Abstract
The rat kidney ablation and infarction (A/I) model of subtotal or 5/6th nephrectomy is the most commonly studied model of nondiabetic chronic kidney disease (CKD). The A/I kidney at 1 wk exhibits reductions in kidney function, as determined by glomerular filtration rate, and diminished metabolic efficiency as determined by oxygen consumption per sodium transport (QO2/TNa). As renoprotective AMPK activity is affected by metabolic changes and cellular stress, we evaluated AMPK activity in this model system. We show that these early pathophysiological changes are accompanied by a paradoxical decrease in AMPK activity. Over time, these kidney parameters progressively worsen with extensive kidney structural, functional, metabolic, and fibrotic changes observed at 4 wk after A/I. We show that induction of AMPK activity with either metformin or 5-aminoimidazole-4-carboxamide ribonucleotide increases AMPK activity in this model and also corrects kidney metabolic inefficiency, improves kidney function, and ameliorates kidney fibrosis and structural alterations. We conclude that AMPK activity is reduced in the subtotal nephrectomy model of nondiabetic CKD, that altered regulation of AMPK is coincident with the progression of disease parameters, and that restoration of AMPK activity can suppress the progressive loss of function characteristic of this model. We propose that induction of AMPK activity may prove an effective therapeutic target for the treatment of nondiabetic CKD.
Keywords: adenosine monophosphate, fibrosis, glomerular filtration rate, kidney metabolic efficiency, oxygen consumption
chronic kidney disease (ckd) is characterized by a progressive loss of kidney function. CKD patients exhibit a high morbidity and mortality primarily due to a high incidence of cardiovascular disease. In spite of improvements in the therapy for hypertension, bone disease, and metabolic acidosis, there are currently no treatment options that effectively control the rate of CKD disease progression. Significant alterations in hemodynamics, structure, and metabolism are components contributing to the progressive deterioration of kidney function. The kidney ablation and infarction (A/I) model of subtotal nephrectomy, also known as 5/6th nephrectomy and remnant kidney, is the most commonly studied nondiabetic model of CKD. It has been widely utilized to investigate different aspects of the pathogenesis of CKD since 1951 (40). In the normal kidney, oxygen consumption (QO2) correlates with changes in total sodium (Na) absorption and, thus, with the energy required for transport. We have shown that A/I results in diminished metabolic efficiency, as determined by oxygen consumption per sodium transport (QO2/TNa). AMPK activity has been associated with beneficial cellular effects in other model systems (4, 9, 15, 20, 36, 53), and as the activity of AMPK also correlates with cellular energy status, we decided to evaluate AMPK in this model.
AMPK is an evolutionarily conserved serine/threonine kinase that is ubiquitously expressed in mammalian tissues and is a major regulator of cellular and whole-body energy homeostasis (3, 48). AMPK functions as an αβγ heterotrimer with phosphorylation at threonine-172 (Thr172) in the α-catalytic subunit, a key mechanism regulating AMPK activation in all tissues (23, 25). An increase in cellular AMP:ATP ratio activates AMPK. This can occur in response to nutritional status or environmental stress, including hypoxia, ischemia, and heat shock. Once activated, AMPK decreases energy demands by downregulating ATP-utilizing pathways and upregulating ATP-generating pathways. This includes decreasing gluconeogenesis and cellular synthesis of fatty acids, cholesterol, and proteins, and increasing glucose uptake and fatty acid oxidation (22, 55). AMPK activation also induces a number of cellular protective mechanisms to suppress oxidative stress, apoptosis, and the accumulation of damaged proteins and organelles (6, 21, 24, 26). Diseased organs expressing decreased activity of AMPK are associated with altered regulation of these networks, such as lipid and protein metabolism, and induction of AMPK exerts protective effects on these organs (1, 9, 20, 31, 45, 53, 56). The impact of AMPK activity in the A/I model has yet to be investigated.
Our previous studies (11, 14) show that the major characteristics of A/I CKD are disorders in energy utilization, i.e., hypoxia and metabolic inefficiency, accompanied by reductions in kidney function and later progression of kidney fibrosis and structural alterations. Two treatments, ANG II blockade with captopril/losartan and hypoxia inducible factor induction with dimethyloxallyl glycine, improved kidney metabolism, and kidney function in A/I CKD (11). As these pathological changes would be expected to affect the energy regulator AMPK, we decided to evaluate AMPK activity in our model. This study examines AMPK activity in A/I kidney to determine whether changes in enzymatic activity correlate with the changes in kidney metabolism and kidney function. We further determine whether restoration of AMPK activity can provide beneficial, renoprotective effects. This is the first investigation of AMPK activity and its impact on the aberrant physiological parameters that characterize this CKD model and offers a novel therapeutic target with the potential of altering disease progression.
MATERIALS AND METHODS
Animal model and treatments.
