Abstract
The volatile compounds from fruits vary based on the spoilage stage. We used FTIR spectroscopy to analyze and to attempt to identify the spoilage process of strawberries. To enhance the sensitivity of the measuring system, we increased the optical pathlength by using multi-reflecting mirrors. The volatile compounds that were vaporized from strawberries in different spoilage stages were tested. We analyzed the spectra and found that the concentrations of esters, alcohols, ethylene, and similar compounds changed with deterioration. The change patterns of the infrared spectra for the volatiles were further examined using 2D correlation spectroscopy. We analyzed the spectral data using PCA and were able to distinguish the fresh, slightly spoiled strawberries from the seriously spoiled strawberries. This study demonstrates that FTIR is an effective tool for monitoring strawberry spoilage and for providing status alerts.
The strawberry fruit is popular worldwide for its special pleasant aroma and nutrients. However, strawberries decay easily during transportation and storage, causing significant economic losses, and this decay can be harmful to human health1. Thus, on-line monitoring of and early warning systems for strawberry spoilage are critical.
Vegetables and fruits vaporize specific types of volatile compounds1. Goff reported that the volatile compounds may indicate to the nutrient content and health information of food2. Some researchers collected and analyzed the volatiles from strawberries and found that the main components of the gas are esters, alcohols, furans, aldehydes, terpenoids, aromatic compounds, ketones, acids, and similar compounds3,4,5. Previous studies have demonstrated that the composition and concentration of such gases varies with freshness, maturity and strawberry variety6,7,8,9,10,11,12,13. However, most of the previous studies used gas chromatography-mass spectrometry (GC-MS) to analyze the volatiles from food, and on-line analysis has seldom been used. Some researchers used E-nose to determine food spoilage stages14,15,16,17,18. These studies have shown the possibility of sensing food decay using volatiles, but E-nose technology is complex and costly, making it unsuitable for real-time monitoring.
Most organic compounds have obvious spectral characteristics in the infrared band, which makes infrared spectroscopy an effective analysis tool for the quantitative and qualitative determination of unknown gases19. Harren used photoacoustic infrared spectroscopy to study the methane, ethane and ethylene that was vaporized from crop leaves and tomatoes20. We measured the volatiles from grapes using Fourier transform infrared spectrometry (FTIR) spectroscopy and found that the ethanol and ethyl acetate content significantly increased during spoilage. However, the sensitivity of the experimental system was somewhat low because of the short optical pathlength21. In this study, we used multi-reflecting mirrors to increase the optical pathlength for testing volatiles, analyzed the changes in the spectral characteristics of the volatiles during the strawberry spoilage process by combining the standard linear spectra with two-dimensional (2D) correction spectroscopy, and discussed the possibility of strawberry grading and spoilage forecasting using spectral recognition. To the best of our knowledge, this study is the first to analyze the fruit decay process by studying fruit volatiles using longpath spectrometry.
