Abstract
The pubertal period is a time of change in an animal’s response to stress, and it is a second period of sexual differentiation of the brain. Recently, it was discovered that particular stressors during the prolonged pubertal period of female mice result in enduring changes in behavioral responsiveness of the brain to estradiol and progesterone. Depending on the behavior, pubertal immune challenge or shipping from suppliers may decrease, eliminate, or even reverse the effects of estradiol. Pubertal immune challenge results in changes in the number of estrogen receptor-immunoreactive cells in key brain areas suggesting a cellular mechanism for this remodeling of the brain’s response to hormones. A hypothesis is put forward that predicts that particular adverse experiences in girls may cause long-term alterations in the brain’s response to estradiol and/or progesterone via activation of the immune system. This could lead to mood disorders or altered response to any behavior influenced by estradiol in humans.
Keywords: Stress, Immune Challenge, Lipopolysaccharide, Depression, Anxiety, Cognitive function, Sexual behavior, Estradiol, Progesterone, Puberty, Females, Mood disorders
Introduction: Estradiol, progesterone, and behavior
The principal hormones secreted by the ovaries1, estradiol and progesterone, have a wide variety of influences on the behavior and physiology of adult animals. Estradiol and progesterone have robust effects on the brain, which then influence virtually every behavior exhibited in adult animals. Perhaps the best-characterized behavior is female sexual behavior (Blaustein, 2009), but estradiol, with or without progesterone, also influences a variety of other behaviors and neuroendocrine functions such as other reproductive behaviors (e.g., maternal behavior), social behaviors, eating, voluntary activity, as well as mood-related behaviors (e.g., anxiety and depression), cognitive function, and the hypothalamicpituitary-adrenal (HPA) axis.
Puberty and adolescence
The distinction between puberty and adolescence is sometimes blurred (Mccormick et al., 2010). However, an understanding of the enduring influences of events that occur specifically during puberty requires a clear definition of puberty. We will use the definitions provided by Sisk and Foster (Sisk and Foster, 2004). That is, puberty refers to the developmental transition to a reproductive state culminating in reproductive competence (Sisk and Foster, 2004; Waylen and Wolke, 2004), while adolescence refers to social and cognitive maturation (Sisk and Foster, 2004). Therefore, although adolescence results from the hormonal changes associated with puberty, puberty and adolescence are distinct.
One source of ambiguity is the operational definition of puberty. Although it often referred to as a singular event, marked in rodents by either vaginal opening or onset of reproductive cycles, the pubertal period is in fact a developmental process. In rodents, puberty is usually considered to begin with the first external sign of ovarian activity (vaginal opening) and terminates with the onset of the first reproductive cycle. In rats, the latency between these events may be quite brief, in some cases within the same day (Ojeda et al., 1976; Parker, Jr. and Mahesh, 1976); in mice, however, this period may last for many weeks depending on housing conditions and other environmental factors (Vandenbergh, 1967; Vandenbergh, 1969). It is important to note that girls also have an extended pubertal period beginning with development of pubic hair and breast budding (Tanner Stage II) and menarche lasting two or three years (Sizonenko, 1989). Unfortunately for attempts to relate animal work to human condition, work in humans often looks at events that occur during the more nebulous period of adolescence, which overlaps, but is not completely coincident, with puberty. In this review, we focus specifically on the pubertal stage of development, because it is likely that reproductive development is most relevant to our findings.
Puberty as an important developmental stage in hypothalamic-pituitary-adrenal (HPA) axis maturation
During pubertal development, the HPA axis matures, and there are dramatic changes in reactivity to stressors (Mccormick et al., 2010; Romeo, 2010). Although basal levels of stress-related hormones do not seem to differ as a function of pubertal development, the time course of release of ACTH and corticosterone in prepubertal animals differs from that of adults depending on the sex of the animals and the particular type of stressor to which the animals are subjected (see (Romeo et al., 2004) and (Mccormick et al., 2010) for reviews.). In particular, in postpubertal rats, blood levels of ACTH and corticosterone return to baseline levels after an acute stressor considerably more rapidly than in prepubertal animals. Furthermore, whereas adult male rats that are exposed to the same stressor repeatedly habituate to the homotypic stressor, prepubertal animals exposed in this way show greater peaks in corticosterone and faster return to baseline levels (Romeo et al., 2006). Therefore it is clear that puberty has a robust influence on the HPA axis.
Influence of early life experiential and hormonal influences on brain and behavior
The pubertal/adolescent period is a critical stage in development of the brain and behavior (Andersen, 2003). This is a period of increase in prevalence of psychiatric disorders (Costello et al., 2003), including those that are influenced by estradiol and/or progesterone (e.g., depression and anxiety) (Angold and Costello, 2006; Hayward and Sanborn, 2002; Paus et al., 2008). Enduring influences of pubertal/adolescent stress have been reported (Mccormick and Mathews, 2007). For example, social stressors during this period in female rats increase behavioral sensitivity to amphetamine (Mccormick et al., 2005) and sensitization to nicotine in adulthood (Mccormick et al., 2004). Unlike the model to be discussed, which focuses specifically on pubertal hormones, the vast majority of experiments on early life stress focus on a range of ages, not restricted specifically to the pubertal period (Isgor et al., 2004) (See (Mccormick and Mathews, 2007) for a summary) as defined above. Finally, in recent years, pubertal hormones have been shown to be responsible for a second phase of sexual differentiation of the brain (Sisk and Zehr, 2005).
Early life stressful events have profound influences on brain development and subsequent neuroendocrine reactivity (Avital and Richter-Levin, 2005; de Kloet et al., 2005; Kaiser et al., 2003; Meaney and Aitken, 1985; Meaney et al., 1985a; Meaney et al., 1985b; Ward, 1984; Weinstock, 2008). Early life stress alters development of the brain (Teicher et al., 2003), and it affects the development of depression and/or anxiety disorders later in life in humans (Heim and Nemeroff, 2001; Heim et al., 2004; Shea et al., 2005; Tarullo and Gunnar, 2006; Teicher et al., 2003), as well as in rodents (Romeo et al., 2003; Sanchez et al., 2001).
Stress during puberty and/or adolescence has profound long-term effects on both brain and behavior. The effects of stress during adolescence on subsequent stress reactivity are mixed (Mccormick et al., 2010), perhaps due to the wide variety of classes of stressor used and precise age at which the stressor exposure occurred. Stressors during puberty/adolescence may increase anxiety and depression later in life (Romeo, 2010), as well as risk-taking and novelty seeking behaviors (Toledo-Rodriguez and Sandi, 2011). Stressor exposure during puberty also has profound effects on some aspects of learning later in life, and the direction of the effect is sexually dichotomous (Hodes and Shors, 2005). Some adolescent/pubertal stressors influence adult response to drugs of abuse. Binge drinking during adolescence in rats affects adult brain and behavior (Gilpin et al., 2012), and conversely, particular stressors during this period may alter sensitivity to drugs of abuse (Kabbaj et al., 2002).
