Abstract
BACKGROUND
New biomarkers are needed to detect pleural mesothelioma at an earlier stage and to individualize treatment strategies. We investigated whether fibulin-3 in plasma and pleural effusions could meet sensitivity and specificity criteria for a robust biomarker.
METHODS
We measured fibulin-3 levels in plasma (from 92 patients with mesothelioma, 136 asbestos-exposed persons without cancer, 93 patients with effusions not due to mesothelioma, and 43 healthy controls), effusions (from 74 patients with mesothelioma, 39 with benign effusions, and 54 with malignant effusions not due to mesothelioma), or both. A blinded validation was subsequently performed. Tumor tissue was examined for fibulin-3 by immunohistochemical analysis, and levels of fibulin-3 in plasma and effusions were measured with an enzyme-linked immunosorbent assay.
RESULTS
Plasma fibulin-3 levels did not vary according to age, sex, duration of asbestos exposure, or degree of radiographic changes and were significantly higher in patients with pleural mesothelioma (105±7 ng per milliliter in the Detroit cohort and 113±8 ng per milliliter in the New York cohort) than in asbestos-exposed persons without mesothelioma (14±1 ng per milliliter and 24±1 ng per milliliter, respectively; P<0.001). Effusion fibulin-3 levels were significantly higher in patients with pleural mesothelioma (694±37 ng per milliliter in the Detroit cohort and 636±92 ng per milliliter in the New York cohort) than in patients with effusions not due to mesothelioma (212±25 and 151±23 ng per milliliter, respectively; P<0.001). Fibulin-3 preferentially stained tumor cells in 26 of 26 samples. In an overall comparison of patients with and those without mesothelioma, the receiver-operating-characteristic curve for plasma fibulin-3 levels had a sensitivity of 96.7% and a specificity of 95.5% at a cutoff value of 52.8 ng of fibulin-3 per milliliter. In a comparison of patients with early-stage mesothelioma with asbestos-exposed persons, the sensitivity was 100% and the specificity was 94.1% at a cutoff value of 46.0 ng of fibulin-3 per milliliter. Blinded validation revealed an area under the curve of 0.87 for plasma specimens from 96 asbestos-exposed persons as compared with 48 patients with mesothelioma.
CONCLUSIONS
Plasma fibulin-3 levels can distinguish healthy persons with exposure to asbestos from patients with mesothelioma. In conjunction with effusion fibulin-3 levels, plasma fibulin-3 levels can further differentiate mesothelioma effusions from other malignant and benign effusions. (Funded by the Early Detection Research Network, National Institutes of Health, and others.)
Despite advances in chemotherapy, radiation therapy, and surgical management for malignant pleural mesothelioma, the median survival remains 12 months.1 Early detection is limited by the long latency period, an inability of imaging to detect the disease at an early stage even when it is used as a screening strategy, and the lack of sensitive and specific blood-based markers.2 Moreover, in patients with undiagnosed pleural effusion, the ability to diagnose mesothelioma is delayed by failure to include the disease in the differential diagnosis and by the lack of noninvasive mesothelioma-specific blood-based markers. Soluble mesothelinrelated protein, the most extensively studied blood-based mesothelioma biomarker, is limited by an overall sensitivity of 47% at 96% specificity.3 A thrombin cleavage site impedes reproducible measurements in serum for osteopontin.4 Using genomic techniques similar to those described for our identification of osteopontin as a mesothelioma marker5 (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org), we assessed whether plasma levels of fibulin-3 have high specificity and sensitivity for discriminating patients with mesothelioma from asbestos-exposed persons and from patients with malignant or benign pleural effusions not due to mesothelioma.
Methods
Study Populations
Plasma and effusion samples from patients with pleural mesothelioma, plasma samples from persons who had been exposed to asbestos but did not have mesothelioma, and plasma and effusion samples from patients with pleural effusions not due to mesothelioma (Table 1) were obtained prospectively (by the first author) at Wayne State University from 1998 through 2005 (Detroit cohort) and at New York University Langone Medical Center from 2005 through 2011 (New York cohort). The EDTA-treated specimens were stored at −80°C.
Table 1.
Demographic and Clinical Characteristics and Fibulin-3 Levels According to Study Cohort.*
| Variable | Detroit Cohort | New York Cohort | Toronto Cohort | |||||
|---|---|---|---|---|---|---|---|---|
| Patients with Mesothelioma (N = 78) |
Asbestos-Exposed Persons (N = 41) |
Patients with Effusions Not Due to Mesothelioma (N = 53) |
Patients with Mesothelioma (N = 64) |
Asbestos-Exposed Persons (N = 95) |
Patients with Effusions Not Due to Mesothelioma (N = 40) |
Patients with Mesothelioma (N = 48) |
Asbestos-Exposed Persons (N = 96) |
|
| Demographic and clinical characteristics | ||||||||
| Age — yr | 65±1 | 64±1 | 63±2 | 65±2 | 59±1 | 62±3 | 64±1 | 65±1 |
| Sex — no. | ||||||||
| Male | 64 | 36 | 33 | 48 | 95 | 15 | 37 | 94 |
| Female | 14 | 5 | 20 | 16 | 0 | 25 | 11 | 2 |
| Race — no.† | ||||||||
| White | 75 | 31 | 39 | 59 | 95 | 36 | NA | NA |
| Other | 3 | 10 | 14 | 5 | 0 | 4 | NA | NA |
| Asbestos exposure — no. (%) | 62 (79) | 41 (100) | NA | 45 (70) | 95 (100) | NA | 32 (67) | 96 (100) |
| Current or former smoker — no. | ||||||||
| Yes | 43 | 31 | 31 | 39 | 59 | 19 | 24 | 62 |
| No | 35 | 10 | 22 | 25 | 36 | 21 | 24 | 34 |
| Fibulin-3 level | ||||||||
| Plasma fibulin-3 — ng/ml | 105.0±7.1 | 13.9±1.2‡ | NA | 112.9±7.6 | 24.3±1.4§¶ | 44.7±3.4§¶ | 66.4±7.2 | 13.9±2.1‖ |
| Effusion fibulin-3 — ng/ml | ||||||||
| Any effusion | 694.4±36.8 | 211.5±25.1‡ | 636.4±92.1§ | 150.6±22.7¶ | ||||
| Benign effusion | 242.3±34.5‡ | 142.2±35.6¶ | ||||||
| Malignant effusion | 181.8±36.1‡ | 159.7±28.9¶ | ||||||
Plus–minus values are means ±SE. NA denotes not available.
