Abstract
Colon has been shown to have a two-layered mucus system where the inner layer is devoid of bacteria. However, a complete overview of the mouse gastrointestinal mucus system is lacking. We now characterize mucus release, thickness, growth over time, adhesive properties, and penetrability to fluorescent beads from stomach to distal colon. Colon displayed spontaneous mucus release and all regions released mucus in response to carbachol and PGE2, except the distal colon and domes of Peyer's patches. Stomach and colon had an inner mucus layer that was adherent to the epithelium. In contrast, the small intestine and Peyer's patches had a single mucus layer that was easily aspirated. The inner mucus layer of the distal colon was not penetrable to beads the size of bacteria and the inner layer of the proximal colon was only partly penetrable. In contrast, the inner mucus layer of stomach was fully penetrable, as was the small intestinal mucus. This suggests a functional organization of the intestinal mucus system, where the small intestine has loose and penetrable mucus that may allow easy penetration of nutrients, in contrast to the stomach, where the mucus provides physical protection, and the colon, where the mucus separates bacteria from the epithelium. This knowledge of the mucus system and its organization improves our understanding of the gastrointestinal tract physiology.
Keywords: mucin, goblet cells, bacteria, mucus adhesiveness, mucus thickness
the gastrointestinal tract (GIT) is subject to massive and continuous physical as well as chemical assault from ingested food; to extract nutrients from it, hydrochloric acid, digestive enzymes, and bile acids are released, together creating a potentially harsh environment for the single layer of columnar epithelial cells (4, 28). Although this varies by region, food and luminal contents contain large numbers of diverse microorganisms that can be potentially harmful if they breach the mucus or epithelial barrier (12). For protection, the gastrointestinal epithelium is covered by mucus in which the main constituent is the secreted gel-forming mucins: in the stomach MUC5AC and in the intestine MUC2, which are also the two most similar of the secreted mucins (15). Mucins are large glycoproteins in which the glycans make up more than 80% of the molecular mass. The central parts have a protein backbone containing sequences rich in proline, threonine, and serine, the so-called PTS-sequences, which are highly O-glycosylated and constitute the mucin domains. The gel-forming mucin MUC2 oligomerizes into large netlike polymers when the COOH- and NH2-termini form disulfide bond-stabilized di- and trimers (1). Mucins are produced, stored and released by specialized cells called goblet cells, characterized by their distended theca containing mucin granules (15). The number of goblet cells relative to enterocytes increases from the proximal to distal intestine, constituting 4, 6, 12, and 16% in the duodenum, jejunum, ileum, and distal colon, respectively (19).
There are relatively few bacteria residing in the stomach and the proximal small intestine (5), but the number of bacteria increases distally: the distal ileum has ∼108 bacteria per milliliter of luminal content and colon 1011. This large amount of bacteria is kept separated from the epithelium by an inner mucus layer physically impenetrable to bacteria (16). The bacteria on the other hand reside and thrive in the outer mucus layer (15). The host benefits from harboring bacteria since they degrade the mucins and ingested complex carbohydrates from the food into simple sugars that are converted into butyrate, propionate, and acetate supplied to the host (3).
Antigens in the bowel content are sampled by lymphoid follicles, which are part of the gut-associated lymphoid tissues of the adaptive immune system (12). In the small intestine the aggregated lymphoid follicles are organized in Peyer's patches, consisting of domes with large numbers of immune cells underneath a single epithelial cell layer called the follicle-associated epithelium (FAE). Interspersed among the FAE enterocytes are the M cells (6, 22), cells specialized for endocytosis and capable of passing antigens from the gut lumen to the underlying immune cells. Recently it has also been suggested that goblet cells can act as sampling sites for dendritic cells (20). Although much is known about the immune cells in the Peyer's patches, it is debated whether the domes of Peyer's patches are covered by mucus (21, 24).
The gastrointestinal mucus system has been relatively poorly explored and understood since it is essentially invisible and collapses upon common formaldehyde fixation. A large step forward was taken by Atuma et al. (2), who could visualize the luminal surface of the mucus by adding charcoal particles and could measure the mucus thickness. These studies were made in rats, but mice are commonly used for studies of gastrointestinal physiology and pathophysiology since these can be easily genetically manipulated. We have developed a methodology and performed studies on the mucus system of the distal colon (10, 16, 17), but to provide a more complete picture of gastrointestinal mucus we are now characterizing the normal mucus system of the whole GIT. Here we characterize the mucus system from stomach to distal colon, including Peyer's patches as mucus release, mucus thickness, growth over time, adhesive properties, and penetrability to fluorescent beads in C57BL/6 mice.
MATERIALS AND METHODS
Animals.
All animal procedures were approved by the local Laboratory Animal Ethics Committee, Gothenburg, Sweden. Mice were kept under specific pathogen-free conditions in individually ventilated cages under controlled temperature (21–22°C), humidity, and 12-h light-dark cycle. They were given standard chow and water ad libitum. Male and female C57BL/6 mice (age 8–16 wk) were euthanized by isoflurane and cervical dislocation.
Explant tissue.
Gastrointestinal tissue was dissected, flushed with ice-cold oxygenated (95% O2-5% CO2) Krebs transport buffer, and mounted in horizontal open Ussing-type chambers with a 4.9-mm2 circular opening as described previously (10). To avoid disturbing the mucus gel, the apical chamber was kept unstirred with a constant volume. Mucus release was stimulated by perfusing the explants basolaterally with a combination of the secretagogues carbachol and prostaglandin E2 (PGE2), 10 μM of each for 40 min.
Mucus thickness measurements.
