Abstract
Throughout the past several decades, studies have uncovered a wealth of information about the neural circuitry underlying fear learning and extinction that has helped to inform treatments for fear-related disorders such as post-traumatic stress and anxiety. Yet, up to 40 percent of people do not respond to such treatments. Adolescence, in particular, is a developmental stage during which anxiety disorders peak, yet little is known about the development of fear-related neural circuitry during this period. Moreover, pharmacological and behavioral therapies that have been developed are based on mature circuitry and function. Here, we review neural circuitry implicated in fear learning and data from adolescent mouse and human fear learning studies. In addition, we propose a developmental model of fear neural circuitry that may optimize current treatments and inform when, during development, specific treatments for anxiety may be most effective.
Keywords: Adolescence, Development, Fear, Extinction, Anxiety, Amygdala, Hippocampus, Prefrontal Cortex, Sensitive Period
Introduction
Throughout the past several decades, advances in psychiatry and neuroscience have shed light on the underlying neural circuitry implicated in anxiety and stress related disorders. The majority of this research has focused on understanding atypical fear responses in the mature adult brain. As a result, pharmacological and behavioral therapies have been developed to target physiologically mature neural circuitry. Although existing therapies and medications offer benefits to adult patients, a comparative lack of knowledge about the development of fear neural circuitry may limit successful treatment outcomes in children and adolescents (Liberman et al., 2006). Many of these disorders have the potential to persist into adulthood (Kim-Cohen et al., 2003; Pine et al., 1998) and become chronic and debilitating when left untreated (Liberman et al., 2006).
Over 75 percent of adults with fear-related disorders met diagnostic criteria as children and adolescents (Kim-Cohen et al., 2003; Pollack et al., 1996). The prevalence of emotional disorders and anxiety disorders specifically, is heightened during adolescent years, occurring in as many as one in ten adolescents (Costello et al., 2005; Kessler et al., 2005; Newman et al., 1996). Due to insufficient or inaccurate diagnosis and a dearth of pediatric and adolescent specialized treatments, fewer than one in five anxious children or adolescents are expected to receive treatment for their disorders (Merikangas et al., 2010), leaving a vast number with inadequate or no treatment (Keller et al., 1992; Liberman et al., 2006).
The increased frequency of anxiety disorders in adolescent populations highlights the importance of understanding the neural mechanisms underlying emotion regulation during this developmental phase. This period of development reflects a transition from dependence on parents to relative independence that begins with pubertal onset (Graber and Brooks-Gunn, 1996) and coincides with significant socio-emotional, psychological, and physical changes (Schulz et al., 2009) related to hormonal fluctuations and sexual maturity (Sisk and Zehr, 2005). Pubertal onset involves a surge in gonadal hormone release, which contributes to development of sexual characteristics and sexual interest (Sisk and Zehr, 2005). The effects of puberty and chronological age are often difficult to disentangle due to the wide variability in pubertal onset across individuals (Doremus-Fitzwater et al., 2012; Rah et al., 2009). Relevant to emotional and anxiety research, pubertal maturation has been associated with elevated physiological reactivity to emotional cues (Silk et al., 2009; Spear, 2009) and alterations in limbic circuitry (Schulz et al., 2009). These findings highlight the importance of understanding the development of neural systems associated with the emergent behaviors of adolescence.
The very nature of adolescent development serves to launch an organism toward reproductive success and survival (Insel and Fernald, 2004) and is associated with increased exploratory behavior and emotional reactivity (Spear, 2000). As such, it is probable that within this developmental transition period exist neural and behavioral characteristics divergent from those of dependent children and independent adults. We present examples of divergent behavior in adolescents in the context of changes in plasticity of neural systems. Plasticity refers to activity-dependent changes in synaptic strength with functional organization of neuronal circuits that enable an organism to adapt its behavior in the face of changing environmental demands. Age-related differences in plasticity may be apparent during transition periods (e.g. adolescence), when previously adaptive behaviors may gradually become incompatible with a new range of experiences that arise across the lifespan. These differences may arise due to maturational constraints of developing brain regions and their connectivity and due to hormonal changes that can alter plasticity, especially under stressful situations (Foy, 2011).
In this review, we outline neural circuitry implicated in fear learning and memory, highlight developmental findings from both human and mouse model studies, and suggest a developmental model of adolescent fear neural circuitry. Specifically, we examine changes in fear learning and memory during the transition into and out of adolescence, a time when anxiety disorders have been shown to peak. We use a neurodevelopmental approach to understand how behavior is translated across species. This approach requires the use of behavioral paradigms that can be used both across development and species. Fear learning paradigms are advantageous in this regard, as they can be used to assess fear responses equivalently in humans and mice and across development. Strong cross-species preservation of the neural circuitry implicated in fear learning is supported by human and nonhuman animal studies, further bolstering the translational credibility of rodent models for studying fear regulation and extinction (Gottfried and Dolan, 2004). Although fear learning and extinction have been examined during infancy and adulthood (Milad and Quirk, 2012; Moriceau and Sullivan, 2006), only recently have they been examined during adolescence. We present evidence from two forms of fear conditioning - cued fear and contextual fear learning. These studies highlight nonlinear changes in fear regulation and extinction and their neural correlates across development.
Neural circuitry involved in fear learning and memory
Acquisition and expression of conditioned fear
During the late 1800’s, it was observed that monkeys with damage to their temporal lobes exhibited aberrations in their emotional reactivity (Brown and Schafer, 1888) and in 1937, Kluver and Bucy demonstrated that monkeys with temporal lobe resections displayed many preternatural behaviors, including complete loss of fear (Kluver and Bucy, 1937). In the late 1950’s, it was discovered that nuclei buried within the temporal lobe were responsible for these changes in fear behavior (Maren, 2001; Phelps and LeDoux, 2005; Weiskrantz, 1956).