Results
Spectral analysis of volatiles during strawberry spoilage
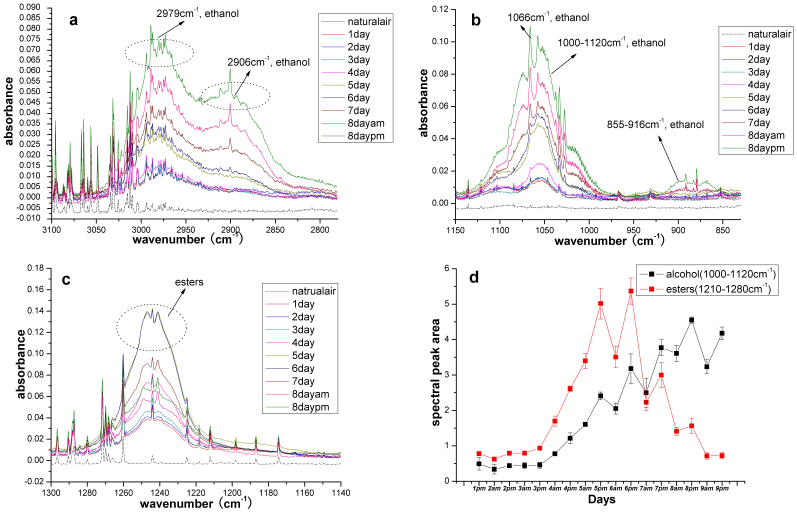
Fig. 1(a), (b) and (c) shows the IR spectra of volatiles from strawberries stored for times ranging from 1 d to 8 d. Three obvious alcohol absorption bands can be observed: 2830–3040 cm−1 (Fig. 1(a)), 1000–1120 cm−1 (Fig. 1(b)) and 855–916 cm−1 22. These bands, which were compared with NIST data, are most likely caused by ethanol. In addition, the narrow absorption bands at 1066, 1058, 1028, 891, 882 and 879 cm−1 also appear to be caused by ethanol22, whereas methanol most likely forms the 1033 cm−1 band22. Fig. 1 shows that fresh strawberries vaporize ethanol, and the concentration of ethanol increases with storage. There is only a slight difference in the spectra for the first 3 days, which indicates that the ethanol vaporization rate is in a stable condition. The bands at 2830–3040 cm−1 and 1000–1120 cm−1 increase significantly during the subsequent days because much more ethanol vaporizes. The narrow bands, such as those at 1066, 1058, 1028, 891, 882, 879 and 1066 cm−1, also change with storage.
Figure 1. Spectral characteristics of major volatiles during strawberry spoilage.
(a) Spectral peaks of ethanol in the range of 2830–3040 cm−1; (b) Spectral peaks of ethanol in the range of 1000–1120 cm−1 and 855–916 cm−1; (c) Spectral peaks of esters in the range of 1210–1280 cm−1; (d) Changes of spectral peaks areas with storage time.
Previous studies have demonstrated that esters, such as ethyl acetate and methyl acetate, are the main components in strawberry volatiles. During the spoilage process, the alcohols transform into esters and then to aliphatic acids1. The 2 main absorption bands of ethyl acetate and methyl acetate occur at 1210–1280 cm−1 and 1730–1790 cm−1. The spectral features at 1210–1280 cm−1 are notable in both the fresh and spoiled strawberries (Fig. 1 (c)), a result that is similar to that of Larsen6. The 1730–1790 cm−1 band is influenced by the absorption of water, for which only a small signal was observed. Our experiments show that the levels of the esters increases with storage, reaches a maximum value on the 5th and 6th days and then decreases on the following days. We think that the amount of esters may decrease as the esters transform into aliphatic acids. However, Larsen only found an increase in esters with storage without a decrease6. This difference may mainly result from the shorter duration of their experiments, resulting in strawberries that were not seriously spoiled. Fig. 1(d) shows the changes in the spectral peaks of the alcohols (1000–1120 cm−1) and esters (1210–1280 cm−1) with storage time, which indicates that their concentration changes with spoilage.