Race was self-reported.
P<0.001 for the comparison with patients with pleural mesothelioma in the Detroit cohort.
P<0.001 for the comparison between the New York cohort and the Detroit cohort.
P<0.001 for the comparison with patients with pleural mesothelioma in the New York cohort.
P<0.001 for the comparison with patients with pleural mesothelioma in the Toronto cohort.
Patients with other cancers were evaluated with regard to marker specificity, including 20 with ovarian cancer, 20 with breast cancer, 20 with glioblastoma, and 31 with prostate cancer (Fig. 1A). A total of 43 healthy controls (defined as persons with benign prostatic conditions and without cancer or exposure to asbestos) were also evaluated (Fig. 1A).
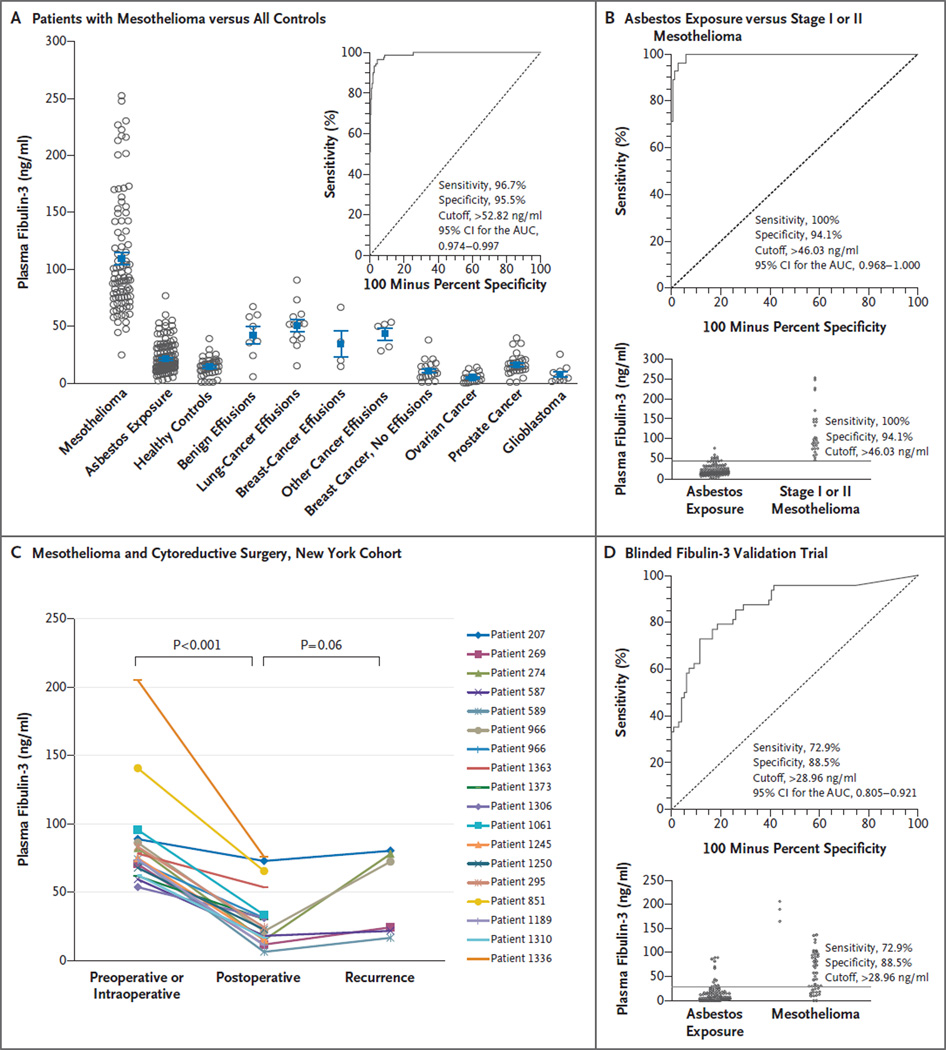
Figure 1. Plasma Fibulin-3 Levels.
Panel A shows plasma fibulin-3 levels in patients with malignant pleural mesothelioma as compared with all other cohorts. Patients for whom matched effusions were available had higher plasma levels than patients for whom effusions were not available. The inset shows the receiver-operating-characteristic (ROC) curve for all patients with mesothelioma versus 290 controls. Panel B shows the ROC curve for patients with stage I or II mesothelioma as compared with all asbestos-exposed persons; the diagram at the bottom shows the cutoff point at highest accuracy. Panel C shows plasma fibulin-3 levels among patients with mesothelioma who underwent cytoreductive surgery and subsequently had disease progression. The fibulin-3 levels fell significantly after surgery and were higher than postoperative levels when progression occurred. Panel D shows the results of blinded fibulin-3 analysis by Princess Margaret Hospital, with an area under the ROC curve (AUC) of 0.87 (top) and the cutoff point at highest accuracy (bottom). CI denotes confidence interval.
