As the roaring of the waves precedes the tempest, so the murmur of rising passions announces the tumultuous change. … Keep your hand upon the helm, or all is lost
(Rousseau, 1762/1911, pp. 172–173).
The notion that the hormonal changes of puberty compromise individuals’ rational decision making has a long and sturdy history in writings on adolescence, as Rousseau’s warning to parents in his 18TH century book Emile, one of the first treatises on this stage of development, aptly illustrates. For as long as individuals have been writing about teenagers, they have described them as victims of their own raging hormones. Even today, popular advice books for parents of teenagers, with titles like Yes, Your Teen is Crazy (Bradley, 2002), continue to portray adolescents’ judgment as hopelessly compromised by the disruptive impact of this period’s endocrinology.
One likely reason for the persistence of this idea is that rates of most forms of risky and reckless behavior do, in fact, increase between preadolescence and middle or late adolescence and decline in early adulthood. This is the case with regard to a wide range of behaviors that are partially or wholly attributable to risk-taking, including violent and non-violent crime (Piquero, 2007), driving crashes and fatalities (Twisk & Stacey, 2007), unprotected sex (CDC, 2012), attempted suicide (Mościcki, 2001), accidental drownings (CDC, 2011), self-inflicted injuries (Kessler, Borges, & Walters, 1999), and initial experimentation with tobacco, alcohol, and illicit drugs (SAMHSA, 2012). Indeed, the leading causes of morbidity and mortality during adolescence are behavioral.
Given the longstanding popular notion that risky and reckless behavior in adolescence is tied to the hormonal changes of puberty, there is a surprising absence of research on the direct links between pubertal maturation and adolescent risk-taking (or adolescent decision making, more generally), with the possible exception of research on the behavioral consequences of early or late pubertal maturation (for a recent review, see Negriff & Susman, 2011). Instead, studies have generally examined the relationship between pubertal development and changes in decision making by way of indirect correlational studies, which often confound the direct influences of puberty with other factors such as chronological age, aspects of emotional, cognitive, and social development that are independent of puberty, and variations in contexts in which decisions are made.
In other words, although the fact that risk-taking increases during the first part of adolescence is consistent with the idea that puberty plays a role in the process, some changes in risk-taking and decision making may coincide with puberty, but be independent of it. For example, normative changes in the context in which individuals live during adolescence may contribute to changes in decision making, and to the increase in risk-taking in particular. Because peers take on increased importance at this time, adolescents may begin to engage in certain types of risky behavior in order to demonstrate or facilitate their affiliation with others. Similarly, because there is typically a weakening of parental supervision as individuals transition from childhood into adolescence, increases in risky behavior may be the consequence of greater opportunity to engage in behaviors that at earlier periods of development had been deterred by the presence of parents.
Our purpose in this article is to examine research on the relation between puberty and risk-taking in adolescence (and, especially, on aspects of decision making that are relevant to our understanding of risky behavior), and to begin sorting out the developmental processes that are likely to be puberty-dependent and puberty-independent. Because there are so few studies of the direct role of pubertal development in adolescent decision making about risk, we approach the issue indirectly and speculatively, describing how decision making changes around the time of puberty, discussing the links between changes in decision making and changes in brain structure and function during adolescence, and, where there is evidence, noting what is known about the ties between the hormonal changes of puberty and changes in brain and behavior and, perhaps more importantly, what is not.
Before proceeding with this discussion, a few caveats are in order. First, because there are relatively few studies that examine the direct links between puberty and decision-making, or risky decision-making in particular, it is hard to draw generalizations from this literature. Our assessment of this body of work is that there are sufficient grounds to advance several informed hypotheses, but not yet grounds to draw firm conclusions. The main aim of this article is not to summarize research on puberty and risky decision-making, but to stimulate more of it by providing readers with some suggestions for further study.
Second, one reason for many apparent inconsistencies and contradictions in this literature is the wide diversity of constructs used and the methods and measures employed. Many different terms are used for constructs that are similar but not exactly identical; for example, sensation-seeking, reward-seeking, novelty-seeking, and thrill-seeking have all been used to refer to the inclination to engage in potentially arousing experiences, although not all of the experiences that are often discussed with respect to sensation-seeking are novel (e.g., riding on a roller coaster that one has ridden previously) or thrilling (e.g., drinking alcohol), and some may not even be immediately rewarding (e.g., self-inflicted cutting). Moreover, researchers use a wide variety of measures and methods to assess the same constructs, some of which may not measure what they purport to measure, or may inadvertently measure multiple phenomena. For instance, although sensation-seeking and impulsivity are entirely different constructs (e.g., one can pursue a novel or exciting goal with a great degree of planning and self-control), self-report measures of these constructs often contain overlapping items (see Steinberg et al., 2008). For example, the Zuckerman Sensation Seeking Scale (Zuckerman, Eysenck, & Eysenck, 1978), perhaps the most widely used self-report measure of sensation-seeking, includes items such as “I often do things on impulse,” “I usually think about what I am going to do before doing it,” and “I am an impulsive person.” As a consequence, studies that link a specific construct to age or puberty might produce results that differ from those that examine a related, but not identical, construct. Apparent inconsistencies in findings may be due to inconsistencies in the constructs and operationalizations employed. In addition, the way that pubertal maturation is measured, defined, and demarcated can vary drastically from one study to another. Commonly used measures of puberty include self-reports, clinician observations, and hormonal assays, which have been scored both continuously and categorically, and there have been many discussions about the validity and reliability of various measures, as well as their intercorrelations (for review see Dorn & Biro, 2011).
Third, studies in this area of inquiry often define the outcome variables of interest at different levels of analysis. For purposes of this paper, we view “reward sensitivity” and “cognitive control” as neurobiological constructs that are measured in studies of brain structure and/or function (see Figure 1). These neurobiological phenomena have psychological manifestations (in our terminology, “sensation seeking” and “self regulation”) that are measured by assessing psychological states or traits through the subjective reports of individuals or their evaluators. For heuristic purposes, we use “sensation-seeking” as an overarching label for a number of interrelated constructs that refer to the inclination to “seek varied, novel, complex, and intense sensations and experiences and the willingness to take physical, social, legal, and financial risks for the sake of such experiences” (Zuckerman, 1994, p. 26). Recruitment of brain regions and systems implicated in reward-processing (e.g., ventral striatum, orbitofrontal cortex) has been linked to measures of sensation seeking in humans and animals (Abler et al., 2006; Leyton et al., 2002; Lind, 2005).
Figure 1.
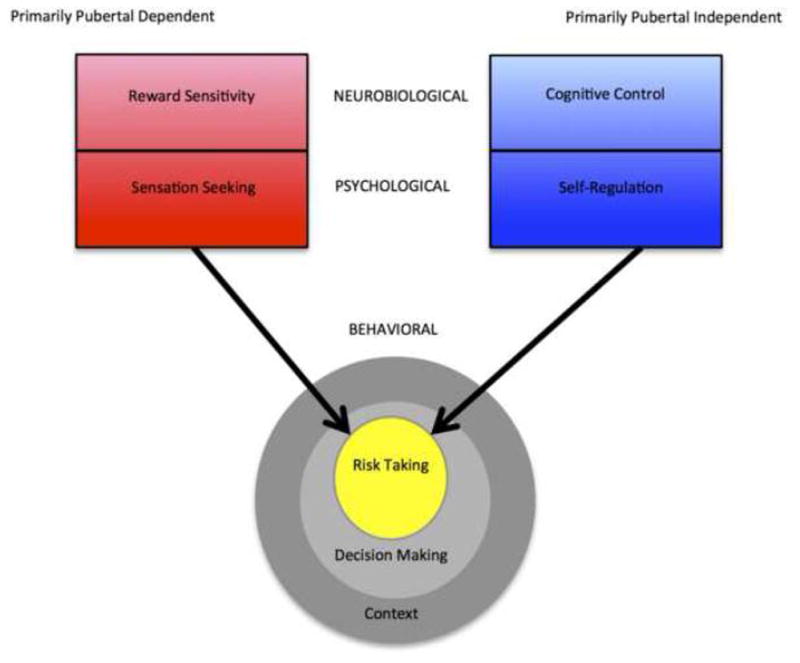
Graphical depiction of constructs implicated in the dual systems model of adolescent risk-taking.
