Abstract
20-Hydroxyeicosatetraenoic acid (20-HETE) is a cytochrome P-450 (Cyp)-derived arachidonic acid metabolite that has been shown to increase smooth muscle contractions and proliferation, stimulate endothelial dysfunction and activation, and promote hypertension. We examined if 20-HETE contributes to microvascular remodeling in hypertension. In Sprague-Dawley rats, administration of the 20-HETE biosynthesis inhibitor HET0016 or the 20-HETE antagonist N-20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (20-HEDE) prevented 5α-dihydrotestosterone (DHT)-induced increases in blood pressure as well as abrogated DHT-induced increases in the media-to-lumen ratio (M/L), media thickness, and collagen IV deposition in renal interlobar arteries. Reserpine prevented blood pressure elevation in DHT-treated rats but did not affect microvascular remodeling (M/L, media thickness, and collagen deposition); under these conditions, treatment with the 20-HETE antagonist attenuated microvascular remodeling, suggesting that 20-HETE contributes to DHT-induced vascular remodeling independent of blood pressure elevation. In Cyp4a14−/− mice, which display androgen-driven and 20-HETE-dependent hypertension, treatment with the 20-HETE antagonist abolished remodeling of renal resistance arteries measured as media thickness (24 ± 1 vs. 15 ± 1 μm) and M/L (0.29 ± 0.03 vs. 0.17 ± 0.01). Moreover, in Cyp4a12 transgenic mice in which the expression of Cyp4a12–20-HETE synthase is driven by a tetracycline-sensitive promoter, treatment with doxycycline resulted in blood pressure elevation (140 ± 4 vs. 92 ± 5 mmHg) and a significant increase in remodeling of renal resistance arteries (media thickness: 23 ± 1 vs. 16 ± 1 μm; M/L: 0.39 ± 0.04 vs. 0.23 ± 0.02); these increases were abrogated by cotreatment with 20-HEDE. This study demonstrated that 20-HETE is a key regulator of microvascular remodeling in hypertension; its effect is independent of blood pressure elevation and androgen levels.
Keywords: 20-hydroxyeicosatetraenoic acid, cytochrome P-450 4A, blood pressure, androgen
vascular remodeling of large and small arteries contributes to the development and complications of hypertension (25). This process renders arteries stiffer and thicker, leading to detrimental effects on blood pressure regulation (14). Structural alterations of the microcirculation, of which the media-to-lumen ratio (M/L) is the most important predictor, are likely to occur in renal microvessels, as previously shown in experimental models of hypertension (7), including spontaneously hypertensive rats (SHRs), DOCA-salt rats, and one-kidney, one-clip Goldblatt hypertensive rats (12). The mechanisms that contribute to arterial remodeling in hypertension are numerous and include stimulation of growth and apoptosis and increased inflammation and fibrosis. Numerous autacoids have been implicated as mediators of arterial remodeling in hypertension. The most prominent is ANG II, which has been shown to exert the capacity of stimulating vasoconstriction, smooth muscle growth, production of inflammatory cytokines and chemokines, and activating extracellular matrix proteins (26), all of which contribute to structural changes and remodeling of the arterial wall in hypertension.
20-Hydroxyeicosatetraenoic acid (20-HETE), a cytochrome P-450 (CYP)-derived arachidonic acid metabolite and a primary eicosanoid in the renal microcirculation, shares much of ANG II's characteristics. It sensitizes smooth muscle cells to constrictor stimuli and contributes to myogenic, mitogenic, and angiogenic responses (17, 38). Several experimental models of hypertension, such as the SHR, display high levels of 20-HETE in the vasculature, and inhibition of its synthesis reduces blood pressure in these models, suggesting that 20-HETE, through its constrictor activity, contributes to increased renal and peripheral vascular resistance and, consequently, hypertension (40). Other mechanisms by which 20-HETE may contribute to hypertension include stimulation of endothelial dysfunction as well as the induction of angiotensin-converting enzyme and activation of the renin-angiotensin system (2, 3, 29, 34). 20-HETE has also been characterized as a proinflammatory mediator; it activates NF-κB, stimulates the production of inflammatory cytokines, and induces NADPH oxidase and superoxide production (8, 13, 28). These characteristics, together with its ability to stimulate vascular smooth muscle migration and growth (19, 30) and cell apoptosis (22), suggest that 20-HETE may be an important mediator of vascular remodeling in hypertension. To this end, studies have suggested that 20-HETE mediates ANG II-induced neointimal thickening in the balloon-injured rat carotid artery (43) and cardiac hypertrophy in Ren-2 transgenic rats (1).
The present study was undertaken to investigate the role of 20-HETE in remodeling of renal resistance arteries in hypertension using experimental models in which hypertension is dependent on increased 20-HETE biosynthesis. These models were 1) androgen-induced hypertension in rats and mice, 2) Cyp4a14 knockout (Cyp4a14−/−) mice, and 3) Cyp4a12 transgenic (Cyp4a12tg) mice. The hypertension in these models was abrogated by treatment with either an inhibitor of 20-HETE biosynthesis or an antagonist of 20-HETE bioactions (41). Here, we show that 20-HETE mediates remodeling of renal resistance vessels in hypertension independent of blood pressure elevation or androgen levels.
