Abstract
During migration, passerine birds typically complete a series of multi-hour flights, each followed by a period of stopover. During flight, rates of respiratory water loss are high, yet these birds show no signs of dehydration after flights. During stopover, birds become hyperphagic to replenish fat reserves, often consuming food with high water content, such as fruit. Thus migratory birds seem to face an osmoregulatory challenge; they must reduce water losses during flight but retain the ability to excrete large quantities of water while maintaining osmotic balance at stopover. Our goal was to measure glomerular filtration rate (GFR) and fractional water reabsorption (FWR) of a migratory bird in free flight, at rest, and during feeding to assess the role of the kidney in maintaining water balance during migration. We used FITC-inulin and one- and two-phase exponential decay models to first validate a technique and then measure GFR in the Swainson's thrush, a small (∼30 g) songbird. Single-phase exponential decay models and the modified slope intercept method overestimated GFR by 26% compared with two-phase exponential decay models. We found no differences in GFR among fed, resting and flying birds, but FWR was significantly higher in resting and flying birds relative to feeding birds. There was no effect of the rate of respiratory water loss on GFR or FWR in flight. These data support the idea that birds in flight do not dramatically alter GFR but rely on increased FWR to minimize excretory water losses.
Keywords: flight, migration, birds, glomerular filtration, water balance
birds have evolved physiological adaptations that allow them to maintain homeostasis in a wide variety of environments. With regard to osmoregulation and water balance, considerable attention has been paid to the adaptations of desert dwelling birds to reduce water losses, focusing on cutaneous water losses (50, 55), respiratory water losses (37, 51, 55), hormonal control of osmoregulation (14, 17, 20), renal function including rates of glomerular filtration (GFR), and fractional water reabsorption (FWR; Ref. 21, 23, 24, 30, 31, 38), as well as the postrenal modification of urine (10, 19).
GFR is the rate at which plasma is filtered across the capillaries of the glomerulus into the urinary space of the Bowman's capsule (19). GFR is primarily modulated by arginine vasotocin and is typically reduced during dehydration in many species of birds (19, 45). FWR refers to water reabsorbed in the kidney, typically in the proximal tubule, descending limb of the loop of Henle, and in the collecting duct after filtration has occurred. Resting values of FWR range from 70 to 90% in birds, and FWR is typically increased during dehydration to reduce excretory water losses (19, 45).
Several studies have examined physiological mechanisms that allow nectar-feeding birds to osmoregulate when excessive quantities of water are consumed and must be eliminated without concomitant losses of osmolites (29–32, 38). These studies have focused primarily on kidney function and have identified many species of birds that have dynamic control over GFR and FWR. During feeding, nectarivorous birds must eliminate large quantities of water and rates of GFR are high, yet at rest and through the night GFR is reduced to minimize water losses (30–32, 38). Typically, nectar feeding birds are small (<10 g), and thus researchers have been forced to develop innovative methodologies for the accurate measurement of GFR, minimizing both the volume of blood taken as well as the number of blood samples required (30–32, 38).
Osmoregulation and water balance are crucial to long distance migration in birds. While in flight, birds may have high rates of water loss and no opportunity to drink. Small passerine migrants typically complete migration through a series of nonstop nocturnal flights, each followed by a period of stopover to replenish fat stores and rebuild lean tissue that was catabolized during the previous flight (13, 40). Flights usually last 6–12 h (53), but sometimes may last for days (16). Flights are fuelled primarily by fat, although considerable catabolism of protein also occurs (13, 26–28, 33, 34, 36, 39, 44). Rates of respiratory water loss are high (11) as a result of the high breathing frequencies and tidal volumes required to sustain this long duration aerobic exercise. Although thousands of migratory birds cross ecological barriers such as the Sahara desert (43) or Gulf of Mexico (49), no studies have convincingly documented dehydration in migratory birds after long flights in the wild (8, 45).
Once arriving at a stopover site, migratory birds become hyperphagic and often consume large quantities of food such as fruit and insects (46), both of which may have high water content (52). Thus migratory birds may face an osmoregulatory challenge during refueling, similar to that faced by avian nectarivores; they must reduce water losses during long duration flight but must retain the ability to excrete large quantities of water while maintaining osmotic balance during stopover refueling. It has been shown that GFR is reduced slightly during flight in pigeons (14), but the renal response to flight has not previously been assessed in any migratory bird.