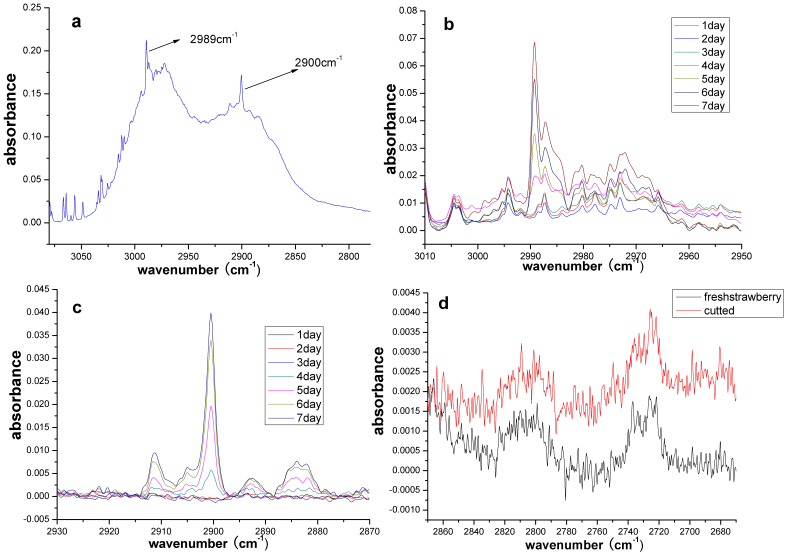
Ethylene is a volatile compound that is vaporized particularly when fruits are mature1. Pelayo demonstrated that the ethylene concentration increased when strawberries were stored in air9. We have observed obvious spectral characteristics near 2989 cm−1 that may be caused by ethylene22. Fig. 2(b) shows the spectral changes at 2989 cm−1 (after baseline correction) during the storage of 6 strawberries. The figure shows that the spectral peak appears after 3 days of storage and increases on the following days. In addition to the features at 2989 cm−1, the spectra also shows significant spectral signatures at 2900 cm−1, 2892 cm−1 and 2884 cm−1, (Fig. 2(c)) that may be related to the C-H stretching of small molecules, such as formaldehyde, ethane, methane, and similar molecules. Pelayo found that the aroma of strawberries is partially produced by benzaldehyde, and its concentration decreased during storage9. We observed very weak spectral peaks near 2806 and 2729 cm−1, which were most likely caused by benzaldehyde (Fig. 2(d))22. However, the changes in these spectral peaks during storage are not obvious. We also cut the strawberry into 4 pieces for testing, but no significant results were found. DMHF (2,5-dimethyl-4-hydroxy-3(2H)-furanone) is a particularly fragrant compound found in strawberries, and its concentration increases during storage10. Blank found that DMHF has several IR spectral peaks at 3435, 2951, 1628, 1680, 1600 and 1100 cm−1 23. However, our experiments did not show obvious features at these bands.
Figure 2. Spectral characteristics of secondary volatiles during strawberry spoilage.
(a) The 2989 cm−1 and 2900 cm−1 spectral peaks. (b) The 2989 cm−1 spectral peak may be from ethylene in detail. (c) The 2900 cm−1 spectral peak in detail. (d) The 2806 cm−1 and 2729 cm−1 spectral peaks.
2D correction spectroscopy analysis of volatiles during strawberry spoilage
More details and subtle variation in spectra can be observed using 2D correction spectroscopy than using standard linear spectroscopy. 2D correlation spectroscopy expands spectra into two dimensions and distinguishes weak peaks, enhancing the information content of spectra24. We used storage time as the disturbance quantity for calculating 2D correction spectra for the 2600–3400 cm−1 and 800–1300 cm−1 bands.
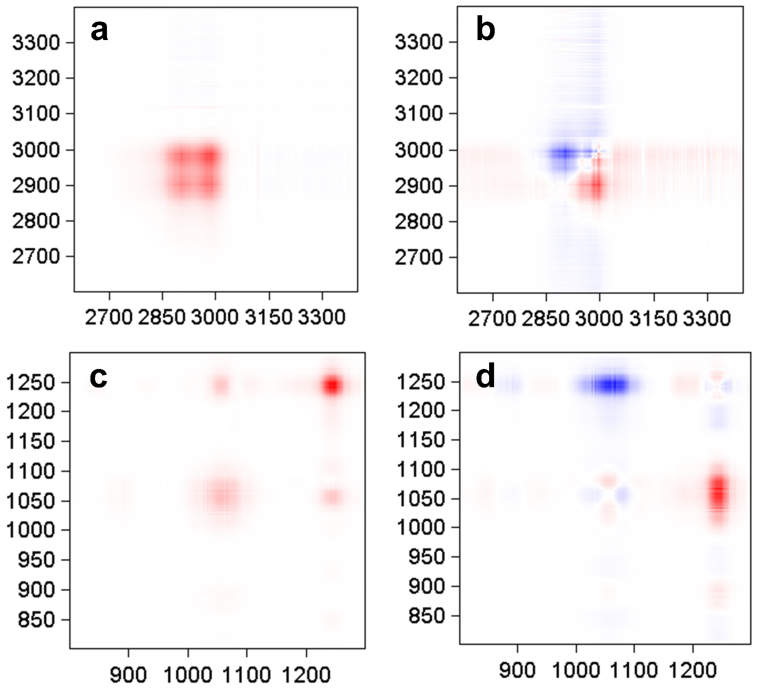
The 2600–3400 cm−1 spectral band: Two self-correlated peaks near 2979 cm−1 and 2906 cm−1 can be observed in the synchronous spectra (Fig. 3(a)), and these peaks are most likely caused by alcohols. There are also several weak cross peaks above 3050 cm−1, and these peaks may be caused by changes in water content. The bands at 2979 cm−1 and 2906 cm−1 are similar in the asynchronous spectra (Fig. 3(b)), which may indicate that the two peaks change synchronously and are related to the same compounds.