Mucus measurements and video recordings were performed as described previously (9, 10). Mucus was aspirated with a Gilson Pipetman P200 (Middleton, WI) set to 150 μl and a yellow tip (no. 70.760.502, Sarstedt, Nümbrecht, Germany). In the small intestine, mucus was measured from the mucus surface to the villus tips, and afterward mucus was removed and the villus height was measured from the epithelium between the villi to the villus tips. Total mucus thickness is presented as the sum of these two measurements. To evaluate thickness of easily aspirated mucus, the whole apical volume was aspirated with a plastic Pasteur pipette (PP-101, outer tip diameter 0.9 mm, inner tip diameter 0.7 mm, maximum volume 800 μl, Cellprojects, Sutton Valence, UK) during approximately 3 s. The remaining mucus thickness was measured after refilling the apical chamber with 150 μl Krebs-mannitol and the addition of new charcoal particles.
Mucus penetrability.
Explants from stomach, small intestine, or colon were mounted in a horizontal imaging chamber and recorded as described previously (10, 13). The chamber was heated to 37°C during a period of 10 min and the apical buffer was removed and a suspension of 0.5 μm (red), 1 μm (far red), and 2 μm (green) fluorescent beads (FluoSpheres, Invitrogen, Carlsbad, CA) was added. The beads were allowed to settle in the mucus for 5 min before new Krebs-mannitol buffer was added and the beads left to sediment for 40 min. Confocal Z-stacks (optical section 2.8 μm, interval 10 μm) were taken to analyze the distribution of the different beads throughout the mucus, using an upright LSM 700 Axio Examiner Z.1 confocal imaging system with a Plan-Apochromat ×20/1.0DIC water objective (Carl Zeiss, Oberkochen, Germany). Volocity 6.0 (PerkinElmer, Waltham, MA) software was used to process images.
Histology and immunostaining.
Stomach tissue was fixed in Karnovsky fixative (2% paraformaldehyde, 2.5% glutaraldehyde in 0.05 M, sodium cacodylate buffer, pH 7.2) for 24 h followed by sequential staining using 1% OsO4 for 4 h, 1% tannic acid for 3 h, and 1% uranyl acetate overnight. Samples were dehydrated and embedded in epoxy resin (Agar 100, Agar Scientific, Stansted, UK). Sections (1 μm) were stained with periodic acid-Schiff. Whole tissue from stomach, duodenum, jejunum, ileum, and proximal and distal colon with content was fixed in methanol-Carnoy's fixative and stained after antigen retrieval (16) with the anti-MUC2C3 antiserum raised against a peptide from a nonglycosylated region (1:500) (16) or mouse monoclonal anti-MUC5AC (45M1, 1:2,000; Invitrogen). As secondary antibodies goat anti-rabbit Alexa 488 or goat anti-mouse Alexa 488 (Invitrogen) were used. DNA was stained by TO-PRO-3 iodide (1 μM, 642/661, Invitrogen) or DAPI (Invitrogen). Images were acquired via a fluorescence microscope, Eclipse E1000 with a Plan-Fluor ×40/0.75 DIC objective (Nikon, Amstelveen, The Netherlands), or an upright LSM 700 Axio Examiner.Z1 laser scanning confocal microscope with a Plan-Apochomat ×40/1.3 Oil DIC objective (Zeiss, Oberkochen, Germany) The pictures were processed uniformly by use of the ZEN 2010 software (Zeiss, Oberkochen, Germany), Volocity 6.0, and Adobe Photoshop.
Drugs.
Carbachol and PGE2 (Sigma, St. Louis, MO), were dissolved in water or a 1:1 mixture of ethanol and DMSO at 10−1 M, respectively. Substances were then further diluted in Krebs-glucose buffer.
Statistical analysis.
Data are presented as means ± SE for n animals. Mann-Whitney test was used to test differences between two groups. Statistical significance was accepted when P < 0.05.
RESULTS AND DISCUSSION
Colonic mucus.