In a similar vein, we use the label “self regulation” to refer to a group of interrelated but distinguishable constructs that refer to the capacity to deliberately modulate one’s thoughts, feelings, or actions in the pursuit of planned goals; among these constructs are impulse control, response inhibition, emotion regulation, and attentional control. Aspects of self-control have been linked to the functioning of brain regions and systems that subserve cognitive control (e.g., lateral prefronal, lateral parietal, and anterior cingulate cortices).
Variations in sensation-seeking and self regulation, in turn, are associated with variations in behaviors, including risk-taking, which can be measured through objective reports or observations. In our model, risk-taking is a subset of many aspects of decision-making that share some, but not all, characteristics in common. Furthermore, as the Figure indicates, all decision-making takes place within a broader context that encourages and enables some acts but discourages and prohibits others.
Fourth, the links among these neurobiological, psychological, and behavioral constructs are imperfect, because they are moderated by other, often unmeasured, individual and contextual variables. An individual might be highly reward-sensitive but might have other qualities that lead him or her to inhibit the pursuit of arousing stimuli (e.g., high trait anxiety). Someone may be high in self-regulation, but in the face of strong peer pressure, might behave more recklessly than one would have predicted on the sole basis of a score on a measure of impulse control. An individual whose neurobiological and psychological inclinations would point to binge drinking will be more likely to drink to intoxication in a context in which alcohol is easily available than in one in which it is much harder to obtain.
Not surprisingly, then, the relation between puberty and reward-sensitivity or cognitive control may be different from that between puberty and sensation-seeking or self regulation, and the relation between puberty and sensation-seeking or self regulation may not be the same as that between puberty and actual risk-taking. In particular, the sharpest increase in risky behavior may occur later in development than the peak in pubertal change or the peak in sensation-seeking, because real-world risk taking is influenced by a wide constellation of factors that may modulate the impact of pubertal hormones or sensation-seeking tendencies on behavior. Puberty, on average, takes place when antisocial peer influence is still relatively weak and parental monitoring is still relatively vigilant (Collins & Steinberg, 2006). Thus, although pubertal hormones may incline the adolescent toward sensation-seeking, it is unlikely that the onset of puberty immediately triggers a rampage of recklessness. Although we argue that puberty and risky behavior are related, it would be foolish to expect that they move together in perfect lockstep.
Finally, as Figure 1 indicates, we hypothesize that reward-sensitivity and its psychological manifestation, sensation-seeking, are more strongly influenced by the biological changes of puberty than are cognitive control and its psychological manifestation, self-regulation. Although we believe that there is some limited support for this hypothesis in the extant literature, we recognize that there are findings that contradict or qualify this proposition (e.g., Perrin et al., 2008). In an effort to advance research on puberty and adolescent risky decision making, we offer this proposition not as a conclusion, but as a bit of cautious speculation that researchers may want to pursue in subsequent study.
A roadmap to this paper may be helpful: We begin by describing decision making as it plays out under “cold” and “hot” circumstances. As we explain, age differences in decision making are far smaller under conditions of low arousal (“cold”) than they are when arousal is high (“hot”), particularly in mid-adolescence, when the gap between sensation-seeking and self-regulation is greatest (Harden & Tucker-Drob, 2011; Shulman, Chein, Harden, & Steinberg, 2013). Having established the importance of these factors in adolescents’ decision making about risk, we describe the processes that might underlie risky decision making during adolescence, noting the ways in which the cognitive and socio-emotional changes of the period interact. Our discussion here is informed by so-called “dual systems” models, which focus on two distinct processes: one that supports reasoned, deliberative decisions and the other that encourages affective, impulsive, reward-focused decisions. We relate these distinct decision making processes to specific brain systems and explore the links among pubertal maturation, behavioral changes, and brain development over the course of adolescence. As we shall argue, asynchrony in the structural and functional maturation of the brain leaves the brain’s affective processing system in a state of relative hypersensitivity during a time when the brain’s deliberative processing system is not yet mature enough to compensate for the heightened affective response, thereby creating a period of vulnerability to affective inputs that encourage risky and reckless behavior. This asynchrony, we argue, may be partially linked to pubertal influences on development and, especially, to pubertal influences on affective processing.
The importance of affective and contextual factors in adolescent risk-taking
The fact that adolescents make notably different decisions about risk than adults, even though they possess the same basic reasoning and information processing skills, presents a challenge to simple rational accounts of adolescents’ behavior. Accordingly, many researchers interested in adolescent decision making have called attention to the importance of other factors that impact decisions about risk, including prior experience, socio-emotional influences, and social context (e.g., Miller & Byrnes, 1997; Fischhoff, 2008; Loewenstein, Weber, Hsee, & Welch, 2001; Reyna & Farley, 2006; Steinberg, 2008).
In recognition of the importance of these factors, researchers have devised ways to examine age differences in decision making in the laboratory by using experimental manipulations designed to induce shifts in the balance between “cold” and “hot” processing. A variety of approaches have proven useful in modulating the balance between these processing pathways, including manipulations of the quality of feedback given to participants during task performance (Figner, Mackinlay, Wilkening, & Weber, 2009), of the emotional content of task stimuli (Somerville, Hare, & Casey, 2011) and of the social conditions under which tasks are performed (Gardner & Steinberg, 2005; Chein, Albert, O’Brien, Uckert, & Steinberg, 2011). Here we give two examples of research in this spirit.
To examine whether the emotional context of decision-making influences the relative involvement of “cold” and “hot” processes differently among adolescents and adults, Figner and colleagues (Figner et al., 2009) manipulated feedback-related arousal in a risky decision-making task. In the task, referred to as the Columbia Card Task, participants were shown a grid of face-down cards. After being informed that the deck contained a specified number of “good” cards that would incrementally increase winnings and a specified number of “bad” cards that would substantially reduce those winnings (and end the round), participants were asked to choose how many cards they wished to turn over. In a “cold” condition, no feedback was provided until the end of game play, long after the participant had committed to turning over a certain number of cards for each round. In the “hot” version, designed to trigger increased involvement of the affective pathway, participants were instructed to turn the cards over one at a time, and were presented with immediate feedback on gains and losses with each card turn. Thus, whereas the “cold” version encouraged participants to rationally determine the optimal choice (based on the known value and probabilities of gain and loss cards), the “hot” version provided continuous affective feedback (e.g., rewards and punishments) to guide the decision-making process.