Figure 3. 2D infrared spectra during strawberry spoilage process.
(a) 2D-IR asynchronous correlation map of 2600–3400 cm−1 spectra. (b) 2D-IR asynchronous correlation map of 2600–3400 cm−1 spectra. (c) 2D-IR asynchronous correlation map of 800–1300 cm−1 spectra. (d) 2D-IR asynchronous correlation map of 800–1300 cm−1 spectra.
The 800–1300 cm−1 spectral band: Three self-correlated peaks can be observed in the synchronous spectra (Fig. 3(c)): 1245 cm−1, 1055 cm−1 and 880 cm−1. Furthermore, the asynchronous spectra (Fig. 3(d)) indicate that the 1055 cm−1 spectral peak can be divided into two individual peaks (1055 cm−1 and 1076 cm−1), which change in opposite directions. The study in the previous chapter assigns the 1245 cm−1 band to esters and the 1055 cm−1 to alcohols, but the 2D correction spectra indicate that the 1055 cm−1 band is not only related to alcohols but also to esters. We think that the 1076 cm−1 band is most likely an ester spectral peak stacked on the 1055 cm−1 band, which is not found in standard linear spectroscopy.
PCA analysis of classification for strawberry spoilage degree
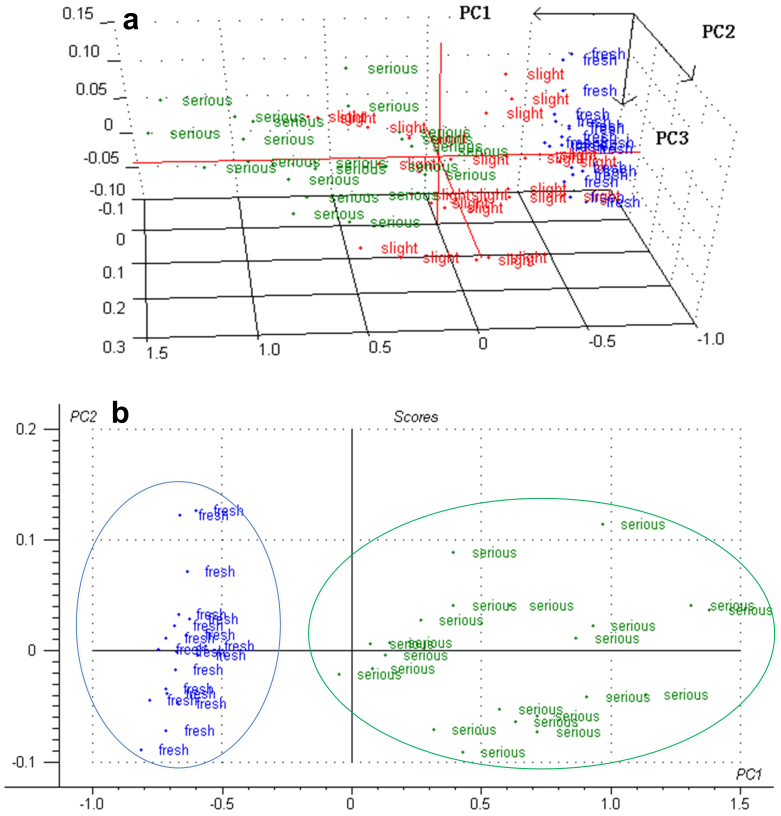
We selected 64 groups of samples for the PCA analysis using the 2800–3100 cm−1 and 850–1150 cm−1 wavelength bands. The strawberries from days 1–3 were defined as “fresh”, while those from days 4–6 were “slight spoilage” and 7–9 days were “serious spoilage”. Fig. 4(a) shows the analysis results (PC = 3). The principal component projections for the three spoilage grades show obvious difference. Two “slight” samples belong to the “serious” area. This discrepancy mainly occurred because the experiments were performed in two containers, thus the spoilage conditions may differ for samples from the same storage day. The differences between fresh and serious spoilage strawberries are much more obvious, which is shown in Fig. 4(b) as two PCs. Thus, it is possible to classify strawberries into different spoilage conditions using a combination of the FTIR spectra of the volatiles and pattern recognition methods.
