Abstract
We examined the effects of increased expression of proximal tubule peroxisome proliferator-activated receptor (PPAR)α in a mouse model of renal fibrosis. After 5 days of unilateral ureteral obstruction (UUO), PPARα expression was significantly reduced in kidney tissue of wild-type mice but this downregulation was attenuated in proximal tubules of PPARα transgenic (Tg) mice. When compared with wild-type mice subjected to UUO, PPARα Tg mice had reduced mRNA and protein expression of proximal tubule transforming growth factor (TGF)-β1, with reduced production of extracellular matrix proteins including collagen 1, fibronectin, α-smooth muscle actin, and reduced tubulointerstitial fibrosis. UUO-mediated increased expression of microRNA 21 in kidney tissue was also reduced in PPARα Tg mice. Overexpression of PPARα in cultured proximal tubular cells by adenoviral transduction reduced aristolochic acid-mediated increased production of TGF-β, demonstrating PPARα signaling reduces epithelial TGF-β production. Flow cytometry studies of dissociated whole kidneys demonstrated reduced macrophage infiltration to kidney tissue in PPARα Tg mice after UUO. Increased expression of proinflammatory cytokines including IL-1β, IL-6, and TNF-α in wild-type mice was also significantly reduced in kidney tissue of PPARα Tg mice. In contrast, the expression of anti-inflammatory cytokines IL-10 and arginase-1 was significantly increased in kidney tissue of PPARα Tg mice when compared with wild-type mice subjected to UUO. Our studies demonstrate several mechanisms by which preserved expression of proximal tubule PPARα reduces tubulointerstitial fibrosis and inflammation associated with obstructive uropathy.
Keywords: peroxisome proliferator-activated receptor, transforming growth factor-β, interleukin-10
recent studies support the notion that patients who develop acute kidney injury (AKI) are at higher risk of developing chronic kidney disease (CKD) (15, 26). The mechanisms that lead to progression from AKI to CKD in humans are not entirely clear, but available studies using numerous animal models suggest that the development of progressive interstitial fibrosis, diminished capillary density, and increased levels of angiotensin II, among other factors, represent mechanistic pathways by which AKI can lead to CKD (6). There is increased evidence suggesting that a sub-family of nuclear receptor transcription factors, known as peroxisome proliferator-activated receptors (PPARs), reduces inflammation and ameliorates tissue fibrosis, but the mechanisms involved in this cytoprotective response have not been elucidated (16, 39, 42, 44). PPARs are metabolic regulators with anti-inflammatory properties in various organs including the kidney (41). Our previous work using PPARα ligands and PPARα transgenic (Tg) mice demonstrated that preserving intact function of proximal tubule epithelial PPARα is cytoprotective during AKI (22–24, 32). PPARα is a nuclear receptor transcription factor expressed predominantly in the proximal tubule and the thick ascending limb of Henle that plays an important role in the modulation of energy utilization through the regulation of fatty acid oxidation (FAO) and oxidant production in mitochondria and peroxisomes (17, 27, 36). The importance of PPARα in modulating FAO by peroxisomes and mitochondria in kidney tissue is underscored by the response of renal PPARα to dietary lipids and by the cytoprotective effects of increasing its expression in the proximal tubule during AKI (23, 24, 35).
In the present study, we extended previous observations made in our laboratory and compared wild-type with PPARα Tg mice using the model of unilateral ureteral obstruction (UUO)-mediated renal fibrosis to define potential cellular mechanisms by which increased expression of proximal tubule PPARα reduces tubulointerstitial fibrosis. Our studies suggest that activation of proximal tubule PPARα may represent a novel therapeutic target to reduce renal fibrosis and ameliorate progression to CKD.
METHODS
Generation of proximal tubule PPARα Tg mice.
Proximal tubule-specific expression of PPARα under the control of the androgen-sensitive KAP (kidney androgen-regulated protein) promoter was achieved with KAP2-PPARα Tg mice, which were generated and characterized as previously described (23). Eight- to ten-week-old, age- and weight-matched PPARα Tg and wild-type (C57 BL/6) female mice were used for the studies. To investigate the effects of increased expression of proximal tubule PPARα, wild-type and PPARα Tg mice received a 5-mg subcutaneous testosterone pellet (Innovative Research of America, Sarasota, FL) and after 9 days were subjected to surgery (see scheme of experimental time course in Fig. 1A). The animals were housed at the Veterinary Medical Unit at the Central Arkansas Veterans Healthcare System (Little Rock, AR). When appropriate, animals were painlessly euthanized according to methods of euthanasia approved by the Panel on Euthanasia of the American Veterinary Medical Association. Our animal study protocols were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System.
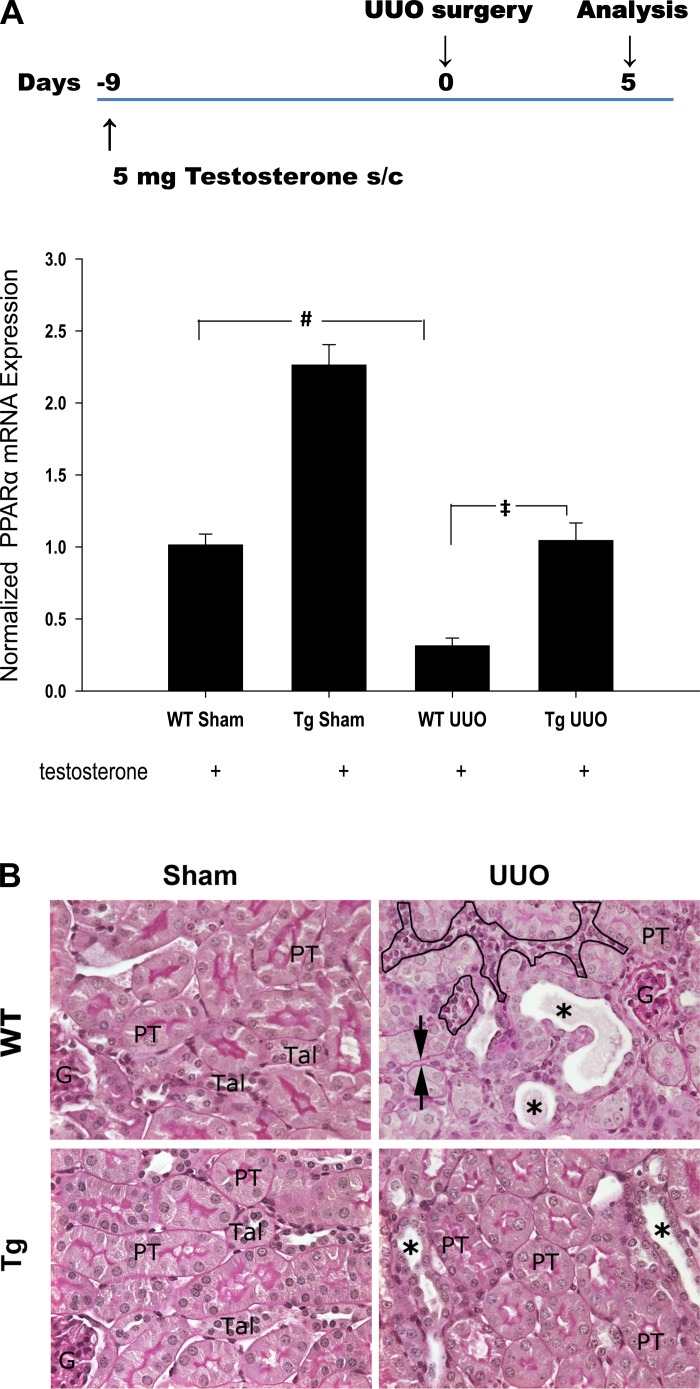
Fig. 1.