MATERIALS AND METHODS
Animal experiments.
All experimental protocols were performed following an Institutional Animal Care and Use Committee-approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Sprague-Dawley male rats (6–7 wk old, Charles Rivers, Wilmington, MA) were treated with 5α-dihydrotestosterone (DHT; 56 mg·kg−1·day−1 ip) or its vehicle (20% benzyl alcohol in corn oil ip) for 21 days. The indicated dose of DHT has been previously shown to induce hypertension in normotensive rats (21, 28). In some experiments, rats were given the CYP4A-selective inhibitor N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine [HET0016; 10 mg·kg−1·day−1 in 10% (wt/vol) lecithin in saline ip] (18) or the 20-HETE antagonist N-20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (20-HEDE; 10 mg·kg−1·day−1 in 5% ethanol in saline ip) (39) together with DHT. Some rats were also given reserpine (100 μg·kg−1·day−1 in DMSO ip). Male C57BL/6 mice (6–8 wk old) were implanted with placebo and DHT pellets (5 mg/day sc, Innovative Research America, Sarasota, FL) for 21 days. Some of the DHT-treated mice were cotreated with the 20-HETE antagonist N-[20-hydroxyeicosa-6(Z),15(Z)-dienoyl]glycine (20-HEDGE; 10 mg·kg−1·day−1 in 5% ethanol in saline ip). 20-HEDE and 20-HEDGE differ in their substitution at the carboxyl group but exhibit similar 20-HETE antagonistic activity in bioassays; both (at 1 μM) reduce the constrictor activity of phenylephrine in renal interlobar arteries by two- to threefold and inhibit 20-HETE angiogenic activity in human microvessel endothelial cells by 90% at 100 nM. Male wild-type and Cyp4a14−/− mice (6 mo old) (10) were treated with or without 20-HEDGE (10 mg·kg−1·day−1 in 5% ethanol in saline ip) for 12 days. Male and female Cyp4a12tg mice (8–14 wk old) were administered doxycycline (DOX; 1 mg/ml in drinking water) for 42 days. In some experiments, 20-HEDE or 20-HEDGE (10 mg·kg−1·day−1 in 5% ethanol in saline ip) were administered for the indicated time period. For blood pressure measurement, animals were acclimated for 7 days before the start of experiments. Systolic blood pressure was determined by the tail-cuff method (Kent Scientific, Torrington, CT). Animals were placed on a far infrared heating pad for 7–10 min. Systolic blood pressure measurements were recorded after five cycles of acclimatization. At the end of experiments, animals were anesthetized with xylazine-ketamine (25/50 mg/kg ip), and a laparotomy was performed. The kidneys were removed, and renal interlobar arteries from rats and preglomerular microvessels (PGMVs) from mice were microdissected for biochemical and functional experiments.
Measurements of 20-HETE.
Rat renal interlobar arteries and mouse PGMVs were incubated in oxygenated Krebs biocarbonate buffer (pH 7.4) with 1 mM NADPH for 1 h at 37°C with gentle shaking. 20-HETE was extracted and quantified by liquid chromotography-tandem mass spectroscopy (Applied Biosystems, Foster City, CA) as previously described (11).
Measurements of media thickness, M/L, and medial cross-sectional area.
The same segments of renal resistance arteries, renal interlobar arteries from rats and PGMVs from mice, were dissected to minimize the difference along the arteries. Arteries were mounted on a pressurized myograph and equilibrated for 1 h in oxygenated Krebs buffer at 37°C. The operator was blinded to treatments except for the blood pressure range of the animal. Lumen diameters from normotensive animals were determined at 100 mmHg and hypertensive animals at 140 mmHg. Measurements of outer diameter (OD) and inner diameter (ID) under passive conditions were used to calculate media thickness [(OD − ID)/2], M/L [M/L = (OD − ID)/ID], and medial cross-sectional area [mCSA = (π/4) × (OD2 − ID2)].
Immunofluorescence.
Dissected arteries were washed twice with PBS, fixed in 4% paraformaldehyde at 4°C overnight, and embedded in OCT compound (Sakura Finetek, Torrence, CA). Cryostat sections were cut transversely into 5-μm sections. Immunofluorescence staining was performed using the following antibodies: anti-collagen IV antibody (ab6586, Abcam, Cambridge, MA) and anti-collagen I antibody (ab34710, Abcam). Briefly, frozen vessel sections were fixed in acetone at 4°C for 20 min and blocked in 5% goat serum in PBS for 45 min at room temperature. Sections were then incubated with primary antibody at 4°C overnight, washed, and further incubated with Cy3-conjugated secondary antibody (111-165-144, Jackson Immnuoresearch, West Grove, PA) for 2 h at room temperature. To further verify cellular entity, sections were washed and counterstained for nuclei with 4′,6-diamino-2-phenylindole for 15 min. Immunofluorescence was visualized using a Zeiss Axioplan-2 fluorescent microscope. Images were captured and analyzed using AxioVision 2 multichannel image processing software (Zeiss, Gottingum, Germany).