Figure 4. PCA chart of FTIR spectra of strawberries under different storage days.
(a) 3 groups of samples (fresh, slight, and serious), (b) 2 groups of samples (fresh and serious).
Discussion
The above analysis verifies that the volatiles from strawberries show significant spectral characteristics that distinguish various spoilage conditions because the composition and concentration of alcohols, esters, and similar compounds regularly change during spoilage. The IR spectra for different storage times reveal the changing properties of the volatiles, while the 2D correction spectra further explain the changes for some weak peaks. The PCA analysis indicates that the IR spectra of volatiles can classify strawberries from different spoilage conditions.
This study is significant not only for its observations of the chemical process of strawberry spoilage but also because this method provides a basis for monitoring and classifying strawberries during storage and transportation. IR spectroscopy has many advantages over GC-MS and E-nose because it is fast and on-line and does not require contact or sampling. To provide stable experimental conditions, we used a pump to exhaust the volatiles into a gas cell and used multi-reflecting mirrors to lengthen the optical path. For practical applications, an open-path measurement method can be used by placing the light source and spectrometer on opposite sides of the measured objects (e.g., objects in cold storage), ensuring a sufficiently long optical path. For there are infrared emissions form nature background, the passive remote sensing method can also be used to detect spoilage conditions by placing the system far from the stored strawberries25,26. Furthermore, this study may also support the use of simplified optical detection methods, such as tunable diode laser absorption spectroscopy (TDLAS). We think that the narrow and weak spectral peaks, such as those at 1058, 1028, 1033, 2989 cm−1, are most likely suitable for detection using a TDLAS system. A modulated DFB laser with a specific wavelength can be placed on one side of the measuring area, and the derivative spectra can be directly detected on the other side. Using this type of setup, the concentration of certain volatiles can be measured, and the strawberry spoilage conditions can be identified27,28. Using TDLAS or non-dispersive infrared sensors29, the method proposed can also be simplified to a hand-held instrument which can measure the decay of strawberries in real-time.
Methods
Experimental materials
Strawberry fruits of the cultivar “fengxiang” were used in our study. The fruits were obtained from a greenhouse in the Changping district, Beijing, China. The fruits were placed in open containers without washing after they were harvested. The fruits samples were then divided into 10 groups. For groups 1–6, 3 strawberries were placed in each container, and 6 strawberries were placed in each container for groups 7–10. The samples from all 10 groups were stored and tested at room temperature (293 K). The experiment lasted for 9 days with testing twice a day (10:00 and 18:00). The strawberries were slightly decayed by the 4th day, when the aroma and luster decreased. On the 7th day, the fruits became seriously decayed, and microorganisms were observable on their surfaces.