A, top: schematic diagram showing the experimental time course. Animals received a subcutaneous pellet of 5 mg testosterone for 9 days before unilateral ureteral obstruction (UUO) surgery. Kidneys were harvested 5 days after UUO. A, bottom: effect of UUO on renal peroxisome proliferator-activated receptor (PPAR)α mRNA expression in both wild-type (WT) and transgenic (Tg) mice. Level of PPARα was determined by qPCR. Data are expressed as means ± SE. #P < 0.005 when comparing WT sham vs. UUO mice. ‡P < 0.005 when comparing WT UUO vs. PPARα Tg UUO mice by unpaired Student's t-test. B: representative photographs of periodic acid Schiff (PAS)-stained sections of sham-operated and 5 days after UUO kidneys from WT and PPARα Tg animals. The sham-operated WT and PPARα Tg mice showed normal kidney architecture, whereas WT mice subjected to UUO showed dilated distal nephron segments, with casts (*), interstitial expansion (circled area), and thickening of basement membrane (arrow head). In PPARα Tg mice 5 days after UUO only some tubular dilation was observed (*). PT, proximal tubule; TAL, thick ascending limbs; G, glomerulus. Magnification: ×488.
Renal fibrosis model of UUO.
KAP2-PPARα Tg and wild-type mice, 8–10 wk old, were assigned to four treatment groups. Either UUO or sham surgeries were performed on both wild-type and PPARα Tg mice. All four groups of animals received 5-mg (21-day release) subcutaneous testosterone pellets 9 days before surgery. The left kidney was exposed through a midline incision under sterile conditions; the ureter was dissected and securely tied at two places with 6–0 silk sutures. Volume depletion was prevented by administration of ∼0.1 ml saline into the peritoneal cavity. The midline incision was closed; the mice were returned to their cages and allowed free access to food and water for 5 days. As control, sham surgery was performed the same way as UUO without tying the ureter. After 5 days, the mice were euthanized and the left kidneys from UUO and sham mice were collected for protein, RNA isolation, and histological evaluation.
Gene expression studies.
PPARα, transforming growth factor-β1 (TGF-β1), α-smooth muscle actin (α-SMA), collagen type I, α1 (Col1A1), fibronectin (Fn), collagen type IV α1 (Col4A1), laminin β, interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), arginase 1, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), CD86 mRNA levels, and miRNA21 (miR21) mRNA levels were determined by quantitative RT-PCR. Total RNA was extracted from cells or mouse kidney tissue and treated with RNase-free DNase before RT reaction. Real-time PCR was carried out using the StepOnePlus real-time PCR system (Invitrogen, Foster City, CA) with iTaqSYBR Green Supermix with Rox (Bio-Rad, Hercules, CA). In each experiment, triplicates of 50-ng cDNA (total RNA equivalent) samples were amplified in a 20-μl reaction. Specificity of the amplified product was confirmed by melting curve analysis and agarose gel electrophoresis. For relative quantification, a standard curve was generated from a six-step cDNA dilution series. Samples were amplified with primers for PPARα, TGF-β1, α-SMA, Col1A1, Fn, Col4A1, laminin β, IL-1β, IL-6, IL-10, TNF-α, arginase 1, MCP-1, MIP-1α, CD86, miR21, and 18S RNA. The relative expression of genes was calculated from the standard curve. Relative quantity was calculated by the ratio of the gene-specific and the appropriate 18S rRNA expression. The primer sequences in the RT-PCR were the following: for PPARα, 5′-AAA GAG GCA GAG GTC CGA TT-3′ (forward), 5′-AGC AAG GTG ACT TGG TCG TT-3′ (reverse); for TGF-β1, 5′-CGA GGC GGT GCT CGC TTT GT-3′ (forward), 5′-CAT AGA TGG CGT TGT TGC GGT CCA-3′ (reverse); for α-SMA, 5′-TCG CTG TCA GGA ACC CTG AGA CG-3′ (forward), 5′-ATC ATC ACC AGC GAA GCC GGC-3′ (reverse); for fibronectin 1, 5′-TCC ACA GCC ATT CCT GCG CC-3′ (forward), 5′-GTT CAC CCG CAC CCG GTA GC-3′ (reverse); for Col1A1, 5′-GCC CCA AGG GTC CTT CCG GT-3′ (forward), 5′-AGG ACC AGG GCT GCC AGG AC-3′ (reverse); for Col4A1, 5′-GGT ATT CAG GGA GAC CGT GG-3′ (forward), 5′-ACC CTT GTG CAC CCC TAG AT-3′ (reverse); for laminin β, 5′-CTA CTG TAA GCG CCT GGT GA-3′ (forward), 5′-CCT CGG AGC AGC TAT TGT TCA-3′ (reverse); for IL-1β, 5′-GAC CCC AAA AGA TGA AGG GCT-3′ (forward), 5′-TGT GCT GCT GCG AGA TTT GA-3′ (reverse); for IL-6, 5′-TCC GGA GAG GAG ACT TCA CA-3′ (forward), 5′-TTG CCA TTG CAC AAC TCT TTT CT-3′ (reverse); for IL-10, 5′-GCA TGG CCC AGA AAT CAA GG-3′ (forward), 5′-AGG GGA GAA ATC GAT GAC AGC-3′ (reverse); for TNF-α, 5′-ATG GCC TCC CTC TCA TCA GT-3′ (forward), 5′-CTT GGT GGT TTG CTA CGA CG-3′ (reverse); for arginase 1, 5′-GCC GAT TCA CCT GAG CTT TG-3′ (forward), 5′-CTG AAA GGA GCC CTG TCT TGT-3′ (reverse); for MCP-1, 5′-AGC TGT AGT TTT TGT CAC CAA GC-3′ (forward), 5′-GAC CTT AGG GCA GAT GCA GT-3′ (reverse); for MIP-1α, 5′-GCC AGG TGT CAT TTT CCT GAC TA-3′ (forward), 5′-GGC ATT CAG TTC CAG GTC AGT-3′ (reverse); for CD86, 5′-TCA AGG ACA TGG GCT CGT ATG-3′ (forward), 5′-AGG TTC ACT GAA GTT GGC GAT-3′ (reverse); for 18S rRNA, 5′-AGG AGT GGG CCT GCG GCT TA-3′ (forward), 5′-AAC GGC CAT GCA CCA CCA CC-3′ (reverse). Quantitative PCR for miR21 or the housekeeping small nucleolar RNA Sno234 was performed using the reverse universal primer specific to the annealed polyA tail and the specific forward primer to the miR21 (5′-GG TAG CTT ATC AGA CTG ATG TTG A-3′) or Sno234 (5′-GCG CGG AAC TGA ATC TAA GTG ATT TAA CAA-3′).
Protein expression by Western blotting.
