Abstract
Zymogen granule (ZG) formation in acinar cells involves zymogen cargo sorting from trans-Golgi into immature secretory granules (ISGs). ISG maturation progresses by removal of lysosomal membrane and select content proteins, which enter endosomal intermediates prior to their apical exocytosis. Constitutive and stimulated secretion through this mechanism is termed the constitutive-like and minor-regulated pathways, respectively. However, the molecular components that control membrane trafficking within these endosomal compartments are largely unknown. We show that tumor protein D52 is highly expressed in endosomal compartments following pancreatic acinar cell stimulation and regulates apical exocytosis of an apically directed endolysosomal compartment. Secretion from the endolysosomal compartment was detected by cell-surface antigen labeling of lysosome-associated membrane protein LAMP1, which is absent from ZGs, and had incomplete overlap with surface labeling of synaptotagmin 1, a marker of ZG exocytosis. Although culturing (16–18 h) of isolated acinar cells is accompanied by a loss of secretory responsiveness, the levels of SNARE proteins necessary for ZG exocytosis were preserved. However, levels of endolysosomal proteins D52, EEA1, Rab5, and LAMP1 markedly decreased with culture. When D52 levels were restored by adenoviral delivery, the levels of these regulatory proteins and secretion of both LAMP1 (endolysosomal) and amylase was strongly enhanced. These secretory effects were absent in alanine and aspartate substitutions of serine 136, the major D52 phosphorylation site, and were inhibited by brefeldin A, which does not directly affect the ZG compartment. Our results indicate that D52 directly regulates apical endolysosomal secretion and are consistent with previous studies, suggesting that this pathway indirectly regulates ZG secretion of digestive enzymes.
Keywords: TPD52, constitutive-like pathway, minor-regulated pathway, endolysosomal secretion
pancreatic acinar cells are a traditional model for studying membrane and protein trafficking within the secretory pathway of mammalian cells (53) because they mediate the constitutive and regulated secretion of digestive enzymes necessary for nutrient digestion in the intestine. Pancreatic acini are arranged in clusters of 8–12 cells with their apical plasma membranes forming the terminal end of pancreatic ductules. Secretagogue stimulation at the basolateral membrane occurs through G protein-coupled receptors to activate PLC and/or adenylyl cyclase pathways that induce the exocytosis of large (∼1 μm diameter) zymogen granules (ZGs). Acutely isolated acini are typically used to study secretion because they are highly sensitive to secretagogues and remain polarized. However, these features are lost in culture through unknown mechanisms, making use of mRNA silencing techniques to reduce protein expression difficult.
In addition to the classic ZG secretory pathway, two unique parallel secretory pathways have been identified in acinar cells from the pancreas and parotid glands known as the constitutive-like (CLP) and minor regulated (MRP) pathways (1, 10, 11, 33, 35). Newly synthesized secretory proteins are selectively secreted through the CLP and MRP, whereas the secretion from ZGs contains proteins that have been stored following synthesis. Secretion from the CLP and MRP, but not ZGs, is acutely inhibited by brefeldin A (BFA), an inhibitor of guanine nucleotide exchange factors for class 1 ADP-ribosylation factors ARF1 and ARF3 that function in vesicle formation from trans-Golgi and endosomal compartments (49). The CLP and MRP were shown to originate from immature secretory granules (ISGs) as small vesicles that move through endosomal intermediates prior to secretion into the acinar lumen (35, 72). The CLP was proposed to enter an apical recycling compartment before undergoing constitutive exocytosis, whereas the MRP traffics from sorting endosomes to the apical membrane upon low-level secretagogue stimulation. Although the CLP and MRP release relatively small amounts of zymogens (<5% of total secretion following a meal), they play an important role in ZG formation/maturation and, moreover, are proposed to indirectly regulate ZG exocytosis by delivering t-SNARE regulatory proteins to the apical membrane necessary for ZG docking and fusion (9).
In addition to acinar cells, similar CLP and MRP pathways have been described in beta cells that mediate the release of unprocessed insulin and C peptide both constitutively and in response to low-level stimulation (2, 43, 44). Other studies have characterized CLP and MRP pathways in endothelial, endochromaffin, and hematopoietic cells (22, 26–27, 45, 65). Unlike the numerous studies aimed at understanding the classic ZG pathway, little is known regarding the molecular identity of the endosomal compartments of the CLP and MRP or the regulatory proteins that control these pathways.
Tumor protein D52 (also known as CRHSP-28 and CSPP28) is a Ca2+-regulated serine phosphoprotein isolated from gastric mucosa and pancreatic acinar cells (28, 54). D52 mRNA was first identified due to its overexpression in human breast carcinomas (8) and belongs to a small family of proteins (6). Although prevalent in many carcinomas, D52 is uniquely abundant in polarized secretory cells containing large secretory granules including acinar cells from the pancreas, lacrimal and salivary glands, chief cells, goblet cells, Paneth cells and granule cells of the hippocampus (15, 29). D52 is a peripheral membrane protein that contains a coiled-coil motif that mediates homomeric and heteromeric interactions among D52 family members (62). D52 also undergoes a Ca2+-dependent interaction with annexin VI, a Ca2+-dependent membrane and cytoskeletal binding protein implicated in endocytosis (67), and MAL2, a proteolipid necessary for apical membrane trafficking and polarization of epithelial cells (51, 74).
We have shown that D52 modulates secretion from permeabilized acinar cells (69) and when expressed in CHO-K1 cells, directly regulated exocytosis of the lysosome-associated membrane protein 1 (LAMP1) in a Ca2+- and phosphorylation-dependent manner (68). Although D52 regulated acinar secretion, it did not copurify or colocalize with ZGs, but rather was associated with trans-Golgi, ISGs (also referred to as condensing vacuoles), and endosomal compartments (29, 70), leading us to explore whether it regulates trafficking within the CLP and MRP to modulate secretion. Here we demonstrate that D52 is dynamically localized within endolysosomal compartments (early endosomes, late endosomes, and lysosomes) and apical recycling endosomes in acini. Surprisingly, expression of D52 and other endolysosomal regulatory proteins rapidly declines during acinar culture. Adenoviral replacement of D52 prevented the loss of these proteins and strongly augmented both digestive enzyme secretion and the exocytosis of LAMP1, indicating that the CLP/MRP represent an endolysosomal secretory pathway separate from ZGs.
MATERIALS AND METHODS
Antibodies.
Anti-D52 polyclonal antibodies were previously described (29). Anti-hemagglutinin mouse monoclonal antibody (cat no. 2367) was purchased from Cell Signaling Technology (Danvers, MA). Anti-trans-Golgi-network (TGN) 38 was a kind gift from Dr. Kathryn E. Howell at the University of Colorado, School of Medicine. Anti-lysosomal LAMP1 (used for immunofluorescence) (cat. no. VAM-EN001) was purchased from Assay Designs (Ann Arbor, MI). Anti-vesicle-associated membrane protein (VAMP) 2 (cat. no. 104 211), anti-syntaxin 2 (cat. no. 110 022), anti-syntaxin 3 (cat. no. 110 032) and anti-syntaxin 4 (cat. no. 110 042) were purchased from Synaptic Systems (Gottingen, Germany). Anti-early endosomal antigen 1 (EEA1) (cat. no. 610456) was purchased from BD Transduction Laboratories (San Jose, CA). The anti-MAL-2 antibody was previously described (21). Anti-VAMP7 (cat. no. ab36195), anti-LAMP1 (cat. no. AB24170) (used for immunoblotting), and anti-Rab11A (cat. no. ab78337) were purchased from Abcam (Cambridge, MA). GRAMP92 was a generous gift from Dr. J. David Castle (48). Anti-synaptotagmin 1 (cat. no. S2177) was purchased from Sigma (St. Louis, MO). Alexa-conjugated rabbit and mouse secondary antibodies were purchased from Invitrogen (Carlsbad, CA). Peroxidase-conjugated secondary antibodies were purchased from GE Healthcare Bio-Sciences (Piscataway, NJ). All antibodies were characterized before Western blotting and immunofluorescence labeling studies by serial dilutions to determine optimal conditions and negative controls (usually secondary antibody alone) to ensure specificity (data not shown).
Other reagents.
Phadebas Amylase Assay kit was purchased from Magle Life Sciences (Cambridge, MA). Cholecystokinin (CCK-8) was purchased from Research Plus (Barnegat, NJ). Dulbecco's minimal essential medium, essential amino acids, fetal bovine serum, TrypLE Express, penicillin and streptomycin, Alexa-conjugated phalloidin, Image-iT FX Signal Enhancer, and ProLong Gold antifade reagent with 4,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen. Bovine serum albumin, 12-O-tetradecanoylphorbol-13-acetate (TPA), and a protease inhibitor cocktail containing AEBSF, aprotinin, EDTA, leupeptin, and E64 were purchased from Calbiochem (EMD Chemicals, Gibbstown, NJ). Protein determination reagent and nonfat dry milk were purchased from Bio-Rad Life Science Research (Hercules, CA). DNA Maxi and Mini-prep kits and PCR reagents were purchased from Promega (Madison, WI). The QuikChange XL Site-Directed Mutagenesis Kit, AdEasy Adenoviral Vector System, and HEK-293 cells were purchased from Stratagene (Santa Clara, CA). Fast Link DNA-ligation kit and BFA were purchased from Epicentre Biotechnologies (Madison, WI). Restriction enzymes were purchased from Fermentas (Glen Burnie, MD). Trypsin substrate Boc-Glu-Ala-Arg-MCA was purchased from Peptides International (Louisville, KY). Collagen Type IV Cellware six-well plates were purchased from BD Biosciences (Bedford, MA). Formaldehyde and Tissue Tek were purchased from Electron Microscopy Sciences (Hatfield, PA). Goat serum, cold-water fish skin gelatin, 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate sodium salt (CPT-cAMP), soybean trypsin inhibitor, Triton X-100, Tween 20, dimethylsulfoxide (DMSO), phenylmethanesulfonyl fluoride (PMSF), thapsigargin (TG), benzamidine, amphotericin B, EGF, NaCl, Trizma base, and IBMX were purchased from Sigma-Aldrich (St. Louis, MO). SuperSignal West Femto Chemiluminescent Substrate was purchased from Thermo Scientific (Rockford, IL).
Tissue fractionation.
