Abstract
The mouse intestinal mucus is mainly made up by the gel-forming Muc2 mucin and the stomach surface mucus Muc5ac, both extensively O-glycosylated. The oligosaccharide diversity provides a vast library of potential recognition sites for both commensal and pathogenic organisms. The mucin glycans are thus likely very important for the selection and maintenance of a stable intestinal flora. Here we have explored the O-glycan patterns of the mouse gastrointestinal tract mucins. The mucins from the mucus of the distal and proximal colon, ileum, jejunum, duodenum, and stomach of conventionally raised wild-type (C57BL/6) mice were separated by composite gel electrophoresis. The O-linked glycans were released by reductive elimination and structurally characterized by liquid chromatography-mass spectrometry. The mucins glycans were mostly core 2 type [Galβ1–3(GlcNAcβ1–6)GalNAcol], but also core 1 (Galβ1–3GalNAcol). In the stomach about half of the Muc5ac mucin O-glycans were neutral and many monosulfated, but with a low grade of sialylation and fucosylation. Mouse ileum, jejunum, and duodenum had similar glycan patterns dominated by sialylated and sulfated core 2 glycans, but few fucosylated. Colon was on the other hand dominated by highly charged fucosylated glycans. The distal colon is different from the proximal colon because different biosynthetic pathways are utilized, although sialylated and sulfated glycans were highly abundant in both parts. The sulfation was higher in the distal colon, whereas sialic acid was more common in the proximal colon. Many fucosylated glycans were found in both the proximal and distal colon. Thus the mucin O-glycans vary along the mouse gastrointestinal tract.
Keywords: glycomics, Mucin, Muc2, Muc5ac, mass spectrometry, oligosaccharide, glycan
about half of the proteins in nature are glycosylated and the glycans play important roles in many different biological processes, for example as adhesion partners for microorganisms. The complexity in carbohydrates is reflected by a high diversity between species, individuals, and also different tissues within the same individual. The selective pressure generated by the interaction between host and microorganisms during evolution is believed to be an important reason for its high glycan diversity (12). Mucins are the largest molecular carrier of glycans because they are heavily O-glycosylated proteins that make up the core of the mucus gel that covers the gastrointestinal tract (GIT). In the colon and small intestine, the MUC2 mucin dominates, whereas MUC5AC is the main mucin in the stomach (6, 14, 38). The stomach epithelium is covered by a two-layered mucus with an inner attached layer, whereas the small intestine is covered by a loosely attached mucus layer (3, 10). Colon has a two-layered mucus with an inner layer devoid of bacteria, whereas the outer layer is the habitat of commensal bacteria (24). MUC2 forms dimeric COOH-termini and trimeric NH2-termini, which together create large polymeric netlike structures that make up the stratified inner mucus layer (1, 23).
The mucin O-glycans constitute up to 80% of the total mucin mass and a fully glycosylated MUC2 monomer reaches ∼2.5 MDa in mass. An important function of the mucin glycans is to cover the protein backbone and by this protect the mucin from digestive and bacterial proteases. Just as important is the mucins capacity to bind water mediated by these glycans, something that is necessary for the gel-forming mucins to form mucus gels. O-glycosylation takes place in the Golgi apparatus by the attachment of GalNAc residues to the hydroxyl group of serine (S) and threonine (T) of the protein backbone to form GalNAcα1-O-S/T (also called Tn antigen). This glycan is then further elongated on position C3 or C6 of GalNAc to form what is called core 1, 2, 3, and 4 structures (22). These are then further elongated with short or long lactosamine chains terminated by fucose, sialic acid, sulfate groups, or ABO or Lewis-type histo-blood group epitopes. The formation of O-glycans is catalyzed by many different glycosyltransferases with different specificities, responsible for the transfer of a single sugar unit from a nucleotide sugar to the glycan (7). The human intestinal tract has a gradient of O-glycan types from ileum to the distal colon (37). Charged glycans are more predominant in the distal colon, as is the Sd/CAD epitope [GalNAcβ1–4(NeuAcα2–3)Galβ1−] (9). A reverse gradient is found for fucosylated glycans because ABO histo-blood group antigens are more abundant in the proximal parts of the intestine. This suggests specific functional properties of the glycans that could be important for the selection of a specific microbiota. This idea is additionally supported by the observation of regiospecific bacterial colonization of certain species in different parts of the GIT (43). We have previously shown that the human sigmoid colon MUC2 O-glycans have a complex glycosylation pattern but are relatively uniform and constant between individuals and not affected by their blood groups (18). Since this is in contrast to all other mucosal surfaces, we hypothesized that this could be related to selection of the commensal flora in the distal colon and that O-glycans generally could be involved in such microbial selection processes. In fact, such a model was suggested by Rawls et al. (35) studying cross-species transplantation of microbiota between mouse and zebrafish where the host selected its innate bacterial habitants. Others have also suggested that mucin O-glycans can act as attachment sites for different bacteria (26, 28). Commensal bacteria typically produce glycosidases and can thus also use the glycans as food source, giving a mutualistic relationship between the host and its bacteria (5, 20). The commensal bacteria further suppress pathogenic microorganisms by occupying all the niches available. The mucin O-glycosylation is a dynamic system and glycan patterns are commonly altered during diseases like cancer, and as a secondary effect due to inflammation, for example in ulcerative colitis (17). These patients also have an altered bacterial composition and also a decreased microbial diversity, something that could be related to the observed changes in mucin O-glycan pattern (41).