Control and experimental (UUO) kidneys were lysed in 1× lysis buffer (Cell Signaling) containing 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1× protease inhibitor cocktail (Calbiochem, San Diego, CA), and 1 mM PMSF. Protein concentration was measured with the BioRad Protein Assay Kit (Pierce, Rockford, IL). After protein concentration was determined, 70 μg protein per lane were loaded with 4× SDS loading buffer, resolved on 15% polyacrylamide (PAGE) gel (Bio-Rad), and transferred onto a PVDF membrane using a Bio-Rad mini-blot apparatus. The membranes were blocked in 5% nonfat milk solution for 1 h and then incubated with primary antibodies against TGF-β1 (dilution 1:1,000; cat. no. SC-146, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Membranes were washed extensively, incubated with anti-rabbit secondary antibodies (1:5,000 dilution; Abcam, Cambridge, MA), and then washed again. Detection was performed with the Amersham ECL Western blotting analysis kit (Thermo Scientific, West Palm Beach, FL). The membranes were stripped and then probed with GAPDH as loading control. Signals on the blots were visualized by autoradiography and quantified by densitometry using the ImageQuant image analysis system (Agfa Duoscan HiD Scanner; Hamrick Software, Phoenix, AZ).
In situ determination and quantitative analysis of fibrotic markers in kidney tissues.
Formalin-fixed, paraffin-embedded 5-μm kidney sections from sham and UUO kidneys were stained with picro-sirius red for 1 h. Positive collagen staining in the interstitium was detected by light microscopy using circularly polarized light. Photographs from the entire cross-sections of kidneys were analyzed by Image J software (http://rsbweb.nih.gov/ij/download.html) to quantify the collagen accumulation. Five-micrometer sections from the same kidneys were stained with periodic acid Schiff reagent and used to evaluate the morphologic changes by light microscopy. The same sections were used for immunohistochemistry to determine TGF-β1 localization in the kidney section from 5-day UUO and sham-operated animals. The sections were pretreated with mild saponin digestion and TGF-β1 was detected with rabbit polyclonal antibody (1:200 dilution from Santa Cruz Biotechnology). Antibody reaction was visualized using the ABC Elite Vectastain Kit (Vector Laboratories, Burlingame, CA).
Proximal tubule cell culture and aristolochic acid treatment.
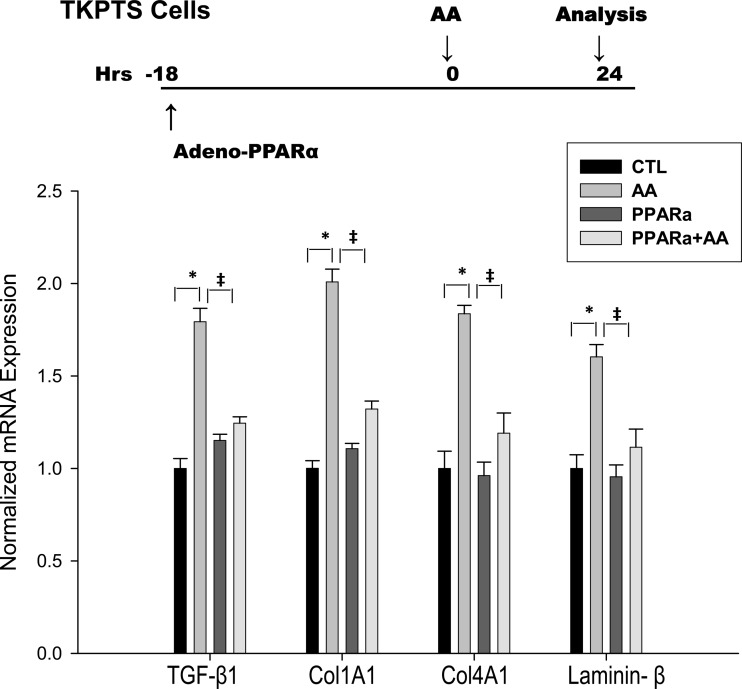
To examine in vitro mechanisms by which increased expression of proximal tubule PPARα reduces fibrosis, we used mouse kidney proximal tubule cells (TKPTS) in culture and exposed them to aristolochic acid (AA). AA is known to cause progressive renal fibrosis (37, 46). Mouse kidney proximal tubule cells (TKPTS) (9) were grown at 37°C with 5% CO2 in DMEM+Ham's F12 medium supplemented with 50 μU/ml insulin and 7% FBS. Cells were grown for 30 h after being split, and PPARα adenovirus was then added to a final multiplicity of infection of 100, which resulted in an approximately threefold increase of PPARα protein expression. Where indicated, cultures were treated with AA at 0.5 μg/ml concentration and grown for an additional 24 h. At that time, cells were harvested and total RNA was isolated using TRIzol reagent (Invitrogen). RNA samples were used for RT-PCR to analyze TGF-β1, Col1A1, Col4A1, and laminin β as described above.
Kidney tissue processing and flow cytometry analysis of inflammatory cells.
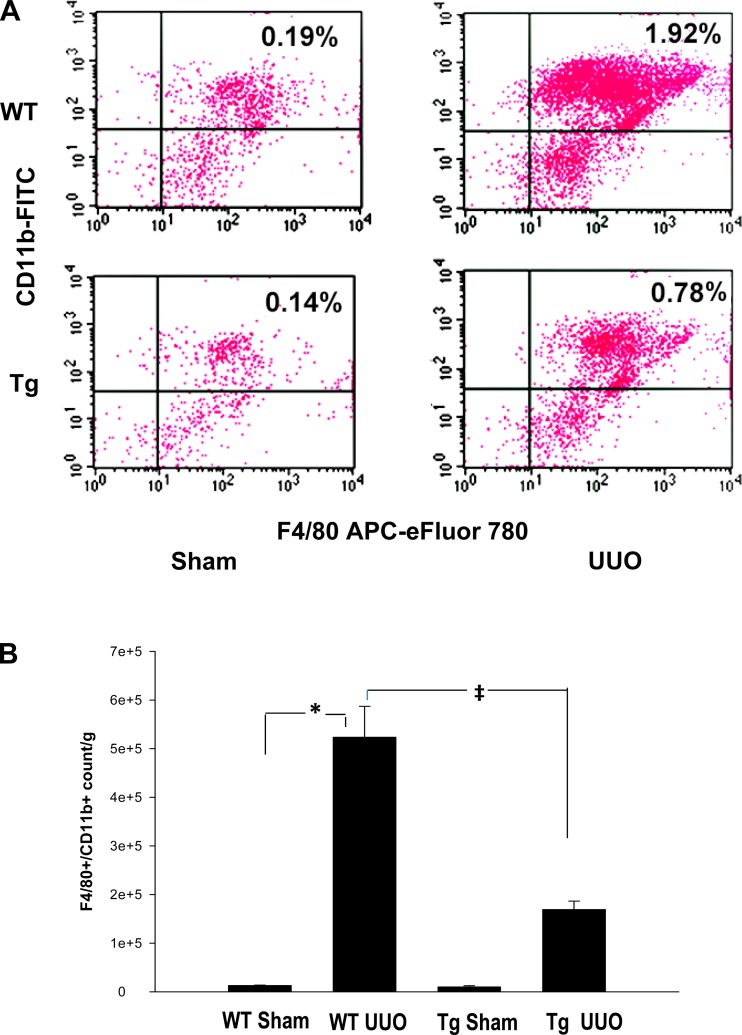
To study the kidney CD45+/CD11b+/F4/80+ macrophage population in UUO and sham-operated wild-type and PPARα Tg mice, we prepared a cell suspension of kidney tissue following the protocols published by Li et al. (20, 21). Briefly, kidneys were perfused with PBS, weighed, minced, and incubated with collagenase enzyme type IA (10 μg/ml; Sigma, St. Louis, MO, catalog no. C9891) in cold Dulbecco's PBS buffer containing 2 mM EDTA for 15 min at 37°C. After digestion, cell suspension was passed through a 100-μm BD Falcon cell-strainer (Fisher Scientific, Pittsburgh, PA) and pellet by centrifugation at 1,200 rpm for 10 min. The cell pellet was washed in FACS buffer (1% BSA in PBS containing 0.1% sodium azide) and resuspended again in FACS buffer. Individual sample volume was adjusted based on the kidney weight. After nonspecific Fc binding with anti-mouse CD16/32 antibodies was blocked, an aliquot of cells was immunostained with CD45-PE alone or in combination with CD11b-FITC, F4/80-APC eFluor 780. Cells were also stained with 7-aminoactinomycin D (7-AAD; 2 μg/ml; Invitrogen Life Technologies-Molecular Probes, Grand Island, NY) to gate out dead cells. Antibodies were purchased from eBioscience (San Diego, CA) and immunostaining protocols were carried out on ice and in the dark.