Puberty as a vulnerable period in development of sexual behavioral response to estradiol and/or progesterone
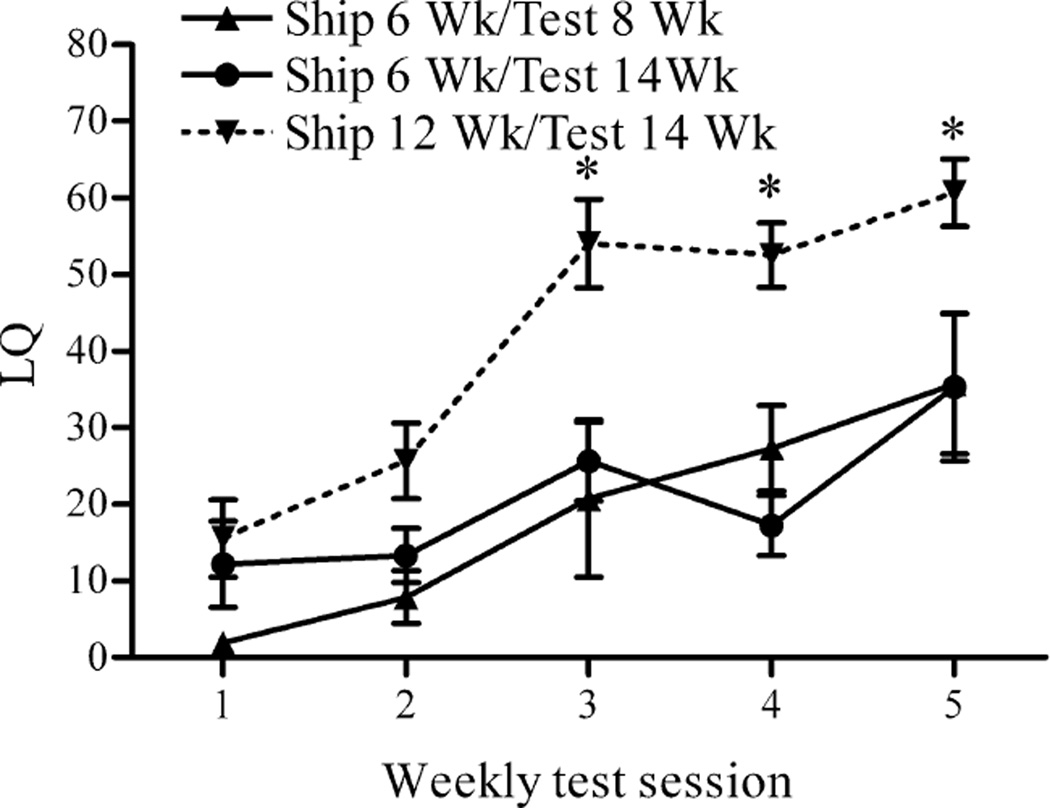
Several years ago, we made a serendipitous discovery. Others had shown that ovariectomized (OVX) mice, unlike rats, guinea pigs, and hamsters, require multiple weekly estradiol and progesterone injections followed by mating tests before they express high levels of sexual receptivity (Blaustein, 2009). While in the middle of a very extensive, parametric experiment in which we were examining the effects of a wide variety of doses of estradiol and progesterone on female sexual behavior in C57Bl/6J mice obtained from Jackson Laboratories, many shipments of mice failed to respond to the estradiol and progesterone injections with the typical level of expression of sexual behavior. Although we were unsuccessful in determining the cause of the lack of response in those animals, mice from two other suppliers expressed a typical level of behavioral response. However, in attempting to eliminate a variety of factors as the source of the problem, we discovered that, although mice shipped at 12 weeks show typical behavioral response, mice shipped at six weeks old do not, even if ovariectomy and hormone replacement are delayed until adulthood (testing at 14 weeks old) (Laroche et al., 2009b) (Figure 1). This result has been replicated over 20 times in the course of various experiments, and it has been observed with mice from two different suppliers.
Figure 1.
Lordosis quotient (LQ) (mean ± SEM) of C67Bl6 female mice shipped during the peripubertal period. Statistically significant differences in receptivity were observed between mice shipped at 6 wk and tested at 14 wk, and those shipped at 12 wk and tested at 14 wk during test sessions 3–5. (*, p < 0.05). Reprinted from (Laroche et al., 2009b) Copyright 2009, The Endocrine Society.
When C57Bl/6 mice were shipped from the supplier (Charles River Laboratories) at either 3, 4, 5, 6, 7, 8, 9, 10 or 12 weeks old, an interesting pattern was revealed. Although mice shipped at three weeks old responded to estradiol and progesterone in adulthood in a manner typical of mice that were shipped in adulthood, mice shipped at 4, 5, or 6 weeks did not; the mice shipped at six weeks showing the lowest levels of behavioral response to estradiol and progesterone. The most fascinating finding was that mice shipped at seven weeks old responded just like adults. In other words, there is a sensitive period, which terminates quite abruptly between six and seven weeks in this strain of mouse during which the stress of shipping results in an enduring change in behavioral responsiveness to estradiol and progesterone.
Shipping is a complex, multifactor stressor. Besides vibrations, changes in temperature, possible exposure to predator odors, the animals are moved from their home cages to a shipping crate without an established social hierarchy and then into new cages in the laboratory. Further, they typically transition from a light dark cycle with lights on early in the morning to a reversed light dark cycle used in laboratories in which there is behavioral testing (lights off late morning; lights on early evening), and their normal circadian rhythm is further disrupted by being exposed to light and darkness in a random manner during transport. In order to provide a controlled stressor in the laboratory to substitute for shipping, mice were either subjected to a standard restraint stress paradigm, food restriction for 36 hours, or a combination of heat, light and restraint (Laroche et al., 2009b). Surprisingly, none of these stressors duplicated the effects of shipping.
In response to a suggestion by the late Steven Zalcman, we injected the bacterial endotoxin, lipopolysaccharide (LPS) (Laugero and Moberg, 2000), which causes a robust stress and immune response and induces the display of sickness behavior for less than two days (Anisman, 2009; Dantzer, 2001), at various ages (Laroche et al., 2009a). LPS activates the innate immune system by its interaction with toll-like receptor 4 (TLR4) (Rivest, 2009). LPS has access to the brain via the circumventricular organs and choroid plexus, where it may interact with microglial cells and macrophages expressing TLR4 (Dantzer, 2001; Rivest, 2009). These in turn may activate the synthesis of pro-inflammatory cytokines. Alternatively, LPS can induce proinflammatory cytokines via peripheral cytokines acting on afferent neural pathways (Dantzer, 2001).