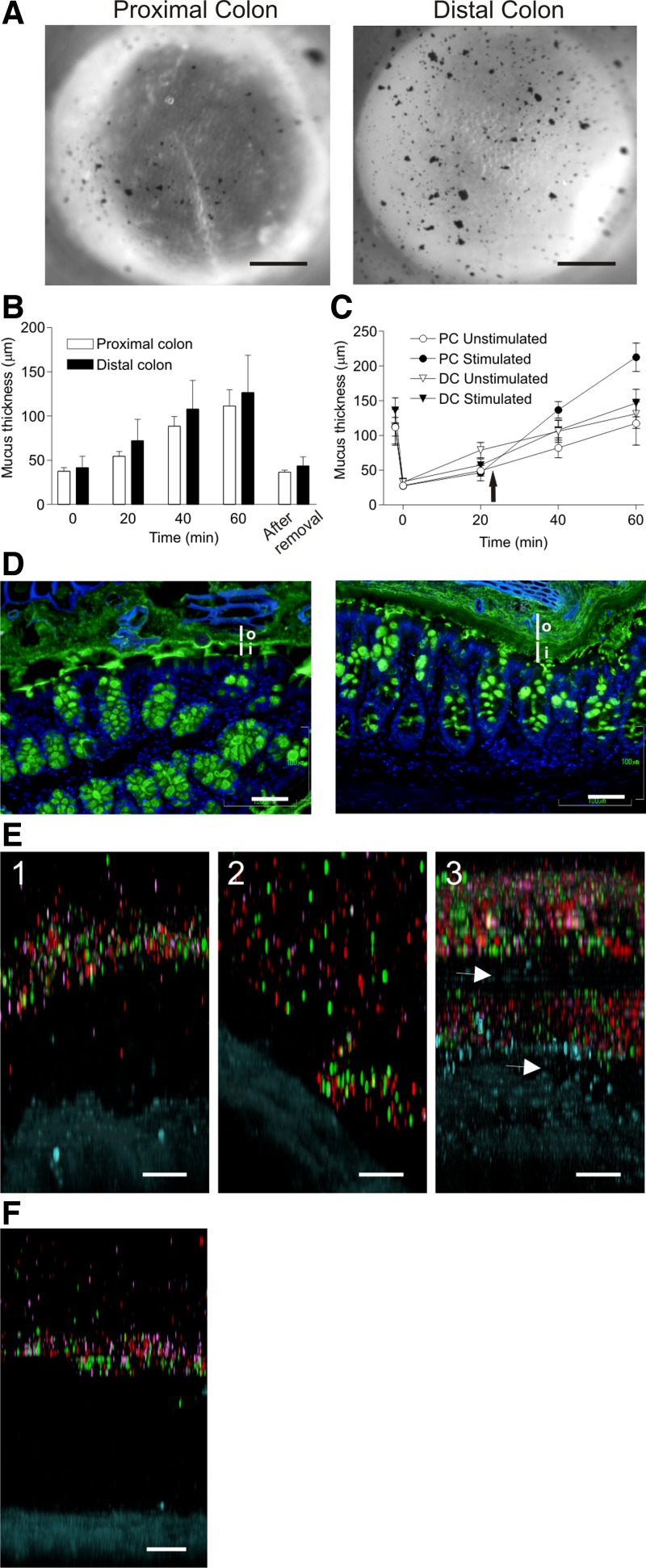
To study the mucus in mouse proximal and distal colon, explants were mounted in a horizontal perfusion chamber and viewed through a stereomicroscope (10). The transparent mucus was visualized by charcoal particles sedimented onto the mucus surface (Fig. 1A). The macroscopic morphology of the proximal colon is different from the distal because the proximal part has diagonal striations that are observed as epithelial folds in the microscope. After mounting, the outer mucus layer was aspirated and the remaining mucus thickness was measured (time 0, Fig. 1, B and C). This thickness represents the inner colon mucus layer (16). The mucus was then allowed to grow and the thickness was measured after 20, 40, and 60 min. During this hour, the mucus in proximal colon increased by 75 ± 19 μm and in the distal colon by 87 ± 14 μm. After this, the outer mucus was aspirated again (after removal, Fig. 1B) and the inner mucus layer was measured 37 ± 2.4 μm for the proximal and 44 ± 3.9 μm for the distal colon. The inner mucus layer thicknesses of these two sections recorded at time 0 and after removal at 60 min was identical, indicating that the outer layer is formed from the inner mucus and that the thickness of this inner layer is maintained constant. To illustrate that the outer mucus layer can be aspirated in both the proximal and distal colon, movies showing the mucus aspiration were recorded (Supplemental Videos S1 and S2; initial frames are shown in Fig. 1A).
Fig. 1.
Colonic mucus forms 2 layers where the inner is a barrier to bacteria. A: explant tissue from the proximal (left) and distal (right) colon mounted in the perfusion chamber with the mucus surface visualized by charcoal and mucus aspirated (Supplemental Videos S1 and S2, first frame in A). B: mucus thickness measured in colon explants from proximal (open bars; n = 10) and distal (solid bars; n = 10). Time 0; initial inner mucus layer. At 60 min, mucus was aspirated and the inner mucus layer thickness measured (after removal). C: mucus thickness measured in proximal (PC) and distal colon (DC), outer loose mucus removed (inner mucus layer, time 0), and mucus thickness measured (20 min). Paired explants, unstimulated (n = 6) or stimulated (n = 6) with carbachol and PGE2 (10 μM of each; arrow); mucus thickness measured every 20 min. The difference between stimulated and unstimulated mucus thickness was significant in the proximal colon (P = 0.04), but not the distal colon (P = 0.59). D: immunostaining of Carnoy-fixed proximal (left) and distal (right) colon for Muc2 (anti-MUC2C3 and Alexa 488 anti-rabbit Ig, green) and DNA (DAPI, blue). Inner mucus layer (i) excludes bacteria (blue), but bacteria are present in the outer mucus layer (o). E: representative confocal Z-stacks of explants (tissue, blue) of proximal colon (n = 7) showing penetrability of mucus to fluorescent beads (red, 0.5 μm; purple, 1 μm; green, 2 μm). E1: between folds. E2: on top of folds. E3: explant stimulated with carbachol and PGE2 (10 μM of each) to induce release of new mucus and show uneven mucus with penetrable and impenetrable region (arrow). F: representative confocal Z-stack visualizing penetrability to fluorescent beads, distal colon. Bar in A = 0.5 mm; bars in D, E, and F = 50 μm.
A combination of carbachol and PGE2 has been shown to stimulate mucus release, especially in the small intestine (10, 11, 25, 30). To test whether it is possible to stimulate colon mucus secretion, explants were stimulated with carbachol and PGE2 and mucus thickness measured every 20 min (Fig. 1C). The mucus thickness increase in the stimulated explants was 185 ± 21 μm in the proximal colon (P = 0.04) and 113 ± 18 μm in the distal colon (P = 0.59, not significant). Thus stimulation caused increased mucus thickness (2.5 times) in the proximal, but not in the distal colon.