Multicolor flow cytometry analysis was carried out using FACS Calibur. Total leukocytes in each sample were estimated by using Caltag counting beads (Invitrogen) followed by CD45+ absolute count per kidney using the method and formula by the manufacturer's recommendation (21). Total leukocyte count and macrophage subset analyses were carried out by using lineage-specific markers. Leukocytes and macrophages within the kidney were quantified by analyzing at least 200,000 cells from each sample. Our results were confirmed by using ImageStreamX flow cytometry and IDEAS software (Amnis, Seattle, WA).
Statistical analysis.
Results are presented as means ± SE. Statistical analysis was performed using an unpaired Student's t-test. A P value of <0.05 was considered to be statistically significant.
RESULTS
PPARα expression downregulated after UUO is attenuated in PPARα Tg mice.
PPARα expression was increased in the Tg mice by 2.3-fold (Fig. 1A) in sham-operated mice when compared with wild-type mice. Five days after UUO, PPARα expression was reduced by ∼75% in wild-type mice. Although it was also reduced in PPARα Tg mice subjected to UUO, the level of expression was similar to that observed in sham-operated wild-type mice.
Increased proximal tubule PPARα expression ameliorates UUO-induced morphological damage.
Sham-operated kidneys from wild-type and Tg animals showed normal architecture with no tubular dilation or interstitial changes. Five days after UUO, kidneys from wild-type mice showed significant dilation of the distal nephron segments with cast formation and epithelial atrophy. Occasional loss of brush border was observed in some proximal tubules. In some areas the interstitial space was wider and hypercellular, and the thickening of the tubular basement membrane could also be seen. PPARα Tg mice subjected to UUO also revealed tubular dilatation and some brush-border loss; however, the interstitial changes were rather similar to those seen in the sham-operated groups (Fig. 1B).
Increased proximal tubule PPARα expression reduces UUO-mediated increased expression of fibrogenic genes.
We found that Tgfβ1, Col1a1, Acta2 (α-SMA), and Fn1 transcripts were upregulated by UUO in wild-type mice. There was a 4.1-, 10.4-, 12.6-, and 10.1-fold increased level of mRNA expression for Tgfβ1, Col1a1, Acta2, and Fn1, respectively (P < 0.001), after 5 days of UUO compared with sham-operated mice, as shown in Fig. 2A. Prior overexpression of PPARα in the Tg mice significantly attenuated the UUO-induced upregulation of Tgfβ1, Col1a1, Acta2, and Fn1 transcripts when compared with wild-type mice (P < 0.05).
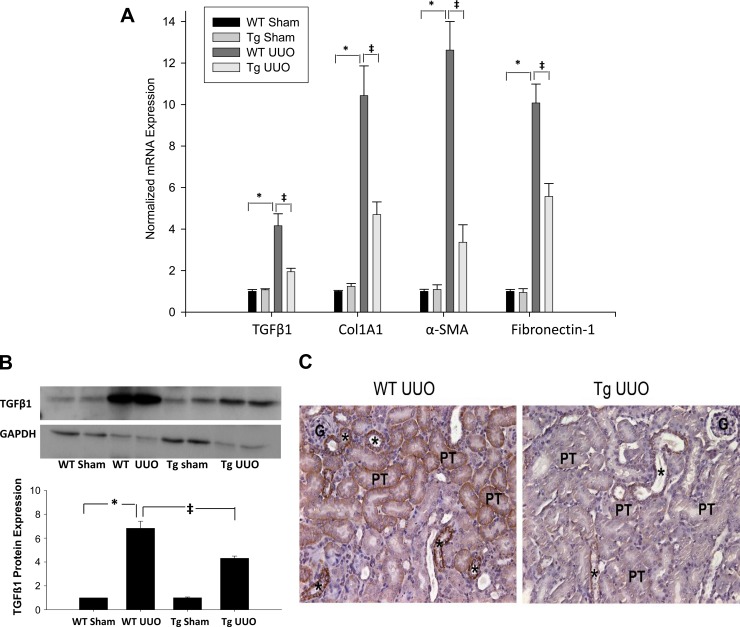
Fig. 2.
A: effect of UUO on renal fibrogenic gene transcripts transforming growth factor (TGF)-β1, collagen type I, α1 (Col1A1), α-smooth muscle actin (SMA), and fibronectin. Bars represent means ± SE mRNA levels for at least 4 mice in each group. *P < 0.001 when comparing WT sham vs. WT-UUO mice. ‡P < 0.05 when comparing WT UUO vs. PPARα Tg UUO mice. B: TGF-β1 protein expression in kidney tissue of WT and PPARα Tg mice subjected to sham and 5 days UUO. B: densitometry and quantification of TGF-β1 signals, normalized to GAPDH levels from Western blot analysis; GAPDH was used as a loading control. Data are expressed as means ± SE. *P < 0.005 when comparing WT sham vs. WT UUO mice. ‡P < 0.005 when comparing WT UUO vs. PPARα Tg UUO mice by unpaired Student's t-test. C: representative photomicrographs of TGF-β1 immunostaining in WT and Tg mice 5 days after UUO. Positive staining is obvious in the cortical thick ascending limbs and proximal convoluted tubules of WT animals. The positive staining is reduced from Tg UUO kidney. *, thick ascending limb of loop of Henle; PT, proximal convoluted tubules. Magnification: ×244.
TGF-β1 protein expression increased sevenfold in wild-type mice 5 days after UUO. This increased expression was significantly reduced in PPARα Tg mice when compared with wild-type mice (Fig. 2B). Formalin-fixed, paraffin-embedded kidney sections were also used for the in situ localization of TGF-β1. Indistinguishable positive immune reaction could be detected in medullary and cortical thick ascending limbs in both wild-type and Tg kidneys. Five days after UUO showed a strong immunostaining for TGF-β in cortical thick ascending limbs, but also a positive staining could be seen in the basolateral surfaces of convoluted proximal tubules (S1-S2 segments) of the wild-type kidneys. The positive TGF-β1 immunoreaction was almost completely diminished from the PPARα Tg mouse kidneys 5 days after UUO. Only occasional weak staining could be found in some cortical thick ascending limbs. There was no obvious staining in proximal tubule segments in the Tg UUO group (Fig. 2C).