The study was approved by the ethics committees of all participating institutions, and all study participants provided written informed consent. The first author vouches for the accuracy and completeness of the data and analysis and the fidelity of the study to the technological and biostatistical protocols.
Patients with Pleural Mesothelioma
In the Detroit cohort, 78 patients had mesothelioma. Plasma was obtained from 37 of these patients, and pleural effusions were obtained from the other 41. In the New York cohort, 64 patients had mesothelioma. Plasma was obtained from 55 of these patients; it was obtained from 18 patients 2 weeks to 18 months after cytoreductive surgery, and plasma from 6 patients was available at the time of documented disease progression. Pleural effusions were available from 33 of the 64 patients with mesothelioma, of whom 12 had matching plasma samples.
The median survival among the 142 patients with mesothelioma and complete follow-up through November 2011 (28 months for patients with stage I or II disease and 8 months for patients with stage III or IV disease) (Fig. S1 in the Supplementary Appendix) was consistent with that in other studies using the International Mesothelioma Interest Group staging system.6
Cancer-free Persons with Asbestos Exposure
Of the 41 asbestos-exposed persons in the Detroit cohort with plasma samples available,4 32 had occupational exposure to asbestos for at least 5 years (78%), 5 had occupational exposure for less than 5 years (12%), and 4 had radiographic evidence of asbestos exposure despite reporting only passive exposure (10%). In this cohort, 10 persons were foundry workers, 5 were pipe fitters, 4 were in building and construction, 4 had passive exposure in the construction business or from contact with a family member, 4 were involved in brake assembly or repair, 3 were involved in boiler repair, 2 had exposure to vermiculite insulation, 2 were plumbers, 2 were ship builders, 1 was a machinist, 1 was a tool and die worker, 1 was a millwright, 1 was a brick layer, and 1 was an electrician. Radiographic evidence of fibrosis was found in 13 persons (32%), and pleural scarring or plaques were found in 30 (73%).
The New York cohort included 95 steamfitters, who provided plasma samples and underwent computed tomographic scanning between September 2010 and March 2011 at Mount Sinai Medical Center in New York.7 Four of the steamfitters (4%) did not report an occupational exposure to asbestos nor did they have pleural plaques or parenchymal fibrosis. The other 91 had exposure of more than 5 years (range, 6 to 58). Sixty steamfitters (63%) had pleural scarring, 23 (24%) had plaques, and 4 (4%) had parenchymal changes.
Validation Cohorts
The Carotene and Retinol Efficacy Trial (CARET)8 contributed deidentified, blinded serum samples (no plasma samples were available) to the University of California, Los Angeles, and New York University; the samples were obtained from 49 asbestos-exposed persons in whom mesothelioma developed and 96 asbestos-exposed, cancer-free controls. Fibulin-3 was measured in the blinded serum, and the results were then unblinded and analyzed by CARET coinvestigators. Princess Margaret Hospital in Toronto contributed deidentified, blinded plasma samples collected from 48 patients with mesothelioma and 96 asbestos-exposed, cancer-free persons as part of an approved mesothelioma screening trial.9 Fibulin measurements in blinded samples were performed at New York University, with subsequent data unblinding and analyses by the Toronto coinvestigators.
Patients with Pleural Effusions Not Due to Mesothelioma
The Detroit cohort included 53 controls with effusions not due to mesothelioma. Of these patients, 1 had an asbestos-related inflammatory effusion, 2 had chronic inflammation, 1 had congestive heart failure, 2 had spontaneous hydropneumothorax, 2 had an effusion after immunotherapy or chemotherapy, 17 had a postoperative benign effusion, 12 had adenocarcinoma of the lung, 4 had squamous carcinoma of the lung, 8 had lung cancer not otherwise specified, 2 had renal-cell cancer, 1 had breast cancer, and 1 had lymphoma.
The New York cohort included 40 controls with effusions not due to mesothelioma. Of these patients, 2 had an asbestos-related inflammatory effusion, 4 had chronic inflammation, 1 had congestive heart failure, 7 had a reactive pleural effusion, 7 had adenocarcinoma of the lung, 2 had squamous carcinoma of the lung, 3 had lung cancer not otherwise specified, 3 had gastrointestinal adenocarcinoma, 1 had renal-cell cancer, 1 had sarcoma, 1 had cancer of an unknown primary site, 5 had breast cancer, 2 had small-cell carcinoma, and 1 had lymphoma. Matching plasma samples were available from 30 of the 40 controls in the New York cohort.
Immunohistochemical Analysis
Immunohistochemical analysis was performed on 4-µm sections of a formalin-fixed, paraffin-embedded tissue microarray from a previously constructed 26-patient tissue microarray (with tissue samples obtained from randomly selected patients with mesothelioma for whom 2 to 4 cores of tumor and 2 cores of non-neoplastic pleural tissue, lung tissue, or both were available) with the use of mouse antihuman fibulin-3 antibody, clone mab3–5 (Santa Cruz Biotechnology) (see the Supplementary Appendix). For each core, both nuclear staining and cytoplasmic staining for fibulin-3 were scored (with the scorer unaware of the tissue microarray key) for the proportion of cells stained (on a scale from 0 to 5, with higher scores indicating a greater proportion of stained cells) and intensity of staining (on a scale from 0 to 3, with higher scores indicating a greater intensity of staining). A total score (range, 0 to 15, with higher scores indicating more cells positive for mesothelioma) was given for mesothelioma cells and the non-neoplastic mesothelial and submesothelial pleural tissue.