Typically, the determination of GFR in small birds utilizes either a single bolus injection of a marker or surgical implantation of an osmotic minipump to deliver a marker, usually [14C]inulin, or l-[1-14C]glucose. These are ideal markers for the determination of GFR as they typically remain unbound in the plasma, are not metabolized, are freely filtered at the glomerulus, and are not reabsorbed or secreted by the kidney (14, 21, 31, 38). In the case of a single bolus injection, the rate of elimination of the marker from the plasma can be used to calculate GFR, as it is assumed there is a single pool in which the marker is diluted. The rate of inulin clearance, traditionally using constant infusion, and more recently using a single bolus injection followed by serial blood sampling, has been held as the gold standard for the determination of GFR (42). This technique poses several methodological challenges, especially when applied to small animals. Quantification of inulin was initially difficult, and thus the use of radiolabeled inulin has become widespread. Recently, fluorescein-labeled inulin (FITC-inulin) has been used to measure GFR, which eliminates typical concerns associated with using radioisotopes (42), particularly in testing facilities that may be difficult to decontaminate, such as our wind tunnel (see below).
After a single bolus injection of a marker, the modified slope intercept method is commonly used for the determination of GFR. This method requires only a single blood sample in conjunction with serial urine collection and has been particularly successful in small animals (12). This technique assumes the rate of disappearance in the plasma of the marker is matched by the rate of appearance in the urine. As long as the clearance of inulin follows first order exponential decay, this technique yields an accurate determination of GFR in very small (∼5 g) birds and mammals (29–31, 38).
In some cases, inulin kinetics appear to follow a second order exponential decay model (42, 48), where the initial rapid decay phase represents distribution of the marker throughout the plasma volume and interstitial space, and the slow phase of the curve represents clearance of the marker from the plasma at the glomerulus. It has been shown that the slope intercept method tends to overestimate GFR (42, 48), but in very small animals such as hummingbirds, the rapid-phase of the second order decay curve appears to be negligible, yielding accurate determinations of GFR using only first order kinetics.
Here we present novel methodology for the accurate determination of GFR using a single bolus injection of FITC-inulin followed by two blood samples in a small bird. We determined that using first order kinetics does not allow for accurate determination of GFR in these birds. Therefore, we use two-pool models with an experimentally derived constant estimating the initial rapid phase of the model to yield a more accurate and globally comparable measure of GFR in a small bird, and use single-pool models only to compare GFR among physiological states within this study.
Our primary goal was to measure GFR and FWR of a migratory bird in flight, at rest, and during feeding to test the hypothesis that renal function is dynamically regulated during these different phases of migration. We predicted that due to the high rates of respiratory water losses experienced in flight, GFR would be reduced as a means to conserve water. During active feeding, GFR should be increased and FWR should be reduced to eliminate excess water. To more fully understand the role of renal function in the maintenance of water balance in long duration flight, we also investigated the renal response to both high and low rates of respiratory evaporative water loss in flight.
MATERIALS AND METHODS
Animal care.
Twenty-four Swainson's thrushes (Catharus ustulatus) were caught at Long Point, ON, Canada, during the spring and fall of 2011 and kept in two large indoor aviaries (2.3 × 2.4 × 3.5 m). Four of these birds were in captivity from a previous study (13) and were included in the study. Birds captured in spring (n = 7) were used for validation of the techniques and were maintained on a 16L:8D light cycle throughout the summer. An additional (n = 13) birds were captured in early fall of 2011 and were also maintained on the 16L:8D light cycle until December, when birds were placed on a 12L:12D winter cycle. In March 2012, birds were again placed on a 16L:8D light cycle to induce migratory fattening and restlessness as in Owen and Moore (41). All data was collected under the same long day light cycle, while birds were in migratory disposition. Birds were fed a semisynthetic maintenance banana mash diet as described in Gerson and Guglielmo (13). All birds maintained healthy weight while in captivity. All animal care protocols followed the Canadian Council on Animal Care guidelines and were approved by the University of Western Ontario Council on Animal Care and the Animal Use Subcommittee (Protocol No. 2006–011-04). Birds were captured and held under a permit from the Canadian Wildlife Service (CA-0256).
Wind tunnel.