Fixed tissue sections from proximal and distal colon were stained for Muc2 by using the anti-MUC2C3 antibody (green) and nuclei (DAPI, blue, Fig. 1D). The inner layer (i) can be observed in both the proximal (left) and distal (right) colon but generally appears thicker and more continuous in the distal colon. The inner layer in both proximal and distal colon looks stratified. The inner mucus layer in distal colon does not show any DNA staining indicative of bacteria. This layer thus acts as a barrier limiting bacterial exposure to the epithelium. In the proximal colon, the innermost mucus separates the epithelium from most bacteria.
Previously we have shown that beads the size of bacteria do not penetrate the distal inner mucus layer (10, 13). To study the penetrability of beads also in the proximal colon, explants were mounted in the horizontal Ussing-type chamber and fluorescent beads with diameters of 0.5 (red), 1 (purple), and 2 (green) μm were allowed to sediment into the mucus for 40 min, after which confocal Z-stacks were acquired (Fig. 1E, tissue stained blue). Three different conditions are shown: 1) between folds, 2) on top of a fold, and 3) after stimulation with carbachol and PGE2 (10 μM each) for 10 min. The mucus penetrability in the proximal colon thus varied depending on where the Z-stacks were acquired. Between the folds, the mucus accumulated and was impenetrable to beads, whereas on top of the folds the mucus tended to form a thinner and more penetrable layer. Penetrability of secretagogue-stimulated, newly secreted mucus was not different from spontaneously released mucus in the proximal colon (Fig. 1E). In the distal colon, the distance separating the lowest beads and the stained tissue (blue) was ∼200 μm (Fig. 1F). Thus the inner layer measured as bead separation was considerably thicker than was measured with the charcoal method.
Although it is not fully understood how the inner mucus layer of the colon becomes impenetrable to bacteria (16), our studies using fluorescent beads with sizes typical for bacteria suggest that the inner mucus layer is acting as a molecular sieve (10, 13). In fact, our current experience is that bead penetrability in the colon directly reflects bacterial penetrability. There is, however, a discrepancy in the thickness of the inner mucus layer measured by the charcoal method compared with the thickness of the mucus impenetrable to fluorescent beads measured in the confocal Z-stacks. These should both correspond to mucus impenetrable to bacteria. The reason for this difference is unknown, but we speculate that the outer layer, although it is released from its attachment to the cells, is still denser close to the inner-outer transition.
Small intestinal mucus.
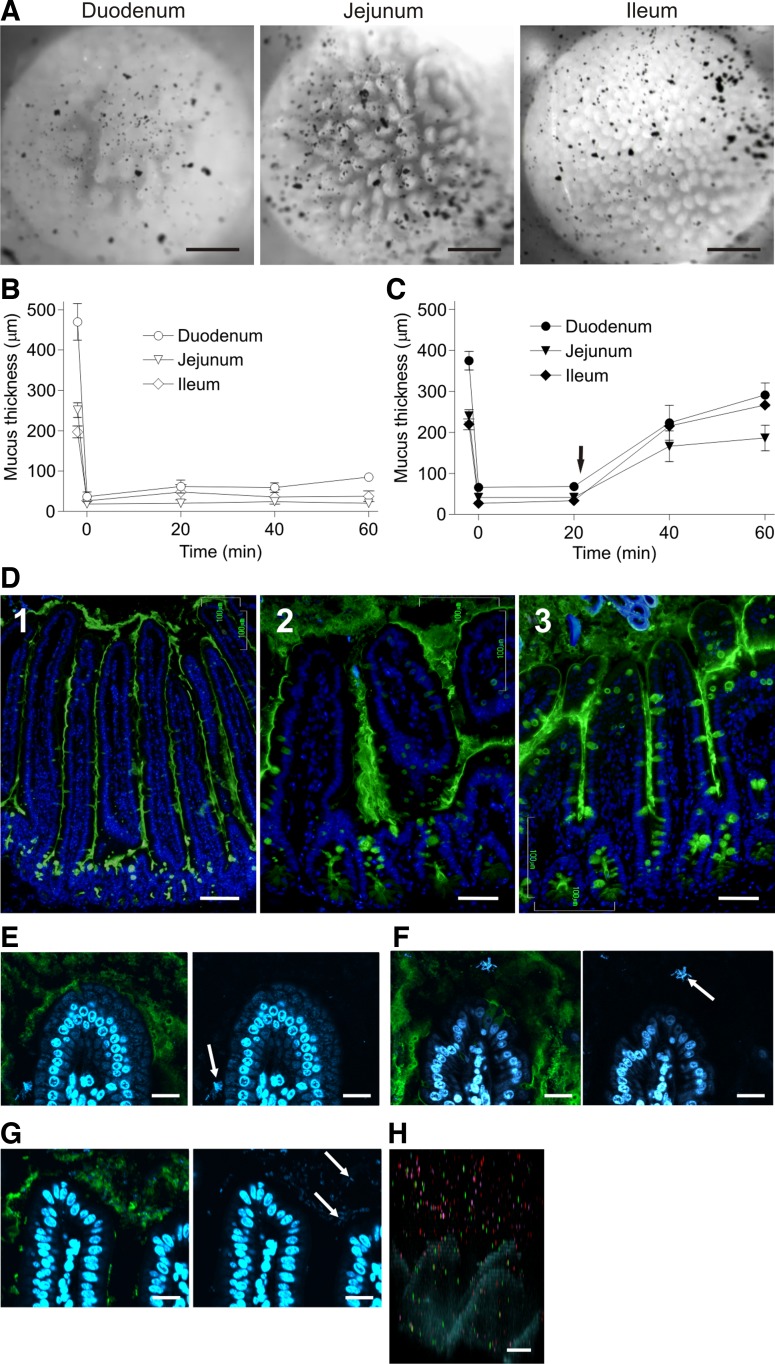
Duodenal, jejunal, and ileal explants were mounted in the horizontal chamber and charcoal particles were added to visualize the transparent mucus (Fig. 2A). The mucus was then aspirated as shown in the movies (Supplemental Video S3, duodenum; Supplemental Video S4, jejunum; Supplemental Video S5, ileum; initial frames are shown in Fig. 2A). The charcoal was initially localized on the mucus surface above the villi, which can be observed protruding toward the viewer through the mucus. The duodenum and jejunum mucus was intermixed with fat droplets and other food particles. The foodstuff could not be removed before mucus thickness measurements and thus the measured mucus thickness in the proximal parts of the small intestine represents both mucus and intermixed food. The mucus was more homogeneous in the more distal parts. The mucus in all three parts can be easily aspirated with a pipette, and charcoal added after mucus removal sediments down between the villi and comes close to the crypt openings, indicating that there was no mucus left. In contrast to the colon, charcoal particles could sediment through the mucus (after 20 min). The small intestinal mucus network built by the Muc2 mucin polymers is more sparse than in the colon, showing that the same Muc2 gene product can build mucus with remarkably different properties.
