Abstract
The CFTR High Expresser (CHE) cells express eightfold higher levels of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel compared with neighboring enterocytes and were first identified by our laboratory (Ameen et al., Gastroenterology 108: 1016, 1995). We used double-label immunofluorescence microscopy to further study these enigmatic epithelial cells in rat intestine in vivo or ex vivo. CHE cells were found in duodenum, most frequent in proximal jejunum, and absent in ileum and colon. CFTR abundance increased in CHE cells along the crypt-villus axis. The basolateral Na+K+Cl− cotransporter NKCC1, a key transporter involved in Cl− secretion, was detected at similar levels in CHE cells and neighboring enterocytes at steady state. Microvilli appeared shorter in CHE cells, with low levels of Myosin 1a, a villus enterocyte-specific motor that retains sucrase/isomaltase in the brush-border membrane (BBM). CHE cells lacked alkaline phosphatase and absorptive villus enterocyte BBM proteins, including Na+H+ exchanger NHE3, Cl−/HCO3− exchanger SLC26A6 (putative anion exchanger 1), and sucrase/isomaltase. High levels of the vacuolar-ATPase proton pump were observed in the apical domain of CHE cells. Levels of the NHE regulatory factor NHERF1, Na-K-ATPase, and Syntaxin 3 were similar to that of neighboring enterocytes. cAMP or acetylcholine stimulation robustly increased apical CFTR and basolateral NKCC1 disproportionately in CHE cells relative to neighboring enterocytes. These data strongly argue for a specialized role of CHE cells in Cl−-mediated “high-volume” fluid secretion on the villi of the proximal small intestine.
Keywords: cystic fibrosis transmembrane conductance regulator, Na+K+Cl− cotransporter 1, camp-regulated traffic, acetylcholine, villus enterocyte
the cystic fibrosis conductance regulator (CFTR) chloride (Cl−) channel is critical to fluid secretion in epithelial cells. In crypt cells throughout the small and large intestine, and in small intestinal villus enterocytes, CFTR is present on the apical membrane and subapical recycling endosomes and is recruited to the apical membrane by cAMP and Ca2+ agonists (2, 7, 28, 30). The prolonged increase of active CFTR channels at the membrane leads to excessive outward movement of water and the anions Cl− and HCO3− into the lumen, resulting in secretory diarrhea (5, 20). Mutated or absent CFTR leads to cystic fibrosis, altered luminal pH, and mucoviscidosis (16–18, 21, 24). The cellular distribution of CFTR is heterogeneous and consistent with the observations that mechanisms of CFTR regulation are tissue and cell specific (14–15, 23, 37, 47).
Our laboratory identified a subpopulation (∼2.5%) of epithelial cells with enterocyte-like morphological features in rat and human small intestine that express very high levels of apical membrane CFTR in association with a prominent cytoplasmic vesicle pool, and we named these cells CFTR High Expresser (CHE) cells (4). This prominent subapical vesicular compartment was shown to be critical for agonist-dependent CFTR vesicle recruitment in CHE cells (6). In duodenal sections, the mean apical CFTR fluorescence intensity (FI) in CHE cells was found to be approximately eightfold higher compared with that for neighboring crypt cells, indicating that CHE cells express ∼30% of total duodenal apical surface CFTR under basal conditions in the rat (6). The eightfold higher CFTR content in the CHE cells compared with other CFTR-expressing cells suggested that CHE cells possess the capacity for intense secretory activity. The CHE cells present a challenge for conducting functional investigations because of their distribution, low abundance, and lack of cellular markers to facilitate isolation/enrichment.
The present study further elucidates morphological and cell biological features to aid our understanding of the functional role of the CHE cells in the rodent intestine. The study aimed to address specific questions: What is the distribution pattern of the CHE cells? Are there unique markers that support functional specificity of the CHE cells? Are there molecular components that are unique to this intestinal cell type, or differential expression of molecular markers in CHE cells? Is the Na+K+/Cl− cotransporter NKCC1, a key transporter involved in Cl− secretory function at the basolateral membrane of epithelial cells, present in CHE cells? How does secretory agonist stimulation (cAMP or the Ca2+ agonist acetylcholine, ACh) alter the transporter trafficking in CHE cells compared with neighboring enterocytes? What characteristics are shared by CHE and non-CHE cells? The distribution profiles of CFTR, NKCC1, Na+/H+ exchanger 3 (NHE3), Cl−/HCO3− exchanger SLC26A6 (i.e., putative anion exchanger 1, PAT1), Vacuolar-ATPase (V-ATPase), Myosin 1a, intestinal alkaline phosphatase (ALP), Na+/H+ exchanger regulatory factor 1 (NHERF-1), Na-K-ATPase, and Syntaxin 3 were examined in detail in the CHE cells in double immunolabeling experiments. The data provide further support indicating a specialized role for CHE cells in Cl−-mediated “high-volume” fluid secretion in the proximal small intestine.
MATERIALS AND METHODS
Reagents and antibodies.
Three independent anti-CFTR antibodies were used in this study. AME4991 is an affinity-purified, rabbit polyclonal antibody raised against the carboxy-terminal peptide of rat CFTR (3). The anti-CFTR mouse monoclonal antibody 217 was obtained from Dr. John Riordan (University of North Carolina-Chapel Hill, and Cystic Fibrosis Foundation Therapeutics). The monoclonal mouse antibody M3A7 raised against the COOH terminus of human CFTR was purchased from Chemicon International (Temecula, CA). The polyclonal rabbit antibody K1A raised against electrogenic Na+/bicarbonate cotransporter NBCe1 was a gift from Dr. Walter Boron (Case Western Reserve University). The human monoclonal antibody (T4) generated against the human Na+K+/Cl− cotransporter 1 (NKCC1) was acquired from the Developmental Hybridoma Bank (University of Iowa). The polyclonal vATPase antibody raised against the Voa3 subunit was generated by Dr. Beth Lee (Ohio State University). Two independent anti-NHE3 antibodies were used as follows: 1) the monoclonal mouse NHE3 antibody (N12920/611776) (BD Biosciences, San Jose, CA) was diluted at 1:500 (30); 2) the polyclonal NHE3 antibody made in rabbit (NHE31-A; Alpha Diagnostics, San Antonia, TX) was diluted at 1:100. The β-catenin monoclonal mouse antibody (610154, lot 76283; BD Transduction Laboratories, Lexington, KY) was diluted at 1:1,000. The PAT1 (SLC26A6) polyclonal rabbit antibody (a-hA6; a gift from Dr. Brett Thompson) was diluted at 1:500. The downregulated in adenoma (DRA, SLC26A3) polyclonal rabbit antibody (gift from Dr. C. Schweinfest) was diluted at 1:500. The NHERF-1 (EBP50) polyclonal rabbit antibody raised against the Ezrin-ERM-binding phosphoprotein of 50 kDa (EBP50; PA1-090; Affinity BioReagents, Golden, CO) was diluted at 1:200. The monoclonal mouse Na-K-ATPase antibody (ABR MA3-924; Affinity BioReagents) was diluted at 1:500. The β-actin monoclonal mouse antibody (A5316; Sigma, St. Louis, MO) was diluted at 1:10,000. The ALP polyclonal rabbit antibody (BYA11911; Accurate Chemical & Scientific, Westbury, NY) was diluted at 1:500. Fluorescent-tagged secondary antibodies were purchased from Invitrogen (Carlsbad, CA) and Jackson ImmunoResearch Laboratories (West Grove, PA). All other drugs, reagents, and chemicals were purchased from Sigma unless otherwise stated.
Animals.
The Institutional Animal Care and Use Committee of Yale University School of Medicine approved the study. Male Sprague-Dawley rats (200–250 g body wt; Charles River Laboratories, Wilmington, MA) were fasted overnight but allowed free access to drinking water and anesthetized with Inactin (120 mg/kg) administered by intraperitoneal injection. Body temperature was maintained with a heating pad, and the animals were euthanized at the end of experiments.
In vivo studies on rat ligated intestinal loops.
Rat jejunal and duodenal loops (∼2.5 cm length) were created with ligatures. Each loop was instilled with either 0.2 ml warm (37°C) normal saline (pH 7.4), pH 2.0 saline acidified with HCl, or 100 μM 8-Br-cAMP diluted in saline for 20 or 30 min.
Ex vivo studies on rat duodenal and jejunal explants.
