Abstract
Expression of genes for lipid biosynthetic enzymes during initiation of lactation in humans is unknown. Our goal was to study mRNA expression of lipid metabolic enzymes in human mammary epithelial cell (MEC) in conjunction with the measurement of milk fatty acid (FA) composition during secretory activation. Gene expression from mRNA isolated from milk fat globule (MFG) and milk FA composition were measured from 6 h to 42 days postpartum in seven normal women. Over the first 96 h postpartum, daily milk fat output increased severalfold and mirrored expression of genes for all aspects of lipid metabolism and milk FA production, including lipolysis at the MEC membrane, FA uptake from blood, intracellular FA transport, de novo FA synthesis, FA and glycerol activation, FA elongation, FA desaturation, triglyceride synthesis, cholesterol synthesis, and lipid droplet formation. Expression of the gene for a key lipid synthesis regulator, sterol regulatory element-binding transcription factor 1 (SREBF1), increased 2.0-fold by 36 h and remained elevated over the study duration. Expression of genes for estrogen receptor 1, thyroid hormone-responsive protein, and insulin-induced 2 increased progressively to plateau by 96 h. In contrast, mRNA of peroxisome proliferator-activated receptor-γ decreased severalfold. With onset of lactation, increased de novo synthesis of FA was the most prominent change in milk FA composition and mirrored the expression of FA synthesis genes. In conclusion, milk lipid synthesis and secretion in humans is a complex process requiring the orchestration of a wide variety of pathways of which SREBF1 may play a primary role.
Keywords: fat globule, milk production, metabolism, microarray, GC-MS
human milk lipids are comprised of 98% triglycerides (TG) and provide ≈50% of an infant's energy intake (15, 36). Milk TG contain fatty acids (FA) derived from three sources: mobilized maternal fat stores, maternal dietary lipids and de novo synthesis within the mammary gland (MG) (15, 24). In addition to liver and adipose tissue, the MG is recognized as one of three major lipid-synthesizing organs in the body (77). The unique feature of FA metabolism within the MG is the synthesis of medium-chain FA because the mammary epithelial cell (MEC) expresses a special enzyme, thioesterase II (37, 52, 78), that terminates FA synthesis at a carbon length of 8–16 carbons. In contrast, longer-chain FA in milk have been shown to originate mainly from either maternal diet or from mobilized adipose tissue (72).
During initiation, or secretory activation, “the onset of copious milk secretion” (2, 9), dramatic changes in milk volume and composition occur (56, 57, 70). Studies over the past decades have improved our understanding of the regulation of the synthetic and secretory pathways of milk proteins and lipids in a variety of animal models (2, 5, 65, 69). We have recently reported the temporal coordination of changes in gene expression of carbohydrate metabolic pathways in human MEC transcriptome obtained from milk fat globules (MFG) (54). In that study, we demonstrated that genes controlling the conversion of glucose to UDP-galactose (UGP2 and PGM1) and UDP-galactose transport into the Golgi (SLC35A2) may be rate limiting in lactose synthesis. We speculated that progesterone withdrawal following delivery may be the most likely signal. Decreasing plasma progesterone as reflected in milk progesterone concentrations was associated with a sharp increase in expression of the prolactin receptor and STAT5, which may in turn induce UGP2 and SLC35A2 expression.
To date, no studies have investigated expression of the genes for lipid biosynthetic enzymes and lipid production in humans during secretory activation of lactation. Furthermore, the majority of studies investigating MG gene regulation in animal models have focused on milk protein gene expression, and, by comparison, little attention has been paid to lipid biosynthesis (2, 6, 66). The present studies describe the temporal coordination of changes in expression of genes in the lipid metabolic pathways and milk lipid content in healthy women during secretory activation and through the first six weeks of lactation.
MATERIALS AND METHODS
Subjects
Following approval from the Institutional Review Board at Baylor College of Medicine (Houston, TX), written informed consent was obtained from seven healthy, lactating women with an average age of 30 ± 1 yr (mean ± SE) and mean pre-pregnancy height and weight of 165 ± 2 cm and 61 ± 4 and BMI of 22 ± 1 kg/m2. Details on inclusion and exclusion criteria and more details about the study design involving these same subjects and samples have already been published (54).
In brief, breast milk was collected 6 h following delivery, q12 h for the first 4 days, and then weekly for a total of 6 wk. About 0.5–1.0 ml of whole milk was kept at −70°C for fat analysis. The remainder of the milk sample was transferred into sterile, RNAse-free, tightly sealed tubes containing 20 μl of RNAse inhibitor (SUPERase IN 20 U/μl, Abmion Applied Biosystems), and then centrifuged (Sorvall Legend T) at 3,000 rpm for 15 min at 4°C. The supernatant fat layer was transferred using a sterile spatula to a new tube. TRIzol (2 ml; Invitrogen Life Technology, Carlsbad, CA) was added prior to storage at −80°C.
Milk FA Analyses
Milk FAs were measured using GC-MS after derivatization to the corresponding pentaflurobenzyl (PFB) derivative. FA standards (C4:0, C6:0, C7:0, C8:0, C9:0, C10:0, C12:0, C13:0, C14:0, C15:0, C16:0, C16:1, C17:0, C18:0, C18:1, C18:2, C18:3, C20:0, C20:1, C20:2, C20:3, C20:4, C20:5, C22:0, C22:1, C22:2, C22:3, C22:4, C22:5, C22:6, C24:0, C24:1, and C24:2) were obtained from Sigma-Aldrich, (St. Louis, MO). The uniformly deuterium-labeled (atom 99%) even-chain saturated FAs (C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0) were obtained from Cambridge Isotope Laboratory (Andover, MA) and used as internal standards. Acetone, hexane, dichloromethane, ethanol, and all solvents were HPLC grade. O-(2,3,4,5,6-pentafluorbenzyl) bromide (PFB-Br) and tetrabutylammonium hydrogen sulfate (TBA) were obtained from Aldrich Chemicals, (Milwaukee, WI).
FAs were converted to their PFB ester derivative following alkaline hydrolysis (23, 76). Briefly, after warming milk samples to 37°C for 15 min, 100μl of milk was pipetted into a 4-ml Teflon-lined screw cap vial containing the labeled even-chain FAs (10 pmol of each of C4:0, C6:0 and C8:0; 100 pmol of each C10:0, C12:0 and C14:0; and 250 pmol of each C16:0 and C18:0). Alkaline hydrolysis was performed by adding 100 μl of 2.0 M KOH in 50% ethanol to each tube. Tubes were tightly sealed with Teflon caps, vortexed briefly (10 s), and heated at 85°C for 2 h. Tubes were then refrigerated at 4°C for 30 min, and 100 μl of 2 N HCl and 250 μl of 0.1 M TBA counter ion solution were added and vortexed for 5 min. An aliquot of 400 μl of 0.13 M PFB-Br in dicholoromethane was added to each tube and vortexed vigorously for 10 min. Tubes were kept at room temperature overnight to complete the derivatization reaction. On the following morning, 1 ml of hexane (containing 10% ethanol) was added, and tubes were vortexed for 5 min. Tubes were subsequently centrifuged for 15 min at 3,000 rpm at 4°C, and the supernatant (organic layer), which contained the PFB-FA esters, was transferred to a clean GC-MS vial and injected to the GC-MS. A set of FA external standards covering the range of FA concentrations in the milk samples was prepared (including adding internal standards), derivatized, and run simultaneously with each single batch of samples.
The derivatized samples and standards were analyzed using a Hewlett-Packard GC-MS (GC 6890, MS 5973) with an SP-1701 column (30 m × 0.25 mm × 0.25 μM; Supelco, Bellefonte, PA). The conditions for the GC were as follows: injector, 250°C (splitless injection of samples); oven, 60°C for 1.0 min; ramp, 20°C/min to 280°C; and hold at 280°C for 10 min. Methane negative chemical ionization analyses were performed as the reagent gas. Data were acquired in selective ion monitoring (SIM) mode. Table 1 provides the mass fragments of different FA-PFB esters. Peak areas of the analyte or of the standards were measured, and the ratio of the area from the analyte-derived ion to that from the internal standard was calculated. The ratios were then compared with the calibration curves for the analyte prepared from the standards to determine the concentration of individual FA. For long-chain FAs (≥18 carbons), for which we did not have labeled internal standards, standard curves were generated by plotting the concentration of the external standards vs. area of the signal normalized to that of d35-stearate. Individual FAs were grouped as de novo synthesized (DNS_FAs; <16 carbons), total saturated (SAT_FAs), monounsaturated (MUS_FAs), polyunsaturated (PU_FAs), omega 6 (ω6_FAs), omega 3 (ω3_FAs), and very-long-chain (VLC_FAs; ≥22 carbons) and were expressed as concentrations (g/100 ml) and percentage of total FA. The daily total milk and individual FA output were calculated by multiplying the FA concentration times an estimate of milk volumes derived from previous publications (56, 57, 70).
Table 1.
Pentafluorobenzyl (PFB) derivative's major fragment of different fatty acids (FAs) measured using negative chemical ionization (NCI) technique and methane as a reagent gas
| Monitored Ions m/z |
|||||
|---|---|---|---|---|---|
| FA | Weight Formula | NCI Fragment Structure | m0 | m + 1 | m + 2 |
| Butanoic (C4:0) | 88.11 | C4H7O2 | 87 | 88 | 89 |
| Butanoic-d7 (C4:0) | 95.15 | C4d7O2 | 94 | 95 | |
| Hexanoic (C6:0) | 116.16 | C6H11O2 | 115 | 116 | 117 |
| Hexanoic-d11 (C6:0) | 127.23 | C6d11O2 | 126 | 127 | |
| Octanoic (C8:0) | 144.21 | C8H15O2 | 143 | 144 | 145 |
| Octanoic-d15 (C8:0) | 159.21 | C8d15O2 | 158 | 159 | |
| Decanoic (C10:0) | 172.26 | C10H19O2 | 171 | 172 | 173 |
| Decanoic-d19 (C10:0) | 191.19 | C10H19O2 | 190 | 191 | |
| Dodecanoic (C12:0) | 200.32 | C12H23O2 | 199 | 200 | 201 |
| Dodecanoic-d23 (C12:0) | 223.32 | C12d23O2 | 222 | 223 | |
| Tetradecanoic (C14:0) | 228.37 | C14H27O2 | 227 | 228 | 229 |
| Tetradecanoic-d27 (C14:0) | 255.37 | C14d27O2 | 254 | 255 | |
| Pentadecanoic (C15:0) | 242.40 | C15H29O2 | 241 | 242 | 243 |
| Hexadecanoic (C16:0) | 256.42 | C16H31O2 | 255 | 256 | 257 |
| Hexadecanoic-d31 (C16:0) | 287.42 | C16d31O2 | 286 | 287 | |
| Hexadec-9-enoic (C16:1) | 254.41 | C16H29O2 | 253 | 254 | 255 |
| Octadecanoic (C18:0) | 284.48 | C18H35O2 | 283 | 284 | 285 |
| Octadecanoic-d35 (C18:0) | 319.48 | C18d35O2 | 318 | 319 | |
| Octadecenoic (C18:1) | 282.46 | C18H33O2 | 281 | 282 | 283 |
| Linoleic (C18:2) | 280.45 | C18H31O2 | 279 | 280 | 281 |
| Linolenic (C18:3) | 278.43 | C18H35O2 | 277 | 278 | 279 |
| Eicosanoic (C20:0) | 312.53 | C20H39O2 | 311 | 312 | 313 |
| Eicosenoic (C20:1) | 310.51 | C20H37O2 | 309 | 310 | 311 |
| Eicosadienoic (C20:2) | 308.50 | C20H35O2 | 307 | 308 | 309 |
| Eicosatrienoic (C20:3) | 306.48 | C20H33O2 | 305 | 306 | 307 |
| Eicosatetraenoic (C20:4) | 304.47 | C20H31O2 | 303 | 304 | 305 |
| Eicosapentaenoic (C20:5) | 302.45 | C20H29O2 | 301 | 302 | 303 |
| Docosanoic (C22:0) | 340.58 | C22H43O2 | 339 | 340 | 341 |
| Docosenoic (C22:1) | 338.57 | C22H41O2 | 337 | 338 | 339 |
| Docosadienoic (C22:2) | 336.55 | C22H39O2 | 335 | 336 | 337 |
| Docosatrienoic (C22:3) | 334.54 | C22H37O2 | 333 | 334 | 335 |
| Docosatetraenoic (C22:4) | 332.52 | C22H35O2 | 331 | 332 | 333 |
| Docosapentaenoic (C22:5) | 330.50 | C22H33O2 | 329 | 330 | 331 |
| Docosahexaenoic (C22:6) | 328.49 | C22H31O2 | 327 | 328 | 329 |
| Tetracosanoic (C24:0) | 368.64 | C24H47O2 | 367 | 368 | 369 |
| Tetracosenoic (C24:1) | 366.62 | C24H45O2 | 365 | 366 | 367 |
Molecular Analysis
For RNA isolation, cRNA amplification, and expression microarray, total RNA was isolated from TRIzol-treated milk fat as previously described (54). The RNA used in the present report was part of the expression array data previously reported (54). However, none of the specific data in this report were presented or discussed.
Data Analysis
Details about raw intensity data analysis and normalization are presented in our previous publication (54). These data have been deposited in NCBI's Gene Expression Omnibus (14) and are accessible through GEO Series accession no. GSE36936 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE36936). It was these data that underwent further analyses. The repeated-measures test was used to determine transcripts that statistically differed from the initial milk sample (6 h) using the Benjamini-Hochberg false discovery rate (B-H FDR) correction for multiple analyses. Of 16,622 probes, 12,400 probes were different (P < 0.05) from the baseline sample (6 h) (54). The gene ontology (GO) option on GeneSpring GX11.5 was utilized to determine the most significant biological processes (corrected P < 0.05) represented in the MFG transcriptome as described earlier (54). The MFG gene list relating to fat metabolic pathway (610/16,622 probes) containing gene accession numbers and symbols were uploaded into the Ingenuity Pathways Analysis application (IPA, Ingenuity Systems, www.ingenuity.com). Top significant networks and canonical pathways representing lipid metabolic pathways were calculated as described previously (45, 54). Additionally, IPA provides upstream regulator analysis based on prior knowledge of expected effects between transcriptional regulators and their target genes stored in the IPA Knowledge Base. The analysis examines how many known targets of each transcription regulator are present in the dataset and compares their direction of change (i.e., expression in the experimental samples relative to control) to what is expected from the literature in order to predict likely relevant transcriptional regulators. If the observed direction of change is mostly consistent with a particular activation state of the transcriptional regulator (“activated” or “inhibited”), then a prediction is made about that activation state based on Z scores (≥2.0 = activated; ≤−2.0 = inhibited). For our IPA analyses, a P value <0.05 was considered significant.