Under “cold” conditions, adolescents (split into groups of 13–16 and 17–19 year olds) and adults (20 years and older) demonstrated similar behavior (i.e., opted, on average, to turn over the same number of cards), with both groups approximating the statistically optimal pattern of behavior based on the value and ratio of “good” versus “bad” cards in the deck. Regardless of age, participants increased their rate of risk-taking in the “hot” version of the task, but the effect of the feedback manipulation was greatest among the adolescent groups, who turned over significantly more cards in the “hot” version than did the adults. The results indicate the increased sensitivity of adolescent participants to the affective manipulation, and the consequent increase in the tendency to engage in risky decision making.
A second illustration of the impact of context on age differences in decision making is a risky driving study completed in our lab (Chein et al., 2011). In a simulated driving game (The “Stoplight Game”) players tried to reach a destination as quickly as possible by making decisions as their car proceeded through a series of traffic intersections. At each intersection, the player could play it safe by hitting the brakes, resulting in a short delay at the light, or could take a chance by running the changing light in the hopes of moving on without delay, but risking a costly crash (and suffering a long delay). Participants were told that how quickly they made it to the end destination would influence a monetary bonus given at the end of the study session. In this task, peers (same-aged, same-sex friends) were watching via computer interface from a neighboring room, so no direct distraction was present. Overall rates of risk-taking (and subsequent crashes) did not differ between adolescents and adults when the game was played alone (the “cold” setting). However, when tested with a peer audience (the “hot” setting), adolescents showed a significant increase in risk-taking, whereas adults did not.
The relevance of socio-emotional context in adolescents’ decisions about risk is a central aspect of “dual systems” models of decision making, which characterize decision making as the byproduct of an interaction between processes that support controlled, reasoned, and deliberative behavior and those that drive reactive, emotional, and reward-sensitive responding. Indeed, the ability of dual-process models to accommodate the interacting influences of cognitive and affective inputs to decision making has elevated this class of models to the forefront of research on adolescent cognitive development (for a full review see, Steinberg, 2010, although see Pfeifer & Allen, 2012, for a recent critique).
The dual systems account
Dual-systems models offer a straightforward account for why adolescent decision making about risk often seems to parallel that of adults when studied under typical laboratory conditions but deviates substantially from that of adults under “hot” conditions and in many real-world contexts. Specifically, many laboratory studies of age differences in risky decision making purposefully minimize socio-emotional and situational factors that are present in everyday situations. Accordingly, these studies only observe the type of decision making that results from relatively slow, “cold,” analytical processes, in the absence of strong affective inputs. Though some of the mental machinery that supports “cold” processing continues to mature well beyond adolescence (e.g., lateral prefrontal cortex: O’Donnell, Noseworth, Levine, & Dennis, 2005; Asato, Terwilliger, Woo & Luna, 2010; Giedd, 2008,) the developmental state of this system during adolescence is generally adequate to support reasoned decision making in minimally arousing situations (Chein et al., 2011; Eshel, Nelson, Blair, Pine, & Ernst, 2007; Figner et al., 2009; Gardner & Steinberg, 2005; Van Leijenhorst, Westerberg, & Crone, 2008).
However, real-world decision making is often conducted under conditions of heightened arousal – e.g., when opportunities for reward and the presence of others are highly salient features of the decision-making environment. According to the dual-systems account, the reactive, “hot,” pathway is in a state of hypersensitivity during adolescence, and socio-emotional contextual factors provide a drive to activation of this hypersensitive system. In other words, increased risk-taking during adolescence can be explained by an increased tendency for adolescents to utilize the “hot” rather than the “cold” pathway when affective contextual influences are present (Gerrard, Gibbons, Houlihan, Stock, & Pomery, 2008; Casey, Getz & Galvan, 2008; Chambers, Taylor, & Potenza, 2003; Dahl, 2004; Steinberg, 2008, 2010). Although adults also encounter decision-making situations in which emotional arousal and social influence are present, adults are less sensitive to these affective inputs both because the responsiveness of the affective pathway may be attenuated in adulthood, and because adults possess a more fully matured capacity for cognitive control that allows impulsive, reactive, responses to be held in check (Chein et al., 2011). The extent to which the maturation of cognitive control itself dampens the responsiveness of the affective pathway is an important, but unstudied, question.
The cognitive and affective pathways discussed in dual-systems accounts are not merely useful theoretical constructs, but have been associated with two specific, interacting, brain systems that are implicated in decision making. Research on the neural mechanisms that subserve deliberative processing has generally focused on a network of interacting brain centers including the lateral prefrontal cortex most prominently, but also regions of the posterior parietal cortex and areas of the dorsal prefrontal midline (especially the dorsal anterior cingulate cortex) (Casey et al., 2001; Casey et al., 2008; Eshel et al., 2007; Luna, Padmanabhan, & O’Hearn, 2010). In the cognitive neuroscience literature, this “cognitive control” network of brain regions is often described with respect to its involvement in self-regulation, the ability to withhold prepotent responding in favor of deliberative, planned, goal-directed, and contextually appropriate actions (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Chein & Schneider, 2005, 2012; Miller & Cohen, 2001). The affective pathway described in dual-systems models is most closely associated with a network of limbic and paralimbic areas involved in emotion- and reward-related processing, most notably the ventral striatum (which subsumes the nucleus accumbens), but also the orbitofrontal cortex, ventromedial prefrontal cortex, amygdala, and superior temporal sulcus (Adolphs, 2003; Knutson & Cooper, 2005; Robbins & Everitt, 1996; Singer, Kiebel, Winston, Dolan, & Frith, 2004; Spear, 2009,). This network, which governs reward sensitivity, among other functions, is thought to influence the suite of inclinations we refer to as “sensation-seeking.”
The influence of puberty on development of the cognitive and affective pathways
As we discuss next, there is both behavioral and brain-related evidence suggesting that adolescence is an important time of change for certain cognitive and affective processes. However, the developmental changes occurring in these systems follow different timelines. At a basic, and admittedly simplistic, level of description, the cognitive mechanisms associated with deliberation follow a linear and protracted developmental trajectory that extends into adulthood (Davidson, Amso, Anderson, & Diamond, 2006; Steinberg et al., 2008; Steinberg, Cauffman, Woolard, Graham, & Banich, 2009), well beyond the most striking period in pubertal development, whereas the processes that underlie affective responding exhibit an inverted-U shaped pattern of developmental change that is most dramatic during the first part of adolescence (Steinberg, Cauffman, et al., 2009; Cauffman et al., 2010), the most significant period of pubertal development. These differential temporal correlations between cognitive development, affective development, and pubertal change provide clues that puberty plays a differential role in the development of each pathway, with puberty having an especially strong impact on the development of the affective pathway. Below we review this evidence in greater detail, beginning first with evidence from studies exploring the behaviors that are most closely tied to the deliberative and affective pathways, and then shifting our attention to explorations of the relation between brain development and pubertal development. To foreshadow, although there are some interesting and potentially informative inconsistencies in the literature, the weight of the available evidence seems to suggest that puberty plays a causal role in producing the imbalance between “cold” and “hot” processing that characterizes adolescent decision making about risk, mainly through its impact on processes subserved by the affective pathway, especially, reward-sensitivity.