Experiments were performed using ∼1.5-mm thick “ring”-shaped intestinal tissue explants dissected from the rat duodenum and jejunum. The explants were maintained in a viable state for up to 4 h, in Dulbecco's modified Eagle medium (DMEM) and left untreated, or treated ex vivo with 0.2 ml normal saline (pH 7.4) or acidified saline (pH 6.0) that was added to the ∼1 ml culture solution for 10 or 20 min at 37°C in 5% CO2/90% air atmosphere incubator. Some explants were treated with 8-Br-cAMP (100 μM or 1 mM), or ACh (10 or 100 μM) for 2 min, 5 min, 10 min, 20 min, 30 min, 45 min, 75 min, and 100 min. Tissues were fixed in 2% paraformaldehyde for 1 h, thoroughly rinsed, and processed for immunolabeling.
Tissue preparation/immunofluorescence labeling.
Preparation of rodent intestinal tissues by fixation, embedding, and sectioning and the use of tissue arrays for immunolabeling, microscopy, and densitometric image analysis were performed as described before (30). For cell distribution analysis, tissues were taken from the proximal duodenum, distal duodenum, proximal jejunum, midjejunum, distal jejunum, ileum, proximal colon, and distal colon. Cryostat sections were rehydrated in PBS. To minimize autofluorescence, sections were treated with 1% sodium borohydride for 20 min and then washed with PBS. Sections were blocked in 10% goat serum, 0.5% bovine serum albumin, 0.15% glycine, and 0.1% Triton X-100 for 1 h, followed by washes with PBS. Sections were incubated with primary antibodies overnight at 4°C, with the exception of NKCC1 antibodies that were incubated for 15 min at room temperature. Control sections were labeled with the respective nonspecific IgG antibodies. The following day, sections were washed with PBS, incubated with the appropriate secondary antibodies for 1 h at room temperature, stained with 1% Hoechst nuclear stain (DAPI), and mounted in Slow Fade medium (Molecular Probes, Eugene, OR).
Morphometric analysis of CHE cell distribution.
The percentage of CHE cells vs. total number of epithelial cells in the rat proximal duodenum, distal duodenum, proximal jejunum, midjejunum, distal jejunum, ileum, proximal colon, and distal colon was determined. In the adult rat, the small intestine is ∼100–120 cm long, consisting of duodenum (∼8–10 cm), jejunum (∼80–100 cm), and ileum (∼8–10 cm). For morphometric analysis, within the duodenum, sections were taken from the proximal duodenum (defined as the first ∼1.5-cm segment), midduodenum (∼2-cm central segment with the pancreatic duct junction), and distal duodenum (∼2-cm segment just proximal to the ligament of Treitz, the suspensory muscle that connects the duodenum to the diaphragm and is considered the structure that defines the beginning of the jejunum). Within the jejunum, sections were taken from the proximal jejunum (first ∼10-cm segment), midjejunum (central segment, ∼40 cm distant from the proximal segment), and distal jejunum (last ∼10-cm segment, adjoining the ileum). Ileum sections were from the ∼5-cm segment proximal to the ileocecal junction. Out of the (∼12–16 cm long) rat colon, sections were taken from the proximal colon (first ∼3-cm segment) and distal colon (last ∼3-cm segment). A minimum of five low-magnification (10×) images taken from randomly selected sections was analyzed from each region. The distinctly intense apical CFTR immunolabeling was used to identify CHE cells. The total number of cells in the images was determined by counting of nuclei (labeled with DAPI). All measured values are presented as means ± SE.
FI image analysis.
Microscopy and densitometric image analysis was performed as described before (30). CFTR and NKCC1 FIs were determined. Briefly, data from six to twelve selected areas were averaged in each image, six to eight images were analyzed for each measurement group in one animal, and data were collected from four animals. Analyses were performed as previously described using Adobe Photoshop CS4 (30). Fluorescence intensity levels were measured by selecting regions of interest in the apical membrane for CFTR, the basolateral membrane for NKCC1, and the intracellular apical pole for both transporters. To select the region of interest, the circular brush tool was set at 100% hardness, and 2.0-μm-thick areas were traced on the apical or basolateral membrane. For intracellular membrane fluorescence, 3.0-μm-thick areas were traced closely to the basolateral membrane. Using the advanced “Analysis and Record Measurements” tools in Adobe Photoshop CS4 Extended, the pixel intensities (mean gray value) were recorded for the selected areas. Statistical significance between two individual measurement groups was determined by unpaired t-test. Differences among groups were determined using one-way ANOVA and the Tukey's post hoc method of multiple comparisons. The level of significance was set at P < 0.05.
RESULTS
Proximal-distal distribution of CHE cells along the rat intestine.
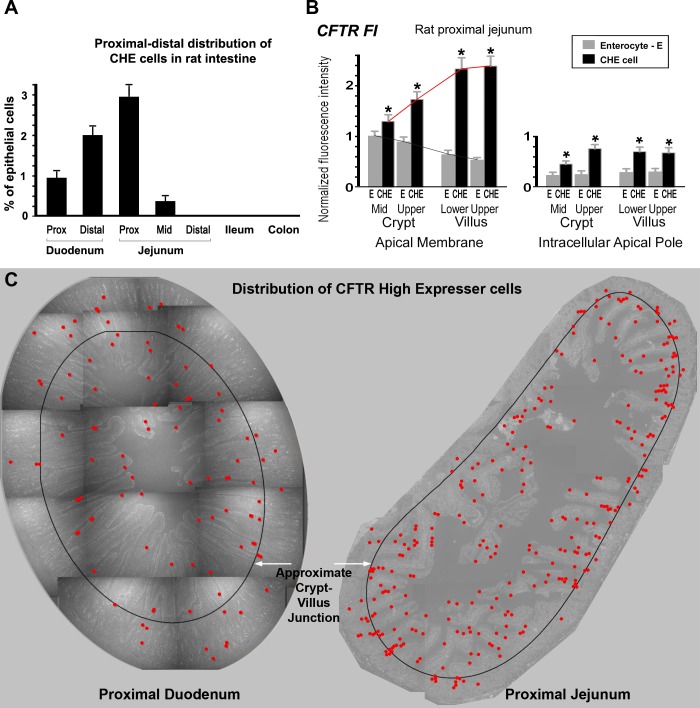
Previous studies from this laboratory localized the CHE cells to the rat small intestine, but the details of their distribution patterns were not documented (4). The ratio of CHE cells vs. total number of epithelial cells along the rat intestine was determined (Fig. 1A). CHE cells accounted for 0.92% of all epithelial cells in the proximal duodenum, 2.1% in distal duodenum, 3% in proximal jejunum, and 0.38% in midjejunum. CHE cells were not found in distal jejunum, ileum, proximal colon, or distal colon. A representative distribution map of CHE cells in proximal duodenum and proximal jejunum is shown in Fig. 1C.
Fig. 1.
A: distribution of cystic fibrosis transmembrane conductance regulator (CFTR) High Expresser (CHE) cells along the rat intestine. Graph shows the distribution of CHE cells relative to all epithelial cells along the different segments of the rat intestine. All values are presented as means ± SE. B: densitometry of normalized CFTR fluorescence intensity (FI) at apical membranes and subapical compartments of enterocytes (E) and CHE cells in untreated rat proximal jejunum. Cells were analyzed from the midcrypts and upper crypts, and lower and upper third of villi. Left: CFTR FI levels at apical membranes. Right: CFTR FI levels at intracellular subapical compartments. *Significant difference of the CHE cell FI values from the enterocyte FI values. Red line shows trend for CFTR FI in CHE cells; grey line shows trend for CFTR FI in non-CHE enterocytes. C: distribution map of CHE cells in proximal duodenum and proximal jejunum. Images at ×10 magnification were taken from a 6-μm-thick cross-section of a ring of duodenal or jejunal tissue immunolabeled for CFTR. Images were constructed as a montage. All CHE cells (red dots) were mapped. Left: representative proximal duodenum ring contains 96 CHE cells. Right: proximal jejunum ring possesses 295 CHE cells. A black ring denotes the approximate crypt-villus junction to indicate that the majority of CHE cells are located in the villus region.
CFTR FI in CHE cells along the crypt-villus axis.