A repeated-measures test was used to determine the changes in milk FA concentrations, proportions, and daily production over time using SPSS software (v. 19; SPSS, Chicago, IL). GraphPad Prism software (v. 4; GraphPad Software) was used to generate the figures for milk FA composition and florescence intensity of the mRNA expression.
RESULTS
Milk FA Concentrations and Daily Production
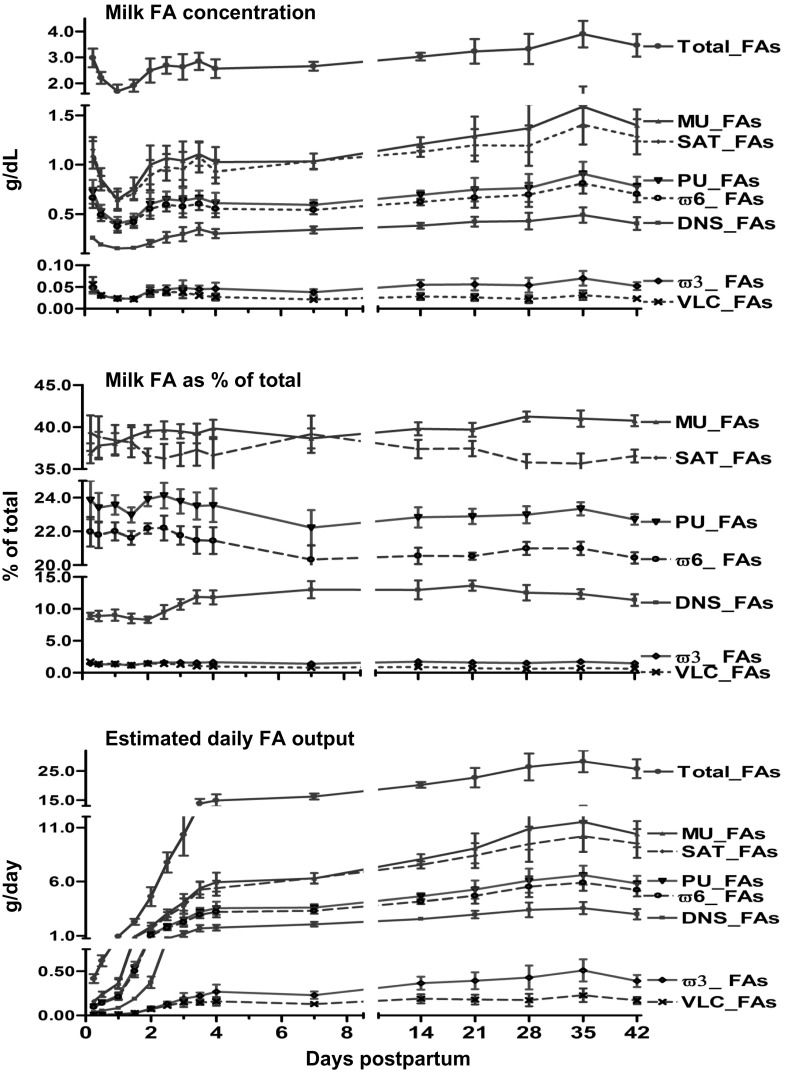
Total milk fat concentration in the initial 6-h sample was 3.0 ± 0.4 g/dl and declined to 1.7 ± 0.3 g/dl by 24 h and subsequently progressively increased (P < 0.01), reaching a value similar to the initial concentration by 60 h (Fig. 1). Following 24 h, the concentrations of the different groups of FAs (DNS_FAs, SAT_FAs, MUS_FAs, and PU_FAs, ω6_FAs, and ω3_FAs) increased (P < 0.05) by 60 h (Fig. 1). Individual FA concentrations (g/dl) from 6 h postpartum are presented in Table 2. DNS_FAs as percentages of total FAs increased (P < 0.01) following day 2 from 8.2 ± 0.5% to reach a plateau of 13.0 ± 1.3% by day 7. Similarly, %MU_FAs increased between 6 h and day 7 (P < 0.01) from 36.7 ± 1.2 to 39.8 ± 0.7% of total FAs (Fig. 1). However, percentages of other FA groups, including SAT_FAs, PU_FAs, ω6_FAs, and VLC_FAs, decreased (P < 0.05) with the duration of lactation. The estimated daily total milk fat output sharply increased (P < 0.001) 21-fold from day 1 to day 4 and then continued to rise slowly, reaching a plateau of 40-fold by day 28. Compared with day 1, the daily output of DNS_FAs, SAT_FAs, MUS_FAs, PUF_As, ω3_FAs, ω6_FAs, and VLC_FAs increased (P < 0.01) 15- to 30-fold by day 4 (Fig. 1). Daily production of individual FAs and groups of FAs (g/day) are presented in Table 3.
Fig. 1.
This figure depicts concentration (g/dl), percentages of total (%), and daily production (g/day) of milk FAs from 6 h to 42 days post partum in 7 normal lactating human volunteers. FAs are classified and labeled as de novo synthesized (C4-C15, DN_FAs), total saturated (SAT_FAs), monounsaturated (MU_FAs), polyunsaturated (PU_FAs), omega 6 (ω6_FAs), omega 3 (ω3_FAs), and very long chain with ≥20 carbons of FAs (VLC_FAs). Values represent means ± SE; n = 7. P values (repeated measures and Bonferroni correction for post hoc) for overall effect of time on sum of each FA groups <0.01.
Table 2.
Concentration of individual and sum of FA groups (g/dl) between 6 h and 42 days of lactation
| Day | 0.25 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 7.0 | 14 | 21 | 28 | 35 | 42 | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 2.986 | 2.220 | 1.693 | 1.911 | 2.500 | 2.699 | 2.642 | 2.860 | 2.577 | 2.670 | 3.041 | 3.249 | 3.339 | 3.915 | 3.483 | <0.01 |
| C4 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | <0.01 |
| C6 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | <0.01 |
| C8 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.003 | 0.003 | 0.006 | 0.009 | 0.010 | 0.011 | 0.013 | 0.010 | <0.01 |
| C10 | 0.006 | 0.004 | 0.003 | 0.003 | 0.004 | 0.008 | 0.012 | 0.017 | 0.017 | 0.025 | 0.035 | 0.042 | 0.042 | 0.052 | 0.041 | <0.01 |
| C12 | 0.079 | 0.059 | 0.047 | 0.049 | 0.064 | 0.092 | 0.106 | 0.131 | 0.118 | 0.144 | 0.164 | 0.177 | 0.189 | 0.209 | 0.162 | <0.01 |
| C14 | 0.158 | 0.119 | 0.094 | 0.098 | 0.127 | 0.154 | 0.168 | 0.187 | 0.156 | 0.157 | 0.164 | 0.180 | 0.176 | 0.201 | 0.175 | <0.01 |
| C15 | 0.008 | 0.005 | 0.004 | 0.004 | 0.005 | 0.007 | 0.009 | 0.009 | 0.007 | 0.006 | 0.008 | 0.011 | 0.007 | 0.011 | 0.011 | <0.01 |
| ∑DNS | 0.256 | 0.189 | 0.150 | 0.155 | 0.202 | 0.263 | 0.296 | 0.348 | 0.302 | 0.339 | 0.382 | 0.422 | 0.428 | 0.490 | 0.403 | <0.01 |
| C16 | 0.674 | 0.481 | 0.363 | 0.428 | 0.544 | 0.546 | 0.498 | 0.556 | 0.486 | 0.532 | 0.568 | 0.564 | 0.584 | 0.655 | 0.637 | <0.01 |
| C18:0 | 0.205 | 0.153 | 0.120 | 0.116 | 0.139 | 0.155 | 0.152 | 0.168 | 0.135 | 0.158 | 0.172 | 0.204 | 0.175 | 0.250 | 0.235 | <0.01 |
| C20:0 | 0.006 | 0.004 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | 0.004 | 0.004 | <0.01 |
| C22:0 | 0.004 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.003 | 0.002 | <0.01 |
| C24:0 | 0.006 | 0.004 | 0.003 | 0.003 | 0.004 | 0.004 | 0.003 | 0.003 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | <0.01 |
| ∑SAT | 1.152 | 0.833 | 0.640 | 0.707 | 0.894 | 0.973 | 0.954 | 1.079 | 0.929 | 1.034 | 1.128 | 1.195 | 1.192 | 1.403 | 1.282 | <0.01 |
| C16:1 | 0.067 | 0.047 | 0.037 | 0.035 | 0.057 | 0.060 | 0.072 | 0.074 | 0.069 | 0.073 | 0.081 | 0.094 | 0.085 | 0.114 | 0.117 | <0.01 |
| C18:1 | 0.955 | 0.757 | 0.572 | 0.688 | 0.895 | 0.958 | 0.922 | 0.988 | 0.921 | 0.927 | 1.086 | 1.161 | 1.257 | 1.430 | 1.244 | <0.01 |
| C20:1 | 0.071 | 0.046 | 0.036 | 0.031 | 0.043 | 0.045 | 0.044 | 0.042 | 0.033 | 0.031 | 0.040 | 0.034 | 0.026 | 0.043 | 0.037 | <0.01 |
| C24:1 | 0.004 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | <0.01 |
| ∑MUFA | 1.097 | 0.852 | 0.646 | 0.756 | 0.998 | 1.065 | 1.040 | 1.105 | 1.025 | 1.032 | 1.208 | 1.289 | 1.369 | 1.588 | 1.398 | <0.01 |
| C18:3 (n-3) | 0.019 | 0.014 | 0.011 | 0.012 | 0.017 | 0.021 | 0.021 | 0.025 | 0.023 | 0.020 | 0.026 | 0.031 | 0.033 | 0.039 | 0.031 | <0.01 |
| C20:5 (n-3) | 0.003 | 0.002 | 0.002 | 0.001 | 0.003 | 0.003 | 0.004 | 0.003 | 0.005 | 0.004 | 0.007 | 0.006 | 0.005 | 0.008 | 0.005 | <0.01 |
| C22:5 (n-3) | 0.011 | 0.006 | 0.004 | 0.004 | 0.008 | 0.008 | 0.007 | 0.006 | 0.006 | 0.005 | 0.006 | 0.006 | 0.005 | 0.007 | 0.006 | <0.01 |
| C22:6 (n-3) | 0.014 | 0.008 | 0.006 | 0.005 | 0.013 | 0.012 | 0.015 | 0.011 | 0.012 | 0.009 | 0.015 | 0.013 | 0.011 | 0.016 | 0.010 | <0.01 |
| ∑n-3 PUFA | 0.047 | 0.029 | 0.023 | 0.022 | 0.041 | 0.045 | 0.048 | 0.045 | 0.046 | 0.037 | 0.054 | 0.056 | 0.054 | 0.070 | 0.052 | <0.01 |
| C18:2 (n-6) | 0.478 | 0.376 | 0.285 | 0.337 | 0.440 | 0.478 | 0.467 | 0.500 | 0.464 | 0.467 | 0.545 | 0.592 | 0.626 | 0.719 | 0.642 | <0.01 |
| C18:3 (n-6) | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.003 | 0.003 | 0.003 | 0.004 | 0.004 | <0.01 |
| C20:2 (n-6) | 0.069 | 0.044 | 0.035 | 0.030 | 0.041 | 0.045 | 0.044 | 0.043 | 0.036 | 0.026 | 0.023 | 0.027 | 0.021 | 0.031 | 0.023 | <0.01 |
| C20:3 (n-6) | 0.056 | 0.035 | 0.026 | 0.023 | 0.032 | 0.034 | 0.034 | 0.033 | 0.029 | 0.028 | 0.030 | 0.033 | 0.030 | 0.040 | 0.032 | <0.01 |
| C20:4 (n-6) | 0.069 | 0.042 | 0.032 | 0.029 | 0.045 | 0.048 | 0.048 | 0.047 | 0.043 | 0.039 | 0.047 | 0.050 | 0.041 | 0.057 | 0.046 | <0.01 |
| C22:4 (n-6) | 0.017 | 0.009 | 0.007 | 0.006 | 0.009 | 0.010 | 0.008 | 0.007 | 0.005 | 0.004 | 0.003 | 0.004 | 0.003 | 0.004 | 0.003 | <0.01 |
| ∑n-6 PUFA | 0.690 | 0.505 | 0.385 | 0.426 | 0.568 | 0.615 | 0.601 | 0.631 | 0.577 | 0.566 | 0.651 | 0.709 | 0.725 | 0.855 | 0.751 | <0.01 |
| ∑PUFA | 0.737 | 0.535 | 0.407 | 0.448 | 0.609 | 0.660 | 0.649 | 0.676 | 0.624 | 0.604 | 0.705 | 0.765 | 0.779 | 0.925 | 0.803 | <0.01 |
| ∑VLCFA | 0.056 | 0.031 | 0.024 | 0.021 | 0.038 | 0.038 | 0.038 | 0.030 | 0.027 | 0.021 | 0.028 | 0.025 | 0.022 | 0.031 | 0.023 | <0.01 |
Values are means from 7 subjects. DNS, de novo synthesized; SAT, saturated including DNS; MUFA, monounsaturated; n-3 PUFA, omega3 polyunsaturated; n-6 PUFA, omega 6 polyunsaturated; PUFA sum of n-3 PUFA and n-6 PUFA and VLCFA, very-long-chain FA (≥20 carbons). Overall P values for repeated-measure analysis were <0.01 for all measured parameters. Following 24 h, concentration of C8, C10, C12, C14, C15, ∑DNS, C16:0, C18:0, ∑SAT, C16:1, C18:1, ∑MUFA, C18:3 (n-3), C20:5 (n-3), C22:6 (n-3), ∑n-3 PUFA, C18:2 (n-6), C18:3 (n-6), ∑n-6 PUFA, and ∑PUFA all increased (P < 0.05) by day3 compared with 24 h. Concentrations of C4 and C6 increased (P < 0.05) by day7 compared with 24 h. However following 24 h, concentration of C20:0, C22:0, C24:0, C22:5 (n-3), C20:2 (n-6), C20:3 (n-6), C20:4 (n-6), C22:4 (n-6), and VLCFA generally decreased (P < 0.05) compared with 6 h.
