Abstract
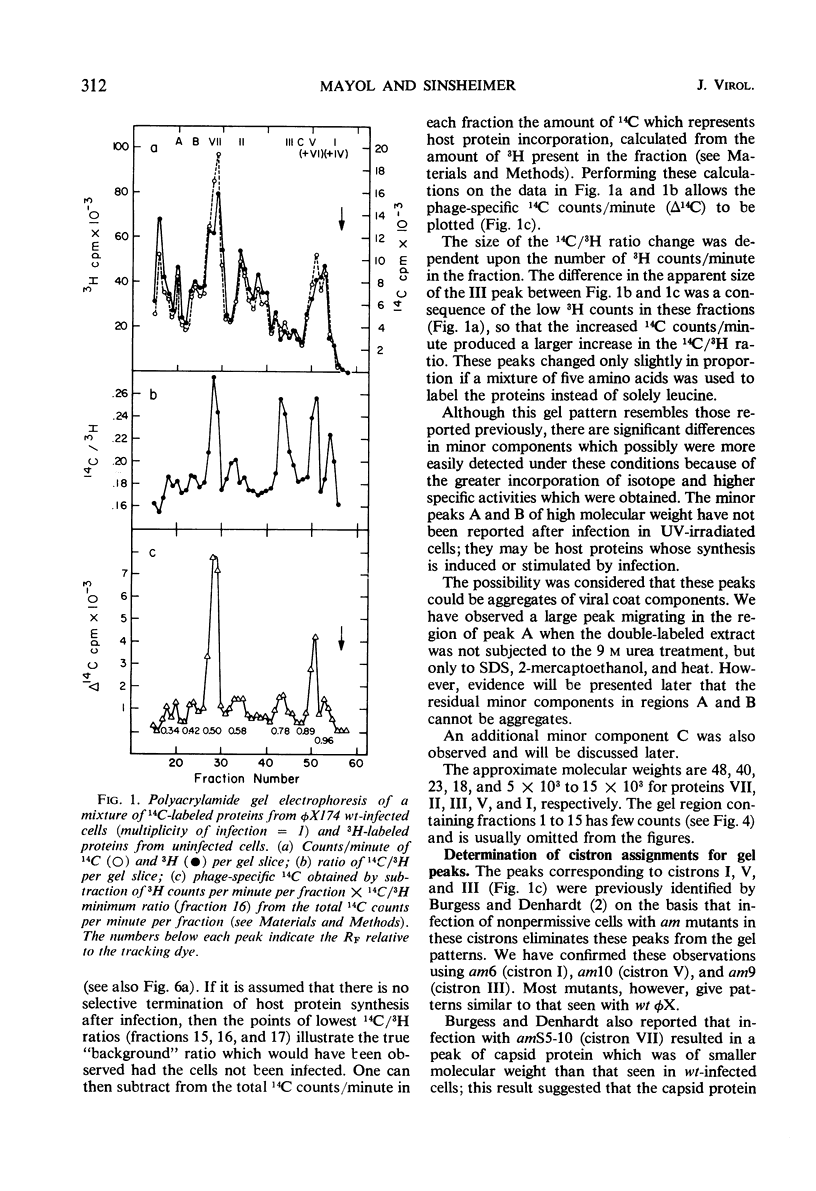
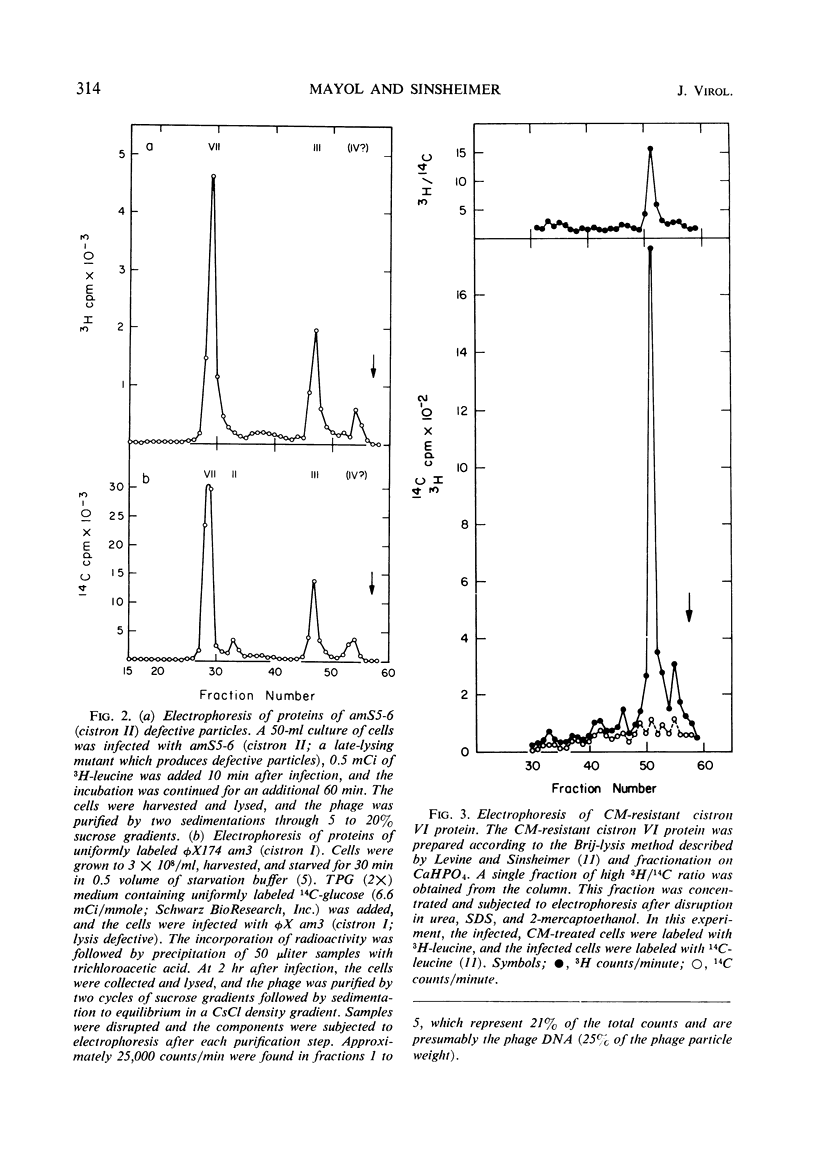
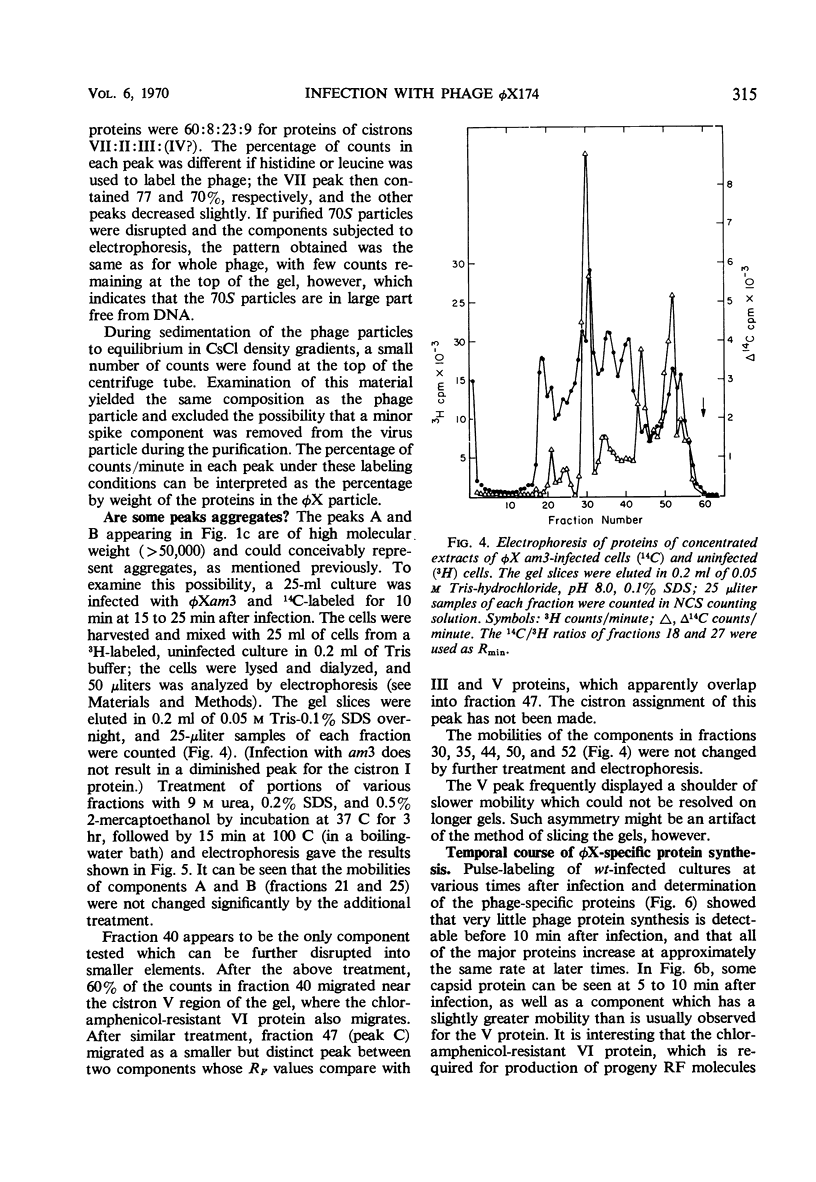
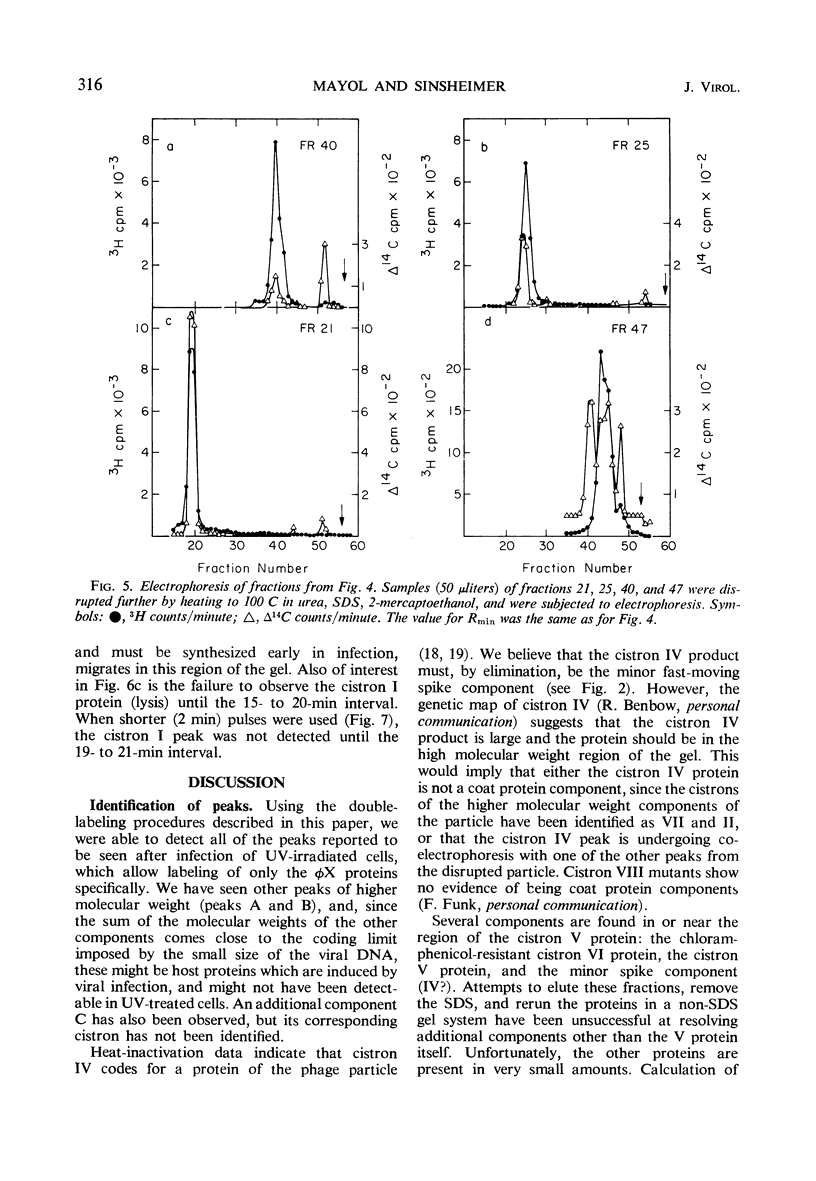
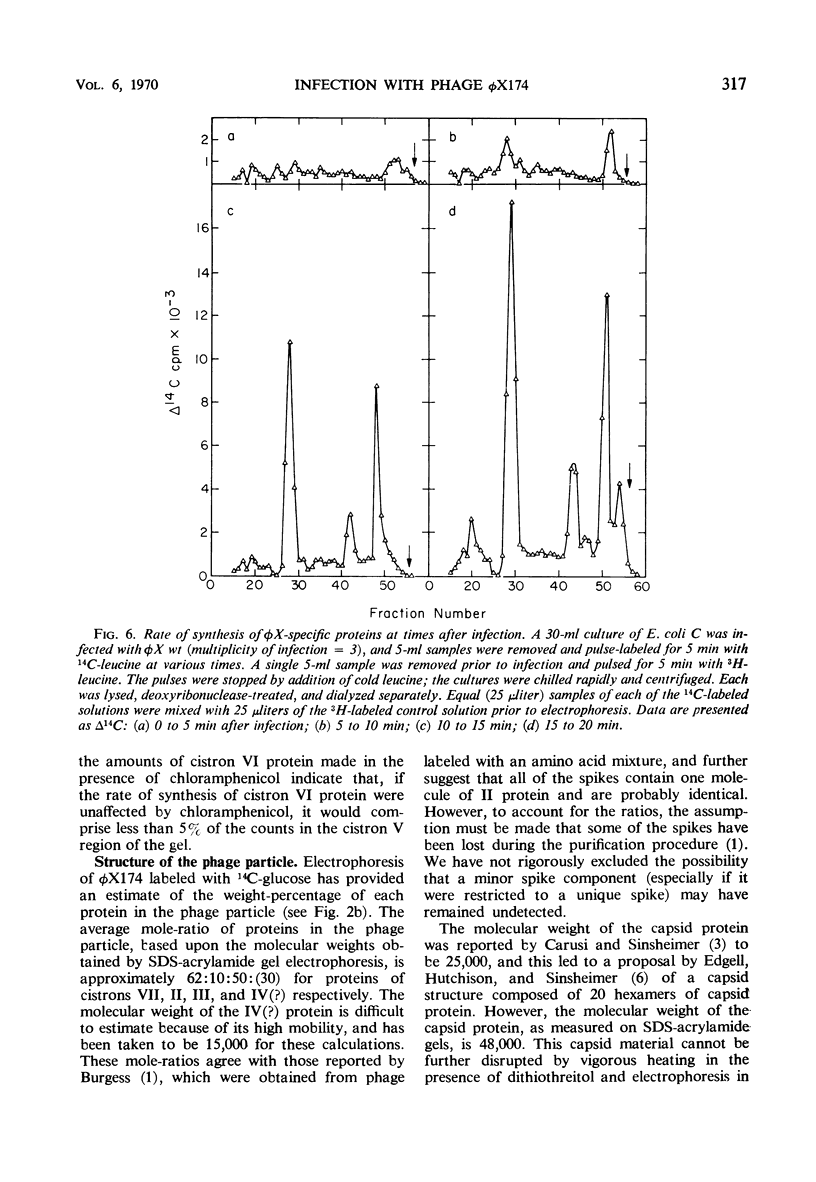
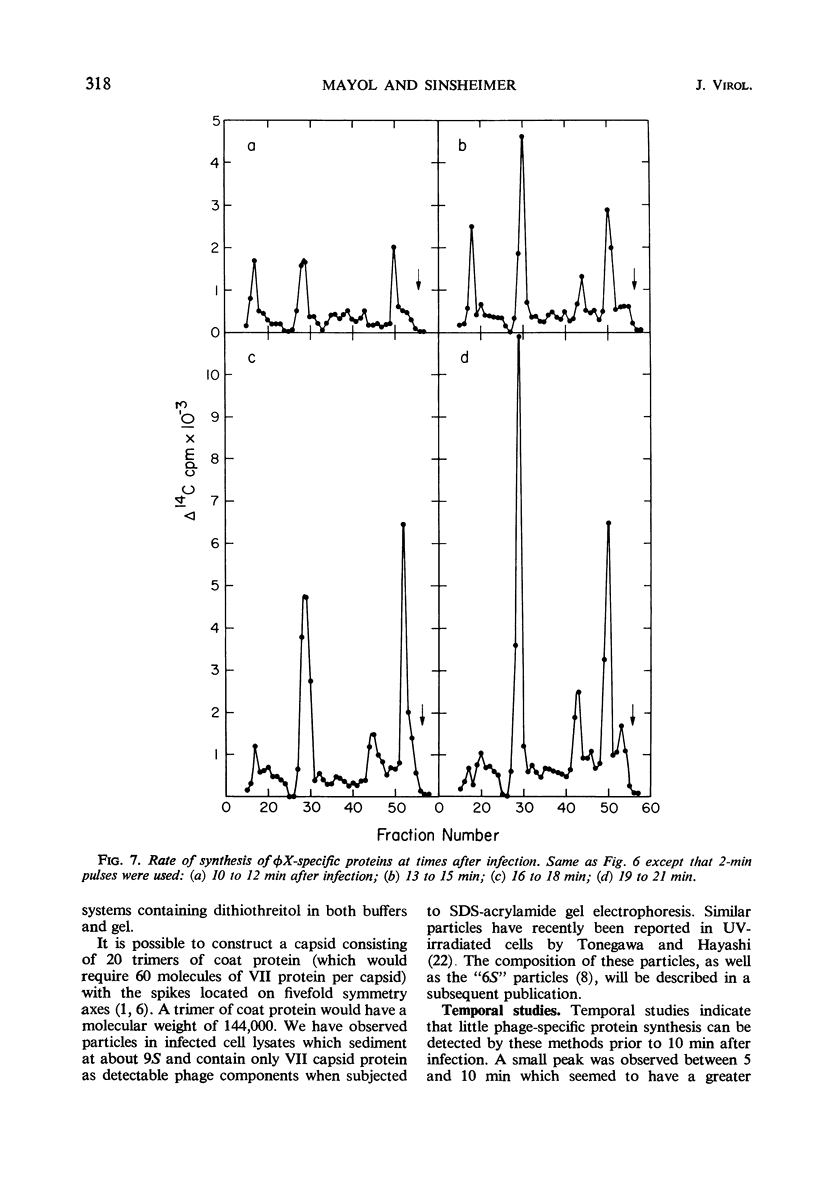
Double-labeling techniques in which 14C-labeled, φX174-infected cells and 3H-labeled, uninfected cells were used permitted the identification of the virus-specific proteins after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis without prior inhibition of host-cell protein synthesis by ultraviolet irradiation. It was also possible to detect previously undescribed components of high molecular weight which may represent induced