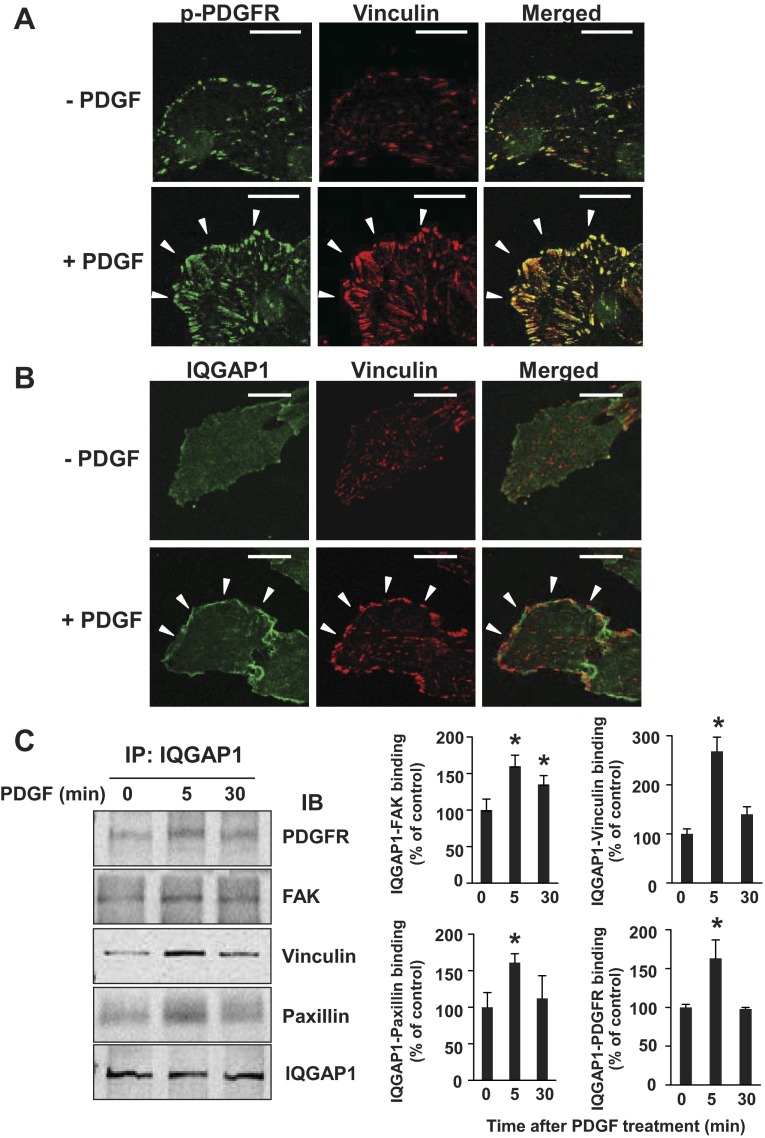
We next performed immunofluorescence analysis with confocal microscopy to determine the subcellular localization of p-PDGFR and IQGAP1 in PDGF-stimulated VSMCs. Figure 4A shows that PDGF stimulation for 5 min increased p-PDGFR, which colocalized with vinculin, a marker of FAs. In parallel, PDGF stimulation promoted colocalization of IQGAP1 with vinculin (Fig. 4B). These results suggest that both activated PDGFR and IQGAP1 localize at FAs in PDGF-stimulated VSMCs.
Fig. 4.
PDGF stimulation promotes localization of activated PDGFR and IQGAP1 at focal adhesions as well as IQGAP1 association with PDGFR and focal adhesion proteins in VSMCs. A and B: growth-arrested RASMs were stimulated with 50 ng/ml PDGF for 5 min. RASMs were double-stained with anti-pPDGFR (green) and vinculin (red) antibodies (A) or with anti-IQGAP1 (green) and vinculin (red) antibodies (B). White arrowheads point to the focal adhesion at the leading edge. All fluorescence images were taken at 5 different fields/well, and the cell images are representative of 3 different experiments. Bar represents 20 μm. C: growth-arrested RASMs were stimulated with 50 ng/ml PDGF for indicated times (in minutes). Lysates were IP with anti-IQGAP1 antibody, followed by IB with anti-PDGFR, anti-focal adhesion kinase (FAK), anti-vinculin, anti-paxillin, and anti-IQGAP1 antibodies. Values represent means ± SE of 3 independent experiments. *P < 0.05 vs. control cells.