Human and mouse GIT Muc2 O-glycosylation have been analyzed in a few previous studies, but not with a full coverage of all parts and with less informative techniques (18, 19, 29, 47). Recently, we published a description of the Muc2 O-glycans in the distal colon of wild-type (WT) and Core1- and Core3-deficient mice (48). The glycans found in the GIT total tissue from mouse had been previously analyzed with matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS), an approach less efficient in detecting sulfated glycans common in intestinal mucins (21). In the present study, we have focused on the O-glycans of the Muc2 and Muc5ac mucins, since they make up most of the glycome of the GIT mucus layers and are important for the interaction between commensal bacteria and the host. We performed a structural characterization of the mucin O-glycans of the stomach, duodenum, jejunum, ileum, and proximal and distal colon of WT mice (C57BL/6) with capillary (graphitized carbon) liquid chromatography-mass spectrometry (LC-MS). This approach has been proved sensitive and specific for mucin O-glycans.
MATERIALS AND METHODS
Animals and mucin sample preparation.
Conventional C57BL/6 mice (Taconic) were in-house bred (10). Five WT mice were euthanized; mucosal scrapings from 2-cm sections of stomach, duodenum, jejunum, ileum, proximal colon, and distal colon were collected; and the mucins were semipurified as previously described (18). Briefly, mucosal scrapings were repeatedly extracted with guanidinium hydrochloride (6 M with 5 mM EDTA, 0.01 M NaH2PO4) in the presence of protease inhibitors (Complete, Roche), followed by reduction (50 mM dithiothreitol) and alkylation (125 mM iodoacetamide) of the Muc2-containing pellet. The solubilized material was dialyzed against water and lyophilized.
SDS-agarose composite gel electrophoresis.
A gel containing a gradient of agarose (0.5–1%), acrylamide (0–6%), and glycerol (0–10%) was prepared (42). The samples (10%) were boiled in sample buffer, loaded, and separated in borate/Tris buffer (192 mM boric acid, 1 mM EDTA, 0.1% SDS, pH 7.6 with Tris base) at 12 mA/gel in ice overnight. The gels were stained with Alcian blue, periodic acid-Schiff (PAS), or Direct blue 71 stain (Sigma).
Identification of proteins by proteomics.
Alcian blue and Direct blue 71-stained bands were excised and destained with 50% acetonitrile and 25 mM ammonium bicarbonate. Gel pieces were dried, reduced with dithiothreitol (10 mM), alkylated with iodoacetamide (55 mM), and digested with 10 μg/ml trypsin (Promega) in 25 mM ammonium bicarbonate (37°C overnight). Peptides were eluted in 50% acetonitrile-2% trifluoroacetic acid and reextracted with 50% acetonitrile-0.2% trifluoroacetic acid. Eluted peptides were pooled, dried, and resuspended in 0.1% formic acid (18 μl) and analyzed by LC-MS using an LTQ Orbitrap XL mass spectrometer (Thermo Scientific) (38, 39). Searches were performed against an in-house database containing mucin sequences (www.medkem.gu.se/mucinbiology/databases), the UniProt-SwissProt mouse database, and a decoy database.
Mucin glycan release.
Mucins were separated on SDS-agarose composite gels and semidry blotted at 24 W (60–70 mA) for 1.5 h to Immobilon (PVDF PSQ) membranes (15). The bands were visualized with Alcian blue or Direct blue 71 stains, and oligosaccharides were released by reductive β-elimination (0.5 M NaBH4 in 50 mM KOH, 50°C overnight), desalted, lyophilized, and resuspended in H2O (15 μl).
Capillary LC/MS analysis of mucin O-glycans.
Porous graphitized carbon (PGC, 5-μm particles, Hypercarb, Thermo Hypersil, Keystone, CO) HPLC columns were packed (10 cm × 250 μm ID) and used at flow rates of 7 μl/min. The mobile phase gradient was 0–45% acetonitrile/water (80/20) with addition of 10 mM ammonium bicarbonate. The instrument was an LTQ (linear trap quadrupole) mass spectrometer (Thermo Fisher Scientific, San Jose, CA) (18, 48). The glycans from the stomach were analyzed in both negative and positive ion mode, whereas the intestinal samples were analyzed only in the negative ion mode. A normalized collision energy of 35 eV was used. Obtained MSn spectra were interpreted manually by aid of comparison to published reference spectra (48) and an in-house database (www.medkem.gu.se/mucinbiology/databases).
Reagents.
HPLC grade acetonitrile and analytical grade acetic acid and methanol were from Merck (Darmstadt, Germany). All other chemicals, unless otherwise stated, were purchased from Sigma (St. Louis, MO). Maxymum recovery 1.7-ml tubes (Axygen, Union City, CA) were used throughout the procedure.
RESULTS AND DISCUSSION
Composite gel electrophoresis and identification of mucins.