host proteins. The gel regions specifically corresponding to cistron II protein and the chloramphenicol-resistant VI protein were identified, and a third new, small peak of unknown origin was detected. Studies of the rate of synthesis of virus-specific proteins at various times after infection indicated that the product of cistron I (lysis) is made only late in infection, but the other proteins seemed to be synthesized at the same relative rates throughout infection (although in different amounts). Studies of the proteins obtained from uniformly labeled φX virus particles indicated that all of the spikes are identical and allowed a formulation of the structure of the phage capsid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess A. B., Denhardt D. T. Studies on phiX174 proteins. I. Phage-specific proteins synthesized after infection of Escherichia coli. J Mol Biol. 1969 Sep 28;44(3):377–386. doi: 10.1016/0022-2836(69)90367-2. [DOI] [PubMed] [Google Scholar]
- Burgess A. B. Studies on the proteins of phi X174. II. The protein composition of the phi X coat. Proc Natl Acad Sci U S A. 1969 Oct;64(2):613–617. doi: 10.1073/pnas.64.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARUSI E. A., SINSHEIMER R. L. THE PHYSICAL PROPERTIES OF A PROTEIN ISOLATED FROM BACTERIOPHAGE PHI-X174. J Mol Biol. 1963 Oct;7:388–400. doi: 