Statistics.
Data are presented as means ± SE. Statistical significance (P < 0.05) between the experimental groups was determined by the Fisher method of analysis for multiple comparisons. For comparison between treatment groups, the null hypothesis was tested by single-factor ANOVA (Dunnett's multiple-comparison test) for multiple groups or an unpaired t-test for two groups.
RESULTS
20-HETE mediates vascular remodeling in DHT-treated Sprague-Dawley rats.
Administration of DHT to normotensive rats significantly increased systolic blood pressure (153 ± 8 vs. 104 ± 4 mmHg) at the end of the 21-day treatment. Cotreatment with the 20-HETE biosynthesis inhibitor HET0016 or with the 20-HETE antagonist 20-HEDE prevented the increase in blood pressure (106 ± 9 and 113 ± 5 mmHg, respectively; Fig. 1A). Treatment with DHT increased media thickness, M/L, and mCSA in renal interlobar arteries by twofold compared with vehicle (Fig. 1, B–D). Cotreatment with either 20-HEDE or HET0016 abrogated the increase in media thickness, M/L, and mCSA to levels not different from renal interlobar arteries of vehicle-treated rats (Fig. 1, B and C). We (28, 39) have previously shown that 20-HETE levels in interlobar arteries from DHT-treated rats increase by twofold compared with rats treated with vehicle. Moreover, administration of HET0016 to DHT-treated rats resulted in a 90% decrease in renal interlobar 20-HETE levels, whereas these levels remained unchanged in vessels from rats cotreated with 20-HEDE (28, 39). We (42) have also demonstrated that DHT-mediated increases in blood pressure and vascular 20-HETE production are inhibited by cotreatment with flutamide, suggesting that these effects of DHT are mediated through the androgen receptor.
Fig. 1.
Androgen-driven 20-hydroxyeicosatetraenoic acid (20-HETE)-dependent hypertension and vascular remodeling in Sprague-Dawley rats. Rats were treated with vehicle, 5α-dihydrotestosterone (DHT), DHT + N-20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (20-HEDE), and DHT + HET0016 for 21 days. A: systolic blood pressure (BP) on day 21. The media thickness (B), media-to-lumen ratio (M/L; C), and medial cross-sectional area (mCSA; D) of renal interlobar arteries were measured using a pressurized myograph. n = 4–6. *P < 0.05 vs. the vehicle-treated group.
Inhibition of 20-HETE biosynthesis or action attenuates collagen IV deposition in renal interlobar arteries.
Collagen IV is primarily localized in the basal lamina of the vessels. A previous study (12) has shown that in vascular remodeling, collagen IV levels increase and contribute to the increased stiffness of the vessels. As shown in Fig. 2, treatment with DHT for 21 days increased collagen IV deposition in renal interlobar arteries by 3.1-fold. Administration of either 20-HEDE or HET0016 to rats treated with DHT negated the increase in collagen IV deposition (Fig. 2).
Fig. 2.
Collagen IV deposition in renal interlobar arteries of DHT-treated rats. Rats were treated with vehicle, DHT, DHT + 20-HEDE, and DHT + HET0016 for 21 days. A: representative immunofluorescence images of collagen IV (red) in renal interlobar arteries with 4′,6-diamino-2-phenylindole (DAPI) staining (blue). B: fold changes of immunofluorescence intensity relative to the vehicle-treated group. n = 4–6. *P < 0.05 vs. the vehicle-treated group.
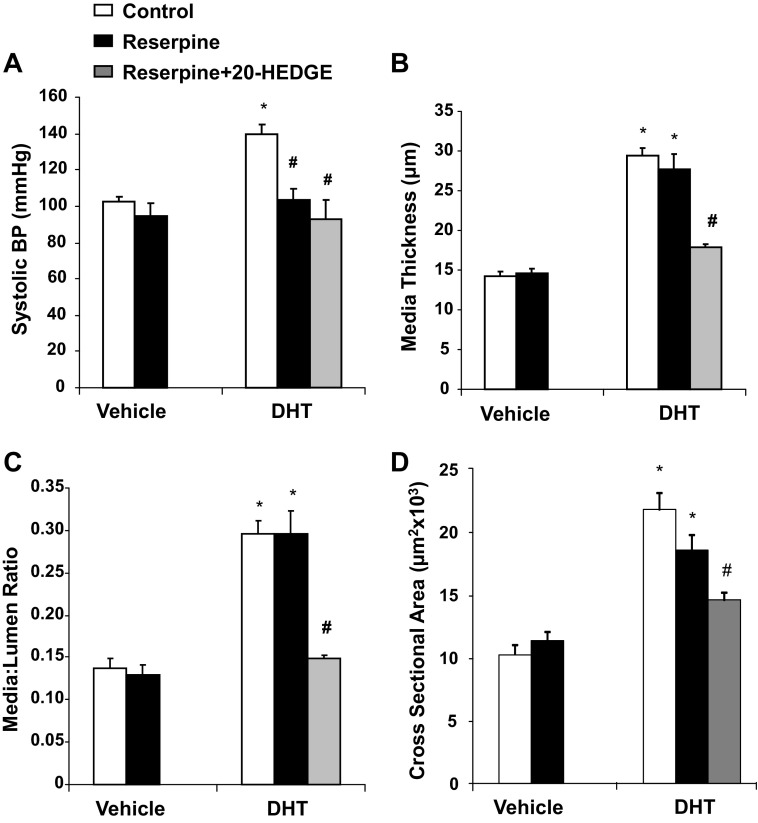
Reserpine abrogates DHT-induced high blood pressure without altering vascular remodeling of renal interlobar arteries.