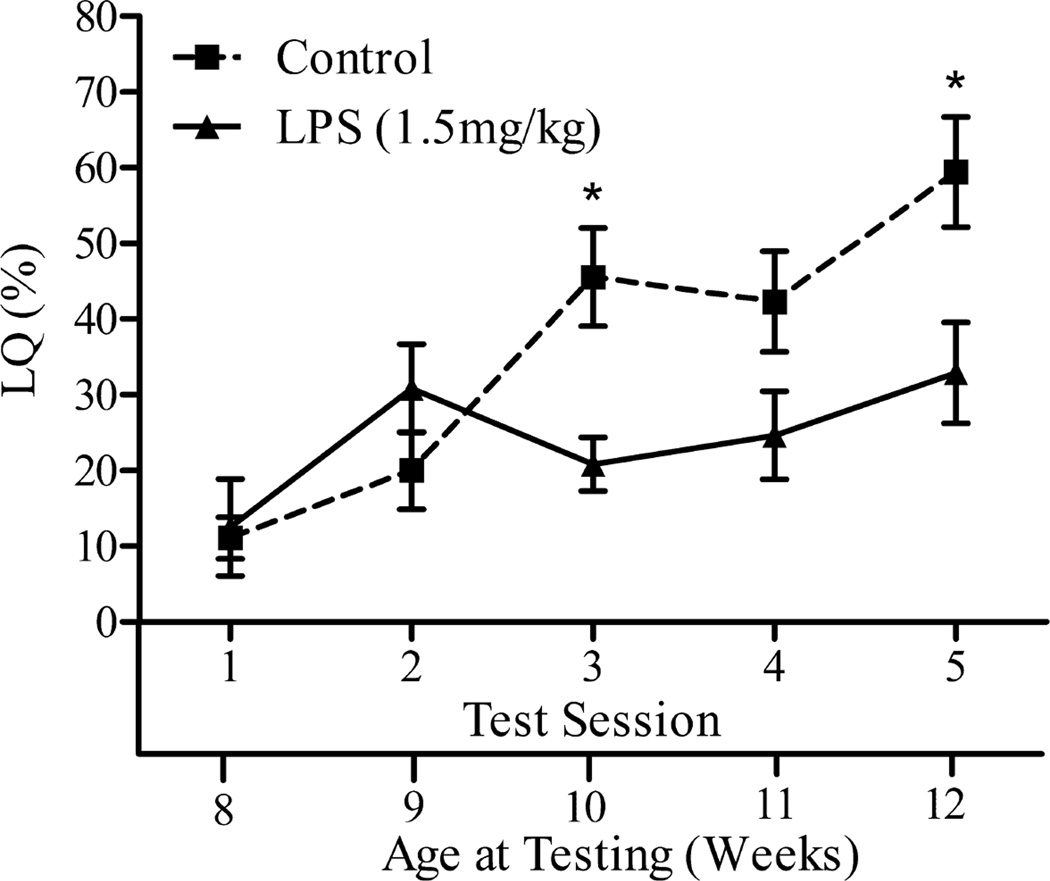
Although neither restraint, nor food deprivation, nor combined heat/light/restraint resulted in decreased behavioral response to estradiol and/or progesterone in adulthood, immune challenge was effective (Laroche et al., 2009a). Mice injected with LPS at 4, 5, or 6 weeks, but not 3, 7, 8, or 10 weeks old expressed lower levels of female sexual behavior in adulthood compared with saline-injected controls. This is the same developmental pattern that was seen with mice shipped at various ages (Figure 2). We do not yet know what shipping and immune challenge by a bacterial endotoxin have in common; however, the parsimony principle of Occam’s Razor suggests that the long-term influences of shipping or LPS treatment on behavioral response to estradiol and progesterone have a common etiology.
Figure 2.
Lordosis quotient (LQ) (mean ± SEM) of C57Bl/6 female mice exposed to a 1.5 mg/kg dose of LPS at 6 wk old. The lower x-axis refers to the animal’s age at time of testing. (*, P < 0.05; **, P < 0.01; ***, P < 0.001; a, no significant difference among these groups). Reprinted from (Laroche et al., 2009a) Copyright 2009, The Endocrine Society.
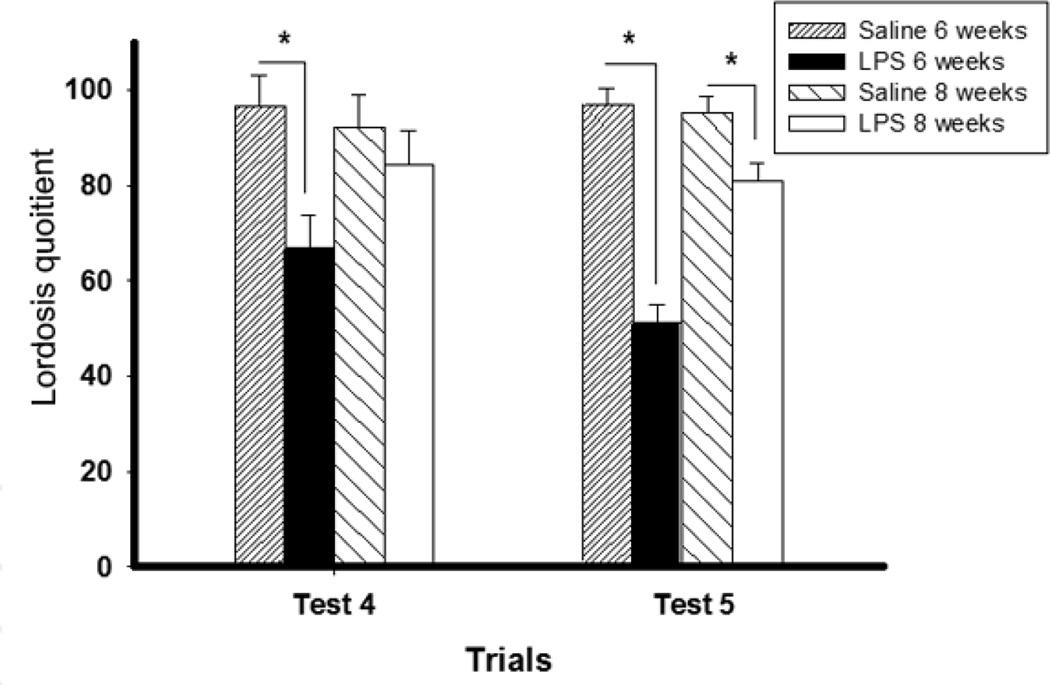
The enduring effects of a pubertal stressor are not idiosyncratic to the inbred C57Bl/6 strain of mouse. Nearly identical results were obtained with the outbred CD1 strain of mouse with two exceptions: (1) the sensitive period did not begin until after four weeks old, as opposed to the four week old onset in the C57Bl/6 mice, and (2) in some cases mice that were 8 weeks old, but not 10 weeks old, were still sensitive to the enduring effects of LPS or shipping on behavioral response to estradiol and progesterone (Ismail et al., 2011) (Figure 3).
Figure 3.
Lordosis quotient (mean ± SEM) of CD-1 female mice shipped at 3, 4, 6, 8 and 10 weeks old on Test 4 and Test 5. a, lordosis quotient significantly lower than females shipped at 3 weeks old (p < 0.05); b, significantly lower than females shipped at 4 weeks old (p < 0.05); c, significantly lower than females shipped at 10 weeks old (p < 0.05). Reprinted from (Ismail et al., 2011) with permission of Elsevier, Copyright 2011.