Proximal tubule PPARα reduces UUO-mediated interstitial fibrosis.
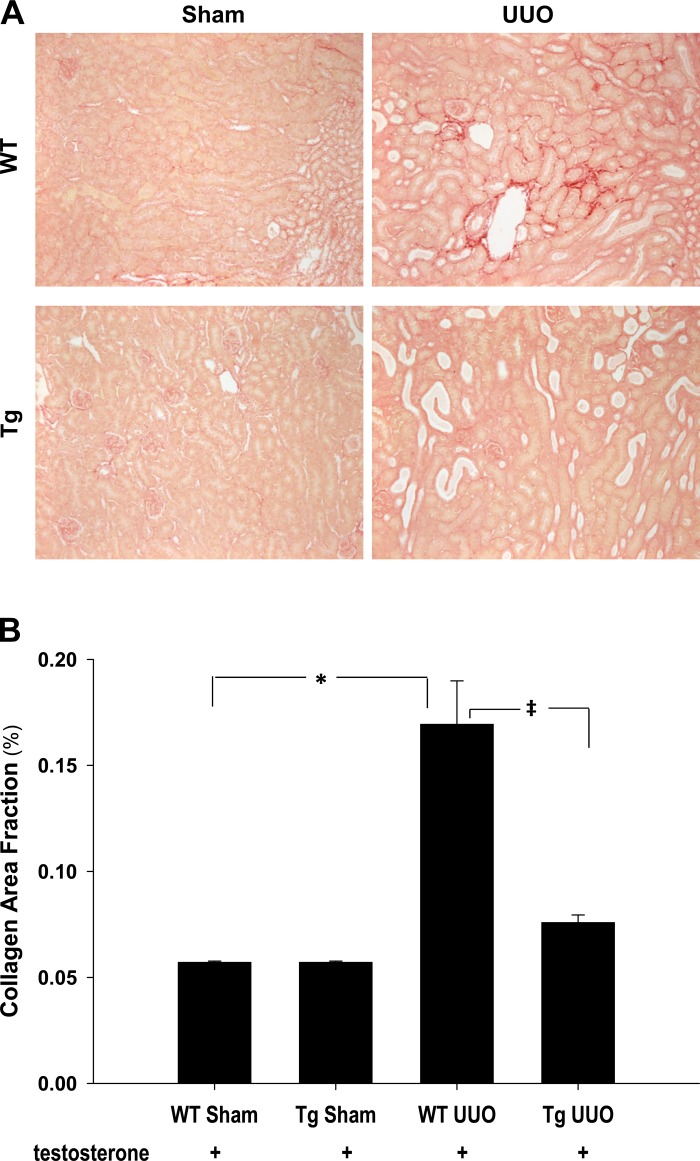
To quantify the amount of collagen produced in the interstitium in wild-type and PPARα Tg mice subjected to UUO, kidney sections were stained with picro-sirius red staining. Positive red stain in the interstitium was increased 5 days after UUO in wild-type kidneys compared with sham ones (P < 0.05), and it was significantly reduced (P < 0.05) in PPARα Tg mice as shown in Fig. 3, A and B, indicating that PPARα upregulation ameliorated UUO-induced kidney fibrosis.
Fig. 3.
A: representative photomicrographs of picro-sirius red-stained sham and 5 days UUO kidney sections from WT and Tg animals. Collagen accumulation can be seen, indicated by the red staining in the WT UUO section. No significant positive staining was seen in shams and Tg UUO kidneys. Magnification: ×122. B: quantitative analysis of picro-sirius red staning in kidney sections from sham and 5 days UUO WT and Tg animals. Collagen accumulation was significantly increased in WT UUO kidneys, and it was unchanged in the Tg UUO samples compared with sham-operated ones. *P < 0.05 when comparing WT sham vs. WT UUO mice. ‡P < 0.05 when comparing WT UUO vs. Tg UUO mice in unpaired Student's t-test.
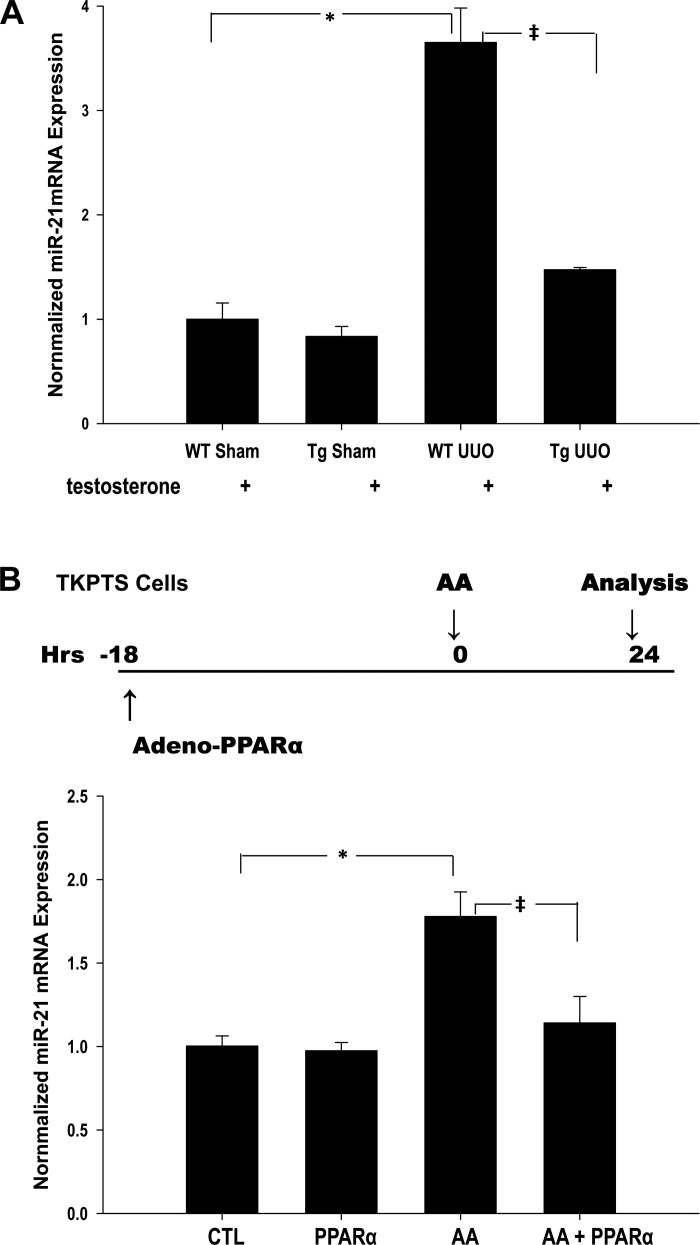
Increased proximal tubule PPARα reduces UUO-mediated increased miR21 expression.
miR21 directly suppresses expression of PPARα and silences other genes in the PPARα downstream signaling pathway in mitochondria and peroxisomes (5). As expected, miR21 expression determined by qPCR was significantly upregulated after UUO (3.5-fold) in wild-type mice (P < 0.05). Overexpression of PPARα in the kidney tubule after UUO, however, reduced miR21 levels close to those in normal kidneys (Fig. 4A). This observation was recapitulated in vitro in TKPTS cells injured by the tubular cell toxin AA (see Fig. 4B).