Experimental setup
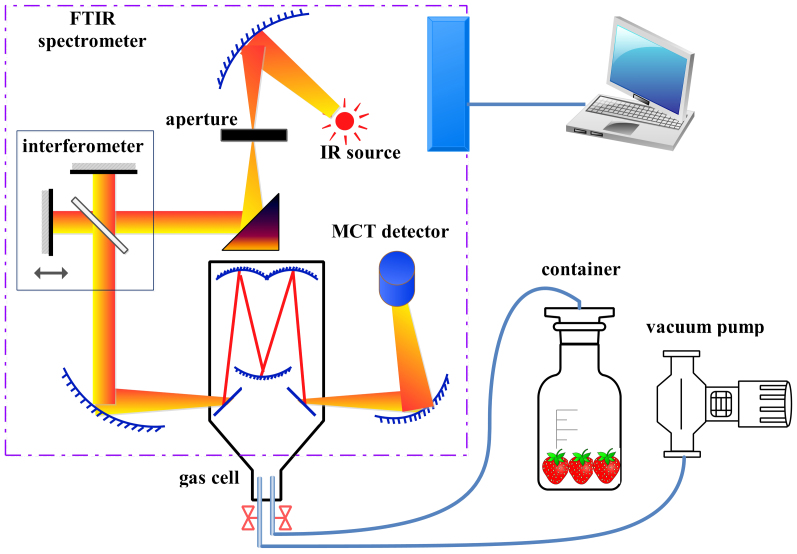
Fig. 5 shows the layout of the experimental system. The container was sealed half an hour before the test. The air inlet of the gas cell was connected to the container, while the air outlet was connected to an air pump. The valve of the air outlet was first opened to exhaust the air from the gas cell using an air pump. Then, the air outlet was closed, and the air inlet was opened. Subsequently, the volatiles from the container would automatically fill the gas cell. Six reflecting mirrors were used to enhance the optical pathlength of the gas cell to 2 m. The light inlet of the gas cell was aligned with the infrared light source of the FTIR spectrometer, and the light outlet was aligned with the detector. The spectrometer in this study was a Vertex 70 (Bruker Ltd., Germany), which used a liquid-nitrogen-cooled MCT detector. The light source was an air-cooled ceramic MIR/NIR light inside the spectrometer. The spectral range for the experiment was 600–4000 cm−1, with a resolution of 0.5 cm−1. The gas cell was a Cyclone™ C2 (Specac Ltd., UK). A 1 L vacuum pump, FY-1H 1(ALUE Ltd., Shenyang, China), was used in this experiment.
Figure 5. The layout of the experimental system.
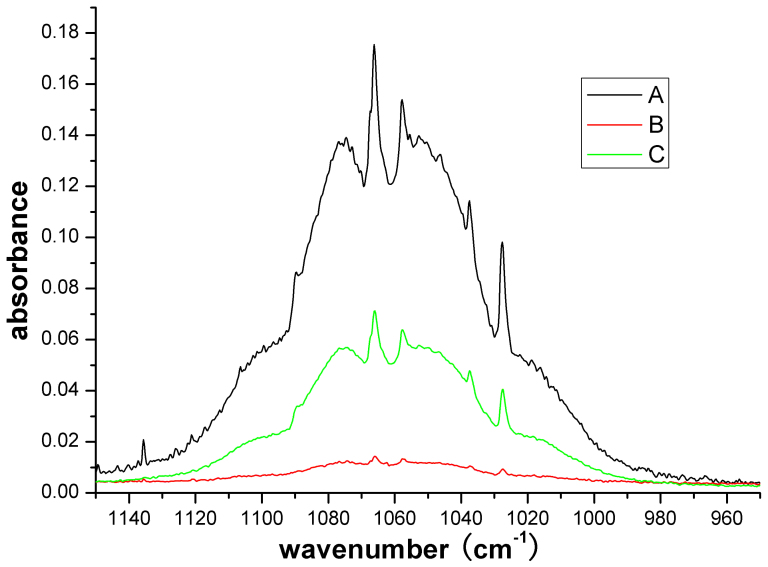
As shown in Tab. 1, the main components in strawberry volatiles have absorbance bands in the range of 600–4000 cm−1, that is why we used optical components and collected spectra in this band. Fig. 6 shows a compare between longpath and normal FTIR, where spectrum A is the method used in this experiment, while B and C are traditional methods. Spectrum A was obtained by putting 100 μL solution (1% ethanol) into the container and then filled into the gas cell (as described above), while B and C were obtained by putting 500 μL solution (1% and 10% ethanol, respectively) into a 5 L container. The latter container was equipped by Vertex 70, and the spectra were measured without gas cell. It can be studied from Fig. 6 that the sensitivity of the method used in this experiment is much better than the normal FTIR.