Pubertal influences on behavior
Many basic cognitive, information-processing, and reasoning skills reach adult-like levels before mid-adolescence, and do not change substantially thereafter (Steinberg, Cauffman et al., 2009). Accordingly, the maturation of these foundational skills does not likely explain the large differences between adolescents’ and adults’ decision making about risk. However, one important cognitive skill, the capacity for self-regulation, exhibits a much more protracted period of maturation that extends across adolescence and into early adulthood (Davidson et al., 2006; Steinberg et al., 2008; Steinberg, Cauffman, et al., 2009). Given that the protracted development of this ability does not parallel the course of pubertal development, it is perhaps unsurprising that few studies have explored the direct relationship between pubertal maturation and the development of self-regulation. We are aware of just two relevant studies, and these studies have conflicting results. First, in a study of self-reported impulse control and pubertal stage, Warren and Brooks-Dunn (1989) reported a curvilinear trend, in which impulse control is heightened during early and late puberty but low at intermediate stages. They found that this effect was further increased when chronological age was controlled. When the relationship between impulse control and the development of secondary sexual characteristics (i.e., breast development) was assessed, more advanced physical maturation was related to higher levels of impulse control, although this relationship was not significant once age was controlled. In contrast, Steinberg and colleagues found that impulsivity, as indexed both by self-report and performance on a behavioral task, was correlated with chronological age but not pubertal status (Steinberg et al., 2008). The discrepancy between these studies may be due to the use of different operationalizations of impulse control; Warren and Brooks-Gunn used items from the Impulse Control subscale of the Offer Self-Image Questionnaire (Offer, Ostrov, & Howard, 1981), on which more than half of the items are questionable indices of impulsivity, and appear to be more reflective of negative affectivity [e.g., “I fear something constantly,” “At times I have fits of crying and/or laughing,” “I carry many grudges,” and “Even under pressure I manage to remain calm”], whereas Steinberg et al. used items from the Barratt Impulsivity Scale, which has better face validity as a measure of impulse control. (Interestingly, studies directly examining puberty and negative affectivity find that they are positively correlated among females (Negriff & Susman, 2011), which may account for the Warren and Brooks-Gunn finding.)
While there is little research on the direct impact of pubertal processes on the development of the self-regulatory abilities involved in “cold” decision making, there is evidence pointing to a more direct link between pubertal maturation and developmental changes in the “hot,” affective processing pathway. To begin, the recruitment of “hot” processes during adolescent decision making is consistent with a peak in reward-sensitivity. Heightened sensitivity to reward is implicated in increased sensation- and novelty-seeking, attention to reward, emotional arousal, and attention to social information. These behaviors are more prevalent among adolescents compared to both children and adults. Evidence for curvilinear developmental changes in psychological and behavioral manifestations of reward-sensitivity is demonstrated in a study examining age differences in reward processing, risk taking, and psychosocial maturity in a large sample of individuals ranging from 10 to 30 years old (for a complete review of study findings, see Steinberg, Cauffman, et al., 2009). This study provided evidence for peaks (followed by declines) in early-to-middle adolescence of self-reported risk preference (Steinberg, 2009) and sensation-seeking (Steinberg, Albert, et al., 2008), as well as behavioral indicators of reward sensitivity (on a modified version of the Iowa Gambling Task; Cauffman et al., 2010) and preference for immediate over delayed rewards (Steinberg, Graham, et al., 2009).
The direct influence of pubertal hormones on reward-seeking behavior has been demonstrated in a series of studies using animal models. Specifically, administrations of testosterone and/or estradiol have been tied to increased conditioned place preference (Alexander, Packard, & Hines, 1994), lever pressing for drug and saccharin administration (Clark, Lindenfield, & Gibbons, 1996; Miele, Rosellin, & Svare, 1988), and reward-related brain stimulation (Bless et al., 1997). It is posited that androgens have reinforcing effects that increase the salience of rewarding stimuli (Wood, 2004).
The reinforcing effects of androgens also have been demonstrated in humans, both with naturally elevated and artificially administered testosterone (Stanton, Liening, & Schultheiss, 2011; VanHonk et al., 2004). Using the Iowa Gambling Task (IGT), Stanton et al (2011) examined salivary levels of testosterone and task performance in young adults. They found that individuals with naturally high levels of testosterone exhibited elevated reward-seeking and decreased punishment avoidance, resulting in disadvantageous decision making. In a similar study, testosterone was artificially administered to young women prior to completing the IGT (VanHonk et al., 2004). Following testosterone administration, participants showed increased reward-seeking, again resulting in disadvantageous decision making, similar to effects seen in studies of naturally high levels of testosterone. Hormone-dependent increases in reward sensitivity thus may bias an individual towards risk-taking.
Sensation-seeking is likely influenced by reward-sensitivity and follows a similar developmental trajectory, peaking during adolescence and declining through adulthood (Steinberg et al., 2008; Zuckerman et al., 1978). A recent analysis of cross-sectional data from more than 7500 individuals between the ages of 12 and 24 found that self-reported sensation-seeking followed an inverted-U shaped function, peaking in mid-adolescence, whereas impulsivity declined linearly over the course of adolescence and into young adulthood (Harden & Tucker-Drob, 2011). Importantly, sensation-seeking during adolescence is more closely tied to pubertal maturation than chronological age (Steinberg, et al., 2008). High levels of sensation seeking behavior are also closely tied to hormone levels during adolescence. For example, Martin and colleagues (2002) found that self-reported sensation seeking was positively correlated with pubertal maturation in a group of early adolescents, an effect that was present even when controlling for chronological age. Additionally, sensation seeking mediated the relationship between puberty and risky behaviors, such as substance abuse. Sensation seeking and substance use have also been closely tied to testosterone release in males (Bauman et al., 1989; Daitzman & Zuckerman, 1980) and estrogen release in females (Daitzman et al., 1978; Martin et al., 1999).
The fact that post-pubertal levels of sex hormones remain high long after sensation-seeking begins to decline indicates that the relation between puberty and sensation-seeking is more complicated than a simple one-to-one correspondence between the two. Several possibilities come to mind. First, sensation-seeking, although influenced by reward-sensitivity, may also be affected by other factors that follow a different developmental trajectory. As we noted earlier, research has not examined the extent to which maturation of cognitive control, which we hypothesize is independent of puberty and which continues long after pubertal maturation is complete, may dampen sensation-seeking. Second, it may be the degree of change in pubertal hormones, rather than their absolute levels, that influence reward-sensitivity; sensation-seeking may therefore peak in adolescence because of the heightened rate of hormonal change. Finally, situational factors may again moderate the relationship: 25-year-old males may have testosterone levels comparable to those of 16-year-olds, but 25-year-old males are more likely to have wives and jobs, both of which put a damper on risky behavior (Sweeten, Piquero, & Steinberg, 2013).
Overall, then, behavioral research is consistent with the hypothesis that changes in behaviors subserved by the “hot,” affective pathway are likely to be linked to the hormonal changes of puberty. There is insufficient research on the relation between pubertal maturation and cognitive control to draw any definitive conclusions. From an evolutionary standpoint, it makes good sense that the hormonal changes of puberty are linked to increases in reward-sensitivity, sensation-seeking, and risk taking, since these changes increase the likelihood that adolescents will leave the natal environment to mate outside the family (Steinberg, 2008). Further research should explore the possibility that pubertal maturation exerts a stronger influence on reward-sensitivity than on cognitive control.
Pubertal influences on brain development
Studies exploring the ways in which the human brain changes – at a global level, but also within the two specific brain networks thought to underlie deliberative and affective processing – during the period from preadolescence into adulthood have fundamentally informed our understanding of the forces that influence adolescent decision making. Of particular importance is the idea that brain development does not follow a single global pattern, but instead, that the different brain systems involved in cognitive control and reward processing seem to undergo asynchronous periods of change evincing a pattern consistent with speculation that changes in systems that subserve reward processing may be more influenced by pubertal maturation than changes in systems that subserve cognitive control.