As interest in the study of emotion regulation grew, it was observed that humans with sustained damage to the amygdala and hippocampus, resulting from unilateral anteromedial temporal lobe resection, experienced impaired fear acquisition compared to control subjects (LaBar et al., 1995). Healthy subjects elicit a fear response as evidenced by increased skin conductance response to a neutral stimulus previously paired with an aversive one. Temporal lobectomy patients are unable to elicit a similar fear response, despite the fact that their verbal description of the conditioned stimulus (CS)–unconditioned Stimulus (US) connection is intact (LaBar et al., 1995).
Non-human animal studies have validated structural-functional relationships observed in patient populations. These studies show that unilateral amygdala damage in fear-conditioned rats results in attenuated fear expression, as evidenced by decreased levels of freezing behavior (LaBar and LeDoux, 1996). Lesion, inactivation, electrophysiological, molecular, and pharmacological studies have further confirmed a key role of the amygdala in fear acquisition (Fanselow and Kim, 1994; Maren et al., 1996; Quirk et al., 1997; Rogan et al., 1997; Sigurdsson et al., 2007).
Figure 1 provides a schematic representation of limbic circuitry implicated in fear learning and memory. During standard cued fear conditioning paradigms, both sensory and auditory thalamic inputs converge on the lateral nucleus of the amygdala simultaneously or in close temporal proximity (Collins and Pare, 2000; Quirk et al., 1995; Sotres-Bayon et al., 2006). After repeated pairings, the conditioned stimulus presentations alone are capable of eliciting activity within the lateral nucleus. The basal and lateral nuclei make up the primary sensory interface involved in fear learning and acquisition, while the central nucleus is the amygdala’s interface to fear expression systems (Maren, 2001).
Figure 1. Neural circuitry of fear expression and extinction.
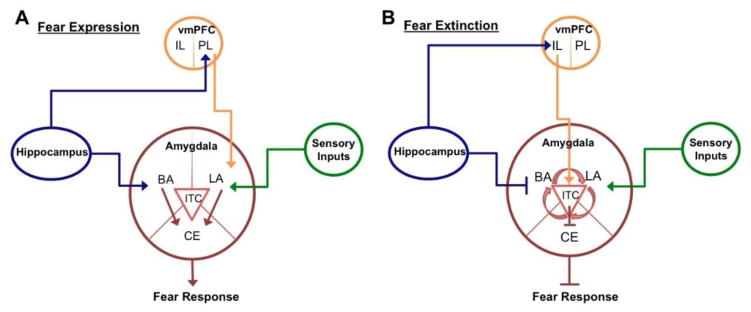
(A) During acquisition and expression of conditioned fear, projections from PL and thalamic nuclei (mediating converging sensory information) excite LA neurons, while hippocampal projections (mediating contextual inputs) lead to excitation of BA neurons directly or indirectly via connections with PL. LA and BA neurons activate CE output neurons, which project to downstream brainstem and hypothalamic nuclei responsible for mediating physiological responses, resulting in fear expression. (B) During extinction of conditioned fear, hippocampal projections (mediating contextual inputs) lead to divergent excitation of IL neurons and inhibition of BA neurons. IL projections directly activate GABAergic ITC cells within the amygdala. Integration of ITC, BA, and LA inputs during extinction results in a suppression of CE output neurons, resulting in a lack of physiological response and suppression of fear expression. Arrowheads delineate pathway excitation; straight ends delineate pathway inhibition. (BA, basal amygdala; LA, lateral amygdala; CE, central amygdala; vmPFC, ventromedial prefrontal cortex; PL, prelimbic cortex; IL, infralimbic cortex; ITC, intercalated cells). *For simplicity, connection arrows are delineated as being unidirectional, although bidirectional projections exist.
Detailed characterization of limbic circuitry in rodents, has demonstrated that once integrated by the basolateral amygdala, relevant sensory information responsible for eliciting a behavioral or autonomic response is relayed to the central nucleus both directly and indirectly (Pitkanen et al., 2000; Sotres-Bayon et al., 2006). After the intra-amygdaloid message has been conveyed from the basal lateral nuclei to the central nucleus, the central nucleus elicits various responses via its divergent projections to downstream efferent structures. These projections target hypothalamic and brainstem nuclei responsible for engaging autonomic responses such as heart rate variability, alterations in blood pressure, respiration, freezing behavior, acoustic startle, and glucocorticoid release (Maren, 2011).
Importantly, context has a profound effect on the acquisition of conditioned fear and the resulting fear memory. Projections from the hippocampus, specifically the CA1 region, to the basal nucleus of the amygdala are implicated in contextual information processing during fear acquisition (Bouton et al., 2006; Phelps and LeDoux, 2005) and lesions to dorsal hippocampus disrupt both acquisition and expression of contextual fear (Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Selden et al., 1991). By detecting environmentally relevant cues, the hippocampus can alter contextual fear directly and also indirectly influence amygdala-specific cued fear, as animals are capable of using contextual cues to retrieve the meaning of a CS appropriate to a given context (Fanselow and Dong, 2010; Maren, 2001). Via its projections to the basal nucleus of the amygdala, the hippocampus plays an important role in determining the emotional salience of an isolated cue. Whether a cue is experienced in a safe or threatening context, the hippocampal-basal amygdala network can influence subseuent fear responses via projections to the amygdala’s central nucleus, by either enhancing or dampening fear behavior.
Whereas patients with selective amygdala damage lack the prototypical autonomic response associated with fear-conditioning (LaBar et al., 1995), patients with selective damage to the hippocampus are unable to verbally describe any CS-US association, despite being able to elicit appropriate autonomic responses to the CS (Bechara et al., 1995). This double dissociation of fear conditioning and declarative knowledge involving the hippocampus and amygdala in humans parallels findings observed in non-human animal models which show impaired performance on fear conditioning tasks after amygdala and hippocampal lesions (LeDoux, 2000).