Fibulin-3 Enzyme-Linked Immunosorbent Assay
Levels of fibulin-3 in plasma and pleural effusions were measured in duplicate wells and quantified in nanograms per milliliter with the use of the human fibulin-3 enzyme-linked immunosorbent assay (USCN Life Science).
Statistical Analysis
Kaplan–Meier survival plots and log-rank tests were used to assess differences in survival according to the stage of disease in the patients with mesothelioma and according to levels of fibulin-3 in plasma and effusions in all participants. The ability of plasma fibulin-3 levels to distinguish cohorts was evaluated by means of descriptive statistics — specifically, the Mann–Whitney test for independent samples, without correction for multiple comparisons, and receiver-operating-characteristic (ROC) curves.10,11 The area under the ROC curve (AUC) was calculated, and 95% confidence intervals were used to test the hypothesis that the AUC is 0.5.11,12 We calculated differences between groups by using analysis of variance and multiple regression analysis in a stepwise fashion, entering only variables with a P value of less than 0.05 in the model. Spearman’s rank-correlation coefficients were calculated to assess the correlation between groups. All statistical analyses were performed with the use of MedCalc software. No adjustment of P values for multiple comparisons was planned; when such an adjustment was made, the results were unchanged.
Results
Plasma Fibulin-3 Levels
Mean plasma fibulin-3 levels differed significantly between asbestos-exposed, cancer-free persons and patients with mesothelioma (Table 1) in both cohorts, and plasma fibulin-3 levels in patients with mesothelioma in the Detroit cohort were similar to those in patients with mesothelioma in the New York cohort (mean [±SE], 105.0±7.1 ng per milliliter and 112.9±7.6 ng per milliliter, respectively; 95% confidence interval [CI] for the difference, −14.8 to 28.6; P = 0.63). Plasma fibulin-3 levels did not differ significantly between 44 patients with mesothelioma who received preoperative chemotherapy and 48 who did not (117.9±8.1 ng per milliliter and 101.1±6.9 ng per milliliter, respectively; 95% CI for the difference, −37.8 to 4.3; P = 0.12).
No relationship was seen between plasma fibulin-3 levels and duration of asbestos exposure or radiographic score in patients in the New York cohort or those in the Detroit cohort; the mean (±SE) duration of exposure in the two cohorts was 34±1 years and 21±2 years, respectively. Plasma fibulin-3 levels were also not influenced by the patient’s age or sex or by the histologic subtype of mesothelioma. Plasma fibulin-3 levels in patients with stage I or II mesothelioma were similar to those in patients with stage III or IV disease (Fig. S2 in the Supplementary Appendix) and did not differ significantly according to overall survival (data not shown). Simultaneously obtained serum and plasma samples from 20 patients had similar fibulin-3 levels, with a correlation coefficient of 0.94 (95% CI, 0.84 to 0.98), although serum values were significantly lower than plasma values (87.3+17.6 ng per milliliter vs. 110.8+21.1 ng per milliliter, P = 0.006).
ROC Curves
In the Detroit cohort, the AUC for asbestos-exposed, cancer-free persons as compared with patients with mesothelioma was 1.00. A cutoff value of 32.9 ng per milliliter had the highest accuracy (minimal false negative and false positive results) for mesothelioma detection (sensitivity, 100% [95% CI, 90.5 to 100]; specificity, 100% [95% CI, 91.4 to 100]). These data were independently confirmed in the New York cohort, with an AUC of 0.99 at a cutoff level of 52.8 ng per milliliter for the highest accuracy (sensitivity, 94.6% [95% CI, 84.9 to 98.9]; specificity, 95.7% [95% CI, 89.6 to 98.8]). When the Detroit and New York cohorts were combined, the AUC was 0.99 at a cutoff level of 52.8 ng per milliliter for the highest accuracy in a comparison of plasma samples from 92 patients with mesothelioma with plasma samples from all 290 controls (Fig. 1A). Plasma fibulin-3 levels differentiated patients with mesothelioma from patients with benign or malignant effusions not due to mesothelioma and also from patients who had other cancers without effusions (Table 2).
Table 2.
Area under the ROC Curve (AUC), Sensitivity, and Specificity According to Study Cohort.*
| Comparison | No. of Participants |
AUC (95% CI) | Cutoff | Sensitivity at 100% Specificity |
Specificity at 100% Sensitivity |
|---|---|---|---|---|---|
| ng/ml | percent | ||||
| Patients with mesothelioma vs. all controls | 92 vs 290 | 0.99 (0.974–0.997) | 52.8 | 51.09 | 74.48 |
| Patients with mesothelioma vs. asbestos-exposed persons | 92 vs. 136 | 0.99 (0.971–0.999) | 52.8 | 71.32 | 69.57 |
| Asbestos-exposed persons vs. healthy persons without asbestos exposure | 136 vs. 43 | 0.64 (0.565–0.710) | 21.1 | 11.00 | 9.30 |
| Patients with mesothelioma vs. patients with benign effusions | 92 vs. 8 | 0.95 (0.889–0.984) | 67.1 | 82.61 | 25.00 |
| Patients with mesothelioma vs. patients with malignant effusions not due to mesothelioma | 92 vs. 22 | 0.94 (0.876–0.974) | 66.6 | 51.09 | 13.64 |
| Patients with mesothelioma vs. all patients with effusions not due to mesothelioma | 92 vs. 30 | 0.94 (0.884–0.976) | 67.1 | 51.09 | 16.67 |
| Patients with mesothelioma vs. controls without effusions | 92 vs. 259 | 0.99 (0.982–1.000) | 44.4 | 69.57 | 81.10 |
CI denotes confidence interval.