To determine whether vascular remodeling in DHT-treated rats occurs in the absence of hypertension, the antihypertensive drug reserpine was administered together with DHT. As shown in Fig. 3, administration of reserpine to DHT-treated rats prevented DHT-induced hypertension. At the end of the 21-day treatment, the blood pressure of rats cotreated with DHT and reserpine was significantly lower than those treated with DHT alone (104 ± 6 vs. 140 ± 6 mmHg). Reserpine alone had no significant hypotensive effect (Fig. 3). Administration of 20-HEDGE to rats treated with DHT and reserpine had no additional blood pressure-lowering effect (Fig. 3).
Fig. 3.
Reserpine abrogates DHT-induced increases in BP. Rats were treated with vehicle, DHT, DHT + reserpine, reserpine, and DHT + reserpine + N-[20-hydroxyeicosa-6(Z),15(Z)-dienoyl]glycine (20-HEDGE) for 21 days. BP was monitored throughout the duration of the experiment. n = 4–6. *P < 0.05 vs. the vehicle-treated group.
Whereas treatment with reserpine lowered blood pressure in DHT-treated rats, it did not altered vascular remodeling (Fig. 4). DHT treatment increased media thickness, M/L, and mCSA in renal interlobar arteries by twofold compared with the vehicle-treated group. Remodeling in arteries from rats treated with reserpine and DHT remained unchanged compared with arteries from rats treated with only DHT (Fig. 4). Importantly, administration of 20-HEDGE to rats treated with DHT and reserpine inhibited vascular remodeling, bringing media thickness, M/L, and mCSA to levels not different from those in arteries of vehicle- or reserpine-treated rats (Fig. 4).
Fig. 4.
Reserpine does not affect remodeling of renal interlobar arteries in DHT-treated rats. Rats were treated with vehicle, DHT, DHT + reserpine, reserpine, and DHT + reserpine + 20-HEDGE for 21 days. A: BP measured on day 21. The media thickness (B), M/L (C), and mCSA (D) of renal interlobar arteries were measured using a pressurized myogragh at the end of the experiment. n = 4–6. *P < 0.05 vs. the vehicle-treated group; #P < 0.05 vs. the DHT-treated group.
Similar results were obtained with regard to collagen I and IV deposition in renal interlobar arteries. DHT treatment increased collagen I (Fig. 5) and collagen IV (Fig. 6) deposition by 2.5- and 5-fold, respectively. Administration of reserpine had no significant effect on DHT-induced collagen I and IV deposition, demonstrating 2- and 4.5-fold increases, respectively, compared with the vehicle-treated group. Reserpine alone had no effect on collagen deposition (Figs. 5 and 6). Importantly, basal and DHT-stimulated 20-HETE production in renal interlobar arteries were not affected by reserpine (Fig. 7).
Fig. 5.
Reserpine does not affect collagen I deposition in renal interlobar arteries from DHT-treated rats. Rats were treated with vehicle, DHT, DHT + reserpine, and reserpine for 21 days. A: representative immunofluorescence images of collagen IV (red) in renal interlobar arteries with DAPI staining (blue). B: fold changes of immunofluorescence intensity relative to the vehicle-treated group. n = 4–6. *P < 0.05 vs. the vehicle-treated group.
Fig. 6.
Collagen IV deposition in renal interlobar arteries. Rats were treated with vehicle, DHT, DHT + reserpine, and reserpine for 21 days. A: representative immunofluorescence images of collagen IV (red) in renal interlobar arteries with DAPI staining (blue). B: fold changes of immunofluorescence intensity relative to the vehicle-treated group. n = 4–6. *P < 0.05 vs. the vehicle-treated group.
Fig. 7.
Reserpine does not affect 20-HETE production in renal interlobar arteries from DHT-treated mice. Rats were treated with vehicle, DHT, DHT + reserpine, and reserpine for 21 days. 20-HETE levels in renal interlobar arteries were measured at the end of the experiment as described in the text. n = 4. *P < 0.05 vs. the vehicle-treated group.