PDGF stimulation promotes association of IQGAP1 with PDGFR and FA proteins in VSMCs.
We also performed coimmunoprecipitation analysis to examine the complex formation of IQGAP1, PDGFR, and FA proteins in VSMCs. Figure 4C shows that PDGF stimulation increased the association of IQGAP1 with PDGFR, FAK, vinculin, and paxillin within 5 min. These results suggest that PDGF promotes formation of the IQGAP1/PDGFR complex with FA proteins in VSMCs.
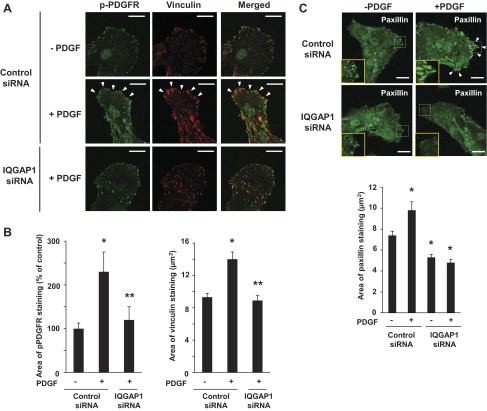
IQGAP1 is required for PDGFR activation in FAs and formation of FAs in VSMCs.
To determine the role of IQGAP1 in PDGFR activation in FAs, we examined the effect of IQGAP1 knockdown on p-PDGFR formation in FAs in VSMCs. Immunofluorescence studies revealed that IQGAP1 siRNA markedly reduced the PDGF-induced increase in p-PDGFR in FAs, which was associated with a decrease in vinculin staining at the leading edge (Fig. 5, A and B). We then examined the role of IQGAP1 in formation of FAs using immunofluorescence analysis for paxillin and found that depletion of IQGAP1 with siRNA significantly reduced basal and PDGF-stimulated paxillin staining (Fig. 5C). Of note, PDGF stimulation or IQGAP1 siRNA did not affect the expression of vinculin or paxillin (data not shown). These results suggest that IQGAP1 is required for PDGF-induced PDGFR activation in FAs and formation of FAs at the leading edge in VSMCs.
Fig. 5.
IQGAP1 is required for PDGFR autophosphorylation in focal adhesions in VSMCs. RASMs were transfected with IQGAP1 or control siRNAs for 48 h and then stimulated with 50 ng/ml PDGF for 5 min. A: cells were double-stained with anti-pPDGFR (green) and vinculin (red) antibodies. White arrowheads point to the focal adhesion in the leading edge. B: quantitative analysis of pPDGFR and vinculin staining. C: cells were stained with anti-paxillin (green) antibody. Graph shows the area of paxillin staining. Bar represents 20 μm. Values represent means ± SE of 3 independent experiments. *P < 0.05 or **P < 0.05 vs. control siRNA cells without or with PDGF treatment, respectively.