The University of Wisconsin Committee on Use and Care of Animals approved all studies involving animals. ZGs, cytosol, and microsomes were purified from a male Harlan Sprague Dawley rat pancreas by a method described previously (67). All steps were carried out at or in buffer at 4°C. The pancreas was homogenized in buffer containing (in mM) 10 MOPS (pH 6.8), 250 sucrose, 0.1 MgSO4, 1 benzamidine and 0.1 PMSF by using a Teflon pestle with clearance of 0.5–0.1 mm. A postnuclear supernatant (PNS) was obtained by a 10 min, 500 g centrifugation. The PNS was further centrifuged for 10 min at 1,300 g to produce a pellet enriched in ZGs and mitochondria. ZGs were further purified by Percoll gradient. The 1,300 g supernatant was centrifuged at 100,000 g for 45 min to separate microsomal and cytosolic fractions. Microsomal fractions were layered on a sucrose gradient composed of (in M) 0.16, 0.86, 0.5, 0.25 sucrose then centrifuged at 160,000 g for 1 h. Fractions were lysed by sonication in buffer containing (in mM) 50 Tris (pH 7.4), 150 NaCl, 1 benzamidine, 0.1 PMSF, 0.2% TX-100, and supplemented with Protease inhibitor cocktail. Equal amounts of protein were separated by SDS-PAGE and immunoblotted with indicated antibodies.
Adenovirus production.
The recombinant adenovirus production was carried out by using AdEasy Adenoviral Vector System (Stratagene). Briefly, the coding region of human D52 wild-type (WT) including an NH2-terminal HA-tag was subcloned into the pShuttle-CMV vector (Stratagene) by using Sal1 and EcoRV restriction sites. All point mutations of D52 in the pShuttle-CMV plasmid were generated by substituting serine residue 136 with glutamate (phosphomimetic) or alanine (phospho-null) with use of the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene) and were confirmed by DNA sequencing. The virus was harvested from AD-293 cells and concentrated by cesium chloride gradient centrifugation. Adenovirus titer was determined by plaque assay using an agarose overlay on AD-293 cells.
Short-term culture of pancreatic acini and lobules.
Pancreatic acini were isolated from male Harlan Sprague Dawley rats by collagenase digestion as previously described (69). Lobules were prepared by injecting the dissected pancreas with DMEM containing indicated adenovirus immediately before mincing the gland into 1-mm3 explants. Isolated acini or lobules from one rat were cultured in eight to ten 100 × 15 mm plastic Petri dishes in 20 ml DMEM supplemented with 0.5% FBS, 0.02% soybean trypsin inhibitor (SBTI), penicillin, and streptomycin for 18 h, unless otherwise noted, at 37°C, 5% CO2 in a humidified atmosphere. Adenoviruses were added to acini at specified titers in the culture medium for 18-h incubation. Under these conditions greater than 99% of acini expressed green fluorescent protein (GFP) (data not shown).
Immunofluorescence studies in pancreatic lobules.
Freshly prepared or cultured lobules were treated as indicated and fixed in 2% formaldehyde in 1× PBS, 2 h at room temperature. Following a series of sucrose dehydrations in 1× PBS at 4°C, tissue was embedded in Tissue Tek and flash frozen in liquid nitrogen cooled isopentane.
Immunofluorescence.
The buffer used for blocking and incubation steps contained 1× PBS, 3% bovine serum albumin, 2% goat serum, 0.7% cold-water fish skin gelatin, and 0.2% Triton X-100. Cryostat sections (9 μm) from fresh or adenovirally infected lobules were rinsed with 1× PBS and then treated with Image-iT FX according to Invitrogen instructions. Primary antibodies were added simultaneously for 2 h at room temperature. Tissue was washed with 1× PBS (3 × 5 min) then secondary antibodies were added simultaneously for 1 h at room temperature and cells were washed with 1× PBS (3 × 5 min). If indicated, tissue was next incubated with an Alexa-conjugated phalloidin (to label actin) according to Invitrogen's instructions, rinsed with 1× PBS (3 × 5 min), and mounted with coverslips and ProLong Gold antifade reagent with DAPI to label nuclei. In Figs. 1 and 2, D52 (1:100) immunoreactivity was detected by using Alexa Fluor 546-conjugated anti-rabbit (1:500). TGN38, MAL2, EEA1, LAMP1, AP-3 (all at 1:100), VAMP2 (1:20), and Rab11a (1, 50) immunoreactivities were detected by use of Alexa Fluor 488-conjugated anti-mouse IgG (1:250). In Fig. 6, D52 immunoreactivity was detected with Alexa Fluor 488-conjugated anti-rabbit (1:500). Rab11a, GRAMP92, EEA1, and LAMP1 immunoreactivities were detected by use of Alexa Fluor 647-conjugated anti-rabbit IgG (1:100) or Alexa Fluor 546-conjugated anti-mouse IgG (1:250). Pseudocolors, were applied postcollection. Tissues were analyzed by bright-field microscopy.
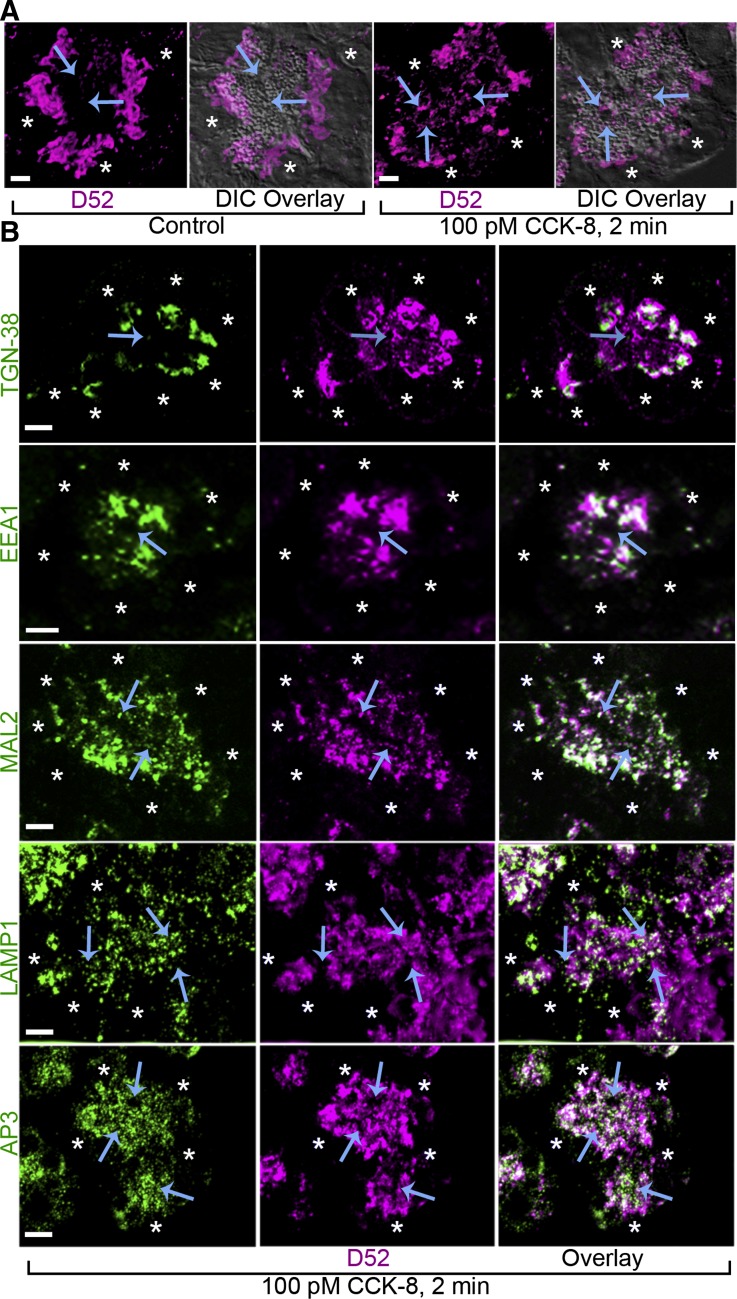
Fig. 1.
D52 accumulates in endosomal compartments upon secretagogue stimulation. A: D52 viewed by bright-field immunofluorescence of cryostat sections from lobules under basal conditions is present in supranuclear regions and accumulates to apical regions with 2-min cholecystokinin (CCK-8) stimulation. B: colocalization of D52 with TGN-38, EEA1, MAL2, LAMP1, or AP-3 in CCK-8-treated lobules. Arrows denote apical plasma membrane. Asterisks denote nuclei. Scale bars, 7 μm. Each image is a reconstructed z-series from a representative experiment performed on at least 3 separate tissue preparations. DIC, differential interference contrast.
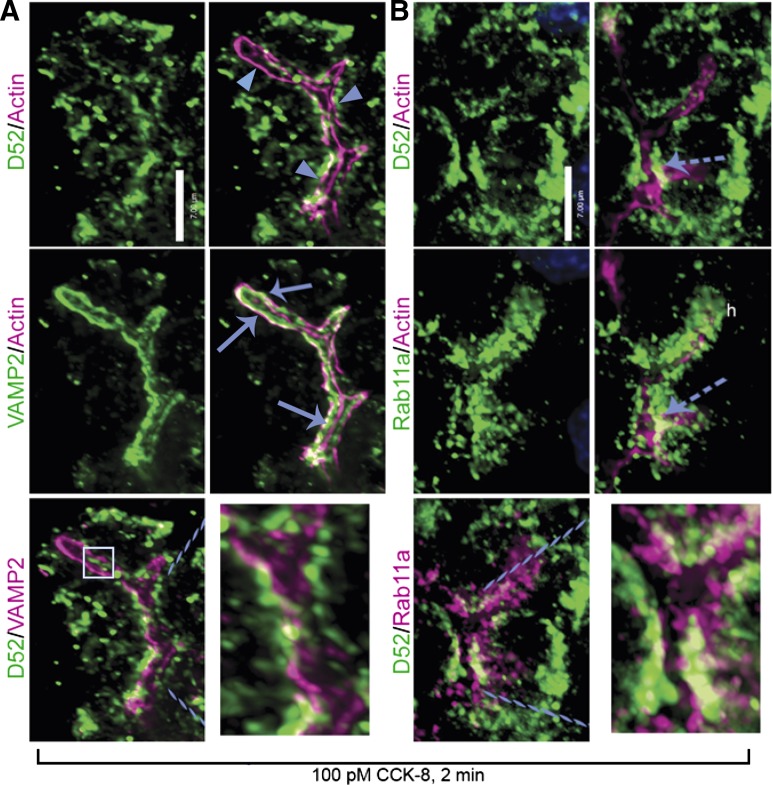
Fig. 2.