Table 3.
Estimated daily output (g/day) of individual and sum of total and FA groups between 6 h and 42 days of lactation
| Time (days) | 0.25 | 0.50 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 7.0 | 14 | 21 | 28 | 35 | 42 | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 0.418 | 0.622 | 0.948 | 2.303 | 4.625 | 7.799 | 10.384 | 13.915 | 14.944 | 16.286 | 20.314 | 22.840 | 26.513 | 28.424 | 25.875 | <0.01 |
| C4 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.002 | 0.003 | 0.003 | 0.005 | 0.008 | 0.009 | 0.012 | 0.013 | <0.01 |
| C6 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.002 | 0.003 | 0.008 | 0.011 | 0.012 | 0.017 | 0.016 | <0.01 |
| C8 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.005 | 0.008 | 0.014 | 0.018 | 0.036 | 0.059 | 0.073 | 0.084 | 0.097 | 0.077 | <0.01 |
| C10 | 0.001 | 0.001 | 0.002 | 0.004 | 0.008 | 0.024 | 0.045 | 0.082 | 0.098 | 0.152 | 0.236 | 0.294 | 0.335 | 0.375 | 0.301 | <0.01 |
| C12 | 0.011 | 0.017 | 0.026 | 0.059 | 0.119 | 0.265 | 0.416 | 0.636 | 0.686 | 0.880 | 1.095 | 1.243 | 1.503 | 1.516 | 1.203 | <0.01 |
| C14 | 0.022 | 0.033 | 0.052 | 0.118 | 0.234 | 0.446 | 0.659 | 0.911 | 0.902 | 0.957 | 1.097 | 1.264 | 1.396 | 1.456 | 1.297 | <0.01 |
| C15 | 0.001 | 0.001 | 0.002 | 0.004 | 0.010 | 0.019 | 0.034 | 0.044 | 0.040 | 0.039 | 0.051 | 0.074 | 0.056 | 0.081 | 0.084 | <0.01 |
| ∑DNS | 0.036 | 0.053 | 0.084 | 0.187 | 0.374 | 0.761 | 1.165 | 1.691 | 1.750 | 2.070 | 2.551 | 2.968 | 3.395 | 3.555 | 2.992 | <0.01 |
| C16 | 0.094 | 0.135 | 0.203 | 0.516 | 1.006 | 1.577 | 1.955 | 2.705 | 2.820 | 3.245 | 3.798 | 3.966 | 4.634 | 4.759 | 4.735 | <0.01 |
| C18:0 | 0.029 | 0.043 | 0.067 | 0.140 | 0.257 | 0.449 | 0.597 | 0.815 | 0.783 | 0.964 | 1.150 | 1.435 | 1.391 | 1.813 | 1.749 | <0.01 |
| C20:0 | 0.001 | 0.001 | 0.002 | 0.003 | 0.006 | 0.010 | 0.012 | 0.015 | 0.014 | 0.015 | 0.019 | 0.018 | 0.024 | 0.031 | 0.027 | <0.01 |
| C22:0 | 0.001 | 0.001 | 0.001 | 0.002 | 0.003 | 0.006 | 0.007 | 0.008 | 0.007 | 0.008 | 0.010 | 0.010 | 0.013 | 0.019 | 0.016 | <0.01 |
| C24:0 | 0.001 | 0.001 | 0.002 | 0.003 | 0.007 | 0.011 | 0.013 | 0.013 | 0.012 | 0.008 | 0.007 | 0.006 | 0.005 | 0.006 | 0.005 | <0.01 |
| ∑SAT | 0.161 | 0.233 | 0.358 | 0.852 | 1.653 | 2.813 | 3.749 | 5.248 | 5.386 | 6.309 | 7.534 | 8.403 | 9.462 | 10.183 | 9.523 | <0.01 |
| C16:1 | 0.009 | 0.013 | 0.021 | 0.042 | 0.106 | 0.174 | 0.284 | 0.360 | 0.400 | 0.444 | 0.544 | 0.662 | 0.676 | 0.827 | 0.866 | <0.01 |
| C18:1 | 0.134 | 0.212 | 0.320 | 0.829 | 1.656 | 2.768 | 3.624 | 4.807 | 5.343 | 5.657 | 7.251 | 8.160 | 9.978 | 10.383 | 9.241 | <0.01 |
| C20:1 | 0.010 | 0.013 | 0.020 | 0.038 | 0.080 | 0.131 | 0.172 | 0.204 | 0.193 | 0.187 | 0.268 | 0.237 | 0.210 | 0.310 | 0.273 | <0.01 |
| C24:1 | 0.001 | 0.001 | 0.001 | 0.002 | 0.004 | 0.006 | 0.007 | 0.008 | 0.006 | 0.005 | 0.005 | 0.004 | 0.005 | 0.008 | 0.006 | <0.01 |
| ∑MUFA | 0.154 | 0.239 | 0.362 | 0.911 | 1.846 | 3.079 | 4.086 | 5.378 | 5.942 | 6.294 | 8.068 | 9.062 | 10.870 | 11.527 | 10.387 | <0.01 |
| C18:3 (n-3) | 0.003 | 0.004 | 0.006 | 0.015 | 0.032 | 0.062 | 0.084 | 0.122 | 0.135 | 0.121 | 0.172 | 0.218 | 0.260 | 0.283 | 0.230 | <0.01 |
| C20:5 (n-3) | 0.000 | 0.001 | 0.001 | 0.002 | 0.006 | 0.010 | 0.016 | 0.016 | 0.029 | 0.024 | 0.049 | 0.041 | 0.042 | 0.058 | 0.040 | <0.01 |
| C22:5 (n-3) | 0.002 | 0.002 | 0.002 | 0.005 | 0.014 | 0.023 | 0.029 | 0.030 | 0.033 | 0.027 | 0.040 | 0.044 | 0.036 | 0.053 | 0.045 | <0.01 |
| C22:6 (n-3) | 0.002 | 0.002 | 0.003 | 0.006 | 0.024 | 0.036 | 0.060 | 0.052 | 0.072 | 0.056 | 0.102 | 0.090 | 0.090 | 0.115 | 0.073 | <0.01 |
| ∑n-3 PUFA | 0.007 | 0.008 | 0.013 | 0.027 | 0.076 | 0.130 | 0.189 | 0.220 | 0.268 | 0.228 | 0.364 | 0.392 | 0.428 | 0.509 | 0.389 | <0.01 |
| C18:2 (n-6) | 0.067 | 0.105 | 0.159 | 0.406 | 0.814 | 1.380 | 1.834 | 2.435 | 2.692 | 2.851 | 3.640 | 4.164 | 4.974 | 5.223 | 4.772 | <0.01 |
| C18:3 (n-6) | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.003 | 0.005 | 0.006 | 0.011 | 0.017 | 0.021 | 0.025 | 0.032 | 0.030 | <0.01 |
| C20:2 (n-6) | 0.010 | 0.012 | 0.019 | 0.036 | 0.076 | 0.129 | 0.171 | 0.207 | 0.207 | 0.160 | 0.154 | 0.192 | 0.168 | 0.222 | 0.169 | <0.01 |
| C20:3 (n-6) | 0.008 | 0.010 | 0.015 | 0.028 | 0.059 | 0.098 | 0.132 | 0.161 | 0.166 | 0.171 | 0.201 | 0.233 | 0.238 | 0.291 | 0.240 | <0.01 |
| C20:4 (n-6) | 0.010 | 0.012 | 0.018 | 0.034 | 0.083 | 0.140 | 0.188 | 0.228 | 0.248 | 0.238 | 0.312 | 0.348 | 0.326 | 0.411 | 0.342 | <0.01 |
| C22:4 (n-6) | 0.002 | 0.003 | 0.004 | 0.008 | 0.017 | 0.028 | 0.032 | 0.033 | 0.028 | 0.023 | 0.023 | 0.026 | 0.022 | 0.027 | 0.023 | <0.01 |
| ∑n-6 PUFA | 0.097 | 0.141 | 0.215 | 0.513 | 1.050 | 1.777 | 2.360 | 3.069 | 3.348 | 3.455 | 4.348 | 4.983 | 5.754 | 6.206 | 5.577 | <0.01 |
| ∑PUFA | 0.103 | 0.150 | 0.228 | 0.540 | 1.127 | 1.907 | 2.549 | 3.289 | 3.616 | 3.683 | 4.712 | 5.375 | 6.181 | 6.715 | 5.965 | <0.01 |
| ∑VLCFA | 0.008 | 0.009 | 0.013 | 0.026 | 0.070 | 0.110 | 0.148 | 0.144 | 0.157 | 0.128 | 0.187 | 0.178 | 0.171 | 0.227 | 0.167 | <0.01 |
Values are means from 7 subjects. Estimated daily production of total FA, C8, C10, C12, C14, C15, C16, C16:1, C18:0, C18:2 (n-6), C18:1, C18:3 (n-3), C20:0, C20:4, C20:5 (n-3), C20:3 (n-6), C20:2 (n-6), C20:1, C22:5 (n-3), DNS, SAT, MUFA, and PUFA all increased (P < 0.05) by 24 h compared with 6 h. Estimated daily production of C18:3 (n-6), C22:0, C22:6 (n-3), C22:4 (n-6), C24:0, C24:1, and VLCFA increased (P < 0.05) by 36 h. However, C4 and C6 increased (P < 0.05) by 60 h compared with 6 h. Daily total and individual milk FA outputs were calculated by multiplying the FA concentration Table 2) times an estimate of milk volumes derived from previous publications (40, 41, 48).
Microarray Data Analysis
Transport of circulating FAs into MEC.
This process involves MEC gene expression of membrane FA and lipid transporter, lipase genes, and intracellular FA binding proteins.
MEMBRANE FA AND LIPID TRANSPORTER GENES.
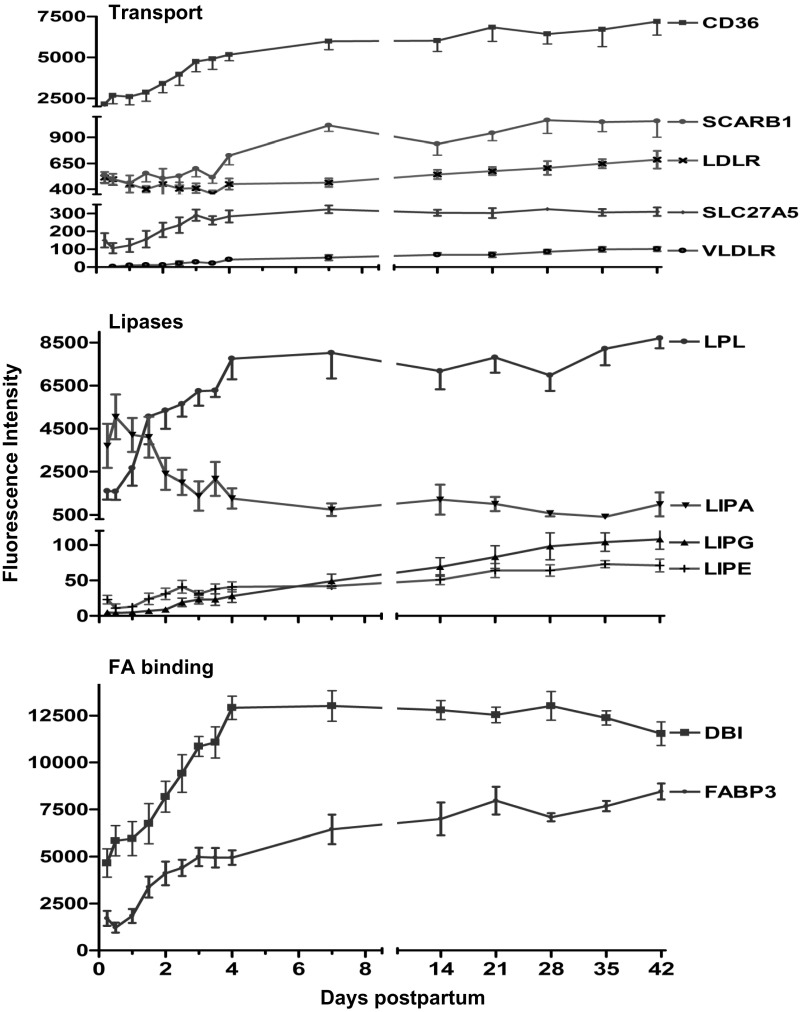
The mRNA of solute carrier family 27 (FA transporter) member 5 (SLC27A5) increased threefold (P < 0.01) by day 4 (Fig. 2). However, the mRNA of FA transporters SLC27A1 and SLC27A3 decreased (−3.0- and −10.0-fold, respectively, P < 0.01) over the first 4 days (data not shown). Expression of genes for both thrombospondin receptor (CD36) and very-low-density lipoprotein receptor (VLDLR) increased (P < 0.01) 2.7- and 22-fold, respectively, by day 4. Expression of the gene for LDL receptor (LDLR) decreased −1.2-fold by day 4 and then increased slightly over the entire study duration to 1.3 (P > 0.05) above the baseline (Fig. 2). However, the mRNA of scavenger receptor class B member 1 (SCARB1 or HDL receptor) increased (P < 0.01) 2.0-fold by day 7 (Fig. 2).