Detailed molecular, biochemical, and electrophysiological studies in the rodent have uncovered a wealth of knowledge about the underlying neural mechanisms for fear learning. Increased spike-firing in amygdala neurons (Quirk et al., 1995), and long-term potentiation (LTP), or enduring synaptic plasticity, have been shown to occur at synapses in the hippocampus and amygdala during fear conditioning and expression (McKernan and Shinnick-Gallagher, 1997; Rogan and LeDoux, 1995; Rogan et al., 1997; Tsvetkov et al., 2002). Glutamate receptor signaling in the amygdala and hippocampus, through both NMDA and AMPA receptors, has also been shown to be crucial for fear acquisition and expression (Kiyama et al., 1998; Maren et al., 1996; Milad and Quirk, 2012; Miserendino et al., 1990; Tsien et al., 1996), further confirming the role of these two structures in fear acquisition and expression. Disruption of downstream signaling cascades, including protein kinase A (PKA) (Bourtchouladze et al., 1998), mitogen activated protein kinase (MAPK), and phosphatidylinositol 3 kinase (PI3K) in the amygdala and hippocampus has also been shown to disrupt learning in both contextual and auditory fear conditioning paradigms (Chen et al., 2005; Mahan and Ressler, 2011; Schafe et al., 1999). The vast knowledge base of physiological, molecular, and electrophysiological correlates of fear acquisition provide researchers with a foundation to examine underlying mechanims of typical fear learning, and also to study potential abberations in these mechanims during atypical fear learning as seen in anxiety and stress disorders. Understanding typical as well as atypical fear learning, from a systems level view to the fine molecular details, may help uncover not only how abnormal fears are formed but also how they may be ameliorated or extinguished.
Extinction of conditioned fear
Once a given CS-US fear association has been acquired, it can be consolidated from an initial short-term association to a more permanent long-term memory. The fear expression of this initial CS-US association can be modified through various manipulations. One such manipulation is to present repeated presentations of the CS alone, in the absence of any US. These unpaired CS presentations lead to the formation of a new memory trace, residing in parallel with the initial memory. When strong enough, this new memory trace, serves to suppress fear expression. This new learning requires a reappraisal of the once-threatening CS, shifting the cue from one of danger to one of safety. The previously fearful behavioral response becomes extinguished after multiple presentations without the US resulting in a diminished conditioned response. This extinction process requires top-down prefrontal control and interactions between both cortical and subcortical limbic regions (LeDoux, 2000; Maren and Quirk, 2004; Morgan et al., 1993).
Cortical regions implicated in extinction of conditioned fear include areas within prefrontal cortex that are important for appropriately adjusting behaviors when the emotional significance of a given cue changes (Sotres-Bayon et al., 2006). The ventromedial prefrontal cortex (vmPFC), in particular, has been shown to be important for making the switch from fear expression to fear suppression during fear extinction learning and retention (Milad and Quirk, 2002; Pare et al., 2004; Santini et al., 2004). Distinct subregions within the vmPFC have been differentially implicated in the expression and extinction of conditioned fear (Santini et al., 2008; Sierra-Mercado et al., 2011; Sotres-Bayon and Quirk, 2010). Specifically, the dorsally located prelimbic cortex (PL) is associated with production of conditioned fear responses and expression of conditioned fear behaviors (Corcoran and Quirk, 2007), whereas the more ventrally located infralimbic cortex (IL) is associated with suppression of conditioned fear responses (Burgos-Robles et al., 2009; Hefner et al., 2008; Knapska and Maren, 2009; Milad and Quirk, 2012; Milad et al., 2004). The infralimbic cortex can dampen fear responses via projections to a cluster of inhibitory intercalated cells located within the amygdala. These inhibitory intercalated cells modulate activity in the central nucleus, thereby effecting the central nucleus’s projections to downstream brainstem targets and their resulting autonomic responses (Milad and Quirk, 2012).
During extinction learning, threat cues are rarely experienced in isolation and are often presented in an environment that can either enhance or reduce a cue’s potential threat. Depending on previous experience with a context, whether safe or dangerous, the aversive nature of a potential threat cue can be modulated. Importantly, the ventromedial prefrontal-hippocampal network can modulate extinction learning by detecting contextual cues present in the surrounding environment, and therefore, priming the extinction positively or negatively (Hugues and Garcia, 2007; Kalisch et al., 2006; Laurent and Westbrook, 2009). With continued presentations of a given CS in the absence of a US during extinction, the vmPFC suppresses amygdala circuitry, particularly through its excitatory glutamatergic projections to intra-amygdala inhibitory GABAergic interneurons (Pare et al., 2004). These inhibitory interneurons can be activated by PL or LA neurons during fear conditioning to ultimately lead to decreased fear expression and associated autonomic responses. During extinction learning, however, a subset of intercalated cells can be activated by inputs from infralimbic cortex which is modulated by the hippocampus, and lead to downstream active inhibition of output neurons in the central nucleus, thus eliminating fear expression and the associated physiological responses.
This hippocampally-mediated prefrontal control of amygdala responses increases an organism’s flexibility to respond appropriately to danger cues across different environments as measured by context dependent fear conditioning. Responses to potential threat cues in the amygdala and medial prefrontal cortex are inversely related (Hare et al., 2008; Kim et al., 2003) and decreased connectivity between the two regions has been associated with anxiety in adults (Pezawas et al., 2005; Shin et al., 2004). Post-traumatic stress disorder (PTSD) populations, with persistently generalized and inappropriate fear responses, tend to show reduced mPFC and hippocampal volume and activity and exaggerated amygdala reactivity (Bremner, 1999; Milad et al., 2009; Rauch et al., 2006). The complex interactions between the vmPFC, amygdala, and hippocampus during extinction of previously conditioned fear memories is necessary for adjusting behavioral and autonomic responses in rapidly changing environments. Impaired distinction between a danger versus safety cue, or generalization of both, can result in inappropriate fear responses often observed in psychiatric disorders such as PTSD or anxiety (Mahan and Ressler, 2011).