During adolescence there are hormonally-driven organizational and activational changes in the brain (Schulz, Molenda-Figueira, & Sisk, 2009; Sisk & Zehr, 2005). Activational changes are considered transient, and the brain systems affected by them often return to their pre-activated state when hormone levels subside; in contrast, organizational changes are permanent changes in the structure and organization of brain regions that are initiated by the release of hormones but not dependent on continuing hormone release (for full review see Sisk & Zehr, 2005). Historically, organizational/activational theories posited that perinatal hormone exposure resulted in organizational changes that were then reactivated during hormone release in adolescence (Phoenix, Goy, Gerall, & Young, 1959). However, recent studies have demonstrated that there are organizational changes occurring during adolescence as well, and, moreover, that these organizational changes may be critical for activational changes to take place (e.g. Meek et al., 1997; Schulz et al., 2004; for full review see Sisk & Zehr, 2005). For example, prepubescent rodents do not exhibit reproductive behaviors when administered gonadal hormones, suggesting that this system is reorganized during puberty to allow activation of adult behaviors (Sato et al, 2008). The extent to which puberty-related changes in brain structure and function during adolescence are due to the activational effects of gonadal hormones, organizational effects, or some combination of the two, is not known.
Adolescence is characterized by two critical structural changes in the brain: decreases in gray matter volume (neuronal bodies) and increases in white matter volume (myelinated axons). Gray matter loss during adolescence is not spatially uniform across the brain, and instead, occurs at different times and rates in different brain regions (for recent reviews see Giedd & Rapoport, 2010; Gogtay and Thomson, 2010). The initiation of gray matter loss in the frontal cortex during adolescence is considered a hormone-dependent process (Giedd et al., 1999; Gogtay et al., 2004; Peper et al, 2009; Sowell et al., 2001). In contrast, increases in white matter have traditionally been considered a hormone-independent process, occurring at the same rate from childhood through adolescence, with no significant changes in the acceleration of growth during puberty (Paus et al., 2001). This relatively linear pattern of development has been taken as evidence that white matter development is relatively independent of puberty (since it continues long after pubertal maturation is complete) and must be driven in part by non-puberty-related forces, including learning and experience. However, in light of recent research indicating that pubertal hormones may in fact play a role in white matter development (Perrin et al., 2008, 2009), more studies of the effect of pubertal hormones on white matter development during adolescence are needed.
Research on the development of cognitive control has focused mainly on changes in the lateral prefrontal cortex (PFC) (Casey, Getz, & Galvan, 2008, Luna, Padmanabhan, & O’Hearn, 2010). In line with behavioral studies showing protracted maturation of self-regulation beyond adolescence, structurally, this brain area is among the last to reach adult-like cortical composition (Giedd, 2006). For example, cortical thinning (associated with decreases in gray matter and increases in white matter) in the PFC is evident throughout adolescence and well into the second decade of life, indicating that this system continues to mature even beyond late adolescence (Asato, Terwilliger, Woo & Luna, 2010; Giedd, 2008; O’Donnell, Noseworth, Levine, & Dennis, 2005).
The proposed link between structural brain maturation and gains in self-regulation is further supported by convergent evidence from functional neuroimaging studies of developmental differences in the neural correlates of self-regulation (Andrews-Hanna et al., 2011; Luna & Sweeney, 2001; Rubia, et al., 2006; Rubia et al 2007; Tamm, Menon, & Reiss, 2002). Imaging studies utilizing a variety of self-regulation paradigms (e.g., Go-No/Go, Stroop, flanker tasks, antisaccade) suggest that adolescents recruit the cognitive control network – especially the lateral PFC – less efficiently than do adults (Adleman et al., 2002; Bunge, et al., 2002; Casey et al., 1997; Casey et al., 2008; Durston et al 2006; Luna et al., 2001). In general, adolescents show stronger activation than children of the lateral PFC while performing cognitive control tasks, consistent with structural maturation of the region in early adolescence (e.g., Luna et al., 2001). In contrast, between adolescence and adulthood, differences in activation appear to reflect a process of refinement in the recruitment and coordination of structurally mature regions, rather than gross differences in level of activation (Tamm, Menon, & Reiss, 2002). Specifically, adolescents show increasingly focal engagement of task-relevant regions supporting cognitive control, a functional advance that may reflect the increased integrity and efficiency of inter-regional connections (Durston et al., 2006). In fact, increased inter-region connectivity, as measured by DTI, is positively correlated with self-reported impulse control during adolescence (Silveri et al., 2006) and with self-reported resistance to peer influence (Paus et al., 2008). Studies have also reported weaker activation in the PFC in adolescents compared to adults during risky decision making, consistent with the notion that heightened risk taking in adolescence may be due in part to immaturity in cognitive control brain systems (Bjork et al., 2007; Chein et al., 2011). We could find no studies that explicitly examined the relationship between frontal lobe function and pubertal maturation, but there is little evidence in either the behavioral or neuroimaging literatures that gains in self-regulation are attributable to a hormone-dependent process.
One of the most important structural and functional brain changes during adolescence is the maturation of limbic and paralimbic areas associated with reward processing (including the amygdala, ventral striatum, orbitofrontal cortex, medial prefrontal cortex, and superior temporal sulcus). This network is centrally involved in the processing of social and emotional stimuli (e.g., face recognition, social judgments, social reasoning; Adolphs, 2003) and, importantly, includes neural circuits that mediate reward-sensitivity (Spear, 2009). Moreover, there is considerable overlap within this network between regions showing activation in response to social stimuli and regions that are differentially activated in response to variations in reward magnitude (e.g., the ventral striatum and medial frontal areas) (Steinberg, 2008). However, the majority of reward-processing studies have focused on the ventral striatum as key player in motivated behaviors.
Findings from studies of age-dependent functional activation of the ventral striatum map nicely onto evidence showing heightened reward sensitivity during adolescence relative to childhood and adulthood. For example, several recent functional neuroimaging studies of age differences in reward processing have found increased BOLD activation in the striatum among adolescents in response to reward anticipation, compared to adults and children (Ernst et al., 2005; Galvan et al., 2006; Geier et al., 2010). In adolescents, striatal response to reward is also magnitude dependent, wherein activation increases as the magnitude of the reward increases (Galvan et al., 2006). Although opposite results were found in an fMRI study of age differences in reward anticipation (rather than receipt), with adolescents showing decreased ventral striatum activation relative to adults (Bjork et al., 2004; Bjork et al., 2010), a recent review of research on developmental changes in reward sensitivity, as indexed by activation of ventral areas in response to rewarding stimuli, concluded that there is considerable support for the notion that reward sensitivity is higher in adolescence than before or after (Galvan, 2010).
Importantly, during risky decision-making tasks, adolescents show increases in striatal activation during receipt of reward compared to children and adults, suggesting sensitivity to feedback (Ernst et al., 2005; Van Leijenhorst, Moor et al., 2010, Van Leijenhorst, Zanolie et al., 2010). While some studies examining striatal activation during decision making have found increased striatal activation in adolescents compared to adults (Chein, et al., 2011; Van Leijenhorst, Zanolie et al., 2010), others have found no developmental differences (Eshel et al., 2007; Van Leijenhorst, Moor et al., 2010). However, the magnitude of striatal activity during decision making has been found to be correlated with self-reported risk-taking (Galvan et al., 2006) and to be predictive of whether or not a risk will be taken (Chein et al., 2011). Differences in developmental accounts of striatal activation during decision-making tasks may be due to nuances in experimental manipulations, but quite consistently highlight the selectivity of the striatum during the decision-making process.