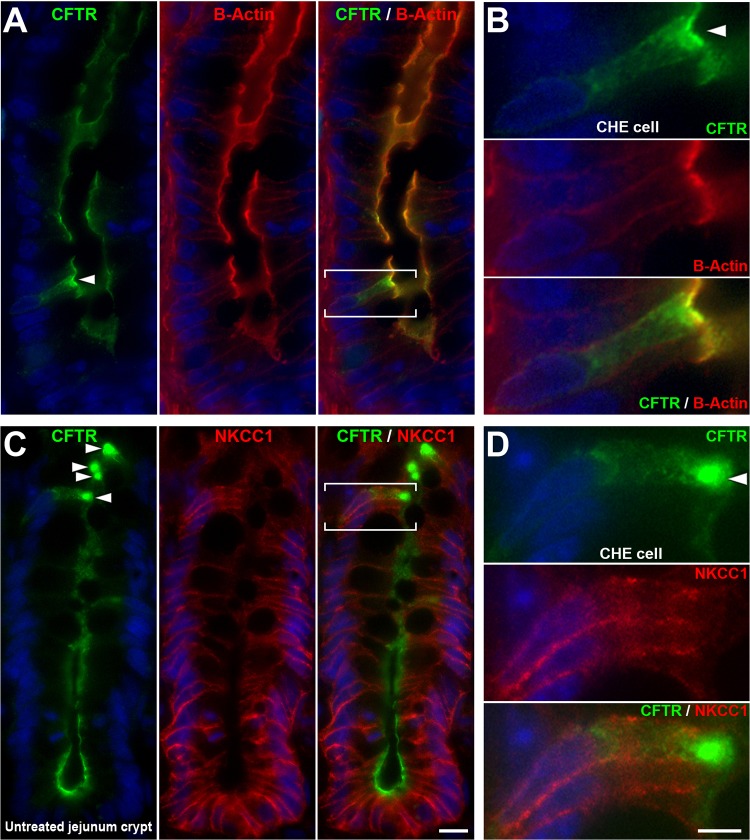
To understand differences in CFTR subcellular distribution between enterocytes and CHE cells, we analyzed CFTR FI at apical membranes and subapical compartments of enterocytes and CHE cells in untreated rat proximal jejunum (Fig. 1B). In the lowest crypt cells, both apical and intracellular CFTR FI were relatively homogenous, thus CHE cells could not be distinguished. Along the crypt-villus axis, CHE cells were first clearly recognizable just above the proliferative zone. Therefore, cells were analyzed from the midcrypt and upper third of crypts and lower and upper third of villi. Starting from the midcrypts, CFTR FI was significantly higher in CHE cells than that in enterocytes (see Fig. 1B, left). Furthermore, apical CFTR FI values of CHE cells increased toward the villus tip (see red trend line). In contrast, apical CFTR FI gradually decreased in enterocytes from midcrypt to upper crypts, lower villi, and upper villi (see grey trend line). Corresponding intracellular CFTR FI values in CHE cells compared with neighboring enterocytes are shown in Fig. 1B, right. Figure 2 shows representative images of CHE cells in the midcrypt region (Fig. 2, A and B) and in the upper crypt region (Fig. 2, C and D).
Fig. 2.
Colocalization of CFTR/β-actin and CFTR/Na+K+/Cl− cotransporter 1 (NKCC1) in CHE cells in the crypt epithelium of rat jejunum. Untreated rat jejunum sections were double immunolabeled for CFTR (green) and β-actin (red) (A and B) or CFTR and NKCC1 (red) (C and D). A: CFTR/β-actin double-labeled CHE cell localized in the lower midcrypt region. B: CHE cell (bracketed in A) is shown enlarged. C: CFTR/NKCC1 double-labeled CHE cells localized in the upper crypt epithelium. D: CHE cell (bracketed in C) is shown enlarged. The CFTR FI of the CHE cells (arrowheads) appears higher than neighboring crypt cells. In untreated condition, NKCC1 FI levels of CHE cells appear similar to those of neighboring crypt cells. Scale bars: A and C, 10 μm; B and D, 5 μm.
Colocalization of CFTR and NKCC1 in CHE cells in the villus epithelium of rat jejunum.
The presence of two key proteins involved in transcellular Cl− transport in epithelial cells, the basolateral NKCC1 and apical CFTR, in enterocytes along the crypt-villus axis has been shown (20, 30), but whether NKCC1 is present on the basolateral membranes in CHE cells was unknown. CFTR/NKCC1 double labeling revealed that NKCC1 is present in all CHE cells along the crypt villus axis, supporting its role in fluid secretion (Figs. 2 and 3). In the untreated condition, the NKCC1 FI levels appeared similar to that of neighboring enterocytes (Figs. 2 and 3, A and B).
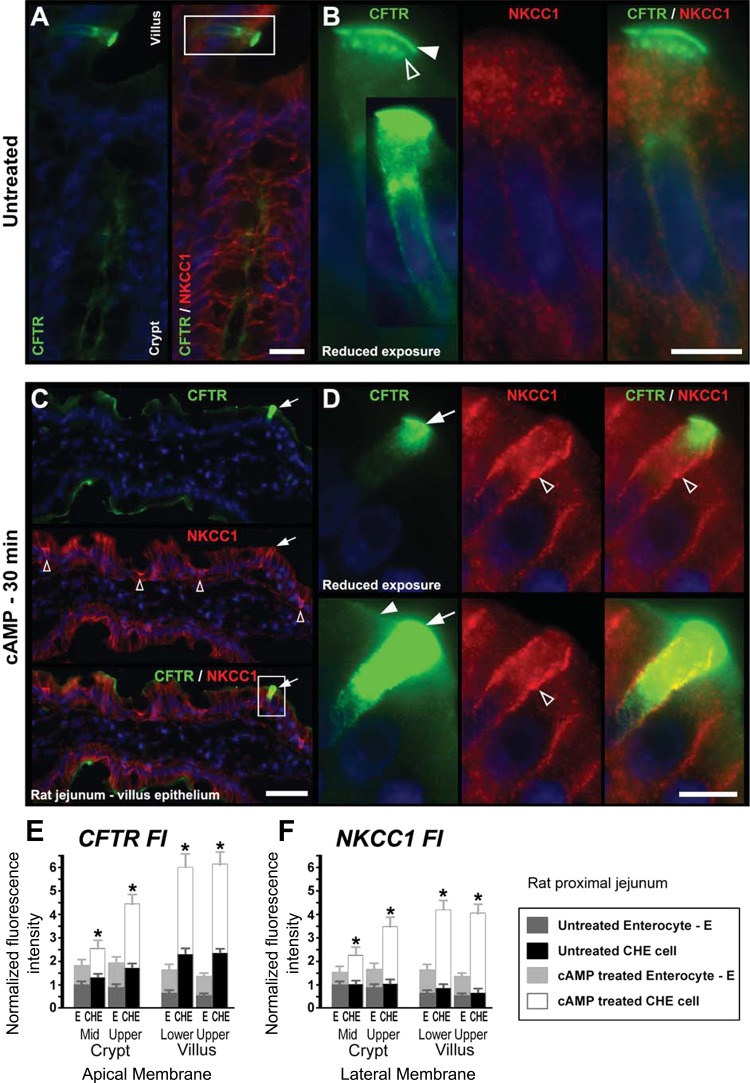
Fig. 3.
cAMP-induced redistribution of CFTR and NKCC1 in CHE cells in the villus epithelium of rat jejunum. Representative images from untreated (A and B) or 8-Br-cAMP-treated (C and D) tissues double labeled to detect CFTR (green) and NKCC1 (red). A: CHE cell at the villus base in untreated jejunum. B: high-magnification-enlarged image of CHE cell. White arrow, apical CFTR labeling; open arrow, CFTR-positive subapical endosomes. In untreated tissues, the distribution of NKCC1 is largely intracellular. C: CFTR/NKCC1 double-labeled villus epithelium with a CHE cell, after a 30-min cAMP treatment. Top: arrow, CFTR-labeled CHE cell. Middle: arrow, NKCC1-labeled CHE cell; open arrows, NKCC1-labeled goblet cells. D: enlarged CHE cell (bracketed in C). Top: CHE cell with CFTR labeling at reduced exposure shows detailed intracellular distribution of CFTR within CHE cell. Bottom: CHE cell with original exposure shows apical CFTR label in neighboring cells. Arrow, apical CFTR in CHE cell; open arrowhead, basolateral NKCC1 label in CHE cell. Arrowhead, apical CFTR label in neighboring non-CHE enterocyte. Images represent the results of 4 experiments (n = 4). E: densitometry of normalized CFTR FI at the apical membrane of CHE cells and non-CHE enterocytes in untreated and cAMP-treated jejunum. F: normalized NKCC1 FI at the lateral membrane of CHE cells and enterocytes in untreated and cAMP-treated jejunum. Cells were analyzed from the midcrypts and upper crypts and lower and upper third of villi. *Significant difference of the cAMP-treated CHE cell FI values from the cAMP-treated enterocyte (E) FI values. Scale bars: A, 25 μm; B, 5 μm; C, 50 μm; D, 10 μm.
cAMP-induced membrane recruitment of CFTR and NKCC1 in CHE cells in rat jejunum.