In adult PTSD patients, the failure to consolidate extinction memory has been correlated with a reduction in vmPFC volume and vmPFC activity, as well as hyperactivity in the amygdala (Liberzon and Sripada, 2008; Shin et al., 2004). Importantly, vmPFC-hippocampal connections are bidirectional and alterations in one region likely result in improper feedback and regulation of the other regions, highlighting the importance of employing integrated treatment approaches that equally utilize vmPFC and hippocampal inputs to the amygdala. Re-exposing an individual to specific threat cues during extinction training, while also incorporating hippocampal-dependent contextual elements may be one way to better target the vmPFC-hippocampal-amygdala circuit.
Adolescent fear – behavioral and molecular findings in mice and humans
Advances in the developmental neurobiology of emotion regulation have yielded substantial evidence of protracted development of prefrontal regions relative to phylogenetically older regions (Casey et al., 2005; Giedd et al., 1999; Giedd et al., 1996; Gogtay et al., 2004). Consistent with developmental changes in structural maturation, immature prefrontal functioning and top down control of subcortical regions has been observed in adolescents relative to adults during emotional contexts (Eshel et al., 2007; Hare et al., 2008; Monk et al., 2003). Healthy adolescents exhibit increased generalization of threat cues during fear conditioning tasks (Lau et al., 2011) and enhanced amygdala reactivity to threat occurs in adolescent populations with anxiety disorders compared to non-anxious individuals (Guyer et al., 2008; Lau et al., 2008; Monk et al., 2008).
The investigation of fear acquisition and extinction across development in humans has been limited due to the nature of aversive conditioning paradigms. These paradigms typically use electric shock as the US, which are not deemed appropriate for use with pediatric populations. As such, investigators have used loud tones (Craske et al., 2008), aversive air puffs to the larynx (Grillon et al., 1998), or air puffs paired with loud screams and aversive faces (Schmitz et al., 2011). The use of a naturalistic cue, that has come to be associated with the presence of a threat with experience (e.g., a frightened face, a scream), for a US may confound developmental studies as our experiences over a lifetime are not equivalent but rather limited by age and opportunity for such experiences. For example, a child may have fewer experiences of dangerous situations or threats than an adult, and an anxious child may have many more experiences of threat than a non-anxious child. These experiences will differentially impact fear-related circuitry (Casey et al., 2013). Because variations with US delivery and behavioral assessment in humans may yield variability in results (Glenn et al., 2011), animal models have been helpful in studying developmental effects of fear learning.
Studies examining the mechanisms of hippocampal-dependent contextual fear and amygdala-dependent cued fear acquisition and extinction in rodent models have traditionally focused on early developmental stages (Moriceau and Sullivan, 2006; Rudy, 1993; Rudy and Morledge, 1994) using pre-weaned or pre-adolescent rodents and adult rodents. Recently, developmentally intermediate ages have been investigated due to the translational relevance of studying fear during adolescent development, when increased prevalence of fear related disorders typically emerges.
Development of Cued Fear Learning and Extinction
A number of studies have begun to examine amygdala-dependent, cued fear extinction in rats across development taking advantage of what is known about developmental differences in brain maturation in humans. These studies show that inactivation of the mPFC fails to disrupt long-term extinction in pre-adolescent, postnatal day 17 (P17) rats, yet inactivation of mPFC does disrupt this memory in P24 rats (Kim et al., 2009). Extinction training in these young age groups leads to increased levels of pMAPK in both the prelimbic and infralimbic cortices, suggestive of non-specific, global mPFC activity, as opposed to the inverse pattern of IL/PL activity typically seen with successful adult extinction retention. In both postnatal day 24 fear-conditioned rats, a single block of CS presentations leads to robust freezing, but does not lead to increased pMAPK in either the IL or PL, contrary to the expected enhancement of PL activity typically seen in fear memory recall (Kim et al., 2009).
Developmental studies of innate fear regulation in rodents demonstrate that during expression of innate, or unconditioned, fear, the mPFC of infant rats is neither active nor responsive while the PL becomes active in pre-adolescence but does not yet regulate freezing behavior. In contrast, the PL in adolescents becomes functionally connected with the amygdala and its downstream brainstem targets, as shown by its ability to begin regulating fear expression and freezing behavior (Chan et al., 2011). These developmentally altered patterns in the mPFC activation are independent of amygdala activity, which suggest that mPFC neural circuitry develops enhanced capacities for fear regulation as an animal matures (Chan et al., 2011). In addition, injections of anterograde tracers placed into the BLA of developing rats show that amygdalo-cortical connectivity is late maturing, with fiber density reaching a plateau circa P45, thus confirming that maturation of this circuit continues into adolescence (Cunningham et al., 2002).
In classical conditioning experiments, adolescent mice have been shown to exhibit increased acquisition of cued fear compared to pre-adolescent and adult mice, despite having equal levels of anxiety-like behavior, as assessed by open field (Hefner and Holmes, 2007). Fear responses during adolescence, typically defined in the rodent as the phase surrounding the 10 days prior to sexual maturation at postnatal day P40, (Adriani et al., 1998; Laviola et al., 1999; Spear, 2000), have been found to be resistant to extinction (McCallum et al., 2010; Pattwell et al., 2012). Adolescent rats require either twice as many extinction trials or a pharmacological intervention, such as the NMDA-agonist D-cycloserine, to achieve reductions in fear expression comparable to younger or older rats (McCallum et al., 2010). This blunted fear extinction during adolescence is associated with a lack of activity in prefrontal cortex, specifically IL, as assessed by pMAPK immunohistochemistry (Kim et al., 2011) or c-Fos immunohistochemistry (Pattwell et al., 2012) compared to younger and older ages. Electrophysiological recordings at IL and PL synapses across development reveal that a fear-conditioning induced potentiation of PL synapses present in adult mice is absent in adolescent mice, Furthermore, extinction-induced enhancement of IL synaptic plasticity in adult mice, is lacking in adolescent mice (Pattwell et al., 2012).
