Abstract
Glucagon-like peptide-1 (GLP-1) receptors (GLP-1R) expressed in the nucleus tractus solitarius (NTS) are physiologically required for the control of feeding. Recently, NTS GLP-1R-mediated suppression of feeding was shown to occur via a rapid PKA-induced suppression of AMPK and activation of MAPK signaling. Unknown are the additional intracellular signaling pathways that account for the long-term hypophagic effects of GLP-1R activation. Because cAMP/PKA activity can promote PI3K/PIP3-dependent translocation of Akt to the plasma membrane, we hypothesize that hindbrain GLP-1R-mediated control of feeding involves a PI3K-Akt-dependent pathway. Importantly, the novel evidence presented here challenges the dogmatic view that PI3K phosphorylation results in an obligatory activation of Akt and instead supports a growing body of literature showing that activation of cAMP/PKA can inhibit Akt phosphorylation at the plasma membrane. Behavioral data show that inhibition of hindbrain PI3K activity by a fourth icv administration of LY-294002 (3.07 μg) attenuated the food intake- and body weight-suppressive effects of a fourth icv administration of the GLP-1R agonist exendin-4 (0.3 μg) in rats. Hindbrain administration of triciribine (10 μg), an inhibitor of PIP3-dependent translocation of Akt to the cell membrane, also attenuated the intake-suppressive effects of a fourth icv injection of exendin-4. Immunoblot analyses of ex vivo NTS tissue lysates and in vitro GLP-1R-expressing neurons (GT1–7) support the behavioral findings and show that GLP-1R activation decreases phosphorylation of Akt in a time-dependent fashion. Current data reveal the requirement of PI3K activation, PIP3-dependent translocation of Akt to the plasma membrane, and suppression in phosphorylation of membrane-bound Akt to mediate the food intake-suppressive effects of hindbrain GLP-1R activation.
Keywords: glucagon-like peptide-1, phosphatidylinositol-3 kinase, obesity, protein kinase B, nucleus tractus solitarius
pharmaceutical targets of the glucagon-like peptide-1 (GLP-1) system are efficacious in the treatment of type 2 diabetes mellitus (see Refs. 4 and 43 for review) and may hold promise for the treatment of obesity (25, 30, 40). GLP-1 receptor (GLP-1R) agonists [e.g., exendin-4 (Ex-4), liraglutide] produce improvements in blood glucose regulation and reductions in food intake and body weight in both humans and animal models (9, 25, 28, 54, 55, 60, 65). Recent evidence indicates that the intake-suppressive effects of GLP-1R agonists are mediated in part through direct GLP-1R signaling in the central nervous system (CNS) following either peripheral or central administration (5, 33, 57, 64). Indeed, stimulation of central GLP-1Rs results in many of the same behavioral and physiological responses that are observed following peripheral GLP-1R ligand administration (e.g., inhibition of feeding, increased insulin secretion) (28, 38, 39, 59). Perhaps the best evidence supporting the physiological relevance of the CNS GLP-1 system in food intake and body weight regulation are reports showing that 1) chronic blockade of CNS GLP-1R by forebrain administration of the selective antagonist exendin-(9–39) results in increased food intake and body weight compared with controls (5, 45) and 2) targeted viral knockdown of GLP-1-producing preproglucagon neurons results in hyperphagia and elevated weight gain (5). Although these findings demonstrate that the CNS GLP-1 system as a whole is required for body weight regulation, the data leave unresolved a systematic evaluation of the specific CNS populations of GLP-1R-expressing nuclei that are physiologically required for energy balance control and the neuronal and intracellular signaling mechanisms mediating the intake-suppressive effects following activation of specific GLP-1R-expressing nuclei.
Although many CNS nuclei relevant for energy balance express GLP-1R, only a select few have been demonstrated to be physiologically required for the normal control of feeding (3, 14, 24, 59). Among these, GLP-1Rs in the nucleus tractus solitarius (NTS) stand out as a critical population for food intake control (21, 24, 26, 28). Recently, using a combination of in vivo and in vitro techniques with the GLP-1R agonist exendin-4, we have shown that the intake-suppressive effects of NTS GLP-1R activation occur through a coordinated intracellular signaling cascade that involves protein kinase A (PKA)-mediated suppression of adenosine 5′-monophosphate protein kinase (AMPK) activity and simultaneous activation of p44/42 mitogen-activated protein kinase (p44/42 MAPK; a.k.a. ERK-1/2) signaling (26). These effects may promote Ca2+-dependent depolarization of the GLP-1R-expressing neurons as well as promote for additional intracellular signaling cascades and long-term changes in gene transcription and protein synthesis.
It is highly plausible that GLP-1R-mediated suppression of food intake involves modulation of phosphatidylinositol-3 kinase (PI3K)/Akt (also known as PKB) signaling, as these intracellular signaling molecules are both direct and indirect downstream targets of cAMP/PKA. Indeed, GLP-1R activation has been shown in vitro to engage PI3K/Akt to promote peripheral tissue/cell differentiation and proliferation (17, 18) and to prevent apoptosis via a PKA/PI3K/Akt-dependent pathway (16). Within the CNS, PI3K/Akt signaling has been implicated in mediating the intake-suppressive effects of insulin and the adipose tissue-derived hormone leptin (48, 50, 51). Given that CNS GLP-1R and leptin receptor signaling interact to control for energy balance (68), it is possible that the PI3K/Akt pathway may serve as a point of convergence to mediate the additive suppression of intake by hindbrain leptin and GLP-1R signaling. However, there have been no reports examining the role of PI3K/Akt signaling in mediating the intake-suppressive effects of CNS GLP-1R signaling. This dearth of information is likely due in part to common misconceptions regarding the cAMP/PKA-PI3K-Akt pathway.