Table 1. The absorbance bands of the main components in strawberry volatiles22.
| Components | Absorbance spectra bands (cm−1) |
|---|---|
| ethanol | 2700–3070, 947–1165, 1320–1530, 1165–1320 |
| methanol | 910–1130, 2720–3120, 1174–1590 |
| ethyl acetate | 1170–1310, 1670–1800, 990–1150, 1340–1425, 2830–3100 |
| methyl acetate | Similar with ethyl acetate |
| ethylene | Several narrow peaks in the range of 2930–3200 and 810–1110 |
Figure 6. The compare between longpath and normal FTIR.
Spectra collection, processing and analysi
The spectra were collected using OPUS 6.5 software (Bruker Ltd., Germany). For absorbance measurements, the background was determined using the vacuum gas cell after exhausting air. Baseline corrections were also performed using OPUS 6.5. The 2D correction spectra were calculated using 2Dshige version 1.3. The PCA analysis of the spectra was performed using Unscrambler 9.7.
Author Contributions
D.D. wrote the main manuscript text, C.Z. and W.Z. designed the project, W.W. and D.D. did the experiment, X. Z. and L.J. did the calculations. All authors reviewed the manuscript.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 61134011, 31101748 & 31271614).
References
- Hui Y. H. Handbook of fruit and vegetable flavors. Wiley. Hoboken, New Jersey (2010). [Google Scholar]
- Goff S. A. & Klee H. J. Plant Volatile Compounds: Sensory Cues for Health and Nutritional Value. Science. 311, 815–819 (2006). [DOI] [PubMed] [Google Scholar]
- Forney C. F., Kalt W. & Jordan M. A. The composition of strawberry aroma is influenced by cultivar, maturity and storage. HortScience. 35, 1022–1026 (2000). [Google Scholar]
- Fukuhara K., Li X. X., Yamashita S., Hayata Y. & Osajima Y. Analysis of volatile aromatics in “ Toyonoka ” strawberries (Fragraria × ananassa) using Porapak Q column extracts. Acta Hortic. 708, 343–348 (2006). [Google Scholar]
- Zabetakis I. & Holden M. A. Strawberry flavour: Analysis and biosynthesis. J. Sci. Food Agric. 74, 421–434 (1997). [Google Scholar]
- Larsen M. & Watkin C. B. Firmness and concentration of acetaldehyde, ethyl acetate and ethanol in strawberries stored in controlled and modified atmospheres. Postharvest Biol. Technol. 5, 39–50 (1995). [Google Scholar]
- Ayala-Zavala J. F., Wang S. Y., Wang C. Y. & Gonzalez-Aguilar G. A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. Lebensm.-Wiss. u.-Technol. 37, 687–695 (2004). [Google Scholar]
- Williams A., Ryan D., Guasca A. O., Marriott P. & Pang E. Analysis of strawberry volatiles using comprehensive two-dimensional gas chromatography with headspace solid-phase microextraction. J. Chromatogr. B 817, 97–107 (2005). [DOI] [PubMed] [Google Scholar]
- Pelayo C., Ebeler S. E. & Kader A. A. Postharvest life and flavor quality of three strawberry cultivars kept at 5°C in air or air +20 kPa CO2. Postharvest Biol. Technol. 27, 171–183 (2003). [Google Scholar]
- Perez A. G., Olias R., Sanz C. & Olias J. M. Furanones in Strawberries: Evolution during Ripening and Postharvest Shelf Life. J. Agric. Food Chem. 44, 3620–3624 (1996). [Google Scholar]
- Du X., Song M. & Rouseff R. Identification of New Strawberry Sulfur Volatiles and Changes during Maturation. J. Agric. Food Chem. 59, 1293–1300 (2011). [DOI] [PubMed] [Google Scholar]