Because cAMP activates fluid transport by CFTR and NKCC1 traffic in enterocytes, the distribution of both transporters was examined in CHE cells following treatment of rat proximal jejunal tissues with saline or 100 μM 8-Br-cAMP. To examine the short-term response of CHE cells to a secretory stimulus, 8-Br-cAMP was injected into ligated jejunum loops in vivo for 30 min (Fig. 3). In the untreated condition, CFTR was partially apical (Fig. 3B, arrowhead) and partially intracellular in subapical endosomes (Fig. 3B, open arrowhead), as shown before (4, 6). In the untreated condition, NKCC1 FI was low and NKCC1 labeling was largely intracellular in CHE cells (Fig. 3, A and B). After cAMP treatment, both apical CFTR and basolateral NKCC1 FI levels increased in villus enterocytes (Fig. 3C), as shown before (28, 30). Interestingly, the secretagogue-induced increases in both CFTR and NKCC1 in CHE cells were particularly robust compared with neighboring enterocytes (Fig. 3, C and D). To appreciate the magnitude of CFTR FI differences in CHE cells vs. non-CHE enterocytes, CFTR labeling is shown at reduced and original exposure levels in Fig. 3D. In Fig. 3D, top, CFTR labeling is presented at reduced exposure to show subcellular CFTR distribution in CHE cells; at this exposure level, CFTR labeling in neighboring enterocytes is undetectable. In Fig. 3D, bottom, the original exposure level reveals CFTR in neighboring enterocytes. Quantitative densitometry confirmed these observations (Fig. 3, E and F). In particular, the quantitative data revealed that the cAMP-induced CFTR and NKCC1 FI increases were both disproportionately higher in CHE cells than in non-CHE enterocytes along the crypt-villus axis.
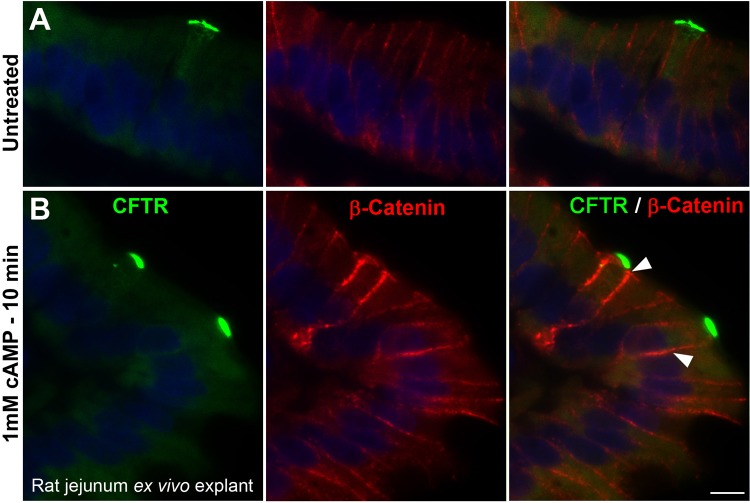
cAMP-induced increase in β-catenin levels in CHE cells.
β-Catenin is part of a complex that constitutes adherens junctions that are necessary for maintenance of the epithelial cell layer and adhesion between cells. Because CFTR is regulated by the PKA/cAMP pathway and PKA activity is known to regulate β-catenin levels at cell borders (29), we examined β-catenin levels in CHE cells in response to cAMP. As a second approach to examine the short-term response of CHE cells to a secretory stimulus, 8-Br-cAMP was added to the tissue culture medium containing an ex vivo jejunum explant tissue ring for 10 min. Tissues were fixed and double immunolabeled to detect CFTR and β-catenin (Fig. 4). In untreated tissues (Fig. 4A), apical and subapical CFTR labeling was present in CHE cells, and basolateral β-catenin labeling was present in both CHE cells and non-CHE enterocytes at relatively equivalent levels (FI). After 10-min cAMP treatment (Fig. 4B), both apical CFTR FI levels and basolateral β-catenin FI levels significantly increased in CHE cells, compared with non-CHE enterocytes. In Fig. 4, (as in Fig. 3D), CFTR labeling again is presented at reduced exposure to show subcellular CFTR distribution in CHE cells. At this exposure level, the apical CFTR labeling was undetectable in non-CHE enterocytes.
Fig. 4.
cAMP-induced redistribution of CFTR and β-catenin in CHE cells in the villus epithelium of proximal jejunum explant ex vivo. Tissue sections were double immunolabeled for CFTR (green) and β-catenin (red). A: untreated tissue. B: cAMP-treated (10 min) tissue. FI for apical CFTR and basolateral β-catenin (arrowheads) increased in CHE cells after treatment, n = 3 experiments. Scale bar = 10 μm.
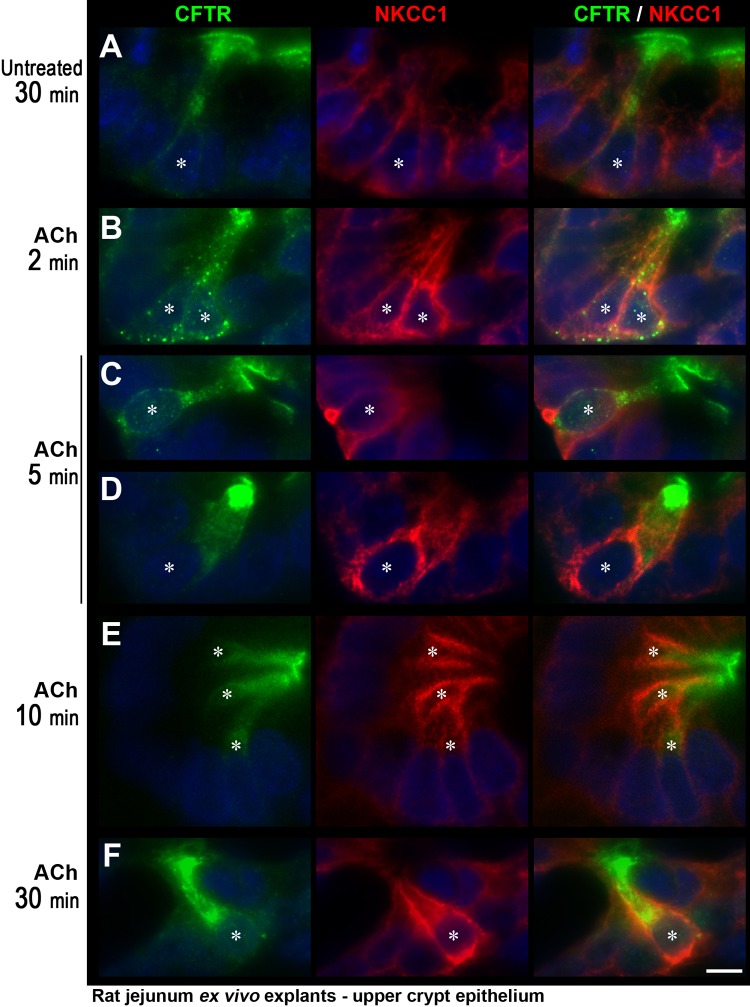
ACh induced redistribution of CFTR and NKCC1 in CHE cells of the upper crypt region in rat jejunum explant ex vivo.
To examine the short-term response of CHE cells to a cholinergic stimulus, jejunum tissue explants maintained in DMEM tissue culture medium (see materials and methods for detail) were exposed to ACh at 10 μM (Fig. 5) or 100 μM (not shown) for 2, 5, 10, or 30 min. The tissue sections were double labeled for CFTR and NKCC1 to identify redistribution patterns. Because the villus epithelia were more sensitive to the ex vivo condition, the crypt regions were examined in detail. In particular, the upper crypt epithelia were analyzed because CHE cells are clearly detectable in this region. In the untreated condition, at all time points, the distribution of CFTR and NKCC1 in CHE cells showed the typical pattern that was observed in vivo. A representative image of a CHE cell is shown at the 30-min untreated condition (Fig. 5A). In this condition, CFTR labeling was in part apical and in part subapical, and the basolateral NKCC1 labeling was at a similar FI level in CHE cells and non-CHE enterocytes. ACh treatment induced a dramatic change in the distribution pattern of both CFTR and NKCC1 that was detected as early as 2 min (Fig. 5B). At 2 min, CFTR label appeared to accumulate in endosomes in the subapical region and basolateral domains, and basolateral NKCC1 FI intensity appeared to distinctly intensify in CHE cells, compared with non-CHE enterocytes. At later time points (from 5 min), the endosomal accumulation of CFTR was reduced, with corresponding increase in apical label. At 5–10 min (Fig. 5, C–E), CFTR label accumulated at the apical and subapical pole, and basolateral NKCC1 FI remained higher in CHE cells than in non-CHE enterocytes. (Note that both representative images of CHE cell in Fig. 5, C and D, are from the upper crypt, but CFTR label appears more pronounced in Fig. 5D, which reflects an en face view of CFTR label on the brush border due to the orientation of the section.) At 30 min (Fig. 5F), both CFTR and NKCC1 FI remained higher in CHE cells but were increasingly intracellular.
