Abstract
In the present study, the role of 5-HT3 receptors in pudendal neuromodulation of bladder activity and its interaction with opioid receptors were investigated in anesthetized cats. The bladder was distended with either saline to induce normal bladder activity or with 0.25% acetic acid (AA) to induce bladder overactivity. Pudendal afferent nerves were activated by 5-Hz stimulation at multiples of the threshold (T) intensity for the induction of anal twitching. AA irritation significantly reduced bladder capacity to 16.5 ± 3.3% of saline control capacity, whereas pudendal nerve stimulation (PNS) at 1.5–2 and 3–4 T restored the capacity to 82.0 ± 12% (P = 0.0001) and 98.6 ± 15% (P < 0.0001), respectively. Cumulative doses (1–3 mg/kg iv) of ondansetron, a 5-HT3 receptor antagonist, eliminated low-intensity (1.5–2 T) PNS inhibition and reduced high-intensity (3–4 T) PNS inhibition of bladder overactivity. During saline distention, PNS at 1.5–2 and 3–4 T significantly increased bladder capacity to 173.2 ± 26.4% (P = 0.036) and 193.2 ± 22.5% (P = 0.008), respectively, of saline control capacity, but ondansetron (0.003–3 mg/kg iv) did not alter PNS inhibition. Ondansetron (0.1–3 mg/kg) also significantly (P < 0.05) increased control bladder capacity (50–200%) during either AA irritation or saline distention. In both conditions, the effects of low- and high-intensity PNS were not significantly different. After ondansetron (3 mg/kg) treatment, naloxone (1 mg/kg iv) significantly (P < 0.05) decreased control bladder capacity (40–70%) during either AA irritation or saline distention but failed to affect PNS inhibition. This study revealed that activation of 5-HT3 receptors has a role in PNS inhibition of bladder overactivity. It also indicated that 5-HT3 receptor antagonists might be useful for the treatment of overactive bladder symptoms.
Keywords: serotonin, 5-HT3 receptor, overactive bladder, opioid receptor, pudendal, neuromodulation
overactive bladder (OAB) affects 16–17% of the general population and >50% of nursing home residents, causing a significant negative impact on patients' quality of life (19, 30). OAB-related costs add up to around $12.6 billion per year (21). Antimuscarinic drugs are currently the first line of therapy, but their adverse effects and poor efficacy result in a high patient dropout rate (1, 2, 16). Sacral neuromodulation (InterStim, Medtronic, Minneapolis, MN) is an effective US Food and Drug Administration-approved alternative for patients with refractory OAB (34), and recent studies (22, 23) have suggested that pudendal neuromodulation is superior to sacral neuromodulation. However, the mechanisms underlying neuromodulation therapies are currently unknown.
Our previous studies in cats revealed that opioid receptors play a major role in the inhibition by tibial nerve stimulation of irritation-induced bladder overactivity (31, 32) and that tramadol (a strong opioid receptor agonist and a reuptake inhibitor for 5-HT and norepinephrine) can significantly enhance the inhibition of tibial nerve stimulation (37). In contrast, opioid receptors play a minor role in pudendal nerve stimulation (PNS)-induced inhibition of normal isovolumetric bladder activity and are not involved in the PNS-induced increase in bladder capacity of the normal or irritated bladder (4, 17). We also discovered that metabotropic glutamate 5 receptors contribute in part to the PNS inhibition in cats (15). The limited involvement of opioid and glutamatergic receptors and additional unknown mechanisms in PNS inhibition indicates that pudendal neuromodulation is complex and depends on multiple neurotransmitters that warrant further investigation. This study was aimed at identifying the contribution of serotonergic 5-HT3 receptor mechanisms to pudendal neuromodulation.
Previous studies (25, 29) in rats have demonstrated that spinal 5-HT receptors are important for the neuromodulation of somatic pain and that the analgesic effect induced by low-frequency transcutaneous electrical nerve stimulation can be suppressed by administration of a selective 5-HT3 antagonist. Therefore, in this study, we examined the role of 5-HT3 receptors in the PNS inhibition of bladder overactivity induced by intravesical infusion of dilute (0.25%) acetic acid (AA), which irritates the bladder and activates nociceptive bladder C-fiber afferents. PNS was used to inhibit bladder overactivity (i.e., increase bladder capacity) and mimic the clinical application of pudendal neuromodulation. Ondansetron (a 5-HT3 receptor antagonist) and naloxone (an opioid receptor antagonist) were administered intravenously to determine the role of these receptors in the PNS inhibition of bladder overactivity. Understanding the neurotransmitter mechanisms underlying neuromodulation therapies is important for the development of new drugs or improvement of current neuromodulation therapies for OAB (1, 2, 16).
METHODS
All protocols involving the use of animals in the present study were approved by the Animal Care and Use Committee of the University of Pittsburgh.
Experimental setup.
Experiments were conducted in a total of 28 adult cats (15 female cats and 13 male cats between 2.4 and 3.8 kg) under α-chloralose anesthesia (65 mg/kg, supplemented as necessary) after induction with isoflurane (2–3% in O2). Heart rates and blood oxygen levels were monitored with a pulse oximeter (9847V, Nonin Medical, Plymouth, MN), which was attached to the tongue. Systemic blood pressure was monitored via a catheter in the right carotid artery. Drugs and fluids were administered through a catheter in the right cephalic vein, and airway access was secured with a tracheostomy tube. Ureters were accessed through a midline abdominal incision and drained externally. The bladder was cannulated through the urethra with a double lumen catheter to infuse (1–2 ml/min) saline or 0.25% AA via one lumen and to measure bladder pressure via another lumen. A ligature was tied around the urethra to prevent leakage. A tripolar cuff electrode (NC223pt, MicroProbes for Life Sciences, Gaithersburg, MD) was attached to the left pudendal nerve via an incision between the tail and sciatic notch and connected to a stimulator (S88, Grass Technologies, West Warwick, RI).
Stimulation protocol and drug administration.