D52 does not translocate to apical plasma membrane following acinar stimulation. All images are D52 localization following 2-min CCK-8 stimulation. A: D52 is on the cytoplasmic side of apical actin filaments (arrowheads) and absent from plasma membrane. Minimal colocalization of D52 is seen with the zymogen granule (ZG) protein VAMP2, which accumulates within actin filaments and along the apical membrane (arrows). Box in bottom left panel shows apical D52 that it is actually at the bottom of the acinus rather than on apical membrane (Supplemental Movie S1). B: D52 partially colocalizes with the recycling endosomal protein Rab11a on the cytoplasmic side of actin filaments. Dashed arrow indicates D52 and Rab11a above the plane of the actin filaments. Dashed lines in bottom panels designate enlarged areas. Scale bars, 7 μm. Images are reconstructed z-series from a representative experiment performed on 3 separate tissue preparations.
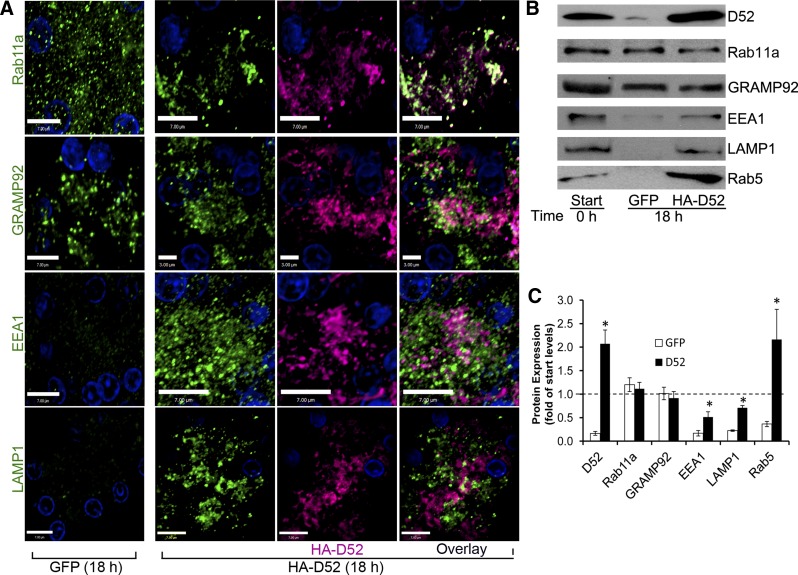
Fig. 6.
Expression of HA-D52 maintains a polarized organization and expression of apical endolysosomal regulatory proteins. A: bright-field microscopy of lobules cultured (18 h) in GFP or HA-D52 adenovirus (106.5). Rab11a, GRAMP92, EEA1, and LAMP1 in GFP-expressing lobules show dispersed punctate staining throughout basal and apical cytoplasm whereas HA-D52 expression maintains a condensed organization of endolysosomal compartments in the apical cytoplasm. Also note the pronounced colocalization of Rab11a with D52. Each image is a reconstructed z-series representative of multiple determinations performed on at least 3 separate tissue preparations. D52 immunoreactivity was detected with Alexa Fluor 488-conjugated anti-rabbit (1:500). Rab11a, GRAMP92, EEA1, and LAMP1 immunoreactivities were detected by use of Alexa Fluor 546-conjugated anti-mouse (1:250). Postcollection, pseudocolors were applied. Bars are 3 or 7 μm as indicated. B: expression of indicated proteins (50 μg/lane) was analyzed by immunoblotting whole-cell lysates prepared from fresh (0 h) or cultured (18 h) acini. C: graph shows means and SE of protein expression quantified from 3 experiments each from different tissue preparations. *P < 0.01 compared with respective GFP controls.
Cell surface labeling of LAMP1 and syt1.
For external cell surface labeling no Triton X-100 was used. Fresh acini or acini cultured with HA-D52 WT or GFP virus were treated with and without 10 μg/ml BFA for 30 min followed by secretagogues at indicated concentrations for specified times at 37°C. Cells were gently pelleted on ice and resuspended with ice-cold fresh media containing anti-LAMP1 (1:20) or anti-synaptotagmin 1 (syt1; 1:20) (individually in Figs. 5B and 7A or concurrently in Fig. 7C) and gently mixed on a wheel for 2 h at 4°C. Cells were then fixed using 2% formaldehyde in 1× PBS for 10 min at room temperature, rinsed one time with 1× PBS, pelleted, and resuspended in blocking buffer devoid of Triton X-100 containing indicated secondary antibodies at 1:100 (LAMP1) or 1:250 (syt1) for 1 h at room temperature. Cells were rinsed one time and resuspended with 1× PBS and placed on a slide for “whole-mount” confocal evaluation. In Fig. 7C, LAMP1 and syt1 immunoreactivities were detected by use of Alexa Fluor 488-conjugated anti-mouse IgG or Alexa Fluor 546-conjugated anti-rabbit IgG, respectively. Pseudocolors were applied as indicated.
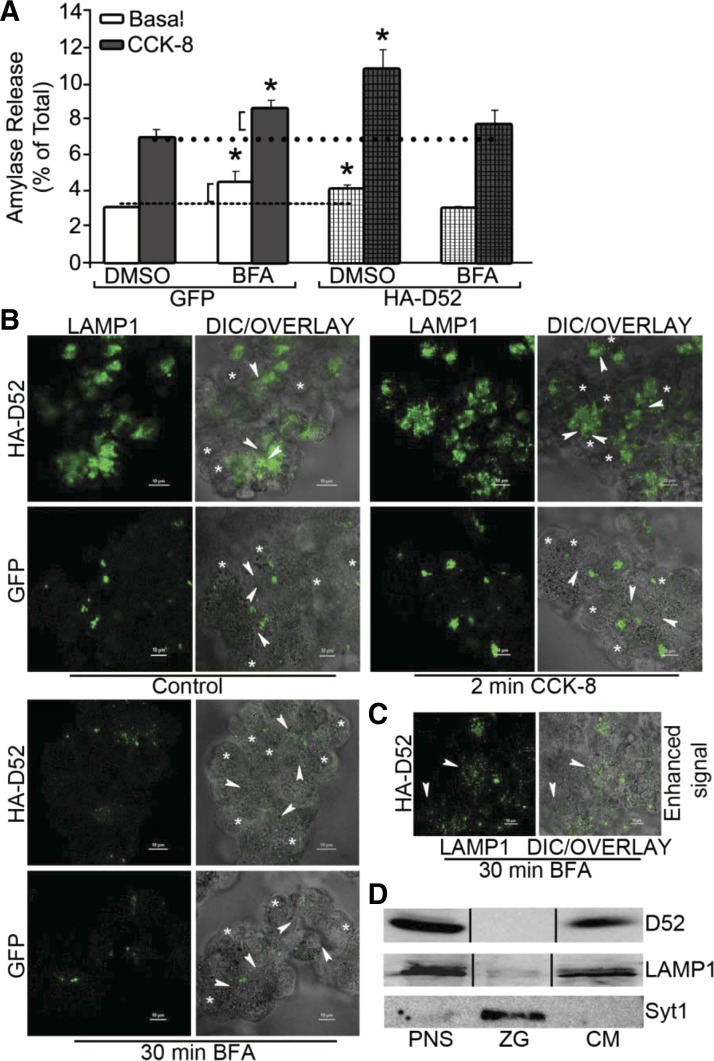
Fig. 5.
Brefeldin A (BFA) inhibits ability of HA-D52 to augment amylase secretion and LAMP1 externalization. A: cultured acini (18 h) expressing GFP or HA-D52 (106.5 pfu/ml) were pretreated with 0.01% DMSO vehicle or BFA (10 μg/ml, 30 min) and amylase secretion was determined under basal or CCK-8-stimulated conditions. Note BFA enhanced basal and stimulated secretion in GFP cells to the same extent (brackets) whereas it fully abolished the secretory effects of HA-D52. Data are means and SE of 3 independent experiments performed in triplicate. *P < 0.05 compared with GFP-expressing DMSO controls. B: LAMP1 surface labeling was measured in intact acini following 18 h culture with GFP or HA-D52. C: HA-D52 expressing acini treated with BFA as in B but with increased excitation showing residual LAMP1 labeling (arrowheads) has spread to lateral and basal regions of acini. Each image is a reconstructed z-series obtained by confocal microscopy of acinar cell whole mounts and is representative of multiple determinations performed on at least 2 separate tissue preparations. Bars, 10 μm. D: D52, LAMP1, and synaptotagmin 1 (syt1) expression in acinar postnuclear supernatant (PNS), zymogen granule (ZG), and microsomal (CM) fractions (100 μg/lane) was analyzed by immunoblotting.
Fig. 7.
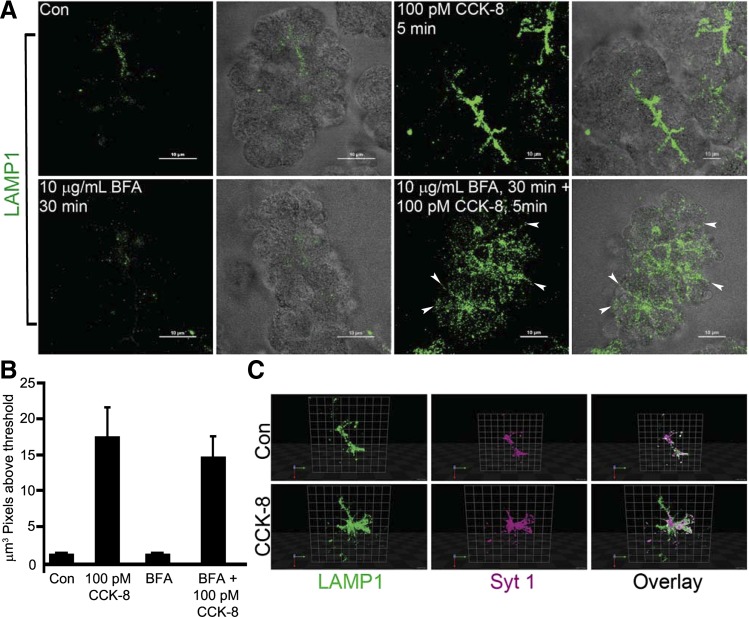
Characterization of the constitutive-like (CLP) and minor-regulated (MRP) pathways by LAMP1 externalization in freshly prepared acini. A: freshly prepared acini were treated as control or with CCK-8 (100 pM) for 5 min. Where indicated, acini were preincubated with BFA (10 μg/ml) for 30 min followed by CCK-8 (100 pM) for 5 min. Externalized LAMP1 labeling was conducted as in Fig. 5. Each image is a reconstructed z-series and representative of multiple determinations from at least 3 separate tissue preparations. Bars, 10 μm. B: quantitative analysis of the volume of LAMP1 immunoreactivity in acini acquired from multiple reconstructed z-series images of acinar clusters from 3 separate tissue preparations. Data are means and SE (n = 8 for each experimental condition). C: cells were processed as in A except they were costained with LAMP1 and syt1 and presented here in a reconstructed 3D format using Volocity software. Blue, red, and green arrows indicate direction in the z, y, and x planes, respectively. Con, control.