When the 28 patients with stage I or II mesothelioma were compared with asbestos-exposed controls, the AUC was 0.99 at a cutoff level of 46.0 ng per milliliter (sensitivity, 100% [95% CI, 87.7 to 100]; specificity, 94.1% [95% CI, 88.7 to 97.4]) (Fig. 1B). Fibulin-3 levels fell after cyto-reductive surgery in 18 of 18 patients with mesothelioma (Fig. 2C), with a trend toward increased levels at the time of confirmed histologic or cytologic progression in 6 patients.
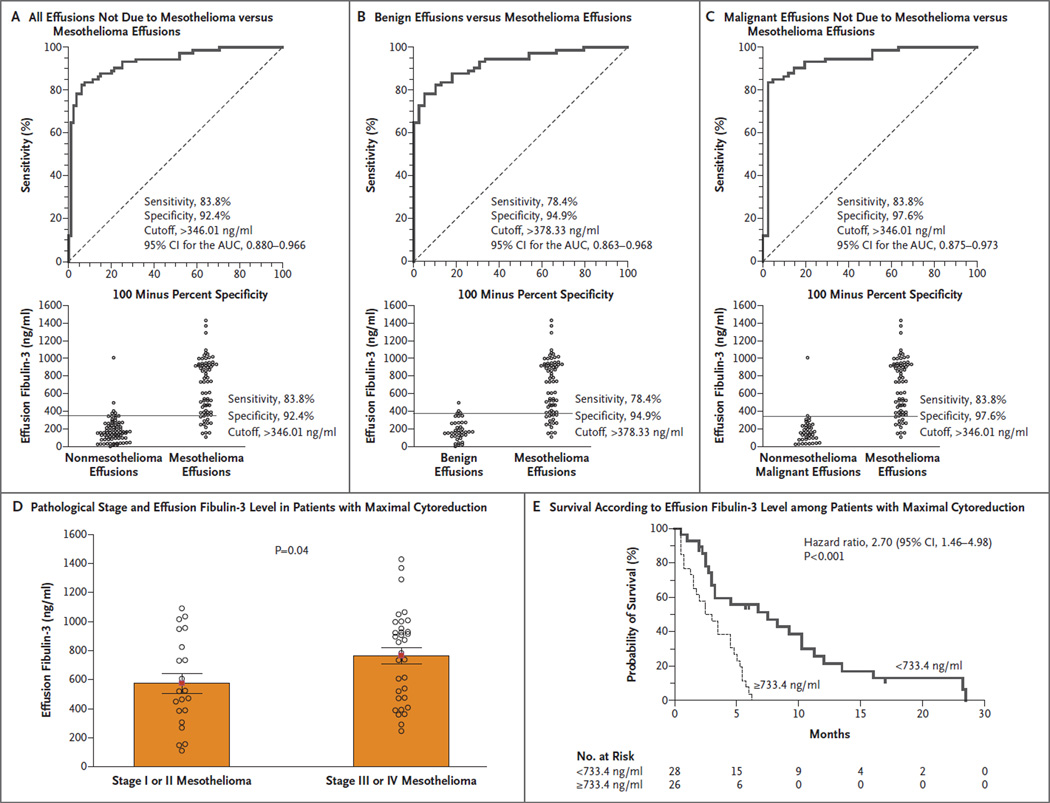
Figure 2. Effusion Fibulin-3 Levels.
Panel A, B, and C show the ROC curves for patients with mesothelioma as compared with all patients with effusions not due to mesothelioma, patients with benign effusions, and patients with malignant effusions not due to mesothelioma, respectively; the diagram at the bottom of each panel shows the cutoff point at highest accuracy. Panel D shows effusion fibulin-3 levels according to pathological stage in patients with mesothelioma who underwent cytoreductive surgery. Unlike plasma fibulin-3 levels, effusion fibulin-3 levels differed significantly between patients with early-stage mesothelioma and those with late-stage mesothelioma. Panel E shows effusion fibulin-3 levels and survival among patients with mesothelioma who underwent cytoreductive surgery. Higher fibulin-3 levels were associated with significantly poorer survival.
Validation Studies
The validation study with serum samples obtained from CARET participants failed to discriminate between patients with and those without malignant pleural mesothelioma (AUC, 0.56 [University of California, Los Angeles] and 0.52 [New York University]). Serum samples from CARET participants were collected from 1985 through 1996, and we noted that even in fresh specimens collected simultaneously and stored for less than 2 years, the serum levels were significantly lower than the plasma levels. We were concerned that serum fibulin-3 measurements, particularly in old, archived specimens, might not be accurate, especially since analysis of the structure of fibulin-3 revealed at least two thrombin cleavage sites13 similar to those of fibulin-2.14 Hence, we performed another blinded validation study using plasma samples collected at Princess Margaret Hospital. Excellent discrimination between the 96 controls and the 48 patients with mesothelioma was found (AUC, 0.87). At a specificity of 100%, a sensitivity of 33% was achieved (Fig. 2D).
Effusion Fibulin-3 Levels
There was a surprisingly poor correlation between fibulin-3 levels in matched samples of plasma and pleural effusions from 17 patients with mesothelioma (correlation coefficient, −0.007; 95% CI, −0.486 to 0.475; P = 0.98) as well as in samples of plasma and pleural effusions from 15 patients with effusions not due to mesothelioma (correlation coefficient, 0.305; 95% CI, −0.245 to 0.707; P = 0.27). Mesothelioma effusions had significantly higher fibulin-3 levels than either benign effusions or malignant effusions not due to mesothelioma (Table 1). The effusion fibulin-3 levels did not differ significantly between 22 patients who received preoperative chemotherapy and 52 who did not (617.4±72.5 ng per milliliter and 703.6±42.6 ng per milliliter, respectively; 95% CI for the difference, −74.7 to 246.9; P = 0.54).