Few neuroimaging studies have attempted to study the relationship between pubertal development and functional activation in reward-related regions. One exception is a recent study by Forbes and colleagues (2010), which compared reward processing in pre/early pubertal adolescents, mid/late pubertal adolescents, and post-pubertal adults. Importantly, all adolescents were within the same age group (girls aged 11–12, and boys aged 12–13). During receipt of reward, mid/late pubertal adolescents exhibited weaker striatal response and stronger PFC response compared to pre/early pubertal adolescents. The mid/late pubertal adolescent group did not differ from the adult group, suggesting that a near mature reward processing system is in place by the end of puberty. In addition, testosterone levels were negatively correlated with striatal response to reward outcome (keep in mind that testosterone levels increase steadily during puberty and, unlike reward sensitivity, do not decline during the transition from adolescence to adulthood). However, in boys testosterone was positively correlated with reward anticipation. These findings suggest that testosterone may influence the striatum differentially during the anticipatory and consummatory phases of reward processing and that sex differences may be particularly important during this period of development. Nonetheless, this study provides evidence that reward sensitivity decreases during the later stages of pubertal maturation, perhaps suggesting desensitization of the activational effects of gonadal hormones on the reward system in the later stages of pubertal development.
Another recent publication, by Op de Macks and colleagues (2011), examined the relationship between reward-related activation and pubertal hormones in a sample of 10- to 16-year-olds during a risk-taking task. Contrary to Forbes et al., (2010), Op de Macks found that testosterone levels were positively associated with striatal activation to receipt of reward among both male and female adolescents, suggesting that testosterone continues to have an activational effect on reward processing in mid-adolescence. Differences between these two studies may be due to task-related differences or differences in participant ages (as we discuss later in this article, the interpretation of correlations between pubertal indices and behavioral outcomes is difficult when the age range of the participants is wide). However, the Op de Macks finding is in line with animal studies indicating the reinforcing behavioral effects of pubertal hormones throughout the lifespan (Alexander, Packard, & Hines, 1994; Clark, Lindenfeld, & Gibbons, 1996). In addition, positive relationships between testosterone/estrodiol levels and striatal response to reward are also seen in adults who are artificially administered testosterone (Hermans et al., 2010) and women in the follicular phase of the menstrual cycle, when estrogen levels are high (Dreher et al., 2007), suggesting that the activational effects of pubertal hormones on reward responsivity (but perhaps not their organizational effects) continue into adulthood.
In further exploration of the influences of gonadal hormones on brain structure, one line of research has focused on the pattern of proliferation and pruning of dopamine receptors in the striatum and prefrontal cortex (PFC) during adolescence as an underlying mechanism of increased functional activation of the reward-related system during this period (Sisk & Foster, 2004). Developmental changes in the mesocorticolimbic dopamine system, in particular, appear to coincide with shifts in reward-related behavior (Spear, 2009). Briefly, this system includes dopamine neurons projecting from the midbrain (substantia nigra and ventral tegmental area) to the striatum (including the nucleus accumbens) and PFC. Converging evidence points to changes around the time of puberty in dopamine receptor density and distribution and in subsequent neurotransmission in the striatum and PFC. Dopamine receptor binding in the rat striatum peaks in adolescence at levels that are 30–45% greater than those observed in adulthood (e.g., Teicher et al., 1995). Furthermore, excitatory dopamine input to the PFC shows adolescent peaks in both rodent (Spear, 2009) and non-human primate populations (Rosenberg & Lewis, 1995). Despite evidence for lower basal levels of dopamine release in adolescent (relative to adult) rats, adolescent rats evince greater dopamine release than adults in response to certain rewarding stimuli (Laviola, Pascucci, & Pieretti, 2001).
Dopaminergic remodeling and associated changes in the reward system have not been directly linked to puberty-related gonadal hormones. Research with gonadectomized rodents demonstrates normative patterns of dopaminergic proliferation and pruning, indicating that such neural development is not dependent on organizational changes in hormones at puberty (Spear, 2009). It is important to keep in mind, however, that many neuroendocrinological changes that take place around the time of puberty are linked to hormonal events that organize the brain long before adolescence, and that are not reflected in changes in gonadal hormones at puberty (Sisk & Foster, 2004). The impact of pubertal hormones on dopamine functioning may thus reflect activational rather than organizational processes. So, puberty-coincident changes in dopaminergic systems may result from steroid-dependent processes (some of which are organized pre- or peri-natally and other of which are activated peripubertally), steroid-independent processes, or interactions among these processes (Steinberg, 2008).
Taken together, these various results illustrate just how complicated the relationship between reward-processing and puberty is – findings vary as a function of the index of puberty employed, the age of the participants, the task used to measure reward sensitivity, and the phase of reward processing (i.e., anticipation or receipt) studied. While functional studies have highlighted the role of pubertal hormones in striatal function, dopaminergic restructuring of associated brain regions is currently considered independent of hormonal change at puberty (although likely dependent on the organizational effects of early exposure to sex hormones). One way in which further research might clarify current findings is by examining whether puberty differentially influences the regulation of specific dopamine receptor subtypes whose distribution is non-uniform across cognitive and affective pathways.
Our understanding of the interactions between reward and control processing systems is also informed by studies examining whether performance on measures of control can be affected by variations in the availability of rewards (Geier, et al., 2010; Hardin et al., 2007; Jazbec, et al., 2006). The findings are somewhat mixed, but provide evidence that developmental differences in control can be reduced (or eliminated) when successful performance is directly and adequately rewarded. For example, Jazbec and colleagues (2006) demonstrated that during a rewarded saccade task adolescents’, but not adults’, antisaccade performance was modulated by reward contingences. More specifically, as reward contingencies increased adolescents committed less antisaccade errors, eliminating developmental differences in control.
Finally, although we have written about the affective and cognitive control systems as separate entities, over the course of adolescence anatomical and functional connections between structures within the affective and cognitive brain systems, specifically the striatum and PFC, increase (Dosenbach et al., 2010, Sommerville, Hare, & Casey, 2011). Increased fronto-striatal circuitry is often implicated in increases in cognitive control (Casey et al., 2007; Durston et al., 2002; Marsh et al., 2006; van den Bos et al., 2011), but it is also plausible that declines in reward sensitivity after adolescence are the result of increased connectivity between these systems. For example, in a recent study by Somerville, Hare, and Casey (2011), children (ages 6–12), adolescents (ages 13–17) and adults (ages 18–29) completed an emotional version of a Go/No-Go task, a classic measure of response inhibition. In this version of the task participants were presented with a sequence of neutral and happy faces. In the neutral (“cold”) condition, participants were asked to withhold a response when neutral faces were presented, whereas the affective (“hot”) condition required withholding a response for happy faces. In both conditions, adolescents, but not children or adults, exhibited greater striatal response to happy compared to neutral faces, demonstrating a hypersensitivity to affective stimuli. More important, during the “hot” trials, adolescents had significantly more false alarms (i.e., inability to withhold a response) than any other age group. These results imply that the adolescents were unable to suppress impulsive behaviors in the face of salient, emotionally arousing stimuli. Significantly, the ability to suppress a response in adults was correlated with increased frontostriatal connectivity, suggesting that the increased connectivity between reward and self-regulation regions may be responsible for decreases in risk-taking behavior into adulthood. Recent research indicates that functional connectivity increases steadily throughout adolescence, reaching a plateau around age 22 (Dosenbach et al., 2010), around the time that rates of most forms of risky behavior have already begun to decline.