Fig. 2.
Fluorescence intensity (means ± SE, n = 7) for genes involved in lipid and FA transport, lipases, and FA binding proteins from 6 h to 42 days of lactation. P values for repeated measures after H-B FDR multiple testing corrections revealed an overall increase (P < 0.01) in mRNA of thrombospondin receptor (CD36), scavenger receptor class B #1 (SCARB1 or HDL receptor), solute carrier family 27 #5 (SLC27A5), very-low-density lipoprotein receptor (VLDLR), lipoprotein lipase (LPL), hormone-sensitive lipase E (LIPE), endothelial lipase G (LIPG), FA binding protein-3 (FABP3), and diazepam binding inhibitor (acyl-CoA binding protein, DBI). LDL receptor (LDLR) increased only to 1.3-fold (P < 0.05) by day 42.
LIPASE GENES.
The mRNA of carboxyl ester lipase or CEL (bile salt-stimulated lipase) increased (P < 0.01) progressively, reaching a plateau of 2.5-fold by day 7. Although the change in the gene expression was modest, CEL expression represents one of the 10 top abundant transcripts of the entire array (data not shown). Similarly, the gene expression of CEL pseudogene (CELP) increased (P < 0.01) 4.4-fold by day 4. Expression of the gene for lipoprotein lipase (LPL) increased (P < 0.01) 6.0-fold by day 4. However, expression of the genes for hormone-sensitive lipase E (LIPE) and endothelial lipase G (LIPG) increased progressively over the entire 42 days of study (4.0- and 27.0-fold respectively, above baseline) (Fig. 2). Expression of the gene for lipase A (cholesterol esterase, LIPA) decreased by day 4 (−4.0-fold, P < 0.01) and remained suppressed over the 42 days of study (Fig. 2).
INTRACELLULAR FA BINDING PROTEIN GENES.
Expression of the genes for FA binding proteins FABP4 and FABP5 decreased sharply (>−10.0-fold, P < 0.01) by day 3 (data not shown). Expression of the genes for FABP3 and diazepam binding inhibitor (acyl-CoA binding protein, DBI) increased (P < 0.01) 4.0- and 3.0-fold, respectively, by day 4 (Fig. 2). Expression of the gene for FABP7 increased 12.0-fold over the first 4 days and then declined to reach baseline values by day 42 (data not shown).
FA de novo synthesis and gene modification.
TCA CYCLE GENE EXPRESSION.
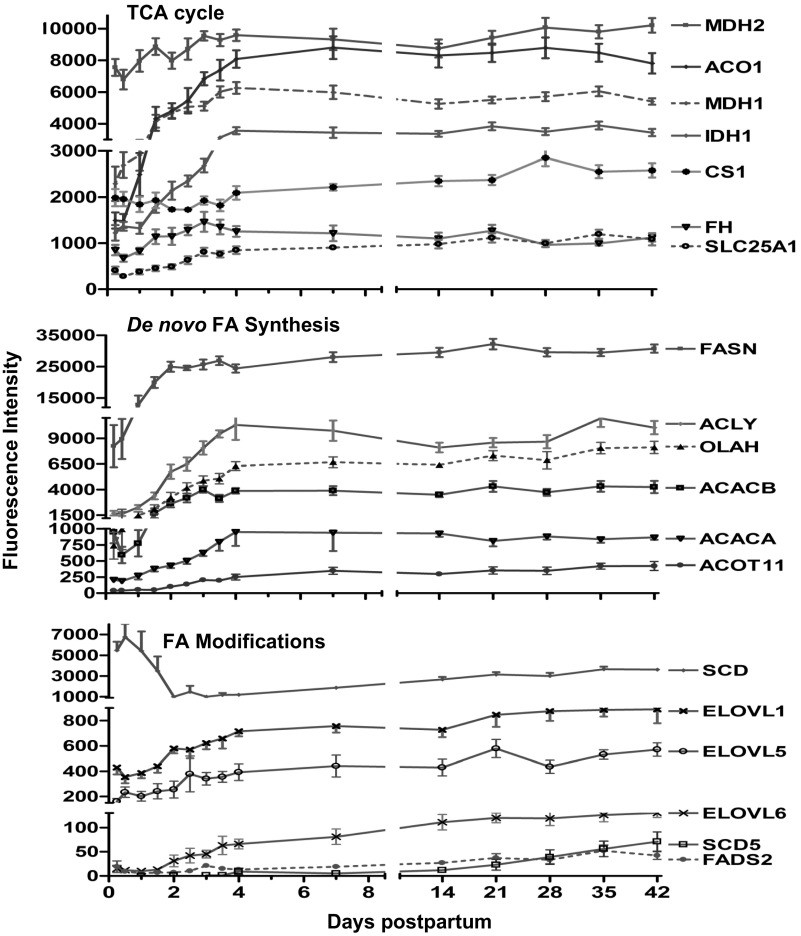
The TCA cycle provides both substrate and energy for de novo synthesis and therefore is an important substrate source for de novo FA synthesis. Expression of the genes for many enzymes of the citric acid cycle increased after 6 h (P < 0.01), reaching a relative plateau by day 4 (Fig. 3). The highest abundance among TCA genes over the study duration was malate dehydrogenase-2 (MDH2, 1.5-fold). However, the greatest fold change over the first 4 days were aconitase-1 (ACO1, 5.9-fold), malate dehydrogenase-1 (MDH1, 3.5-fold), isocitrate dehydrogenase-1 and -2 (IDH1/2, 3.3- and 2.3-fold, respectively), succinate dehydrogenase complex subunit C (SDHC, 2.0-fold), and solute carrier family 25 #1 (mitochondrial carrier; citrate transporter; SLC25A1, 2.4-fold) (Fig. 3). In contrast, expression of the gene for citrate synthase (CS) remained relatively constant (data not shown).
Fig. 3.
Fluorescence intensity (means ± SE, n = 7) for genes involved in TCA cycle, de novo FA synthesis, and FA modification from 6 h to 42 days of lactation. P values for repeated measures after H-B FDR multiple testing corrections revealed an overall increase (P < 0.01) in mRNA of malate dehydrogenase 2 (MDH2) aconitase-1 soluble (ACO1), malate dehydrogenase1 (MDH1), isocitrate dehydrogenase1 (IDH1), fumarate hydratase (FH), solute carrier family 25 (mitochondrial carrier; citrate transporter 1, SLC25A1), ATP citrate lyase (ACLY), acetyl-CoA carboxylase-α and -β (ACACA & B), FA synthase (FASN), S-acyl FASN thioesterase medium chain (OLAH), acyl-CoA thioesterase-11 (ACOT11), elongation of long-chain FAs (ELOVL) member 5 (ELOVL5) and 6 (ELOVL6), and very-long-chain FA-like 1 (ELOVL1), FA desaturase 2 (FADS2), and stearoyl-CoA desaturase-5 (SCD5). However, mRNA of stearoyl-CoA desaturase (Δ9-desaturase, SCD) decreased (P < 0.01) overtime.
FA SYNTHESIS AND THIOESTERASE GENES.
Expression of the genes for ATP citrate lyase (ACLY), acetyl-CoA carboxylase-α (ACACA) and acetyl-CoA carboxylase-β (ACACB) increased (P < 0.01) progressively to 4.0-, 6.0-, and 5.0-fold, respectively, by day 4 (Fig. 3). Expression of the gene for FA synthase (FASN) increased 4.0-fold (P < 0.01), reaching a plateau by day 2 and also represented one of top 10 abundant transcripts of the entire array (Fig. 3). Similarly, expression of the gene for S-acyl fatty acid synthase thioesterase, medium chain (thioesterase II or oleoyl-acyl-carrier protein hydrolase, OLAH) increased progressively, reaching a plateau of 12.0-fold by day 4. The mRNA of acyl-CoA thioesterase (ACOT) family members 1, 2, 4, 7, 8, 9, and 11 were detected. ACOT11 had the highest increase (8.0-fold, P < 0.01) over the duration of study (Fig. 3), while the mRNAs of ACOT1, -2, and -8 increased (2.9-, 2.3-, and 3.8-fold, respectively, P < 0.01); in contrast, those of ACOT4, -7, and 9 decreased (−1.4-, −5.6-, and −106-fold, respectively, P < 0.01) over the study duration (data not shown). The mRNA of thioesterase superfamily member 2 [THEM2, currently defined as ACTO13 (30)], despite being more abundant than ACOT11, was unchanged over the study duration (data not shown).
FA ELONGATION AND DESATURATION GENES.
Expression of the genes for elongation of long-chain FAs (ELOVL) member 5 (ELOVL5), -6 (ELOVL6), and very-long-chain FA-like 1 (ELOVL1) increased 9.0-, 8.0-, and 2.0-fold, respectively, by day 4. Expression of the gene for fatty acid desaturase-2 (FADS2) was the only member of the FA desaturases that increased (>2.0-fold, P < 0.01) over the first 7 days. Expression of the gene for FADS3 decreased (−2.0-fold, P < 0.01), whereas that of FADS1 was not detected (data not shown). Expression of the gene for stearoyl-CoA desaturase (Δ9-desaturase, SCD) decreased (−10.0-fold, P < 0.01) by day 3 (Fig. 3). Expression of the gene for SCD5 was very low in abundance compared with SCD but increased 3.0-fold (P < 0.01) by day 4 (Fig. 3).
Glycerol and FA activation and TG assembly genes.
GLYCEROL BACKBONE ACTIVATION.
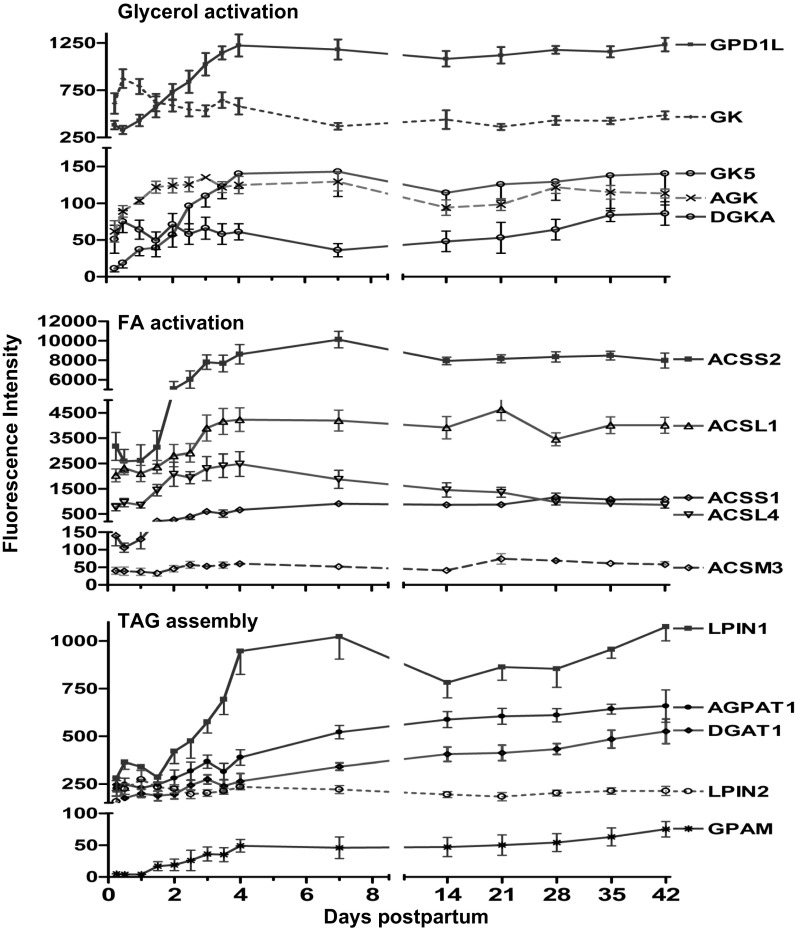
Expression of the gene for glycerol kinase (GK) decreased −2.0-fold (P < 0.01) by day 7. In contrast, expression of the genes for glycerol-3-phosphate dehydrogenase-1-like (GPD1L), GK5, acylglycerol kinase (AGK), and diacylglycerol kinase-α (DGKA) increased (3.5-, 20.0-, 3.0-, and 2.2-fold, respectively, P < 0.01) over the first 4 days (Fig. 4).
Fig. 4.
Fluorescence intensity (means ± SE, n = 7) for genes involved in glycerol activation, FA activation, and triacylglycerol (TG) synthesis from 6 h to 42 days of lactation. mRNA of glycerol-3-phosphate dehydrogenase 1-like (GPD1L), glycerol kinase-5 (GK5), acylglycerol kinase (AGK), and diacylglycerol kinase-α (DGKA), acyl-CoA synthetase short-chain family #1 and 2 (ACSS1&2), acyl-CoA synthetase long-chain family #1 and 4 (ACSL1&4), acyl-CoA synthetase medium-chain family #3 (ACSM3), lipin 1 and 2 (LPIN1&2), 1-acylglycerol-3-phosphate O-acyltransferase-1 (AGPAT1), glycerol-3-phosphate acyltransferase (mitochondrial, GPAM); all increased by day 4 (P < 0.01, repeated-measures analysis after H-B multiple testing corrections). While GK decreased (P < 0.01) by day 7, diacylglycerol O-acyltransferase-1 (DGAT1) increased (P < 0.01) only after day 7.
FA ACTIVATION.
Expression of the genes for acyl-CoA synthetase short-chain family member 1 (ACSS1) and ACSS2 increased (P < 0.01) 5.0- and 3.0-fold, respectively, by day 4. Expression of the genes for ACS long-chain family member 1 (ACSL1), ACSL4, and ACS medium-chain family member 3 (ACSM3) increased (P < 0.01) 2.1-, 6.2-, and 1.8-fold, respectively, over the first 4 days and remained relatively constant over the duration of study (Fig. 4).
ACYLTRANSFERASES AND TG ASSEMBLY.