Fig. 2.
Small intestine has one type of mucus, which is not attached. A: explant tissue from the duodenum (left), jejunum (middle), and ileum (right) mounted and the transparent mucus visualized with charcoal and mucus aspirated showing nonattached mucus (Supplemental Videos S3, S4, and S5, respectively). Mucus in duodenum and jejunum was intermixed with food particles and lipid droplets. B and C: mucus thickness of paired explants from the duodenum, jejunum, and ileum measured (n = 6) every 20 min (B) or stimulated with carbachol and PGE2 (10 μM of each; arrow in C). Stimulation gave a significantly thicker mucus layer in all segments, P = 0.0022, 0.0022, and 0.004 in duodenum, jejunum, and ileum, respectively. After the initial mucus thickness measurement, mucus was removed and remaining mucus thickness was measured (both time 0). D: immunostainings of Carnoy-fixed tissue sections for Muc2 (anti-MUC2C3 and Alexa 488 anti-rabbit Ig, green) and DNA (DAPI, blue). Because of mucus shrinkage caused by fixation and staining, the mucus appears threadlike. E–G: bacterial DNA (blue; arrows) and Muc2 mucin (green) show a few bacteria on the villus tip and between the villi, but no bacteria close to the crypt openings. E: duodenal villus tip. F: jejunal villus tip. G: ileal villus tip. H: fluorescent beads penetrate the mucus in ileum; experiment as in Fig. 1, E and F. Bar in A = 0.5 mm; bar in D1 = 100 μm; bars in D2, D3, and H = 50 μm; bars in E, F, and G = 10 μm.
Small intestinal explants do not spontaneously secrete mucus when mounted in the chamber (Fig. 2B), in contrast to colonic explants. However, upon stimulation with carbachol and PGE2 (Fig. 2C, arrow), new mucus is secreted and after 40 min it almost reaches the same thickness as before removal. The mucus is significantly thicker after stimulation compared with unstimulated samples (Fig. 2C). This mucus can be aspirated in all sections (data not shown).
Immunostainings for Muc2 (green) in Carnoy-fixed tissue sections from duodenum, jejunum, and ileum revealed thin, threadlike mucus between the villi [Fig. 2D, duodenum (1), jejunum (2), and ileum (3)]. The Muc2-stained mucus does not fill the space between the villi, likely an artifact due to shrinking of the mucus gel during fixation and processing. In most instances, the mucus covered the villus tips. DNA-stained bacteria [blue; arrows in Fig. 2, duodenum (E), jejunum (F), and ileum (G)] could be observed in the Muc2-stained mucus (green) especially in the distal small intestine. The bacteria were found in proximity to the villus tips, but seldom in direct contact with the epithelium. Very few bacteria were found between the villi, and no bacteria were observed close to the crypt openings.
The penetrability to fluorescent beads was tested as for the colonic explants. The ileal mucus was completely penetrable to the fluorescent beads as shown in Fig. 2H. The tall villi in the duodenum and jejunum do not stand perpendicular to the tissue when mounted in the chamber and consequently the beads do not easily sediment down between the villi. Thus it was not possible to obtain a correct picture of mucus penetrability in these sections, but whenever the beads were not stopped by tilted villi they penetrated the mucus. We thus conclude that the mucus in all parts of the small intestine was penetrable to beads the size of bacteria.
A majority of the nutrients from ingested food are taken up in the small intestine, and it is thus not difficult to accept that the small intestinal mucus is different from that in colon. Instead of a physical barrier as in colon, the bacteria are likely kept at bay by the antibacterial peptides and proteins secreted by the epithelial cells and the Paneth cells in the crypts (4, 31). It can be suggested that the mucus decreases the diffusion rate of both bacteria and antibacterial compounds and consequently generates a gradient limiting the contact between bacteria and the epithelium (14). The small intestinal mucus fills out most of the space between the villi and usually also covers the villus tips. However, bacteria can be observed relatively close to the villus tips, especially in the distal small intestine. In the ileum, the mucus is denser and forms a more continuous mucus layer than in more proximal parts. We observed very few bacteria between the villi and never close to the crypt openings. Most likely this is because most of the mucins are secreted at the crypt opening, and as this is mixed with the antibacterial peptides and proteins [e.g., defensins, lysozyme, and DMBT1 (deleted in malignant brain tumors 1)] from the Paneth cells, there is a flow of mucus that efficiently hinders bacteria from entering the crypts.