All experimentation was conducted according to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Protocols were approved in advance by the Department of Veterans Affairs San Diego Healthcare System Institutional Animal Care and Use Committee. Male Wistar rats weighing 225–250 g were purchased from Harlan. The rat kidney A/I surgery was performed under sterile conditions. After anesthesia with pentobarbital sodium (50 mg/kg ip), the hair on two flanks was removed, and surgical areas were cleaned with 10% povidone-iodine solution followed by 75% ethanol. The rat was placed with the left side on a heating pad with medium heat, and a right flank skin incision (1 to 1.5 cm long) was made. The muscle was then clamped by inserting two small surgical hemostats inserted through a small hole before cutting to prevent bleeding. The right kidney was carefully exposed with a small forceps. After separating the adrenal gland, the right kidney pedicle was ligated with 4–0 silk suture, and the right kidney was then removed. The muscle layer of the wound was sewn, and the skin was closed with chromic gut suture. The rat's position was changed with the right side now on the heating pad. A left flank incision was made and the left kidney was retracted out of the peritoneal space to expose the renal artery. Two branches of the left renal artery were ligated with 4–0 silk sutures. The left kidney was then gently retracted back into the body, and the incision was closed. Sham-operated rats underwent anesthesia and manipulation of the kidney pedicles on both sides, without removal of any kidney mass. The rats were kept warm until ambulatory.
Four groups of rats were used for the 7-day study: 1) normal (n = 6), 2) untreated A/I rats (n = 8), 3) A/I rats (n = 8) treated with metformin (250 mg·kg−1·day−1 gavage), and 4) A/I rats (n = 8) treated with 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR; 500 mg·kg−1·day−1 ip injection). All of the treatments started on the A/I surgery day. For the 30-day study, the drug dose for metformin was also 250 mg·kg−1·day−1 via gavage.
Kidney function measurement and oxygen consumption calculation.
In vivo kidney function and kidney oxygen consumption were measured as previously described (11–14). Briefly, rats were anesthetized with Inactin (100 mg/kg ip) and placed on a temperature-controlled table to maintain body temperature at 37°C. After cannulation of trachea, left jugular vein, left femoral artery, and urinary bladder, the left renal blood flow (RBF; ml/min) was monitored with a perivascular ultrasonic transit time flow probe (Transonics T420; Ithaca, NY). Systemic blood pressure and RBF were recorded after the animals were allowed 60 min for stabilization with the flow probe in place. Glomerular filtration rate (GFR) was measured by clearance of [3H]inulin in Ringer solution (111.23 mM NaCl, 4.69 mM KCl, and 29.76 mM NaHCO3) at 12 μCi·1.5 ml−1·h−1. Blood samples were taken from the femoral artery and renal vein for measurements of total arterial blood hemoglobin (tHb), O2Hb, Po2, Pco2, pH, [Na+], [K+], and [HCO3−] with a color spectrophotometer, 682 CO-Oximeter (Instrumentation Laboratory, Lexington, MA). O2Content (O2Ct) was calculated by this formula: O2Ct (ml/ml blood) = (1.39 × tHb × O2Hb + Po2 × 0.003)/100. The total left kidney (QO2) was calculated as RBF × (arterial O2Ct − renal venous O2Ct). Sodium transported (TNa) equals total sodium filtered (GFR × PNa) minus the sodium (Na+) excreted in the urine.
Protein expression and immunoblotting.
Kidney cortical tissues were homogenized in buffer [10 mM Tris, pH 8, 5 mM EDTA, 5 mM EGTA, 150 mM NaCl, 0.1% NP-40 and 1 mM PMSF] with complete protease inhibitor cocktail (Roche, Indianapolis, IN) and phosphatase inhibitors followed by incubation on ice for 10 min. After centrifugation, the supernatant was collected. The protein concentrations were measured by Bio-Rad protein assay reagents (Bio-Rad, Hercules, CA). Samples were denatured in Laemmli buffer and subjected to SDS-PAGE. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA), which were incubated in blocking buffer (5% milk, 20 mmol/l Tris·HCl, pH 7.5, 150 mmol/l NaCl, 0.1% Tween 20) for 1 h and then incubated overnight in cold room with primary antibodies, anti-p-AMPK-α (Thr172, cell signaling), anti-AMPK-α1 (Cell Signaling Beverly, MA), anti-pACC and anti-ACC (Cell Signaling), respectively, followed by the secondary antibody incubation for 1 h at room temperature. Signals were detected by a chemiluminescence method (ECL plus; Amersham Biosciences, Piscataway, NJ) using horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Evaluating expressions of GAPDH, or β-tubulin or β-actin on the same membrane was utilized for normalization of gel loading and transfer efficiency. Quantification of protein expression was performed using Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD).
Histologic analysis.
Kidneys were perfused with cold PBS (∼30 ml) to flash the blood out, fixed with 4% paraformaldehyde in situ by cannulation of the abdominal aorta, suspended in 4% formaldehyde in PBS for 24 h, and then embedded in paraffin. Sections (5 μm thick) were used for Masson's trichrome stain to determine the extent of glomerulosclerosis and tubulointerstitial fibrosis. Quantification of tissue fibrosis was based on 20 fields (10 from the kidney cortex and 10 from the kidney medulla) taken at random from each kidney slide of six separate kidney samples per group (60 images per group). The percentage of glomerular sclerosis was counted on each image (numbers of glomeruli sclerosed/total numbers of glomeruli × 100%). Percentage of cortical or medulla fibrosis was counted in each image with a grid sheet (total area in each image as 100).
Statistical analysis.
Results were shown as means ± SE. Statistics was done by one-way ANOVA followed by Bonferroni t-test using SPSS Software. The null hypothesis was rejected when P < 0.05.
RESULTS
A/I affects rat kidney AMPK activity.