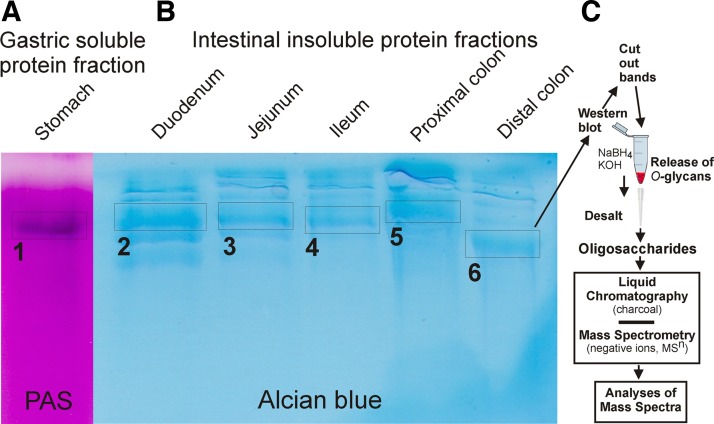
The mucins from mucosal scrapings of the stomach, duodenum, jejunum, ileum, and distal and proximal colon of WT C57BL/6 mice were semipurified by guanidinium hydrochloride (GuHCl) extraction. The GuHCl-insoluble mucin fractions, which reflect the intracellular stored mucin and the inner mucus layer of colon from the intestinal tract (containing >80% of the Muc2), were reduced, alkylated, and separated by composite gel electrophoresis. The gel was stained by Alcian blue for negatively charged mucins, revealing several bands due to Muc2 mono- (lower bands, estimated size 2.5 MDa) and oligomers (Fig. 1, Alcian blue) (4, 16). From the stomach, both the GuHCl-insoluble and -soluble fractions were separated on composite gels and stained with PAS stain, revealing that the GuHCl-soluble fraction contained a large glycosylated protein band (Fig. 1, PAS).
Fig. 1.
SDS-polyacrylamide-agarose composite gel electrophoresis of the guanidinium hydrochloride (GuHCl)-soluble fraction of mucosal scrapings from mouse stomach (A), and the GuHCl-insoluble fraction from mouse duodenum, jejunum, ileum, proximal colon, and distal colon (B). Staining with periodic acid-Schiff (PAS) in (A) and Alcian blue in (B). Bands 1–6 denote the glycoprotein bands used for glycan release on the corresponding blotted membranes. Band 1 contains Muc5ac and bands 2–6 contain mainly Muc2. C: schematic figure explaining the preparation and analysis procedure for the Muc2 mucin O-glycans.
Stained protein bands were excised and trypsinized, the peptides were eluted from the gel pieces, and the bands were analyzed by mass spectrometry (Fig. 1, A and B). The PAS-stained band from the stomach, band 1, contained only Muc5ac peptides. All other protein bands, including bands labeled 2–6, contained mainly Muc2 peptides, but also a few peptides from Fcgbp (Fc-gamma binding protein), which is known to be covalently attached to Muc2 (25). Protein bands 2, 4, and 5 also contained trace amounts of the Muc13 mucin.
Release and analysis of the mucin oligosaccharides.
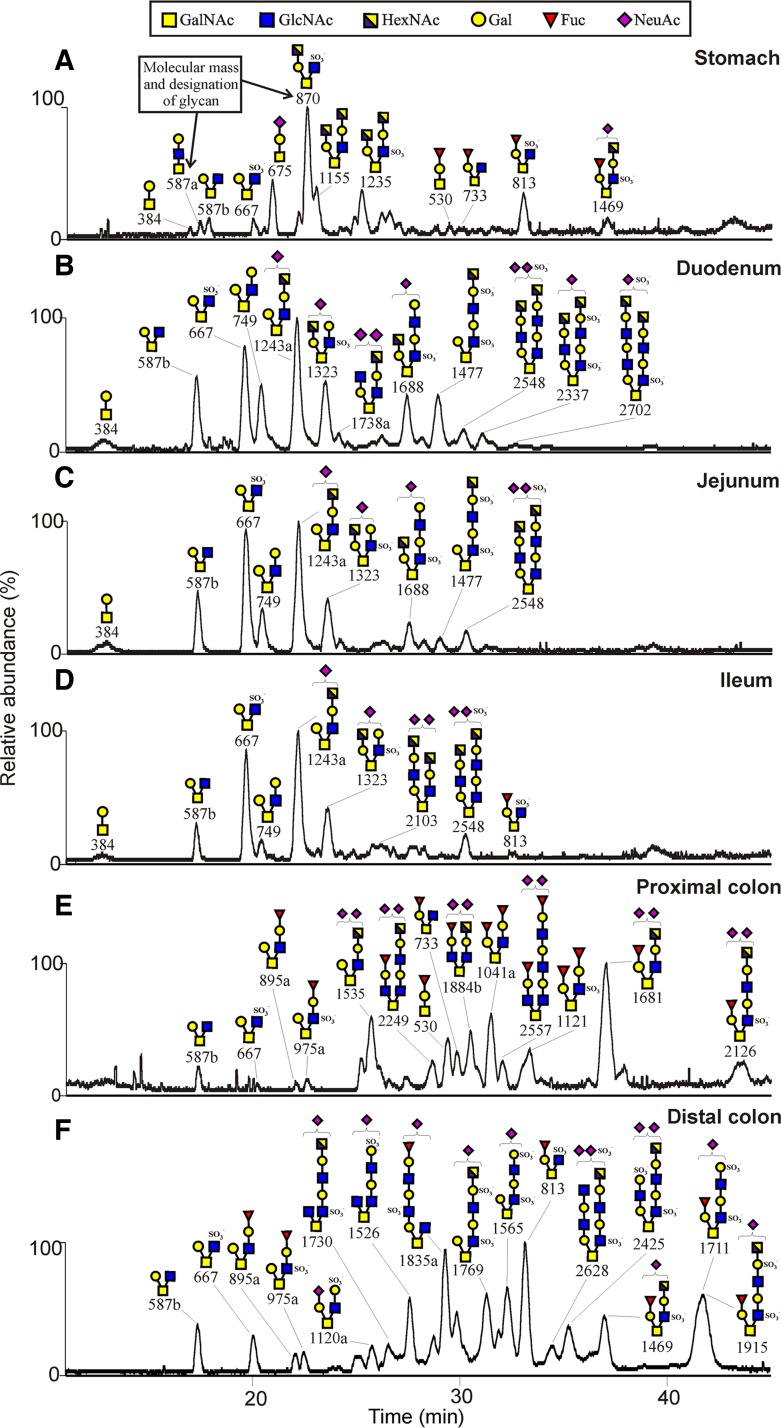