ROC Curves
Fibulin-3 levels in effusions discriminated between patients with mesothelioma and participants without the condition in both the Detroit and New York cohorts, with AUCs of 0.95 and 0.91, respectively, and the cutoffs for maximum sensitivity and specificity were similar (378 ng per milliliter and 346 ng per milliliter) (Table S1 in the Supplementary Appendix). As shown in Figures 2A, 2B, and 2C, fibulin-3 levels discriminated effusions from patients with mesothelioma from effusions from all other participants (AUC, 0.93), whether they had benign effusions (AUC, 0.93) or malignant effusions (AUC, 0.94).
Among 54 patients who underwent cytoreductive surgery and pathological staging, effusion fibulin-3 levels differed significantly between the 21 patients with stage I or II mesothelioma and the 33 patients with stage III or IV disease (576±67 ng per milliliter vs. 765±55 ng per milliliter, P = 0.04) (Fig. 2D). Moreover, when we used as a cutoff value the median effusion fibulin-3 level (733.4 ng per milliliter) in all 69 patients with malignant pleural mesothelioma who underwent cytoreductive surgery and for whom survival information was available, survival differed significantly according to the effusion fibulin-3 level at the time of surgery (Fig. 2E). A multivariate model revealed that the cutoff effusion fibulin-3 level, stage of disease, and histologic subtype were independently predictive of survival (Table S2 in the Supplementary Appendix). Additional prognostic cutoff-point modeling is described in the Supplementary Appendix.
Immunohistochemical Studies
Nuclear expression of fibulin-3, cytoplasmic expression of fibulin-3, or both were seen in the mesothelioma cells of 26 out of 26 specimens, and the staining-intensity scores were similar when the epithelial histologic subtype (mean score, 7.7±0.6) was compared with the mixed epithelial-and-sarcomatoid biphasic histologic subtype (6.9±0.8; 95% CI for the difference, −1.2 to 3.0; P = 0.87) or with purely sarcomatoid histologic subtypes (6.6±1.1; 95% CI for the difference, −2.6 to 4.9; P = 0.62). The total staining score (for both nuclear and cytoplasmic fibulin-3 staining) was consistently higher for the neoplastic mesothelioma components than for the non-neoplastic pleura (7.4±0.5 [range, 0 to 15] vs. 2.4±0.8 [range, 0 to 12]; 95% CI for the difference, −6.9 to −3.1; P<0.001) (Fig. S4 in the Supplementary Appendix).
Discussion
The results of our study suggest that levels of fibulin-3 in plasma and effusions may aid in determining the diagnosis and prognosis of pleural mesothelioma. The specificity and sensitivity of fibulin-3 in discriminating between asbestos-exposed persons, as well as patients with effusions not due to mesothelioma, and patients with mesothelioma are superior to those of other published markers, and fibulin-3 levels are not influenced by the duration of asbestos exposure. In addition, high levels of fibulin-3 in effusions have a high positive predictive value for the presence of mesothelioma and appear to reflect the prognosis.
Fibulin-3 is a highly conserved member of the extracellular glycoprotein fibulin family encoded by the gene epidermal growth factor–containing fibulin-like extracellular matrix protein 1 (EFEMP1) on chromosome 2p16.15 Gene expression is low in normal tissues, with the highest expression in the thyroid.16 Fibulin-3 is expressed in condensing mesenchyme, giving rise to bony and cartilaginous structures.17 It mediates cell-to-cell and cell-to-matrix communication, is inversely related to cell growth, and has variable angiogenic effects.18,19 Inactivation of EFEMP1 due to DNA hypermethylation has been reported in lung,20,21 prostate,22 colorectal,23 breast,24 nasopharyngeal,25 and hepatocellular26 carcinomas. In contrast, fibulin-3 is up-regulated in pancreatic adenocarcinoma metastases,27 and there are conflicting opinions about whether the elevated expression of fibulin-3 enhances or suppresses invasion of glioblastomas.28,29
A single study of differences in gene expression between ovarian or primary peritoneal serous carcinomas and diffuse malignant peritoneal mesothelioma showed that EFEMP1 was overexpressed in all the mesotheliomas but in none of the ovarian or serous carcinomas.30 In hereditary maculopathy, overexpression of fibulin-3 is associated with a mutation in EFEMP1.31,32 We have not detected any mutations in the EFEMP1-coding exons of 20 pleural mesotheliomas (data not shown).