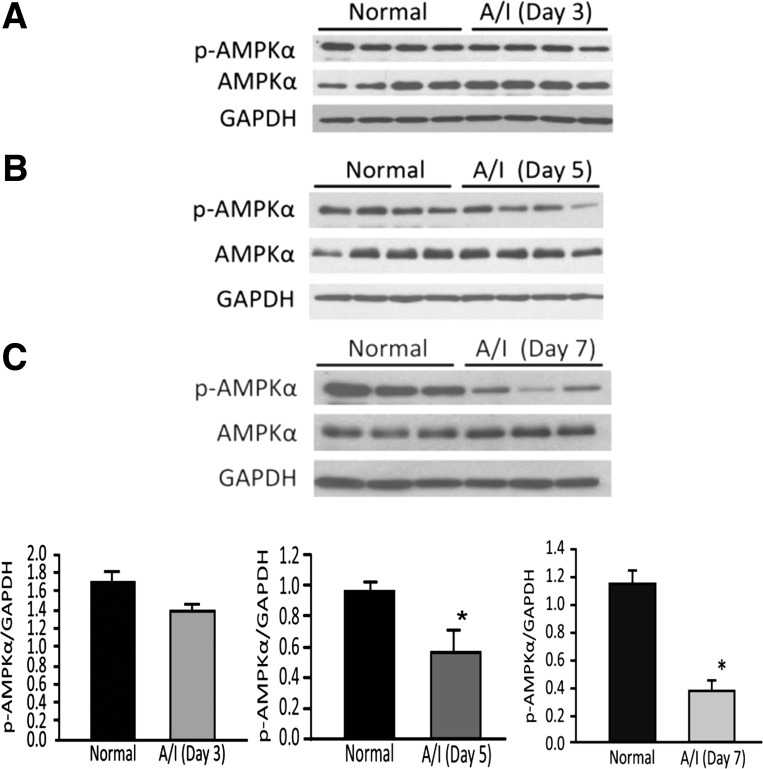
Because AMPK activity has been associated with metabolic changes and cellular stress in other model systems (20, 57), we first evaluated the status of AMPK expression and activity in the A/I model. To determine AMPK activity, we evaluated the activated, phosphorylated state of the enzyme and total AMPK expression using immunoblotting. AMPK phosphorylated at threonine-172 (p-AMPK) was temporally evaluated from 3, 5, and 7-day A/I animals relative to their normal, non-A/I counterparts. We did not observe a significant reduction in p-AMPK in the A/I kidneys by day 3 (Fig. 1A), but the decrease became significant by day 5 (Fig. 1B) and decreased further by day 7 after subtotal nephrectomy (Fig. 1C). Total AMPK values did not significantly change.
Fig. 1.
Reduction of AMPK activity in ablation and infarction (A/I) kidney cortex. Each immunoblot was sequentially probed with three antibodies to p-AMPKα (Thr172), AMPKα1, and anti-GAPDH, respectively. AMPK phosphorylation at threonine-172 (Thr172) in the α-subunit is a key regulator of AMPK activation and is an established marker for activated AMPK. The anti-AMPKα antibody recognizes total AMPKα protein. Anti-GAPDH or anti-β-actin antibodies were used for normalization of sample loading and transfer efficiency. AMPK activity tends to decrease in the A/I kidney relative to the normal kidney by day 3 after A/I (A), but is not significant. This decrease becomes significant by day 5 (B) after the injury and progresses in day 7 (C). Respective graphs are below the immunoblots. Both p-AMPK and the p-AMPK/AMPK ratio yielded similar results. Data are expressed as means ± SE. *P < 0.01 vs. normal; n = 3 or 4.
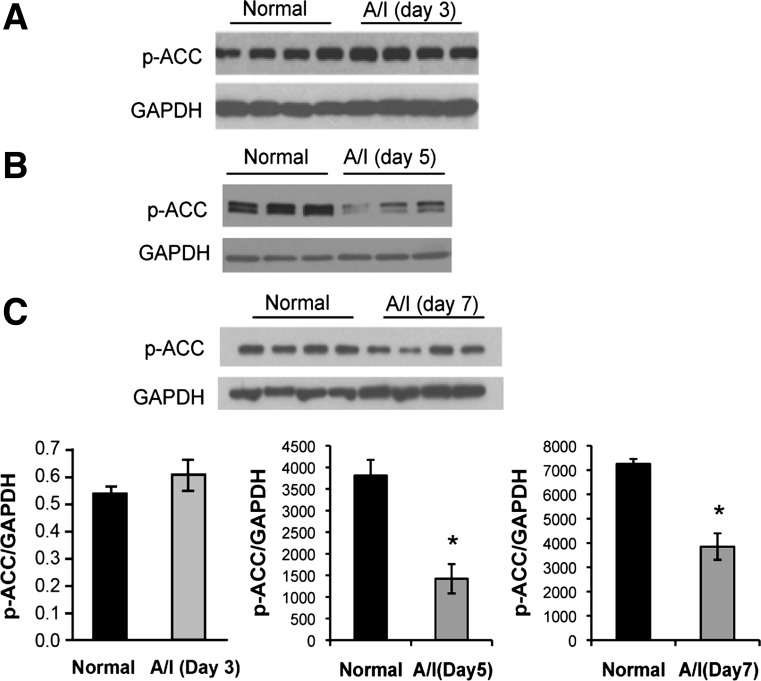
Acetyl CoA carboxylase (ACC) is a downstream target of AMPK. Thus, we evaluated phosphorylated acetyl CoA carboxylase (p-ACC) to confirm p-AMPK expression as representative of enzyme activity. At day 3 A/I, we observed no changes in p-ACC (Fig. 2A), yet at day 5 A/I, where we observed significance in p-AMPK, p-ACC is also reduced (Fig. 2B). Thus, the phosphorylation of AMPK corresponds with the activity of the enzyme in our model system.