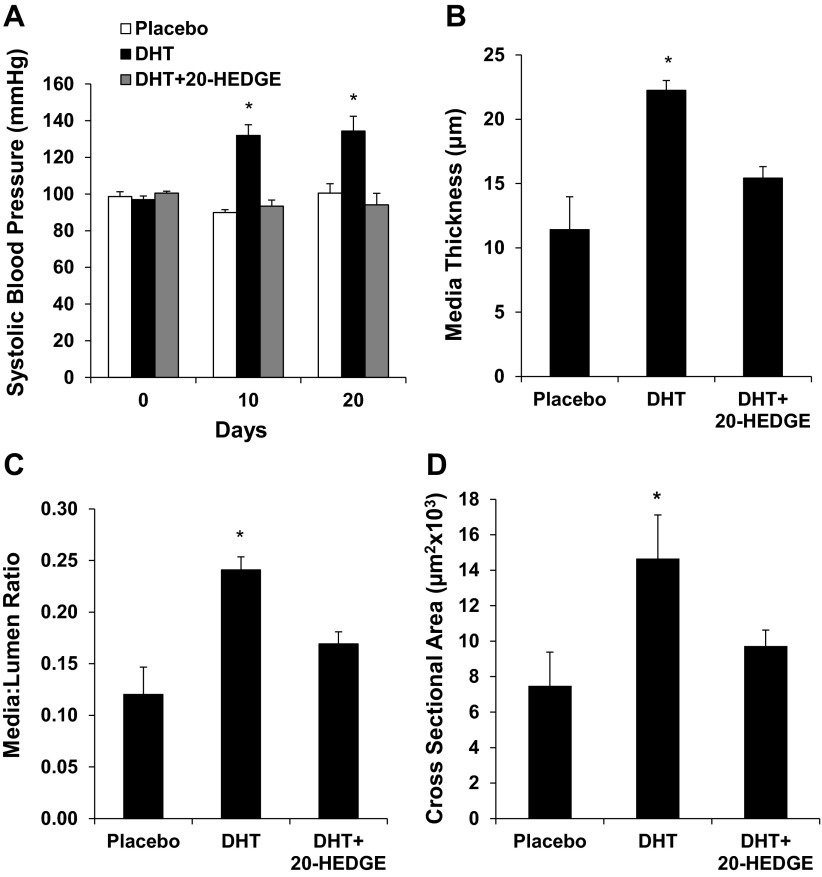
Vascular remodeling in androgen-driven hypertension in mice is mediated by 20-HETE.
Administration of DHT pellets to C57BL/6 mice significantly increased systolic blood pressure (134 ± 8 vs. 100 ± 5 mmHg) at the end of 21-day treatment. Cotreatment with the 20-HETE antagonist 20-HEDGE prevented the increase in blood pressure (94 ± 6 mmHg; Fig. 8A). Treatment with DHT increased media thickness and M/L of preglomerular arteries by twofold compared with the placebo-treated group. Cotreatment with 20-HEDGE attenuated the increase in media thickness and M/L of PGMVs (Fig. 8, B and C). Similar to what was observed in DHT-treated rats, preglomerular arteries from DHT-treated mice also displayed 20-HETE-dependent increases in mCSA (7.5 ± 1.9, 14.7 ± 1.0, and 9.7 ± 0.9 × 103 μm2 in placebo-, DHT-, and DHT + 20-HEDGE-treated groups, respectively, n = 5–6, P < 0.05), suggesting hypertrophic remodeling.
Fig. 8.
Vascular remodeling in preglomerular arteries of DHT-treated C57BL/6 mice. A: systolic BP was measured in C57BL/6 mice treated with vehicle, DHT, and DHT + 20-HEDGE for 21 days. Media thickness (B) and M/L (C) were measured using a pressurized myograph at the end of the experiment. n = 5–6. *P < 0.05 vs. the vehicle-treated group.
Cyp4a14−/− mice display male-specific hypertension that is associated with androgen-driven induction of Cyp4a12 expression and 20-HETE biosynthesis (10). Male Cyp4a14−/− mice are hypertensive compared with their counterpart wild-type mice (153 ± 4 vs. 120 ± 1 mmHg) without any pharmacological intervention (Fig. 9A). Administration of 20-HEDGE resulted in a gradual decrease in blood pressure, reaching that of wild-type mice after 12 days of treatment (122 ± 3 vs. 120 ± 1 mmHg). Renal preglomerular arteries from male Cyp4a14−/− mice displayed a 60% and 40% increase in media thickness (Fig. 9B) and M/L (Fig. 9C), respectively, compared with wild-type mice. The increases in media thickness and M/L were abrogated by the administration of 20-HEDGE. Similarly, treatment with 20-HEDGE reduced mCSA to levels observed in wild-type mice (7.7 ± 0.3, 14.5 ± 1.8, and 8.6 ± 0.5 × 103 μm2 in wild-type, Cyp4a14−/−, and 20-HEDGE-treated Cyp4a14−/− mice, respectively, n = 4, P < 0.05).
Fig. 9.

Vascular remodeling of preglomerular arteries in cytochrome P-450 (Cyp)4a14 knockout (Cyp4a14−/−) mice. A: systolic BP was measured in Cyp4a14−/− mice treated with or without 20-HEDGE for 12 days. Media thickness (B) and M/L (C) were measured using a pressurized myograph at the end of the experiment. n = 4. *P < 0.05 vs. the vehicle-treated group.