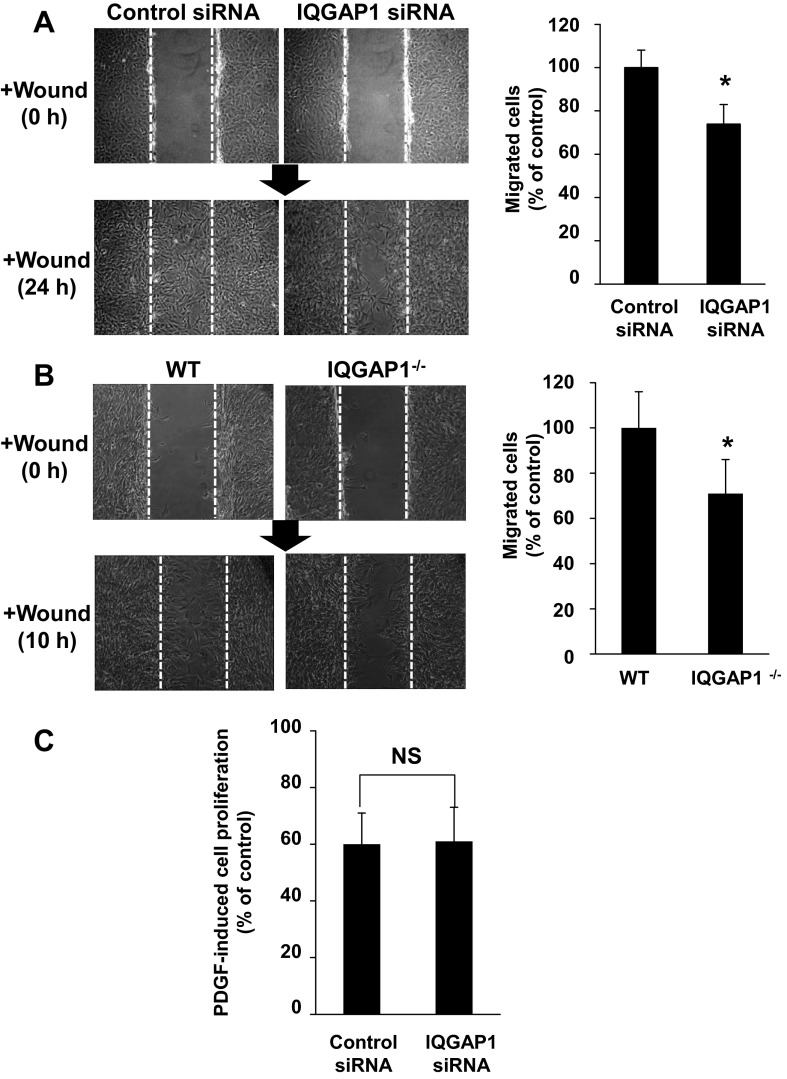
IQGAP1 is involved in PDGF-stimulated VSMC migration.
To determine the functional role of IQGAP1 in PDGFR signaling and FA formation, we examined the role of IQGAP1 in PDGF-stimulated VSMC migration. Wound scratch assay of confluent rat VSMCs in the presence of PDGF showed that IQGAP1 siRNA significantly inhibited directional cell migration (Fig. 6A). Similarly, wound injury-induced cell migration was significantly inhibited in VSMCs derived from IQGAP1−/− mice compared with those from WT mice (Fig. 6B). In contrast, IQGAP1 siRNA did not have a significant effect on PDGF-stimulated VSMC proliferation (Fig. 6C). These results suggest that IQGAP1 is specifically involved in PDGF-stimulated VSMCs migration.
Fig. 6.
IQGAP1 is involved in PDGF-stimulated VSMC migration, but not proliferation. RASMs were transfected with IQGAP1 or control siRNAs for 48 h (A and C), or mouse aortic SMCs were isolated from wild-type (WT) or IQGAP1−/− mice (B). A and B: wound-scratch assay was performed in confluent monolayers of VSMCs in the presence of PDGF (50 ng/ml). Images were captured immediately after rinsing at 0 h and at 24 h (A) or 10 h (B) after the wounding in the cells. C: cell proliferation stimulated with or without 50 ng/ml PDGF was determined by cell number after cells were plated in 0.1% bovine serum containing culture medium for 72 h. Values represent means ± SE of 3 independent experiments. *P < 0.05 vs. control siRNA or WT cells. NS, not significant.
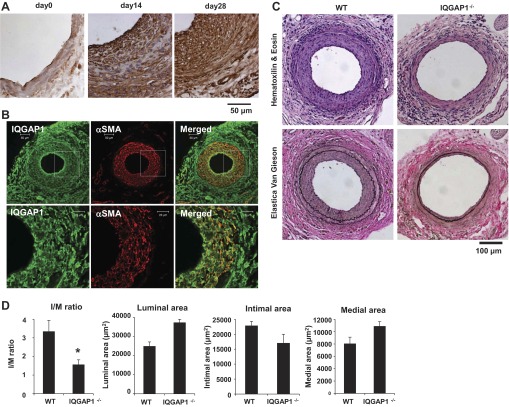
IQGAP1 is involved in neointimal formation in response to vascular injury in vivo.