Birds were flown in a wind tunnel at the Advanced Facility for Avian Research at the University of Western Ontario. This wind tunnel was specifically designed for bird flight and allows the researcher to independently control humidity and temperature within the range of 0–90% relative humidity at any temperature up to 30°C, while also controlling wind speed between 0 and 20 m/s. The wind tunnel has a plenum (4.0 × 5.0 m) surrounding the working section of the tunnel. Resting birds were held in a rolling flight cage (dimensions 1.2 × 0.7 × 1.8 m) within the plenum, beside the working section, and were exposed to the same temperature and humidity conditions as the bird in flight. For a technical description of the tunnel, see Gerson and Guglielmo (13).
Preparation and measurement of FITC-inulin.
One-hundred milligrams of FITC-inulin (Sigma F-3272) was dissolved in 2 ml of 0.9% NaCl by heating the solution in a boiling water bath. Once cooled to room temperature, the solution was dialyzed against 0.9% NaCl in well-rinsed benzoylated dialysis tubing with a nominal molecular mass cutoff of 2,000 kDa (Sigma D7884) at room temperature in the dark for 24 h to remove any unbound FITC from solution. The dialyzed solution was then sterile filtered through a 0.22-μm filter into a sterile vacutainer and stored in the dark at room temperature until use (42).
The fluorescence of FITC is significantly affected by pH (42). Three microliters of plasma, urine, or 100-fold diluted stock solution were pipetted into 147 μl of 500 mM HEPES buffer, pH 7.4 at room temperature, and vortexed for 5 s. Then, 50 μl of this solution were loaded onto a 96-well black plate in duplicate. Plates were read at an excitation wavelength of 485, and an emission wavelength of 538 using a Spectramax M2e plate reader (Molecular Devices) at room temperature as in (42).
Experimental design.
Birds were organized into two cohorts to minimize the risk of feather damage from being kept in the rolling flight cages for too long. Birds in the first cohort were maintained in six rolling flight cages for ∼3 wk, in pairs while they participated in the study, after which they were moved back to the large aviary. These rolling flight cages allowed easy transport to and from the wind tunnel. A second cohort was then moved from the large aviary into the rolling flight cages. Each bird was tested in the wind tunnel to identify birds with the natural inclination to fly in the wind tunnel. Each “flying” bird was then paired up with a “resting” bird. Each experimental day, a flying/resting pair of birds had food removed at 1100 and at 1300 were moved into the wind tunnel, which was preset to either high evaporative water loss (HEWL: 18°C, 2.0 g H2O/m) or low evaporative water loss conditions (LEWL: 18°C, 12.0 g H2O/m) as in Gerson and Guglielmo (13). At this time each bird was weighed and injected in the left pectoralis muscle with a ∼3% solution of FITC-inulin in 0.9% saline, in a preweighed syringe at a dose rate of ∼2.0 mg inulin/g body mass (Mb). A small aliquot of the injected solution was saved each experimental day for the calculation of the initial fluorescence for the calculation of Qi (below). The empty syringe was weighed, and the difference in weight was recorded as the amount injected. Birds were then allowed a minimum of 45 min for equilibration of the inulin to occur. Birds were weighed and scanned using a quantitative magnetic resonance body composition analyzer (QMR; Echo Medical Systems) to quantify fat mass, lean mass, and total body water (25), and a small blood sample was taken via brachial puncture in resting birds or from a toe-nail clip in flying birds. This equilibration period was determined to be sufficient during preliminary validations. Once this blood sample was taken, resting birds were placed in a covered rolling flight cage located in the plenum of the wind tunnel where they sat for 2 h. Flying birds were released directly into the wind tunnel and flew for a maximum duration of 2 h. This duration was predetermined to be short enough to avoid complete washout of the FITC-inulin marker while still having the bird reach a physiologically relevant steady state. After the 2-h flight or resting period, birds were immediately bled from the brachial vein, scanned using QMR, and weighed. All blood samples were immediately centrifuged at 2,000 g (Galaxy Mini Microcentrifuge; VWR International), and plasma was stored on ice in the dark until the experiment was complete, and then all samples were stored at −80°C in the dark until analysis. All samples were analyzed within 2 wk. A uretal urine sample was collected by inserting a blind-ended polyethylene sampling tube (Intramedic PE 160; Becton Dickinson) into the cloaca as described by Goldstein and Braun (18). Urine was centrifuged at 2,000 g to remove any precipitated uric acid. To measure GFR and FWR in fed birds, we used the same protocol, except we left birds in their rolling flight cages in the animal room with normal access to food and water, and we did not scan using QMR. All fed birds were bled via a brachial puncture.
Calculation of GFR and FWR.