20-HETE mediates vascular remodeling in the absence of androgen.
To examine whether 20-HETE contributes to vascular remodeling in hypertension in the absence of androgen, we used Cyp4a12tg mice. These mice were developed to overexpress Cyp4a12 under the control of a tetracycline-sensitive promoter (Tet-on). A recent study from our laboratory showed that renal preglomerular arteries from Cyp4a12tg mice treated with DOX demonstrated increased Cyp4a12 protein and 20-HETE levels compared with Cyp4a12tg mice placed on water alone. The blood pressure elevation as well as the increased 20-HETE production in DOX-treated Cyp4a12tg mice were negated by the administration of 20-HEDGE (41). To examine whether vascular remodeling takes place in this 20-HETE-mediated hypertensive model, mice were treated with vehicle, DOX, and DOX + 20-HEDGE for 42 days. After 42 days of treatment, systolic blood pressure in the DOX-treated group rose to 140 ± 4 mmHg, whereas the blood pressure in mice cotreated with DOX and 20-HEDGE remained unchanged (89 ± 1 mmHg; Fig. 10A). Treatment of Cyp4a12tg mice with DOX for 6 wk resulted in a 73% increase in M/L (0.39 ± 0.04 vs. 0.23 ± 0.02). There was also a 50% increase in media thickness (23 ± 1 vs. 16 ± 1 μm). In the DOX + 20-HEDGE-treated group, both media thickness and M/L were attenuated (18 ± 2 μm and 0.28 ± 0.03, respectively; Fig. 10, B and C). Similar results were obtained with regard to mCSA (5.2 ± 1.0, 9.3 ± 1.3, and 6.2 ± 1.2 × 103 μm2 in vehicle-, DOX-, and DOX + 20-HEDGE-treated Cyp4a12tg mice, respectively, n = 6–8, P < 0.05).
Fig. 10.
Remodeling of preglomerular arteries in Cyp4a12 transgenic (Cyp4a12tg) mice. A: systolic BP was measured in Cyp4a12tg mice treated with water, doxycycline (DOX), and DOX + 20-HEDGE for 6 wk. Media thickness (B) and M/L (C) were measured using a pressurized myograph at the end of the experiment. n = 6–8. *P < 0.05 vs. the vehicle-treated group.
DISCUSSION
This study identifies 20-HETE as a key mediator of vascular remodeling of renal resistance arteries in hypertension independent of blood pressure elevation. Remodeling of microvessels occurs in hypertension and involves structural, functional, and mechanical changes that result in decreased lumen size, increased M/L, and increased thickness. These vascular changes are believed to be important contributors of cardiovascular complications associated with hypertension, including end-organ damage. Numerous autacoids, including bioactive peptides and lipids, have been implicated in the process of vascular remodeling. 20-HETE is a CYP-derived eicosanoid whose synthesis and actions have been linked to the pathogenesis of hypertension in experimental models and humans (38, 42). Numerous reports have demonstrated that the vascular synthesis of 20-HETE increases in experimental models of hypertension; vascular overexpression of CYP4A2–20-HETE synthase leads to hypertension, and inhibition of 20-HETE synthesis results in lower blood pressure (11, 28, 29, 34). 20-HETE exerts powerful biological activities in the vasculature; it constricts smooth muscles, stimulates smooth muscle proliferation and migration, inhibits endothelial nitric oxide synthase, and activates the NF-kB-mediated inflammatory program in the vascular endothelium (3, 13, 17). However, its contribution to vascular remodeling in the microcirculation is unknown.
Here, we demonstrated a significant remodeling of renal resistance arteries in experimental models in which blood pressure elevation is largely dependent on an increase in 20-HETE synthesis and action. These animal models include androgen-treated rats and mice, Cyp4a14−/− mice, and Cyp4a12tg mice (41). Treatment with a 20-HETE synthesis inhibitor or an antagonist of 20-HETE action prevented the rise in blood pressure in response to androgen, normalized blood pressure in androgen-driven hypertensive CYP4a14−/− mice, and abrogated the rise in blood pressure in response to DOX in Cyp4a12tg mice. These results clearly indicated that hypertension in these models is largely dependent on increases in 20-HETE biosynthesis and actions (41). The present study also showed that hypertension in these models is associated with marked remodeling of renal resistance arteries, i.e., renal interlobar arteries, as exemplified by structural and biochemical changes that are indicative of this process. Thus, structural changes, including media thickness, M/L, and mCSA, significantly increased in all three models, and, in DHT-treated rats, remodeling was also evidenced by marked increases in collagen I and V deposition. Importantly, remodeling in all of these hypertensive models was fully abrogated by either inhibition of 20-HETE biosynthesis or blockade of 20-HETE actions. These findings clearly implicate 20-HETE as an important mediator of vascular remodeling in hypertension.