The plasma fibulin-3 level was significantly elevated in patients with mesothelioma in the two separate geographic cohorts (Detroit and New York), and these elevations were confirmed in a blinded validation with the use of specimens from Toronto. The characteristics of the patients with mesothelioma in the Detroit and New York cohorts were similar, including their mean plasma fibulin-3 levels. Plasma fibulin-3 levels discriminated between stage I or II mesothelioma and asbestos exposure without mesothelioma, at a specificity of 94% and a sensitivity of 100%, and the similarity of plasma fibulin-3 levels in early- and late-stage disease suggests that fibulin-3 may be associated with early events in mesothelial transformation. Surprisingly, effusion fibulin-3 levels did not correlate with plasma levels. Cavitary levels of fibulin-3 may reflect the biology of mesothelioma more accurately than plasma levels, because an advanced stage of disease was associated with higher effusion fibulin-3 levels, and effusion fibulin-3 levels (in contrast to plasma levels) were independently prognostic in patients who underwent complete staging at the time of cytoreductive surgery. Only effusion levels of vascular endothelial growth factor (VEGF) have been associated with prognosis in patients with mesothelioma33; however, VEGF levels cannot discriminate between effusions due to mesothelioma and effusions not due to mesothelioma.34
The data from this study cannot support a conclusion that fibulin-3 is an early detection marker for mesothelioma, owing to the lack of prospective, plasma-based longitudinal collections. Our CARET data suggest that plasma, not serum, should be used for fibulin measurement, because of the possibility of uncontrolled thrombin cleavage. Further validation studies of plasma fibulin-3, as well as the prognostic implications of an elevated fibulin-3 level, must be performed as part of an international effort to investigate the management of a rising plasma fibulin-3 level and to determine the number of years before clinical onset that plasma fibulin-3 can be used to detect mesothelioma. Moreover, the role of fibulin-3 as a monitoring biomarker after treatment for the disease should be prospectively validated, since we report that plasma fibulin-3 levels fell dramatically after surgical cytoreduction and rose at the time of progression. The precision of the cutoff points defined in this study for maximal sensitivity and specificity in the detection of disease will also need to be examined in further studies. Although the values for plasma fibulin-3 were similar in our cohorts, the cutoffs varied between the two geographic cohorts. This could be due to heterogeneity of the cohorts (specifically, the health of asbestos-exposed persons as compared with that of patients with cancer) or the effects of batching assays. Finally, fibulin-3 levels must be measured in more asbestos-exposed patients with benign effusions, which will require a prospective trial.
Future investigations should also explore why fibulin-3 is selectively elevated in mesothelioma as compared with other cancers and should address the question of whether this is an epigenetic-based phenomenon either through methylation or microRNA control. These studies could potentially clarify the role of fibulin-3 in mesothelioma growth, invasion, and metastasis formation and determine whether the molecule might be targeted for specific cytotoxic or biologic therapies.
Supplementary Material
Acknowledgments
Supported by the Princess Margaret Hospital Foundation and the Princess Margaret Hospital Mesothelioma Research Program (funded by the Masters Insulators Association of Ontario, International Association of Heat and Frost Insulators and Asbestos Workers, Local 793, and other unions, and the Imperial Oil Charitable Foundation) for plasma banking; by the M. Qasim Choksi Chair in Lung Cancer Translational Research (held by Dr. Tsao), the Alan B. Brown Chair in Molecular Genetics (held by Dr. Liu), and the Ontario Ministry of Health and Long-Term Care; and by donations from Belluck and Fox, the Simmons Foundation, Levi Phillips and Konigsberg, the Stephen E. Banner Fund for Lung Cancer Research, the Rosenwald Family, and the Anderson Family and grants from the Early Detection Research Network, National Institutes of Health, to the New York University Thoracic Oncology Research Laboratories (U01 CA-111295) and to Beth Israel Deaconess Medical Center (U01 CA-113913).
We thank Joseph Levin, Laura Linker, Ryan Harrington, Jean Reiss, and Stephanie Krauter for technical assistance; Mike Mehan for statistical consultation; and our collaborators who provided control plasma samples for our studies: Dr. David Zagzag, Neuropathology Department, New York University, New York; Dr. Paul Engstrom and JoEllen Weaver, Fox Chase Cancer Center, Philadelphia; Dr. Sylvia Formenti, Dr. Leonard Liebes, and Michelle Malanga, NYU Cancer Institute, New York; and Dr. Martin Sanda and Jonathan Noel, Beth Israel Deaconess Medical Center, Boston.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 2.Pass HI, Carbone M. Current status of screening for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009;21:97–104. doi: 10.1053/j.semtcvs.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Hollevoet K, Reitsma JB, Creaney J, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J Clin Oncol. 2012;30:1541–1549. doi: 10.1200/JCO.2011.39.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 5.Pass HI, Liu Z, Wali A, et al. Gene expression profiles predict survival and progression of pleural mesothelioma. Clin Cancer Res. 2004;10:849–859. doi: 10.1158/1078-0432.ccr-0607-3. [DOI] [PubMed] [Google Scholar]
- 6.Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. Chest. 1995;108:1122–1128. doi: 10.1378/chest.108.4.1122. [DOI] [PubMed] [Google Scholar]
- 7.Selikoff IJ, Seidman H. Use of death certificates in epidemiological studies, including occupational hazards: variations in discordance of different asbestos-associated diseases on best evidence ascertainment. Am J Ind Med. 1992;22:481–492. doi: 10.1002/ajim.4700220403. [DOI] [PubMed] [Google Scholar]