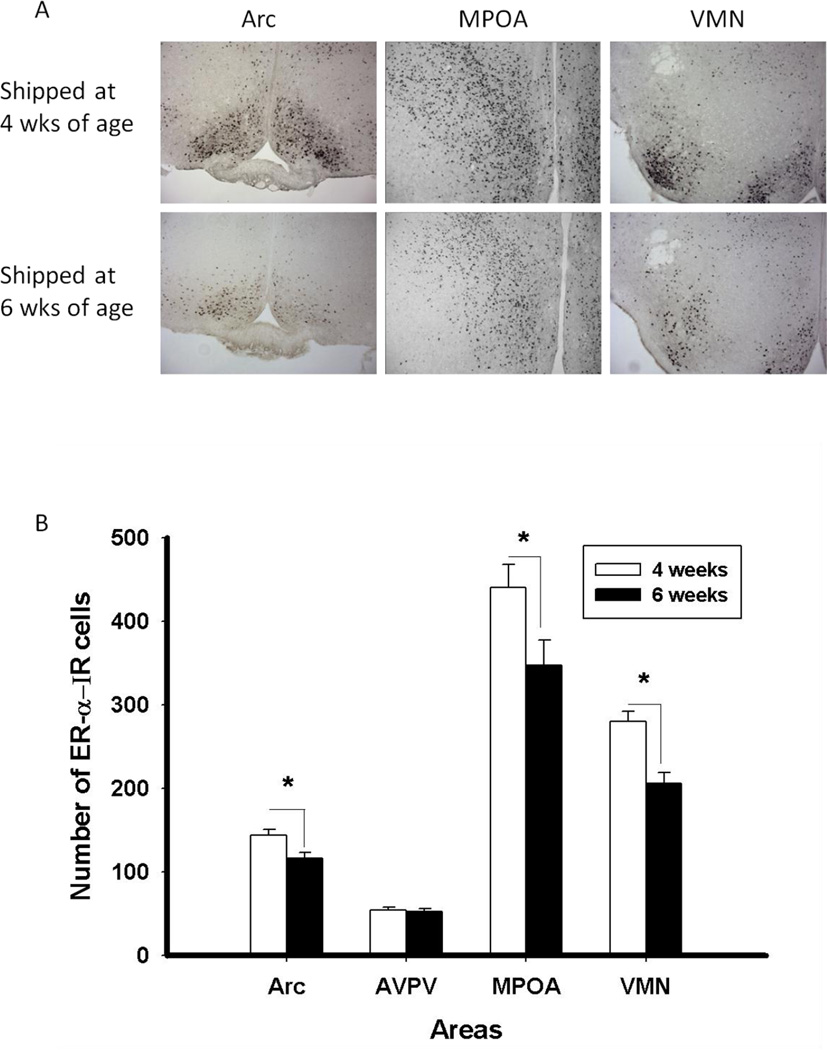
Many of the effects of estradiol on the brain are mediated via interaction with intracellular estrogen receptors (ERs). In the case of the effects of estradiol on female sexual behavior, ERα is essential (Musatov et al., 2006; Rissman et al., 1999). To test the hypothesis that the altered response to estradiol can be referred to a change in the concentration of ERs, mice of the CD-1 strain that were shipped at four or six weeks and tested twice to confirm depressed behavioral response to estradiol and progesterone were euthanized, brain sections were immunostained for ERα, and brains were analyzed for the number of ERα-immunoreactive (-ir) cells in four brain areas. Shipping at six weeks old (contrasted with four weeks) resulted in decreases in ERα-ir staining (i.e., the number of ER-ir cells) in the arcuate nucleus, medial preoptic area and ventromedial hypothalamic area, but not the anteroventral periventricular nucleus, suggesting a cellular basis for the decreased behavioral response to estradiol and progesterone after pubertal shipping (Figure 4). Preliminary results show a similar decrease in the medial preoptic area and ventromedial hypothalamic area after pubertal immune challenge (Ismail, Fitzpatrick, DiGloria and Blaustein, unpublished observations).
Figure 4.
A) Photomicrographs and B) Number of ER-α IR (Mean ±SEM) cells in females shipped at four or six weeks old in the arcuate nucleus (Arc), anteroventral paraventricular nucleus (AVPV), medial preoptic area (MPOA) and ventromedial nucleus of the hypothalamus (VMN). (*, p < 0.05). Reprinted from (Ismail et al., 2011) with permission of Elsevier, Copyright 2011.
Puberty as a vulnerable period in development of the antidepressive and anxiolytic effects of estradiol or estradiol and progesterone
Ovariectomy (OVX) increases depression-like (Bekku and Yoshimura, 2005; Bernardi et al., 1989; Koss et al., 2012; Okada et al., 1997; Stoffel and Craft, 2004; Suda et al., 2008) behaviors, and estradiol ameliorates the effects (Bekku and Yoshimura, 2005; Bernardi et al., 1989; Estrada-Camarena et al., 2006; Galea et al., 2001; Lagunas et al., 2010; Okada et al., 1997; Rachman et al., 1998), although there are strain differences, and specific hormone treatment can be a factor (Koss et al., 2012). Also, female aromatase knockout mice that are deficient in estradiol synthesis show greater depression-like symptoms than wild-type mice (Dalla et al., 2004).
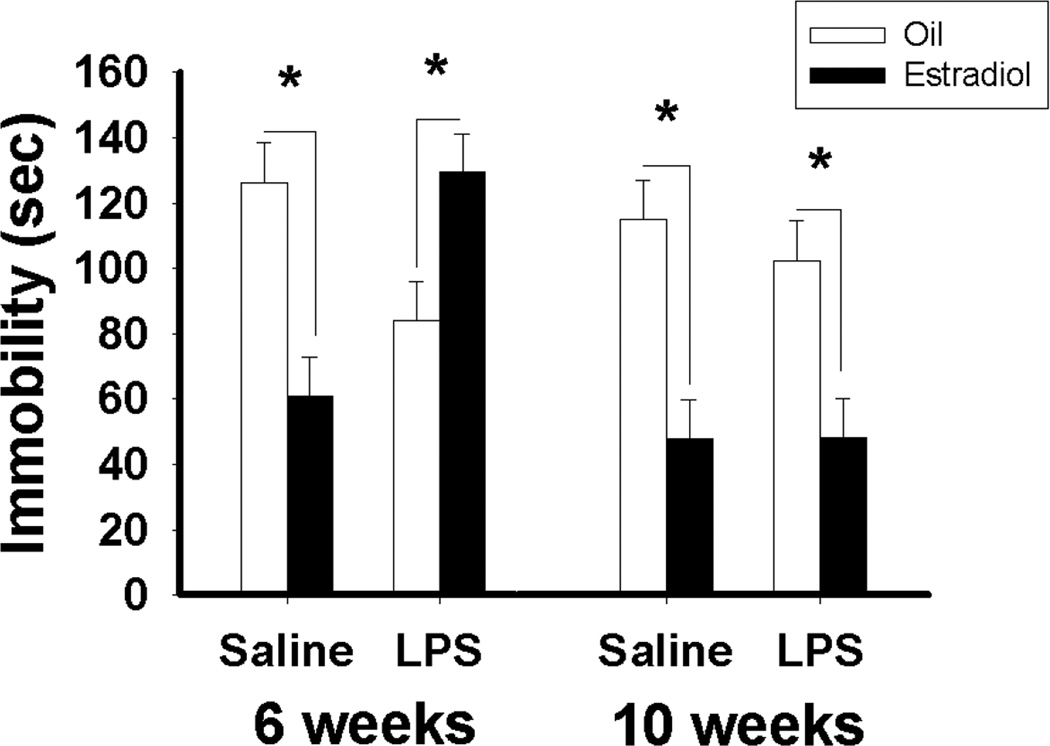
Because estradiol has antidepressive effects in classic tests of rodent depression-like behaviors (e.g., forced swim test, tail suspension test), the effects of pubertal immune challenge on the antidepression-like effects of estradiol were assessed (Ismail et al., 2012). One week exposure to estradiol via a Silastic© implant resulted in a robust decrease in these depression-like behaviors. It was predicted that peripubertal immune challenge would suppress the antidepressive effects of estradiol (Figure 5). However, the effects of estradiol were actually reversed. More specifically, OVX mice treated with saline control at six or eight weeks old or LPS at eight weeks old responded to estradiol with a decrease in depression-like behaviors. However, pubertally immune-challenged mice expressed higher levels of depression-like behavior when treated with estradiol in adulthood. That is to say that the pubertal immune challenge reversed the effects of estradiol on these behaviors in adulthood. Pubertal immune challenge had very similar effects in both C57Bl/6 and CD1 strains of mice.