To determine the functional significance of IQGAP1 in VSMC migration in vivo, we examined the role of IQGAP1 in neointimal formation using a mouse femoral artery wire injury model with IQGAP1−/− and WT mice. Immunohistochemical (Fig. 7A) and immunofluorescence (Fig. 7B) analysis showed that IQGAP1 protein expression was weakly expressed in uninjured vessels and robustly increased and colocalized with α-smooth muscle actin (αSMA) in neointima in the injured vessels. Figure 7C shows that neointimal formation in the femoral artery at 3 wk after the injury was markedly inhibited in IQGAP1−/− mice compared with WT mice. Quantitative morphometric analysis of the injured vessels revealed a marked decrease in intimal area and intimal-to-medial ratio (I/M) with no significant changes in medial area or overall vessel size in IQGAP1−/− mice (Fig. 7D). These results suggest that IQGAP1 plays an important role in neointimal formation in part by promoting vascular migration in vivo.
Fig. 7.
IQGAP1 is involved in neointimal formation in response to vascular injury in vivo. A and B: IQGAP1 is highly expressed in neointimal VSMCs of wire-injured mouse femoral arteries. Immunohistochemical (A) or immunofluorescence (B) analysis of injured arteries stained with anti-IQGAP1 antibody (day 0, 14, 28; A) or costained with anti-IQGAP1 (green) and α-smooth muscle actin (αSMA; red) antibodies (day 21; B). C and D: H&E and elastica van Gieson (delineates elastic laminae) staining of femoral arteries obtained from WT and IQGAP1−/− mice at 3 wk after injury. In D, quantitative morphometric analysis is shown of vessel remodeling in WT and IQGAP1−/− mice (means ± SE of three sections from each of six vessels). *P < 0.05 vs. WT mice.
DISCUSSION
IQGAP1 has been implicated as a regulator for cell motility; however, its role in VSMC migration as well as neointimal formation in response to injury remains unknown. In the present study, we provide the first evidence that 1) PDGF stimulation of VSMCs promotes IQGAP1 binding to PDGFR and FA signaling proteins, which is associated with tyrosine phosphorylation of IQGAP1; 2) IQGAP1 plays a critical role in VSMC migration at least in part through increasing PDGFR autophosphorylation in FAs and FA formation at the leading edge; and 3) IQGAP1 expression is markedly increased in the neointima of wire-injured femoral arteries, which is colocalized with α-smooth muscle actin-positive (αSMA+) VSMCs. Moreover, mice lacking IQGAP1 show decreased neointimal formation after vascular injury.
We demonstrated previously that IQGAP1 directly binds to VEGFR2 to transmit VEGFR2 signal to promote endothelial migration (33) and postischemic neovascularization (27). Others have shown that IQGAP1 associates with FGFR1 (2) and EGFR (16) in nonvascular cells. However, the role of IQGAP1 in PDGFR signaling in VSMCs was virtually unexplored. The present study shows that PDGF stimulation rapidly promotes association of IQGAP1 with PDGFR, which is cotemporaneous with an increase in tyrosine phosphorylation of PDGFR in VSMCs. The gain- and loss-of-function approach demonstrates that IQGAP1 positively regulates PDGFR autophosphorylation. Consistent with our result, McNulty et al. (16) reported that EGFR coimmunoprecipitates with IQGAP1 and that EGFR tyrosine phosphorylation is inhibited in IQGAP1-null mouse embryonic fibroblasts. These findings suggest that IQGAP1 binding to growth factor receptors is required for their activation in various systems. The mechanisms by which IQGAP1 regulates PDGFR phosphorylation in VSMCs remain unclear. We previously reported that reactive oxygen species (ROS) are important for VEGFR2 autophosphorylation (28) and that IQGAP1 is involved in VEGF-induced ROS production in endothelial cells (33). However, ROS are not involved in PDGF-induced p-PDGFR in VSMCs (31) and we found that IQGAP1 siRNA has no effects on PDGF-induced ROS production in VSMCs (Kohno T and Ushio-Fukai M, unpublished observations). It is shown that EGF-induced phosphorylation of IQGAP1 on Ser1443 is involved in EGFR activation (16), while we could not detect serine phosphorylation of IQGAP1 in PDGF-stimulated VSMCs using current available pSer antibody (Kohno T and Ushio-Fukai M, unpublished observations). In contrast, we found that PDGF rapidly increases IQGAP1 tyrosine phosphorylation in VSMCs, as reported in endothelial cells stimulated with VEGF (17, 33), which correlates with an increase in PDGFR autophosphorylation. Whether tyrosine phosphorylation of IQGAP1 regulates PDGFR activation requires further investigation.