Fig. 4.
PPARα repressed UUO-mediated upregulation of miRNA21 (miR21) in kidney tissue and also reduces aristolochic acid (AA)-stimulated miR21 expression in TKPTS cells. Level of miR21 mRNA was determined by qPCR. A: quantitative analysis of miR21 expression of WT and PPARα Tg mice subjected to sham and UUO surgery. *P < 0.05 when comparing changes in miR21 expression between WT sham and WT UUO mice. ‡P < 0.05 when comparing changes in miR21 expression between WT UUO and Tg UUO mice in unpaired Student's t-test. B: TKPTS cells were incubated with PPARα adenovirus for 18 h before being treated with AA and grown for an additional 24 h. Bars represent means ± SE mRNA levels for at least 4 independent experiments in each group. *P < 0.05 when comparing untreated cells (control) vs. AA-treated cells. ‡P < 0.05 when comparing AA-treated cells vs. AA in the presence of PPARα (AA+PPARα) in unpaired Student's t-test.
Overexpression of PPARα in proximal tubule (TKPTS) cells reduces TGF-β1 production and collagen 1a1, collagen 4, and laminin expression.
Release of active TGF-β by injured epithelial cells is implicated in the pathogenesis of epithelial injury and interstitial fibrogenesis in the kidney. Moreover, injured epithelial cells are believed to secrete increased tubule basement membrane proteins including collagen IV and laminins. To evaluate the effect of PPARα on cultured epithelial cells, we overexpressed PPARα in TKPTS cells by adenoviral transduction and injured epithelial cells with AA. Whereas AA stimulated epithelial TGF-β production, as well as degradation of collagen 1, collagen IV, and laminin, PPARα overexpression blunted these effects. These results suggest that PPARα reduces tubular epithelial cell injury by preventing AA-mediated degradation of tubule basement membrane (Fig. 5).
Fig. 5.
PPARα inhibited AA-stimulated TGF-β1, Col1A1, Col4A1, and laminin-β mRNA expression in TKPTS cells. Levels of mRNA were determined by qPCR. TKPTS cells were incubated with PPARα adenovirus for 18 h before being treated with AA and grown for an additional 24 h. *P < 0.05 when comparing untreated cells (control) vs. AA-treated cells. ‡P < 0.05 when comparing AA-treated cells vs. cells treated with AA in the presence of PPARα (AA+PPARα) in unpaired Student's t-test.
Increased proximal tubule PPARα reduces infiltration of kidney tissue macrophages.
Single cell preparations from whole kidney were initially analyzed by flow cytometry for CD45+, 7-AAD-negative cells to identify the total live leukocyte population. A significant increase in the total CD45+ population indicated a major influx of leukocytes into kidneys was seen as early as 48 h but persistent accumulation was seen after 5 days of UUO in wild-type mice (Fig. 6, A–B), whereas the sham-operated mice did not show similar perturbation. These results were confirmed by Image StreamX flow cytometry (data not included). After ascertaining that the mononuclear phagocytes were one of the major infiltrating cells in the UUO kidney, we investigated the role of PPARα-mediated protection by flow cytometry analysis of inflammatory mononuclear cells. CD45+/CD11b+/F4/80+ cells were considered proinflammatory monocyte/macrophages based on previous studies (11). Tg overexpression of PPARα in proximal tubules markedly blunted this response such that leukocyte recruitment was reduced by more than 50% as shown in Fig. 6, A and B.
Fig. 6.
Reduced infiltration of inflammatory mononuclear phagocytes after UUO in PPARα Tg mice. Kidney cell suspensions obtained from mice subjected to 4 experimental conditions were analyzed by flow cytometry as described in methods. Inflammatory macrophages were identified as F4/80+/CD11b+ cells. Comparisons were made between WT and PPARα Tg mice. A: flow histogram of inflammatory macrophages identified as F4/80+/CD11b+ cells. B: quantification of macrophage accumulation in kidney tissue that was significantly reduced in PPARα Tg mice subjected to UUO when compared with WT mice. Data represent means ± SE of 3 independent experiments. *P < 0.05 when comparing WT sham vs. WT UUO mice. ‡P < 0.05 when comparing WT UUO vs. Tg UUO mice using unpaired Student's t-test.
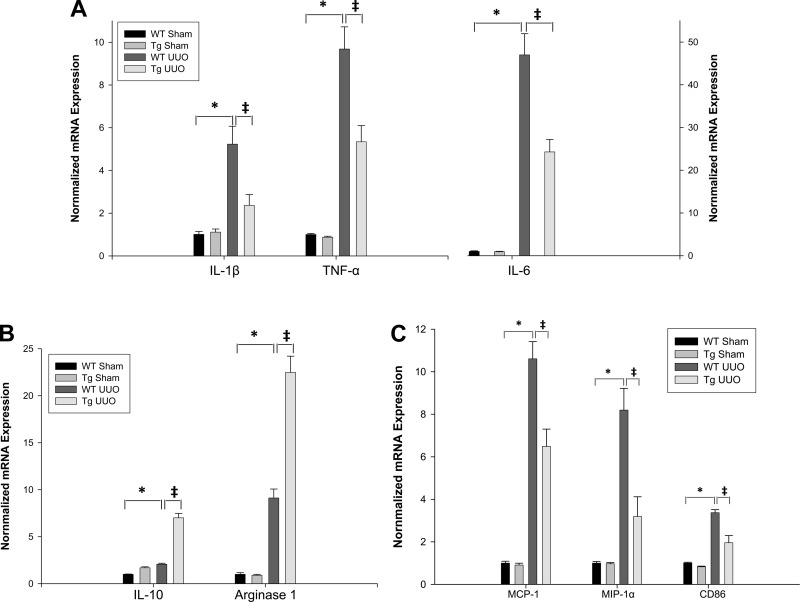
PPARα increases IL-10 expression and suppresses renal UUO-induced proinflammatory cytokines/chemokines IL-1β, IL-6, TNF-α, MCP-1, and MIP-1α.
To investigate the mechanisms by which increased expression of proximal tubule PPARα ameliorated UUO-mediated renal fibrosis, mRNA expression levels of pro- and anti-inflammation cytokines/chemokines were investigated. As shown in Fig. 7, there were 5.2-, 9.7-, 46.9-, 2.1-, 10.6-, and 8.2-fold increases of mRNA expression for IL-1β, TNF-α, IL-6, IL-10, MCP-1, and MIP-1α, respectively (P < 0.001), 5 days after UUO surgery in wild-type mice when compared with sham-operated mice. Overexpression of PPARα in Tg mice significantly attenuated the UUO-induced upregulation of proinflammation cytokines/chemokines IL-1β, IL-6, TNF-α, MCP-1, and MIP-1α mRNA expression when compared with wild-type mice (P < 0.05). Anti-inflammatory cytokine IL-10 was further increased (7-fold) in Tg mice 5 days after UUO surgery compared with wild-type sham mice and was also significantly elevated (P < 0.05) when compared with wild-type UUO mice (Fig. 7B).