An increase in cAMP/PKA activity can promote phosphorylation of PI3K (11), but despite the common assertion that PI3K activation increases Akt phosphorylation, this is not an obligatory outcome (7, 37, 42). Rather, PI3K activation converts phosphatidylinositol (4,5)-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 subsequently recruits pleckstrin homology (PH) domain-containing proteins like Akt to the plasma membrane (32), where Akt can then be phosphorylated or dephosphorylated at two critical sites, Thr308 and Ser473, the former by phosphoinositide-dependent kinase 1 (2, 7). Akt Ser473 phosphorylation can occur by a number of kinase pathways (7, 42) and is independent of Thr308 phosphorylation (1). Thus, for the neural control of food intake and energy balance, much of the misconception regarding PI3K/Akt signaling has resulted from the mislabeling of pharmacological inhibitors of PI3K activation (i.e., wortmannin or LY-294002) as inhibitors of Akt phosphorylation. Although this may be true for certain hormonal systems and cell types like insulin and pancreatic β-cells (see Refs. 15 and 31 for review), a more conservative, and perhaps more appropriate, interpretation would be that PI3K inhibitors block any change in Akt activity by blocking PIP2-dependent conversion to PIP3, as PIP3 activity would be required to recruit Akt to the plasma membrane (7).
Given that GLP-1R activation increases cAMP/PKA activity to reduce food intake (26) and that an increase in cAMP/PKA activity can promote PI3K/PIP3-dependent translocation of Akt to the plasma membrane, we hypothesize that hindbrain GLP-1R-mediated control of food intake involves a PI3K/Akt-dependent pathway. However, whether CNS GLP-1R activation would promote phosphorylation or dephosphorylation of Akt at the membrane remains unknown, as an increase in cAMP/PKA can either stimulate or inhibit Akt activity via a Rap1b-dependent mechanism (19, 37, 42, 63, 66). Therefore, using a combination of in vivo and in vitro techniques, here we evaluate the role of the PI3K/Akt pathway in mediating the food intake- and body weight-suppressive effects of hindbrain GLP-1R activation. A combination of behavioral, ex vivo, and in vitro data reveal that GLP-1R-mediated suppression of intake involves an inhibition of membrane-bound Akt phosphorylation in the NTS. Additionally, hindbrain administration of either the classic PI3K inhibitor LY-294002 or the Akt translocation inhibitor triciribine (TCN; inhibits PIP3/PH translocation of Akt to cell membrane) and attenuates the intake- and body weight-suppressive effects of hindbrain GLP-1R activation.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed individually in hanging wire mesh cages in a temperature- and humidity-controlled environment. Rats were maintained on a 12:12-h light-dark cycle with food and water available ad libitum. All experimental procedures received approval from the University of Pennsylvania Institutional Animal Care and Use Committee.
Drugs
Ex-4 (American Peptide) was dissolved in artificial cerebrospinal fluid (aCSF; Harvard Apparatus) for central injections. TCN (EMD Millipore) and LY-294002 (Cell Signaling Technology) were dissolved in 50% DMSO in aCSF for central injections. Doses for drugs were selected from the literature (49, 52, 61).
Surgery
Rats were anesthetized using a mixture of ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) (KAX cocktail) and placed into a stereotaxic apparatus. Each rat was stereotaxically implanted with a guide cannula (26-gauge; Plastics One, Roanoke, VA) with its tip positioned 2.0 mm above the fourth ventricle (coordinates: on the midline, 2.5 mm anterior to the occipital suture and 5.2 mm ventral to the skull, with injector aimed 7.2 mm from skull) (24, 26, 27, 35). Cannulae were attached to the skull with dental acrylic and jeweler's screws. For all surgeries, analgesia was provided (2 mg/kg meloxicam). At least 5 days after surgery, a fourth icv injection placement was assessed by measurement of the sympathoadrenal-mediated hyperglycemic response to the cytoglucopenia induced by 5-thio-d-glucose (210 μg) dissolved in aCSF (24, 56). Only data from rats showing at least a twofold increase in blood glucose level in response to this treatment were included in the analyses.
Feeding Behavior
Hindbrain inhibition of PI3K activity.
Twenty minutes prior to the onset of the dark cycle, ad libitum-fed rats (n = 15) received counterbalanced fourth icv injections of the PI3K inhibitor LY-294002 (3.07 μg) or vehicle (1 μl of 50% DMSO in aCSF). Ten minutes later, rats received counterbalanced fourth icv injections of Ex-4 (0.3 μg) or vehicle (1 μl of aCSF). At the onset of the dark cycle, rats were presented with preweighed chow, and cumulative food intake was measured to the nearest 0.1 g at 1, 3, 6, and 24 h post-food presentation, accounting for spillage. Body weight was recorded 24 h post-food presentation. Experimental conditions were separated by ≥48 h.
Hindbrain inhibition of PI3K/PIP3-dependent Akt translocation.
Twenty minutes prior to the onset of the dark cycle, a separate group of ad libitum-fed rats (n = 17) received a counterbalanced fourth icv injection of the Akt translocation inhibitor TCN (10 μg) or vehicle (1 μl of 50% DMSO in aCSF). Ten minutes later, rats received counterbalanced fourth icv injections of Ex-4 (0.3 μg) or vehicle (1 μl of aCSF). At the onset of the dark cycle, rats were presented with preweighed chow, and cumulative food intake was measured to the nearest 0.1 g at 1, 3, 6, and 24 h post-food presentation, accounting for spillage. Body weight was recorded 24 h post-food presentation. Experimental conditions were separated by ≥48 h.
Analysis of Intracellular Akt Signaling
Ex vivo tissue collection.
Shortly after the onset of the dark cycle, rats received a fourth icv injection of Ex-4 (0.3 μg) or aCSF vehicle (1 μl). Following injection, rats were anesthetized with the KAX cocktail and euthanized by decapitation at 2 min (n = 10), 30 min (n = 12), 3 h (n = 6), or 6 h (n = 6) postinjection. Brains were rapidly removed and flash-frozen as either a whole brain or, more specifically, as a microdissected dorsal vagal complex (26, 27) in −70°C isopentane and stored at −80°C until processing. For whole brains, micropunched tissue from the caudal medial NTS (mNTS) was collected bilaterally using a cryostat, as described previously (26, 29). All DVC or mNTS tissue was subsequently processed for immunoblot analysis. Tissue from the 2-min, 3-h, and 6-h times were used to assess whole protein content; tissue collected at 30 min was used to measure protein in the membrane-bound and cytoplasmic compartments.
In vitro GLP-1R-expressing immortalized GT1–7 cells.