Figure 5.
Duration of immobility (Mean ± SEM) during the tail suspension test. (*, p < 0.05). During five minutes, mice were suspended 60 cm above the ground by a piece of adhesive tape placed within 1cm from the tip of the tail. The duration of immobility (absence of any body movement) was recorded as a measure of behavioral despair. Reprinted from (Ismail et al., 2012) with permission of Elsevier, Copyright 2012.
Estradiol and progesterone also influence anxiety-like behaviors. OVX increases anxiety-like behavior in rats (Diaz-Veliz et al., 1989; Pandaranandaka et al., 2008; Picazo et al., 2006) and mice (Ogawa et al., 1998), and hormone replacement reduces these behaviors in OVX rats (Diaz-Veliz et al., 1989, 1991, 1997a, 1997b; Mora et al., 1996; Olivera-Lopez et al., 2008; Pandaranandaka et al., 2006, 2008). In OVX mice, chronic treatment with low doses of estradiol reduce anxiety-like behavior (Tomihara et al., 2009), while high doses may be anxiogenic (Morgan and Pfaff, 2001, 2002; Tomihara et al., 2009). Female mice with knockout of either the estrogen receptor α (Choleris et al., 2003, 2006; Ogawa et al., 1998) or estrogen receptor β (Imwalle et al., 2005; Krezel et al., 2001) gene display more anxiety-like behavior than wild-types.
In studies of anxiety-like behavior, an injection of estradiol benzoate (EB, 2 µg sc) followed 44 hours later by an injection of progesterone (100 µg sc), similar to the treatment used in tests of female sexual behavior, causes a decrease in anxiety-like behavior, assessed by the elevated plus maze, light-dark box and marble burying test (Olesen et al., 2011). Pubertal immune challenge had very similar effects in both C57Bl/6 and CD1 strains of mice. In general, the hormone treatments were anxiolytic in mice that were injected with saline at 6 or 8 weeks old and in mice injected with LPS at 8 weeks old. However, in mice given the immune challenge at 6 weeks old during the pubertal period, hormone treatment had either an anxiogenic effect or no effect (Olesen et al., 2011), suggesting that pubertal immune challenge caused a major change in response to estradiol and progesterone. It should be noted that there was a tendency for LPS to reduce anxiety-like behavior in both the light-dark box and the elevated plus maze. This is consistent with previous research in which adolescent stressors or prenatal LPS treatment reduced anxiety-like behavior (Conrad and winder, 2012; Wang, Meng, Ning, Zhao, Wang, Liu, Zhang, Zhang, Chen, Xu, 2010). Nevertheless, in all cases, in mice injected with LPS at six weeks old, hormone treatment was either without effect or caused an increase in anxiety-like behavior.
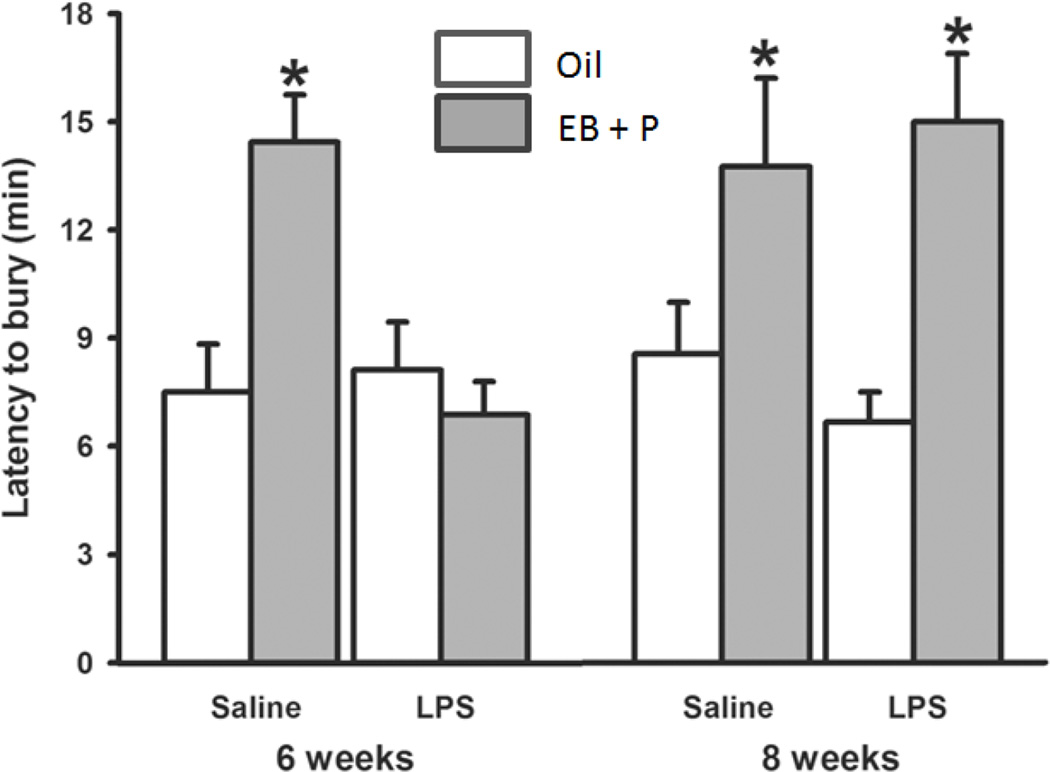
There are limitations to all tests for human mental disorders in mice. For example, there is question of whether the marble-burying test is a better test for anxiety or compulsive behavior (Li, Morrow, Witkin, 2006). Nevertheless, the results of all three tests suggested that pubertal immune challenge fundamentally alters the brain’s response to estradiol and progesterone later in life. (Figure 6).
Figure 6.
Latency to bury marbles in the marble burying test. (*, p < 0.05). Mice were placed in the center of an arena containing ten evenly spaced marbles with bedding underneath. The latency to cover three marbles at ¾ or more with bedding was assessed to examine anxiety-like behavior. Reprinted from (Olesen et al., 2011) with permission of Elsevier, Copyright 2011.