Biochemical cell fractionation analysis shows that IQGAP1 and activated PDGFR are localized in both caveolin-enriched caveolae/lipid rafts and paxillin-enriched non-caveolae/lipid raft fractions in PDGF-stimulated VSMCs. We found that IQGAP1 knockdown significantly inhibits PDGF-induced p-PDGFR in non-lipid rafts, but not caveolae/lipid rafts. This result is supported by the immunofluorescence study showing that both IQGAP1 and activated PDGFR localize at FAs, which are an important plasma membrane signaling complex containing paxillin, vinculin, and FAK (6, 26), in PDGF-stimulated VSMCs. Moreover, coimmunoprecipitation analysis supports that PDGF stimulation promotes association of IQGAP1 with FA signaling proteins and PDGFR. Functionally, depletion of IQGAP1 inhibits PDGF-induced PDGFR activation in FAs as well as formation of FAs. The mechanisms by which IQGAP1 regulates PDGF-induced FA formation remain unclear. We found that IQGAP1 siRNA has no effects on tyrosine phosphorylation of paxillin induced by PDGF (Kohno T and Ushio-Fukai M, unpublished observations). Growth factor receptors are shown to couple to integrins to regulate FAs assembly and turnover, which play an important role in cell migration (5, 6, 26). It has been shown that IQGAP1 binds to active Rac1 to keep it inactive (8, 10) and that IQGAP1 associates with Rac-bound β1-integrin through protein tyrosine phosphatase (PTP)2A to regulate F-actin assembly (24). EGF stimulation dissociates PTP2A-IQGAP1 complex from the Rac-bound β1-integrin to maintain β1-integrin-mediated cell adhesion in epithelial cells (25). Thus, it is plausible that IQGAP1 binding to Rac1 and integrins may be involved in PDGF-stimulated formation of FAs in VSMCs.
VSMC migration is a key component of neointimal formation and vascular remodeling in injured vessels. The present study using an IQGAP1 knockdown or knockout approach demonstrates that IQGAP plays a critical role in PDGF-induced VSMC migration. It has been shown that IQGAP1 regulates cell motility by regulating actin assembly (2, 4, 11) or by linking adenomatous polyposis coli to Rac1, Cdc42, and actin filaments (19, 30) in nonvascular cells. It is also reported that IQGAP1 mediates cell proliferation through a CDC42-mammalian target of rapamycin (mTOR) pathway in nonvascular cells (29) or endothelial cell migration and proliferation (9, 17, 33) through ROS production (9, 33). Given that IQGAP1 is not involved in PDGF-induced ROS production in VSMCs, it is likely that IQGAP1 specifically links PDGFR signal to FAs, which stimulates VSMC migration. It is important to identify the tyrosine phosphorylation sites or PDGFR-binding sites in IQGAP1 and determine the functional significance of phosphorylation and scaffold function of IQGAP1 in FA formation and VSMC migration in future study.
To determine the functional role of IQGAP1 in VSMC migration in vivo, we used a mouse wire injury model. IQGAP1 protein is weakly expressed in uninjured vessels and is robustly induced in αSMA+ neointimal lesions after vascular injury. Of note, IQGAP1 protein expression is observed in cultured quiescent VSMCs, which is not changed after PDGF stimulation for 0–24 h (Kohno T and Ushio-Fukai M, unpublished observations). The discrepancy in the extent of IQGAP1 expression in quiescent VSMCs between in vitro and in vivo conditions may be due to the possibility that cultured VSMCs undergo phenotypic changes after the passage. Addressing the mechanism for IQGAP1 upregulation in response to injury and the role of IQGAP1 in VSMCs differentiation and phenotypic change are important subjects for future study. In the present study, we also provide direct evidence that IQGAP1-deficient mice exhibit impaired neointimal formation, suggesting that IQGAP1 plays an important role in vascular pathophysiologies such as vascular remodeling in response to injury. It is important to note that the reduction of neointimal formation in the knockout mouse could be attributed to lack of IQGAP1 in not only VSMCs but also endothelial cells or immune cells, and that this is a limitation of the study. Previous studies using IQGAP1−/− mice demonstrate that IQGAP1 is required for angiogenesis in response to hindlimb ischemia (27), vascular barrier protection during acute lung injury (3), and reorganization of the gastric epithelium (12). These findings suggest the physiological and pathological importance of IQGAP1 in vivo.