GT1–7 cells immortalized from murine hypothalamic neuroblastoma cells (generous gift from Dr. Sangwon Kim, University of Pennsylvania) express GLP-1R (6, 26, 67). Cells were maintained at 37°C in 5% CO2 and were cultured in 1× DMEM with 4.5 mg/ml glucose (Invitrogen), 10% (vol/vol) fetal bovine serum (Thermo Fisher Scientific), and 2% penicillin (10,000 IU/ml)-streptomycin (10,000 μg/ml) solution (Mediatech). Overnight serum-starved (18 h) GT1–7 cells were treated with 10 nmol Ex-4 for 15, 30, or 60 min (26). At the end of the incubation, cells were washed in ice-cold 0.1 M PBS, and lysates were prepared by scraping cells in 500 μl of NP-40 lysis buffer containing protease and phosphatase inhibitors through a 26-gauge needle. Lysates were further treated with a hand homogenizer and then incubated at 4°C for 30 min to further solubilize proteins. This was followed by 10 min of centrifugation (12,000 g) at 4°C to pellet out insoluble material. Protein concentrations were determined immediately using BCA assay. Samples were further analyzed via immunoblot for Akt activity using p-Akt-Ser473 rabbit monoclonal antibody (Cell Signaling Technology) normalized to total Akt (Cell Signaling Technology). All immunoblots were quantified using NIH Image J software.
Immunoblot analyses on whole protein content from NTS lysates.
mNTS-enriched tissue was collected from rats euthanized 2 min, 3 h, or 6 h after a fourth icv injection and homogenized in NP-40 lysis buffer containing protease and phosphatase inhibitors. Homogenized tissue and GT1–7 cell lysates were subjected to SDS-PAGE and transferred to PVDF membranes for immunoblot analysis. Akt activity was measured using pAkt-Ser473 rabbit monoclonal antibody normalized to β-actin (Cell Signaling Technology).
Immunoblot analyses on membrane-bound and cytoplasmic protein from NTS lysates.
Caudal mNTS tissue, collected from rats euthanized 30 min post-fourth icv injection, was gently homogenized in a glass Dounce homogenizer in a mild lysis buffer containing DTT and protease and phosphatase inhibitors. The homogenate was centrifuged at 150,000 g at 4°C. The supernatant was removed and saved as the cytosolic fraction, and the pellet was resuspended in a harsher lysis buffer containing 1% Triton X-100 and protease and phosphatase inhibitors. Protein concentrations for the supernatant and the pellet were determined immediately using BCA assay, and samples were analyzed further via immunoblot for Akt activity. For this experiment, p-Akt was normalized to total Akt due to the lack of an appropriate housekeeping protein for use in both cell fractions.
Data Analysis and Statistics
Data for each experiment were analyzed separately and are expressed as means ± SE. All statistical analyses were made using Statistica (version 7; StatSoft). Comparisons between treatment means were analyzed by one- or two-way mixed-design ANOVA tests. The α-level for all tests was set at P < 0.05. Statistically significant main effects and interactions were probed using Student-Newman-Keuls post hoc analyses.
RESULTS
Suppression of Food Intake by Hindbrain GLP-1R Activation Requires PI3K Signaling
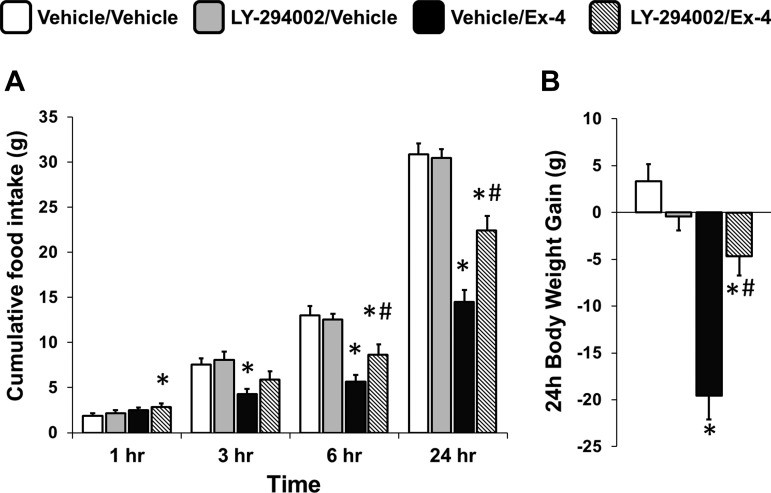
To investigate whether PI3K signaling is necessary for the food intake- and body weight-suppressive effects of hindbrain GLP-1R activation, rats were given fourth icv injections of the PI3K activity inhibitor LY-294002 (10 μg) or its vehicle (1 μl of 50% DMSO in aCSF) in combination with a fourth icv injection of Ex-4 (0.3 μg) or its vehicle (1 μl aCSF). As expected, Ex-4 reduced food intake significantly at 3, 6, and 24 h postinjection (all ANOVAs F1,14 ≥ 11.36, P ≤ 0.005, vehicle/vehicle vs. vehicle/Ex-4, all P < 0.04; Fig. 1A); this intake-suppressive effect was attenuated at 6 and 24 h by LY-294002 pretreatment (all ANOVAs F1,14 ≥ 5.90, P ≤ 0.03, vehicle/Ex-4 vs. LY-294002/Ex-4, all P < 0.02). Additionally, the Ex-4-mediated reduction in 24-h body weight gain was attenuated by LY-294002 (F1,14 = 27.60, P = 0.0001, vehicle/Ex-4 vs. LY-294002/Ex-4, P < 0.001; Fig. 1B). Together, these results indicate that PI3K activity is required for the full expression of the food intake- and body weight-suppressive effects of hindbrain GLP-1R activation.
Fig. 1.
Cumulative chow intake (A) and 24-h body weight change (B) following 4th icv delivery of the phosphatidylinositol 3-kinase (PI3K) inhibitor LY-294002 [3.07 μg, vehicle, 1 μl of 50% DMSO in artificial cerebrospinal fluid (aCSF)] and the glucagon-like peptide 1 receptor (GLP-1R) agonist exendin-4 (Ex-4; 0.3 μg, vehicle, 1 μl of aCSF) alone or in combination. PI3K inhibition attenuated the Ex-4-mediated reductions in food intake at 6 and 24 h as well as 24-h body weight gain. Data are means ± SE. *P < 0.05 from vehicle/vehicle; #P < 0.05 from vehicle/Ex-4.