By day 4, expression of the genes for glycerol-3-phosphate acyltransferase mitochondrial (GPAM) and 1-acylglycerol-3-phosphate O-acyltransferase-1 (AGPAT1) increased (9.0- and 1.8-fold, respectively, P < .0.1; Fig. 4). Expression of the gene for diacylglycerol O-acyltransferase (DGAT1) increased (1.5-fold, P < 0.01) only following day 7. Expression of the genes for lipin 1 (LPIN1) and LPIN2 increased (P < 0.01) 4.0- and 2.0-fold, respectively, by day 4 (Fig. 4).
Cholesterol transport and synthesis genes.
CHOLESTEROL TRANSPORT GENES.
Expression of the genes for ATP-binding cassette, subfamilies A1 (ABCA1) and G1 (ABCG1) decreased (P < 0.01) by day 4 (−40.0- and −48.0-fold, respectively; Fig. 5). However, ABCG2, which was detected at 24 h, increased to 7-fold by day 7 and continued to increase progressively over the study duration. ABCA7 was detected in very low abundance (∼1/10 of ABCA1) and similarly decreased by −3.0-fold by day 4 (data not shown).
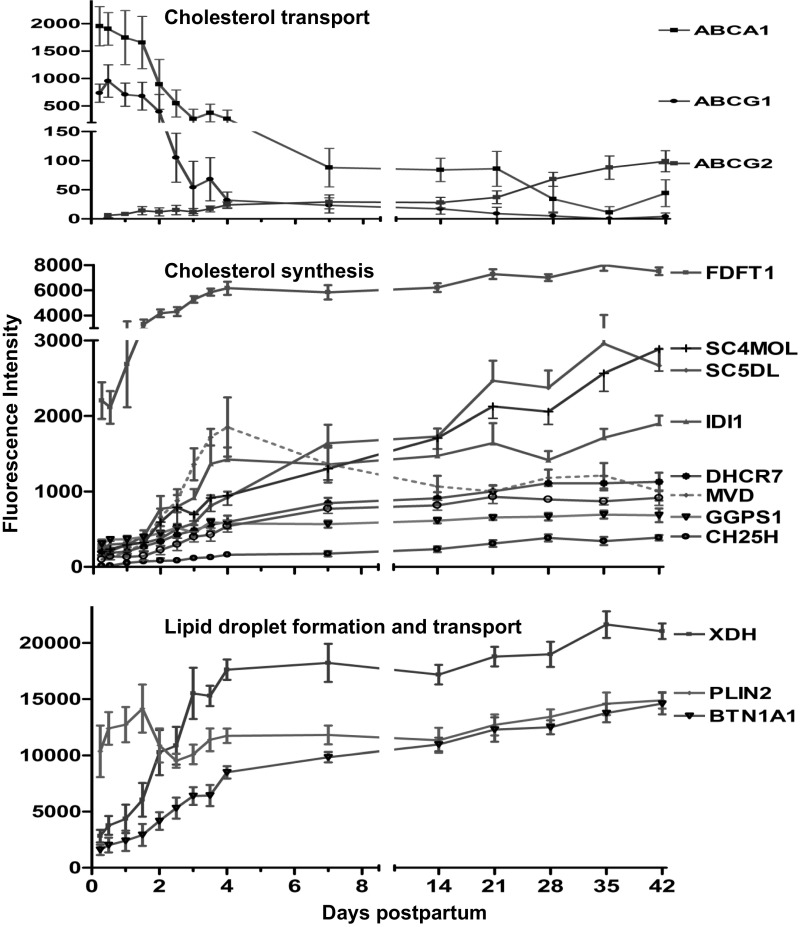
Fig. 5.
Fluorescence intensity (means ± SE, n = 7) for genes involved in cholesterol transport and biosynthesis and lipid droplet synthesis from 6 h to 42 days of lactation. P values represent repeated-measures analysis after H-B multiple testing corrections. mRNA of ATP-binding cassette subfamily A #1 (ABCA1) and subfamily G #1 (ABCG1) decreased (P < 0.01), whereas ABCG2 increased (P < 0.01) by day 4. Cholesterol synthesis genes included farnesyl-diphosphate farnesyltransferase -1 (FDFT1), sterol-C4-methyl oxidase-like (SC4MOL), sterol-C5-desaturase like (SC5DL), isopentenyl-diphosphate delta isomerase-1 (IDI1), mevalonate (diphospho) decarboxylase (MVD), cholesterol 25-hydroxylase (CH25H), 7-dehydrocholesterol reductase (DHCR7), geranylgeranyl diphosphate synthase-1 (GGPS1), and cholesterol 25-hydroxylase (CH25H); all increased severalfold (P < 0.01) over study duration. Expression of genes for xanthine dehydrogenase (XDH) and butyrophilin, subfamily 1# A1 (BTN1A1) increased severalfold (P < 0.01) by day 4, whereas perilipin 2 (PLIN2) had little or no change over time.
CHOLESTEROL SYNTHESIS AND METABOLISM GENES.
By day 4, the mRNA of genes responsible for cholesterol synthesis increased more than 2.0-fold (Fig. 5). The genes included farnesyl-diphosphate farnesyltransferase-1 (FDFT1), sterol-C4-methyl oxidase-like (SC4MOL) sterol-C5-desaturase like (SC5DL), isopentenyl-diphosphate delta isomerase-1 (IDI1), mevalonate (diphospho) decarboxylase (MVD), cholesterol 25-hydroxylase (CH25H), 7-dehydrocholesterol reductase (DHCR7), geranylgeranyl diphosphate synthase-1 (GGPS1), and cholesterol 25-hydroxylase (CH25H); all increased 3.0- to 18.0-fold (P < 0.01; Fig. 5).
LIPID DROPLET SYNTHESIS GENES.
Expression of the genes for xanthine dehydrogenase (XDH), perilipin 2 (PLIN2), and butyrophilin subfamily 1 member A1 (BTN1A1) increased (8.0-, 1.3-, and 7.5-fold, respectively, P < 0.01) by day 4 (Fig. 5). Expression of the gene for PLIN5 started with the same expression level of BTN1A1 but decreased (−8.0-fold, P < 0.01) by day 4 (data not shown).
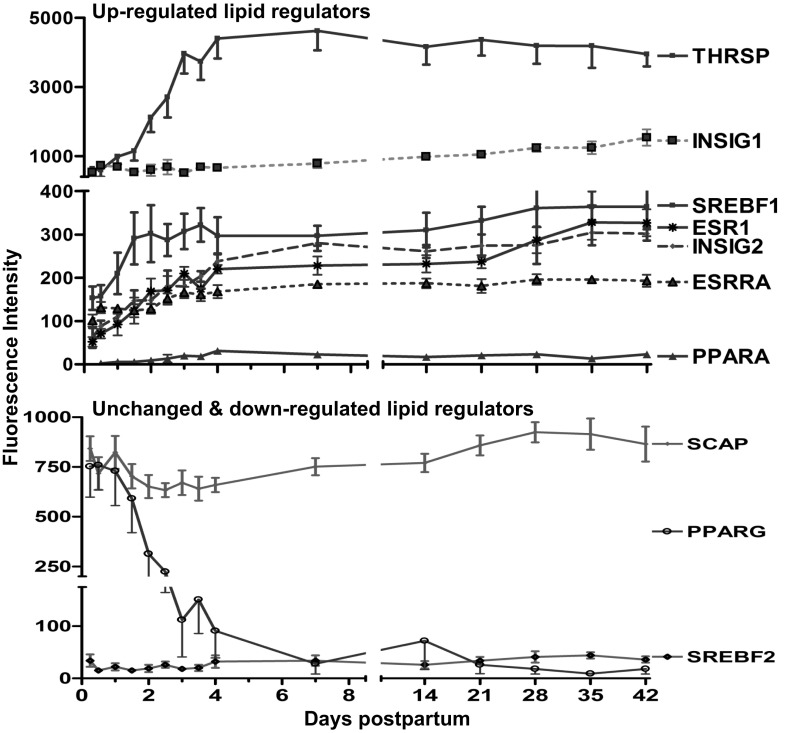
Regulators and transcription factor genes.
Expression of the gene for SREBF1 increased 2.0-fold by 36 h and then plateaued (Fig. 6). Other regulatory elements increased more slowly, reaching a relative plateau of 2.5- to 15-fold (P < 0.01) by day 4: thyroid hormone-responsive (THRSP or Spot 14), estrogen receptor 1 (ESR1), estrogen receptor related-α (ESRRA), INSIG2, and PPARA (Fig. 6). In contrast, expression of the gene for INSIG1 continued to increase slowly (2.6-fold, P < 0.01) over the entire 42 days of study. However, by day 4, expression of the genes for SREBF, SREBF chaperone (SCAP), and PPARγ decreased (−1.2, −1.2-, and −33.0-fold, respectively, P < 0.01) (Fig. 6).
Fig. 6.
Fluorescence intensity (means ± SE, n = 7) for upregulated lipid regulator genes between 6 h and 42 days of lactation. P values represent repeated-measures analysis after H-B multiple testing corrections. mRNA of thyroid hormone-responsive protein (THRSP), sterol-regulatory element binding transcription factor 1 (SREBF1), insulin-induced genes 2 (INSIG2), estrogen receptor 1 (ESR1), estrogen receptor related-α (ESRRA), and peroxisome proliferator-activated receptorα (PPARA) all increased (≥2.0-fold, P < 0.01) by day 4. However, - mRNA of PPAR-γ, (PPARG), SREBF chaperone (SCAP) and SREBF2 decreased (P < 0.01) by day 4.
Ingenuity Pathway Analysis
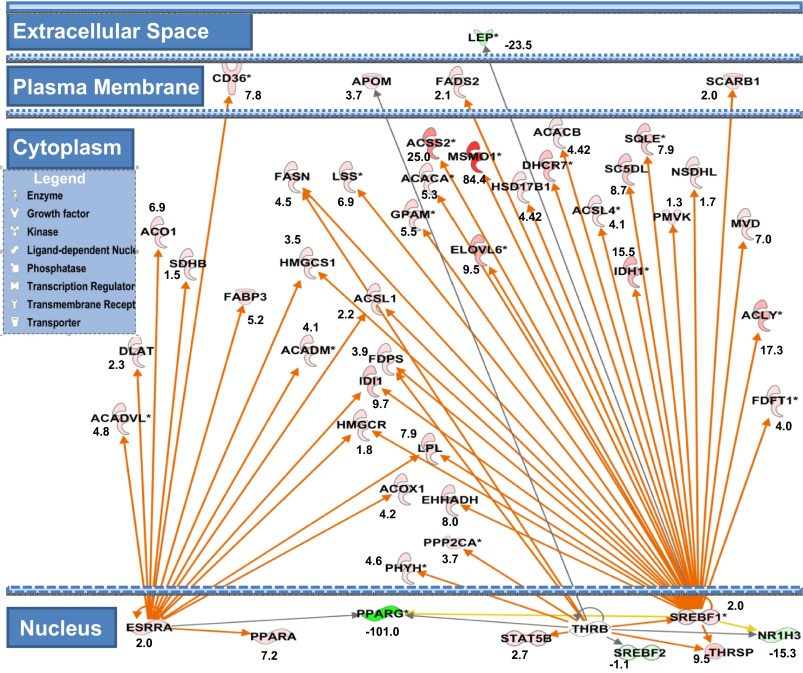
Ingenuity pathway analysis for the 610 transcripts representing the gene ontology (GO) of lipid metabolic processes revealed several significant canonical pathways. In descending order of significance, the top five canonical pathways were: FA β-oxidation, 3-phosphoinositide biosynthesis, TCA cycle, stearate biosynthesis, phospholipases, and others. IPA upstream regulator analysis showed that among lipid transcription regulators SREBF1 was predicted as the top significant regulator to be activated by 48 h (31/48 genes have expression direction consistent with activation of SREBF1, Z score = 3.4, and Overlap P = 1.6 E−38) and has been shown to play a central role in the regulation of many genes related to lipid metabolism (Fig. 7). Thyroid hormone receptor-β (THRB) was also predicted to be activated at 48 h (8/13 genes have expression direction consistent with activation of THRB, Z score = 2.1, Overlap P = 9.5E−6). ESRRA was the earliest (24 h) upstream regulator predicted to be activated (17/28 genes have expression direction consistent with activation of ESRRA, Z score = 2.8, Overlap P = 7.4 E−18) (Fig. 7).
Fig. 7.
Cell compartment view for network of lipid metabolic genes highlighting the role of SREBF1, THRSP, and ESRRA curated by ingenuity pathway analysis (IPA). Green nodes indicate downregulated genes; red indicates upregulated genes, and numbers represent fold changes at 72 h vs. 6 h. Orange arrows indicate predicted activation, yellow indicate inconsistent findings with state of the downstream gene, and gray indicate that the relationship is not predicted. Activation of SREBF1 leads to increase in expression of the genes for lipases (LPL), HDL transporter (SCARB1), TCA cycle (IDH), de novo FA synthesis (ACACA&B, ACLY, FASN), FA activation (ACSS1&2, ACSL1&4), FA elongation (ELVOL6), FA desaturation (FADS2, SC5DL), TG synthesis (GPAM), whereas the remaining genes are primarily involved in sterol biosynthesis. Downstream of ESRRA activation is the induction of the transcription factor (PPARA), FA transporter (CD36), lipase (LPL), FA binding (FABP3), TCA (SDHB, ACO1), FA modification (ACOX1, ACADM, ACADVL), FA activation (ACSL1), and others for cholesterol synthesis (HMGCS1, HMGCR, DLAT). Activation of THRB upregulates transcription factor (STAT5B) and de novo FA synthesis regulator (THRSP) as well as FA synthesis (FASN), FA activation (ACSL1), FA oxidation (PHYH, EHHADH), protein phosphatase (PPP2CA), and sterol synthesis (HMGCR, IDI1).