Fig. 5.
Acetylcholine (ACh) induced redistribution of CFTR and NKCC1 in CHE cells in the upper crypt region of rat jejunum explant ex vivo. Tissue explants maintained in DMEM medium were untreated or treated with 10 μM ACh for 2–30 min. Tissue sections were double immunolabeled for CFTR (green) and NKCC1 (red). Representative images of untreated tissue (A), or tissues treated with ACh for 2 min (B), 5 min (C and D), 10 min (E), or 30 min (F). *CHE cell. The images are representative of 3 experiments (n = 3). Scale bars = 10 μm.
Absence of the Na+/H+ exchanger NHE3 in CHE cells.
Our previous studies indicated that CHE cells on the villus epithelium were distinguished by high levels of CFTR but lacked absorptive hydrolases that are normally present on the brush border of villus enterocytes (4). The pathogenesis of CFTR-mediated diarrhea is intimately linked to the Na+/H+ exchanger NHE3, which is present in the brush border of mature villus enterocytes. In secretory diarrhea, cyclic nucleotides simultaneously increase CFTR abundance and anion secretion on the brush-border membrane (BBM) while inhibiting Na+ absorption by decreasing NHE3 levels and function to result in net fluid secretion (19, 28, 30, 33). In addition to our investigations of secretory transport proteins in CHE cells, we examined whether CHE cells may possess fluid absorptive functions. CFTR/NHE3 double labeling of rat duodenum and jejunum tissues indicated that the CHE cells distinctly lack NHE3 in their BBM (Fig. 6A), whereas non-CHE enterocytes coexpress CFTR and NHE3 at their apical domain, as shown before (28, 30). Short-term acidic conditions are known to stimulate exocytic insertion and surface expression of CFTR while inducing a partial endocytosis of NHE3, particularly in the upper villus (28). After stimulation with HCl saline (Fig. 6B), the FI level of CFTR dramatically increased in the BBM of villus CHE cells, whereas NHE3 was undetectable.
Fig. 6.
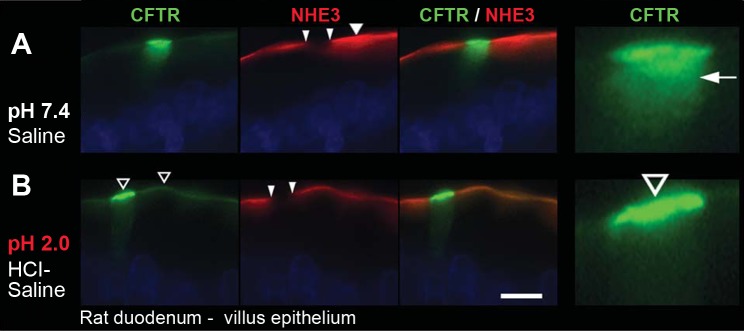
Localization of CFTR and lack of Na+/H+ exchanger 3 (NHE3) in CHE cells in the villus epithelium of rat duodenum. Ligated duodenum loops were treated with normal saline pH 7.4 (A) or acidic HCl saline pH 2.0 (B). Tissue sections were double immunolabeled for CFTR (green) and NHE3 (red). A: wide arrowhead, NHE3 label in saline-treated enterocytes. Small arrowheads point to absence of NHE3 at the apical pole of CHE cells. Arrow, subapical CFTR in enlarged CHE cell. B: wide open arrowheads, intense CFTR label in CHE cells and non-CHE enterocytes in HCl-saline-treated enterocytes. Images are representative of 3 experiments (n = 3). Scale bars = 10 μm.
Elevated levels of vacuolar-ATPase in CHE cells.
Vacuolar-ATPase proton pumps are essential for homeostatic regulation of intracellular and extracellular pH in epithelial and nonepithelial cells (11, 34, 39). V-ATPase pumps play an important role in regulating luminal pH in the kidney and epididymis, but little is known regarding V-ATPase in the intestine (40–41). Our laboratory recently identified endogenous V-ATPase expression in native enterocytes in the rodent intestine (our unpublished observations). Because the Na+/H+ exchanger NHE3, which is involved in the extrusion of protons on the enterocyte BBM, was absent from CHE cells, we examined whether an alternate mechanism for proton extrusion was present. V-ATPase/CFTR double labeling revealed the presence of V-ATPase proton pump in CHE cells, at higher levels than in neighboring enterocytes (Fig. 7).
Fig. 7.
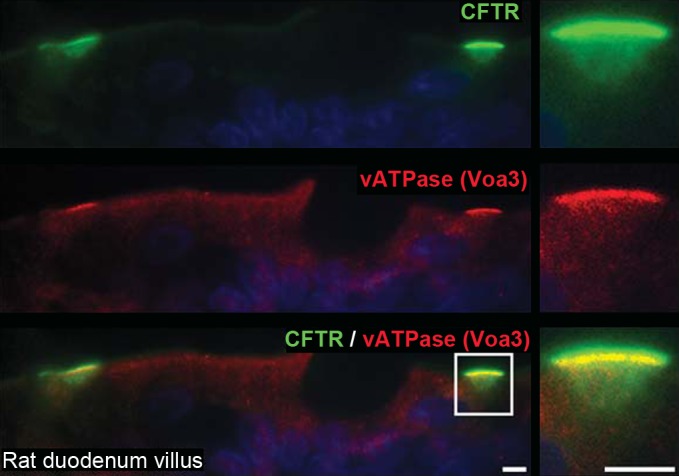
Localization of V-ATPase in CHE cells in the villus epithelium of the rat duodenum. Tissue sections were double immunolabeled for CFTR (green) and V-ATPase Voa3 subunit (red). Left: apical CFTR and V-ATPase fluorescence are distinctly high in 2 CHE cells. Right: enlarged CHE cell (bracketed on left). Images represent the results of 3 experiments (n = 3). Scale bars = 5 μm.
Absence of the Cl−/HCO3− anion exchanger PAT1 (SLC26A6) in CHE cells.
Villus enterocytes in the small intestine regulate HCO3− secretion from the apical BBM by signaling through CFTR and chloride/bicarbonate exchangers (44, 49). To investigate a role for CHE cells in apical HCO3− transport, we examined whether the Cl−/HCO3− anion exchangers PAT1 (SLC26A6) and DRA (SLC26A3) were present in the CHE cells. Apical PAT1 immunofluorescence labeled duodenal and jejunal villus epithelia strongly (data not shown). CFTR/PAT1 double immunolabeling revealed that PAT1 was absent in the CHE cells, whereas neighboring non-CHE enterocytes coexpressed CFTR and PAT1 at their apical domain (Fig. 8). In the rat proximal colon, we reported strong apical DRA immunolabeling (29), but, in small intestinal tissues (processed identically and collected from the same rat), we consistently detected weak and patchy DRA labeling (data not shown). In mice, a similarly patchy DRA immunolabeling pattern was described in duodenal villi (54), and modest DRA labeling was reported in jejunum villi relative to the surface epithelium of the cecum (56), using a different antibody. In our CFTR/DRA double-labeling experiments, DRA was not detected in CHE cells (data not shown). It is possible that low levels of DRA may be present in CHE cells, but this could not be confirmed due to low levels of DRA labeling.
Fig. 8.
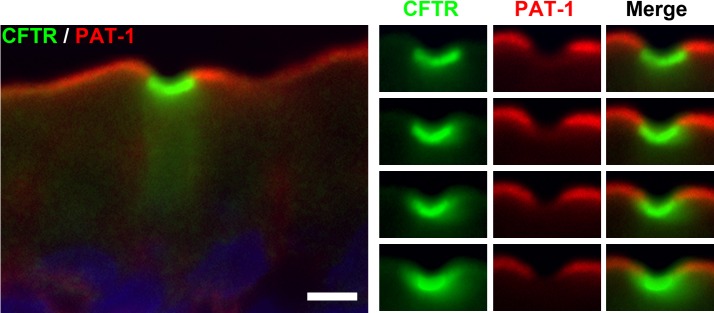
Localization of CFTR and putative anion exchanger 1 (PAT-1) (SLC26A6) in CHE cells in the villus epithelium of the rat jejunum. Tissue sections were double immunolabeled for CFTR (green) and PAT-1 (red). Left: CHE cell and neighboring enterocytes. Right: apical pole of the CHE cell. Images were taken at 4 different planes of foci throughout the 6-μm-thick tissue section. Images represent the results of 4 experiments (n = 4). Scale bar = 5 μm.
Absence of intestinal ALP in CHE cells.