The fact that the small intestinal mucus is easy to aspirate and remove is in contrast to mice with a nonfunctional Cftr ion channel (10) where the mucus is attached. This was attributed to the bicarbonate transporting property of the Cftr channel as the attached mucus was detached when secreted into buffers with high amounts of bicarbonate. Mice with a nonfunctional Cftr channel show distal ileal obstruction and ileal bacterial overgrowth (7, 8, 23). This is similar to cystic fibrosis patients who frequently have intestinal obstruction problems (DIOS, distal intestinal obstruction syndrome). This illustrates the importance of having nonattached mucus that can trap bacteria and move these distally with the fast small intestinal peristalsis.
Mucus on Peyer's patches.
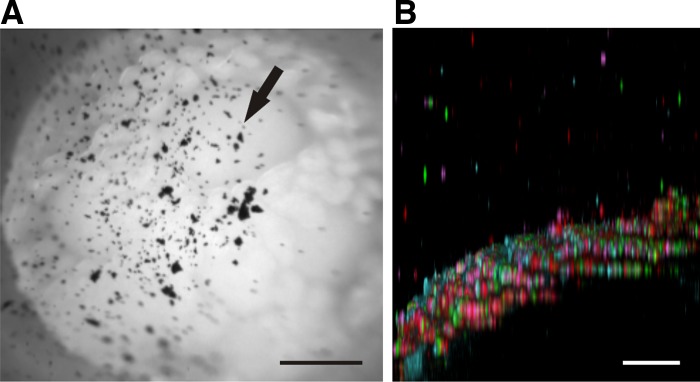
To address whether Peyer's patches are covered with mucus, mouse ileal explants containing Peyer's patches were mounted in the horizontal chamber and movies illustrating the gentle aspiration of mucus visualized by charcoal were recorded. The epithelial surfaces of all analyzed Peyer's patches were covered with mucus as shown in Fig. 3A and corresponding video (Supplemental Video S6; initial frame in Fig. 3A). Four domes are found in this video with villi in between. The presence of mucus is evident from the charcoal localized above the dome epithelium and the fact that aspiration can remove the charcoal-containing mucus. Charcoal added after aspiration sedimented down onto the epithelium, suggesting that all mucus was removable. Like mucus in the small intestine, the mucus on Peyer's patches was penetrable to fluorescent beads (Fig. 3B, 40-min sedimentation). Most beads sedimented to the epithelium, but some remain suspended in the mucus, further supporting the notion that there is a mucus layer on top of the domes.
Fig. 3.
The domes of Peyer's patches are covered by a penetrable mucus layer. A: mucus visualized with charcoal (arrow) on ileal explants containing 4 Peyer's patches. Nonattached mucus demonstrated in the movie (Supplemental Video S6). B: mucus on domes penetrable to fluorescent beads as shown in Fig. 1, E and F. The epithelium (blue) of the dome is convex (viewed slightly tilted) and completely covered by beads. Bar in A = 0.5 mm; bar in B = 50 μm.
It has been controversial whether there is mucus on top of Peyer's patches. When we analyzed our explants for mucus we observed, as has been done previously in rats (18), that there is mucus on top of the patches. Since this mucus is readily removable and also penetrable to beads the size of bacteria, it will not hinder bacteria from reaching the domes.
Stomach mucus.
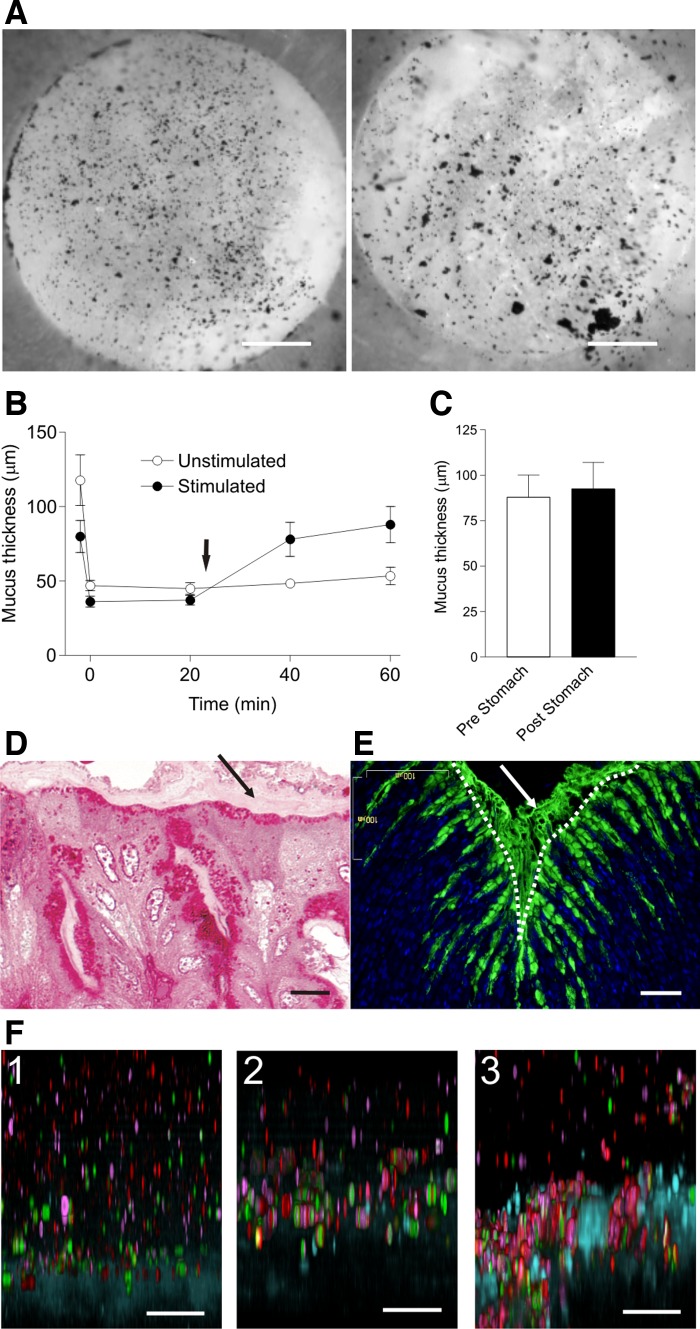
Mouse stomach corpus was mounted in the perfusion chamber (Fig. 4A, right) and the mucus surface visualized with charcoal. As shown in the Supplemental Video S7, only small amounts of the charcoal could be removed by gentle aspiration and also most of the outer mucus is still attached. This is in contrast to the colon where the outer layer is easily removed. The inner mucus layer was firmly adherent, as illustrated in Supplemental Video S8 (Fig. 4A, left). The inner mucus layer could only be removed after application of strong force that damaged the epithelium. This suggests that the inner mucus layer of the stomach is more firmly attached to the epithelium than in the colon. Mucus thickness was measured after mounting the stomach explants in the chamber and after the outer mucus was aspirated. Remaining mucus thickness, corresponding to the inner mucus layer, was 45 ± 3 μm directly after removal (time 0, Fig. 4B), a thickness almost identical to in vivo measurements (27). No spontaneous increase in mucus thickness was observed during the 60 min (○), but when the tissue was stimulated with carbachol and PGE2 (arrow), the mucus thickness increased to 88 ± 11 μm (Fig. 4B; ●), mucus being significantly thicker after stimulation than in unstimulated explants. The stomach mucus thickness was measured after stimulation of mucus release (Fig. 4C, Pre) and then after gentle aspiration (Fig. 4C, Post). No mucus could be removed, illustrating that stomach mucus is firmly attached to the epithelium also after secretion and not converted into easily aspirated mucus as in the colon.