- 8.Barnhart S, Keogh J, Cullen MR, et al. The CARET asbestos-exposed cohort: baseline characteristics and comparison to other asbestos-exposed cohorts. Am J Ind Med. 1997;32:573–581. doi: 10.1002/(sici)1097-0274(199712)32:6<573::aid-ajim1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Roberts HC, Patsios DA, Paul NS, et al. Screening for malignant pleural mesothelioma and lung cancer in individuals with a history of asbestos exposure. J Thorac Oncol. 2009;4:620–628. doi: 10.1097/JTO.0b013e31819f2e0e. [DOI] [PubMed] [Google Scholar]
- 10.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 11.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [Erratum, Clin Chem 1993;39:1589.] [PubMed] [Google Scholar]
- 12.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 13.Tran H, Mattei M, Godyna S, Argraves WS. Human fibulin-1D: molecular cloning, expression and similarity with S1–5 protein, a new member of the fibulin gene family. Matrix Biol. 1997;15:479–493. doi: 10.1016/s0945-053x(97)90021-4. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Mann K, Murphy G, Chu ML, Timpl R. Different susceptibilities of fibulin-1 and fibulin-2 to cleavage by matrix metalloproteinases and other tissue proteases. Eur J Biochem. 1996;240:427–434. doi: 10.1111/j.1432-1033.1996.0427h.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Marmorstein LY. Focus on molecules: fibulin-3 (EFEMP1) Exp Eye Res. 2010;90:374–375. doi: 10.1016/j.exer.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi N, Kostka G, Garbe JH, et al. A comparative analysis of the fibulin protein family: biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 17.Moore RM, Redline RW, Kumar D, et al. Differential expression of fibulin family proteins in the para-cervical weak zone and other areas of human fetal membranes. Placenta. 2009;30:335–341. doi: 10.1016/j.placenta.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segade F. Molecular evolution of the fibulins: implications on the functionality of the elastic fibulins. Gene. 2010;464:17–31. doi: 10.1016/j.gene.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Albig AR, Neil JR, Schiemann WP. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 2006;66:2621–2629. doi: 10.1158/0008-5472.CAN-04-4096. [DOI] [PubMed] [Google Scholar]
- 20.Kim EJ, Lee SY, Woo MK, et al. Fibulin-3 promoter methylation alters the invasive behavior of non-small cell lung cancer cell lines via MMP-7 and MMP-2 regulation. Int J Oncol. 2012;40:402–408. doi: 10.3892/ijo.2011.1191. [DOI] [PubMed] [Google Scholar]
- 21.Yue W, Dacic S, Sun Q, et al. Frequent inactivation of RAMP2, EFEMP1 and Dutt1 in lung cancer by promoter hypermethylation. Clin Cancer Res. 2007;13:4336–4344. doi: 10.1158/1078-0432.CCR-07-0015. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Yoon HY, Kim SK, et al. EFEMP1 as a novel DNA methylation marker for prostate cancer: array-based DNA methylation and expression profiling. Clin Cancer Res. 2011;17:4523–4530. doi: 10.1158/1078-0432.CCR-10-2817. [DOI] [PubMed] [Google Scholar]
- 23.Tong JD, Jiao NL, Wang YX, Zhang YW, Han F. Downregulation of fibulin-3 gene by promoter methylation in colorectal cancer predicts adverse prognosis. Neoplasma. 2011;58:441–448. doi: 10.4149/neo_2011_05_441. [DOI] [PubMed] [Google Scholar]
- 24.Sadr-Nabavi A, Ramser J, Volkmann J, et al. Decreased expression of angiogenesis antagonist EFEMP1 in sporadic breast cancer is caused by aberrant promoter methylation and points to an impact of EFEMP1 as molecular biomarker. Int J Cancer. 2009;124:1727–1735. doi: 10.1002/ijc.24108. [DOI] [PubMed] [Google Scholar]
- 25.Hwang CF, Chien CY, Huang SC, et al. Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol. 2010;222:367–379. doi: 10.1002/path.2776. [DOI] [PubMed] [Google Scholar]
- 26.Nomoto S, Kanda M, Okamura Y, et al. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1, EFEMP1, a novel tumor-suppressor gene detected in hepatocellular carcinoma using double combination array analysis. Ann Surg Oncol. 2010;17:923–932. doi: 10.1245/s10434-009-0790-0. [DOI] [PubMed] [Google Scholar]
- 27.Seeliger H, Camaj P, Ischenko I, et al. EFEMP1 expression promotes in vivo tumor growth in human pancreatic adenocarcinoma. Mol Cancer Res. 2009;7:189–198. doi: 10.1158/1541-7786.MCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 28.Hu B, Thirtamara-Rajamani KK, Sim H, Viapiano MS. Fibulin-3 is uniquely up-regulated in malignant gliomas and promotes tumor cell motility and invasion. Mol Cancer Res. 2009;7:1756–1770. doi: 10.1158/1541-7786.MCR-09-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Pioli PD, Siegel E, et al. EFEMP1 suppresses malignant glioma growth and exerts its action within the tumor extracellular compartment. Mol Cancer. 2011;10:123. doi: 10.1186/1476-4598-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson B, Stavnes HT, Holth A, et al. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from breast carcinoma in effusions. J Cell Mol Med. 2011;15:535–544. doi: 10.1111/j.1582-4934.2010.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaelides M, Jenkins SA, Brantley MA, Jr, et al. Maculopathy due to the R345W substitution in fibulin-3: distinct clinical features, disease variability, and extent of retinal dysfunction. Invest Ophthalmol Vis Sci. 2006;47:3085–3097. doi: 10.1167/iovs.05-1600. [DOI] [PubMed] [Google Scholar]
- 32.Roybal CN, Marmorstein LY, Vander Jagt DL, Abcouwer SF. Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of the unfolded protein response and VEGF expression. Invest Ophthalmol Vis Sci. 2005;46:3973–3979. doi: 10.1167/iovs.05-0070. [DOI] [PubMed] [Google Scholar]
- 33.Hirayama N, Tabata C, Tabata R, et al. Pleural effusion VEGF levels as a prognostic factor of malignant pleural mesothelioma. Respir Med. 2011;105:137–142. doi: 10.1016/j.rmed.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Fiorelli A, Vicidomini G, Di DM, et al. Vascular endothelial growth factor in pleural fluid for differential diagnosis of benign and malignant origin and its clinical applications. Interact Cardiovasc Thorac Surg. 2011;12:420–424. doi: 10.1510/icvts.2010.250357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.