Intestinal ALP is found on the apical BBM of mature enterocytes, where it plays an important role in intestinal barrier functions (46) and affects HCO3− secretion and surface microclimate pH in rat duodenum (1). To further investigate the BBM characteristics of CHE cells, we examined whether the enzyme ALP was present. CFTR/ALP double immunolabeling revealed that, in contrast to non-CHE enterocytes that coexpressed CFTR and ALP on the BBM, ALP was absent from CHE cells (Fig. 9).
Fig. 9.
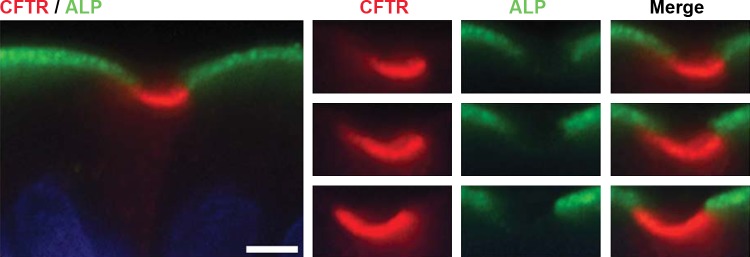
Localization of CFTR and alkaline phosphatase (ALP) in CHE cells in the villus epithelium of rat jejunum. Tissue sections were double immunolabeled for CFTR (red) and ALP (green). Left: CHE cell and neighboring enterocytes. Right: apical pole of the CHE cell. Images were taken at 3 different planes of foci throughout the 6-μm-thick tissue section. The images represent the results of 4 experiments (n = 4). Scale bar = 5 μm.
The lack of apical transport proteins involved in HCO3− mediated regulation of pH balance at the epithelial surface (NHE3, SLC26A6, and ALP) in CHE cells prompted us to investigate the presence of basolateral HCO3− entry transporters. The electrogenic Na+/bicarbonate cotransporter NBCe1 is present on the basolateral membranes of CFTR expressing villus enterocytes of the small intestine, where it contributes to CFTR-mediated HCO3− transport (13, 28, 30, 49). CFTR/NBCe1 double-label studies suggested NBCe1 labeling along the basolateral membranes of CHE cells, but it could not be confirmed with confidence. Furthermore, following cAMP stimulation, basolateral NBCe1 FI increased in villus cells as described before (28), but, in contrast to the robust increase in NKCC1, no specific changes in NBCe1 could be ascribed to the CHE cells (not shown).
Low levels of Myosin 1a in CHE cells.
Our earlier ultrastructural studies indicated that the microvilli of CHE cells were disordered and less packed compared with neighboring enterocytes (7). Because the plus-end myosin motor in the enterocyte, Myosin 1a, is critical for normal brush border structure (52) and CFTR localization in mature enterocytes (31), we examined Myosin 1a localization in the CHE cells. CFTR/Myosin 1a double immunolabeling revealed that Myosin 1a was present in the BBM of CHE cells, but Myosin 1a FI levels were very low compared with neighboring enterocytes (Fig. 10).
Fig. 10.
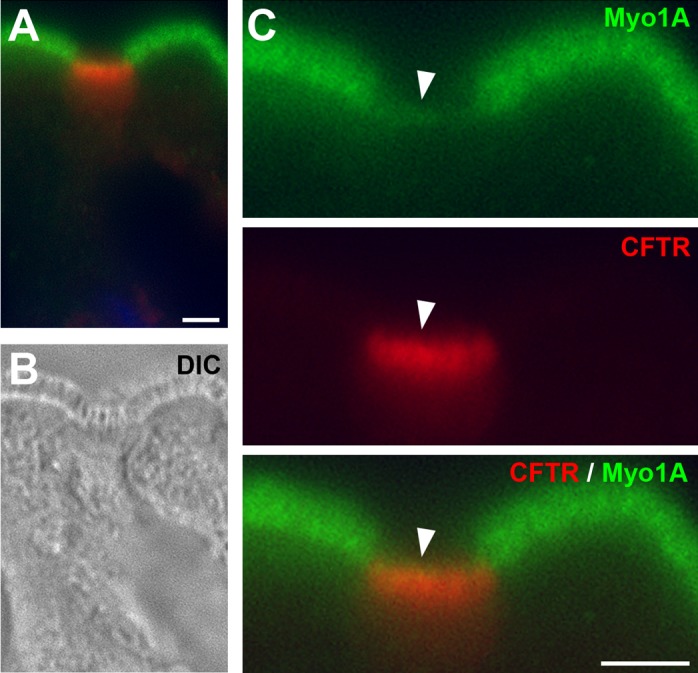
Myosin 1a (Myo1a) expression is low in CHE cells in the villus epithelium of the rat jejunum. Tissue sections were double immunolabeled for Myo1a (green) and PAT-1 (red). A: Myo1a/CFTR double-labeled CHE cell and neighboring enterocytes. B: diffraction interference contrast (DIC) image. C: enlarged apical pole of CHE cell and neighboring enterocytes. Arrowhead, brush border region of CHE cell. Images represent the results of 4 experiments (n = 4). Scale bar = 5 μm.
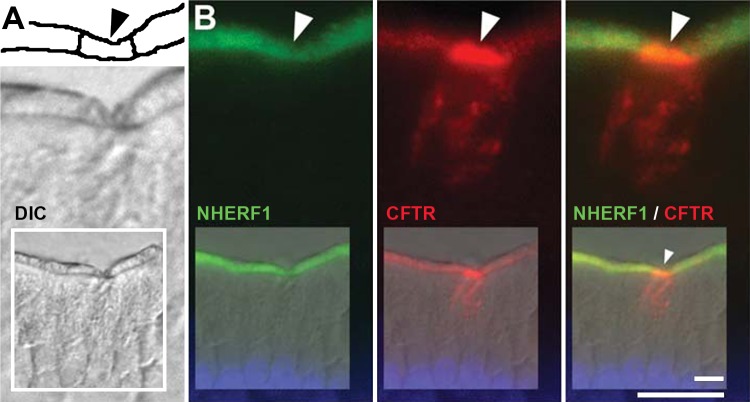
Localization of NHERF-1 (EBP50) in CHE cells.
We further investigated whether the postsynaptic density and zonnula occludens-1 adaptor protein NHERF-1 was present in CHE cells because NHERF-1 is a regulator of NHE3 and CFTR (25–26). CFTR/NHERF-1 double immunolabeling revealed that NHERF-1 was present in the BBM of CHE cells, at FI levels similar to neighboring enterocytes (Fig. 11).
Fig. 11.
Localization of Na+/H+ exchanger regulatory factor 1 (NHERF-1) (EBP50) in CHE cells in the villus epithelium of the rat jejunum. Tissue sections were double immunolabeled for NHERF-1 (green) and CFTR (red). A, top: drawing of brush border region. Black arrowhead, brush border of CHE cell. Bottom: DIC image. B: NHERF-1/CFTR double-labeled CHE cell. Arrowheads, brush border region of CHE cell. Insets: lower-power image of CHE cell and neighboring enterocytes. The images represent the results of 4 experiments (n = 4). Scale bars = 10 μm.
Other biological markers relevant to epithelial functions including syntaxin 3, protein kinase A, and Na-K-ATPase (not shown) were tested in double-labeling studies and found to be present at the same levels in CHE cells and neighboring enterocytes.
Disorganized and short microvilli are a characteristic feature of CHE cells.
Detailed diffraction interference contrast (DIC) image analysis of the CHE cells at high magnification of DIC during the course of the study confirmed earlier observations from this laboratory (4, 7) that the brush border microvilli of CHE cells were more disorganized compared with neighboring villus enterocytes (see Figs. 10 and 11).
DISCUSSION
Disproportionately robust secretagogue-induced membrane recruitment of CFTR and NKCC1 in CHE cells.
The results of the present study demonstrate for the first time that both apical CFTR and basolateral NKCC1 disproportionately increase in the CHE cells compared with neighboring enterocytes after stimulation with the secretagogues cAMP or the Ca2+ agonist ACh. In the intestine, both cAMP and Ca2+ agonists regulate fluid secretion by inserting CFTR Cl− channels from subapical endosomes into the apical BBM and NKCC1 Cl− transporter from intracellular endosomes to the basolateral membrane of enterocytes (7, 28, 30). Contrary to the constitutively higher CFTR levels in CHE cells, NKCC1 expression in CHE cells was similar to other enterocytes at steady-state condition. However, the NKCC1-trafficking response to the secretory stimuli of cAMP and ACh was much more robust in CHE cells compared with other enterocytes. One possible explanation for this observation is that a larger proportion of intracellular NKCC1 is recruited to the basolateral membrane in CHE cells following stimulation than neighboring enterocytes. Because a robust CFTR- and NKCC1-trafficking response is consistent with elevated transcellular Cl− transport, this further strengthens the notion that the CHE cells are enriched with molecular machinery to elicit robust Cl− secretory functions. A decade ago, we identified a subpopulation of highly fluorescent villus cells following loading rat duodenum segments in vivo with the Cl− indicator dye LZQ. These preliminary observations suggested that some villus cells, possibly the CHE cells, have uniquely high intracellular Cl− concentration (N. Ameen, unpublished observations). However, the lack of Cl− indicator dyes retainable upon fixation has precluded double-label experiments with CFTR antibodies to confirm that this subpopulation is CHE cells. Due to the lack of ALP in CHE cells, ALP as a negative marker could be another promising alternative to identify CHE cells. Thus, theoretically, it is possible that a few ALP-negative “gap” cells (of 4–5-μm thickness) among ALP-positive villus epithelial cells could be “matched” with Cl− dye accumulating (Cl− concentrating) cells in the same tissue with the combined use of Cl− indicator dyes and ALP markers; however, such an approach is technically unfeasible at present.