Uniphasic rectangular pulses (5-Hz frequency, 0.2-ms pulse width) were delivered to the intact pudendal nerve via the cuff electrode. The stimulation intensity threshold (T) was defined as the minimal intensity to induce an observable anal sphincter twitch. During the experiment, PNS was applied at multiples of the threshold intensity.
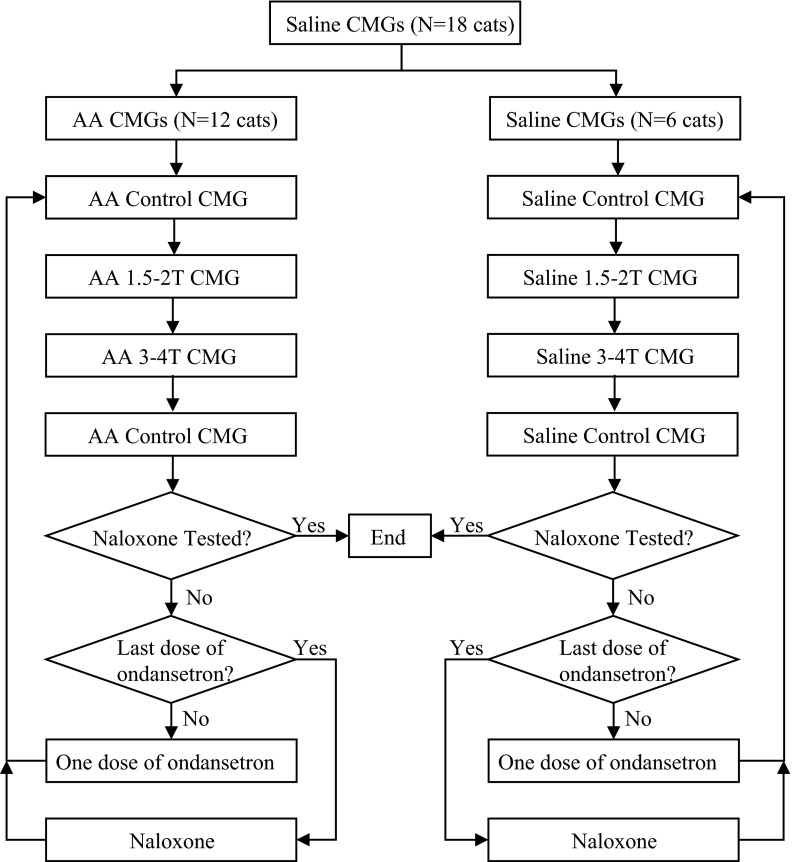
The timeline of the experimental protocol is shown in Fig. 1. The initial bladder capacity was determined during a cystometrogram (CMG) by slowly infusing the bladder with saline. Bladder capacity was defined as the threshold bladder volume to induce a micturition contraction of large amplitude (>20 cmH2O). Multiple CMGs were performed to determine reproducibility of the saline control capacity. Ondansetron (Hospira, Lake Forrest, IL) was then tested in two experimental groups. In the first group (n = 12 cats), repeated CMGs were performed by infusing 0.25% AA intravesically to irritate the bladder, activate nociceptive bladder afferent C-fibers, and induce bladder overactivity (12). The bladder capacity became stable at ∼20% of the saline control after 30–60 min of AA irritation. In the second group (n = 6 cats), repeated CMGs were performed by the intravesical infusion of saline to distend the bladder, activate non-nociceptive bladder afferent Aδ-fibers, and induce normal reflex bladder activity (12).
Fig. 1.
The timeline of the experimental protocol. CMGs, cystometrograms; AA, acetic acid; T, stimulation intensity threshold.
In both experimental groups, the bladder capacity was first determined during four CMGs: 1) control CMG without stimulation, 2) CMG with PNS at 1.5–2 T, 3) CMG with PNS at 3–4 T, and 4) control CMG without stimulation to determine any poststimulation effects. Increasing cumulative doses of ondansetron were then administered (0.003, 0.01, 0.03, 0.1, 0.3, 1.0, and 3.0 mg/kg iv). Ten minutes after the administration of each dose of ondansetron, the four CMGs were performed again in a period of ∼20–50 min under the four different conditions (control, 1.5- to 2-T PNS, 3- to 4-T PNS, and poststimulation control) to determine drug effects on bladder capacity. After the last dose of ondansetron (3 mg/kg) was tested, naloxone (1 mg/kg iv, Sigma-Aldrich, St. Louis, MO) was administered. Five minutes after naloxone administration, the four CMGs were repeated. The bladder was emptied after each CMG with a 3- to 5-min rest period to allow the distended detrusor to recover. The entire experiment was performed in a period of 4–8 h.
Vehicle control experiments were conducted in a separate group of cats during repeated saline CMGs (n = 5 cats) or during repeated AA CMGs (n = 5 cats) using the same stimulation and drug administration protocols as described above.
Data analysis.
For the repeated CMG recordings, bladder capacity was normalized to the initial saline control capacity in the same animal to allow comparisons between animals. Capacity measurements under the same conditions were averaged and are reported as means ± SE. Statistical significance (P < 0.05) was detected by one-way or two-way ANOVA and linear regression analysis.
RESULTS
Effect of ondansetron on bladder overactivity and PNS inhibition.