Challenges for future research
Both behavioral and neuroimaging research have demonstrated significant changes in self-regulation and sensation-seeking across adolescence (Steinberg, 2008). Within the dual systems framework, heightened attention to reward in the context of immature cognitive control makes adolescents particularly vulnerable to risk taking. Within this model, mature decision making is reached when the influences of affective states are in balance with processes that allow for reasoned decision making, perhaps related to increased fronto-striatal connectivity.
As evidenced in this review, the complicated interactions between pubertal maturation and changes in reward-sensitivity and cognitive control remain largely unstudied. We believe that the possibility that changes in reward sensitivity are relatively more puberty-dependent than are changes in cognitive control is worthy of systematic investigation. Although more research on the links between puberty, brain maturation, socio-emotional context, and decision making in adolescence is clearly needed, it is important to note several methodological challenges that must be considered.
First, chronological age and puberty are confounded, and examining the impact of one while controlling for the impact of the other is not necessarily the best strategy to pursue. If one is interested in the relation between puberty and some aspect of decision making, for example, controlling for chronological age changes the research question into one about pubertal timing, rather than pubertal status (Steinberg, 1987). A long history of behavioral research demonstrates that many of the effects of pubertal maturation are conditioned on the age at which puberty occurs. Whether the links between puberty and either cognitive control or reward sensitivity are also moderated by age is not known, although there is reason to think that the impact of pubertal hormones on risk taking might be stronger among early maturers than on-time or late maturers, in part because early maturers experience these hormonal changes at younger ages, when their self-regulatory competencies are less mature (Steinberg, 2008). In order to examine the effects of puberty independent of age, one can investigate a sample in which there is variation in pubertal status within a narrowly bounded chronological age group (Dahl, 2004), but again, pubertal timing and pubertal status are confounded in such samples. Conversely, in order to isolate the effects of chronological age that are independent of puberty, one needs a sample with a wide age range but little variation in pubertal status.
Second, it is important to keep in mind that puberty may influence brain development through many different mechanisms. Although we have focused the present discussion on the possible direct effects of pubertal hormones on brain structure and function, puberty may also affect adolescent decision-making indirectly, by influencing the ways in which an adolescent whose outward appearance is changing interacts with others. For example, early maturing adolescents may be given increased responsibilities at home and in the classroom, which in turn may contribute to the development of self-regulatory competence.
Third, there is accumulating evidence that the timing of puberty itself is influenced by social experience. Several studies have shown, for example, that puberty is accelerated in family contexts characterized by parent-adolescent conflict and or father absence (for a review, see Ellis, 2000). It is therefore conceivable that variations in adolescents’ experiences that are linked to the development of decision making may influence pubertal maturation, rather than the reverse.
Finally, because puberty coincides with many other changes in adolescent functioning, it is essential that researchers explore the ways in which puberty interacts with other factors to influence decision making. In addition to possible variability in the impact of puberty as a function of timing, it is likely that the ways in which puberty affects decision making vary as a function of the context in which the adolescent develops. Puberty may be associated with risky decision making in the laboratory, but whether this plays out in the real world depends on opportunities for engaging in risky behavior. A combination of laboratory experiments, animal studies, and nonexperimental studies outside the laboratory will be most informative.
Highlights.
Adolescent risky decision making (DM) has both pubertal and non-pubertal influences.
Pubertal influences on DM are examined within the dual systems framework.
We propose that a pubertal-dependent affective system impacts adolescent DM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31(2):790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Adleman N. A Developmental fMRI Study of the Stroop Color-Word task. NeuroImage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Is the human amygdala specialized for processing social information? Annals New York Academy of Sciences. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Packard MG, Hines M. Testesterone has rewarding affective properties in male rats: Implications for the biological basis of sexual motivation. Behavioral Neuroscience. 1994;108(2):424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: Neural underpinnings and relation to self-report behaviors. (S. Gilbert, Ed.) PLoS ONE. 2011;6(6):e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: A DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman KE, Foshee VA, Koch GG, Haley NJ, Downton MI. Testosterone and cigarette smoking in early adolescence. Journal of Behavioral Medicine. 1989;12(5):425–433. doi: 10.1007/BF00844876. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knuston B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: Comparing motivational neurocircuitry recruitment using fMRI. In: Lauwereyns J, editor. PLoS ONE. 7. Vol. 5. 2010. p. e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. Journal of Neuroscience. 2007;27(18):4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bless EP, McGinnis KA, Mitchell AL, Hartwell A, Mitchell JB. The effects of gonadal steroids on brain stimulation reward in female rats. Behavioural Brain Research. 1997;82:235–244. doi: 10.1016/s0166-4328(96)00129-5. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen J. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley MJ. Yes, your teen is crazy! Loving your kid without losing your mind. Gig Harbor, WA: Harbor Press, Inc; 2002. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CH, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008a;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, et al. Sensitivity of prefrontal cortex to changes in target probability: A functional MRI study. Human Brain Mapping. 2001;13:26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Hinshaw S, Epstein JN, Greenhill LL, Buhle J, Shafritz KM, Liston C, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. American Journal of Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Casey B, Jones RM, Hare T. The adolescent brain. Annals New York Academy of Sciences. 2008b;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of cognitive neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46(1):193–207. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics. Washington, CDC: 2011. [Google Scholar]
- Centers for Disease Control and Prevention. Surveillance Sumaries. 4. Vol. 61. Washington, CDC: 2012. Youth Risk Behavior Surveillance Survey—United States, 2011. [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatry. 2003;160(6):1041. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Cognitive Brain Research. 2005;25(3):607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. The brain’s learning and control architecture. Current Directions in Psychological Science. 2012;21(2):78–84. [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Lindenfield RC, Gibbons CH. Anabolic-androgenic steroids and brain reward. Pharmacology, Biochemistry, and Behavior. 1996;53(3):741–745. doi: 10.1016/0091-3057(95)02082-9. [DOI] [PubMed] [Google Scholar]
- Collins WA, Steinberg L. Adolescent development in interpersonal context. In: Eisenberg N, Damon W, Lerner R, editors. Social, emotional, and personality development. Handbook of Child Psychology. New York: Wiley; 2006. pp. 1003–1067. [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021(1):1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Daitzman RJ, Zuckerman M, Sammelwitz P, Ganjam V. Sensation seeking and gonadal hormones. Journal of Biosocial Science. 1978;10(4):401–408. doi: 10.1017/s0021932000011895. [DOI] [PubMed] [Google Scholar]
- Daitzman R, Zuckerman M. Disinhibitory sensation seeking, personality and gonadal hormones. Personality and Individual Differences. 1980;1:103–110. [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Biro FM. Puberty and its measurement: A decade in review. Journal of Research on Adolescence. 2011;21:180–195. [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. PNAS. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey B. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug A, Zimmerman R, Casey B. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. [Google Scholar]
- Ellis B, Garber J. Psychosocial antecedents of variation in girls’ pubertal timing: Maternal depression, stepfather presence, and marital and family stress. Child Development. 2000;71(2):485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: Age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(3):709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Fischhoff B. Assessing adolescent decision-making competence. Developmental Review. 2008;28(1):12–28. [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck S, Worthman CM, Moyles DL, Tarr JA, et al. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(2):162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4(6):1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology. 2005;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescents. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard M, Gibbons FX, Houlihan AE, Stock ML, Pomery EA. A dual-process approach to health risk decision making: The prototype willingness model. Developmental Review. 2008;28(1):29–61. [Google Scholar]