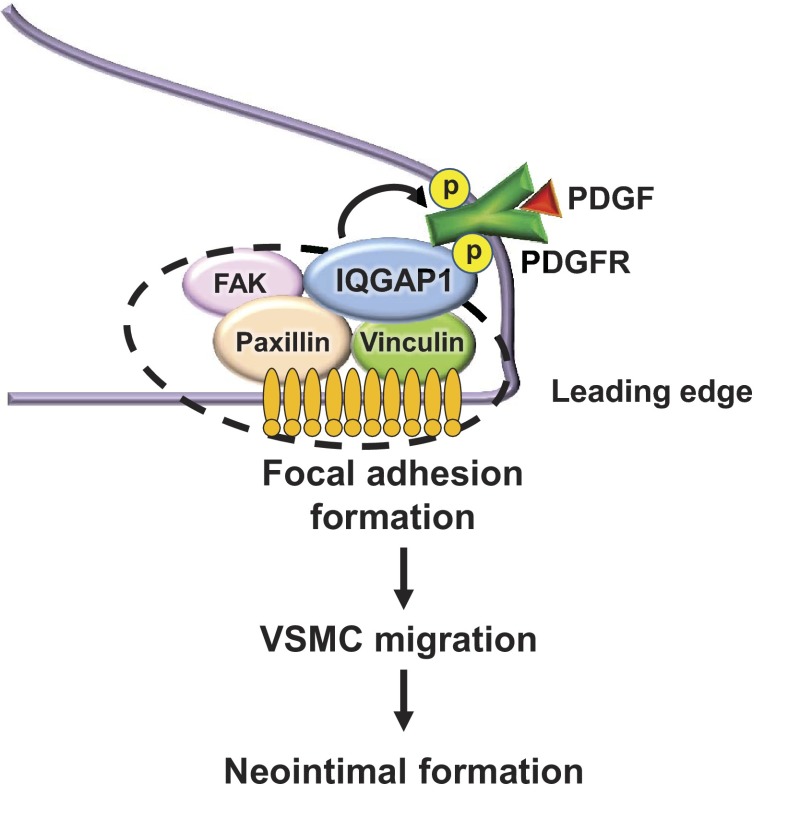
In summary, the present study demonstrates that IQGAP1 plays an important role in VSMC migration and neointimal formation after vascular injury by promoting association with PDGFR and FA proteins, PDGFR activation in FAs, and formation of FAs (Fig. 8). These findings provide novel insight into IQGAP1 as a potential therapeutic target for VSMC migration-related vascular diseases, such as atherosclerosis and postangioplasty restenosis.
Fig. 8.
Proposed model for the role of IQGAP1 in PDGF-induced focal adhesion formation and VSMC migration, leading to neointimal formation after injury. PDGF stimulation promotes the formation of multiple IQGAP1-bound PDGFR/FAK/paxillin/vinculin complexes. IQGAP1 is involved in PDGF-induced PDGFR autophosphorylation in focal adhesions as well as focal adhesion formation at the leading edge, thereby promoting VSMC migration and neointimal formation.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01 HL-077524 and HL-077524-s1 (to M. Ushio-Fukai) and R01 HL-070187 (to T. Fukai), Department of Veterans Affairs Merit Review Grant 1I01BX001232 (to T. Fukai), American Heart Association (AHA) Scientist Development Grant 12SDG12060100 (to N. Urao), AHA Postdoctoral Fellowships 09POST2250151 (to N. Urao), 12POST12050692 (to T. Kohno), and 11POST5740006 (to V. Sudhahar), and a Grant for Studying Overseas from the Japanese Circulation Society (to T. Kohno).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.K., T.F., and M.U.-F. conception and design of research; T.K., N.U., T.A., V.S., H.I., M.Y.-T., and R.D.M. performed experiments; T.K. and N.U. analyzed data; T.K., T.F., and M.U.-F. interpreted results of experiments; T.K. prepared figures; T.K. drafted manuscript; T.K., N.U., T.A., V.S., H.I., M.Y.-T., R.D.M., T.F., and M.U.-F. approved final version of manuscript; M.U.-F. edited and revised manuscript.