The procedure is explained in Fig. 1C. GuHCl-insoluble mouse mucin fractions from the colon and small intestine, the GuHCl-soluble fractions from the stomach were separated on composite gels as shown in Fig. 1, and the proteins were blotted to a PVDF membrane. From the stained membranes, the blotted protein bands corresponding to bands 1–6 in Fig. 1 were excised and the O-glycans were released by reductive β-elimination (42). The glycans were desalted and analyzed by graphitized carbon liquid-chromatography coupled to a mass spectrometer. The glycans were detected as singly, doubly, or triply charged ions depending on the number of charged substituents, further fragmented by collision-induced dissociation, and recorded as MS2, MS3, and MS4 spectra. The glycan structures were interpreted from the obtained spectra as done previously (17, 29, 36, 48). For the structural interpretations the following assumptions were made: hexose is Gal, deoxyhexose is Fuc, internal N-acetylhexosamine is GlcNAc, and reduced N-acetylhexosamine is GalNAcol (before release attached to the serine or threonine of the mucin protein core). When the N-acetylhexosamine was terminal and its identity less clear, it was assigned as HexNAc. Fig. 2 shows the chromatograms of the analysis of the released mucin O-glycans with the major glycans annotated from the stomach (A), duodenum (B), jejunum (C), ileum (D), proximal colon (E), and distal colon (F). The chromatographic profiles reveal complex glycan patterns that differ substantially between stomach, small intestine, and colon, as discussed below. Supplemental Table S1 shows the detailed structures of all identified O-glycans. The characteristic structural features of the glycans on mouse Muc5ac and Muc2 mucins in the different parts of the GIT are summarized in Fig. 3.
Fig. 2.
Chromatograms of released O-glycans from C57BL/6 mouse mucins (Muc5ac in stomach and Muc2 in the intestinal tract) analyzed by liquid chromatography-mass spectrometry as explained in Fig. 1C. Stomach (A), duodenum (B), jejunum (C), ileum (D), proximal colon (E), and distal colon (F). The proposed glycan structures of the major components are annotated as their mass (m/z, z = 1). Supplemental Table S1 contains the full list of all components identified. The graphs show the base peak chromatograms. The bottom GalNAc in each structure has been attached to the protein core and is recorded as a reduced GalNAc (alditol) formed by reduction (NaBH4) during release.
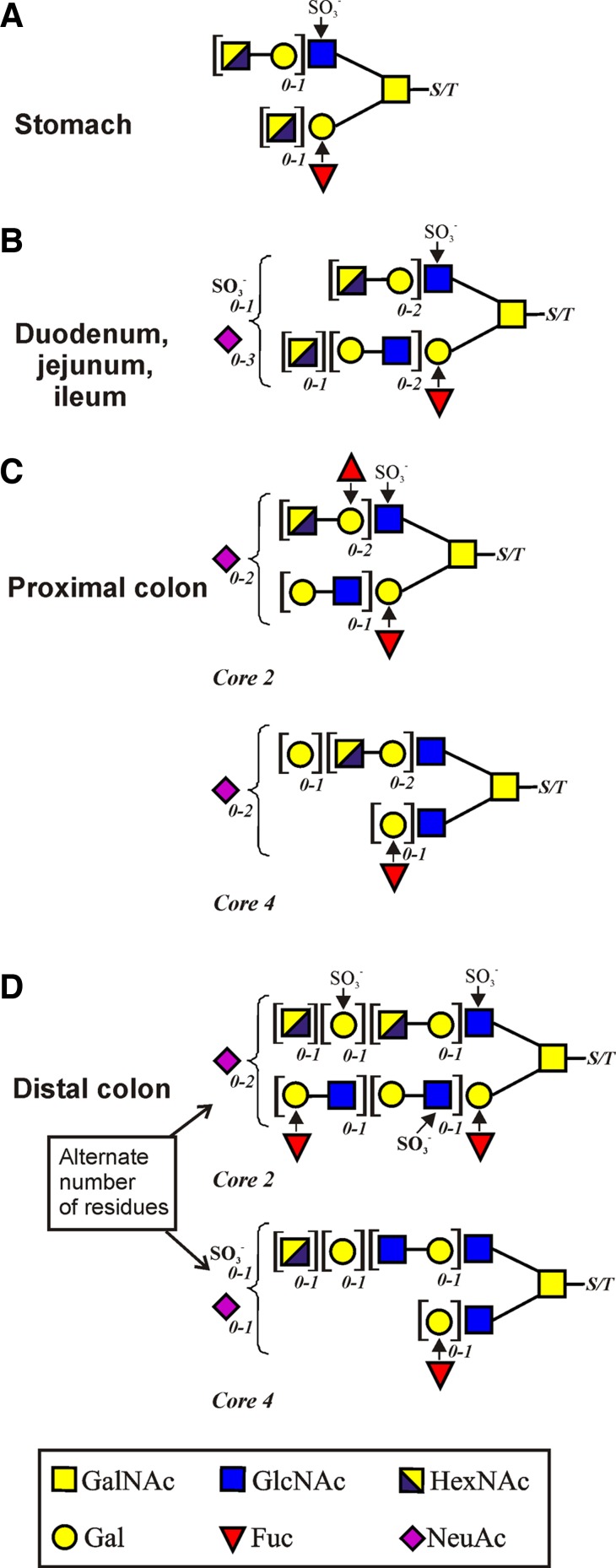
Fig. 3.