Studies with human subjects also show age-dependent differences in fear reactivity across adolescent development. Tasks using fearful or screaming faces result in heightened amygdala activity (Hare et al., 2008) and fear learning (Glenn et al., 2011) in human adolescents compared to younger children. Diminished fear extinction, relative to children and adults, has been shown in human adolescents, during a task of cued fear conditioning involving aversive sounds (Pattwell et al., 2012) and parallels rodent findings as shown in Figure 2. Taken together, these studies reveal a non-linear pattern in fear extinction learning with blunted regulation of amygdala-dependent fear responses during fear extinction in adolescents.
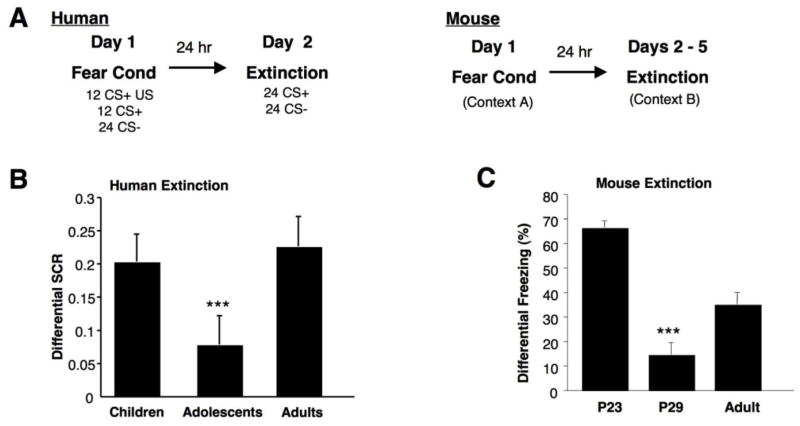
Figure 2. Cued extinction learning and spontaneous recovery across development in mice and humans.
(A) Behavioral paradigms for parallel fear conditioning experiments in humans and mice. (B) Analysis of extinction indices [(Averaged first two extinction trials) – (Averaged last two extinction trials)] reveals a main effect of age group for humans, such that adolescents display attenuated fear extinction learning compared to children and adults, [adolescent .05916 ± 0.06904; children .25435 ± 0.04839; adults 0. 22510 ± 0.05931). (C) A lack of extinction learning and retention of extinction memory in is observed in adolescent mice, as displayed by a significantly decreased differential extinction indices [(Day 1, Tone 1) – (Day 4, Tone 5)] compared to older and younger ages, [(P23 66.5% ± 2.75; P29 14.72% ± 4.79; P70 35.17% ± 4.89). Adapted from Figure 1 of Pattwell et al., 2012.
These findings of diminished fear extinction may help provide insight into the heightened prevalence of anxiety disorders during adolescence. Furthermore, this adolescent extinction data may help inform current treatment approaches, as the major non-pharmacologic therapy for anxiety disorders, cognitive-behavioral therapy (CBT) relies on extinction principles. If the capacity for successful extinction learning is attenuated during specific developmental period, therapies relying primarily on extinction techniques may prove ineffective for treating anxiety disorders in adolescent populations.
Impact of Early-life Stress on fear learning and extinction
Early-life stress, as induced by maternal separation in young rat pups, causes a shift in the typically attenuated adolescent extinction curve (Callaghan and Richardson, 2012). Specifically, rats that were previously subjected to early life stress show typical extinction learning during adulthood, yet exhibit attenuated extinction learning earlier in development, during pre-adolescent ages. These findings suggest fine-tuning of the circuitry with these early experiences may map onto human forms of childhood anxiety. In the rodent experiments, a shift in the developmental window of extinction failure, typically observed during adolescence, can occur in younger, pre-adolescent rats subjected to early-life stress. These data suggest that maladaptive experiences, such as early-life stress, may have the potential to increase susceptibility for anxiety by shifting a developmental window associated with increased fear reactivity
Role of the Hippocampus and mPFC in Cued Extinction Learning
The importance of the mPFC in cued extinction learning and extinction retention is widely accepted and inactivation of the mPFC alone is enough to impair the retrieval of cued extinction memory (Sierra-Mercado et al., 2006). Importantly, and often disregarded, however, is that inactivation of the hippocampus alone before extinction training also leads to impaired retrieval of cued extinction memory the following day (Corcoran et al., 2005) suggesting that mPFC may be an important target of the hippocampus for modulating extinction learning and recalling extinction memory in rodents and humans (Kalisch et al., 2006; Quirk and Mueller, 2008). Furthermore, contextual modulation of amygdala activity requires the hippocampus (Maren and Hobin, 2007). It is important to note that in cases where cued extinction retention and the degree of successful extinction are assessed in the same context as where conditioning took place, it may be difficult, even impossible, to claim that poor extinction learning solely results from insufficient vmPFC regulation.
Many of the extinction paradigms in the existing literature fail to account for important hippocampal contributions associated with contextual information. Distinct populations of neurons exist in the BA for triggering the activation of neuronal circuits responsible for integrating sensory and contextual information (Herry et al., 2008). These populations of “fear neurons,” and “extinction neurons,” as they are called, are differentially connected with the hippocampus and mPFC. Particularly, hippocampal inputs to BA preferentially target the “fear neurons,” over the “extinction neurons,” suggesting that hippocampal input to these neurons may override the retrieval of cued or contextual extinction memory, a likely contributor to the phenomenon of fear renewal (Hobin et al., 2006).
Fear renewal, or the fear that returns upon experiencing a reminder outside of the extinction context, remains a major obstacle for clinical treatment of anxiety disorder in humans (Milad et al., 2005; Rodriguez et al., 1999) and may be the result of tipping the balance between activation between specific neuronal circuits in the hippocampus and amygdala (Herry et al., 2008). This clinical observation lends support for finding better treatment methods, perhaps through further investigation of hippocampally-mediated techniques, such as contextual-based extinction.