Puberty as a vulnerable period in development of the positive cognitive effects of estradiol
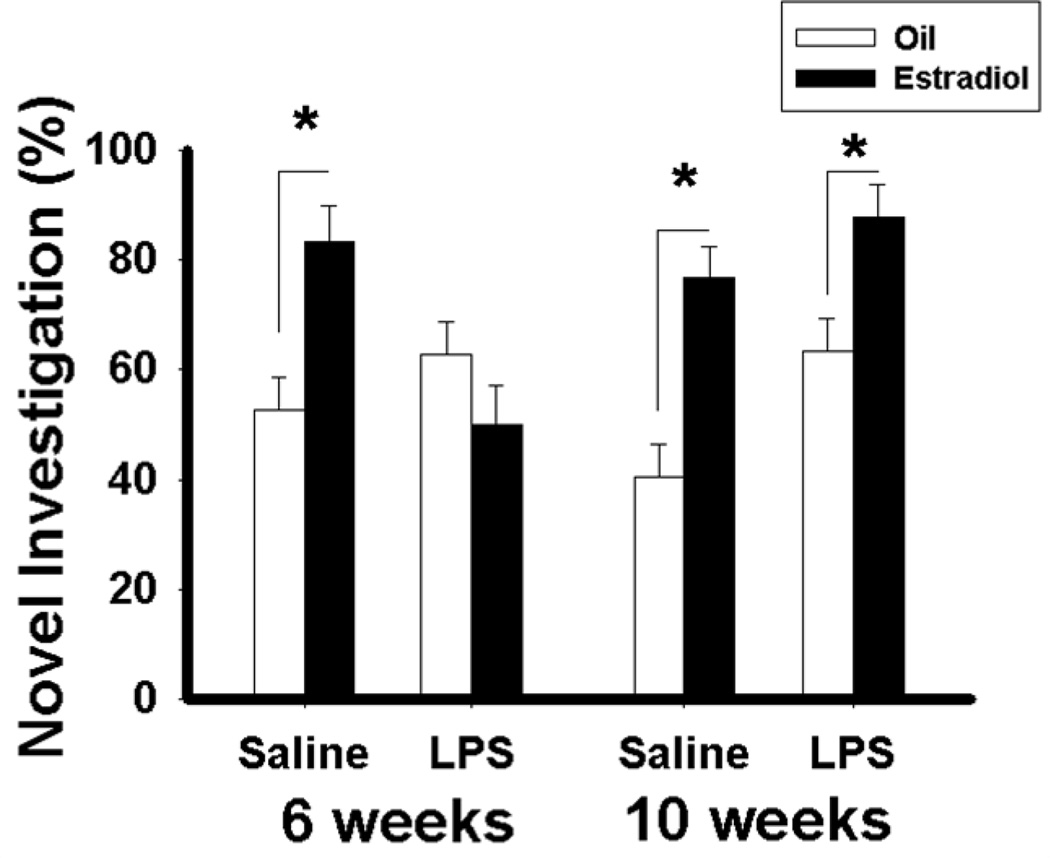
Other behaviors that is influenced by estradiol in adulthood are those associated with cognitive function. Because estradiol also has positive effects on a variety of learning tasks, including spatial and recognition memory (Luine, 2008), the effects of pubertal immune challenge on some of the cognitive effects of estradiol were assessed. Estradiol improves cognitive function in a variety of tasks in which the hippocampus, an estradiol-sensitive brain area, is involved. These include social discrimination and recognition tests as a measure of social memory, and object recognition and placement tests as a measure of object memory. In these four tests, estradiol improved cognitive function in adulthood in mice that had received saline control injections during puberty or post-puberty, as well as in mice that were immune challenged after puberty (Ismail and Blaustein, 2012) (Figure 7). However, in mice that received the immune challenge at six weeks old (during pubertal development), estradiol was without any effect on these four cognitive tasks.
Figure 7.
Percentage of time spent investigating the novel object (Mean ± SEM) during the object recognition test. (*, p < 0.05). Mice were placed in an open arena with two different objects placed at opposite corners of the arena for three minutes. Thirty minutes later, mice were again placed in the square arena and were presented with the original object from the previous trial and a novel object at opposite corners of the arena for three minutes. The time spent exploring each object was recorded. Increased investigation of the novel object is indicative of enhanced cognitive function. Reprinted from (Ismail and Blaustein, 2012) with permission of Elsevier, Copyright 2012.
Role of Corticosterone
An early hypothesis was that the stress of shipping or the immune challenge activated the HPA axis resulting in an increase in corticosterone secretion, and that this is the proximate cause of the altered response. However, doses from 0.2 – 1.5 mg LPS/kg bw induce similar levels of corticosterone levels at two hours after injection, and the ineffective stressors, such as restraint, food deprivation or combined heat/light/restraint all induce similar levels of corticosterone, but only shipping and higher doses of LPS (0.5 – 1.5 mg/kg bw) cause the enduring changes in response to estradiol and progesterone. Therefore, this hypothesis is unlikely to be tenable (Laroche et al., 2009a).
Another hypothesis that was tested is that the effective pubertal stressors induce long-term changes in the response of the HPA axis to further stress. This too has been ruled out; although there are changes in corticosterone release in response to stress in adulthood in mice given a pubertal immune challenge, they are in the wrong direction. It was predicted that these mice would show increased corticosterone release in response to a stressor (e.g., restraint) or removal from the home cage and exposure to the testing arena, because it is well-known that corticosterone can inhibit sexual receptivity in rodents (DeCatanzaro et al., 1981). However, in all cases, the pubertal stressor causes a decrease, not an increase in corticosterone secretion (Laroche et al., 2009b).
Finally, we can ask if the effects of immune challenge are due to release of a hormone that then defeminizes or in some way remodels the behavioral response of the brain. However, neither an estrogen, progestin, androgen, glucocorticoid, or mineralocorticoid antagonist administered prior to the immune challenge altered the long-term response to the immune challenge (Laroche, 2008).
Role of ovarian hormones in conferring sensitivity to the long-term effects of pubertal immune challenge
In order to determine if the proximate cause of the long-term effect of pubertal immune challenge is related to the ovarian secretions that accompany puberty (or a consequence of the ovarian secretions), five-week-old (i.e., one week before immune challenge) mice were either OVX or sham-OVX (Rappleyea, Ismail and Blaustein, unpublished obs). They were then immune-challenged or injected with saline control. At seven weeks old, they were sham-OVX or OVX, so that all animals were OVX at the time of start of hormonal treatments one week later. Although LPS caused the typical decrease in hormonal induction of sexual receptivity in mice that were sham-OVX at the time of immune challenge, in mice that were OVX at the time of immune challenge, the effects of the LPS were completely blocked. The reason for this is not that removal of the ovaries decreased the sickness behavior resulting from the immune challenge; on the contrary, OVX prior to the LPS treatment actually increased the severity of sickness behavior slightly. Therefore, either ovarian hormones or a sequela of ovarian hormone secretion between five and six weeks old confer sensitivity to the long-term effects of immune challenge on response to estradiol and progesterone. It is interesting to note that in adult female mice, estradiol acting through ERαis required for the neuroimmune response to LPS (Soucy et al., 2005).