Quantification of immunofluorescence images.
Multiple (10–15) bright-field, z-series images from at least three separate tissue preparations were analyzed by using Image J software with the colocalization threshold plug-in. Threshold values for each image were automatically determined by the software and, therefore, were unbiased and provided conservative estimates. These values were collated and statistically analyzed with Excel software. Data reported are means and SE, and P values were calculated by an unpaired Student's t-test.
Acinar secretory assays.
Cultured acini were washed and resuspended in HEPES buffer containing (in mM) 10 HEPES, 137 NaCl, 4.7 KCl, 0.56 MgCl2, 1.28 CaCl2, 0.6 Na2PO4, 5.5 d-glucose, 2 l-glutamine, and essential amino acids, gassed with 100% O2, supplemented with 0.1 mg/ml SBTI and 1 mg/ml BSA, with pH adjusted to 7.48. Acini were stimulated with CCK-8 or other indicated cellular secretagogues for 30 min in a 37°C water bath with gentle agitation. After the 30-min incubation, acini were immediately transferred to an ice bath and centrifuged 1 min at 12,000 g, and the medium was collected. Amylase content in the medium was determined by use of a Phadebas assay kit. Secretion was calculated as percent of total cellular amylase present at the start of the experiment. For BFA studies, acini were preincubated in HEPES buffer containing 10 μg/ml BFA for 30 min in a 37°C water bath with gentle agitation before secretory assays were carried out in the presence of BFA or vehicle (DMSO). All data are means and SE of at least three separate tissue preparations performed in triplicate. P values were calculated by a paired Student's t-test.
Preparation of ZGs from cultured acini.
ZGs were purified by a method described previously (12). Tissue preparation and centrifugations were carried out in buffer at 4°C. In brief, cultured acini harvested from one adult rat pancreas were homogenized by using a Teflon pestle with clearance of 0.5–0.1 mm in 3 ml of homogenization buffer containing in mM, 25 PIPES (pH 6.8), 300 sucrose, 0.2 CaCl2, 0.2 MgCl2, 2 EGTA, 1 benzamidine, 0.1 PMSF, and 0.02% SBTI. Homogenates were centrifuged at 500 g for 10 min to generate a PNS. The PNS was centrifuged at 1,000 g for 10 min to generate ZG and particulate fractions. The supernatant was further centrifuged at 100,000 g for 45 min to generate cytosolic and microsomal fractions. The ZG and particulate fraction were resuspended in 0.5 ml homogenization buffer with an equal volume Percoll and centrifuged at 100,000 g for 20 min with the resulting white lower band containing the ZG.
Immunoblotting.
SDS-PAGE and immunoblotting were conducted as previously described (38). Anti-D52 (5 μg/ml)-HA (1:1,000), -syntaxin 2–4 (1:1,000), -VAMP2 (1:2,000), and -VAMP8 (1:1,000), -syt1 (1:2,000), and -AP-3 (1:1,000) immunoreactivities were detected by using appropriate horseradish peroxidase-conjugated secondary antibodies. Immunoreactivity was quantified by densitometry.
Microscope image acquisition.
Bright-field images were captured by using a Nikon Eclipse TE2000 microscope, a PlanApo ×100 oil objective with a numerical aperture of 1.4, and a Hamamatsu Orca camera. After collection, images were deconvolved with Volocity software and were processed with Volocity, Image J, or Photoshop software. Postcollection, in some instances, pseudocolors were applied. Confocal images were captured by use of an inverted Nikon Eclipse Ti-E microscope, a Plan Apo ×100 oil objective with a numerical aperture of 1.4, a side-mounted scanhead, argon gas, diode-pumped solid-state and diode lasers, and a work station running NIS-Elements C software. Images were processed with Volocity, Image J, NIS-Elements AR, or Photoshop software. Postcollection pseudocolors were applied. The z-series images from Fig. 8 and Supplemental Fig. S2 (supplemental material for this article is available online at the Journal website) were reconstructed into a 3D format with Volocity software.
Fig. 8.
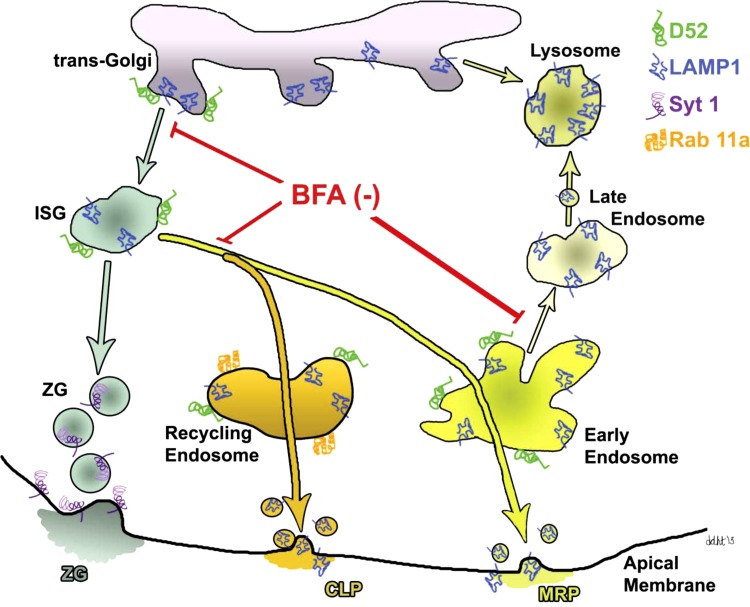
Model of the CLP and MRP in acinar cells. The CLP and MRP originate from immature secretory granules (ISGs) containing lysosomal membrane and content proteins and a fraction of newly synthesized zymogens captured by bulk flow. The CLP enters Rab11a-positive recycling endosomes prior to secretion. The MRP is activated by secretagogues and involves Rab5- and EEA1-positive early endosomes and may also include late endosomes where LAMP1 is most concentrated. BFA was previously shown to inhibit vesicle trafficking from trans-Golgi, ISGs, and endosomes to lysosomes (49). Although not indicated, these pathways are necessary for the trafficking and recycling of important regulatory proteins on the apical membrane necessary for ZG exocytosis.
RESULTS
D52 is present within apical endolysosomal compartments in acini.
We previously showed by colocalization and subcellular fractionation that D52 is absent from purified ZGs and is present in light microsomal fractions containing endosomal and trans-Golgi markers (70). Under basal conditions, D52 localizes to supranuclear regions of cytoplasm and rapidly accumulates within apical regions following 2-min stimulation with CCK-8 (Fig. 1A). Within supranuclear regions, D52 colocalizes with the trans-Golgi marker TGN38, whereas in more apical regions it extensively colocalizes with EEA1 and MAL2 (Fig. 1B). Similar to its localization when expressed in CHO-K1 cells (68), D52 also significantly colocalizes with the clathrin adaptor complex AP3 present on ISGs and early endosomes and LAMP1 present on late endosomes (Fig. 1B). Quantification of fluorescence colocalization for AP3 or LAMP1 revealed 48 ± 5 and 49 ± 10% overlap of each protein with D52, respectively. The inverse analysis showed D52 colocalized with 18 ± 2 and 21 ± 3% (means and SE, n = 10) of each marker, indicating that D52 resides in multiple compartments.
The presence of D52 on endosomes (and not ZGs), yet having acute effects on ZG secretion, indicates that it could translocate to the apical membrane to modulate ZG exocytosis. To examine this, D52 was localized with actin filaments located immediately beneath the apical membrane (Fig. 2A). Following 2-min CCK-8 stimulation, D52 was present along the cytoplasmic edge of actin filaments, but absent from the apical membrane. Previous studies using various antibodies have demonstrated that VAMP2-positive ZGs are present within the most apical aspects of the acinar cytoplasm (7, 19, 20, 73) and rapidly accumulate within apical actin filaments and on apical plasma membranes following CCK-8 stimulation (73). In stimulated cells, D52 showed only minimal colocalization with VAMP2 in areas confined to the cytoplasmic side of actin filaments (Fig. 2B). Sparse punctuate D52 staining infrequently appeared in the center of the acinar lumen independent of VAMP2 (boxed area). However, examination of reconstructed 3D images showed D52 was actually on the cytoplasmic side of the actin filaments lining the bottom of the acinar lumen and absent from the plasma membrane (Supplemental Movie S1). The absence of D52 on the apical membrane implies that it does not directly regulate ZG exocytosis but could modulate the endosome-derived delivery of proteins needed for ZG exocytosis. Thus we further evaluated the localization of D52 within recycling endosomes, which typically occupy the most apical aspects of epithelia.
Rab11a is a primary component of apical recycling endosomes and plays a pivotal role in formation and maintenance of the apical lumen (46, 75). In acini, Rab11a was detected at the very apical aspects of the cytoplasm and, like D52, was present on the cytoplasmic side of the actin filaments (Fig. 2B). Significant colocalization of Rab11a and D52 on a subset of Rab11a-positive vesicles was clearly detected; however, the majority of each protein occupied separate compartments. Although trace amounts of Rab11a copurify with ZGs (data not shown) (13), the absence of D52 on these organelles indicates that colocalization was occurring within endosomes. Colocalization of Rab11a and VAMP2 was precluded because both were detected with monoclonal antibodies. These findings indicate that during physiological stimulation, D52 accumulates in subapical compartments composed of early and late endosomes as well as Rab11a-positive recycling endosomes. Having identified a dynamic localization of D52 within apical endosomal compartments immediately following cell stimulation, we initiated studies to further evaluate the role of D52 in digestive enzyme secretion.
D52 regulates acinar amylase secretion.