PI3K/PIP3-Dependent Translocation of Akt to the Plasma Membrane is Required for Suppression of Food Intake by Hindbrain GLP-1R Activation
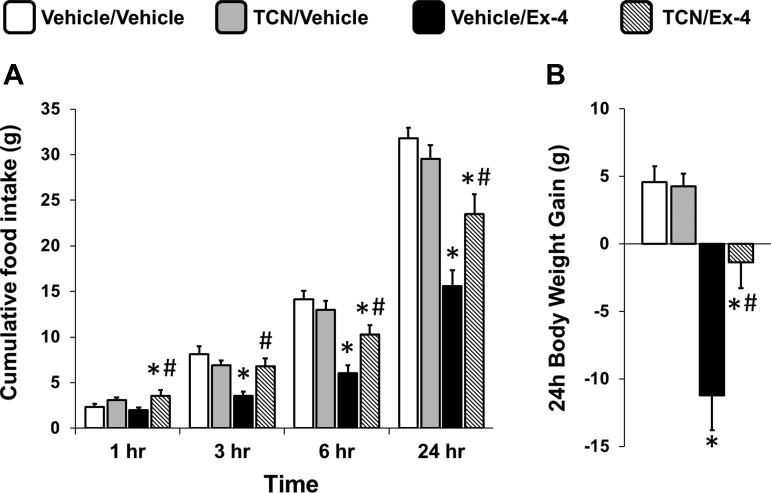
Given the requirement of PI3K signaling for the intake- and body weight-suppressive effects of hindbrain Ex-4 administration, its downstream target Akt might also be involved. To test whether translocation of Akt from the cytoplasm to the cell membrane is required for the energy balance effects of hindbrain GLP-1R activation, fourth icv-targeted injections of TCN (inhibitor of PIP3/PH translocation of Akt to the cell membrane; 10 μg) or its vehicle (1 μl 50% DMSO in aCSF) were given just prior to the fourth icv injection of Ex-4 (0.3 μg) or its vehicle (1 μl of aCSF). TCN alone increased food intake at 1 h post-food presentation (main effect of TCN at 1 h, F1,16 = 10.28, P < 0.01; Fig. 2A). Ex-4 significantly reduced 3-, 6-, and 24-h food intake (all ANOVAs, F1,16 ≥ 12.64, P ≤ 0.003, vehicle/vehicle vs. vehicle/Ex-4, all P < 0.001; Fig. 2A); this effect was attenuated by TCN (all ANOVAs F1,16 ≥ 9.35, P ≤ 0.008, vehicle/Ex-4 vs. TCN/Ex-4, all P < 0.005 at 3, 6, and 24 h). Finally, 24-h body weight gain was reduced by Ex-4 (F1,16 = 57.11, P < 0.001, vehicle/vehicle vs. vehicle/Ex-4, P < 0.001; Fig. 2B); TCN attenuated the Ex-4-mediated suppression of body weight gain (F1,16 = 9.99, P = 0.006, vehicle/Ex-4 vs. TCN/Ex-4, P < 0.003). These results indicate that PI3K/PIP3-dependent translocation of Akt from the cytoplasm to the cell membrane, and thus a change in Akt activity state (i.e., either phosphorylation or dephosphorylation of Akt), is required for the food intake- and body weight-suppressive effects of hindbrain GLP-1R activation.
Fig. 2.
Cumulative chow intake (A) and 24-h body weight change (B) following 4th icv delivery of the inhibitor of Akt translocation triciribine (10 μg, vehicle, 1 μl of 50% DMSO in aCSF) and GLP-1R agonist Ex-4 (0.3 μg, vehicle, 1 μl of aCSF) alone or in combination. Blocking the translocation of Akt to the plasma membrane attenuated the food intake- and body weight-suppressive effects of Ex-4. Data are means ± SE. *P < 0.05 from vehicle/vehicle; #P < 0.05 from vehicle/Ex-4.
GLP-1R Activation Reduces Akt Phosphorylation in Caudal mNTS Tissue
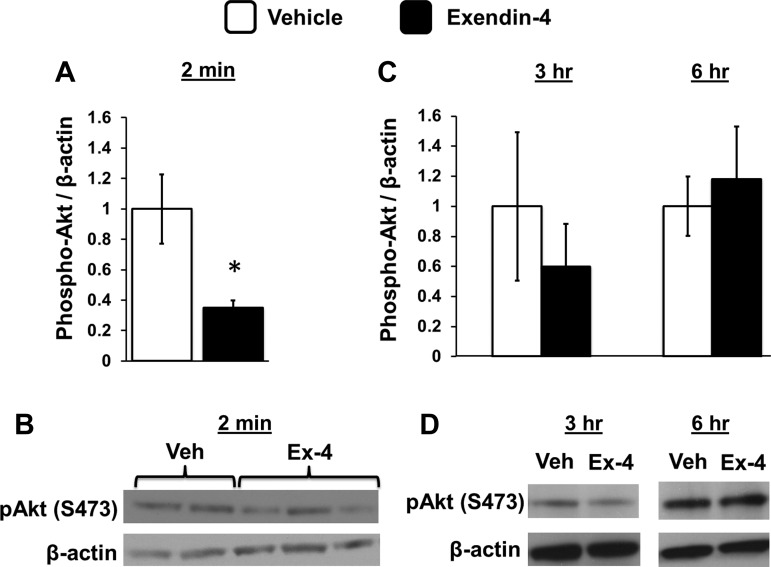
Hindbrain GLP-1R activation by fourth icv Ex-4 suppressed phosphorylation of Akt (p-Akt) at Ser473 in mNTS tissue at 2 min compared with p-Akt levels in aCSF-treated rats (data presented as phosphorylated Akt/β-actin levels; F1,8 = 7.76, P < 0.03; Fig. 3, A and B). In a separate experiment, fourth icv Ex-4 had no effect on Akt phosphorylation in mNTS tissue at 3 or 6 h postadministration (data presented as phosphorylated Akt/β-actin levels; ANOVAs F1,4 ≤ 1.27, P ≥ 0.32; Fig. 3, C and D), times at which fourth icv Ex-4 robustly suppresses food intake in behavioral studies. Together, these results suggest that phosphorylation of Akt at Ser473 is reduced by Ex-4 and that this suppression of Akt activation precedes and is required for the behavioral changes in feeding.
Fig. 3.