Summary of effects of pubertal stressors on adult, behavioral response to estradiol with or without progesterone
Collectively, with all behaviors studied – female sexual behavior, depression-like, anxiety-like and cognitive, pubertal shipping and/or immune challenge changes response to hormones in adulthood. Arguments can be made that any particular test is not a valid test for a human condition. For example, the behavioral despair that is inferred from an animal stopping swimming (Porsolt et al., 1977) may not be a valid proxy for assessment of the despair in depression in humans. Likewise, aversion to the open arms of an elevated plus maze may not be an ideal proxy for assessments of anxiety in humans. Nevertheless, depending on the behavior that is examined, the effects of the pubertal stressor on behavioral response to estradiol or estradiol and progesterone is to decrease, eliminate or reverse response to the hormones in adulthood. The effects of estradiol and progesterone on hormone-induced female sexual behavior is decreased, the antidepressant-like effects of estradiol are reversed, the anxiolytic effects of estradiol and progesterone are either eliminated or reversed, and the cognitive enhancing effects of estradiol are eliminated. In all cases, these can be considered negative consequences of pubertal stressors on response. Therefore, it is reasonable to conclude that particular pubertal stressors have enduring, negative effects on a wide variety of estradiol- or estradiol plus progesterone-influenced behaviors in female mice.
Relevance to humans: Some questions and a hypothesis
The notion that estradiol and/or progesterone play an important role in mood in humans is supported by sex differences in the prevalence of mood disorders, changes in symptoms of various mental health disorders associated with circulating levels of estradiol and/or progesterone, and the efficacy of estradiol in treating these disorders (Osterlund et al., 2005; Payne, 2003). Further, the sex difference in depression, which results in women having a 4:1 higher incidence than men, emerges during puberty (Ge et al., 2001), and trauma during this period increases the incidence of mood disorders later in life (Ge et al., 2001). Clinical studies have stressed the importance of estrogens and/or progestins in depression-related, affective disorders in women, such as premenstrual dysphoric symptoms, postpartum depression, and peri- and post-menopausal depression (Amin et al., 2005; Bloch et al., 2003; Bloch et al., 2000; Harsh et al., 2009; Rachman et al., 1998; Rocca et al., 2008; Rubinow and Schmidt, 1995; Schmidt, 2005; Schmidt et al., 2000).
Does the phenomenon of pubertal stress/immune challenge causing long-term changes in response to estradiol and progesterone have relevance to humans? Could particular classes of immune activation during puberty cause enduring changes in the immune system that then lead to altered behavioral responses to estrogen or progestins? The answer to these questions is that we do not know, but we can make a prediction, once we lay out what is known.
First, stress during puberty/adolescence in humans influences mental health later in life (Danese and McEwen, 2012; Danese et al., 2009), and inflammation has been implicated in the etiology of some depressive disorders (Miller et al., 2009; Raison and Miller, 2011). Similarly, particular stressors during early childhood or adolescent development may induce short- and long-term immune changes (Danese et al., 2008; Danese et al., 2007; Lemieux et al., 2008; Pace et al., 2011). Interestingly, children (12 year olds), who are maltreated and depressed, have higher levels of the inflammatory biomarker, C-reactive protein (CRP), levels in childhood (Danese et al., 2011). More importantly, adults maltreated in childhood (Danese et al., 2007) and adult women who experienced sexual abuse during adolescence (Bertone-Johnson et al., 2012) have elevated CRP and/or interleukin-6 levels in plasma, Further, women with childhood abuse-related PTSD have increased activity in the peripheral NF-κB pathway (Pace et al., 2011), one of the molecular signaling transduction pathways important for immune response. These results collectively suggest that the human immune system responds to particular adverse experiences during critical developmental stages, and that the immune system may remain activated into adulthood. Although, it has yet to be studied during puberty, immune challenge during another developmental stage – neonatal -- in rodents causes long-term changes in the immune system (Spencer et al., 2006a; Spencer et al., 2010; Spencer et al., 2006b).
Unfortunately, there are many dots that need to be connected in the case of humans; as discussed earlier, most studies in humans have looked at the effects of events generally during “childhood” (Danese et al., 2007) or “adolescence,” so it is not possible to directly correlate the work in humans with the mouse studies discussed in this review. Nevertheless, it is tempting to speculate that, similar to the peripubertal, sensitive period in mice, there is a sensitive period in girls between the first stages of puberty and menarche that confers vulnerability to particular stressors, such as abuse or maltreatment that influence the immune system. These immune changes in turn could adversely influence mental health directly through inflammatory pathways (Miller et al., 2009; Raison and Miller, 2011). The work in mice suggests that the possibility should be considered that adverse pubertal events that influence the immune system may exert some of their long-term effects on the brain and behavior by altering response to estradiol and/or progesterone.
Perhaps LPS can be considered a useful proxy in mice for the immune responses in humans that result from maltreatment (e.g., (Danese et al., 2011)) and possibly other stressors. Perhaps the results of experiments in which mice were immune challenged during puberty predict a heretofore unstudied phenomenon in humans. We suggest that particular types of trauma in girls very specifically during the pubertal period may result in altered response to estradiol and/or progesterone, and that this altered response is conveyed by the immune system. For example, as estradiol is antidepressive in humans as well as in mice, the mouse studies in which pubertal immune challenge results in an opposite behavioral response to estradiol on tests of depression-like behaviors suggest that in girls exposed to trauma (e.g., abuse, maltreatment), the acute inflammatory response predisposes them to respond in an opposite manner to estradiol later in life. Thus, perhaps estradiol increases the likelihood of depression rather than being antidepressive in these individuals.
In summary, we have described a robust influence of particular pubertal stressors on all behaviors that have been studied to date. We have also described changes in the amount of ER-ir in some brain areas that may be the proximate cause for the altered adult behavioral response. There are, however, many unknowns, including the specific physiological events (perhaps particular aspects of immune activation) that transduce the effects of pubertal stressor to adulthood. And finally, we have suggested a novel hypothesis that may point to a mechanism for some of the adverse outcomes of particular pubertal/adolescent stressors in girls that focuses specifically on changes in the brain’s response to estradiol and/or progesterone.
Highlights.
Pubertal stressors alter response to estradiol and progesterone later in life.
Pubertal shipping or immune challenge alters behavioral response to hormones.
The authors propose that similar effects could occur in humans.
Acknowledgments
This work from the authors’ laboratory was supported by grants NS 19327, MH093854, and training grant T32 MH020051 from the National Institutes of Health, IOS 1050179 from the National Science Foundation, and an Isis grant from the Society for Women's Health Research. We thank Dr. Mary Holder for helpful comments on the manuscript, the late Dr. Steven Zalcman for his suggestion that we consider the use of an immune challenge as a pubertal stressor, and the members of the Stress, Gender Drugs and the Brain network of the Society for Women’s Research for many thoughtful discussions in the early stages of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have traditionally referred to estradiol and progesterone as “ovarian hormones,” because they are secreted from the ovaries. However, we will not use this terminology in this review because; although both are secreted from the ovaries, they both can be synthesized de novo by the brain, as well as by the adrenal gland. Referring to them as “ovarian hormones” or “sex hormones” may predispose readers to think of them as exclusively ovarian in origin.