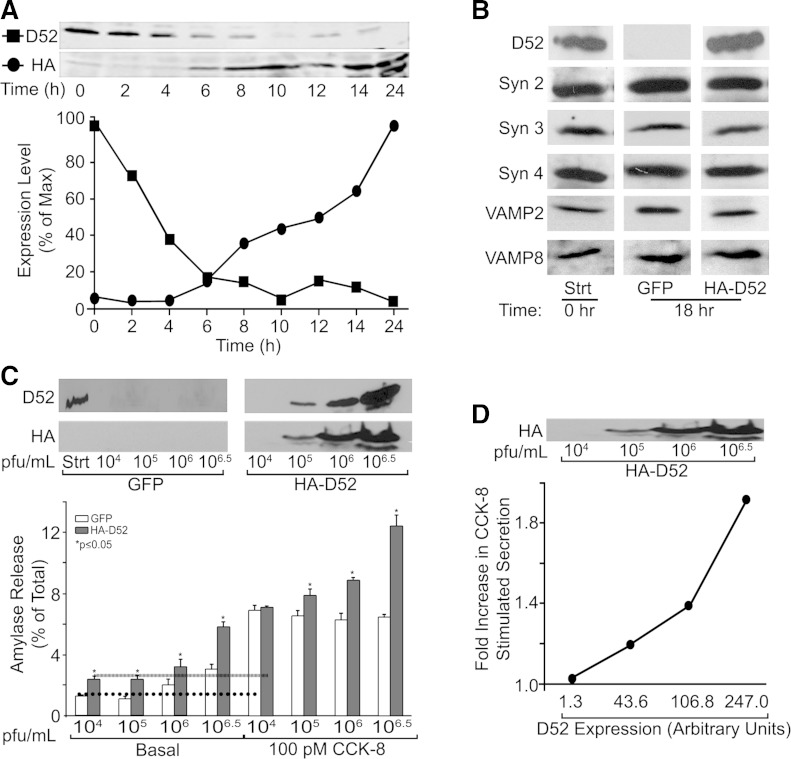
A gradual (∼2–24 h) loss of acinar cell ZG exocytosis has long been recognized as a consequence of acinar cell culture. Similarly, we observed that endogenous D52 levels rapidly declined to levels 20% of freshly isolated acini by 6 h and were nearly undetectable after 10 h of culture (Fig. 3A). In contrast, after 18 h of culture there was little change in SNARE proteins (syntaxin 2, 3, and 4 and VAMP 2 and 8) that mediate acinar cell secretion (24, 25, 73) (Fig. 3B). To maintain D52 expression, we generated an adenovirus coding for HA-tagged human D52 allowing its rapid expression in cultured acini. HA-D52 expression was detected by 6 h and continued to increase over 24 h. A typically less intense lower molecular weight HA-D52 band was sometimes detected between 14 and 24 h that may be a slightly degraded form of the protein. A similar band is also sometimes seen for endogenous D52 in fresh acini. After 18 h, levels of HA-D52 expression strongly corresponded with an enhancement of basal and CCK-8-stimulated amylase secretion (Fig. 3, C and D). The 18-h time point was chosen because 1) acini retain a stimulated secretory response, 2) minimal endogenous D52 is present, and 3) strong HA-D52 expression was achieved. Incubation of cells with control GFP adenovirus at 104 and 105 pfu/ml had no effects on basal secretion, which was very low (≤1.3% of total). However, at 106 or 106.5 pfu/ml, basal secretion was elevated in GFP controls to 2.0 and 3.1%, respectively. Although basal secretion was mildly elevated, suggesting some cellular stress in response to the higher virus titers, these levels remained on the low side of typical acinar secretory studies. Supporting this, high levels of GFP adenovirus had no effects on CCK-8-stimulated secretion. Expression of HA-D52 doubled basal secretion compared with respective GFP controls at all adenovirus concentrations. Moreover, HA-D52 augmented CCK-8-stimulated secretion in a concentration-dependent manner starting at 105 pfu/ml when HA-D52 was first detectable (Fig. 3D). On the basis of a comparison of endogenous D52 in freshly isolated acini with the adenovirally expressed protein, it was estimated that endogenous levels are achieved when infecting acini with 105-106 pfu/ml adenovirus at 18 h. This is likely a conservative estimate since immunoblotting was conducted with a polyclonal antibody directed against full-length human D52 and therefore potentially of higher affinity for adenovirally expressed human D52 than endogenous rat protein.
Fig. 3.
HA-D52 expression augments acinar secretion. A: acini were cultured 18 h with or without HA-D52 adenovirus (106.5 pfu/ml). Endogenous D52 and HA-D52 levels (50 μg/lane) were quantified by immunoblotting and densitometry. B: the acinar SNARE proteins syntaxin (Syn) and VAMPs were analyzed in freshly prepared acini (0 h) or after 18 h culture with green fluorescent protein (GFP) or HA-D52 adenovirus. Data are representative of 3 separate tissue preparations. C, top: D52 expression in freshly prepared acini (Strt) or following adenoviral expression for 18 h at indicated concentrations. C, bottom: amylase secretion in basal or CCK-8-stimulated acini infected with GFP or HA-D52 adenovirus (18 h). Note HA-D52 significantly augmented basal and CCK-8-stimulated secretion (D) in proportion to its expression levels. Data are means and SE of 3 independent experiments performed in triplicate.
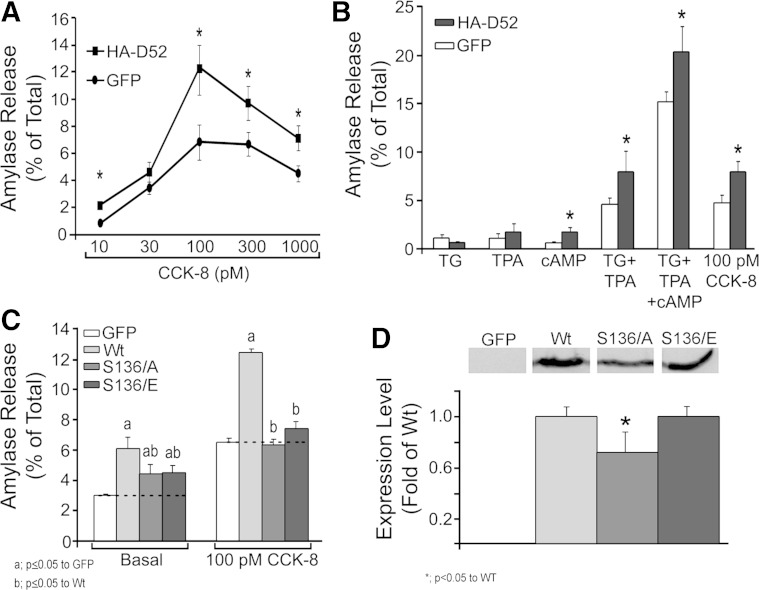
CCK-8 induces a biphasic secretory response with high concentrations of hormone (>100 pM) reducing secretion below maximal responses. HA-D52 expression augmented secretion over a full range of CCK-8 concentrations with a maximal twofold increase at 100 pM (Fig. 4A) and maintained the biphasic secretory response observed in freshly isolated acinar cells. Data in Fig. 4, A and B, represent basal secretion subtracted from stimulated values demonstrating that HA-D52 elevated stimulated secretion independent of its effects on basal. To rule out effects on CCK receptor activation or immediate downstream signaling events that can affect secretion, acini were stimulated with pharmacological agents including TG to elevate cellular Ca2+; phorbol ester (TPA) to mimic diacylglycerol; or CPT-cAMP to activate protein kinase A and EPAC signaling pathways (Fig. 4B). Expression of HA-D52 augmented secretion in response to TG plus TPA to the same extent as CCK-8 but did not significantly enhance secretion in response to TG or TPA alone. In contrast, HA-D52 modestly but significantly enhanced secretion in response to CPT-cAMP, which by itself had minimal effects. Combined incubation with all three agents induced a potentiated response that was augmented by HA-D52 expression. These data demonstrate that HA-D52 effects are not exclusive to a single intracellular signaling pathway and that it likely functions at a step downstream of receptor activation to augment secretion.
Fig. 4.
HA-D52 effects are present with pharmacological stimulation of acini and are dependent on serine 136. A: amylase secretion from cultured acini (18 h) expressing GFP or HA-D52 (106.5 pfu/ml) measured in response to a full range of CCK-8 concentrations (30 min). Basal secretion (GFP = 1.5 ± 0.2%, D52 = 2.4 ± 0.4%) was subtracted from stimulated values. B: cultured acini (18 h) expressing GFP or HA-D52 (106.5) were treated with 2 μM thapsigargin (TG), 1 μM 2-O-tetradecanoylphorbol-13-acetate (TPA), 100 μM 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate (CPT-cAMP), 100 pM CCK-8, or indicated combinations (30 min). Basal secretion [GFP = 1.75 ± 0.2%, HA-D52 wild type (WT) = 3.0 ± 0.4%] was subtracted from stimulated values. C: basal or CCK-8-stimulated amylase secretion in acini expressing GFP, HA-D52 WT, S136A, or S136E mutants (106.5 pfu/ml). D: expression levels of HA-D52 WT, S136A, and 136E following 18 h culture. All experiments are means and SE of 3 independent experiments performed in triplicate.
D52 serine 136 phosphomutants fail to augment stimulated secretion.
D52 has been shown to undergo rapid and transient Ca2+-dependent phosphorylation at serine 136 (S136) in gastric mucosal, colonic T84, HEK293, and CHO-K1 cells (15, 68). Furthermore, S136 phosphorylation was shown to regulate LAMP1 exocytosis and endomembrane trafficking necessary for cytokinesis in CHO-K1 cells (66, 68). We therefore hypothesized that a D52 phospho-null mutant would lack secretagogue-stimulated effects, whereas a phosphomimetic mutant would more fully augment secretion independent of stimulation. Expression of S136A phospho-null or S136E phosphomimetic mutants each augmented basal secretion to approximately half of that seen for HA-D52 WT (Fig. 4C). However, neither mutant was able to augment stimulated secretion. This was unlikely due to protein expression since S136A levels were only 20% lower than WT or S136E, which were equivalent (Fig. 4D). Like endogenous D52, the WT, S136A, and S136E proteins distributed equally to cytosolic and membrane fractions of lysates and had similar localization by immunofluorescence (data not shown). The explanation for the lack of a phosphomimetic effect remains unclear (see discussion), but these results clearly support an important role for S136 in mediating D52 secretory function.
D52 regulates vesicle trafficking through the CLP.