A: phosphorylation of Akt is reduced in nucleus tractus solatarius (NTS)-enriched tissue 2 min after 4th icv delivery of Ex-4 (0.3 μg) compared with vehicle (1 μl of aCSF). B: representative immunoblots for phosphorylated Akt and β-actin are shown. C: no changes in Akt phosphorylation were observed in NTS-enriched tissue 3 or 6 h after 4th icv delivery of Ex-4 (0.3 μg) compared with vehicle (1 μl of aCSF). D: representative images of these immunoblots are depicted. Data are means ± SE. *P < 0.05 from vehicle.
Ex-4 Suppresses Phosphorylation of Akt in GLP-1R-Expressing GT1–7 Neurons
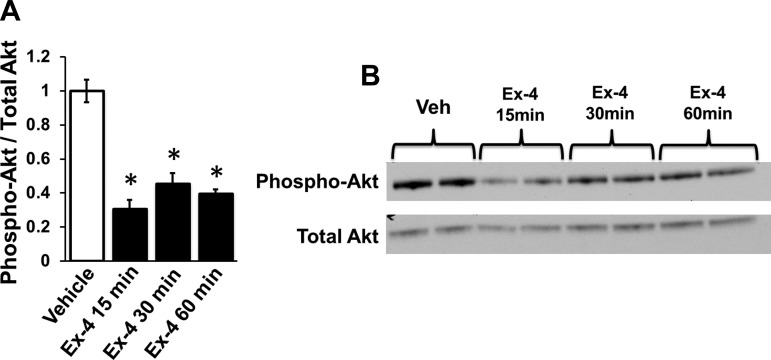
To determine whether the reduction in Akt phosphorylation by GLP-1R activation occurs within a GLP-1R-expressing neuron, phosphorylated and total Akt levels were analyzed in GLP-1R-expressing GT1–7 neuronal cells after 15, 30, or 60 min of 10 nM Ex-4 exposure. Ex-4 significantly reduced Ser473-p-Akt at all three times compared with vehicle treatment (aCSF), with the lowest levels of p-Akt observed at 15 min post-Ex-4 application (data presented as phosphorylated/total Akt levels, F3,4 = 32.43, P < 0.003, vehicle vs. Ex-4, P < 0.005 at 15, 30, and 60 min; Fig. 4).
Fig. 4.
A: Akt phosphorylation is decreased in GLP-1R-expressing GT1–7 neuronal cells after 15, 30, or 60 min of exposure to Ex-4 (10 nM). B: representative immunoblots are shown. Data are means ± SE. *P < 0.05 from vehicle (aCSF).
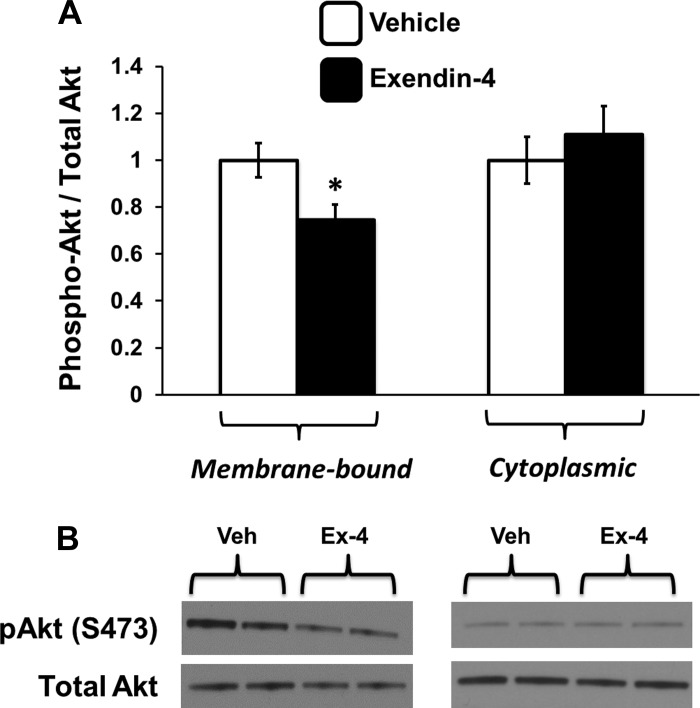
Hindbrain GLP-1R Activation Reduces Phosphorylation of Membrane-Bound Akt at Ser473
To test directly the hypothesis that hindbrain GLP-1R activation inhibits phosphorylation of membrane-bound Akt, high-speed centrifugation was used to separate the cytosolic and cell membrane components of caudal mNTS tissue so that each compartment could be analyzed separately by immunoblot. As shown in Fig. 5, in this preparation, fourth icv Ex-4 reduced Ser473 phosphorylation of Akt (F1,9 = 6.70, P = 0.03) in the membrane fraction. However, fourth icv Ex-4 had no effect on Akt activity in the nonmembrane (i.e., cytoplasmic) fraction (F1,10 = 0.48, P = 0.50). These findings indicate that hindbrain GLP-1R activation specifically reduces Ser473 phosphorylation of Akt at the cell membrane.
Fig. 5.
A: phosphorylation of Akt is selectively reduced in the membrane fraction but not the cytoplasm of medial NTS tissue 30 min after 4th icv delivery of Ex-4 (0.3 μg) compared with vehicle (1 μl of aCSF). B: representative immunoblots for the membrane (left) and cytoplasmic (right) fractions. Data are means ± SE. *P < 0.05 from vehicle (Veh).
DISCUSSION
Central GLP-1R signaling is physiologically required for the normal control of energy balance (3, 5, 14, 24, 45, 59). Despite the importance of forebrain GLP-1R signaling for food intake regulation (3, 13, 14, 38, 41, 44), neural processing restricted to the hindbrain is sufficient to mediate the food intake-suppressive effects of GLP-1R agonists administered peripherally or centrally (28). Within the hindbrain, GLP-1R signaling in the mNTS is physiologically relevant for the normal control of food intake (24), and following direct exogenous mNTS activation of GLP-1R, cumulative food intake is robustly suppressed for more than 24 h (26, 68). Previous findings indicate that NTS GLP-1R-mediated suppression of food intake requires a cAMP/PKA-dependent activation of p44/42-MAPK and simultaneous suppression of AMPK (26). Because the intake-suppressive effects of hindbrain GLP-1R activation are so robust and long-lasting, additional cAMP/PKA-dependent intracellular signaling pathways are likely required to mediate the suppression of intake by GLP-1R activation. Here, a combination of behavioral, ex vivo, and in vitro data reveal that hindbrain GLP-1R-mediated suppression of food intake involves a PI3K/Akt-dependent pathway. Interestingly, the data support the hypothesis that GLP-1R-mediated suppression of intake involves a PI3K/PIP3-dependent translocation of Akt to the plasma membrane and subsequent suppression of Akt phosphorylation at Ser473. Indeed, the GLP-1R agonist Ex-4 decreased phosphorylation of Akt significantly in NTS-enriched tissue and in GLP-1R-expressing neuronal cell lysates. Additionally, hindbrain administration of the classic PI3K inhibitor LY-294002 or of TCN, an inhibitor of the PIP3-dependent translocation of Akt to the cell membrane, attenuates the intake- and body weight suppressive-effects of fourth icv Ex-4. Together with our previous report (26), the current results suggest the importance of cAMP/PKA-dependent activation of the PI3K/Akt signaling cascade in mediating the hypophagic effects of hindbrain GLP-1R activation.