DISCUSSION
To date, no studies have investigated lipid biosynthetic enzyme gene expression and lipid production in humans during secretory activation of lactation. Furthermore, the majority of studies investigating MG gene regulation in animal models have focused on milk protein gene expression, and, by comparison, little attention has been paid to lipid biosynthesis during the secretory activation phase of lactation (2, 6). In the present study, we utilized our established model of the RNA isolated from human MFG, a reflection of gene expression in MECs (44–46), to study lipid metabolic enzyme mRNA expression profiles in conjunction with the measurement of milk FA composition during secretory activation and through the first 6 wk of lactation. We demonstrated that the initiation of lactation in human MECs is a very complex process associated with upregulation in expression of genes representing all aspects of lipid metabolism and milk FA production. These included lipolysis at the MEC membrane, FA uptake from blood, intracellular FA transport, FA and glycerol activation, de novo FA synthesis, FA elongation, FA desaturation, synthesis of TG, cholesterol synthesis and metabolism, and lipid droplet formation. We propose a central role for SREBF1 in controlling expression of the genes responsible for lipid synthesis in the human MECs. Decreased progesterone concentration and increased prolactin receptor signaling may be the primary trigger for the activation of SREBF1. Activation of ESRRA seems to play an important role in the transport and trafficking of FA and cholesterol synthesis genes within the MEC. Since milk fat is mostly TG (98%), our focus in this study was the metabolic processes associated with TG synthesis and packaging.
Lipolysis and transport of circulating TG and NEFA into MEC
MECs have the ability to take up efficiently LCFA from albumin-bound circulating FAs (NEFA) and lipoproteins. Moreover, lipoprotein lipase (LPL) in the MEC, compared with other tissues, is very effective in the hydrolysis of TG derived from VLDL or chylomicrons (16). In our present study, we observed not only a severalfold upregulation in expression of the gene for LPL but also in the genes encoding for a variety of other lipases including LIPE and LIPG (Fig. 1). Of particular interest was the mRNA expression of CEL, representing one of the most abundant transcripts of the entire arrays and its pseudogene CELP. CEL is known as a lipolytic enzyme capable of hydrolyzing cholesteryl esters, tri-, di-, and monoacylglycerols, phospholipids, lysophospholipids, and ceramide, allowing it to potentially complement other lipolytic enzymes for complete hydrolysis and assimilation of lipids (29). However, CEL (and probably its evolutionary derivative CELP), does not represent a gene that can be active in the MG but is considered a secreted enzyme that is activated in the infant's GI tract for efficient fat digestion (17). Interestingly, the magnitude of increase in LPL mRNA expression in human MECs is an intermediate between that observed in bovine (6) and rodent models (32, 66). This would suggest that the primary source of FA in the murine model is de novo synthesis within the MEC, whereas in bovine it is the circulating FA with human intermediate between these two models.
Recent evidence points to the VLDLR as an essential component of LPL activity (75). In our study, VLDLR expression was upregulated dramatically over the first 4 days but continued to increase over the 6 wk of the study. The concomitant increase in expression of the genes for lipases and VLDLR suggest a central role for these two proteins in milk fat transport during the initiation and maintenance of lactation (75). The very subtle change in the observed mRNA expression of LDLR indicates that circulating LDL may not be a significant source for milk FA; however, the upregulation of the HDL receptor (SCARB1) suggests that its core lipids may be. On the basis of these observations, we would speculate the existence of selectivity in the circulating lipoproteins as a source for milk lipids.
Passive diffusion of FA across membranes plays a minor role compared with protein-mediated FA uptake and the “flip-flop mechanism” (55). The main proteins involved in FA uptake in nonruminant cells include FA translocator FAT/CD36 (CD36) and FA transport proteins (FATP or SLC27A5) (13). CD36 mRNA in our study was the highest in abundance among all FA transporter genes and parallels observation in the bovine model (6). Our data show that human mammary tissue expresses most of the known FA transporters (SLC27A isoforms) at lower levels, and only expression of the gene for SLC27A5 was upregulated, suggesting limited uptake of NEFA (4). CD36 has been found to colocalize with FATP (6), ACSL, and FABP (74), supporting the concept of cooperation among these proteins to facilitate FA uptake. Clearly, FA uptake by mammary cells in humans as well as in other species is a complex and coordinated process that requires further elucidation.
Once hydrolysis is complete, lipid transporters transfer the FAs to the cytoplasm where they must be bound to FABP or acylated and bound to fatty acyl binding proteins (ACBP or DBI). FABP and DBI are the main intracellular FA transporters in ruminant (6) and nonruminant MEC (48). Such appears to be the case in humans as well, since expression of the genes for both DBI and FABP3 were upregulated severalfold. Expression of the gene for DBI is the highest in mRNA abundance and fold change among this category of genes (Fig. 2), contributing >50% of all FABP genes. These data suggest that a significant portion of FAs is delivered to specific organelles in the MEC in the active acylated form. It is interesting to note that in bovine, FABP3 is the second most abundant transcript, contributing ∼16% of total RNA as a result of 80-fold upregulation during lactation (6). In contrast, bovine DBI mRNA abundance was <0.2% of total RNA and had only a small increase (1.5-fold) during lactation (6). A similar pattern has been seen in mice (66). Thus, in contrast to bovine and murine animals in which LCFAs are mostly shuttled intracellularly in the nonacylated form, in humans this would occur in the activated acylated form.
De Novo FA Synthesis and FA Modification
The other source for milk FAs is de novo synthesis within the MG (24). In our study, the increase in FAs representing mammary de novo FA synthesis (<16 carbons) was the most prominent change in the milk FA profile (whether expressed as g/dl, %total, or g/day). This change in FA composition was mirrored in expression of the genes for enzymes involved in de novo synthesis within the MG. The mRNA expression of the pertinent rate-limiting step in de novo FA synthesis, ACACA (which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA), and that of FASN were upregulated severalfold during the secretory activation. Substrates for de novo FA synthesis are most likely derived from the TCA cycle. The upregulation in expression of the genes for many enzymes of the TCA cycle and the citrate transporter SLC25A1 observed in our study suggest a role for the mitochondria in the generation and export of citrate to the cytoplasm, an initial step in the synthesis of both FA and cholesterol (66). Similarly, the increase in expression of the genes in the malate shunt (66) and the pentose phosphate shunt (54) would provide a mechanism for the generation of NADPH for de novo FA synthesis during secretory activation. Generally, the contribution of de novo synthesis of FA ≥16 carbons in humans is very low (less than 2–5% for C16:0, using stable isotopic tracers, unpublished data). Similar observations were reported for limited capacity of the human MG to synthesize C16:0 and C18:0 FA, even on a stringent low-fat diet (24). This is not the case in rodents and probably bovine as well. Therefore, we did not consider C16:0 in the class of de novo synthesized FA. Interestingly, we have detected the odd-chain C15:0 FA (as well as trivial amounts of other odd-chain FA from C3 to C13). Since, FASN is unlikely to synthesize an odd-chain FA unless primed with an odd carbon precursor, suggesting that mitochondrial propionyl-CoA may be utilized.
It is well established that the production of medium-chain FA by the mammary FA synthetase is regulated by thioesterase II, the key enzyme that is expressed specifically in MEC of mammalian species (8, 37, 38, 52, 59, 73, 78, 82) by hydrolyzing medium-chain acyl moieties from thioester linkage to the FASN system. Our findings indicated that expression of the gene for S-acyl fatty acid synthase thioesterase, medium-chain (OLAH) was severalfold upregulated (12.0-fold) during the secretory activation. Additionally, we have detected an array of several ACOT genes, including ACOT1, 2, 4, 7, 8, 9, 11, and 13. ACOT family members catalyze the cleavage of acyl-CoAs into free CoA and free FAs and have the potential to regulate the intracellular concentrations of these FA molecules (7). The increase in ACOT1 in human MEC is contrary to what is observed in the mouse (68). Furthermore, ACOT11 had the highest increase (8.0-fold) among ACOT genes over the study duration. Interestingly, expression of ACOT11 gene in a mouse homolog in brown adipose tissue is induced by low temperatures and is repressed by warm temperatures (1), and the increase in expression of the protein has been associated with obesity resistance in mice (1). Generally, the increase in expression of the genes for ACOT1 and 11 may indicate that human MEC rely more on FA oxidation for their energy source compared with mice. This speculation is supported by our finding that FA β-oxidation is the top significant canonical pathway in lipid metabolic genes of this dataset. However, further studies are mandated to elucidate the role of ACOT genes in the MEC.
Despite the observation that there was a severalfold upregulation in expression of the gene for elongation of FA, this was not reflected in milk FA composition. Elongation of palmitate to stearate (proportion of C18/C16) was the only evidence of elongation (1.3-fold increase, P < 0.05), but the individual and sum of very-long-chain FA (VLCFA, >20 carbons) whether expressed as concentrations or as percentages of total were decreased over the study duration. We observed little evidence of desaturation in milk FA, which is in agreement with expression of the genes for desaturases. In bovine, SCD mRNA plays a crucial role in TG synthesis in lactating MG based on its activity (34), the mRNA abundance (23% of total) and upregulation (>40-fold) during lactation (6). However, in our study, the mRNA expression of SCD decreased over the first 72 h (−10.0-fold) and then gradually increased (3.0-fold from this nadir) by day 21 (Fig. 3), suggesting a less important role for SCD in human MECs during secretory activation.
FA and Glycerol Activation and TG Assembly
Internalized FAs must be esterified with CoA in the inner face of the plasma membrane via acyl-CoA (ASC) (47) prior to participating in further metabolism. In humans, mRNA of acyl-CoA long-chain family member 1(ACSL1) was predominant among ACSL isoforms and was up-regulated several fold during the onset of lactation, in a agreement with expression data from murine (50) and bovine mammary tissue (4, 50). Collectively these observations highlight the importance of ACSL1 for milk TG synthesis in the MECs. Interestingly, the mRNA of acyl-CoA synthetase medium-chain family member 3 (ACSM3) was present in very low abundance in the initial milk sample but was up-regulated during the onset of lactation. Expression of genes for the two enzymes involved in activation of short chain FAs, acyl-CoA synthetase short-chain family member 1(ACSS1, mitochondrial) and member 2 (ACSS2, cytosolic), were detected, and both increased severalfold with the onset of lactation. However, ACSS2 mRNA abundance was 10-fold greater than ACSS1. In bovine ACSS1 activates acetate toward oxidation (18), whereas ACSS2 correlates with mammary de novo FA synthesis (50). ACSS2 was also shown to direct acetate toward FA synthesis in humans in vitro (42).Thus, abundances of ACSS members and their increase at the onset of lactation suggest the proteins encoded by these genes provide activated acetate for de novo FA synthesis and carbon source for energy generation within the MECs (22). Therefore, it is likely that the protein products of ACSM3 (and the ACSS2) might be utilized to incorporate medium- and short-chain acyl-CoAs into the TG synthesis pathway.
To synthesize TG, both fatty acyl-CoAs and glycerol 3-phosphate must be readily available (21). Liver and adipose tissue are the classical lipogenic tissues. The major steps in the pathway of TG synthesis in MG from animal models have been elucidated (3, 12, 25, 27, 35, 39, 58, 81). Glycerol can enter the MECs from the plasma to be phosphorylated by glycerol kinase (GK). Although expression of the gene for GK decreased during the onset of lactation, an alternative gene, the putative glycerol kinase-5 (GK5) increased 20-fold during the onset of lactation, indicating that GK5 may be crucial in glycerol activation within the MEC. There was also upregulation in expression of the genes for mono-acylglycerol kinase (AGK) and diacylglycerol kinase-α (DGKA) indicating activation of the glycerol carbon backbone in these substrates for further acylation. Another potential significant source for glycerol is glucose via glycolysis. We have shown that glycolysis was the top significant canonical pathway activated during initiation of lactation (54).
The ultimate synthesis of TG involves the transfer of acyl-FA to the activated glycerol backbone. This is accomplished by an array of acyltransferases. Surprisingly, the abundance and change in expression of the three acyltransferases involved in the formation of mono-, di-, and triacylglycerol [glycerol-3-phosphate acyltransferase, mitochondrial (GPAM), 1-acylglycerol-3-phosphate O-acyltransferases (AGPAT), and diacylglycerol O-acyltransferase (DGAT1)] were not proportional to their expected roles, as has been reported in bovine tissue (5, 21, 71). Additionally, the mRNA of LPIN1 (encodes a phosphohydrolase enzyme that catalyzes the dephosphorylation of phosphatidic acid to yield diacylglycerol) was the most abundant among this group of genes (LPIN1 > AGPAT1 > DGAT1 > GPAM), and their respective mRNA expressions were upregulated 4-, 2.5-, 1.2-, and 9.0-fold, respectively, during the onset of lactation. The opposite has been seen in the bovine model, where GPAM was the most abundant gene (GPAM > AGPAT6 > DGAT1 > LPIN1), and the respective increases were 10-, 15-, 1.0-, and 20-fold, respectively, by day 60 postpartum (6). It is interesting to note that DGAT1 was not upregulated during the postpartum period in bovines and only slightly increased in humans. Since DGAT1 is involved in the last step of TG synthesis by converting diacylglycerol to TG, its low expression would theoretically result in increase in di- but not tri-acylglycerol in milk. Since this is not the case, it would suggest either an increase in the protein activity of DGAT1 and/or the existence of another pathway for TG synthesis. However, the fact remains that DGAT1 is one of many proteins comprising the TG synthesis pathway (10), and a lack in functionality of any gene in this pathway can likely reduce the efficiency of TG synthesis (6).
Cholesterol and Lipid Droplet Synthesis
The presence and severalfold upregulation in the mRNA of many enzymes of the cholesterol synthesis pathway over the entire study duration (Fig. 5) may suggest that cholesterol is synthesized within the human MG. These findings are consistent with the observations from mice (66) and goats (41), in which ∼20% of the cholesterol in milk is synthesized within the MG, the remainder being imported from the plasma. Yet, the dramatic downregulation in the mRNA of the very abundant cholesterol transporters (ABCA1 and -7 and ABCG1) and the upregulation occurring only in ABCG2 (4.0-fold increase but its abundance was trivial) during the onset of lactation in our study underestimates the contribution from circulating cholesterol. These findings are consistent with bovine (6) and murine models in which the mRNA abundances for major cholesterol transporters ABCA1, ABCG1, and ABCA7 were decreased during lactating compared with nonlactating MG (6, 43). Collectively, these observations suggest that cholesterol transport via ABC transporters into the MECs during lactation may play a minor role in cholesterol influx. Whether cholesterol is delivered into the MECs within the core of the HDL and VLDL particles via their receptors, which were both upregulated, requires further investigation.