Digestive enzyme secretion through the classic ZG secretory pathway vs. the CLP and MRP can be distinguished by the sensitivity of the CLP and MRP to acute inhibition by BFA (11). We previously reported that BFA reversibly disrupts D52 localization in acinar cells (70). BFA has acute effects on Golgi and endosomal compartments but does not inhibit ZG exocytosis and in fact modestly enhances acinar secretion (9, 33). Consistent with these studies, preincubation of GFP-control acini with BFA for 30 min modestly increased basal and stimulated secretion to the same extent; accordingly, no effect on stimulated secretion was apparent (Fig. 5A). In comparison, BFA completely blocked HA-D52 effects on basal and CCK-8-stimulated secretion. We also examined the ability of HA-D52 to modulate LAMP1 externalization as an indicator of endolysosomal exocytosis, as we reported in CHO-K1 cells (68). Like D52, LAMP1 is not present on purified ZGs, which contain syt1 (Fig. 5D). Incubation of intact GFP-control acini with an antibody that recognizes the intravesicular domain of LAMP1 revealed very low levels of externalized LAMP1 labeling under basal and CCK-8-stimulated (100 pM) conditions (Fig. 5B). Strikingly, HA-D52 expression induced robust LAMP1 externalization along the apical membrane under basal conditions, although CCK-8 stimulation (2 min) did not further enhance LAMP1 intensity. Paralleling the effects of BFA on HA-D52-induced amylase secretion, LAMP1 labeling was strongly inhibited by BFA under basal conditions. Enhanced excitation of LAMP1 fluorescence to identify residual labeling in BFA-treated cells revealed minor residual punctuate staining that was no longer confined to apical regions and was distributed to basolateral regions (Fig. 5C). Because the CLP and MRP control secretion through BFA-sensitive endosomal compartments, these results strongly support a major role for D52 in regulating the CLP.
HA-D52 expression maintains expression and apical polarity of endolysosomal proteins in cultured acini.
Mechanically dissected pancreatic lobules were infected with GFP or HA-D52 to evaluate the effects of HA-D52 expression on acinar morphology (Fig. 6A). Lobules were used because they better maintain acinar structure in culture and have greater stability during fixation and sectioning than isolated acini. As seen for endogenous D52 (see Fig. 3), GFP-expressing lobules (18 h) showed markedly reduced levels of EEA1 and LAMP1 and highly dispersed punctate labeling of Rab11a, and, to a lesser extent, GRAMP92 throughout apical and basal regions of acinar cells, indicating a loss of apical endosomal polarity (compare Figs. 1 and 6). Remarkably, HA-D52 expression prevented much of the loss of EEA1 and LAMP1 and eliminated dispersal of the endosomal compartments to basal cytoplasm. Indeed, LAMP1, EEA1, Rab11a, and GRAMP92 all retained a compact organization within the apical cytoplasm following HA-D52 expression. In contrast to endogenous D52, which showed more limited codistribution with Rab11a (27.1 ± 0.03% colocalization of voxels n = 9), when more highly expressed, HA-D52 demonstrated markedly enhanced colocalization with Rab11a (64.0 ± 0.12% colocalization of voxels n = 9). Immunoblotting confirmed that GRAMP92 and Rab11a levels remained stable during acinar culturing, whereas EEA1, LAMP1, and an additional early endosomal marker Rab5 were greatly diminished. Adenovirally driving HA-D52 expression prevented more than 50% of the loss of EEA1 and LAMP1 and induced the expression of Rab5 to levels comparable to HA-D52 (Fig. 6B).
Endolysosomal exocytosis in freshly prepared acini.
To confirm the presence of the endolysosomal secretory pathway under more physiological conditions, LAMP1 surface labeling was conducted in freshly prepared acini, which express endogenous D52 and other endosomal regulatory proteins. Unlike HA-D52 expression in cultured acini, which mainly enhanced basal and not CCK-8-stimulated LAMP1 surface labeling, LAMP1 labeling was enhanced eightfold following a 2-min treatment with CCK-8 (100 pM). Minimal LAMP1 labeling was detected under basal conditions. Interestingly, 30 min pretreatment with BFA prior to stimulation with 100 pM CCK-8 resulted in highly dispersed LAMP1 externalization extending from apical to lateral and basal aspects of acinar cells but did not reduce total LAMP1 fluorescence, which remained as high as that seen for 100 pM CCK-8 alone (Fig. 7B).
To identify sites of ZG exocytosis under these conditions colocalization studies were conducted by surface labeling syt1 and LAMP1. We previously demonstrated that ZG exocytosis can be analyzed in acinar cell whole preparations by surface labeling with an antibody that recognizes the ZG luminal domain of syt1 (see Fig. 5D), which is externalized upon acinar stimulation and ZG exocytosis (20). Analysis of CCK-8-stimulated acini viewed in a reconstructed 3D format shows incomplete overlap of the syt1- and LAMP1-associated fluorophores in apical regions of acini (Fig. 7C). Notably, LAMP1 labeling was more expanded than syt1. The incomplete overlap of externalized LAMP1 and syt1, together with the presence of syt1 in purified ZGs and absence of LAMP1 in these organelles (see Fig. 5D), clearly supports their presence in separate secretory compartments, ZGs, and endolysosomally derived vesicles, respectively.
DISCUSSION
This work delineates an important functional role for D52, which is uniquely expressed in specialized cells containing large secretory granules, in regulating post-Golgi trafficking and secretion from endolysosomal compartments. Results indicate that the CLP and MRP are integrated within an apical endolysosomal system containing D52, EEA1, Rab5, and LAMP1 as well as Rab11a-positive recycling endosomes (Fig. 8), the latter of which has been shown to play an essential role in development and maintenance of epithelial polarity (46). Adenovirally driving HA-D52 expression in cultured acini, which rapidly lose endogenous D52, augmented both basal and stimulated amylase secretion in a BFA-sensitive manner, supporting that the CLP/MRP plays a role in promoting ZG exocytosis. Findings that HA-D52 expression strongly enhanced constitutive LAMP1 surface labeling in cultured acini together with our identification of constitutive and CCK-8-stimulated LAMP1 exocytosis in freshly isolated acini provides new evidence that the CLP and MRP represent apically directed endolysosomal secretory pathways. Importantly, the molecular identification of these pathways begins to shed light on the relationship between lysosomal and zymogen protein trafficking in acinar cells, which has important implications to pancreatic disease (see Potential significance to pancreatic disease below).
The CLP and MRP mediate lysosome-like secretion in acini.
Like D52, LAMP1 immunoreactivity was not detected in purified ZGs, nor has either protein been reported in proteomic studies of ZG membranes (5, 13, 56). The appearance of LAMP1 on the apical membrane of freshly prepared acini and its robust regulation by CCK-8 is similar to results seen in other cells of hematopoietic lineage and melanocytes, which contain specialized lysosome-related secretory organelles (18). Furthermore, lysosome-like secretory organelles are also present in nonspecialized cultured cells (47).
Trafficking of many lysosomal membrane proteins, including LAMP1, is regulated by AP3, which functions together with BFA-sensitive ARF1 in coated vesicle formation at trans-Golgi and post-Golgi compartments (55, 58). Indeed, secretory granule maturation is both inhibited by BFA (22, 36, 59) and dependent on AP3 expression in PC12 cells (33). We previously demonstrated that D52 is associated with ISGs (also called condensing vacuoles) in acini (70). In agreement with the strong colocalization of D52 with AP3 in acinar cells (Supplemental Fig. S1) and CHO-K1 cells (68), D52 was previously identified in proteomic screens of AP3-derived clathrin coated vesicles from HeLa (4) and PC12 cells (61), although we have not been able to demonstrate a clear interaction of D52 with AP3 subunits in vitro.
In comparison to LAMPs, mannose-6 phosphate-bearing lysosomal hydrolases are removed from trans-Golgi and ISGs in pancreatic acini by AP1-derived clathrin-coated vesicles (40). A number of studies have shown the presence of cathepsin B in the acinar ZG secretory pathway as well as its apical secretion in response to secretagogue stimulation (34, 42, 71), indicating a significant fraction of the immature enzyme escapes removal from ISGs. Similarly, β-hexosaminidase has also been shown in pancreatic secretions (34). However, the relative contribution of CLP/MRP vs. ZG secretion of these enzymes has not been investigated. Interestingly, early studies utilizing cytochemical labeling of acid hydrolases combined with electron microscopy demonstrated an apically directed lysosomal secretory pathway that originates from the periphery of ISGs and functions in parallel to the classic ZG pathway in pancreatic acinar cells (30). We propose that this pathway likely represents the CLP and MRP.
BFA inhibition of the CLP and MRP.
In GFP-control cultured acini, little or no LAMP1 surface labeling was detected owing to the lack of LAMP1 expression and loss of endolysosomal compartments. Conversely, robust constitutive LAMP1 surface labeling was seen in HA-D52-expressing acini and this was strongly inhibited by BFA, suggesting that the CLP originates early on at the level of the trans-Golgi or ISGs. Conversely, in freshly prepared acini where constitutive LAMP1 exocytosis was minimal, BFA did not inhibit CCK-8-stimulated LAMP1 exocytosis, but rather extensively expanded its surface labeling to lateral and basal regions of acini. The original studies identifying the MRP as being sensitive to BFA utilized pulse labeling of secretory proteins, which are rapidly discharged from cells treated with secretagogue (35). Moreover, the timing of BFA addition to pulse-labeled cells was critical to detect inhibition of MRP discharge. Therefore, the BFA resistance of LAMP1 surface labeling seen here at steady state indicates that the LAMP1 secretory compartment remained intact following BFA treatment but presumably had not filled with newly synthesized cargo.
Similar to our results, in polarized MDCK cells, BFA was shown to acutely redirect the apical membrane protein dipeptidyl peptidase IV from trans-Golgi to basal membranes rather than its normal apical targeting (50). BFA treatment has been shown to induce extensive tubulation of early and late endosomal compartments (49) as well as Rab11a-positive recycling endosomes, which express the BFA-inhibited guanine nucleotide exchange factor BIG2 (64). Thus it is possible that BFA-induced tubulation of apical endosomal compartments could promote LAMP1 exocytosis at more lateral and basal regions of the cell following CCK-8 stimulation.
Interplay between the CLP/MRP and the ZG secretory pathway.