10.1016/s0022-2836(63)80032-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- Gelfand D. H., Hayashi M. Electrophoretic characterization of phiX174-specific proteins. J Mol Biol. 1969 Sep 28;44(3):501–516. doi: 10.1016/0022-2836(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Greenlee L. L., Sinsheimer R. L. The process of infection with bacteriophage phi X174. 18. Intracellular antiserum-precipitable components. J Mol Biol. 1968 Mar 14;32(2):321–326. doi: 10.1016/0022-2836(68)90012-0. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Knippers R., Salivar W. O., Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXVI. Transfer of the parental DNA of bacteriophage phiX174 into progeny bacteriophage particles. J Mol Biol. 1969 Feb 14;39(3):641–654. doi: 10.1016/0022-2836(69)90150-8. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XIX. Isolation and characterization of a chloramphenicol-resistant protein from phi-X-infected cells. J Mol Biol. 1968 Mar 28;32(3):567–578. doi: 10.1016/0022-2836(68)90343-4. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXV. Studies with bacteriophage phiX174 mutants blocked in progeny replicative form DNA synthesis. J Mol Biol. 1969 Feb 14;39(3):619–639. doi: 10.1016/0022-2836(69)90149-1. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- MARKERT A., ZILLIG W. STUDIES ON THE LYSIS OF ESCHERICHIA COLI C BY BACTERIOPHAGE PHI-X174. Virology. 1965 Jan;25:88–97. doi: 10.1016/0042-6822(65)90256-4. [DOI] [PubMed] [Google Scholar]
- Mayol R. F., Thayer S. A. Synthesis of estrogen-specific proteins in the uterus of the immature rat. Biochemistry. 1970 Jun 9;9(12):2484–2489. doi: 10.1021/bi00814a014. [DOI] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shleser R., Tessman E. S., Casaday G. Protein synthesis by a mutant of phage S13 blocked in DNA synthesis. Virology. 1969 May;38(1):166–173. doi: 10.1016/0042-6822(69)90139-1. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Hayashi M. Intermediates in the assembly of phi X174. J Mol Biol. 1970 Mar 14;48(2):219–242. doi: 10.1016/0022-2836(70)90158-0. [DOI] [PubMed] [Google Scholar]