The model of DHT-induced hypertension has been identified as a model of 20-HETE-dependent hypertension in which the contribution of 20-HETE to blood pressure elevation consists of promoting vasoconstriction while interfering with vasodilation and, consequently, increases in vascular tone and peripheral vascular resistance (42). An important finding in the present study is that reserpine, a general blood pressure-lowering drug, prevented DHT-induced blood pressure but did not inhibit DHT-induced remodeling of renal resistance arteries. The remodeling that occurred in the presence of reserpine, however, was inhibited by the administration of 20-HEDE, indicating that the 20-HETE-mediated increase in blood pressure is independent of its effect on vascular remodeling. This key finding suggests that 20-HETE exerts effects on the vascular wall other than simply affecting its contractile behavior. Additionally, these effects appear to be independent of androgen since 20-HETE-dependent remodeling of renal microvessels was also observed in DOX-treated Cyp4a12tg mice in which overexpression of Cyp4a12, the major 20-HETE synthase in mice, was driven by a tetracycline-sensitive promoter.
The biochemical changes preceding the structural changes that characterize remodeling include increased extracellular matrix deposition along with enhanced expression of adhesion molecule and growth factors, which, in turn, contribute to the onset of vascular inflammation and smooth muscle migration, hypertrophy, hyperplasia, and apoptosis (12). The findings that the 20-HETE-mediated increase in M/L was also accompanied with an increase in mCSA suggests that 20-HETE-mediated hypertrophic remodeling of these small resistance arteries occurs in all models (25). The mechanisms by which 20-HETE contributes to vascular remodeling are unclear; however, 20-HETE has been implicated in many of the biochemical changes preceding remodeling of the arterial wall. 20-HETE has been shown to contribute to collagen accumulation in kidneys from hypertensive rats overexpressing CYP4A2 protein (11). It has also been shown to exert proinflammatory actions. In vascular endothelial cells, 20-HETE increases ROS, including superoxide (3, 8, 16), and stimulates NF-κB activity (4, 13), resulting in endothelial activation with increased expression of ICAM-1 and IL-8 levels (13). 20-HETE-dependent increases in NADPH oxidase, ROS, and NF-κB activity are also seen in the renal microvasculature of androgen-treated rats (28, 39). Moreover, inhibition of NF-κB activation attenuates androgen-induced 20-HETE-dependent increases in blood pressure (39). In smooth muscle cells, 20-HETE has been shown to act as a mitogen. In cultured vascular smooth muscle cells from adult rabbits (19) and rats (33) as well as in isolated renal arterioles (31), 20-HETE induces the phosphorylation of ERK1/2, a MAPK that plays a pivotal role in the proliferation induced by the activation of receptor tyrosine kinases and G protein-coupled receptors (9). Blockade of the formation of 20-HETE attenuated norepinephrine-induced and ANG II-induced ERK1/2 phosphorylation, suggesting that 20-HETE may serve as a second messenger of these growth factors (20, 24). The same investigators also showed that 20-HETE contributed to ANG II-induced neointimal thickening in the injured carotid artery (43). Stec et al. (30) demonstrated that 20-HETE promotes platelet-derived growth factor-stimulated vascular smooth muscle cell migration via pathways that involve MEK and phosphatidylinositol 3-kinase activation. 20-HETE has also been shown to inhibit apoptosis of pulmonary artery smooth muscle cells (35). Collectively, these actions may underlie, at least in part, the ability of 20-HETE to stimulate remodeling of resistance arteries.
The findings in this study have implications to vascular remodeling in other forms of hypertension. This stems from observations that 20-HETE is a microcirculatory eicosanoid that is often produced in response to stimuli that are highly relevant to the pathogenesis of hypertension, including ANG II and endothelin (5, 23, 32). Increases in vascular 20-HETE are also observed in SHRs (6), a common model for essential hypertension and vascular remodeling, and in models of salt-sensitive hypertension, such as Dahl salt-sensitive rats (15). Several clinical studies (36, 37) have shown increased 20-HETE levels in hypertensive individuals. A recent study by Schuck et al. (27) demonstrated that enhanced plasma levels of 20-HETE are associated with more advanced endothelial dysfunction and vascular inflammation in patients with established atherosclerotic cardiovascular disease. The proinflammatory and progrowth bioactions of 20-HETE in vascular cells together with its increased occurrence in hypertensive patients suggest that it may play a role in microvascular remodeling and contributes to the development and cardiovascular complications of hypertension.
GRANTS
This work was supported in part by Robert A. Welch Foundation Award GL625910 (to J. R. Falck) and a gift from Dr. Cornelius Borman (to M. L. Schwartzman). This work was supported by National Institutes of Health Grants HL-034300 (to M. L. S), HL-097402 (to C. C. Wu), DK-038226 (to J. H. Capdevila and J. R. Falck), and GM-31278 (to J. R. Falck) and by Diversity Supplement Award HL-34300-26A1S1 (to V. Garcia).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.D., C.-C.W., V.G., I.D., A.W., G.J., F.Z., V.L.M., and J.H.C. performed experiments; Y.D., C.-C.W., F.Z., and J.H.C. analyzed data; Y.D., V.G., and F.Z. prepared figures; Y.D. and M.L.S. drafted manuscript; Y.D., V.G., J.R.F., J.H.C., and M.L.S. approved final version of manuscript; C.-C.W., J.H.C., and M.L.S. conception and design of research; J.R.F., J.H.C., and M.L.S. edited and revised manuscript; M.L.S. interpreted results of experiments.