HA-D52 expression only enhanced basal but not CCK-8-stimulated LAMP1 exocytosis (see Fig. 5), yet it strongly increased CCK-8-stimulated amylase secretion (with basal subtracted) by roughly twofold of GFP controls (Fig. 4A). Evidence that both the LAMP1 and amylase secretory effects seen with HA-D52 expression were acutely blocked by BFA pretreatment supports that the enhancement of CLP trafficking indirectly augmented secretion from the ZG pathway, as has been proposed (9). However, stimulated secretion was not fully eliminated with a loss of the endolysosomal regulatory proteins, suggesting that this pathway may play an ancillary role in ZG exocytosis, although further studies are needed to analyze a potential role for D52 in ZG formation and maturation. Other studies of the secretory mechanisms in pancreatic and parotid acinar cells may be relevant to our observations.
In parotid acini, the earliest stage of secretion was shown microscopically to involve a large expansion of the apical membrane and loss of microvilli that is independent of ZG exocytosis (35, 52). Pulse-chase labeling of secretory proteins further demonstrated that the MRP is discharged within the first 3–5 min of acinar stimulation and is independent of ZG exocytosis (9). Supporting this, membrane capacitance studies in both pancreatic and parotid acini revealed an early immediate phase of secretion and apical membrane expansion thought to involve small vesicles <50 nm in diameter, but not individual ZG exocytotic events (14). Finally, Castle et al. (9) further provided evidence that this early phase activation inserts the regulatory t-SNARE syntaxin 3 into the apical membrane, which may then act as a receptor site for ZG exocytosis. However, analysis of t-SNARE translocation to the acinar plasma membrane is difficult to interpret because syntaxins 2, 3, and 4 have all been implicated in ZG exocytosis (23, 73). Further complicating this analysis is that, under basal conditions, syntaxin 3 is present on ZGs, endosomes, and plasma membrane, and syntaxin 4 is on both basolateral and apical membranes. Syntaxin 2 is mainly on the apical membrane and interacts with VAMP2 in vitro, but a clear secretory function for this protein is lacking (24, 32, 73). Nonetheless, studies in various secretory cells have clearly established an important role for the endosomal recycling and/or delivery of SNAREs and other regulatory proteins to the plasma membrane that are necessary for secretory vesicle docking, priming, and fusion (3, 37, 41, 57, 60).
In comparison to the above studies implicating mainly the early discharge of the MRP in enhancing ZG secretion, the present data support that enhanced CLP discharge by HA-D52 expression has a similar effect. These results may reflect high expression levels of adenovirally expressed HA-D52. We conservatively estimate that endogenous D52 levels were achieved at 18 h when using between 105 and 106 pfu/ml (see Fig. 3C and accompanying text), whereas most studies were conducted with 106.5. Compared with the twofold enhancement in secretion seen at 106.5, 106 enhanced secretion by 1.4-fold (see Fig. 3D). Interestingly, we previously reported that low-level D52 expression in CHO-K1 cells was necessary to detect Ca2+-dependent effects on LAMP1 exocytosis since high levels mainly enhanced basal exocytosis. Similar attempts to titrate back adenovirus in acini were less definitive at clearly showing an effect of HA-D52 on the MRP. Thus it is unlikely that rescuing HA-D52 expression to physiological levels will fully reverse the secretory rundown of cultured acini; however, evidence of the loss of endolysosomal proteins during culture and the restoration of these proteins (and presumably their respective compartments) with HA-D52 expression plays an important role in secretory rundown may prove highly significant to understanding the mechanisms of acinar secretory function.
D52 phosphorylation.
D52 phosphorylation is Ca2+ dependent and potentially mediated by the δ6-isoform of CaM kinase II (15, 38). In acinar cells, D52 phosphorylation is maximal within 2 min of secretagogue stimulation and then rapidly declines by 15 min (38–39). When expressed in CHO-K1 cells, the S136A phospho-null mutant enhanced basal but failed to augment Ca2+-stimulated LAMP1 exocytosis, whereas S136E greatly enhanced it independent of elevated Ca2+ (68). The S136A mutant also strongly inhibited endomembrane trafficking necessary for cytokinesis in CHO-K1 cells, whereas WT and phosphomimetic mutants were much less effective (66). These studies indicate that the D52 phosphomutants retain some basal activity but phosphorylation is required for full activation. On the basis of these results, the S136E phosphomimetic mutant was anticipated to augment amylase secretion above basal levels independent of stimulation, but failed to do so. The lack of this response could reflect the transient nature of D52 phosphorylation, which may indicate an activation-deactivation cycle for its function. Alternatively, prolonged 18-h expression of a phosphomimetic could alter the overall activity of the secretory pathway, making it refractory to secretagogue stimulation. Finally, glutamate has a smaller acidic side chain than the bulky phosphoserine and does not accurately reproduce structural changes occurring in all phosphorylated proteins (16). Nevertheless, the loss of secretagogue-stimulated secretion upon S136 mutation clearly supports a pivotal role for serine 136 in mediating D52 secretory function.
Loss of endolysosomal regulatory protein expression during acinar culture.
It is well established that acinar cells cultured on plastic undergo a marked reduction in secretory activity together with a loss of cellular polarity. Intriguingly, along with endogenous D52, we observed a profound loss of expression of the endolysosomal proteins EEA1, Rab5, and LAMP1 during 18 h acinar culture, which for EEA1 and LAMP1 was partially prevented by expression of HA-D52, and in the case of Rab5, induced expression to levels similar to HA-D52. Remarkably, there was no loss of the SNARE proteins required for ZG exocytosis. The mechanism responsible for the selective loss of endolysosomal proteins during acinar cell culture as well as the dramatic effects of HA-D52 on inducing the expression of these proteins, especially Rab5, is currently under further investigation. Our findings suggest that the reduction in membrane trafficking within the CLP, MRP, and/or endolysosomal compartments during acinar culture likely plays an important role in the overall loss in acinar secretory function.
Potential significance to pancreatic disease.
Identification of the CLP/MRP as an endolysosome-like secretory pathway may provide valuable insight into the pathobiology of pancreatic disease. The onset of acute pancreatitis involves the premature activation of proteolytic zymogens by lysosomal hydrolases in a LAMP-1-positive endolysosomal compartment (17). Moreover, evidence that the CLP and MRP are likely involved in the maturation of lysosomes may prove highly significant as abnormalities in lysosomal function needed for cell autophagy have been tied to the onset and progression of acute pancreatitis (31). Finally, demonstration that D52 mediates trafficking through Rab11a-positive recycling endosomes may be highly significant as this compartment plays an integral role in maintaining epithelial polarity (46), the loss of which has been implicated in both the onset of pancreatitis (23) and progression of pancreatic cancer (63).
GRANTS
G. E. Groblewski was supported by NIH DK07088 and USDA/HATCH WIS01583 and F. S. Gorelick by NIDDK RO1 (DK-54021), NIAAAR21 (AA020847), and a Veterans Administration Merit Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.W.M., D.D.T., J.A.B., F.S.G., and G.E.G. conception and design of research; S.W.M., D.D.T., and M.A.F. performed experiments; S.W.M., D.D.T., F.S.G., and G.E.G. analyzed data; S.W.M., D.D.T., J.A.B., F.S.G., and G.E.G. interpreted results of experiments; S.W.M. and D.D.T. prepared figures; S.W.M. drafted manuscript; S.W.M., J.A.B., F.S.G., and G.E.G. edited and revised manuscript; S.W.M., D.D.T., M.A.F., J.A.B., F.S.G., and G.E.G. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Arvan P, Castle JD. Phasic release of newly synthesized secretory proteins in the unstimulated rat exocrine pancreas. J Cell Biol 104: 243–252, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvan P, Halban PA. Sorting ourselves out: seeking consensus on trafficking in the beta-cell. Traffic 5: 53–61, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Benado A, Nasagi-Atiya Y, Sagi-Eisenberg R. Protein trafficking in immune cells. Immunobiology 214: 403–421, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. Comparative proteomics of clathrin-coated vesicles. J Cell Biol 175: 571–578, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borta H, Aroso M, Rinn C, Gomez-Lazaro M, Vitorino R, Zeuschner D, Grabenbauer M, Amado F, Schrader M. Analysis of low abundance membrane-associated proteins from rat pancreatic zymogen granules. J Proteome Res 9: 4927–4939, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Boutros R, Fanayan S, Shehata M, Byrne JA. The tumor protein D52 family: many pieces, many puzzles. Biochem Biophys Res Commun 325: 1115–1121, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Braun JE, Fritz BA, Wong SM, Lowe AW. Identification of a vesicle-associated membrane protein (VAMP)-like membrane protein in zymogen granules of the rat exocrine pancreas. J Biol Chem 269: 5328–5335, 1994. [PubMed] [Google Scholar]
- 8.Byrne JA, Tomasetto C, Garnier JM, Rouyer N, Mattei MG, Bellocq JP, Rio MC, Basset P. A screening method to identify genes commonly overexpressed in carcinomas and the identification of a novel complementary DNA sequence. Cancer Res 55: 2896–2903, 1995. [PubMed] [Google Scholar]
- 9.Castle AM, Huang AY, Castle JD. The minor regulated pathway, a rapid component of salivary secretion, may provide docking/fusion sites for granule exocytosis at the apical surface of acinar cells. J Cell Sci 115: 2963–2973, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Castle JD. Sorting and secretory pathways in exocrine cells. Am J Respir Cell Mol Biol 2: 119–126, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Castle JD, Castle AM. Two regulated secretory pathways for newly synthesized parotid salivary proteins are distinguished by doses of secretagogues. J Cell Sci 109: 2591–2599, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Ernst SA, Williams JA. Dominant negative Rab3D mutants reduce GTP-bound endogenous Rab3D in pancreatic acini. J Biol Chem 278: 50053–50060, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics 5: 306–312, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Warner JD, Yule DI, Giovannucci DR. Spatiotemporal analysis of exocytosis in mouse parotid acinar cells. Am J Physiol Cell Physiol 289: C1209–C1219, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Chew CS, Chen X, Zhang H, Berg EA. Calcium/calmodulin-dependent phosphorylation of tumor protein D52 on serine residue 136 may be mediated by CAMK2δ6. Am J Physiol Gastrointest Liver Physiol 295: G1159–G1172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem 278: 13061–13068, 2003. [DOI] [PubMed] [Google Scholar]