Fig. 2.
AMPK activity is confirmed with phosphorylation of acetyl-CoA carboxylase (p-ACC). ACC is directly phosphorylated by AMPK, which inhibits the enzyme's activity. The phosphorylation of this key substrate is reflective of AMPK activity. There were no changes in ACC phosphorylation in day 3 A/I kidney when compared with the normal (A). p-ACC was significantly decreased at day 5 (B) and day 7 (C) after the injury where the p-AMPK (Fig. 1) was significantly reduced. Respective graphs are below the immunoblots. Data are expressed as means ± SE. *P < 0.01 vs. normal; n = 3 or 4.
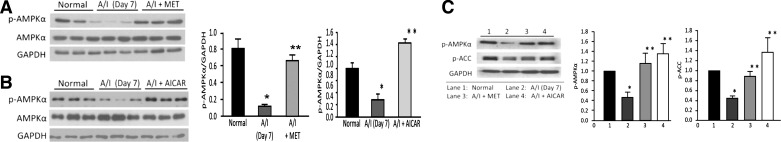
To determine whether we could reinstate AMPK activity in A/I rats, we administered metformin or AICAR, potent activators of AMPK (46, 49, 61). Metformin was selected since it is an approved medication that has been widely used as a first-line therapy for Type 2 diabetes, whereby induction of AMPK activity is required for the suppressive effects of this agent on liver gluconeogenesis (27). AICAR is an adenosine analog that is a more selective AMPK agonist, which binds the enzyme mimicking the effects of AMP on AMPK (7). We administered metformin at an intermediate range, 250 mg·kg−1·day−1 (gavage) and AICAR at 500 mg·kg−1·day−1 ip. In the kidney ischemia-reperfusion model of acute kidney injury, 500 mg/kg concentration of AICAR was effective in correcting kidney physiology and reducing the inflammatory response (31). Metformin is a widely used therapeutic to treat Type 2 diabetes, but the risk of lactic acidosis limits its clinical utilization for CKD patients, even though that concern has been largely confuted (42). A/I rats receiving metformin exhibited no changes in blood pH (normal, 7.43 ± 0.02; A/I, 7.42 ± 0.01) or lactate (normal, 1.56 ± 0.06; A/I, 1.71 ± 0.12 mmol/l). AMPK activity in A/I rats was reinstated to normal levels by administration of AMPK agonists metformin (Fig. 3A) or AICAR (Fig. 3B) at the 7-day time point. We then evaluated p-ACC at this 7-day time point with or without the administration of metformin and AICAR. Phosphorylation of ACC correlated with the p-AMPK values, validating the induction of AMPK activity in A/I kidneys in response to both metformin and AICAR (Fig. 3C).
Fig. 3.
Agonist induction of AMPK activity in A/I kidney cortex. Each immunoblot was sequentially probed with three antibodies to p-AMPKα (Thr172), AMPKα1, and GAPDH, as in Fig. 1. This series was performed at day 7 with treatment groups undergoing A/I. As with Fig. 1, the day 7 A/I kidneys exhibited a substantial decrease in AMPK activity relative to the normal animals. This reduction in AMPK activity was normalized by AMPK activation with metformin (MET) (A), or 5-aminoimidazole-4-carboxamide-1 riboside (AICAR) (B). Respective graphs are below the immunoblots for A and B. C: representative blot showing the correlation of p-AMPK with p-ACC at this 7-day time point to validate agonist induction of AMPK activity in A/I kidneys. Data are expressed as means ± SE. *P < 0.01 vs. normal. **P < 0.01 vs. A/I; n = 3.
Effects of AMPK on kidney metabolic efficiency and function in A/I.
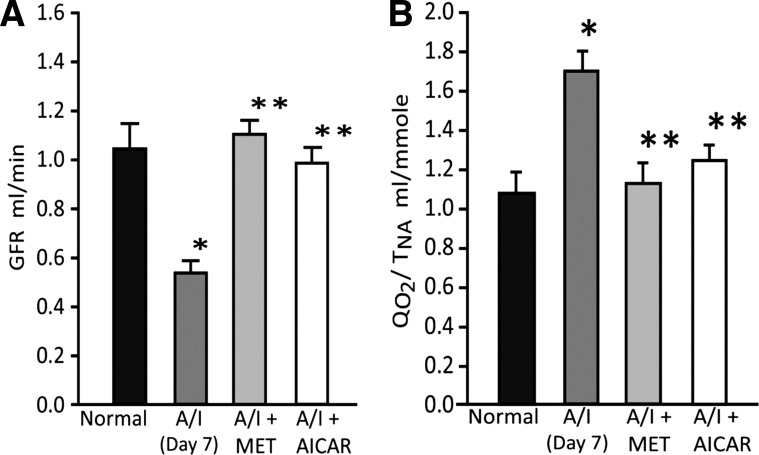
When compared with the normal kidney, 7-day A/I rats demonstrate significant reductions in kidney function, as reflected by declines in GFR, and in kidney metabolic efficiency, as indexed by an elevated QO2/TNa, (Fig. 4, A and B). These findings are consistent with our previous report (11). This reduced metabolic efficiency increases kidney hypoxia, and the requirement for AMPK activity in the A/I kidney would appear even more necessary. To determine whether restoring AMPK activity observed in A/I back to control levels might be renoprotective, we evaluated the effects of induction of AMPK activity on kidney pathophysiology. As demonstrated in Fig. 3, both metformin and AICAR reinstate AMPK activity in A/I animals to control levels. This agonist induction of AMPK activity normalizes both kidney function, GFR (Fig. 4A), and metabolic efficiency, QO2/TNa (Fig. 4B) in 7-day A/I animals. The effects of metformin and AICAR are equivalent.