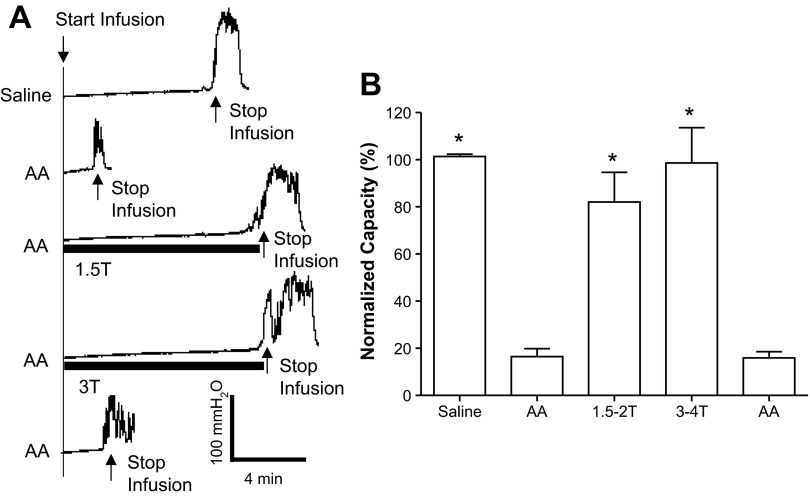
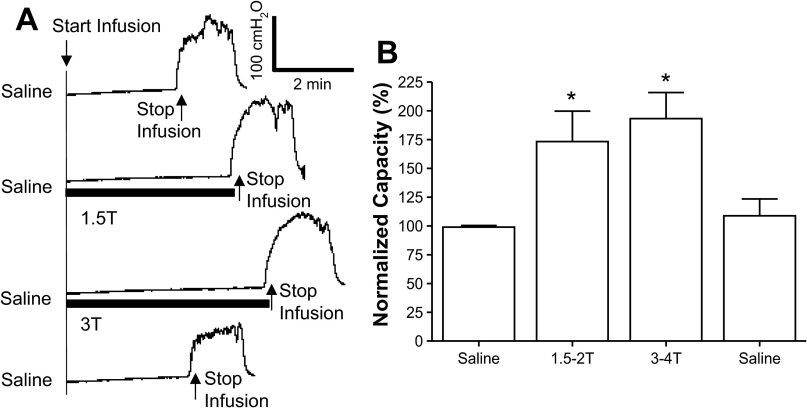
AA irritated the bladder, induced bladder overactivity, and significantly (P < 0.0001) reduced bladder capacity to 16.5 ± 3.3% (1.4 ± 0.3 ml) of the saline control capacity (9.2 ± 1.1 ml, n = 12 cats; Fig. 2). PNS at 1.5–2 and 3–4 T applied during AA CMG significantly increased bladder capacity to 82.0 ± 12% (P = 0.0001) and 98.6 ± 14% (P < 0.0001) of the saline control capacity, respectively. After the stimulation, bladder capacity returned to the prestimulation level (Fig. 2), indicating that there was no poststimulation inhibition.
Fig. 2.
Pudendal nerve stimulation (PNS) inhibited the bladder overactivity caused by 0.25% AA irritation. A: CMG recordings during saline or AA infusion with or without PNS. The PNS duration is indicated by the solid bars under the bladder pressure traces. PNS T was defined as the minimal intensity to induce anal sphincter twitch. T = 0.5 V. Infusion rate = 1 ml/min. B: bladder capacities measured during CMGs under different conditions (n = 12 cats). PNS: 5 Hz, 0.2 ms; T = 0.4–1.4 V. *Significantly different from the AA control before PNS (by one-way ANOVA).