- 17.De Lisle RC. Altered posttranslational processing of glycoproteins in cerulein-induced pancreatitis. Exp Cell Res 308: 101–113, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J 14: 1265–1278, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Falkowski MA, Thomas DD, Groblewski GE. Complexin 2 modulates vesicle-associated membrane protein (VAMP) 2-regulated zymogen granule exocytosis in pancreatic acini. J Biol Chem 285: 35558–35566, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falkowski MA, Thomas DD, Messenger SW, Martin TF, Groblewski GE. Expression, localization and functional role for synaptotagmins in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 301: (2) G306–G316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanayan S, Shehata M, Agterof AP, McGuckin MA, Alonso MA, Byrne JA. Mucin 1 (MUC1) is a novel partner for MAL2 in breast carcinoma cells. BMC Cell Biol 10: 7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez CJ, Haugwitz M, Eaton B, Moore HP. Distinct molecular events during secretory granule biogenesis revealed by sensitivities to brefeldin A. Mol Biol Cell 8: 2171–2185, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaisano HY. A hypothesis: SNARE-ing the mechanisms of regulated exocytosis and pathologic membrane fusions in the pancreatic acinar cell. Pancreas 20: 217–226, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell 7: 2019–2027, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology 136: 2040–2044, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Giblin JP, Hewlett LJ, Hannah MJ. Basal secretion of von Willebrand factor from human endothelial cells. Blood 112: 957–964, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Grimes M, Kelly RB. Intermediates in the constitutive and regulated secretory pathways released in vitro from semi-intact cells. J Cell Biol 117: 539–549, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groblewski GE, Wishart MJ, Yoshida M, Williams JA. Purification and identification of a 28-kDa calcium-regulated heat-stable protein. A novel secretagogue-regulated phosphoprotein in exocrine pancreas. J Biol Chem 271: 31502–31507, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Groblewski GE, Yoshida M, Yao H, Williams JA, Ernst SA. Immunolocalization of CRHSP28 in exocrine digestive glands and gastrointestinal tissues of the rat. Am J Physiol Gastrointest Liver Physiol 276: G219–G226, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Grondin G, Beaudoin AR. Immunocytochemical and cytochemical demonstration of a novel selective lysosomal pathway (SLP) of secretion in the exocrine pancreas. J Histochem Cytochem 44: 357–368, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, Gukovskaya AS. Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol 27, Suppl 2: 27–32, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen NJ, Antonin W, Edwardson JM. Identification of SNAREs involved in regulated exocytosis in the pancreatic acinar cell. J Biol Chem 274: 22871–22876, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Hendricks LC, McClanahan SL, Palade GE, Farquhar MG. Brefeldin A affects early events but does not affect late events along the exocytic pathway in pancreatic acinar cells. Proc Natl Acad Sci USA 89: 7242–7246, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano T, Saluja A, Ramarao P, Lerch MM, Saluja M, Steer ML. Apical secretion of lysosomal enzymes in rabbit pancreas occurs via a secretagogue regulated pathway and is increased after pancreatic duct obstruction. J Clin Invest 87: 865–869, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang AY, Castle AM, Hinton BT, Castle JD. Resting (basal) secretion of proteins is provided by the minor regulated and constitutive-like pathways and not granule exocytosis in parotid acinar cells. J Biol Chem 276: 22296–22306, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Huang XF, Arvan P. Formation of the insulin-containing secretory granule core occurs within immature beta-granules. J Biol Chem 269: 20838–20844, 1994. [PubMed] [Google Scholar]
- 37.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol 182: 355–366, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaspar KM, Thomas DD, Taft WB, Takeshita E, Weng N, Groblewski GE. CaM kinase II regulation of CRHSP-28 phosphorylation in cultured mucosal T84 cells. Am J Physiol Gastrointest Liver Physiol 285: G1300–G1309, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Kaspar KM, Thomas DD, Weng N, Groblewski GE. Dietary and hormonal stimulation of rat exocrine pancreatic function regulates CRHSP-28 phosphorylation in vivo. J Nutr 133: 3072–3075, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol 141: 359–371, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch M, Holt M. Coupling exo- and endocytosis: an essential role for PIP(2) at the synapse. Biochim Biophys Acta 1821: 1114–1132, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Kukor Z, Mayerle J, Kruger B, Toth M, Steed PM, Halangk W, Lerch MM, Sahin-Toth M. Presence of cathepsin B in the human pancreatic secretory pathway and its role in trypsinogen activation during hereditary pancreatitis. J Biol Chem 277: 21389–21396, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Kuliawat R, Arvan P. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol 118: 521–529, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuliawat R, Klumperman J, Ludwig T, Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J Cell Biol 137: 595–608, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kzhyshkowska J, Krusell L. Cross-talk between endocytic clearance and secretion in macrophages. Immunobiology 214: 576–593, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Lapierre LA, Avant KM, Caldwell CM, Oztan A, Apodaca G, Knowles BC, Roland JT, Ducharme NA, Goldenring JR. Phosphorylation of Rab11-FIP2 regulates polarity in MDCK cells. Mol Biol Cell 23: 2302–2318, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laulagnier K, Schieber NL, Maritzen T, Haucke V, Parton RG, Gruenberg J. Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes. Mol Biol Cell 22: 2068–2082, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laurie SM, Mixon MB, Brand SH, Castle JD. A secretion granule membrane protein (GRAMP 92) is found in non-granule membranes including those of the endocytic pathway. Eur J Cell Biol 58: 12–27, 1992. [PubMed] [Google Scholar]
- 49.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67: 601–616, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Low SH, Tang BL, Wong SH, Hong W. Selective inhibition of protein targeting to the apical domain of MDCK cells by brefeldin A. J Cell Biol 118: 51–62, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madrid R, Aranda JF, Rodriguez-Fraticelli AE, Ventimiglia L, Andres-Delgado L, Shehata M, Fanayan S, Shahheydari H, Gomez S, Jimenez A, Martin-Belmonte F, Byrne JA, Alonso MA. The formin INF2 regulates basolateral-to-apical transcytosis and lumen formation in association with Cdc42 and MAL2. Dev Cell 18: 814–827, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci USA 108: 13552–13557, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palade G. Intracellular aspects of the process of protein synthesis. Science 189: 347–358, 1975. [DOI] [PubMed] [Google Scholar]
- 54.Parente JA, Goldenring JR, Petropoulos AC, Hellman U, Chew CS. Purification, cloning, and expression of a novel, endogenous, calcium-sensitive, 28-kDa phosphoprotein. J Biol Chem 271: 20096–20101, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol 164: 1065–1076, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rindler MJ, Xu CF, Gumper I, Smith NN, Neubert TA. Proteomic analysis of pancreatic zymogen granules: identification of new granule proteins. J Proteome Res 6: 2978–2992, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizzoli SO, Bethani I, Zwilling D, Wenzel D, Siddiqui TJ, Brandhorst D, Jahn R. Evidence for early endosome-like fusion of recently endocytosed synaptic vesicles. Traffic 7: 1163–1176, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol 13: 444–453, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Rosa P, Barr FA, Stinchcombe JC, Binacchi C, Huttner WB. Brefeldin A inhibits the formation of constitutive secretory vesicles and immature secretory granules from the trans-Golgi network. Eur J Cell Biol 59: 265–274, 1992. [PubMed] [Google Scholar]
- 60.Rozas JL, Gomez-Sanchez L, Mircheski J, Linares-Clemente P, Nieto-Gonzalez JL, Vazquez ME, Lujan R, Fernandez-Chacon R. Motorneurons require cysteine string protein-alpha to maintain the readily releasable vesicular pool and synaptic vesicle recycling. Neuron 74: 151–165, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Salazar G, Craige B, Wainer BH, Guo J, De Camilli P, Faundez V. Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles. Mol Biol Cell 16: 3692–3704, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sathasivam P, Bailey AM, Crossley M, Byrne JA. The role of the coiled-coil motif in interactions mediated by TPD52. Biochem Biophys Res Commun 288: 56–61, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Shi G, Direnzo D, Qu C, Barney D, Miley D, Konieczny SF. Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene 32: 1950–1958, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shin HW, Morinaga N, Noda M, Nakayama K. BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol Biol Cell 15: 5283–5294, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanley AC, Lieu ZZ, Wall AA, Venturato J, Khromykh T, Hamilton NA, Gleeson PA, Stow JL. Recycling endosome-dependent and -independent mechanisms for IL-10 secretion in LPS-activated macrophages. J Leukoc Biol 92: 1227–1239, 2012. [DOI] [PubMed] [Google Scholar]
- 66.Thomas DD, Frey CL, Messenger SW, August BK, Groblewski GE. A role for tumor protein TPD52 phosphorylation in endo-membrane trafficking during cytokinesis. Biochem Biophys Res Commun 402: 583–587, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas DD, Kaspar KM, Taft WB, Weng N, Rodenkirch LA, Groblewski GE. Identification of annexin VI as a Ca2+-sensitive CRHSP-28-binding protein in pancreatic acinar cells. J Biol Chem 277: 35496–35502, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Thomas DD, Martin CL, Weng N, Byrne JA, Groblewski GE. Tumor protein D52 expression and Ca2+-dependent phosphorylation modulates lysosomal membrane protein trafficking to the plasma membrane. Am J Physiol Cell Physiol 298: C725–C739, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas DD, Taft WB, Kaspar KM, Groblewski GE. CRHSP-28 regulates Ca2+-stimulated secretion in permeabilized acinar cells. J Biol Chem 276: 28866–28872, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Thomas DD, Weng N, Groblewski GE. Secretagogue-induced translocation of CRHSP-28 within an early apical endosomal compartment in acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G253–G263, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Tooze J, Hollinshead M, Hensel G, Kern HF, Hoflack B. Regulated secretion of mature cathepsin B from rat exocrine pancreatic cells. Eur J Cell Biol 56: 187–200, 1991. [PubMed] [Google Scholar]
- 72.von Zastrow M, Castle JD. Protein sorting among two distinct export pathways occurs from the content of maturing exocrine storage granules. J Cell Biol 105: 2675–2684, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weng N, Thomas DD, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J Biol Chem 282: 9635–9645, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Wilson SH, Bailey AM, Nourse CR, Mattei MG, Byrne JA. Identification of MAL2, a novel member of the mal proteolipid family, though interactions with TPD52-like proteins in the yeast two-hybrid system. Genomics 76: 81–88, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Winter JF, Hopfner S, Korn K, Farnung BO, Bradshaw CR, Marsico G, Volkmer M, Habermann B, Zerial M. Caenorhabditis elegans screen reveals role of PAR-5 in RAB-11-recycling endosome positioning and apicobasal cell polarity. Nat Cell Biol 14: 666–676, 2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.