Development of Contextual Fear Learning and Extinction
From a developmental perspective, the notion of hippocampal involvement in mediating both contextual and cued fear processing is a promising one. While the hippocampal cytoarchitecture is well established by 34 weeks in utero in the human, (Arnold and Trojanowski, 1996), development of the structure has been shown to continue through adolescence in both rodents and non-human primates (Benes et al., 1994; Kornack and Rakic, 1999).
Longitudinal scans of children and adolescents, between the ages of four and twenty-five years, reveal that postnatal hippocampal maturation is not homogenous and that distinct maturational profiles exist for specific subregions (Gogtay et al., 2006). While overall hippocampal volume remains constant throughout these ages, posterior subregions of the hippocampus show volumetric enlargement over time while anterior regions undergo substantial volumetric reductions. The cause of these heterogeneous volume changes remains unknown, but it is hypothesized that they may be due to differences in neuronal proliferation, synaptic production and/or pruning, myelination, or glial alterations and may parallel differences in functional development (Gogtay et al., 2006). Of note, the anterior region of the hippocampus, which exhibits decreases in volume as a function of age, is reciprocally connected to the prefrontal cortex (Cavada et al., 2000), amygdala (Petrovich et al., 2001; Pitkanen et al., 2000), and hypothalamic-pituitary-adrenal axis (Bannerman et al., 2004) - regions implicated in fear and anxiety.
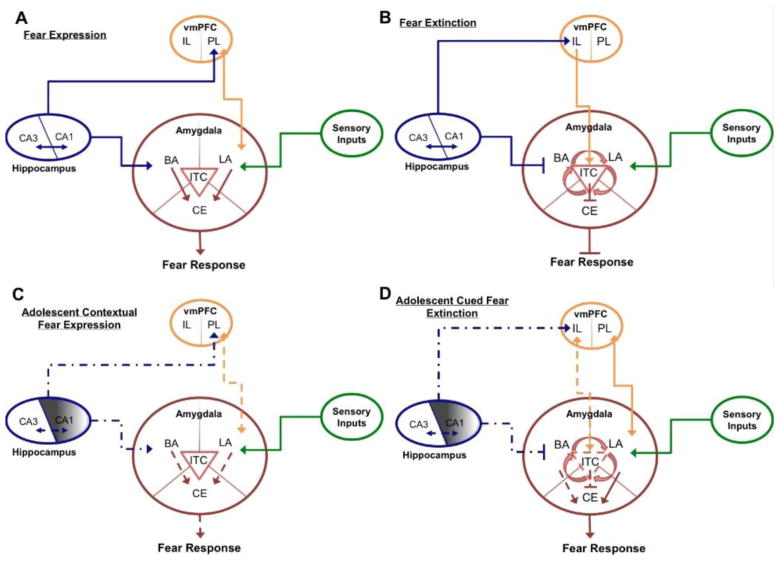
This heterogeneous postnatal development of hippocampal subregions, specifically the volumetric decreases observed in the anterior region, correlates with contextual fear data showing that contextual fear expression during pre-adolescent ages is intact, temporarily suppressed during adolescence, and then reemerges again during adulthood (Pattwell et al., 2011) (Figure 3), supporting the notion that development is not a linear process in which neural maturation occurs uniformly in one direction or another. Rather, an intricate reciprocal balance between neural development in one brain region may lead to alterations in another region. Convergent adolescent and adult rodent contextual fear data further highlight the importance of the developing hippocampus in mediating fear responses. The aforementioned literature on human and non-human primate hippocampal development suggests a developmentally sensitive fear circuitry model, depicted in Figure 4.
Figure 3. Hippocampal-dependent contextual fear memory across adolescent development.
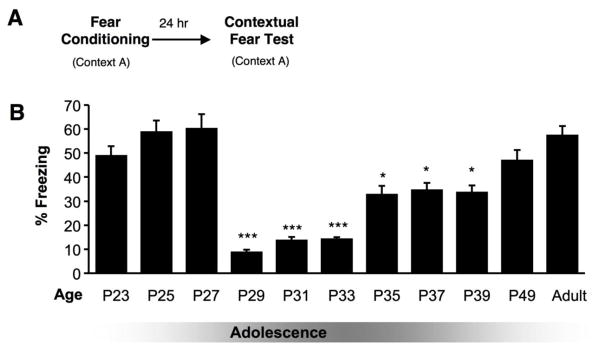
(A) Mice of all ages were fear conditioned (fear cond) with three tone-shock pairings. Twenty-four hr later, they were returned to the conditioning context (Context A) and freezing behavior was scored. (B) Adolescent mice (P29 – P39) froze significantly less than both younger (P23 – P27) and older (P49 – P70) mice. All results are presented as a mean ± SEM. determined from analysis of 7–10 mice per group (fear conditioning) and 28 mice per group (novel object placement), (*p < 0.05, ***p < 0.001). Adapted from Figure 1, Pattwell et al., 2011.
Figure 4. Developmental neural circuitry of fear expression and extinction.
Diagrammatic representation of adult neural circuitry implicated in (A) fear expression and (B) fear extinction. (C) A lack of contextual fear expression in adolescent mice can be traced to immature CA1. Without proper CA1-PL and CA1-BA inputs, there is a lack of downstream activation of CE output neurons, resulting in a diminished or suppressed fear response. (Weak signals and low functional connectivity represented by dotted lines). (D) A lack of CA1 excitatory input to IL combined with a lack of CA1 inhibitory input to BA, typical in cued fear extinction, results in reduced inhibition of CE output neurons, resulting in fear expression and a lack of extinction. Arrowheads delineate pathway excitation; straight ends delineate pathway inhibition. (BA, basal amygdala; LA, lateral amygdala; CE, central amygdala; vmPFC, ventromedial prefrontal cortex; PL, prelimbic cortex; IL, infralimbic cortex; ITC, intercalated cells). *For simplicity, connection arrows are delineated as being unidirectional, although bidirectional projections exist.