REFERENCES
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav. Cogn Neurosci. Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child Adolesc. Psychiatr. Clin. N.Am. 2006;15:919–937. doi: 10.1016/j.chc.2006.05.013. ix. [DOI] [PubMed] [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J. Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology (Berl) 2005;183:300–307. doi: 10.1007/s00213-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Bernardi M, Vergoni AV, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–1068. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am. J. Prev. Med. 2012;43:611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD. Feminine reproductive behavior and physiology in rodents: Integration of hormonal, behavioral and environmental influences. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Philadelphia: Elsevier; 2009. pp. 67–107. [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr. Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Amer. J. Psychiat. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. EurJNeurosci. 2004;20:217–228. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Werts H, Freeman J, Pariante CM, Moffitt TE, Arseneault L. Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatry. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and agerelated disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. SciUSA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-Induced Sickness Behavior: Mechanisms and Implications. Ann NY Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci. Biobehav. Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- DeCatanzaro D, Knipping RP, Gorzalka BB. Antagonism of estrogen-induced lordosis by corticosterone in adrenalectomized-ovariectomized female rats and mice. Pharmacol. Biochem. Behav. 1981;15:761–766. doi: 10.1016/0091-3057(81)90019-8. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Lopez-Rubalcava C, Fernandez-Guasti A. Facilitating antidepressant-like actions of estrogens are mediated by 5-HT1A and estrogen receptors in the rat forced swimming test. Psychoneuroendocrinology. 2006;31:905–914. doi: 10.1016/j.psyneuen.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Galea LAN, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav. Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev. Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS. One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsh V, Meltzer-Brody S, Rubinow DR, Schmidt PJ. Reproductive aging, sex steroids, and mood disorders. Harv. Rev. Psychiatry. 2009;17:87–102. doi: 10.1080/10673220902891877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J. Adolesc. Health. 2002;30:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Ismail N, Blaustein JD. Pubertal immune challenge blocks the ability of estradiol to enhance performance on cognitive tasks in adult female mice. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.11.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm. Behav. 2011;59:565–571. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Kumlin AM, Blaustein JD. A pubertal immune challenge alters the antidepressant-like effects of chronic estradiol treatment in inbred and outbred adult female mice. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.09.047. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Kruijver FPM, Straub RH, Sachser N, Swaab DF. Early social stress in male guineapigs changes social behaviour, and autonomic and neuroendocrine functions. J. Neuroendocrinol. 2003;15:761–769. doi: 10.1046/j.1365-2826.2003.01055.x. [DOI] [PubMed] [Google Scholar]
- Koss WA, Einat H, Schloesser RJ, Manji HK, Rubinow DR. Estrogen effects on the forced swim test differ in two outbred rat strains. Physiol Behav. 2012;106:81–86. doi: 10.1016/j.physbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM. Long-term ovariectomy enhances anxiety and depressive-like behaviors in mice submitted to chronic unpredictable stress. Horm. Behav. 2010;58:786–791. doi: 10.1016/j.yhbeh.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Laroche J. Dissertation. Philadelphia: University of Massachusetts; 2008. Neuroendocrine effects of pubertal stress exposure in the female mouse. United States -- Massachusetts: Dissertations & Theses @ University of Massachusetts @ Amherst; ProQuest Dissertations & Theses A&I. [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009a;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009b;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugero KD, Moberg GP. Summation of behavioral and immunological stress: metabolic consequences to the growing mouse. Am J Physiol Endocrinol Metab. 2000;279:E44–E49. doi: 10.1152/ajpendo.2000.279.1.E44. [DOI] [PubMed] [Google Scholar]
- Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain Behav. Immun. 2008;22:994–1003. doi: 10.1016/j.bbi.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J. Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Mccormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Mccormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Mccormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm. Behav. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Mccormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm. Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav. Neurosci. 1985a;99:765–770. doi: 10.1037//0735-7044.99.4.765. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Dev. Brain Res. 1985b;18:159–164. doi: 10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl. Acad. Sci. USA. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Weaton JE, Jameson HE, McCann SM. The onset of puberty in the female rat: changes in plasma prolactin, gonadotropins, luteinizing hormone-releasing hormone (LHRH), and hypothalamic LHRH content. Endocrinology. 1976;98:630–638. doi: 10.1210/endo-98-3-630. [DOI] [PubMed] [Google Scholar]
- Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn. J. Pharmacol. 1997;73:93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Ismail N, Merchasin ED, Blaustein JD. Long-term alteration of anxiolytic effects of ovarian hormones in female mice by a peripubertal immune challenge. Horm. Behav. 2011;60:318–326. doi: 10.1016/j.yhbeh.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine. 2005;28:235–242. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav. Immun. 2011 doi: 10.1016/j.bbi.2011.07.232. in press. [DOI] [PubMed] [Google Scholar]
- Parker CR, Jr, Mahesh VB. Hormonal events surrounding the natural onset of puberty in female rats. Biol. Reprod. 1976;14:347–353. doi: 10.1095/biolreprod14.3.347. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JL. The role of estrogen in mood disorders in women. Int. Rev. Psychiatry. 2003;15:280–290. doi: 10.1080/0954026031000136893. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain cfos expression in ovariectomized rats subjected to the forced swim test. Proc. Natl. Acad. Sci. USA. 1998;95:13941–13946. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Curr. Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, de AM, Melton LJ., III Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–1059. doi: 10.1097/gme.0b013e318174f155. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev. Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm. Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. The treatment of premenstrual syndrome - forward into the past. New England. Journal. of. Medicine. 1995;332:1574–1575. doi: 10.1056/NEJM199506083322309. (23, JUN 8) [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ. Depression, the perimenopause, and estrogen therapy. Ann. NY. Acad. Sci. 2005;1052:27–40. doi: 10.1196/annals.1347.003. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am. J. Obstet. Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat. Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sizonenko PC. Physiology of puberty. J. Endocrinol. Invest. 1989;12:59–63. [PubMed] [Google Scholar]
- Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Boisse L, Mouihate A, Pittman QJ. Long term alterations in neuroimmune responses of female rats after neonatal exposure to lipopolysaccharide. Brain Behav. Immun. 2006a;20:325–330. doi: 10.1016/j.bbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Field E, Pittman QJ. Neonatal programming by neuroimmune challenge: effects on responses and tolerance to septic doses of lipopolysaccharide in adult male and female rats. J. Neuroendocrinol. 2010;22:272–281. doi: 10.1111/j.1365-2826.2010.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006b;31:1910–1918. doi: 10.1038/sj.npp.1301004. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced "depression" in female rats. Physiol Behav. 2004;83:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol. Psychiatry. 2008;64:311–319. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm. Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress during Adolescence Increases Novelty Seeking and Risk- Taking Behavior in Male and Female Rats. Front Behav. Neurosci. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG. Effect of the presence of a male on the sexual maturation of female mice. Endocrinology. 1967;81:345–349. doi: 10.1210/endo-81-2-345. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinology. 1969;84:658–660. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- Ward IL. The prenatal stress syndrome: Current status. Psychoneuroendocrinology. 1984;9:3–11. doi: 10.1016/0306-4530(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Waylen A, Wolke D. Sex 'n' drugs 'n' rock 'n' roll: the meaning and social consequences of pubertal timing. Eur. J. Endocrinol. 2004;151(Suppl 3):U151–U159. doi: 10.1530/eje.0.151u151. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]