Summary of general structures of the mucin O-glycans in the different parts of the gastrointestinal tract. Stomach (on Muc5ac) (A); duodenum, jejunum, and ileum (on Muc2) (B); proximal colon (Muc2) (C); and distal colon (on Muc2) (D). The right-hand GalNAc is attached to the amino acids serine (S) or threonine (T). Square brackets denote that these residues can appear 0, 1, or 2 times as indicated by the italic subscript numbers.
Gastric Muc5ac O-glycans.
The Muc5ac is the dominating mucin in the stomach. In our study, its O-glycans were released from the mouse Muc5ac band corresponding to band 1 in Fig. 1. All identified structures were based on the core 2 substructure [Galβ1–3(GlcNAcβ1–6)GalNAcol] formed from the core 1 precursor (Galβ1–3GalNAcol) by the enzyme core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT). The gastric O-glycans are largely devoid of sialic acid (NeuAc) substitutions and typically elongated with lactosamine units (Gal-GlcNAc) on both the C3 and C6 branches of GalNAcol (Fig. 2A, Fig. 3, and Supplemental Table S1). A monosulfated glycan series having a sulfate group attached to the inner GlcNAc residue of the core 2 substructure was observed. The monosulfated glycan labeled 870, HexNAc-Gal-3(SO3−GlcNAc-6)GalNAcol, was the major glycan present, and its C6 elongated form (labeled 1235), HexNAc-Gal-3[HexNAc-Gal-(SO3−)GlcNAc-6]GalNAcol, was also relatively abundant. Only a few monosialylated glycans were identified, as well as one abundant fucosylated component (labeled 813), although several minor fucosylated components were also found. The glycan labeled 1469 was carrying both a sulfate group, a fucose, and a sialic acid residue (Fig. 2A and Supplemental Table S1). The gastric mucin O-glycans observed in mouse differ substantially compared with human gastric mucin O-glycans, since human MUC5AC mainly carries mono- and difucosylated glycans forming ABO blood group antigens (40), whereas the glycan labeled 813 is the only highly abundant compound on mouse Muc5ac that carries the blood group H antigen (Fucα1–2Galβ1−). The mouse stomach, like the porcine, has a higher level of sulfation than the human (49). The Muc5ac O-glycan structures were substantially different from the colonic and small intestinal Muc2 O-glycans.
Small intestinal Muc2 O-glycans.
The O-glycans were released from the mouse small intestine monomeric Muc2 band (bands 2–4 in Fig. 1). The three parts of the small intestine are almost identical in their O-glycan profile with a less complex mucin O-glycosylation pattern compared with stomach and colon (Fig. 2, B–D, Fig. 3, and Supplemental Table S1). All components in the small intestine are of core 2 type, as in the stomach. Among the major O-glycans in the three parts are the component 587b [Gal-3(HexNAc-6)GalNAcol], its sulfated equivalent 667 [Gal-3(SO3− GlcNAc-6) GalNAcol], and its Gal elongated form labeled 749 [Gal-3(Gal-GlcNAc-6)GalNAcol] (Fig. 2, B–D and Supplemental Table S1). The 749 glycan is the precursor for the highly abundant glycan 1243a ([NeuAc]Gal-3(HexNAc-Gal-GlcNAc-6)GalNAcol) where the site of sialic acid substitution was not possible to determine (Supplemental Table S1). In addition, a few larger glycans were found being elongated with lactosamine units and appearing as disialylated and monosulfated glycans (labeled 1477, 1688, 2548). The fucosylation grade was very low throughout the small intestine, with only one monofucosylated glycan detected in ileum (glycan 813). This is in contrast to the human small intestine, where the fucosylation is high in ileum, especially compared with colon (36, 37). A majority of the glycans in the mouse small intestine is mono- or disialylated, again differing from the human intestine. The small intestinal Muc2 O-glycan pattern was very different from colon.
Colonic Muc2 O-glycans.
The O-glycans were released from the proximal and distal colon monomeric Muc2 band (bands 5–6 in Fig. 1). Several disialylated glycans were present in the proximal colon, probably owing to an active sialyltransferase in this region (46). The majority of the structures were, as in the other parts of the intestine, based on the core 2 substructure [Galβ1–3(GlcNAcβ1–6)GalNAcol] formed by the core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT), of which the isoforms C2GnT2 and C2GnT1 are known to be highly expressed in mouse colon (45) (Fig. 2, E and F, Fig. 3, and Supplemental Table S1). In both proximal and distal colon, a few core 4 [GlcNAcβ1–3(GlcNAcβ1–6)GalNAcol] glycans were found (Fig. 2, E and F). One core 4 glycan in the mouse proximal colon was the 1884b glycan, which is also present in the human distal colon in three different core 4 isoforms (18). Most abundant in proximal colon were the components 1681, 1041a, and 1535. No core 3 structures were present on the mouse Muc2 mucins, in contrast to the human distal colon, where core 3 dominates (9, 18, 34, 36).