- Giedd JN. The teen brain: Insights from neuroimaging. Journal of Adolescent Health. 2008;42(4):335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of Pediatric Brain Development: What Have We Learned and Where Are We Going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijenbos A, Paus T, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, et al. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, et al. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: Further evidence for a dual systems model. Developmental Psychology. 2011;47(3):739–746. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- Harden MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents. Journal of Child Psychology and Psychiatry. 2007;48(5):446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Bos PA, Ossewaarde L, Ramsey NF, Fernández G, van Honk J. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. NeuroImage. 2010;52(1):277–283. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Scroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174(4):752–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Archives of General Psychiatry. 1999;56(7):617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitizationto D-amphetamine in periadolescent but not in adult rats. Pharmacology, Biochemistry, and Behavior. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C] raclopride study in healthy men. Neuropsychopharmacology. 2002;27(6):1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Lind NM, Gjedde A, Moustgaard A, Olsen AK, Jensen SB, Jakobsen S, Cumming P. Behavioral response to novelty correlates with dopamine receptor availability in striatum of Göttingen minipigs. Behavioural brain research. 2005;164(2):172–177. doi: 10.1016/j.bbr.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychological Bulletin. 2001;127(2):267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: A strategy for testing neurodevelopmental hypotheses. Schizophrenia Bulletin. 2001;27(3):443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz R, Quackenbush G, Royal J, Skudlarski P, Peterson B. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Arch G, Ill M, Curry T, Martin D. Alcohol use in adolescent females: Correlates with estradiol and testosterone. The American Journal on Addictions. 1999;8(1):9–14. doi: 10.1080/105504999306036. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogoli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Hormones and behavior. 1997;31(1):75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Miele J, Rosellini RA, Svare B. Estradiol benzoate can function as an unconditioned stimulus in a conditioned taste aversion paradigm. Hormones and Behavior. 1988;22:116–130. doi: 10.1016/0018-506x(88)90035-9. [DOI] [PubMed] [Google Scholar]
- Miller DC, Byrnes JP. The role of contextual and personal factors in children’s risk taking. Developmental Psychology. 1997;33(5):814–823. doi: 10.1037//0012-1649.33.5.814. [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annu Rev Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mościcki EK. Epidemiology of completed and attempted suicide: toward a framework for prevention. Clinical Neuroscience Research. 2001;1(5):310–323. [Google Scholar]
- Negriff S, Susman EJ. Pubertal timing, depression, and externalizing problems: a framework, review, and examination of gender differences. Journal of Research on Adolescence. 2011;21(3):717–746. [Google Scholar]
- O’Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. NeuroImage. 2005;24(4):948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Offer D, Ostrov E, Howard K. The adolescent: A psychological self-portrait. New York: Basic Books; 1981. [Google Scholar]
- Op de Macks ZAO, Moor BG, Overgaauw S, Güroğlu B, Dahl RE, Crone EA. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Accident Analysis and Prevention. 2011;1(4):506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans A, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Toro R, Leonard G, Lerner J, Lerner R, Perron M, Pike G, Richer L, Steinberg L, Veillette S, Pausova Z. Morphological properties of the action-observation cortical network in adolescents with low and high resistance to peer influence. Social Neuroscience. 2008;3:303–316. doi: 10.1080/17470910701563558. [DOI] [PubMed] [Google Scholar]
- Peper JS, Schnack HG, Brouwer RM, Van Baal GCM, Pjetri E, Székely E, van Leeuwen M, et al. Heritability of regional and global brain structure at the onset of puberty: A magnetic resonance imaging study in 9-year-old twin pairs. Human Brain Mapping. 2009;30(7):2184–2196. doi: 10.1002/hbm.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, et al. Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. Journal of Neuroscience. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, et al. Sex differences in the growth of white matter during adolescence. NeuroImage. 2009;45(4):1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65(3):369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Piquero AR. Taking stock of developmental trajectories on criminal activity over the life course. In: Liberman A, editor. The long view of crime: A synthesis of longitudinal research. New York: Springer; 2007. [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making. Psychological Science in the Public Interes: Implications for Theory, Practice, And Public Policy. 2006;7(1):1–46. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt J. Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. The Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rousseau J. In: Emile: Or, on education. Foxley B, editor. New York: Dutton; 1911. Orig.work published in 1762. [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SM, Schulz KM, Sisk CL, Wood RI. Adolescents and androgens, receptors and rewards. Hormones and behavior. 2008;53(5):647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational activational hypothesis adapted to puberty and adolescence. Hormones and behavior. 2009;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and behavior. 2004;45(4):242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Shulman E, Chein J, Harden KP, Steinberg L. Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood. 2013 doi: 10.1007/s10964-014-0116-9. Manuscript under review. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magnetic Resonance Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41:653–662. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;36(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey B. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of Neuroscience. 2001;21(22):8619–8629. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. New York: W.W. Norton & Co; 2009. [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa Gambling Task. Hormones and Behavior. 2011;59(2):252–256. doi: 10.1016/j.yhbeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Steinberg L. The impact of puberty on family relations: Effects of pubertal status and pubertal timing. Developmental Psychology. 1987;23:451–460. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Should the science of adolescent brain development inform public policy? American Psychologist. 2009;64:739–750. doi: 10.1037/0003-066X.64.8.739. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Cauffman E, Woolard J, Graham S, Banich M. Are adolescents less mature than adults?: Minors’ access to abortion, the juvenile death penalty, and the alleged APA ‘flipflop.’. American Psychologist. 2009;64(7):583–594. doi: 10.1037/a0014763. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age Differences in Future Orientation and Delay Discounting. Child Development. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Sweeten G, Piquero A, Steinberg L. Age and the explanation of crime, revisited. Journal of Youth and Adolescence. 2013 Feb 15; doi: 10.1007/s10964-013-9926-4. Article available online in advance of publication. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-42, HHS Publication No (SMA) 11-4667. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. Results from the 2010 National Survey on Drug Use and Health: Mental Health Findings. [Google Scholar]
- Tamm L, Menon V, Reiss A. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter J. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Twisk DA, Stacey C. Trends in young driver risk and countermeasures in European countries. Journal of Safety Research. 2007;38:245–57. doi: 10.1016/j.jsr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- van den Bos W, Cohen MX, Kahnt T, Crone EA. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2011;22(6):1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG, Hermans EJ, Putman P, Tuiten A, Koppeschaar H. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29(7):937–943. doi: 10.1016/j.psyneuen.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: Age and gender analyses of probability estimation and reward evaluation. Developmental Neuropsychology. 2008;33(2):179–196. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What Motivates the Adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2009;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Warren MP, Brooks-Gunn J. Mood and behavior at adolescence: Evidence for hormonal factors. Journal of Clinical Endocrinology and Metabolism. 1989;69(1):77–83. doi: 10.1210/jcem-69-1-77. [DOI] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiology & Behavior. 2004;83(2):279–289. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. Journal of Consulting and Clinical Psychology. 1978;46(1):139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge university press; 1994. [Google Scholar]