Fig. 7.
Effect of UUO on renal cytokine production. A: changes in IL-1β, TNF-α, IL-6 mRNA levels. B: changes in IL-10 and arginase-1 mRNA levels in WT and PPARα Tg mice subjected to sham or UUO surgery. Levels of mRNA were determined by qPCR. Bars represent means ± SE and mRNA levels were measured using 4 mice in each group. *P < 0.001 when comparing WT sham vs. WT UUO mice. ‡P < 0.05 when comparing WT UUO vs. Tg UUO mice in unpaired Student's t-test. C: effect of UUO on renal monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and macrophage surface marker CD86 mRNA levels in WT and PPARα Tg mice subjected to sham and UUO surgery. Bars represent means ± SE mRNA levels for at least 4 mice in each group. *P < 0.001 when comparing WT sham vs. WT UUO mice. ‡P < 0.05 when comparing WT UUO vs. Tg UUO mice in unpaired Student's t-test.
PPARα increases expression of arginase-1 and repressed expression of macrophage marker CD86.
To study the roles of PPARα on macrophages in UUO-induced renal fibrosis, we examined the mRNA expression of surface markers/receptors of M1/M2 macrophages. As shown in Fig. 7, B and C, there were 9.1- and 3.4-fold increases of mRNA expression for the macrophage markers arginase-1 and CD86, respectively (P < 0.001), 5 days after UUO surgery in wild-type mice when compared with sham mice. Overexpression of PPARα in Tg mice significantly suppressed the UUO-induced upregulation of CD86 mRNA expression when compared with wild-type mice (P < 0.05). In contrast, arginase-1 was further increased (22.5-fold) in Tg mice 5 days after UUO surgery when compared with wild-type sham mice, but arginase-1 levels were further increased when compared with wild-type UUO mice (P < 0.05).
DISCUSSION
Our study is the first to evaluate the role of and mechanisms by which increased expression of proximal tubule PPARα attenuates renal fibrosis. Several important findings are supported by our data. Our studies confirm previous observations demonstrating increased expression of TGF-β in cortical proximal tubules (PCT and PST) of UUO-treated wild-type mice, although there was some level of expression of TGF-β in the thick ascending limb, collecting duct, and also in the interstitial compartment of the obstructed kidney (10). The observed peritubular fibrosis present after 5 days of UUO in wild-type mice was almost absent in the kidney tissue of PPARα Tg mice. Importantly, PPARα Tg mice also produced less proximal tubule TGF-β, which was demonstrated by reduced mRNA, protein, and reduced immunostaining in the basolateral domain of proximal tubules. TGF-β is a pleiotropic cytokine that plays a major role in stimulating extracellular matrix production after UUO, and blocking TGF-β has been shown to reduce tubular apoptosis in several models of chronic kidney injury (25, 30). It contributes to renal fibrosis by several mechanisms including induced loss of phosphatase tensin homolog that contributes to the failure of regenerating epithelial cells to redifferentiate, thereby causing the retention of proliferative signaling and giving rise to profibrotic peptides (19). TGF-β also increases renal fibrosis not only by a direct effect on myofibroblasts but by inducing the production of Notch (1), CTGF (34), and PDGFβ (40). TGF-β also mediates upregulation of osteopontin in injured epithelial cells and triggers a focal inflammatory process with migration of monocytes and macrophages (28). We find that the UUO-mediated increased expression of fibrogenic gene transcripts including Col1A1, α-SMA, and fibronectin in wild-type mice was significantly reduced in PPAR-α Tg mice subjected to UUO. These results indicate that PPARα overexpression in the proximal tubule alone effectively reduces interstitial fibrosis in the model of UUO.
Similar to the in vivo model of UUO, in the in vitro model of fibrosis using AA to injure proximal tubule epithelial cells in culture, increased expression of PPARα directly reduces production of TGF-β and prevented AA-mediated degradation of collagen 4 and laminin, the major components of tubular basement membrane. In addition to injured tubule epithelium, TGF-β can be produced also by interstitial kidney fibroblasts, macrophages, and endothelium (12). Our findings as well as recently published data (3, 43) support the concept that, during injury, the induction of tubulointerstitial fibrosis occurs as a result of altered cross talk mechanisms between tubular epithelial cells and interstitial fibroblasts. We demonstrated in AKI models (23) that proximal tubule PPARα could increase mitochondrial FAO and reduce oxidant production, and we speculate that these cellular mechanisms could affect the production of TGF-β or its effect on transcription of fibrotic genes. Our in vivo studies do not allow us to examine the cellular mechanisms by which PPARα reduces TGF-β expression; however, a recent study suggests that mitochondrial reactive oxygen species derived from complex III of the mitochondrial electron transport chain are required for TGF-β-mediated transcription of profibrotic genes (18).
Another important observation in the present study is the significant inhibition of UUO-induced miR21 expression in kidney tissue of proximal tubule PPARα Tg mice that is also accompanied by reduced fibrosis. miRNAs are a class of ∼20-nucleotide single-strand endogenous RNAs that control the translation of mRNAs by promoting the degradation of target mRNAs or preventing their translation (4, 33). MiRNAs have recently been demonstrated to regulate cell proliferation, differentiation, and apoptosis (29). In a recent study, we described that PPARα expression and activity measured as FAO in kidney tissue were significantly reduced in models of renal fibrosis. In addition, miR21 expression was significantly elevated in mice subjected to an ischemic fibrosing model or UUO (5). Reducing miR21 expression in kidney tissue by using anti-miR21 oligonucleotides or miR21 knockout mice resulted in epithelial cell protection and less interstitial fibrosis in response to kidney injury (5). Moreover, using an array assay we demonstrated that PPARα and many genes regulating fatty acid metabolism are seed-matched targets for miR21 in the kidney indicating that miR21 directly silences PPARα and the downstream signaling pathways mediated by PPARα. The findings here of reduced expression of miR21 when proximal tubule PPARα was increased in Tg mice lend support to the notion of a feedback circuit that modulates miR21 expression in kidney tissue at the transcriptional level via the PPARα/AP1 signaling cascade (45). The reduced expression of miR21 during UUO also could be explained by reduced expression of TGF-β in PPARα Tg mice. TGF-β receptor-mediated signaling has been shown to directly regulate miRNA biogenesis by several mechanisms including direct gene transcription of miRNA genes, direct binding of TGF-β receptor-induced SMAD proteins to specific miRNAs, and by stabilization of miRNA processing machinery (7, 14). The net effect of TGF-β signaling is to upregulate miRNA biogenesis.