The glycans of the distal and proximal colon differed substantially, although some precursor glycans were common for the two parts (glycans 587b, 667, 895a and 975a; Fig. 2, E and F, Fig. 3, and Supplemental Table S1). Two completely different sets of O-glycans are being formed as different biosynthetic pathways are utilized in the two parts of the colon, although there are common features since both sets are sialylated and sulfated. Many mono- and disulfated structures were found in the distal colon, whereas the proximal colon seemed to have lower levels of sulfotransferases, as just a few monosulfated glycans were identified. The GlcNAc6ST-2 transferase has been shown to be a major sulfation enzyme in the murine colon (50). The sulfate group is usually attached to the GlcNAc residue of the core 2 substructure linked to GalNAcol. For the disulfated components, an additional sulfate group is commonly attached to the Gal residue on the repeating lactosamine chain on the C6 branch of GalNAcol. In the distal colon most glycans were elongated on the C6 branch of GalNAcol (glycans 1565, 1711, 1769, and 1915), but there were also glycans elongated on the C3 of the GalNAcol (glycans 1835a) (Fig. 2F and Supplemental Table S1). The fucosylation grade was high in both the distal and proximal colon with a majority of the glycans being monofucosylated. This is in agreement with what is found on the distal human colon MUC2, with many sialylated and sulfated glycans, but also many monofucosylated glycans (18).
Comparison of GIT mucin O-glycans.
In all parts of the mouse GIT, the O-glycans were dominated by the core 2 substructure [Galβ1–3(GlcNAcβ1–6)GalNAcol]. In the stomach, the terminal epitopes were mainly neutral or monosulfated and differed substantially from the more distal parts of the GIT. This is also different compared with the human MUC5AC that carries mainly neutral core 2-glycans with terminal ABO blood group antigens (30, 40). Since the binding sites for Helicobacter pylori are known to be of glycan nature, this can explain the difficulties in infecting mice with this human pathogen (32). The mouse small intestinal Muc2 O-glycans showed a less complex glycosylation pattern, similar in duodenum, jejunum, and ileum. The scarce fucosylation in the small intestine is surprising since this is in sharp contrast to human small intestine, where fucosylation is highly prevalent (36). In contrast, the mouse Muc2 was mono- or disialylated. Most of the mouse colon Muc2 O-glycans were based on the core 2 substructure, but also a few core 4 [GlcNAcβ1–3(GlcNAcβ1–6)GalNAcol] glycans were detected. In the human colon, the core 3 is the most predominant one, but in human sigmoid colon a few core 4 glycans were identified (18), in line with the mouse colon. Interestingly, the glycans of the proximal and distal mouse colon differ considerably, since two completely different sets of O-glycans are formed in the proximal and distal parts. An epitope commonly found on the human colon MUC2 O-glycans is the NeuAc residue linked α2–6 to the core GalNAcol, an epitope absent in mouse colon, where instead NeuAc is commonly linked to internal GlcNAc residues (18, 36, 48).
Importance of mucin O-glycans.
The importance of the mucin glycans has been demonstrated in several mouse models deficient in glycosyltransferases. Both the mice lacking core 1 β1,3-galactosyltransferase (C1GalT1, also called T-synthase) and the core 3 β1,3N-acetylglucosaminyltransferase (C3Gnt) are more prone to develop colon inflammation, especially after dextran sodium sulfate (DSS) challenge (2, 11, 48). In the C1GalT1−/− mice, mostly core 4 [GlcNAcβ1–3(GlcNAcβ1–6)GalNAcol] Muc2 O-glycans are formed, a quite drastic change compared with WT mice. Also the core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT) knockout mice are more sensitive to DSS (21). The Sda/CAD epitope [GalNAcβ1–4(NeuAcα2–3)Galβ1−] is common in human colon, but rare in mouse (9). The last step in the biosynthesis of the Sda/CAD epitope, when a GalNAc residue is added to the sialylated precursor, is catalyzed by the β1,4N-acetylgalactosaminyltransferase 2 (B4Galnt2). The B4Galnt2−/− mouse has been shown to have an altered intestinal microbiota (44). Together these results suggest that the O-glycosylation of the GIT is important for an intact mucus interaction with bacteria. The link between host mucin glycosylation could be bacterial binding, something that has been suggested for a few commensal bacteria, of which the most studied bacterial strains are Lactobacillus rhamnosus, Bacteroides fragilis, and Lactobacillus reuteri, the latter encoding for mucus-binding proteins (8, 26, 27).
Mucin O-glycosylation is a dynamic system and it is common that glycan patterns are changed during inflammation, something that has been demonstrated in murine infection models using the intestinal parasite Nippostrongylus brasiliensis. Transient glycosylation changes could be identified in the small intestine of both rats and mice during the infection cycle, and the responsible glycosyltransferases were identified. In the mouse model the Fut2 transferase was upregulated (19) and in rats a blood group A transferase (33). It can be speculated that the induced glycosyltransferase expressions were due to inflammation, something that is also suggested from the transient glycosylation alterations during ulcerative colitis relapses (17). In fact, it has been observed that certain glycosyltransferases can be regulated by proinflammatory cytokines (13).