ACh is critical in controlling epithelial ion transport and water movement that is necessary for gut hydration. The effect of ACh on intestinal ion transport is mainly an increase in Cl− secretion due to interaction with epithelial M3 and, to a lesser extent, neuronal M1 muscarinic receptors (27). This study revealed a short-term trafficking response of CFTR and NKCC1 in CHE cells in response to ACh. The response was rapid and was evident within 2 min. After 5–10 min, both CFTR and NKCC1 were recruited to their respective membranes. However, the response was biphasic; after 30 min, both transporters were increasingly in an intracellular location. This biphasic mechanism of transporter trafficking is characteristic of the epithelial response to ACh that was described in colon explants (43). We previously reported a carbachol-induced membrane-trafficking response of CFTR and NKCC1 in epithelial cells along the crypt-villus axis (30). The present study revealed that the trafficking responses were disproportionately larger in the CHE cells. Overall, the biphasic responses in distribution are consistent with high-volume fluid secretion by these cells that can be rapidly switched on and off.
Absence of NHE3, SLC26A6, and ALP in CHE cells.
NHE3 is expressed on the BBM of mature enterocytes of the small intestine (10). The finding that villus CHE cells lack NHE3 is an unexpected new finding because CFTR and NHE3 are generally coexpressed in the apical domain of enterocytes of small intestinal villi (30), and both transporters interact to transform the absorptive and secretory functions of the cell (49). The observation that the CHE cells also lack the Cl−/HCO3− exchanger SLC26A6 is another intriguing new finding. Like NHE3, SLC26A6 is predominantly localized to villus cells in the small intestine (48, 55) and colocalizes with CFTR on the brush border of villus enterocytes. SLC26A6 is involved in the NHE3- and CFTR-codependent switch from anion absorptive to HCO3− secretory mode in villus enterocytes (49). Here, SLC26A6 is dependent on proton recycling via NHE3 to operate in the Cl− absorptive mode (49). Indeed, SLC26A6 and NHE3 interact (42), supporting the observed defects in Na+ and Cl− absorption in the small intestine of SLC26A6-null mice (45).
The lack of SLC26A6 and NHE3 in CHE cells indicates that the molecular machinery that enables fluid absorption or the switch to HCO3− secretion (NHE3, SLC26A6) is absent while the machinery necessary for Cl−-mediated fluid secretion (CFTR and NKCC1) is dominant in the CHE cells. Intestinal ALP is a glycosylphosphatidylinositol-anchored ectoenzyme that is highly expressed in the BBM of duodenal epithelial cells, with expression declining along the proximal-caudal axis. ALP activity affects HCO3− secretion and regulates protective surface microclimate pH in rat duodenum (1, 35). The lack of ALP in CHE cells provides further evidence against a major role for HCO3− secretion by CHE cells. Earlier studies from our laboratory demonstrated the absence of the brush border hydrolase sucrase/isomaltase in the CHE cells (4). Future studies should elucidate whether the CHE cells completely lack the absorptive phenotype of villus enterocytes, including fluid and nutrient absorption. The observation that the CHE cells express NHERF-1, but not NHE3, was somewhat unexpected because NHERF-1 was originally thought to be functionally related to NHE3, hence its name. However, NHERF-1 (EBP50) also seems important for the stabilization of apical CFTR (12). NHERF-1 is also expressed in nonepithelial cells like T cells. In these cells, type I PKA is anchored close to the T cell receptor in lipid rafts by NHERF-1, and cAMP activates its recruitment to lipid rafts (36), similar to CFTR. The cAMP-induced increase in the level of β-catenin in CHE cells independently suggests that the PKA/cAMP pathway can be selectively and disproportionately activated in the CHE cells.
High levels of V-ATPase in CHE cells.
The V-ATPase pump is a multisubunit enzyme complex that mediates ATP-driven proton transport across membranes (11, 34, 39), performs housekeeping functions, and serves important intracellular functions, including organelle pH regulation in cells (38–39). The identification of exceptionally high levels of expression of V-ATPase proton pumps in CHE cells compared with neighboring enterocytes is a novel finding. V-ATPase pumps play important roles in regulating extracellular pH and homeostasis in nonintestinal epithelial tissues (40–41). In marine fish intestine, vacuolar-type H+ ATPase was identified in the apical membrane of enterocytes, where it secretes acid and facilitates Cl−/HCO3− exchange (22), but a role for V-ATPase has not been identified in the mammalian intestine. We recently identified a physiological association of V-ATPase with CFTR in native enterocytes of rat jejunum, suggesting that enterocytes can utilize V-ATPase pumps for proton extrusion at the BBM (our unpublished observations). In CHE cells, the association of V-ATPase pumps with CFTR was predominantly observed at the apical domain. The physiological significance of these findings is unclear. The CHE cells possess abundant organelles, including mitochondria, consistent with high metabolic activity (7). V-ATPase pumps may contribute important functions for maintaining organelle homeostasis and pH regulation in the CHE cells. Proton secretion by intestinal epithelial cells is largely a function attributed to NHE Na+-H+- exchanger family members (57). In the small intestine, NHE3 regulates Na+ absorption by a process of Na+-H+ exchange on the apical membrane of enterocytes. The results of the present study suggest that, in the CHE cells, V-ATPase pumps may provide an alternate source of proton extrusion necessary to maintain intracellular pH in the absence of NHE3. The exceptionally high levels of V-ATPase proton pumps in the CHE cells are consistent with the increased demand of intracellular deacidification during the process of intense Cl− secretion. In other unpublished studies, we observed that, in rat Brunner's gland, V-ATPase undergoes robust traffic together with CFTR to the apical membrane of acinar cells in response to cAMP activation. Interestingly, acinar cells also lack NHE3 (32). These observations suggest that V-ATPase pumps in the CHE cells may function similarly in the Brunner's glands acinar cells to balance intracellular and luminal pH in the absence of apical NHE3. The ion transporter profile of the CHE cells appears unique among intestinal epithelial cells; however, there are striking parallels between the CHE cells and the cells of the submucosal Brunner's glands of the duodenum: both cell types have distinctly high levels of CFTR, NKCC1, and V-ATPase and lack NHE3, PAT-1, and sucrase/isomaltase. Both cell types also respond to cAMP stimulation with robust apical CFTR and basolateral NKCC1 trafficking. This suggests that both cell types appear to specialize in high-volume fluid secretion and lack absorptive functions.
Low levels of Myosin 1a in CHE cells.
Myosin 1a is an intestine-specific plus-end motor that plays an important role in brush border assembly (52). Maturing crypt cells possess underdeveloped microvilli and are much shorter and less tightly organized compared with microvilli in mature villus enterocytes. Our recent study in mouse small intestine demonstrated that Myosin 1a is absent in lower crypt cells, is detectable at low levels in upper crypts, and is fully apically distributed in villus enterocytes, where it regulates CFTR BBM trafficking and ion transport (31). In the colon, where villus enterocytes are absent, Myosin 1a levels are also low (31). Our published observations and those presented in the present study indicate that Myosin 1a is an important regulator of CFTR trafficking in mature villus enterocytes but does not appear to be the dominant motor in CHE cells. The microvilli of CHE cells (throughout the entire crypt-villus axis) are shorter and more disorganized than neighboring enterocytes, consistent with our observations that villus CHE cells possess low levels of Myosin 1a compared with neighboring villus enterocytes. In the absence of Myosin 1a, robust apical CFTR traffic in villus CHE cells could be mediated by a number of alternate plus-end motors such as Myosin 1c, 1d, and 1e (8–9, 52).
Moreover, low Myosin 1a expression in CHE cells is consistent with our published observations that CHE cells lack sucrase-isomaltase (4) because Myosin 1a is critical for retention of sucrase-isomaltase in the BBM of villus enterocytes (53). The underdeveloped microvilli and lack of membrane proteins involved in nutrient or fluid absorption are features of the CHE cells that suggest the possibility of an incomplete or “arrested” developmental process for this cell type, compared with the fully mature villus enterocytes that drive fluid and nutrient absorption.