Our studies also show that increased expression of proximal tubule PPARα during UUO is accompanied by reduced infiltration of macrophages. In addition, kidney tissues of PPARα Tg mice had reduced expression of inflammatory cytokines including MCP-1 and MIP-1α, which help recruit macrophages to kidney tissue during UUO. Macrophages and dendritic cells derived from monocytes are abundant in kidney injury and play an important role in inflammation, tissue repair, and fibrosis. Ablative studies in several models of kidney interstitial disease indicate that macrophages significantly contribute to the development of fibrosis (8, 13, 31, 38). Several mechanisms have been proposed to explain monocyte/macrophage function. One is that the way monocytes are activated upon entering the diseased kidney dictates their differentiation and function. Another is that monocytes exist as functionally discrete subsets. We also find that increased expression of proximal tubule PPARα markedly reduced production of proinflammatory cytokines (TNFα, IL-6, and IL-1β) and increased the expression of anti-inflammatory cytokine IL-10. Thus, the increased expression of proximal tubule PPARα seems to affect gene expression of CD11b+/F4/80+ cell subpopulations suggesting that renal inflammation involves at least two phenotypically different monocyte/macrophage subpopulations: CD11b+/F4/80+ monocytes showing M1-type activation and CD11b+/F4/80+ IL-10 and arginase-1 producing M2-type macrophages. In a previous study, we described that the anti-inflammatory effect of PPARα ligand in the cisplatin model of AKI was mediated by inhibition of kidney tissue NF-κB and this could be an additional mechanism for the anti-inflammatory effect of PPARα on renal fibrosis (22). Our results are different from the ones published by a group of investigators that showed that using a PPARα ligand was cytoprotective, but they did not find any effects of the PPARα ligand on renal inflammatory markers using a similar model of UUO (2). We speculate that increased expression of proximal tubule PPARα protein in our PPARα Tg mice (23) as opposed to a nonspecific saturating effect of the PPARα ligand could account for the observed differences between the two studies.
In summary, we describe several mechanisms in this study by which increased expression of proximal tubule PPARα reduces inflammation and interstitial fibrosis including reduced production of TGF-β, reduced expression of miR21, and reduced influx of inflammatory macrophages. Together, these mechanisms contribute to reduced extracellular matrix production. Additional studies are needed to further examine the mechanisms by which PPARα affects production of TGF-β in renal epithelium, inflammatory cells, and/or pericytes, as well as the effects of increased expression of PPARα on UUO-mediated macrophage activation, production of IL-10, and the activation of pericytes to myofibroblasts.
GRANTS
D. Portilla's laboratory is funded by National Institutes of Health Grant DK75976, a Veterans Affairs Merit Award, and a Research Enhancement Award Program award. N. Mariappan was a T32 fellow supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant T32 DK061921.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.L., N.M., J.M., B.S., K.K., P.M.P., and D.P. performed experiments; S.L., N.M., J.M., K.K., P.M.P., and D.P. analyzed data; S.L., N.M., J.M., B.S., K.K., P.M.P., and D.P. interpreted results of experiments; S.L., N.M., J.M., and K.K. prepared figures; S.L., N.M., J.M., B.S., K.K., S.T., P.M.P., J.S.D., and D.P. edited and revised manuscript; S.L., N.M., J.M., B.S., K.K., S.T., P.M.P., J.S.D., and D.P. approved final version of manuscript; D.P. conception and design of research; D.P. drafted manuscript.
REFERENCES
- 1.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120: 4040–4054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boor P, Celec P, Martin IV, Villa L, Hodosy J, Klenovicsova K, Esposito C, Schafer S, Albrecht-Kupper B, Ostendorf T, Heidland A, Sebekova K. The peroxisome proliferator-activated receptor-[alpha] agonist, BAY PP1, attenuates renal fibrosis in rats. Kidney Int 80: 1182–1197, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Campanholle G, Ligresti G, Gharib SA, Duffield JS. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol 304: C591–C603, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffield JS. Macrophages and immunologic inflammation of the kidney. Sem Nephrol 30: 234–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Fukuda K, Yoshitomi K, Yanagida T, Tokumoto M, Hirakata H. Quantification of TGF-β1 mRNA along rat nephron in obstructive nephropathy. Am J Physiol Renal Physiol 281: F513–F521, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gewin L, Zent R. How does TGF-β mediate tubulointerstitial fibrosis? Sem Nephrol 32: 228–235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, Ma FY, Tesch GH, Manthey CL, Nikolic-Paterson DJ. Role of macrophages in the fibrotic phase of rat crescentic glomerulonephritis. Am J Physiol Renal Physiol 304: F1043–F1053, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Hata A, Davis BN. Control of microRNA biogenesis by TGF beta signaling pathway-A novel role of Smads in the nucleus. Cytokine Growth Factor Rev 20: 517–521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwaisako K, Haimerl M, Paik YH, Taura K, Kodama Y, Sirlin C, Yu E, Yu RT, Downes M, Evans RM, Brenner DA, Schnabl B. Protection from liver fibrosis by a peroxisome proliferator-activated receptor δ agonist. Proc Natl Acad Sci USA 109: E1369–E1376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzedine H, Launay-Vacher V, Baumelou A, Deray G. Renal effects of PPARalpha-agonists. Minerva Urol Nefrol 56: 339–342, 2004 [PubMed] [Google Scholar]
- 18.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GRS, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem 288: 770–777, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, Griffin KA, Koesters R, Weinberg JM, Bidani AK, Kriz W, Venkatachalam MA. PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol 302: F1210–F1223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Sem Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol 289: F469–F480, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int 76: 1049–1062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Wu P, Yarlagadda P, Vadjunec NM, Proia AD, Harris RA, Portilla D. PPARα ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol 286: F572–F580, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Ling H, Li X, Jha S, Wang W, Karetskaya L, Pratt B, Ledbetter S. Therapeutic role of TGF-β-neutralizing antibody in mouse cyclosporin A nephropathy: morphologic improvement associated with functional preservation. J Am Soc Nephrol 14: 377–388, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Hernandez FJ, Lopez-Novoa JM. Potential utility of PPARalpha activation in the prevention of ischemic and drug-induced acute renal damage. Kidney Int 76: 1022–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin-a molecule for all seasons. QJM 95: 3–13, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev 15: 563–568, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, Hauswirth WW, Flotte TR, Rodriguez-Iturbe B, Johnson RJ. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol 16: 3651–3660, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int 73: 1374–1384, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z. The microRNA world: small is mighty. Trends Biochem Sci 28: 534–540, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133–143, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Ouali F, Djouadi F, Merlet-Benichou C, Bastin J. Dietary lipids regulate beta-oxidation enzyme gene expression in the developing rat kidney. Am J Physiol Renal Physiol 275: F777–F784, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Portilla D. Energy metabolism and cytotoxicity. Semin Nephrol 23: 432–438, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pozdzik AA, Salmon IJ, Debelle FD, Decaestecker C, Van den Branden C, Verbeelen D, Deschodt-Lanckman MM, Vanherweghem JL, Nortier JL. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int 73: 595–607, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Sugimoto T, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int 79: 871–882, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab 23: 351–363, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Weinberg JM. Mitochondrial biogenesis in kidney disease. J Am Soc Nephrol 22: 431–436, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, Wu TH, Linn GR, Ling H, Wu KD, Tsai TJ, Chen YM, Duffield JS, Lin SL. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 182: 118–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang F, Kong D, Lu Y, Zheng S. Peroxisome proliferator-activated receptor-γ as a therapeutic target for hepatic fibrosis: from bench to bedside. Cell Mol Life Sci 70: 259–276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JYJ, Chiu JJ, Li JYS, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA 108: 10355–10360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Fu P, Huang XR, Liu F, Chung AC, Lai KN, Lan HY. Mechanism of chronic aristolochic acid nephropathy: role of smad3. Am J Physiol Renal Physiol 298: F1006–F1017, 2010 [DOI] [PubMed] [Google Scholar]