Conclusions.
The combined results show that the mucus organization and O-glycans vary considerably along the mouse GIT, whereas the mucus proteome is less variable (10, 38). Although all details of mucus organization and proteome of human GIT are not known, the mouse and human mucus seem to be similar. This is in contrast to the GIT mucin glycome, where the two species differ more. Human mucin O-glycans for example carry substantial amounts of terminal ABO blood group determinants, which are not observed in mouse and could thus be one possible explanation for their large differences in microbiota, since mouse and human only share 15% of their intestinal (cecal) microbiota (31). Thus different species have a relative specific intestinal bacterial flora that is at least partly based on a host selection processes (35). Different bacteria are also known to have specific niches along the GIT. Because the mucin O-glycome varies, whereas the proteome is relatively stable, it is likely that the differences in mucin glycan structures are involved in the bacterial selection process by bacterial adhesin-mucin glycan interactions. The present understanding of the mouse mucin glycome allows manipulation of mice to obtain a humanized mucin O-glycans as well as manipulation of bacterial adhesins, all in attempts to better understand the host-bacterial selection. The mucin O-glycans are likely important for the maintenance of a stable, but regiospecific, GIT flora (43).
GRANTS
This work was supported by the Swedish Research Council (grants 7461, 21027), Swedish Cancer Foundation, Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (LUA-ALF), Wilhelm and Martina Lundgren Foundation, Torsten and Ragnar Söderberg Foundation, Adlerbert Research Foundation, the Sahlgrenska Academy, the National Institute of Allergy and Infectious Diseases (U01AI095473; the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), and the Swedish Foundation for Strategic Research - Mucus, Bacteria, Colitis Center (MBC, Innate Immunity Program).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.H.L., K.A.T., and G.C.H. conception and design of research; J.M.H.L. and A.M.R.-P. performed experiments; J.M.H.L., K.A.T., A.M.R.-P., H.K., and G.C.H. analyzed data; J.M.H.L., K.A.T., A.M.R.-P., H.K., and G.C.H. interpreted results of experiments; J.M.H.L., H.K., and G.C.H. prepared figures; J.M.H.L., A.M.R.-P., and G.C.H. drafted manuscript; J.M.H.L., A.M.R.-P., H.K., and G.C.H. edited and revised manuscript; J.M.H.L., K.A.T., A.M.R.-P., H.K., and G.C.H. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1. Ambort D, Johansson MEV, Gustafsson JK, Nilsson H, Ermund A, Johansson BR, Kock P, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the Gel-forming MUC2 mucin. Proc Natl Acad Sci USA 109: 5645–5650, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med 204: 1417–1429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atuma C, Strugula V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Axelsson MAB, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem 273: 18864–18870, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 307: 1915–1920, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Bara J, Chastre E, Mahiou J, Singh RL, Forguelafitte ME, Hollande E, Godeau F. Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer 75: 767–773, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22: 736–756, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boekhorst J, Helmer Q, Kleerebezem M, Siezen RJ. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology 152: 273–280, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Capon C, Maes E, Michalski JC, Leffler H, Kim YS. Sd(a)-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem J 358: 657–664, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ermund A, Schutte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am J Physiol Gastrointest Liver Physiol. First published July 5, 2013; 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, McDaniel M, Sferra TJ, Turner J, Chen H, Hansson GC, Braun J, Xia L. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis. J Clin Invest 121: 1657–1666, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9: 747–755, 1999. [DOI] [PubMed] [Google Scholar]
- 13. Groux-Degroote S, Krzewinski-Recchi MA, Cazet A, Vincent A, Lehoux S, Lafitte JJ, Van Seuningen I, Delannoy P. IL-6 and IL-8 increase the expression of glycosyltransferases and sulfotransferases involved in the biosynthesis of sialylated and/or sulfated Lewisx epitopes in the human bronchial mucosa. Biochem J 410: 213–223, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem 269: 2440–2446, 1994. [PubMed] [Google Scholar]
- 15. Hayes CA, Nemes S, Issa S, Jin C, Karlsson NG. Glycomic work-flow for analysis of mucin O-linked oligosaccharides. Methods Mol Biol 842: 141–163, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Herrmann A, Davies JR, Lindell G, Martensson S, Packer NH, Swallow DM, Carlstedt I. Studies on the “insoluble” glycoprotein complex from human colon. J Biol Chem 274: 15828–15836, 1999. [DOI] [PubMed] [Google Scholar]
- 17. Holmén Larsson JM, Karlsson H, Gråberg Crespo J, Johansson MEV, Eklund L, Sjövall H, Hansson GC. An altered O-glycosylation profile of the MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis 17: 2299–2307, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Holmen Larsson JM, Karlsson H, Sjovall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology 19: 756–766, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Holmen JM, Olson FJ, Karlsson H, Hansson GC. Two glycosylation alterations of mouse intestinal mucins due to infection caused by the parasite Nippostrongylus brasiliensis. Glycoconj J 19: 67–75, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291: 881–884, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Ismail MN, Stone EL, Panico M, Lee SH, Luu Y, Ramirez K, Ho SB, Fukuda M, Marth JD, Haslam SM, Dell A. High-sensitivity O-glycomic analysis of mice deficient in core 2 β1,6-N-acetylglucosaminyltransferases. Glycobiology 21: 82–98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen PH, Kolarich D, Packer NH. Mucin-type O-glycosylation—putting the pieces together. FEBS J 277: 81–94, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Johansson MEV, Holmen Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108: 4659–4665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johansson MEV, Phillipson M, Petersson J, Holm L, Velcich A, Hansson GC. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johansson MEV, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res 8: 3549–3557, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Juge N. Microbial adhesins to gastrointestinal mucus. Trends Microbiol 20: 30–39, 2012. [DOI] [PubMed] [Google Scholar]