Increased cAMP/PKA signaling by GLP-1R activation engages numerous intracellular signaling mechanisms implicated in energy balance regulation, some of which are established (10, 26, 58) and some that remain untested. The current data offer new evidence demonstrating the importance of PI3K activation and its downstream targets in mediating the food intake- and body weight-suppressive effects of hindbrain GLP-1R activation. The most parsimonious explanation of these data is that the Ex-4-mediated activation of PI3K occurs through a cAMP/PKA-dependent pathway (11). However, because PI3K activity can be modulated directly by a PKA-independent pathway that involves exchange protein directly activated by cAMP signaling (66), we cannot rule out the possibility that PKA signaling may not be upstream of GLP-1R-mediated suppression of membrane-bound Akt signaling. Nonetheless, it is important to reiterate that PKA activity is required for the suppression of intake following hindbrain GLP-1R activation (26). Therefore, the conservative interpretation would be that hindbrain GLP-1R-mediated suppression of intake requires a coordinated set of intracellular signaling pathways that involve modulation of PKA, AMPK, p44/42-MAPK, PI3K, and Akt signaling. Disruption of any one of these signaling pathways is sufficient to attenuate the food intake-suppressive effects of GLP-1R activation.
Given that reductions in food intake are often associated with activation of Akt, our finding that hindbrain GLP-1R activation decreases phosphorylation of membrane-bound Akt may seem at odds with the intake-suppressive effects of GLP-1 signaling. More specifically, this perceived paradox is driven largely by solid evidence demonstrating that hypothalamic leptin and insulin signaling increases Akt phosphorylation and that blockade of PI3K activation by wortmannin or LY-294002 attenuates the energy balance-suppressive effects of hypothalamic leptin or insulin (48, 51, 52). Thus, the common assumption is made that an increase in Akt phosphorylation should lead to behavioral changes that decrease food intake. Yet emerging evidence that complements current data is beginning to challenge this perspective. Recently, Kanoski et al. (34) showed that lateral icv administration of ghrelin (an orexigenic hormone) increases phosphorylation of Akt Ser473 in the ventral hippocampus, a CNS nucleus that these authors also show to be a direct site of action mediating the increase in food intake by ghrelin. Moreover, the increase in food intake by ventral hippocampal ghrelin administration was attenuated by blockade of PI3K activation using LY-294002. In consideration of this recent report (34) and the current findings, it is important to reiterate that PI3K activation does not necessitate an increase in Akt phosphorylation. Rather, PI3K activation converts PIP2 to PIP3, which subsequently recruits Akt to the plasma membrane, where Akt can then be phosphorylated or dephosphorylated (32). Because all phosphorylation changes of Akt must occur at the membrane (7), blockade of PI3K signaling does not unidirectionally lead to a blockade in Akt phosphorylation but instead will block any change in Akt phosphorylation state. Further confirmation of this effect is provided by the current data showing that the intake-suppressive effects of hindbrain GLP-1R activation can be attenuated by a fourth icv administration of not only LY-294002 but also by TCN, which inhibits PIP3-mediated translocation of Akt to the cell membrane. Therefore, in context with reports on hypothalamic leptin/insulin signaling (48, 51, 52) and hippocampal ghrelin signaling (34), a safe assertion is that intracellular signaling pathways mediating energy status signals may not be uniform in their activity across all CNS nuclei and should instead be viewed as one part of a coordinated intracellular signaling cascade, the orchestration of which is required to mediate the energy balance effects of a given hormonal signal.
In addition to the food intake-suppressive effects produced by CNS GLP-1 signaling, there is an increasing appreciation for the physiological requirement of the central GLP-1 system for glycemic control (5, 39, 53). Although increases in PKA, p44/42-MAPK, and PI3K signaling and decreases in AMPK and Akt signaling are all required to mediate the food intake-suppressive effects of hindbrain GLP-1R activation, it remains unknown whether all or part of this same intracellular signaling cascade is required to mediate the glycemic effects of central GLP-1R activation. Indeed, there is precedence for diverging intracellular signaling pathways mediating differential behavioral effects in ingestive behavior (12). Nonetheless, it would stand to reason that some if not all of the aforementioned intracellular signaling pathways are implicated in modulating the glycemic effects of CNS GLP-1R signaling. For example, similar intracellular signaling mechanisms are engaged by other neurohormonal systems acting at the NTS to regulate glycemic control. In addition to GLP-1, NTS neurons process numerous peripheral signals relevant for energy status and glycemic control, including neuroendocrine signals such as insulin (20) and leptin (23, 29), as well as vagally mediated gastrointestinal satiation signals (8, 21, 22, 46). In the case of insulin, Filippi et al. (20) have shown recently that NTS insulin signaling regulates glucose tolerance via a p44/42-MAPK pathway. In line with the current data, this same report indicated that insulin delivery to the hindbrain did not result in a significant increase in phosphorylation of Akt within the dorsal vagal complex despite the usual assumption that insulin receptor signaling leads to an increase in Akt phosphorylation. Because hindbrain GLP-1R activation also activates MAPK signaling (26), it is possible that insulin receptor signaling and GLP-1R signaling may be interacting within the hindbrain through at least a MAPK-dependent mechanism to coordinate regulation of glycemia. Such hypotheses require further examination.