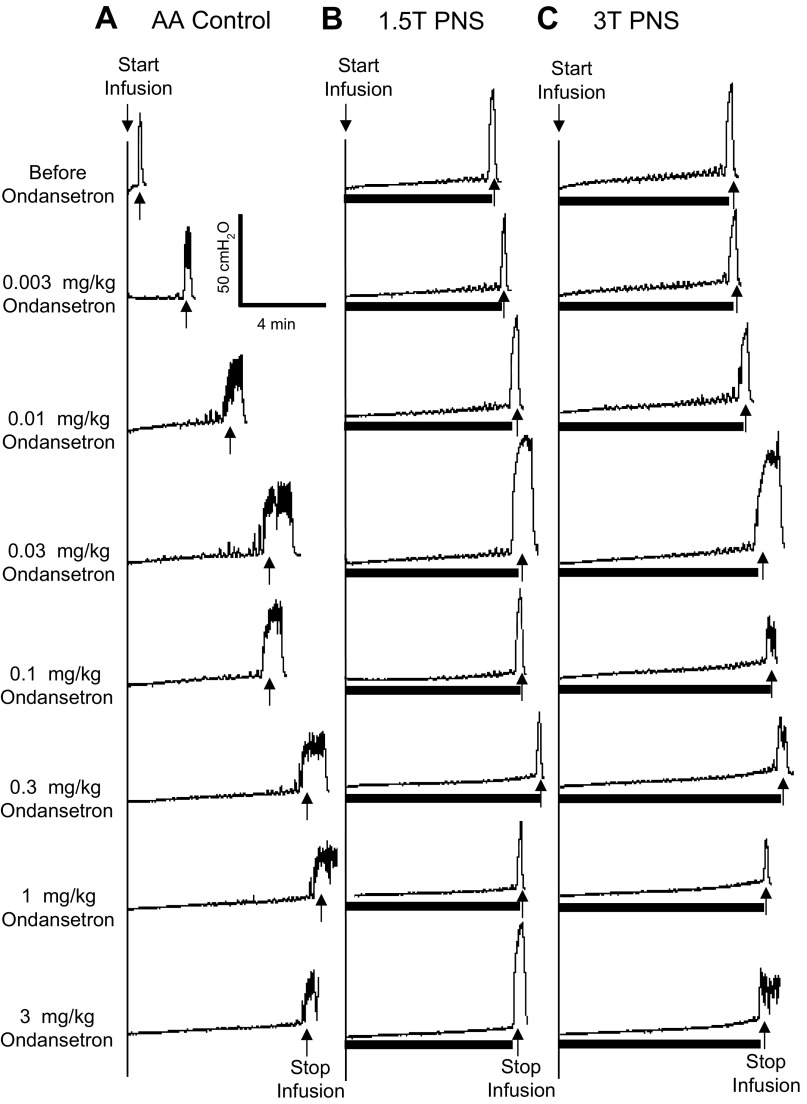
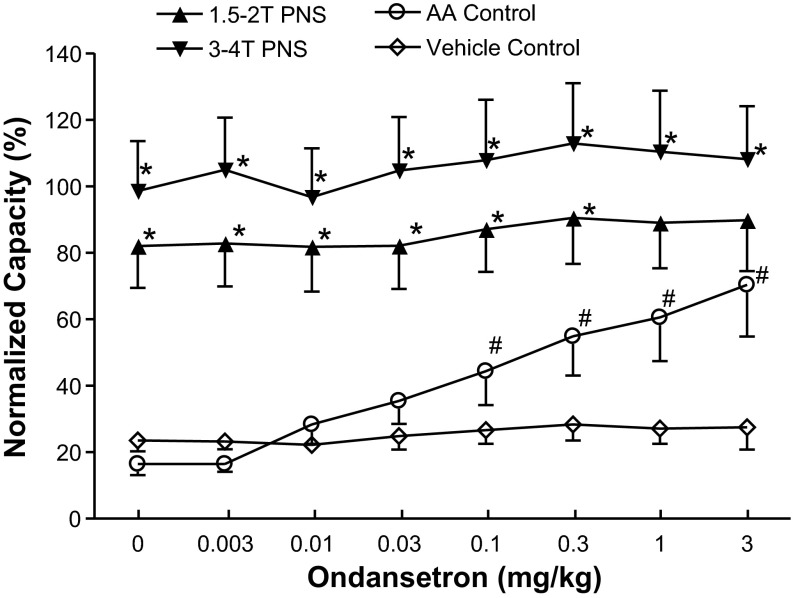
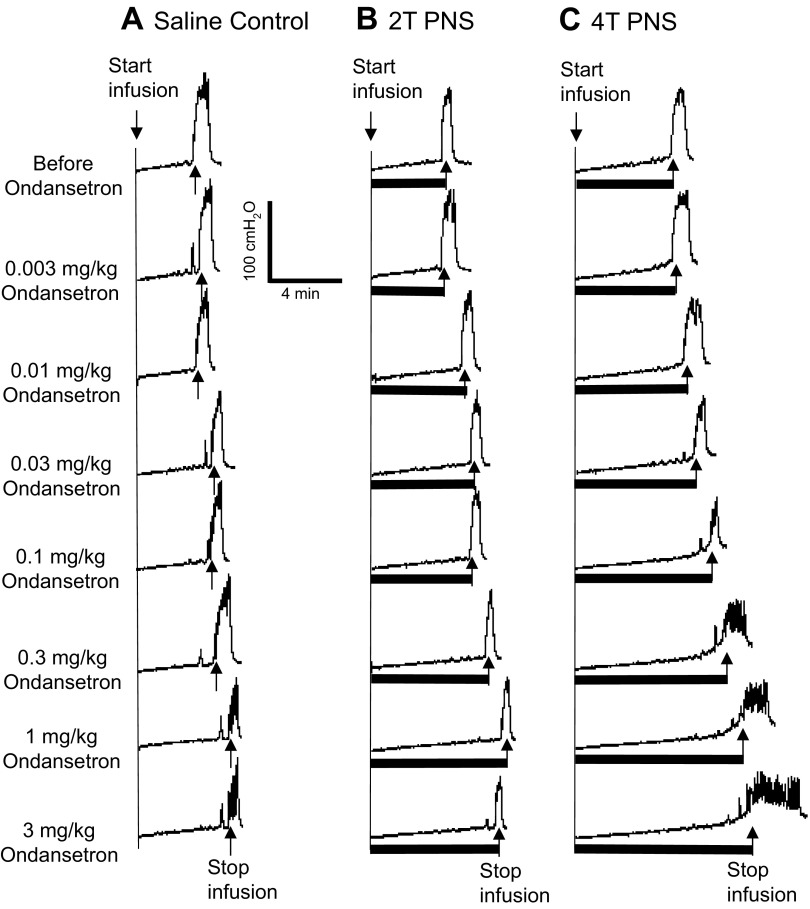
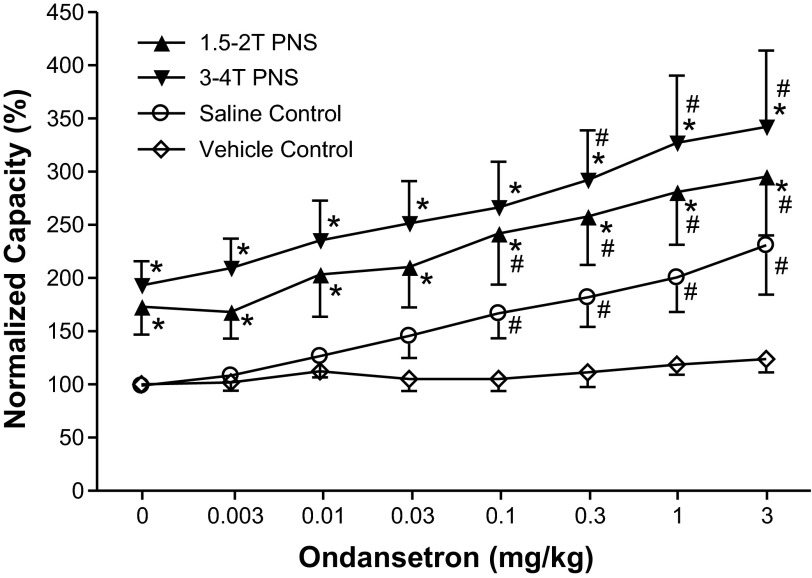
When increasing doses of ondansetron were administered intravenously at 30- to 60-min intervals to obtain cumulative dose-response curves, doses between 0.1 and 3 mg/kg significantly (P < 0.05) increased the control bladder capacity, but the bladder capacity during PNS was not changed (Figs. 3 and 4). However, PNS inhibition (i.e., the difference between the control capacity and the capacity during PNS) was gradually reduced (Figs. 3 and 4). The inhibition induced by low-intensity PNS (1.5–2 T) was lost with ondansetron doses of 1–3 mg/kg (Fig. 4). Although high-intensity PNS (3–4 T) could significantly increase bladder capacity at all doses, the inhibition was progressively and significantly (P < 0.05) reduced at 0.3–3 mg/kg doses of ondansetron (Fig. 4). During vehicle controls, bladder capacity was not changed during the entire period of the experiment if ondansetron was not administered (Fig. 4).
Fig. 3.