A proposed model of adolescent neural circuitry in fear learning and memory
Crosstalk between CA1 and CA3 regions of the hippocampus is required for contextual fear expression and cued fear extinction in the adult brain. The adult brain integrates specific hippocampally-mediated contextual inputs, thalamic sensory inputs, and prefrontal cortical inputs within intra-amygdalar circuits to produce an appropriate behavioral response. As shown in Figure 4C and Figure 4D, the successful retrieval of cued fear memories across pre-adolescence, adolescence, and adulthood (McCallum et al., 2010; Pattwell et al., 2011; Pattwell et al., 2012), suggests that sensory inputs to the lateral amygdala (LA) are functional across postnatal development. As younger, pre-adolescent mice are unimpaired on contextual fear tasks, one possibility is that pre-adolescent mice rely more heavily on available sensory inputs, and are relying solely on elemental cues of odor, texture, etc., to retrieve the contextual memory rather than its more complex configural elements. The successful retrieval of contextual fear memories during early, pre-adolescent ages, as previously shown in Pattwell et al., 2011, suggests that aforementioned age-dependent volumetric decreases in CA1 region of the hippocampus have yet to occur. In pre-adolescence mice, a potential over-abundance of active synapses between CA1-PL and CA1-BA allow for successful retrieval and expression of contextual fear. As the mice transition to adolescence, and reorganization of CA1 connection, combined with developmental synaptic pruning may interfere with ability of the adolescent mouse’s ability to retrieve contextual fear memories.
Rodent data has also shown that bidirectional PL-amygdala synapses mature earlier than IL-amygdala synapses (Chan et al., 2011). This delayed maturation of IL-amygdala connectivity may also account developmental differences in ability to express contextual fear. If the amygdala has not yet received any inhibition from IL inputs during pre-adolescence, PL-amygdala synaptic activation is dominant, allows for expression of fear during contextual tasks. During adolescent development in both rodents and humans, however, vmPFC maturation can be observed (Cunningham et al., 2002; Gogtay et al., 2004) and as IL-amygdala synapses are refined, activity in PL-amygdala circuits must override IL-amygdala inputs, allowing for fear expression. Because CA1-PL connectivity is necessary for this interaction, and because CA1 undergoes volumetric decreases throughout adolescent development (Gogtay et al., 2006), which may be indicative of changes in synaptic pruning or myelination, CA1 inputs to BA and PL are not sufficient for fear expression in adolescent mice (as shown by the shading out of CA1 and the resulting dotted arrows in Figure 4C and Figure 4D). This hypothesis is also supported by blunted responses in synaptic neurotransmission in BA of P29 mice upon contextual fear tests, as shown via electrophysiological experiments (Pattwell et al., 2011) and preliminary data in which there is little c-Fos activity in CA1, PL, and BA in P29 fear-conditioned mice upon contextual fear retrieval compared to control mice (Pattwell et al., unpublished data).
Importantly, CA1 is responsible for retrieval of fear memories, while CA3 is responsible for fear acquisition and encoding of fear memories. Consistent with behavioral findings, adolescent mice acquire and consolidate contextual fear memories, despite exhibiting a lack of fear retrieval and expression. Preliminary c-Fos data suggest that CA3 is active during retrieval in adolescent fear-conditioned mice (Pattwell et al., unpublished data), supporting the notion that the fear memory has been encoded and retrieved, allowing for retrieval at later, post-adolescent ages when the neural circuitry underlying fear learning and memory has reached stabilized, adult-like structural and functional maturation. Further, it has been shown that inactivation of CA1 can interfere with CA3 communication with extra-hippocampal regions (Lee and Kesner, 2004), such as the amygdala. With little or no fear expression and corresponding CA1-PL or CA1-BA inputs, contextual extinction and contextual reconsolidation update appear to work remarkably well in adolescent mice to persistently attenuate fear memories, possibly due to a lack of CA1 inputs, allowing for intra-amygdalar activation of ITC cells with little or no competing input from CA1 or PL. CA1-vmPFC connectivity has also been shown to be important for retrieval of cued fear extinction in and it is possible that the lack of extinction learning and retention of extinction memory in adolescent mice (as described in (McCallum et al., 2010; Pattwell et al., 2012)) may result from a similar mechanism, consistent with the developmental role of CA1 maturation in fear learning and integration with IL/PL circuits.
Concluding Remarks
In this review, we outlined the neural circuitry implicated in fear learning, discussed developmental differences in fear learning during the transitions into and out of adolescence, and suggested a plausible developmental model of fear neural circuitry that may underlie adolescent fear behaviors. In particular, contextual fear is expressed during early, juvenile ages, suppressed during adolescence, and re-emerges during adulthood (Pattwell et al., 2011). Additionally, cued fear extinction learning is intact during early ages in both mice and humans, blunted during adolescence, and intact again in adulthood (McCallum et al., 2010; Pattwell et al., 2012). These findings suggest a nonlinear pattern in the development of fear learning during which adolescent behaviors differ from prototypical fear responses associated with both younger and older ages (Casey et al., 2008).
An evolutionary hypothesis for the suppression of contextual fear is consistent with the demands associated with adolescent development. Adolescence is a time of exploration when one must leave the safety and stability of their environment in search of reproductive success, thus, a suppression of contextual fear may contribute to the fearlessness required for exploring new environments that is typically seen with this age group (Spear, 2000). As specific danger cues remain relevant during this novelty-seeking period, cued fear expression remains intact and is resistant to extinction during adolescence. Combined, these behaviors allow the adolescent to remain both exploratory and cautious, thus optimizing chances for survival and success.