ACKNOWLEDGMENTS
The authors thank Jenifer Brown for assistance in preparing the manuscript.
REFERENCES
- 1.Chabova VC, Kramer HJ, Vaneckova I, Vernerova Z, Eis V, Tesar V, Skaroupkova P, Thumova M, Schejbalova S, Huskova Z, Vanourkova Z, Kolsky A, Imig JD, Cervenka L. Effects of chronic cytochrome P-450 inhibition on the course of hypertension and end-organ damage in Ren-2 transgenic rats. Vasc Pharmacol 47: 145–159, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Cheng J, Garcia V, Ding Y, Wu CC, Thakar K, Falck JR, Ramu E, Schwartzman ML. Induction of angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeicosatetraenoic acid-mediated endothelial dysfunction. Arterioscler Thromb Vasc Biol 32: 1917–1924, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol 294: H1018–H1026, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cheng J, Wu CC, Gotlinger KH, Zhang F, Narsimhaswamy D, Falck JR, Schwartzman ML. 20-Hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IκB kinase-dependent endothelial nitric-oxide synthase uncoupling. J Pharmacol Exp Ther 332: 57–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol 279: F544–F551, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H2455–H2465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassi G, Schiffrin EL. Media-to-lumen ratio as predictor of renal abnormalities in hypertension: new findings, new questions. J Hypertens 28: 1811–1813, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther 321: 18–27, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci STKE 2000: re1, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA 98: 5211–5216, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol 297: F875–F884, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38: 581–587, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-κB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther 324: 103–110, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Lemarie CA, Tharaux PL, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol 48: 433–439, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Lukaszewicz KM, Lombard JH. Role of the CYP4A/20-HETE pathway in vascular dysfunction of the Dahl salt-sensitive rat. Clin Sci (Lond) 124: 695–700, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 294: L902–L911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41: 175–193, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA 95: 12701–12706, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthalif MM, Uddin MR, Fatima S, Parmentier JH, Khandekar Z, Malik KU. Small GTP binding protein Ras contributes to norepinephrine-induced mitogenesis of vascular smooth muscle cells. Prostaglandins Other Lipid Mediat 65: 33–43, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol 284: R1055–R1062, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Nilakantan V, Maenpaa C, Jia G, Roman RJ, Park F. 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol Renal Physiol 294: F562–F570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyekan AO, McAward K, Conetta J, Rosenfeld L, McGiff JC. Endothelin-1 and CYP450 arachidonate metabolites interact to promote tissue injury in DOCA-salt hypertension. Am J Physiol Regul Integr Comp Physiol 276: R766–R775, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Parmentier JH, Muthalif MM, Nishimoto AT, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates angiotensin II-induced phospholipase D activation in vascular smooth muscle cells. Hypertension 37: 623–629, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens 17: 1192–1200, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Schiffrin EL, Touyz RM. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart Circ Physiol 287: H435–H446, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Schuck RN, Theken KN, Edin ML, Caughey M, Bass A, Ellis K, Tran B, Steele S, Simmons BP, Lih FB, Tomer KB, Wu MC, Hinderliter AL, Stouffer GA, Zeldin DC, Lee CR. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis 227: 442–448, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50: 123–129, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension 56: 871–878, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stec D, Gannon KP, Beaird JS, Drummond HA. 20-Hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cells. Cell Physiol Biochem 19: 121–128, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension 33: 414–418, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Tsai IJ, Croft K, Puddey IB, Beilin LJ, Barden AE. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Physiol Heart Circ Physiol 300: H1194–H1200, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Uddin MR, Muthalif MM, Karzoun NA, Benter IF, Malik KU. Cytochrome P-450 metabolites mediate norepinephrine-induced mitogenic signaling. Hypertension 31: 242–247, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res 98: 962–969, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Tang X, Li Y, Leu C, Guo L, Zheng X, Zhu D. 20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur J Pharmacol 588: 9–17, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Ward NC, Puddey IB, Hodgson JM, Beilin LJ, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med 38: 1032–1036, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 51: 1393–1398, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Williams JM, Murphy S, Burke M, Roman RJ. 20-Hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu CC, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of κB kinase. Hypertension 57: 788–794, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12-20-HETE synthase. J Am Soc Nephrol; 10.1681/ASN.2012070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat 96: 45–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaghini FA, Zhang C, Parmentier JH, Estes AM, Jafari N, Schaefer SA, Malik KU. Contribution of arachidonic acid metabolites derived via cytochrome P4504A to angiotensin II-induced neointimal growth. Hypertension 45: 1182–1187, 2005 [DOI] [PubMed] [Google Scholar]