Footnotes
This article is the topic of an Editorial Focus by Michael V. Autieri (1a).
REFERENCES
- 1. Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, Maryon EB, Kaplan JH, Ushio-Fukai M, Fukai T. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res 107: 787–799, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a. Autieri MV. Increasing our IQ of vascular smooth muscle cell migration with IQGAP1. Focus on “IQGAP1 links PDGF receptor-β signal to focal adhesions involved in vascular smooth muscle cell migration: role in neointimal formation after vascular injury.” Am J Physiol Cell Physiol (May 8, 2013). 10.1152/ajpcell.00125.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bensenor LB, Kan HM, Wang N, Wallrabe H, Davidson LA, Cai Y, Schafer DA, Bloom GS. IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci 120: 658–669, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A, Burlingame AL, Matthay MA, Sheppard D. IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia. Am J Physiol Lung Cell Mol Physiol 303: L12–L19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol 16: 242–249, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res 100: 607–621, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Giancotti FG, Ruoslahti E. Integrin signaling. Science 285: 1028–1032, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Griendling KK, Taubman MB, Akers M, Mendlowitz M, Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem 266: 15498–15504, 1991 [PubMed] [Google Scholar]
- 8. Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J 15: 2997–3005, 1996 [PMC free article] [PubMed] [Google Scholar]
- 9. Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol 25: 2295–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem 271: 23363–23367, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Le Clainche C, Schlaepfer D, Ferrari A, Klingauf M, Grohmanova K, Veligodskiy A, Didry D, Le D, Egile C, Carlier MF, Kroschewski R. IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. J Biol Chem 282: 426–435, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol 20: 697–701, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem 272: 7211–7222, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Liu P, Ying Y, Anderson RG. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA 94: 13666–13670, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mateer SC, Wang N, Bloom GS. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil Cytoskeleton 55: 147–155, 2003 [DOI] [PubMed] [Google Scholar]
- 16. McNulty DE, Li Z, White CD, Sacks DB, Annan RS. MAPK scaffold IQGAP1 binds the EGF receptor and modulates its activation. J Biol Chem 286: 15010–15021, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLos One 3: e3848, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res 102: 1182–1191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci 118: 2085–2092, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev 15: 237–254, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 8: 403–409, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest 100: S87–S89, 1997 [PubMed] [Google Scholar]
- 23. Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690–9697, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Suzuki K, Chikamatsu Y, Takahashi K. Requirement of protein phosphatase 2A for recruitment of IQGAP1 to Rac-bound beta1 integrin. J Cell Physiol 203: 487–492, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Takahashi K, Suzuki K. Regulation of protein phosphatase 2A-mediated recruitment of IQGAP1 to beta1 integrin by EGF through activation of Ca2+/calmodulin-dependent protein kinase II. J Cell Physiol 208: 213–219, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Turner CE. Paxillin interactions. J Cell Sci 113: 4139–4140, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Urao N, Razvi M, Oshikawa J, McKinney RD, Chavda R, Bahou WF, Fukai T, Ushio-Fukai M. IQGAP1 is involved in post-ischemic neovascularization by regulating angiogenesis and macrophage infiltration. PLos One 5: e13440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9: 731–739, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Wang JB, Sonn R, Tekletsadik YK, Samorodnitsky D, Osman MA. IQGAP1 regulates cell proliferation through a novel CDC42-mTOR pathway. J Cell Sci 122: 2024–2033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell 7: 871–883, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res 94: 1219–1226, 2004 [DOI] [PubMed] [Google Scholar]
- 32. White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal 24: 826–834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA, Wang N, Kontos CD, Bloom GS, Alexander RW. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species–dependent endothelial migration and proliferation. Circ Res 95: 276–283, 2004 [DOI] [PubMed] [Google Scholar]