Fig. 4.
AMPK agonists restore renal function and renal metabolic efficiency. Compared with the normal animals, untreated A/I rats exhibited a significant decrease in glomerular filtration rate (A), representative of kidney function, and, an increase in the ratio of total renal oxygen consumption factored by the total amount of sodium reabsorbed (QO2/TNa) (B), indicating a decrease in renal metabolic efficiency, at 1 wk after injury. Agonist induction of AMPK activity with metformin (MET) or AICAR, significantly improved renal function (A) and corrected renal metabolic efficiency (B). *P < 0.01 vs. normal. Data are expressed as means ± SE. **P < 0.01 vs. A/I.
Effects of AMPK on kidney histology and fibrosis in A/I.
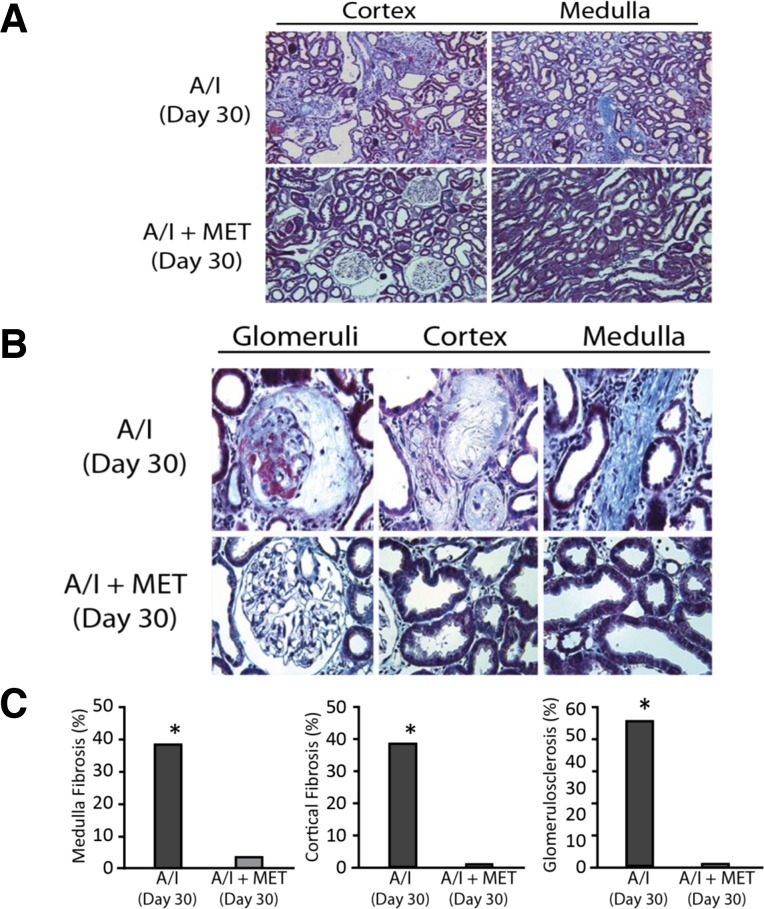
Kidney fibrosis and histologic alterations are principal downstream complications in this model and reflect disease progression. To determine whether correcting early physiological parameters would continue in the longer term and whether downstream complications indicative of disease progression would be affected, we evaluated at a 30-day time point. As shown in Table 1, at 30 days after A/I, kidney function and metabolic efficiency worsened, relative to 7 days, and this correlated with extensive kidney fibrosis, kidney lesions, and morphologic changes (Fig. 5). Metformin normalized GFR and QO2/TNA at this extended time point (Table 1). Trichrome staining shows extensive kidney fibrosis in glomeruli (54.9%), kidney cortex (39%), and medulla (38.7%) (Fig. 5). Restoration of AMPK activity by metformin ameliorates kidney fibrosis, as well as structural and morphologic abnormalities observed in the A/I rats (Fig. 5). The blood pH did not significantly deviate from normal in A/I (7.40 ± 0.015) and A/I animals administered metformin (7.43 ± 0.007) for 30 days.
Table 1.