Possible role of the CHE cells in high-volume fluid secretion.
The CHE cells are a specialized subpopulation of nonabsorptive enterocytes that are confined to the proximal small intestine and are abundant in the villus epithelium, an epithelium that is mainly specialized in the absorption of fluid and nutrients. In this location, the CHE cells, possessing the highest levels of CFTR of any intestinal cell on their apical membrane, are strategically located on the villus edge and face the intervillus luminal spaces. In the absence of tools to investigate the functions of this enigmatic cell population directly, the data provided in this study suggest that the CHE enterocytes on the villus epithelium are specialized for Cl-mediated high-volume fluid secretion. Why might it be necessary to have such a subpopulation of Cl-secretory cells on an absorptive epithelium? CHE cells are present in humans and rats but rarely seen in mice (2, 4, 50–51). The crypt epithelium, the main site of fluid secretion, intermittently liquefies lumen content that, not only helps digestion, but also aids in the clearance of mucus from intervillus spaces to facilitate absorption of ingested food materials. Crypt cells may handle this function adequately in the mouse small intestine, where villi are shorter with wide intervillus spaces. In contrast, rat and human small intestine possess longer villi, larger absorptive surfaces, and tighter intervillus spaces, and cells specialized in fluid secretion are apparently present on the villi themselves, the CHE cells. We speculate that the role of CHE cells is to liquefy lumen content and thus assist in mucus clearance. The proximal jejunum, where CHE cells are most abundant, possesses a particularly low luminal pH that can promote accumulation of firm adherent mucus on epithelial surfaces and intervillus spaces (18, 24, 28). Indeed, CFTR abundance in CHE cells increases as the cells migrate toward the villus tip, suggesting that the most robust secretion emanates from CHE cells in the villus region. CHE cells could generate high-volume fluid and pressure in their local environment to remove adherent mucus. By rapid biphasic on-off-switching mechanisms of the cholinergic Ca2+ signaling pathway, the CHE cells appear to be able to apply high-volume fluid secretion intermittently and locally. Proximal small intestinal villi are the main locus of nutrient absorption. The unique ability of the CHE cells to locally assist in the removal of adherent mucus to clear absorptive epithelial surfaces before nutrient absorption by high-volume fluid secretion may be an important function that is necessary for the villus epithelium to efficiently absorb nutrients.
GRANTS
This study was supported by National Institute of Health Grant R01-DK-077065 to N. Ameen, and by National Institute of Health Grant P30-DK-34989 to the Yale Liver Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.L.J. and N.A.A. conception and design of research; R.L.J. and A.M.C. performed experiments; R.L.J., A.M.C., and N.A.A. analyzed data; R.L.J. and N.A.A. interpreted results of experiments; R.L.J. and A.M.C. prepared figures; R.L.J. drafted manuscript; R.L.J. and N.A.A. edited and revised manuscript; N.A.A. approved final version of manuscript.
REFERENCES
- 1.Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol 293: G1223–G1233, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69–75, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 108: 1016–1023, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Ameen NA, Marino C, Salas PJ. cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo. Am J Physiol Cell Physiol 284: C429–C438, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887–894, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Benesh AE, Nambiar R, McConnell RE, Mao S, Tabb DL, Tyska MJ. Differential localization and dynamics of class I myosins in the enterocyte microvillus. Mol Biol Cell 21: 970–978, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bond LM, Brandstaetter H, Sellers JR, Kendrick-Jones J, Buss F. Myosin motor proteins are involved in the final stages of the secretory pathways. Biochem Soc Trans 39: 1115–1119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest 93: 106–113, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol 292: F1–F10, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Broere N, Hillesheim J, Tuo B, Jorna H, Houtsmuller AB, Shenolikar S, Weinman EJ, Donowitz M, Seidler U, de Jonge HR, Hogema BM. Cystic fibrosis transmembrane conductance regulator activation is reduced in the small intestine of Na+/H+ exchanger 3 regulatory factor 1 (NHERF-1)- but not NHERF-2-deficient mice. J Biol Chem 282: 37575–37584, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Collaco A, Jakab R, Hegan P, Mooseker M, Ameen N. Alpha-AP-2 directs myosin VI-dependent endocytosis of cystic fibrosis transmembrane conductance regulator chloride channels in the intestine. J Biol Chem 285: 17177–17187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaco A, Marathe J, Hoknke H, Kravstov D, Ameen NA. Syntaxin 3 is necessary for cAMP and cGMP-regulated exocytosis of CFTR: implications for enterotoxigenic diarrhea. Am J Physiol Cell Physiol 299: C1450–C1460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field M, Semrad CE. Toxigenic diarrheas,congenital diarrheas and cystic fibrosis:disorders of intestinal ion transport. Annu Rev Physiol 55: 631–655, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266: 107–109, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125: 1148–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Golin-Bisello F, Bradbury NA, Ameen NA. Heat Stable Enterotoxin (STa) and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708–C716, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev 79: S193–S214, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Guffey S, Esbaugh A, Grosell M. Regulation of apical H(+)-ATPase activity and intestinal HCO3− secretion in marine fish osmoregulation. Am J Physiol Regul Integr Comp Physiol 301: R1682–R1691, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA 95: 8496–8501, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, Grinstein S, Lefkowitz RJ. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 392: 626–630, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol 149: 463–479, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakab RL, Collaco AM, Ameen NA. Cell-specific effects of luminal acid, bicarbonate, cAMP, and carbachol on transporter trafficking in the intestine. Am J Physiol Gastrointest Liver Physiol 303: G937–G950, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Dig Dis Sci 57: 2826–2845, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kravtsov DV, Caputo C, Collaco A, Hoekstra N, Egan ME, Mooseker MS, Ameen NA. Myosin Ia is required for CFTR brush border membrane trafficking and ion transport in the mouse small intestine. Traffic 13: 1072–1082, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulaksiz H, Bektas H, Cetin Y. Expression and cell-specific and membrane-specific localization of NHE-3 in the human and guinea pig upper gastrointestinal tract. Cell Tissue Res 303: 337–343, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Li X, Zhang H, Cheong A, Leu S, Chen Y, Elowsky CG, Donowitz M. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol 556: 791–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20: 415–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol 587: 3651–3663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation–overview of mechanisms of action in T cells. Cell Signal 23: 1009–1016, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Naren AP, Di A, Cormet-Boyaka E, Boyaka PN, McGhee JR, Zhou W, Akagawa K, Fujiwara T, Thome U, Engelhardt JF, Nelson DJ, Kirk KL. Syntaxin 1A is expressed in airway epithelial cells, where it modulates CFTR Cl(-) currents. J Clin Invest 105: 377–386, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson N, Harvey WR. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev 79: 361–385, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Nishi T, Forgac M. The vacuolar (H+)-ATPases—nature's most versatile proton pumps. Nat Rev Mol Cell Biol 3: 94–103, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrovic S, Barone S, Wang Z, McDonough AA, Amlal H, Soleimani M. Slc26a6 (PAT1) deletion downregulates the apical Na+/H+ exchanger in the straight segment of the proximal tubule. Am J Nephrol 28: 330–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, Tighe R, Williams MR. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol 582: 507–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossman H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP,cGMP and calcium-dependent HCO3− secretion. J Physiol 505: 411–423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seidler U, Rottinghaus I, Hillesheim J, Chen M, Riederer B, Krabbenhoft A, Engelhardt R, Wiemann M, Wang Z, Barone S, Manns MP, Soleimani M. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflügers Arch 455: 757–766, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Shifrin DA, Jr, McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr Biol 22: 627–631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 20: 2337–2350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl-/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Singh AK, Riederer B, Chen M, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U. The switch of intestinal Slc26 exchangers from anion absorptive to HCO3− secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol 298: C1057–C1065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strong TV, Boehm K, Collins FS. Localization of the cystic fibrosis conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trezise AEO, Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 353: 434–436, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Tyska M, Mackey A, Huang J, Copeland N, NA J, Mooseker M. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 16: 2443–2457, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyska M, Mooseker M. A role for myosin-1A in the localization of a brush border disaccharidase. J Cell Biol 165: 395–405, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL. Functional activity of Pat-1 (Slc26a6) Cl(-)/HCO(3)(-) exchange in the lower villus epithelium of murine duodenum. Acta Physiol (Oxf) 201: 21–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker NM, Simpson JE, Yen P, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3− exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology 135: 1645–1653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–413, 2005 [DOI] [PubMed] [Google Scholar]