Dose-dependent effect of ondansetron on PNS-induced inhibition of bladder overactivity caused by AA irritation. CMGs were performed in sequence from left to right in A–C and from top to bottom. The PNS duration is indicated by the solid bars under the bladder pressure traces. A: control CMGs without PNS. B: CMGs during 1.5-T PNS. C: CMGs during 3-T PNS. PNS: 5 Hz, 0.2 ms; T = 0.4 V. Infusion rate = 1 ml/min.
Fig. 4.
Summarized results of the dose-dependent effects of ondansetron on 1) bladder capacity of the bladder irritated by AA and 2) the PNS-induced increase in bladder capacity at two intensities of stimulation (n = 12 cats). The x-axis indicates intravenous doses of ondansetron and also repeated intravenous injections of vehicle at the same volumes and at the same time intervals as the drug injections (n = 5 cats, vehicle control). Vehicle injections did not change bladder capacity. The bladder capacity was normalized to the saline control capacity before any treatment. PNS: 5 Hz, 0.2 ms; T = 0.4–1.4 V. *Significantly different from the AA control (by two-way ANOVA); #significantly different from the bladder capacity measured before ondansetron treatment (i.e., at 0 mg/kg ondansetron) (by one-way ANOVA). Note that the open symbols represent no PNS during the CMG.
Effect of ondansetron on normal bladder activity and PNS inhibition.
Before ondansetron treatment and during saline infusion, PNS significantly increased bladder capacity to 173.2 ± 26.4% (P = 0.036) at low intensity (1.5–2 T) and 193.2 ± 22.5% (P = 0.008) at high intensity (3–4 T) of the control capacity (6.6 ± 1.3 ml, n = 6 cats; Fig. 5). The bladder capacity returned to the prestimulation level after the termination of PNS (Fig. 5), i.e., no poststimulation effect was observed.
Fig. 5.
PNS inhibited the normal bladder activity induced by saline distention. A: CMG recordings during the saline infusion with or without PNS. The PNS duration is indicated by the solid bars under the bladder pressure traces. T = 0.4 V. Infusion rate = 1 ml/min. B: bladder capacities measured during CMGs under different conditions (n = 6 cats). PNS: 5 Hz, 0.2 ms; T = 0.4–1.4 V. *Significantly different from the saline control before or after PNS (by one-way ANOVA).
Cumulative dosing of ondansetron significantly (P < 0.05) increased the control bladder capacity at doses of 0.1–3 mg/kg but did not change the ability of PNS to inhibit the bladder and increase bladder capacity (Figs. 6 and 7). Compared with the control capacity, PNS significantly (P < 0.05) inhibited the bladder even at the highest dose of ondansetron (3 mg/kg) and increased the bladder capacity to 295.3 ± 55.3% at 1.5–2 T and 342.2 ± 71.5% at 3–4 T. In addition, 3 mg/kg ondansetron significantly (P < 0.05) reduced the amplitude of bladder contractions from 101 ± 10 to 75 ± 4 cmH2O (also see Fig. 6A), whereas 3–4T PNS further reduced the contraction amplitude to 60 ± 6 cmH2O (also see the last row of CMGs in Fig. 6A). A similar effect on contraction amplitude was not observed during AA irritation (Fig. 3). During vehicle controls, bladder capacity was not changed during the entire period of experiment if ondansetron was not administered (Fig. 7).
Fig. 6.
Dose-dependent effect of ondansetron on PNS-induced inhibition of normal bladder activity during saline infusion. CMGs were performed in sequence from left to right in A–C and from top to bottom. The PNS duration is indicated by the solid bars under the bladder pressure traces. A: control CMGs without PNS. B: CMGs during 1.5-T PNS. C: CMGs during 3-T PNS. PNS: 5 Hz, 0.2 ms; T = 0.4 V. Infusion rate = 1 ml/min.
Fig. 7.
Summarized results of the dose-dependent effects of ondansetron on 1) bladder capacity during saline CMGs and 2) the PNS-induced increase in bladder capacity at two intensities of stimulation (n = 6 cats). The x-axis indicates intravenous doses of ondansetron and also repeated intravenous injections of vehicle in the same volumes and at the same time intervals as the drug injections (n = 5 cats, vehicle control). Vehicle injections did not change bladder capacity. PNS: 5 Hz, 0.2 ms; T = 0.4–1.4 V. *Significantly different from the saline control (by two-way ANOVA); #significantly different from the bladder capacity measured before ondansetron treatment (i.e., at 0 mg/kg ondansetron) for different CMG conditions (by one-way ANOVA). Note that the open symbols represent no PNS during the CMG.
Effect of naloxone on bladder activity and PNS inhibition.
Our previous studies (4, 17) have shown that naloxone without ondansetron treatment can significantly reduce bladder capacity during saline infusion but not during AA irritation. In addition, naloxone also plays a minor role in PNS inhibition during saline infusion.