- 27. Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx APA, Lebeer S, De Keersmaecker SCJ, Vanderleyden J, Hamalainen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjärvi T, Auvinen P, de Vos WM. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc Natl Acad Sci USA 106: 17193–17198, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karlsson KA. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem 58: 309–350, 1989. [DOI] [PubMed] [Google Scholar]
- 29. Karlsson NG, Herrmann A, Karlsson H, Johansson MEV, Carlstedt I, Hansson GC. The glycosylation of rat intestinal Muc2 mucin varies between rat strains and the small and large intestine. A study of O-linked oligosaccharides by a mass spectrometric approach. J Biol Chem 272: 27025–27034, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Kenny DT, Skoog EC, Lindén SK, Struwe WB, Rudd PM, Karlsson NG. Presence of terminal N-acetylgalactosamineβ1–4N-acetylglucosamine residues on O-linked oligosaccharides from gastric MUC5AC: involvement in Helicobacter pylori colonization? Glycobiology 22: 1077–1085, 2012. [DOI] [PubMed] [Google Scholar]
- 31. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore ME, Boren T, Solnick JV. Life at the margins: modulation of attachment proteins in Helicobacter pylori. Gut Microbes 2: 42–46, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olson FJ, Johansson MEV, Klinga-Levan K, Bouhours D, Enerback L, Hansson GC, Karlsson NG. Blood group A glycosyltransferase occurring as alleles with high sequence difference is transiently induced during Nippostrongylus brasiliensis parasite infection. J Biol Chem 277: 15044–15052, 2002. [DOI] [PubMed] [Google Scholar]
- 34. Podolsky DK. Oligosaccharide structures of human colonic mucin. J Biol Chem 260: 8262–8271, 1985. [PubMed] [Google Scholar]
- 35. Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127: 423–433, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robbe C, Capon C, Coddeville B, Michalski JC. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem J 384: 307–316, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robbe C, Capon C, Maes E, Rousset M, Zweibaum A, Zanetta JP, Michalski JC. Evidence of regio-specific glycosylation in human intestinal mucins: presence of an acidic gradient along the gastrointestinal tract. J Biol Chem 278: 46337–46348, 2003. [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez-Pineiro AM, Bergstrom JH, Ermund A, Gustafsson JK, Schutte A, Johansson MEV, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am J Physiol Gastrointest Liver Physiol. First published July 5, 2013; 10.1152/ajpgi.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez-Pineiro AM, van der Post S, Johansson MEV, Thomsson KA, Nesvizhskii AI, Hansson GC. Proteomic study of the mucin granulae in an intestinal goblet cell model. J Proteome Res 11: 1879–1890, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossez Y, Maes E, Darroman TL, Gosset P, Ecobichon C, Curt MJC, Boneca IG, Michalski JC, Robbe-Masselot C. Almost all human gastric mucin O-glycans harbor blood group A, B or H antigens and are potential binding sites for Helicobacter pylori. Glycobiology 22: 1193–1206, 2012. [DOI] [PubMed] [Google Scholar]
- 41. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594, 2013. [DOI] [PubMed] [Google Scholar]
- 42. Schulz BL, Packer N, Karlsson NG. Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal Chem 74: 6088–6097, 2002. [DOI] [PubMed] [Google Scholar]
- 43. Sekirov I, Russel SL, Antunes LCM, Finlay B. Gut microbiota in health and disease. Physiol Rev 90: 859–904, 2010. [DOI] [PubMed] [Google Scholar]
- 44. Staubach F, Kunzel S, Baines AC, Yee A, McGee BM, Backhed F, Baines JF, Johnsen JM. Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J 6: 1345–1355, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, Ho SB, Dell A, Fukuda M, Marth JD. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol 29: 3770–3782, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takashima S. Characterization of mouse sialyltransferase genes: their evolution and diversity. Biosci Biotechnol Biochem 72: 1155–1167, 2008. [DOI] [PubMed] [Google Scholar]
- 47. Thomsson KA, Hinjosa-Kurtzberg M, Axelsson KA, Domino SE, Lowe JB, Gendler SJ, Hansson GC. Intestinal mucins from cystic fibrosis mice show increased fucosylation due to an induced Fuca1–2 glycosyltransferase. Biochem J 367: 609–616, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomsson KA, Holmen-Larsson J, Angstrom J, Johansson MEV, Xia L, Hansson GC. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology 22: 1128–1139, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomsson KA, Karlsson H, Hansson GC. Sequencing of sulfated oligosaccharides from mucins by liquid chromatography and electrospray ionization tandem mass spectrometry. Anal Chem 72: 4543–4549, 2000. [DOI] [PubMed] [Google Scholar]
- 50. Tobisawa Y, Imai Y, Fukuda M, Kawashima H. Sulfation of colonic mucins by N-acetylglucosamine-6-O-sulfotransferase-2 and its protective function in experimental colitis in mice. J Biol Chem 285: 6750–6760, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.