Once TGs are formed, they are incorporated with cholesterol and phospholipids into MFG in the ER membrane (28). MFG are then transported to the apical membrane and eventually released into the lacteal (28). However, the precise mechanism of milk fat secretion into the milk compartment is still debated (49). In animal models, there is a growing evidence that milk lipid secretion occurs through a “tripartite” complex among butyrophilin (an integral transmembrane protein, BTN), xanthine dehydrogenase (soluble metabolic enzyme, XDH) and adipophilin (lipid droplet surface protein, ADPH, or perilipin, PLIN2) (28, 49, 63, 64). We confirmed the high abundances in gene expression of XDH, BTN1A1, and ADFP (Fig. 5), supporting this model in humans as has been proposed for murine (49) and bovine (6) models.
SREBF1, THRSP, and ESRRA Activation: Potential Rate-Limiting Step for Lipid Production in Human MEC
Having determined a number of functionally important genes and lipid metabolites that are upregulated at the onset of lactation, we attempted to reveal the hierarchy of regulators for genes controlling lipid synthesis in the human MEC. We examined the temporal profiles and expression levels of categories of genes known to regulate lipid metabolism in liver, adipose, and MG in animal models (6, 66). We recently reported (54) that the concomitant decrease in milk progesterone was associated with an increase in expression of the gene for prolactin receptor (PRLR) and plasma prolactin concentration, suggesting that increased prolactin signaling pathway may be an important trigger for the induction of crucial genes in the lactose synthesis pathway. Similarly, we propose that progesterone withdrawal and PRLR activation may also trigger the lipid synthesis machinery in human MEC, as has been proposed in other animal models (6, 66).
We propose that increased induction and activation of SREBF1 is central to MG lipid synthesis in humans as it has been shown in rodent (66, 69) and bovine (6) models. The change in expression of the genes for SREBF1 and thyroid hormone-responsive protein (THRSP) in this study paralleled the peak of milk prolactin concentration and a sevenfold increase in expression of the gene for PRLR (54). The resulting IPA network (Fig. 7) underscores a central role for SREBF1, THRSP, and ERRA genes in controlling transcription of many of the genes assessed in the present study that regulate milk fat synthesis.
This speculation is in keeping with a substantial body of evidence that prolactin is central to the process of secretory activation in mice (19, 20, 65, 69). Bromocriptine-mediated loss of serum PRL suppresses MEC-specific gene expression of milk protein genes, transporters for glucose and citrate, and both glycolytic and lipogenic enzymes in mice (69). Additionally, SREBF1 isoforms a and c, were two- and threefold decreased in BrCr-Rx mice. Finally, a severalfold decrease was observed in the genes of de novo FA synthesis (FASN, ACLY, ACC1, and SLC25A1) and TG synthesis (DGAT1, SCD2, and FADS) (69). Expression of other genes, namely INSIG2 and PPARA increased progressively to reach a relative plateau by day 4, but their role in the induction of milk fat synthesis remains to be determined. The data from bovine model, although emphasizing the role of SREBF1, supports the notion that is not the only player regulating lipid synthesis in bovine mammary tissue but suggest a putative role of PPARG in the coordination with PPARGC1A and INSIG1 in controlling function/expression of SREBF1(6). It is worthwhile to note that activation of SREBF1 as a transcription factor is under control of both INSIG and SREBF cleavage-activating protein (SCAP) and that the importance of SCAP in this pathway has been demonstrated in mice (67) and bovine (5, 26).
SREBP1 and THRSP (Spot14) have been shown to be involved in the regulation of lipid synthesis in the MG of mice (33) and bovine (26). THRSP is required for de novo lipid synthesis in the lactating MG (83), since in THRSP-null mice milk TG is significantly reduced as a result of decreased MG de novo lipid synthesis. Additionally, THRSP is a component of the lipogenic phenotype observed in aggressive breast cancers (80). In rodents (62) and cows (11), administration of thyroid hormones resulted in an increased milk yield. In addition, administration of TRH to women resulted in elevated serum prolactin and daily milk yield in women with inadequate lactation (60). The connection between THRB and THRSP is not clear in the MG. THRB gene knockout in liver of mice decreased expression of the gene for THRSP (79), which could be explained by DNA-protein interactions as a result of binding of THRSP gene promoter and protein of THRB occurs in a nuclear extract from rat liver (40).
Independently of the mRNA expression pattern, the upstream regulator analysis using IPA has shown that ESRRA was the earliest (24 h postpartum) upstream regulator predicted to be activated (see results). The role of ESRRA activation was directed more toward upregulation of genes responsible for lipolysis, transport, binding, and activation of FA based on studies from rat cardiac myocytes (31) and mouse T lymphocytes (51) and adipocytes (61). In addition, ESRRA is a member of a complex mediating the biogenesis of functional mitochondria (53) in human follicular thyroid carcinoma cell lines. We speculate the same role for ESRRA may apply to the MEC which indeed remain to be determined.
Those authors (6) proposed that a network of transcription regulators and nuclear receptors, including SREBF1, SREBF2, PPARG, INSIG1, and PPARGC1A, coordinate activation of the genes driving the lipid synthesizing machinery. However, these speculations are not supported in our human data, as the mRNA expression for INSIG1 continued to increase very slowly to reach 2.6-fold by day 42, the mRNA expression for SREBF2, PPARG, and SCAP decreased during the onset of lactation. Taken together, our findings allowed us to develop a model of milk fat synthesis regulation in human MEC (Fig. 8). The model incorporates the most recent information available, including our data, on enzymes involved in milk fat synthesis.
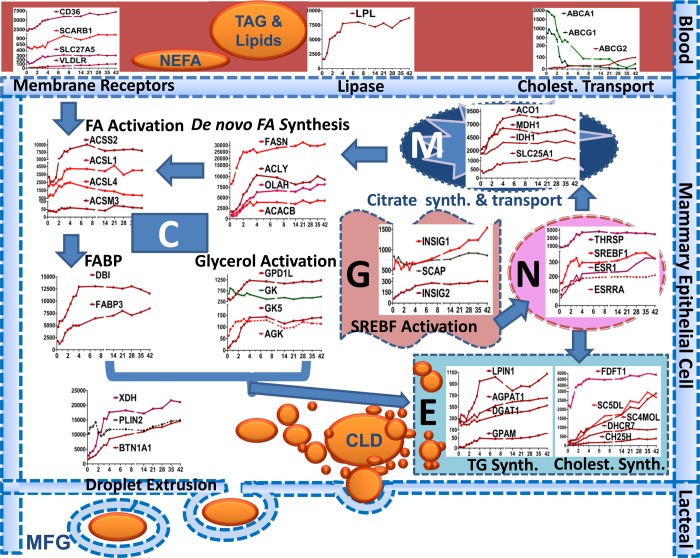
Fig. 8.
This figure depicts a model of the lipid synthesis genes in the human mammary epithelial cell during secretory activation according to cell compartment. C, cytoplasm; M, mitochondria; N, nucleus; E, endoplasmic reticulum; G, Golgi; CLD, cytoplasmic lipid droplet, MFG, milk fat globule. The VLDL-TG core (or chylomicrons) is hydrolyzed by LPL in cooperation with VLDLR and releases LCFA for transport across the endothelium into the extracellular space. LCFA are imported into mammary cells via CD36 in concert with SLC27A5. Once LCFAs cross the membrane, most are activated into an LC-acyl-CoA (LCACoA) primarily via ACSL1 & 4 for subsequent utilization. ACACA & B synthesize malonyl-CoA, and both acetyl-CoA and malonyl-CoA are substrates for FASN. Formation of acetyl-CoA from acetate is carried out by ACSS2. Acetyl CoA can also be derived from citrate by action of ACLY. Mitochondria generate ATP, protons, and citrate as fuel and substrate for de novo FA synthesis. OLAH (thioestrase II) interacts with FASN to produce medium-chain FA (C6-C14) and subsequent activation by ACSM3, which allows FAs to enter into the TG synthesis pathway. Glycerol and acylglycerols are activated by GK, GK5, and AGK. LCACoA bind primarily to DBI and FABP3, which transports them to specific intracellular organelles for serving as substrate for TG synthesis. Activated glycerol and LCACoA form TG via sequential reactions carried out by GPAM, AGPAT1, LIPIN1, and DGAT1. Cholesterol is mainly synthesized within the MEC (overall upregulation of genes responsible for synthesis) and little is imported (downregulation of transporters except ABCG2). Once TG and cholesterol are formed, they are inserted into the ER membrane to form tiny CLD. These fuse to form bigger CLD and are secreted, by an apocrine mechanism, surrounded by apical membrane into the alveolar lumen as MFG through the interaction of PLIN2 with BTN1A1 and XDH. The outer MFG membrane contains, among other membrane proteins derived from the apical membrane, lipid transporters and cholesterol. We propose that a role for SREBF1 and THSRP activation, via prolactin receptor signaling (STAT5B) is crucial in activation of genes involved in lipolysis, de novo synthesis, FA activation, and TG and cholesterol biosynthesis. SREBF1 is not active in the nucleus until cleaved by a process in the Golgi involving SCAP and INSIG. ESRRA activation via PPARA triggers upregulation of genes involved in FA transporter (CD36), lipase (LPL), FA binding (FABP3), and FA activation (ACSL1).
Because of the nature of microarray analysis, we emphasize that our findings are relative gene expressions. However, we have associated the mRNA expression (but not their protein concentrations or enzymatic activities) with the ultimate product (i.e., milk FA). We believe that the relative values over time for genes represented in our study are generally correct. However, it is likely that they are influenced by changes in expression of the major milk protein genes (45). This could account for an underestimation of gene expression, particularly for mRNAs with low abundances. Although we have attempted to cluster genes according to known pathways of TG synthesis (as has been shown in Figs. 2–6), clustering of genes based on their trajectories (65) might also be useful. In this regard, most of the genes involved in FA transport and synthesis are upregulated between delivery and 48 h postpartum, suggesting that they are under the same or similar regulation. Some genes including DBI, FA activation, TG assembly, XDH, and BTN1A1 are upregulated more between 48 and 96 h postpartum, suggesting that their expression may be regulated independently of those induced over the first 48 h. However, other genes showed major upregulation over the entire period of study duration, suggesting yet a third mode of regulation. These later genes include LIPG, FA modification (the ELOVLs and SCD5) and those involved in cholesterol synthesis as well as INSIG1. Some genes (like LDLR, GK, PLIN2, and SCAP) have clear importance in MG lipid synthesis (based on other models) but whose expression did not change over the course of our studies. Finally, other genes (PPARG, ABCA1, ABCG1, and LIPA), known to be integral in lipid and cholesterol metabolism, were profoundly downregulated.
In conclusion, initiation of human lactation is characterized by dramatic upregulation in expression of genes associated with FA uptake from blood (LPL, CD36, SCARB1, and VLDLR) and intracellular transport/channeling (DBI and FABP3). Milk FA profiles mirrored mammary gene expression, since de novo synthesis increased with establishment of lactation. The onset of lactation was associated with upregulation of mRNA for genes involved in activation of FA (ACSL1, ACSS2), de novo synthesis (ACACA, ACLY, FASN, and OLAH), desaturation (FADS2), elongation (ELVOL1/5), glycerol activation (GK5, GPD1L, and AGK), synthesis of TG (AGPAT1, GPAM, and LPIN1), lipid droplet formation (BTN1A1, XDH, and PLIN2), and cholesterol synthesis (FTFD1, MVD, and SC5DL). Activation of SREBF1, together with THRSP and ESRRA via the concomitant decrease in progesterone concentration and increase prolactin signaling, is most likely central for milk lipid synthesis regulation in the human MG.
GRANTS
This project was supported by NIH grants RO1 DK-55478, HD-37857, MO1 RR-00188, and USDA/ARS 6250-5100. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, TX). The contents of this publication do not necessarily reflect the views of policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.M. and M.W.H. conception and design of research; M.A.M. and M.W.H. performed experiments; M.A.M. and M.W.H. analyzed data; M.A.M. and M.W.H. interpreted results of experiments; M.A.M. and M.W.H. prepared figures; M.A.M. and M.W.H. drafted manuscript; M.A.M. and M.W.H. edited and revised manuscript; M.A.M. and M.W.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the volunteers whose participation made this study possible. We gratefully acknowledge and thank our laboratory manager Susan Sharma, PhD, and our research coordinator (Janette Gonzalez) in the execution of these studies. We also thank Dr. Daryll Hadsell for review of this manuscript, insights, and invaluable comments.