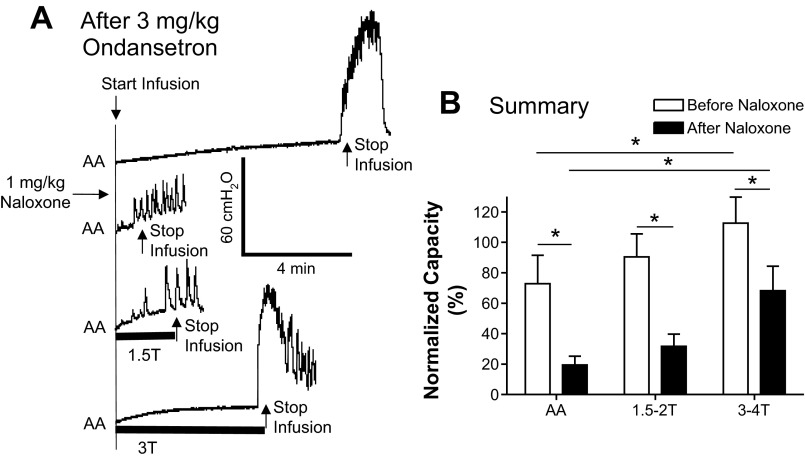
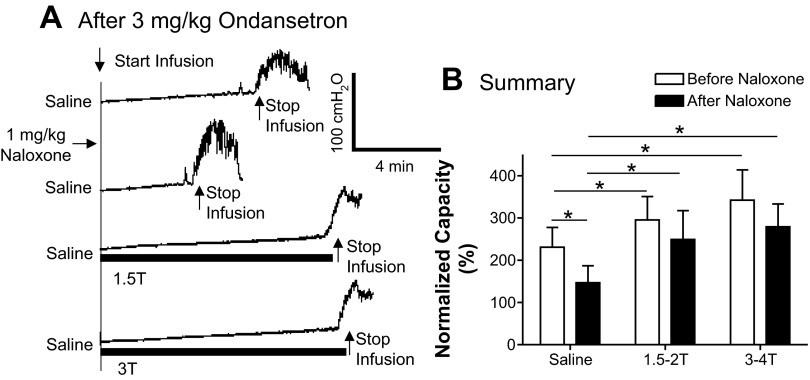
During AA control CMGs after 3 mg/kg ondansetron, naloxone (1 mg/kg) excited the bladder and significantly (P < 0.05) reduced the bladder capacity from 70.3 ± 15.5% to 19.4 ± 5.3% of the saline control capacity (Fig. 8, A and B). In these experiments, PNS significantly (P < 0.05) increased bladder capacity only at high intensity (3–4 T) but not at low intensity (1.5–2 T; Fig. 8A) similar to the effects before naloxone treatment (Fig. 8B). The bladder capacities measured during PNS were significantly (P < 0.05) smaller after naloxone treatment than before naloxone treatment (Fig. 8B), but the percent increase in capacity during high-intensity PNS was not significantly different (40% vs. 49%) before and after naloxone treatment.
Fig. 8.
Effect of naloxone (1 mg/kg) on PNS-induced inhibition of bladder overactivity after 3 mg/kg ondansetron treatment. A: CMG recordings during AA infusion with or without PNS (5 Hz, 0.2 ms; T = 0.5 V). The PNS duration is indicated by the solid bars under the traces. B: bladder capacities measured after 3 mg/kg ondansetron under different CMG conditions before and after naloxone treatment (n = 10 cats). PNS at 1.5–2 T did not significantly change bladder capacity before or after naloxone treatment, whereas PNS at 3–4 T significantly increased bladder capacity before and after naloxone treatment. PNS: 5 Hz, 0.2 ms; T = 0.4–1.4 V. *Significant difference (by one-way ANOVA and two-way ANOVA).
During saline infusion after 3 mg/kg ondansetron, naloxone treatment also excited the bladder and significantly (P < 0.05) reduced the bladder capacity from 230.8 ± 46.6% to 146.6 ± 40.4% of the saline control capacity (Fig. 9, A and B). PNS significantly (P < 0.05) increased bladder capacity at both low (1.5–2 T) and high (3–4 T) intensities (Fig. 9A) similar to the effects before naloxone treatment (Fig. 9B). The bladder capacities measured during PNS were not significantly different before and after naloxone treatment (Fig. 9B).
Fig. 9.
Effect of naloxone (1 mg/kg) on PNS-induced inhibition of normal bladder activity after 3 mg/kg ondansetron treatment. A: CMG recordings during saline infusion with or without PNS (5 Hz, 0.2 ms; T = 1 V). The PNS duration is indicated by the solid bars under the traces. B: bladder capacities measured after 3 mg/kg ondansetron under different CMG conditions before and after naloxone treatment (n = 6 cats). PNS: 5 Hz, 0.2 ms; T = 0.4–1.4 V. *Significant difference (by one-way ANOVA and two-way ANOVA).
DISCUSSION
The effects of ondansetron, a 5-HT3 receptor antagonist, indicate that activation of 5-HT3 receptors is partially involved in the PNS inhibition of AA-induced bladder overactivity (Figs. 3 and 4) but is not involved in the PNS inhibition of normal bladder activity induced by the intravesical infusion of saline (Figs. 6 and 7). Ondansetron also significantly increased bladder capacity in both normal (Figs. 6 and 7) and irritated bladders (Figs. 3 and 4), indicating an excitatory role of 5-HT3 receptors in the micturition reflex pathway. Although naloxone excited the bladder, the PNS-induced increase in bladder capacity remained after naloxone treatment (Figs. 8 and 9), indicating that the activation of opioid receptors does not contribute to the PNS-induced increase in bladder capacity but does have a role in tonic inhibition of the micturition reflex.
A potential problem in interpreting our data arises because ondansetron and PNS both increase bladder capacity. These two effects could occur by the same mechanism, i.e., that both treatments suppress or remove a tonic 5-HT3 receptor-mediated facilitation of the micturition reflex pathway and thereby increase bladder capacity. Thus, the block of PNS inhibition by ondansetron could be explained by the drug eliminating the target for PNS and thereby occluding the effect of PNS. This interpretation of the results would be consistent with the apparent “ceiling effect” shown in Fig. 4 in AA-irritated bladders, where ondansetron dose dependently increased bladder capacity and reduced the change in bladder capacity induced by PNS inhibition while not changing the peak effect of PNS, which remained constant at ∼80% of the saline control capacity. However, several observations led us to conclude that ondansetron suppresses a tonic 5-HT3 receptor-mediated facilitation of the micturition reflex pathway and also independently suppresses a 5-HT3 receptor synaptic mechanism that is essential for PNS inhibition of bladder overactivity.
For example, when the results from AA-irritated bladders in Fig. 4 are evaluated along with data from nonirritated bladders (Fig. 7), it is clear that occlusion does not explain the effects of the drug on PNS inhibition. In cats without bladder irritation, ondansetron more than doubled bladder capacity, presumably by removing a tonic 5-HT3 receptor excitatory input to the micturition pathway but did not suppress PNS inhibition, which increased bladder capacity to 275% of the control capacity. If the effect of ondansetron to increase bladder capacity occluded the response to PNS in AA-irritated bladders, it is reasonable to expect that a similar occlusion would occur in nonirritated bladders; however, it did not occur. The inhibitory effect of ondansetron on bladder capacity suggests that 5-HT3 receptor-mediated tonic facilitation plays an important role in modulating reflex bladder activity under both conditions (irritated and nonirritated), whereas 5-HT3 receptor mechanisms only seem to play a role in PNS inhibition of AA-irritated bladders. This leads us to the conclusion that ondansetron inhibition of reflex bladder activity and PNS inhibition of reflex bladder activity are mediated by different mechanisms and that PNS inhibition of the irritated bladder involves the 5-HT3 receptor.