Fig. 4.
The stomach has mucus layers that are firmly attached to the epithelium and penetrable to fluorescent beads. A: stomach explants from the corpus, mucus visualized with charcoal were gently aspirated (Supplemental Video S7; initial frame shown in A, left). A second movie recorded to illustrate the firm attachment of the outer layer in the stomach (Supplemental Video S8; initial frame shown in A, right). B: mucus thickness of stomach corpus explants measured in paired experiments (n = 5, ○) or with stimulated with carbachol and PGE2 (10 μM each; ●; arrow). Initial mucus thickness was measured, mucus was removed, and remaining mucus thickness was measured (both time 0). Mucus was significantly thicker in explants after stimulation compared with unstimulated samples, P = 0.0048. C: aspiration of mucus on stomach explants after stimulation of secretion by carbachol and PGE2 mucus (n = 5). D: Karnovsky-fixed, epoxy resin-embedded sections stained with periodic acid-Schiff (left), revealing a thin, striated mucus layer on top of the epithelium (arrow). E: Carnoy-fixed paraffin sections stained with anti-Muc5ac (green) and DNA stain (blue) show Muc5ac-positive goblet cells and mucus. Epithelial surface marked with dotted line. F: fluorescent beads sedimented for 60 min into the mucus as described in Fig. 1, E and F: unstimulated tissue (1), tissue stimulated for 20 min with carbachol and PGE2 (10 μM of each) (2), mucus secreted after 10 min stimulation into an apical pH 3 buffer (3). Bars in A = 0.5 mm; bar in D = 10 μm; bars in E and F = 50 μm.
Light microscopy of Karnovsky-fixed, epoxy resin-embedded, and periodic acid-Schiff-stained mouse stomach showed a thin, poorly stained, striated layer on top of the epithelium (Fig. 4D, arrow). This striated structure resembles the colonic inner mucus layer but is very likely considerably dehydrated and maybe also less organized. Carnoy-fixed paraffin sections of the stomach corpus stained for the Muc5ac mucin (green) and DNA (blue) show Muc5ac-positive cells along the surface and at the upper parts of the glands. Attached mucus was also observed on the epithelial surface (Fig. 4E).
The stomach mucus was also tested for bead penetrability. Because the normal pH of the stomach is low, three different experiments were performed: 1) beads were directly applied on the mucus present on the tissue at pH 7.4 (Fig. 4F-1); 2) mucus secretion was stimulated with carbachol and PGE2, and beads were applied at pH 7.4 (Fig. 4F-2); 3) secretion was stimulated into an apical pH 3 buffer to mimic stomach conditions (Fig. 4F-3). Irrespective of experimental conditions, beads penetrated the mucus and reached the epithelial cell surface. Therefore, in contrast to the colon mucus, the stomach mucus is penetrable to fluorescent beads the size of bacteria.
In the stomach, the mechanical stress is high because the ingested food is coarse, with large, hard, undigested pieces that can cause mechanical damage together with the chemical challenge caused by the gastric acid, but the bacterial load is comparatively low. The stomach has, similar to the colon, mucus that is firmly attached to the epithelium. This point is clearly demonstrated in Supplemental Videos S7 and S8. The inner mucus acts as a slow diffusion barrier as it helps create a pH gradient from 1–2 in the lumen to 7 close to the epithelium, by limiting the diffusion of luminal hydrochloric acid and epithelial bicarbonate (26, 29). Our experiments also show that the stomach is relatively stable at low pH, since the mucus formed in apical buffer with pH 7.4 or 3 had similar attachment properties and penetrability.