Kidney function, kidney metabolic efficiency, blood pressure, and body weight in 30-day A/I rats
| GFR, ml/min | RBF, ml/min | QO2, ml/min | QO2/TNa, ml/mmol | BP, mmHg | Body Weight, g | |
|---|---|---|---|---|---|---|
| Normal, n = 5 | 1.63 ± 0.04 | 9.72 ± 0.53 | 0.22 ± 0.01 | 1.03 ± 0.07 | 113 ± 2 | 367 ± 18 |
| 30 Day A/I, n = 5 | 0.34 ± 0.09 | 2.86 ± 0.42 | 0.07 ± 0.01 | 2.03 ± 0.41 | 140 ± 5 | 241 ± 16 |
| P | <0.01 vs. I | <0.01 vs. I | <0.01 vs. I | <0.01 vs. I | <0.1 vs. I | <0.01 vs. I |
| 30 Day A/I + Met, n = 8 | 1.17 ± 0.14 | 7.39 ± 0.84 | 0.24 ± 0.01 | 1.22 ± 0.09 | 130 ± 6 | 317 ± 11 |
| P | <0.05 vs. II | <0.01 vs. II | <0.01 vs. II | <0.05 vs. II | <0.02 vs. II |
All values are expresssed as means ±SE. A/I, kidney ablation and infarction; GFR, glomerular filtration rate; RBF, renal blood flow; QO2, renal oxygen consumption; TNa, amount of sodium reabsorbed (an elevated QO2/TNa reflects a decrease in renal metabolic efficiency); BP, mean blood pressure; Met, metformin.
Fig. 5.
AMPK agonist abrogates fibrosis and kidney lesions at 30 days A/I. A: low power light microscopy of a representative Masson's trichrome stained 30-day A/I kidney reveals extensive kidney fibrosis in both cortex and medulla. B: high-power light microscopy demonstrates fibrosis in glomeruli and interstitium of cortex and medulla in the 30 day A/I kidney, as well as glomerular and tubular lesions. AMPK agonist metformin (MET) prevents fibrosis and lesions in A/I glomeruli and interstitium (A and B). C: graphic representation and quantification of fibrosis. Data are expressed as means ± SE. *P < 0.01 vs. all treated groups.
DISCUSSION
This study demonstrates that the A/I rat kidney exhibits an early and progressive temporal reduction of AMPK activity coincident with reductions in kidney metabolic efficiency and kidney function leading to kidney fibrosis and that agonist induction of AMPK activity improves these pathophysiological alterations. These data imply AMPK dysfunction as a novel pathway involved in the early progression of the A/I model of CKD.
The kidney's unique structure and its characteristics of oxygen consumption should result in constitutive expression of AMPK activity. Under physiological conditions, renal oxygen consumption is an incremental function of Na+ reabsorption. A preglomerular oxygen diffusion shunt produces a reduction in Po2 in the cortex (40–45 mmHg) that is even lower than the values in the renal vein (30, 43, 58). Furthermore, a countercurrent exchange of O2 between the ascending and descending vasa recta vessels located in the medulla leads to a further gradual reduction in Po2 within the medulla with the lowest values for Po2 residing in the papilla (10 mmHg). Additionally, significant active tubular reabsorption in the thick ascending limb, located at the outer medulla, makes this region particularly sensitive to oxidative stress. This distinct kidney structure and physiology place the kidney on the border of hypoxia, which should normally dictate activation of the energy regulator AMPK. Our results confirm that AMPK is expressed in the normal kidney, as previously reported by others (2, 18, 47).
AMPK in diseased organs is associated with dysregulation of lipid and protein metabolism (1, 20, 56), including kidney models of adiponectin deficiency, and diabetes, as well as being associated generally with a high-fat diet (9, 45). Induction of AMPK activity can exert protective effects on these organs. In addition, AMPK activity serves a renoprotective function in podocyte cells (5, 16, 17, 38, 39, 45) and in mesangial cells, AMPK can impact mesangial cell hypertrophy and inflammatory response (15, 37, 62). Whether AMPK contributes to the pathophysiology of kidney disease induced by A/I is not known. Because the A/I kidney is characterized by high oxygen consumption and hypoxia (13, 33, 35), oxidative stress, and inflammation (19, 51, 54), a requirement for augmented AMPK activity appears logical. Such conditions of cellular stress might be expected to increase the activity of AMPK in the A/I kidney, and yet we observe a decrease in enzymatic activity. There is a myriad of other effects impacting the kidney in this model, including, but not limited to, growth factors (29); high glucose, which suppresses AMPK activity in mesangial cells in culture (32); and the renin-angiotensin system in the A/I kidney, which also suppresses enzyme activity in podocytes; and in high-fat diet-stressed kidneys also stressed by salt loading (5, 10), and this is in accord with other observations of ANG II in brain and skeletal muscle lowering AMPK activity (50, 60). Conversely, ANG II administration increased AMPK phosphorylation in kidney and pulmonary artery endothelial cells, the latter attributed to the AT2 receptor (8, 41). The decrease in AMPK activity, therefore, implies an overriding effector(s) suppressing the upregulation of AMPK activity in this model, suggesting a complex and possibly conflicting balance of regulators of AMPK enzymatic activity.