It is true that ondansetron suppresses bladder overactivity at the same time that it suppresses PNS inhibition of bladder overactivity. This is not surprising if the drug is acting by blocking 5-HT3 receptors in the two pathways, but does not imply that the drug produces the two effects by one mechanism to increase bladder capacity, which, in turn, occludes the response to PNS by producing a ceiling effect. In fact, 3- to 4-T PNS further inhibited the bladder (Fig. 4), indicating that at least 1.5- to 2-T PNS did not reach a ceiling effect.
It is also important to recognize that the 5-HT3 receptor mechanism that regulates the micturition reflex is only facilitatory and not essential because the micturition reflex still occurs after a large dose of ondansetron; only bladder capacity is increased. However, PNS inhibition was blocked by ondansetron. This difference also suggests that the two distinct 5-HT3 receptor mechanisms are involved in 1) the micturition excitatory reflex and 2) the PNS inhibitory pathway.
Our result demonstrating the involvement of 5-HT3 receptors in PNS inhibition of nociceptive C-fiber afferent-mediated bladder overactivity agrees well with previous studies investigating the neuromodulation of somatic nociception. Intrathecal administration of a 5-HT3 receptor antagonist prevented the antihyperalgesia induced by transcutaneous electrical nerve stimulation in rats (25). Other studies have also shown that neuromodulation-induced analgesia significantly depends on descending central serotonergic pathways, which is evidenced by enhanced antinociception during neuromodulation after 5-hydroxytryptophan administration (28), increased 5-HT concentrations in the spinal cord after neuromodulation (29), and elimination of neuromodulation-induced analgesia after systemic 5-HT depletion (35). Since AA activates nociceptive bladder C-fiber afferents and triggers a spinal micturition reflex (12), it is possible that PNS drives the descending 5-HT pathway that activates 5-HT3 receptors to inhibit C-fiber afferent-mediated bladder overactivity in the spinal cord. However, since PNS at high intensity can still inhibit bladder overactivity (Fig. 4) and PNS at both low and high intensities fully inhibits normal bladder activity after ondansetron treatment (Fig. 7), receptors other than 5-HT3 receptors either in the spinal cord or in the brain must also be involved in PNS inhibition. This conclusion is supported by our previous study (15) in cats, which showed that metabotropic glutamate 5 receptors partially mediate pudendal inhibition, suggesting that pudendal neuromodulation of bladder overactivity might use multiple neurotransmitter mechanisms to achieve its clinical efficacy in OAB treatment.
It is known that descending raphe-spinal 5-HT pathways produce an overall inhibitory effect on the micturition reflex (6, 26). However, our results suggest that 5-HT3 receptors play an excitatory role in both spinal and spinobulbospinal micturition reflexes (Figs. 4 and 7). Previous studies (8, 9) in cats have reported that intrathecal administration of zatosetron, a 5-HT3 receptor antagonist, excited the bladder and significantly reduced bladder capacity, indicating that tonic activation of 5-HT3 receptors in the spinal cord mediates inhibition of reflex bladder activity. Therefore, the opposite results obtained in the present study after intravenous administration of the 5-HT3 antagonist ondansetron (Figs. 3 and 7) imply that 5-HT3 receptors in the brain have a tonic excitatory effect on the micturition reflex that overrides the inhibitory effect of spinal 5-HT3 receptors. On the other hand, a 5-HT3 mechanism seems to play a minimal role in the micturition reflex in rats. Intrathecal administration of a 5-HT3 antagonist (33) in rats had no effect on bladder capacity and only a slight decrease in micturition pressure. Intracerebroventricular administration of a 5-HT3 agonist had no effect on either bladder capacity or micturition pressure in rats (14). These results indicate that a 5-HT3 mechanism in different species may play a very different role in the micturition reflex.
The results of this study also suggest that 5-HT3 antagonists such as ondansetron might be useful in OAB treatment (Figs. 4 and 7). Urinary retention is listed as one of the common adverse reactions of ondansetron (7, 13), providing clinical evidence that 5-HT3 antagonists suppress voluntary micturition in humans. The idea of using 5-HT3 antagonists to treat C-fiber afferent-mediated bladder overactivity is also supported by the demonstration that 5-HT3 receptors are located on nociceptive primary afferents in the dorsal horn and facilitate the central processing of afferent input, leading to painful behaviors (10, 36). Activation of 5-HT3 receptors also releases substance P and neurokinin A from primary afferents (20, 27). Therefore, it is possible that 5-HT3 receptors in the spinal cord may also facilitate the nociceptive C-fiber afferent pathway that induces bladder overactivity. Clinical evidence of 5-HT3 participation in nociception has been demonstrated by the use of 5-HT3 antagonists to treat many pain syndromes, such as irritable bowel syndrome, fibromyalgia, migraine, and chronic neuropathic pain (3, 5, 11, 20). Since ondansetron is a US Food and Drug Administration-approved drug for nausea that can suppress voiding within its therapeutic dosage (7, 13), it will be feasible for clinicians to safely test its clinical efficacy as a treatment for OAB. Ondansetron also has a favorable side effect profile (18, 24), which further advocates its potential for OAB treatment if found to be clinically effective.
After AA irritation and ondansetron treatment, naloxone significantly reduced bladder capacity (Fig. 8). However, our previous study (32) showed that after AA irritation without ondansetron pretreatment, naloxone did not alter bladder capacity. This difference suggests that tonic enkephalinergic inhibition of bladder overactivity emerges after block of 5-HT3 receptors, indicating that a 5-HT3 mechanism may suppress tonic enkephalinergic inhibition of bladder reflex activity under AA irritation conditions. During normal reflex bladder activity induced by saline infusion, tonic enkephalinergic inhibition is prominent before (4, 17, 32) and after ondansetron treatment (Fig. 7), indicating that interactions between 5-HT3 and opioid receptor mechanisms are not important in the control of the supraspinal micturition pathway activated by non-nociceptive Aδ-fiber afferents. Similarly, an interaction between 5-HT3 and opioid receptor mechanisms is not essential for PNS inhibition because the inhibition of both normal and OAB activity in animals pretreated with ondansetron was not changed after naloxone treatment (Figs. 8 and 9).