Activation of the GLP-1R produces a reduction in Akt phosphorylation that occurs within minutes in both ex vivo NTS-enriched tissue and in vitro GT1–7 neuronal cell lysates. Paradoxically, the behavioral analyses show that the suppression of food intake by hindbrain Ex-4 delivery is not observed until 3 h postadministration, an effect dependent upon an increase in PI3K activation, translocation of Akt to the cell membrane, and presumably the suppression of membrane-bound Akt phosphorylation. Similar time course discrepancies for other intracellular signaling cascades between tissue lysates and physiological/behavioral feeding responses are reported for GLP-1R (26) and for other anorectic ligands acting within the brain (36, 47, 52, 62). Moreover, hindbrain administration of TCN produced an increase in food intake 1 h postinjection (the earliest time point measured), indicating that a basal level of Akt activity within the hindbrain is required to maintain normal homeostatic food intake. Collectively, these findings suggest that although PI3K and Akt signaling are required to mediate the suppression of intake by NTS GLP-1R activation, other downstream signaling cascades and recruitment of additional CNS nuclei are also required to mediate the intake-suppressive effects of hindbrain GLP-1R activation. It is highly likely that the identified intracellular signaling pathways and additional downstream targets are modifying gene transcription and protein synthesis, theoretically making the NTS GLP-1R-expressing neuron more sensitive to other anorectic signals that are processed within the NTS (see Ref. 21 for review). As illustrated in our working model (Fig. 6), likely candidates of these additional downstream intracellular signaling pathways that can modulate gene transcription include, but are not limited to, the mammalian target of rapamycin (mTOR) as well as the transcription factors forkhead box O and cAMP response element-binding protein. Therefore, exploration of each of these signaling pathways in mediating the food intake-suppressive effects of hindbrain GLP-1R activation is highly warranted.
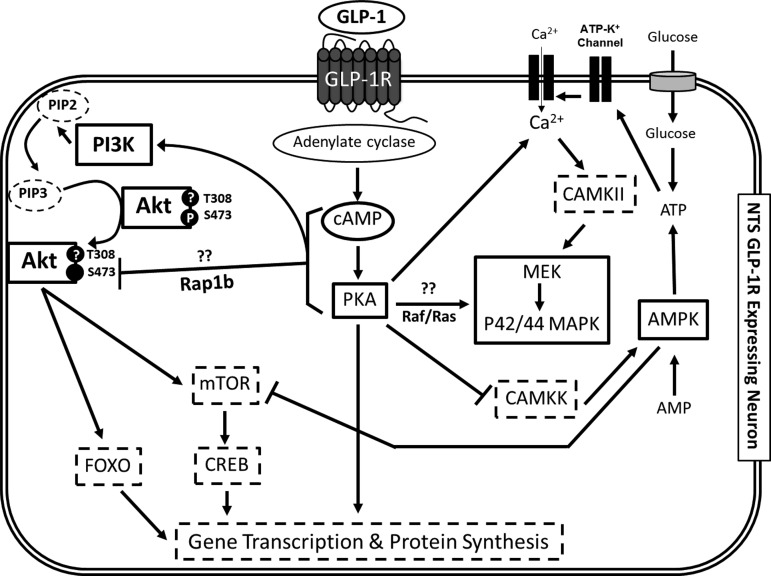
Fig. 6.
Proposed model of the intracellular signals necessary for the GLP-1R activation-mediated suppression of food intake. Our previous work established that activation of hindbrain GLP-1R results in a cAMP-dependent increase in PKA activity, which in turn increases P42/44 MAPK phosphorylation and decreases AMPK activation. Based on the present findings, we hypothesize that cAMP-dependent activation of PKA increases phosphorylation of PI3K, allowing for the phosphatidylinositol (3,4,5)-trisphosphate (PIP3)-dependent translocation of Akt to the plasma membrane and subsequent reduction of Akt phosphorylation at Ser473. We predict that the activation of AMPK and Akt converge on the mammalian target of rapamycin (mTOR)/cAMP response element-binding protein (CREB) pathway and forkhead box O (FOXO), resulting in protein synthesis that may drive the long-lasting effects of GLP-1R-mediated food intake suppression. Dashed lines indicate putative signaling pathways relevant for the control of GLP-1R activation-induced reduction of food intake and body weight. PIP2, phosphatidylinositol (4,5)-bisphosphate.
Although the data reported here provide solid evidence that GLP-1R activation reduces food intake via a PI3K-dependent translocation of Akt to the membrane, there are additional unanswered questions that require further investigation. Specifically, the membrane preparation results indicate that the decreased phosphorylation of Akt by hindbrain GLP-1R activation was occurring specifically at membrane-bound Akt and not through an alteration in cytoplasmic Akt signaling. This is consistent with the requirement that Akt be bound to the membrane for phosphorylation state to change (7). Although we hypothesize that these changes in Akt phosphorylation are taking place at the plasma membrane, the current data cannot rule out the possibility that the observed decrease in Akt phosphorylation may be occurring in other organelle membranes. In addition, it is currently unknown which isoform(s) of Akt (i.e., Akt1, Akt2, or Akt3) is being modulated by hindbrain GLP-1R signaling. Therefore, future studies utilizing transgenic mice with specific Akt isoform deletions are certainly warranted to further explore this question.
Collectively, the current results demonstrate the requirement of PI3K activation, PIP3-dependent translocation of Akt to the plasma membrane, and suppressed phosphorylation of membrane-bound Akt in mediating the food intake- and body weight-suppressive effects of hindbrain GLP-1R activation. The decreased Akt phosphorylation by GLP-1R activation was demonstrated to occur within a GLP-1R-expressing neuron. Together with our previous report (26), these data advance our understanding of the complex and orchestrated intracellular signaling cascade that is required to mediate the energy balance effects of hindbrain GLP-1R signaling and may provide insight for the development of more effective and CNS-directed GLP-1-based pharmacotherapies for the treatment of obesity. More broadly, these data also further our understanding of how NTS neurons process and integrate energy balance signals.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-096139 and DK-085435 (to M. R. Hayes).
DISCLOSURES
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
L.E.R., E.G.M.-B., D.J.Z., L.E.M., D.R.O., and M.R.H. performed the experiments; L.E.R., E.G.M.-B., D.J.Z., and M.R.H. analyzed the data; L.E.R., E.G.M.-B., D.J.Z., and M.R.H. interpreted the results of the experiments; L.E.R., E.G.M.-B., and M.R.H. prepared the figures; L.E.R., E.G.M.-B., and M.R.H. drafted the manuscript; L.E.R., E.G.M.-B., D.J.Z., L.E.M., D.R.O., and M.R.H. edited and revised the manuscript; L.E.R., E.G.M.-B., D.J.Z., L.E.M., D.R.O., and M.R.H. approved the final version of the manuscript; M.R.H. contributed to the conception and design of the research.