REFERENCES
- 1. Adams SH, Chui C, Schilbach SL, Yu XX, Goddard AD, Grimaldi JC, Lee J, Dowd P, Colman S, Lewin DA. BFIT, a unique acyl-CoA thioesterase induced in thermogenic brown adipose tissue: cloning, organization of the human gene and assessment of a potential link to obesity. Biochem J 360: 135–142, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis! Breast Cancer Res 9: 204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernard L, Rouel J, Leroux C, Ferlay A, Faulconnier Y, Legrand P, Chilliard Y. Mammary lipid metabolism and milk fatty acid secretion in alpine goats fed vegetable lipids. J Dairy Sci 88: 1478–1489, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bionaz M, Loor JJ. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J Nutr 138: 1019–1024, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bionaz M, Loor JJ. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform Biol Insights 5: 83–98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bionaz M, Loor JJ. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 9: 366, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brocker C, Carpenter C, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human acyl-CoA thioesterase gene (ACOT) family. Human Genomics 4: 411–420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchbinder JL, Witkowski A, Smith S, Fletterick RJ. Crystallization and preliminary diffraction studies of thioesterase II from rat mammary gland. Proteins 22: 73–75, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Casey TM, Plaut K. The role of glucocorticoids in secretory activation and milk secretion, a historical perspective. J Mammary Gland Biol Neoplasia 12: 293–304, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43: 134–176, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Davis SR, Collier RJ, McNamara JP, Head HH, Sussman W. Effects of thyroxine and growth hormone treatment of dairy cows on milk yield, cardiac output and mammary blood flow. J Anim Sci 66: 70–79, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Dils RR. Comparative aspects of milk fat synthesis. J Dairy Sci 69: 904–910, 1986 [DOI] [PubMed] [Google Scholar]
- 13. Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 21: 259–268, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emken EA, Adlof RO, Hachey DL, Garza C, Thomas MR, Brown-Booth L. Incorporation of deuterium-labeled fatty acids into human milk, plasma, and lipoprotein phospholipids and cholesteryl esters. J Lipid Res 30: 395–402, 1989 [PubMed] [Google Scholar]
- 16. Fielding BA, Frayn KN. Lipoprotein lipase and the disposition of dietary fatty acids. Br J Nutr 80: 495–502, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Fredrikzon B, Hernell O, Blackberg L, Olivecrona T. Bile salt-stimulated lipase in human milk: evidence of activity in vivo and of a role in the digestion of milk retinol esters. Pediatr Res 12: 1048–1052, 1978 [DOI] [PubMed] [Google Scholar]
- 18. Fujino T, Kondo J, Ishikawa M, Morikawa K, Yamamoto TT. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J Biol Chem 276: 11420–11426, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol 229: 163–175, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Golden KL, Rillema JA. Effects of prolactin on galactosyl transferase and alpha-lactalbumin mRNA accumulation in mouse mammary gland explants. Proc Soc Exp Biol Med 209: 392–396, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez-Baro MR, Lewin TM, Coleman RA. Regulation of triglyceride metabolism. II. Function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action. Am J Physiol Gastrointest Liver Physiol 292: G1195–G1199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenbaum AL, Salam A. Regulation of mammary gland metabolism: pathways of glucose utilization, metabolite profile and hormone response of a modified mammary gland cell preparation. Eur J Biochem 87: 505–516, 1978 [DOI] [PubMed] [Google Scholar]
- 23. Hachey DL, Patterson BW, Reeds PJ, Elsas LJ. Isotopic determination of organic keto acid pentafluorobenzyl esters in biological fluids by negative chemical ionization gas chromatography/mass spectrometry. Anal Chem 63: 919–923, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Hachey DL, Silber GH, Wong WW, Garza C. Human lactation. II: Endogenous fatty acid synthesis by the mammary gland. Pediatr Res 25: 63–68, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Hansen HO, Grunnet I, Knudsen J. Triacylglycerol synthesis in goat mammary gland. Factors influencing the esterification of fatty acids synthesized de novo. Biochem J 220: 521–527, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harvatine KJ, Bauman DE. SREBP1 and thyroid hormone responsive spot 14 (S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA. J Nutr 136: 2468–2474, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Harvatine KJ, Boisclair YR, Bauman DE. Recent advances in the regulation of milk fat synthesis. Animal Int J Anim Biosci 3: 40–54, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Heid HW, Keenan TW. Intracellular origin and secretion of milk fat globules. Eur J Cell Biol 84: 245–258, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Hui DY, Howles PN. Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J Lipid Res 43: 2017–2030, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Hunt MC, Yamada J, Maltais LJ, Wright MW, Podesta EJ, Alexson SE. A revised nomenclature for mammalian acyl-CoA thioesterases/hydrolases. J Lipid Res 46: 2029–2032, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24: 9079–9091, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen DR, Gavigan S, Sawicki V, Witsell DL, Eckel RH, Neville MC. Regulation of lipoprotein lipase activity and mRNA in the mammary gland of the lactating mouse. Biochem J 298: 321–327, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kadegowda AK, Connor EE, Teter BB, Sampugna J, Delmonte P, Piperova LS, Erdman RA. Dietary trans fatty acid isomers differ in their effects on mammary lipid metabolism as well as lipogenic gene expression in lactating mice. J Nutr 140: 919–924, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Kinsella JE. Stearyl CoA as a precursor of oleic acid and glycerolipids in mammary microsomes from lactating bovine: possible regulatory step in milk triglyceride synthesis. Lipids 7: 349–355, 1972 [DOI] [PubMed] [Google Scholar]
- 35. Kuhn NJ. Esterification of glycerol 3-phosphate in lactating guinea-pig mammary gland. Biochem J 105: 213–223, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lammi-Keefe CJ, Jensen RG. Lipids in human milk: a review. 2: Composition and fat-soluble vitamins. J Pediatr Gastroenterol Nutr 3: 172–198, 1984 [DOI] [PubMed] [Google Scholar]
- 37. Libertini LJ, Smith S. Purification and properties of a thioesterase from lactating rat mammary gland which modifies the product specificity of fatty acid synthetase. J Biol Chem 253: 1393–1401, 1978 [PubMed] [Google Scholar]
- 38. Libertini LJ, Smith S. Synthesis of long chain acyl-enzyme thioesters by modified fatty acid synthetases and their hydrolysis by a mammary gland thioesterase. Arch Biochem Biophys 192: 47–60, 1979 [DOI] [PubMed] [Google Scholar]
- 39. Lin CY, Abraham S, Smith S. Acyl specificity in triglyceride synthesis by lactating rat mammary gland. J Lipid Res 17: 647–656, 1976 [PubMed] [Google Scholar]
- 40. Liu HC, Towle HC. Functional synergism between multiple thyroid hormone response elements regulates hepatic expression of the rat S14 gene. Mol Endocrinol 8: 1021–1037, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Long CA, Patton S, McCarthy RD. Origins of the cholesterol in milk. Lipids 15: 853–857, 1980 [DOI] [PubMed] [Google Scholar]
- 42. Luong A, Hannah VC, Brown MS, Goldstein JL. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem 275: 26458–26466, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Mani O, Korner M, Sorensen MT, Sejrsen K, Wotzkow C, Ontsouka CE, Friis RR, Bruckmaier RM, Albrecht C. Expression, localization, and functional model of cholesterol transporters in lactating and nonlactating mammary tissues of murine, bovine, and human origin. Am J Physiol Regul Integr Comp Physiol 299: R642–R654, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Maningat PD, Sen P, Rijnkels M, Hadsell DL, Bray MS, Haymond MW. Short-term administration of rhGH increases markers of cellular proliferation but not milk protein gene expression in normal lactating women. Physiol Genomics 43: 381–391, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maningat PD, Sen P, Rijnkels M, Sunehag AL, Hadsell DL, Bray M, Haymond MW. Gene expression in the human mammary epithelium during lactation: the milk fat globule transcriptome. Physiol Genomics 37: 12–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maningat PD, Sen P, Sunehag AL, Hadsell DL, Haymond MW. Regulation of gene expression in human mammary epithelium: effect of breast pumping. J Endocrinol 195: 503–511, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol 17: 274–278, 2006 [DOI] [PubMed] [Google Scholar]
- 48. McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res 40: 1371–1383, 1999 [PubMed] [Google Scholar]
- 49. McManaman JL, Russell TD, Schaack J, Orlicky DJ, Robenek H. Molecular determinants of milk lipid secretion. J Mammary Gland Biol Neoplasia 12: 259–268, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Mellenberger RW, Bauman DE, Nelson DR. Metabolic adaptations during lactogenesis: fatty acid and lactose synthesis in cow mammary tissue. J Mammary Gland Biol Neoplasia 14: 261–268, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Michalek RD, Gerriets VA, Nichols AG, Inoue M, Kazmin D, Chang CY, Dwyer MA, Nelson ER, Pollizzi KN, Ilkayeva O, Giguere V, Zuercher WJ, Powell JD, Shinohara ML, McDonnell DP, Rathmell JC. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci USA 108: 18348–18353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mikkelsen J, Witkowski A, Smith S. Interaction of rat mammary gland thioesterase II with fatty acid synthetase is dependent on the presence of acyl chains on the synthetase. J Biol Chem 262: 1570–1574, 1987 [PubMed] [Google Scholar]
- 53. Mirebeau-Prunier D, Le Pennec S, Jacques C, Gueguen N, Poirier J, Malthiery Y, Savagner F. Estrogen-related receptor alpha and PGC-1-related coactivator constitute a novel complex mediating the biogenesis of functional mitochondria. FEBS J 277: 713–725, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Mohammad MA, Hadsell DL, Haymond MW. Gene regulation of UDP-galactose synthesis and transport: potential rate-limiting processes in initiation of milk production in humans. Am J Physiol Endocrinol Metab 303: E365–E376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol Neoplasia 7: 163–176, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Neville MC, Allen JC, Archer PC, Casey CE, Seacat J, Keller RP, Lutes V, Rasbach J, Neifert M. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr 54: 81–92, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, Allen J, Archer P. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr 48: 1375–1386, 1988 [DOI] [PubMed] [Google Scholar]
- 58. Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr 17: 159–183, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Nolin JM, Thompson BJ, Smith S. Localization of thioesterase II, the chain-length regulatory enzyme of milk fatty acid synthesis, in rat mammary gland epithelial cells. J Endocrinol 94: 251–256, 1982 [DOI] [PubMed] [Google Scholar]
- 60. Peters F, Schulze-Tollert J, Schuth W. Thyrotrophin-releasing hormone–a lactation-promoting agent? Br J Obstet Gynaecol 98: 880–885, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, Czech MP. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest 116: 125–136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quevedo-Corona L, Franco-Colin M, Caudillo-Romero M, Pacheco-Rosado J, Zamudio-Hernandez S, Racotta R. 3,5,3′-Triiodothyronine administered to rat dams during lactation increases milk yield and triglyceride concentration and hastens pups growth. Life Sci 66: 2013–2021, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Reinhardt TA, Lippolis JD. Bovine milk fat globule membrane proteome. J Dairy Res 73: 406–416, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Robenek H, Hofnagel O, Buers I, Lorkowski S, Schnoor M, Robenek MJ, Heid H, Troyer D, Severs NJ. Butyrophilin controls milk fat globule secretion. Proc Natl Acad Sci USA 103: 10385–10390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rudolph MC, McManaman JL, Hunter L, Phang T, Neville MC. Functional development of the mammary gland: use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J Mammary Gland Biol Neoplasia 8: 287–307, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Rudolph MC, McManaman JL, Phang T, Russell T, Kominsky DJ, Serkova NJ, Stein T, Anderson SM, Neville MC. Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics 28: 323–336, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Rudolph MC, Monks J, Burns V, Phistry M, Marians R, Foote MR, Bauman DE, Anderson SM, Neville MC. Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol Endocrinol Metab 299: E918–E927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rudolph MC, Neville MC, Anderson SM. Lipid synthesis in lactation: diet and the fatty acid switch. J Mammary Gland Biol Neoplasia 12: 269–281, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Rudolph MC, Russell TD, Webb P, Neville MC, Anderson SM. Prolactin-mediated regulation of lipid biosynthesis genes in vivo in the lactating mammary epithelial cell. Am J Physiol Endocrinol Metab 300: E1059–E1068, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Saint L, Smith M, Hartmann PE. The yield and nutrient content of colostrum and milk of women from giving birth to 1 month post-partum. Br J Nutr 52: 87–95, 1984 [DOI] [PubMed] [Google Scholar]
- 71. Short VJ, Brindley DN, Dils R. Co-ordinate changes in enzymes of fatty acid synthesis, activation and esterification in rabbit mammary gland druing pregnancy and lactation. Biochem J 162: 445–450, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith S. Mechanism of chain length determination in biosynthesis of milk fatty acids. J Mammary Gland Biol Neoplasia 14: 245–260, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Smith S, Libertini LJ. Specificity and site of action of a mammary gland thioesterase which releases acyl moieties from thioester linkage to the fatty acid synthetase. Arch Biochem Biophys 196: 88–92, 1979 [DOI] [PubMed] [Google Scholar]
- 74. Stahl A. A current review of fatty acid transport proteins (SLC27). Pflügers Arch 447: 722–727, 2004 [DOI] [PubMed] [Google Scholar]
- 75. Tacken PJ, Hofker MH, Havekes LM, van Dijk KW. Living up to a name: the role of the VLDL receptor in lipid metabolism. Curr Opin Lipidol 12: 275–279, 2001 [DOI] [PubMed] [Google Scholar]
- 76. Tomcik K, Ibarra RA, Sadhukhan S, Han Y, Tochtrop GP, Zhang GF. Isotopomer enrichment assay for very short chain fatty acids and its metabolic applications. Anal Biochem 410: 110–117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 50, Suppl: S138–S143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Waltham PC, Carey EM. Acyl-coenzyme A thioesterase activities in lactating mammary gland of the rabbit. Biochem Soc Trans 4: 1147–1148, 1976 [DOI] [PubMed] [Google Scholar]
- 79. Weiss RE, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S. Thyroid hormone action on liver, heart, and energy expenditure in thyroid hormone receptor beta-deficient mice. Endocrinology 139: 4945–4952, 1998 [DOI] [PubMed] [Google Scholar]
- 80. Wells WA, Schwartz GN, Morganelli PM, Cole BF, Gibson JJ, Kinlaw WB. Expression of “Spot 14” (THRSP) predicts disease free survival in invasive breast cancer: immunohistochemical analysis of a new molecular marker. Breast Cancer Res Treat 98: 231–240, 2006 [DOI] [PubMed] [Google Scholar]
- 81. West CE, Bickerstafee R, Annison EF, Linzell JL. Studies on the mode of uptake of blood triglycerides by the mammary gland of the lactating goat. The uptake and incorporation into milk fat and mammary lymph of labelled glycerol, fatty acids and triglycerides. Biochem J 126: 477–490, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Witkowski A, Naggert J, Wessa B, Smith S. A catalytic role for histidine 237 in rat mammary gland thioesterase II. J Biol Chem 266: 18514–18519, 1991 [PubMed] [Google Scholar]
- 83. Zhu Q, Anderson GW, Mucha GT, Parks EJ, Metkowski JK, Mariash CN. The Spot 14 protein is required for de novo lipid synthesis in the lactating mammary gland. Endocrinology 146: 3343–3350, 2005 [DOI] [PubMed] [Google Scholar]