In summary, this study revealed that 5-HT3 receptors are partially involved in PNS inhibition of bladder overactivity, suggesting that pudendal neuromodulation might use multiple neurotransmitters and receptors, including 5-HT3 and metabotropic glutamate 5 receptors (15), to effectively treat OAB. Additionally, we demonstrated that ondansetron, a 5-HT3 receptor antagonist, can inhibit bladder overactivity and may be a potential pharmacotherapy for OAB. Understanding the neurotransmitter mechanisms underlying pudendal neuromodulation may allow the identification of new pharmacological targets or enhance the efficacy of current neuromodulation therapies for OAB (1, 2, 16).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-094905, DK-068566, DK-090006, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. conception and design of research; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urology 63: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Mayer EA, Drossman DA, Heath A, Dukes GE, McSorley D, Kong S, Mangel AW, Northcutt AR. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 13: 1149–1159, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Chen ML, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, Tai C. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol 224: 282–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couturier EG, Hering R, Foster CA, Steiner TJ, Clifford Rose F. First clinical study of the selective 5-HT3 antagonist, granisetron (BRL 43694), in the acute treatment of migraine headache. Headache 31: 296–297, 1991 [DOI] [PubMed] [Google Scholar]
- 6.de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology 59: 30–36, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Epocrates Ondansetron (online). http://www.epocrates.com [22 July 2013]
- 8.Espey MJ, Downie JW. Serotonergic modulation of cat bladder function before and after spinal transection. Eur J Pharmacol 287: 173–177, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Espey MJ, Du HJ, Downie JW. Serotonergic modulation of spinal ascending activity and sacral reflex activity evoked by pelvic nerve stimulation in cats. Brain Res 798: 101–108, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research–evolving concepts in management of pain and inflammation. Eur J Pharmacol 560: 1–8, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Farber L, Stratz TH, Bruckle W, Spath M, Pongratz D, Lautenschlager J, Kotter I, Zoller B, Peter HH, Neeck G, Welzel D, Muller W; German Fibromyalgia Study Group Short-term treatment of primary fibromyalgia with the 5-HT3-receptor antagonist tropisetron. Results of a randomized, double-blind, placebo-controlled multicenter trial in 418 patients. Int J Clin Pharmacol Res 21: 1–13, 2001 [PubMed] [Google Scholar]
- 12.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GlaxoSmithKline Zofran Prescribing Information. Research Triangle Park, NC: GlaxoSmithKline, 2002 [Google Scholar]
- 14.Ishizuka O, Gu B, Igawa Y, Nishizawa O, Pehrson R, Andersson KE. Role of supraspinal serotonin receptors for micturition in normal conscious rats. Neurourol Urodyn 21: 225–230, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol 589: 5833–5843, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazzeri M, Spinelli M. The challenge of overactive bladder therapy: alternative to antimuscarinic agents. Int Braz J Urol 32: 620–630, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189: 1574–1579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marty M, Pouillart P, Scholl S, Droz JP, Azab M, Brion N, Pujade-Lauraine E, Paule B, Paes D, Bons J. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med 322: 816–821, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87: 760–766, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Oehme P, Hilse H, Morgenstern E, Gores E. Substance P: does it produce analgesia or hyperalgesia? Science 208: 305–307, 1980 [DOI] [PubMed] [Google Scholar]
- 21.Onukwugha E, Zuckerman IH, McNally D, Coyne KS, Vats V, Mullins CD. The total economic burden of overactive bladder in the United States: a disease-specific approach. Am J Manag Care 15: S90–97, 2009 [PubMed] [Google Scholar]
- 22.Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int 100: 835–839, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Priestman TJ, Roberts JT, Lucraft H, Collis CH, Adams M, Upadhyaya BK, Priestman S. Results of a randomized, double-blind comparative study of ondansetron and metoclopramide in the prevention of nausea and vomiting following high-dose upper abdominal irradiation. Clin Oncol 2: 71–75, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Radhakrishnan R, King EW, Dickman JK, Herold CA, Johnston NF, Spurgin ML, Sluka KA. Spinal 5-HT2 and 5-HT3 receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain 105: 205–213, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramage AG. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br J Pharmacol 147, Suppl 2: S120–S131, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saria A, Javorsky F, Humpel C, Gamse R. 5-HT3 receptor antagonists inhibit sensory neuropeptide release from the rat spinal cord. Neuroreport 1: 104–106, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Shimizu T, Koja T, Fujisaki T, Fukuda T. Effects of methysergide and naloxone on analgesia induced by the peripheral electric stimulation in mice. Brain Res 208: 463–467, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Sluka KA, Lisi TL, Westlund KN. Increased release of serotonin in the spinal cord during low, but not high, frequency transcutaneous electric nerve stimulation in rats with joint inflammation. Arch Phys Med Rehab 87: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol 20: 327–336, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Tai C, Chen M, Shen B, Wang J, Roppolo JR, de Groat WC. Irritation induced bladder overactivity is suppressed by tibial nerve stimulation in cats. J Urol 186: 326–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Testa R, Guarneri L, Angelico P, Velasco C, Poggesi E, Cilia A, Leonardi A. Effect of different 5-hydroxytryptamine receptor subtype antagonists on the micturition reflex in rats. BJU Int 87: 256–264, 2001 [DOI] [PubMed] [Google Scholar]
- 34.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama A, Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Woolf CJ, Mitchell D, Barrett GD. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain 8: 237–252, 1980 [DOI] [PubMed] [Google Scholar]
- 36.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22: 1010–1019, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, Mally AD, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. Am J Physiol Renal Physiol 302: F1576–F1582, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]