- Archbold D. D., Hamilton-Kemp T. R., Barth M. M. & Langlois B. E. Identifying Natural Volatile Compounds That Control Gray Mold (Botrytis cinerea) during Postharvest Storage of Strawberry, Blackberry, and Grape. J. Agric. Food Chem. 45, 4032–4037 (1997). [Google Scholar]
- Perez A. G., Sanz C., Olias R., Rios J. J. & Olias J. M. Evolution of Strawberry Alcohol Acyltransferase Activity during Fruit Development and Storage. J. Agric. Food Chem. 44, 3286–3290 (1996). [Google Scholar]
- Hui G. et al. Study of peach freshness predictive method based on electronic nose. Food Control. 28, 25–32 (2012). [Google Scholar]
- Gobbi E. et al. Electronic nose and Alicyclobacillus spp. spoilage of fruit juices: An emerging diagnostic tool. Food Control. 21, 1374–1382 (2010). [Google Scholar]
- Papadopoulou O. S., Tassou C. C., Schiavo L., Nychas G. E. & Panagou E. Z. Rapid assessment of meat quality by means of an electronic nose and support vector machines. Procedia Food Science. 1, 2003–2006 (2011). [Google Scholar]
- Keshri G., Magan N. & Voysey P. Use of an electronic nose for the early detection and differentiation between spoilage fungi. Lett. Appl. Microbiol. 27, 261–264 (1998). [DOI] [PubMed] [Google Scholar]
- Magana N. & Evans P. Volatiles as an indicator of fungal activity and differentiation between species, and the potential use of electronic nose technology for early detection of grain spoilage. J. Stored Prod. Res. 36, 319–340 (2000). [DOI] [PubMed] [Google Scholar]
- Griffiths P. & Haseth J. A. D. Fourier Transform Infrared Spectrometry. John Wiley & Sons (2007). [Google Scholar]
- Harren F. J. M. & Cristescu S. M. Online, real-time detection of volatile emissions from plant tissue. AoB Plants. 5, plt003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Z. et al. Analysis of Volatiles during Grape Deterioration Using FTIR. ACTA CHIM. SINICA. 71, 234–238 (2013). [Google Scholar]
- Linstrom P. J. & Mallard W. G. Eds., NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 20899, http://webbook.nist.gov, (retrieved April 9, 2013).
- Blank I., Fay L. B., Lakner F. J. & Schlosser M. Determination of 4-Hydroxy-2,5-dimethyl-3(2H)-furanone and 2(or 5)-Ethyl-4-hydroxy-5(or 2)-methyl-3(2H)-furanone in Pentose Sugar-Based Maillard Model Systems by Isotope Dilution Assays. J. Agric. Food Chem. 45, 2642–2648 (1997). [Google Scholar]
- Sasic S. & Ozaki Y. Statistical Two-Dimensional Correction Spectroscopy: Its Theory and Applications to Sets of Vibrational Spectra. Anal. Chem. 73, 2294–2301 (2001). [DOI] [PubMed] [Google Scholar]
- Ross K. R. & Todd L. A. Field Evaluation of a Transportable Open-Path FTIR Spectrometer for Real-Time Air Monitoring. Appl. Occup. Environ. Hyg. 17, 131–143 (2002). [DOI] [PubMed] [Google Scholar]
- Love S. P., Goff F., Counce D., Siebe C. & Delgado H. Passive infrared spectroscopy of the eruption plume at Popocatepetl volcano, Mexico. Nature. 396, 563–567 (1998). [Google Scholar]
- Wagner S. et al. In situ TDLAS measurement of absolute acetylene concentration profiles in a non-premixed laminar counter-flow flame. Appl Phys. 107, 585–589 (2012). [Google Scholar]
- Zeller W. et al. DFB Lasers Between 760 nm and 16 μm for Sensing Applications. Sensors. 10, 2492–2510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio R. et al. Non-selective NDIR array for gas detection. Sensor Actuat. B- Chem. 127, 69–73 (2007). [Google Scholar]