Our results demonstrate that induction of AMPK activity by two agonists that operate via different mechanisms provide early beneficial effects in the A/I model of CKD. Specifically, these agonists increased AMPK activity and significantly normalized QO2/TNa and GFR at 1 wk A/I. These observations become more critical and relevant if they modify the likelihood for progressive loss of function in this model of CKD. When the longer-term effects of therapy and structural alterations are evaluated 4 wk after creation of the A/I model, we find that metformin prevented the progressive reduction of GFR and RBF. The reduction in metabolic efficiency worsens with time in the untreated A/I kidney, whereas metformin successfully normalizes oxygen consumption at this 4-wk time point as well. In addition to the progression of events monitored from week 1 to week 4, we evaluated histologic and fibrotic changes as downstream consequences in this model to further validate disease progression. The untreated A/I kidney manifested marked interstitial fibrosis, glomerulosclerosis, loss of proximal tubule brush border, and other lesions at this later time. Agonist induction of AMPK activity largely prevented these progressive structural, morphologic, and fibrotic changes. These data imply that damping the early metabolic and functional changes caused by A/I can exert beneficial effects by preventing disease progression. Taken together, these data support the observations that the hemodynamic and metabolic changes predict and parallel the progressive scarring of the A/I kidney and that AMPK activity may be an integral part of this response. The use of two different agonists with differing mechanisms and secondary effects implies that their common factor, activation of AMPK in this study, is the effector. Further validation of this mechanism will require knockout animals. Since AMPK deficiency is lethal, a knockout of a specific subunit isoform or a selective cell type may complicate defining the extent of AMPK involvement. Further studies will also be necessary to determine how the downstream effector pathways of AMPK are involved in the observed attenuation of the pathophysiological functional and metabolic changes wrought by A/I. In all, these results are in accord with the hypothesis of a constitutive and regulatory role of AMPK in the normal kidney.
It has been reported that kidney AMPK is inactivated in both animal models of Types 1 and 2 diabetes, and this activation of AMPK attenuates diabetic glomerular and tubular injury (2, 4, 28, 36, 59). Activation of kidney AMPK by AICAR or metformin also produces beneficial effects in models of adiponectin deficiency, diabetics with high-fat diets (9, 45), polycystic kidney disease (52, 53), and in acute kidney ischemia (34, 44). The present study demonstrates that an early and persistent reduction of AMPK activity closely correlated with reductions of kidney function, kidney metabolic efficiency, and later kidney fibrosis in the A/I CKD model, and different treatments that restore the kidney AMPK activity correct these abnormalities. These data from various kidney-related models suggest that decreased kidney AMPK activity may be a common pathway for the progression of CKD.
In conclusion, our findings demonstrate that AMPK activity is reduced in the A/I kidney coincident with declines in metabolic efficiency and kidney function and later progression to renal lesions and fibrosis. These landmarks of disease progression are prevented by reinstating AMPK activity to normal levels.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.S. and R.C.B. conception and design of research; J.S. and A.D. performed experiments; J.S. analyzed data; J.S. and R.C.B. interpreted results of experiments; J.S. and A.D. prepared figures; J.S., K.S., R.C.B., and A.D. drafted manuscript; J.S., K.S., and R.C.B. edited and revised manuscript; J.S., K.S., and R.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We wish to thank John Reeves for his assistance in preparing this manuscript. This work is supported by National Institutes of Health Grants DK-02920, DK-56248, DK-28602, the UAB-UCSD O'Brien Center for Acute Kidney Injury Research P30DK079337, and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
REFERENCES
- 1.Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res 90: 224–233, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Cammisotto PG, Londono I, Gingras D, Bendayan M. Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol 294: F881–F889, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: nature's energy sensor. Nat Chem Biol 7: 512–518, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Chang CY, Wu YT, Huang JP, Yen TH, Hung LM. Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci 18: 47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JY, Ha TS, Park HY, Ahn HY. Angiotensin II suppresses adenosine monophosphate-activated protein kinase of podocytes via angiotensin II type 1 receptor and mitogen-activated protein kinase signaling. Clin Exp Nephrol 17: 16–23, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Colombo SL, Moncada S. AMPKα1 regulates the antioxidant status of vascular endothelial cells. Biochem J 421: 163–169, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Day RM, Lee YH, Han L, Kim YC, Feng YH. Angiotensin II activates AMPK for execution of apoptosis through energy-dependent and -independent mechanisms. Am J Physiol Lung Cell Mol Physiol 301: L772–L781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 22: 1846–1855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deji N, Kume S, Araki S, Isshiki K, Araki H, Chin-Kanasaki M, Tanaka Y, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun 418: 559–564, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Deng A, Arndt MA, Satriano J, Singh P, Rieg T, Thomson S, Tang T, Blantz RC. Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade. Am J Physiol Renal Physiol 299: F1365–F1373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng A, Miracle CM, Lortie M, Satriano J, Gabbai FB, Munger KA, Thomson SC, Blantz RC. Kidney oxygen consumption, carbonic anhydrase, and proton secretion. Am J Physiol Renal Physiol 290: F1009–F1015, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Deng A, Miracle CM, Suarez JM, Lortie M, Satriano J, Thomson SC, Munger KA, Blantz RC. Oxygen consumption in the kidney: effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int 68: 723–730, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Deng A, Tang T, Singh P, Wang C, Satriano J, Thomson SC, Blantz RC. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int 75: 197–204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding DF, You N, Wu XM, Xu JR, Hu AP, Ye XL, Zhu Q, Jiang XQ, Miao H, Liu C, Lu YB. Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Am J Nephrol 31: 363–374, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem 285: 37503–37512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faour WH, Gomi K, Kennedy CR. PGE2 induces COX-2 expression in podocytes via the EP4 receptor through a PKA-independent mechanism. Cell Signal 20: 2156–2164, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Fraser S, Mount P, Hill R, Levidiotis V, Katsis F, Stapleton D, Kemp BE, Power DA. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am J Physiol Renal Physiol 288: F578–F586, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Goncalves AR, Fujihara CK, Mattar AL, Malheiros DM, Noronha Ide L, de Nucci G, Zatz R. Renal expression of COX-2, ANG II, and AT1 receptor in remnant kidney: strong renoprotection by therapy with losartan and a nonsteroidal anti-inflammatory. Am J Physiol Renal Physiol 286: F945–F954, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Hallows KR, Mount PF, Pastor-Soler NM, Power DA. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am J Physiol Renal Physiol 298: F1067–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie DG. AMPK and autophagy get connected. EMBO J 30: 634–635, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardie DG. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc 70: 92–99, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr 93: 891S–896S, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med 13: 3644–3654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879–27887, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Jia F, Wu C, Chen Z, Lu G. AMP-activated protein kinase inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells. Cardiovasc Drugs Ther 25: 21–29, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, Lee CH, Choi HS. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57: 306–314, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 60: 634–643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komura N, Kihara S, Sonoda M, Maeda N, Tochino Y, Funahashi T, Shimomura I. Increment and impairment of adiponectin in renal failure. Cardiovasc Res 86: 471–477, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Leichtweiss HP, Lubbers DW, Weiss C, Baumgartl H, Reschke W. The oxygen supply of the rat kidney: measurements of intrarenal Po2. Pflügers Arch 309: 328–349, 1969 [DOI] [PubMed] [Google Scholar]
- 31.Lempiainen J, Finckenberg P, Levijoki J, Mervaala E. AMPK activator AICAR ameliorates ischemia reperfusion injury in the rat kidney. Br J Pharmacol 166: 1905–1915, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv ZM, Liu Y, Zhang PJ, Xu J, Jia ZH, Wang R, Wan Q. The role of AMPKα in high-glucose-induced dysfunction of cultured rat mesangial cells. Ren Fail 34: 616–621, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 15: 1277–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Nath KA, Croatt AJ, Hostetter TH. Oxygen consumption and oxidant stress in surviving nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1354–F1362, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Pastor-Soler NM, Hallows KR. AMP-activated protein kinase regulation of kidney tubular transport. Curr Opin Nephrol Hypertens 21: 523–533, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Peairs A, Radjavi A, Davis S, Li L, Ahmed A, Giri S, Reilly CM. Activation of AMPK inhibits inflammation in MRL/lpr mouse mesangial cells. Clin Exp Immunol 156: 542–551, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piwkowska A, Rogacka D, Jankowski M, Angielski S. Extracellular ATP through P2 receptors activates AMP-activated protein kinase and suppresses superoxide generation in cultured mouse podocytes. Exp Cell Res 317: 1904–1913, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepinski JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun 393: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Rather LJ. Studies in subtotal nephrectomy in rats. I. Inter-relationship of blood pressure, urinary protein, and the development of secondary lesions in the renal stump resembling chronic glomerulonephritis. II. Effect of adrenalectomy on proteinuria. III. Adrenal size in moderate renal excretory insufficiency. Stanford Med Bull 9: 207–217, 1951 [PubMed] [Google Scholar]
- 41.Sakamoto A, Hongo M, Saito K, Nagai R, Ishizaka N. Reduction of renal lipid content and proteinuria by a PPAR-γ agonist in a rat model of angiotensin II-induced hypertension. Eur J Pharmacol 682: 131–136, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev CD002967, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Schurek HJ, Jost U, Baumgartl H, Bertram H, Heckmann U. Evidence for a preglomerular oxygen diffusion shunt in rat renal cortex. Am J Physiol Renal Fluid Electrolyte Physiol 259: F910–F915, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, Kashgarian M, Caplan MJ. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol 301: F1346–F1357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271: 611–614, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Stumvoll M, Haring HU, Matthaei S. Metformin. Endocr Res 32: 39–57, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Tabony AM, Yoshida T, Galvez S, Higashi Y, Sukhanov S, Chandrasekar B, Mitch WE, Delafontaine P. Angiotensin II upregulates protein phosphatase 2Cα and inhibits AMP-activated protein kinase signaling and energy balance leading to skeletal muscle wasting. Hypertension 58: 643–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tain YL, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol Renal Physiol 292: F1404–F1410, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Takacs-Vellai K, Vellai T, Puoti A, Passannante M, Wicky C, Streit A, Kovacs AL, Muller F. Inactivation of the autophagy gene bec-1 triggers apoptotic cell death in C. elegans. Curr Biol 15: 1513–1517, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Takiar V, Nishio S, Seo-Mayer P, King JD, Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 108: 2462–2467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaziri ND, Bai Y, Ni Z, Quiroz Y, Pandian R, Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharmacol Exp Ther 323: 85–93, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 196: 81–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol 45: 276–295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S, Song P, Zou MH. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond) 122: 555–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron Po2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 59: 230–237, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki T, Tanimoto M, Gohda T, Ohara I, Hagiwara S, Murakoshi M, Matsumoto M, Kaneko S, Aoki T, Toyoda H, Ishikawa Y, Funabiki K, Horikoshi S, Tomino Y. Combination effects of enalapril and losartan on lipid peroxidation in the kidneys of KK-Ay/Ta mice. Nephron Exp Nephrol 113: e66–e76, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology 153: 1411–1420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J, Cui S, Wei R, Chen X, Cai G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell Physiol Biochem 27: 681–690, 2011 [DOI] [PubMed] [Google Scholar]