ACKNOWLEDGMENTS
Valuable technical assistance was provided by Orianne Montaubin, Frank Wang, Line Stensland, Sky Prestowitz, and Christopher Turner. We thank Drs. Kendra Bence, Heath Schmidt, Sangwon Kim, and Scott Kanoski for valuable intellectual advice.
REFERENCES
- 1. Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 2. Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7: 261–269, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beak SA, Heath MM, Small CJ, Morgan DG, Ghatei MA, Taylor AD, Buckingham JC, Bloom SR, Smith DM. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J Clin Invest 101: 1334–1341, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berndt N, Yang H, Trinczek B, Betzi S, Zhang Z, Wu B, Lawrence NJ, Pellecchia M, Schonbrunn E, Cheng JQ, Sebti SM. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ 17: 1795–1804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berthoud HR. The Caudal Brainstem and the Control of Food Intake and Energy Balance. New York: Plenum, 2004, p. 195–240 [Google Scholar]
- 9. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 wk in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 8: 436–447, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Burmeister MA, Ayala J, Drucker DJ, Ayala JE. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am J Physiol Endocrinol Metab 304: E677–E685, 2013 [DOI] [PubMed] [Google Scholar]
- 11. Cass LA, Summers SA, Prendergast GV, Backer JM, Birnbaum MJ, Meinkoth JL. Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol Cell Biol 19: 5882–5891, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daniels D, Yee DK, Faulconbridge LF, Fluharty SJ. Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology 146: 5552–5560, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14453–14457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elghazi L, Rachdi L, Weiss AJ, Cras-Méneur C, Bernal-Mizrachi E. Regulation of beta-cell mass and function by the Akt/protein kinase B signalling pathway. Diabetes Obes Metab 9, Suppl 2: 147–157, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Erdogdu O, Eriksson L, Xu H, Sjöholm A, Zhang Q, Nyström T. Exendin-4 protects endothelial cells from lipoapoptosis by PKA, PI3K, eNOS, p38 MAPK, and JNK pathways. J Mol Endocrinol 50: 229–241, 2013 [DOI] [PubMed] [Google Scholar]
- 17. Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325: 26–35, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Fang D, Huang Z, Guan H, Liu J, Yao B, Xiao H, Li Y. The Akt/FoxO1/p27 pathway mediates the proliferative action of liraglutide in beta cells. Mol Med Rep 5: 233–238, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Filippa N, Sable CL, Filloux C, Hemmings B, Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol 19: 4989–5000, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filippi BM, Yang CS, Tang C, Lam TK. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab 16: 500–510, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 33, Suppl 1: S11–S15, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100: 503–510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayes MR, Skibicka KP, Bence KK, Grill HJ. Dorsal hindbrain 5′-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology 150: 2175–2182, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Holz GG, Chepurny OG. Diabetes outfoxed by GLP-1? Sci STKE 2005: pe2, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J 315: 709–713, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry 73: 915–923, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62: 1916–1927, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan AM, Ponzio TA, Sanchez-Watts G, Stanley BG, Hatton GI, Watts AG. Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: a proposed role during glycemic challenges. J Neurosci 27: 7344–7360, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim S, Jee K, Kim D, Koh H, Chung J. Cyclic AMP inhibits Akt activity by blocking the membrane localization of PDK1. J Biol Chem 276: 12864–12870, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115: 3554–3563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 64: 4–11, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 138: 4445–4455, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Lou L, Urbani J, Ribeiro-Neto F, Altschuler DL. cAMP inhibition of Akt is mediated by activated and phosphorylated Rap1b. J Biol Chem 277: 32799–32806, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5: 262–269, 2009 [DOI] [PubMed] [Google Scholar]
- 44. McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol 274: R23–R29, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 140: 244–250, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Moran TH. Gut peptide signaling in the controls of food intake. Obesity (Silver Spring) 14, Suppl 5: 250S–253S, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab 297: E202–E210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2: 411–420, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Neasta J, Ben Hamida S, Yowell QV, Carnicella S, Ron D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry 70: 575–582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Niswender KD, Gallis B, Blevins JE, Corson MA, Schwartz MW, Baskin DG. Immunocytochemical detection of phosphatidylinositol 3-kinase activation by insulin and leptin. J Histochem Cytochem 51: 275–283, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 299: E318–E324, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 56: 8–15, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Raun K, von Voss P, Knudsen LB. Liraglutide, a once-daily human glucagon-like peptide-1 analog, minimizes food intake in severely obese minipigs. Obesity (Silver Spring) 15: 1710–1716, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213: 451–452, 1981 [DOI] [PubMed] [Google Scholar]
- 57. Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sandoval D, Barrera JG, Stefater MA, Sisley S, Woods SC, D'Alessio DD, Seeley RJ. The anorectic effect of GLP-1 in rats is nutrient dependent. PLoS One 7: e51870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 284: R1427–R1435, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293: R983–R987, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Shao JL, Wan XH, Chen Y, Bi C, Chen HM, Zhong Y, Heng XH, Qian JQ. H2S protects hippocampal neurons from anoxia-reoxygenation through cAMP-mediated PI3K/Akt/p70S6K cell-survival signaling pathways. J Mol Neurosci 43: 453–460, 2011 [DOI] [PubMed] [Google Scholar]
- 62. Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 146: 3739–3747, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol 21: 1921–1929, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Turton MD. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996 [DOI] [PubMed] [Google Scholar]
- 65. Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges JP, Verhoeven R, Buganova I, Madsbad S. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 30: 1608–1610, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Wang L, Liu F, Adamo ML. Cyclic AMP inhibits extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways by inhibiting Rap1. J Biol Chem 276: 37242–37249, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Yang Y, Zhou LB, Liu SQ, Tang JF, Li FY, Li RY, Song HD, Chen MD. Expression of feeding-related peptide receptors mRNA in GT1–7 cell line and roles of leptin and orexins in control of GnRH secretion. Acta Pharmacol Sin 26: 976–981, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 36: 1522–1528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]