Conclusions.
The separation of bacteria from the epithelium by a nonpenetrable inner mucus layer was previously described for the distal colon (16). Here we demonstrated that the proximal colon also has an inner mucus layer, but this layer is partly penetrable to beads the size of bacteria. The stomach, like colon, has an attached mucus layer, whereas the small intestine only has an easily removable mucus. Thus the mucus of the alimentary tract has fundamentally different secretory mechanisms, appearance, and properties. The two-layered mucus system of colon is also present in germ-free mice (16), but less developed with thinner thickness. Small intestinal mucus differ in being thinner, nonadherent, and more penetrable, indicating differences in the barrier to bacteria.
GRANTS
This work was supported by the Swedish Research Council, The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (LUA-ALF), Wilhelm and Martina Lundgren's Foundation, Torsten och Ragnar Söderbergs Stiftelser, The Sahlgrenska Academy, National Institute of Allergy and Infectious Diseases (U01AI095473; the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), and The Swedish Foundation for Strategic Research — The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.E., A.S., M.E.J., J.K.G., and G.C.H. conception and design of research; A.E., A.S., M.E.J., and J.K.G. performed experiments; A.E., A.S., M.E.J., J.K.G., and G.C.H. analyzed data; A.E., A.S., M.E.J., J.K.G., and G.C.H. interpreted results of experiments; A.E., A.S., M.E.J., and G.C.H. prepared figures; A.E., M.E.J., and G.C.H. drafted manuscript; A.E. and G.C.H. edited and revised manuscript; A.E., A.S., M.E.J., J.K.G., and G.C.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Electron Microscopy Unit and the Centre for Cellular Imaging at the University of Gothenburg for technical help.
REFERENCES
- 1. Ambort D, Johansson MEV, Gustafsson JK, Nilsson H, Ermund A, Johansson BR, Kock P, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA 109: 5645–5650, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atuma C, Strugula V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 307: 1915–1920, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Bevins CL. Paneth cell defensins: key effector molecules of innate immunity. Biochem Soc Trans 34: 263–266, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA 103: 732–737, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol 52: 2–12, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Delisle RC, Roach EA, Norkina O. Eradication of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J Pediatr Gastroenterol Nutr 42: 46–52, 2006. [DOI] [PubMed] [Google Scholar]
- 8. French PJ, vanDoorninck JH, Peters RHPC, Verbeek E, Ameen NA, Marino CR, deJonge HR, Bijman J, Scholte BJ. A Delta F508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature-sensitive processing defect in vivo. J Clin Invest 98: 1304–1312, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel is required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gustafsson JK, Ermund A, Johansson MEV, Schutte A, Hansson GC, Sjovall H. An ex vivo method for studying mucus formation, properties and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol 302: G430–G438, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gustafsson JK, Sjovall H, Hansson GC. Ex vivo measurements of mucus secretion by colon explants. Methods Mol Biol 842: 237–243, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 336: 1268–1273, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson MEV, Gustafsson JK, Sjoberg KE, Pettersson J, Holm L, Sjovall H, Hansson GC. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 5: e12238, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansson MEV, Hansson GC. Keeping bacteria at a distance. Science 334: 182–183, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Johansson MEV, Holmen Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108: 4659–4665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johansson MEV, Phillipson M, Petersson J, Holm L, Velcich A, Hansson GC. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johansson MEV, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res 8: 3549–3557, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Khan J, Iiboshi Y, Cui L, Wasa M, Okada A. Role of intestinal mucus on the uptake of latex beads by Peyer's patches and on their transport to mesenteric lymph nodes in rats. JPEN J Parenter Enteral Nutr 23: 19–23, 1999. [DOI] [PubMed] [Google Scholar]
- 19. Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12: 319–330, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483: 345–349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9: 265–278, 2011. [DOI] [PubMed] [Google Scholar]
- 22. Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell 86: 345–348, 1996. [DOI] [PubMed] [Google Scholar]
- 23. Norkina O, Burnett TG, Delisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun 72: 6040–6049, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Onori P, Franchitto A, Sferra R, Vetuschi A, Gaudio E. Peyer's patches epithelium in the rat: a morphological, immunohistochemical, and morphometrical study. Dig Dis Sci 46: 1095–1104, 2001. [DOI] [PubMed] [Google Scholar]
- 25. Phillips TE, Phillips TH, Neutra MR. Regulation of intestinal goblet cell secretion. III. Isolated intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 247: G674–G681, 1984. [DOI] [PubMed] [Google Scholar]
- 26. Phillipson M, Atuma C, Henriksnas J, Holm L. The importance of mucus layers and bicarbonate transport in preservation of gastric juxtamucosal pH. Am J Physiol Gastrointest Liver Physiol 282: G211–G219, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Phillipson M, Johansson MEV, Henriksnäs J, Petersson J, Gendler SJ, Sandler S, Persson AEG, Hansson GC, Holm L. The gastric mucus layers: constituents and regulation of accumulation. Am J Physiol Gastrointest Liver Physiol 295: G806–G812, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Pott J, Hornef M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep 13: 684–698, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schade C, Flemstrom G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology 107: 180–188, 1994. [DOI] [PubMed] [Google Scholar]
- 30. Specian D, Neutra MR. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol 85: 626–640, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIg promotes the spatial segregation of microbiota and host in the intestine. Science 334: 255–258, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.