As we have outlined throughout this review, the brain of an adolescent is developmentally distinct from that of a child or an adult. As such, the adolescent brain experiences and interprets associations differently than children and adults during daily life. In the event of traumatic situations, it should be noted that the same features of adolescent brain development designed for survival and evolutionary success may contribute to a vulnerability for anxiety disorders that are treatment resistant such as PTSD or anxiety. The lack of fear extinction associated with adolescent development, may hinder responses to traditional psychotherapy, such as CBT. Because CBT desensitizes an individual to anxiogenic stimuli through repeated exposures (i.e. extinction learning), future studies aimed at examining whether this technique is effective during adolescence, when extinction learning is attenuated, may provide insight into optimal treatment strategies for anxious individuals. Clinical studies of youth rarely extend into adulthood or examine age-effects in non-linear terms, so additional research is needed to explore whether anxious adolescents will benefit similarly to children and adults during CBT.
If non-linear development of the neural circuitry implicated in fear learning has evolutionarily primed adolescents to exhibit attenuated fear extinction, resistance to classical extinction therapies may put anxious adolescents at a clinical disadvantage. CBT relying on exposure therapy may need to be reconsidered as a treatment option for developing populations, who lack the ability to extinguish previously acquired associations. At the dawn of individualized psychiatric treatment, when treatments are now being tailored for patients with various genetic polymorphisms (Mahan and Ressler, 2011), new avenues in individualized medicine may also need to incorporate the age of the patient to determine when, during development, specific treatments may be most effective.
Highlights.
Adolescence is a developmental stage when anxiety disorders are peaking.
Current therapies for anxiety disorders rely on fear extinction principles.
Development of neural circuitry underlying extinction learning continues through adolescence.
Parallel human and rodent experiments reveal attenuated extinction during adolescence.
Acknowledgments
This work was supported by the Sackler Institute (B.J.C., S.S.P.); the DeWitt-Wallace Fund of the New York Community Trust (F.S.L.); National Institutes of Health Grants HD055177 (B.J.C. and S.S.P.) and MH079513 (B.J.C. and F.S.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ. Human fetal hippocampal development: I. Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J Comp Neurol. 1996;367:274–292. doi: 10.1002/(SICI)1096-9861(19960401)367:2<274::AID-CNE9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Alterations in brain structure and function associated with post-traumatic stress disorder. Semin Clin Neuropsychiatry. 1999;4:249–255. doi: 10.153/SCNP00400249. [DOI] [PubMed] [Google Scholar]
- Brown S, Schafer EA. An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Phil Trans R Soc Lond B. 1888;179:303–327. [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Early-life stress affects extinction during critical periods of development: An analysis of the effects of maternal separation on extinction in adolescent rats. Stress. 2012 doi: 10.3109/10253890.2012.667463. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Pattwell SS, Glatt CE, Lee FS. Treating the developing brain: implications from human imaging and mouse genetics. Annu Rev Med. 2013:64. doi: 10.1146/annurev-med-052611-130408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chan T, Kyere K, Davis BR, Shemyakin A, Kabitzke PA, Shair HN, Barr GA, Wiedenmayer CP. The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J Neurosci. 2011;31:4991–4999. doi: 10.1523/JNEUROSCI.5216-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Egger H, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:972–986. doi: 10.1097/01.chi.0000172552.41596.6f. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Lindsey Bergman R, Naliboff B, Lipp OV, Negoro H, Ornitz EM. Is aversive learning a marker of risk for anxiety disorders in children? Behaviour research and therapy. 2008;46:954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Barreto M, Spear LP. Age-Related Differences in Impulsivity Among Adolescent and Adult Sprague-Dawley Rats. Behavioral neuroscience. 2012 doi: 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Foy MR. Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiology of learning and memory. 2011;95:134–144. doi: 10.1016/j.nlm.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. The development of fear learning and generalization in 8–13 year-olds. Developmental psychobiology. 2011 doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J. Expectations for and precursors to leaving home in young women. New Dir Child Dev. 1996:21–38. doi: 10.1002/cd.23219967104. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJ, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of general psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annual review of neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Wunder J, Beardslee WR, Schwartz CE, Roth J. Chronic course of anxiety disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1992;31:595–599. doi: 10.1097/00004583-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R. Immunohistochemical analyses of long- term extinction of conditioned fear in adolescent rats. Cereb Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. Am J Physiol. 1937;119:352– 353. [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE. Partial disruption of fear conditioning in rats with unilateral amygdala damage: correspondence with unilateral temporal lobectomy in humans. Behav Neurosci. 1996;110:991–997. doi: 10.1037//0735-7044.110.5.991. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155– 184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear- conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D- aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of general psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109– 113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR. Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol. 1996;64:552–562. [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early- acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci U S A. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early- adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Otto MW, Sabatino S, Majcher D, Worthington JJ, McArdle ET, Rosenbaum JF. Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am J Psychiatry. 1996;153:376–381. doi: 10.1176/ajp.153.3.376. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short- latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Rah JH, Shamim AA, Arju UT, Labrique AB, Rashid M, Christian P. Age of onset, nutritional determinants, and seasonal variations in menarche in rural Bangladesh. J Health Popul Nutr. 2009;27:802–807. doi: 10.3329/jhpn.v27i6.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rodriguez BI, Craske MG, Mineka S, Hladek D. Context-specificity of relapse: effects of therapist and environmental context on return of fear. Behav Res Ther. 1999;37:845–862. doi: 10.1016/s0005-7967(98)00106-5. [DOI] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97– 110. [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Merikangas K, Swendsen H, Cui L, Heaton L, Grillon C. Measuring anxious responses to predictable and unpredictable threat in children and adolescents. J Exp Child Psychol. 2011;110:159–170. doi: 10.1016/j.jecp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: pupillary, behavioral, and subjective evidence during emotional word identification. Dev Psychopathol. 2009;21:7–26. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]