GFR for experimental data was estimated by fitting a first order exponential decay curve to the relationship between concentration of marker in the plasma and time, similar to (31), except because two blood samples were taken the decay curve was determined directly. GFR was calculated using Eq. 1.
| (1) |
where k is the exponent of the exponential decay curve fitted to the concentration of marker in plasma over time, and Sp is the dilution space of the marker that was calculated using equation 2.
| (2) |
In this equation, Qi is the total quantity of marker injected and Ai(0) is the zero time intercept of the exponential decay curve fitted to the concentration of marker in the plasma over time. Units were converted to milliliters per hour. FWR was calculated as in (31) using Eq. 3,
| (3) |
where Pm and Um are the simultaneous concentrations of the marker in plasma and urine, respectively.
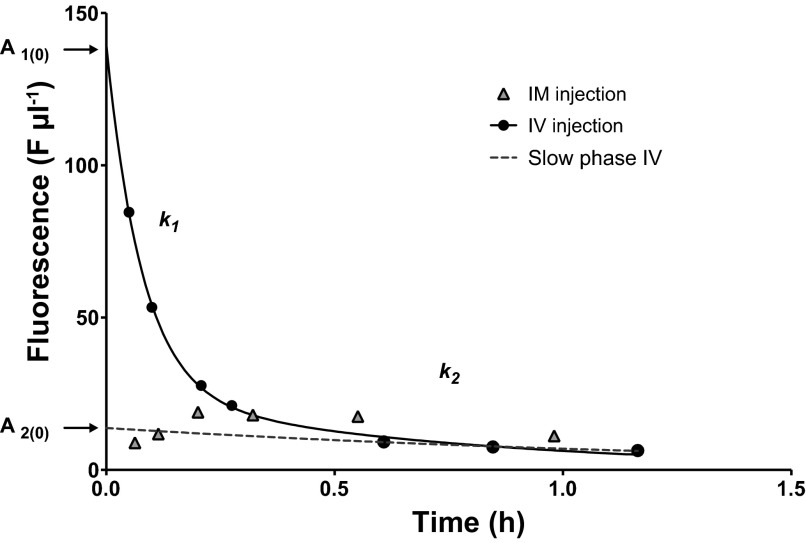
Upon analysis using the single pool model we discovered that the estimated Ai(0) was low, resulting in unrealistically high values for the dilution space for inulin (Sp > Mb) indicating inulin did not follow first order exponential decay, and a second order decay curve would more accurately describe the relationship. To determine the degree to which using first order decay models overestimated GFR, and to develop a correction factor so that the values calculated for GFR using the first order decay kinetics could be comparable outside of this study, the decay of FITC-inulin was determined using intravenous jugular injection followed by serial bleeding as in (42). Intravenous injection minimizes equilibration time and allows an accurate determination of the entire decay curve (Fig. 1).
Fig. 1.
A second order decay curve fit to the concentration of FITC-inulin over time after intravenous injection in a single individual. A1(0) and k1 represent the intercept and slope of the rapid phase of the curve. A2(0) and k2 represent the intercept and slope of the slow phase of the curve. Dotted line represents the slow phase of the curve, which approximates the fit of a first order decay curve to the experimental data. Triangles show the equilibration time and decay of the marker when injected intramuscularly in a single individual.
Five birds were successfully injected with FITC-inulin in the right jugular vein with the same dose as described above. Injection volumes were determined gravimetrically as above. Birds were then bled from the brachial vein at 2, 5, 10, 20, 30, 50, and 70 min postinjection using a 28-gauge needle to minimize blood loss at each bleed interval. Not all birds were bled at all intervals, but enough samples were taken from birds to accurately fit a second order decay curve to data from each individual bird. Once a total of 300 μl of blood was taken, the bird was returned to its cage with food and water. All blood samples were centrifuged as described above, and plasma was saved until analysis. Concentrations of FITC-inulin in plasma were plotted against time since injection and a two-phase exponential decay model was fit using Prism 5 (Fig. 1; Graphpad Software; Ref. 42). GFR using these two-phase models was calculated using equation 4,
| (4) |
where Qi is the total quantity of marker injected, A1(0) is the zero time intercept for the rapid phase of the decay curve, k1 is the exponent of the rapid phase of the decay curve, and A2(0) and k2 are the zero time intercept and the exponent of the slow phase of the decay curve respectively (Fig. 1).
GFR calculation using two-phase exponential decay.
With the use of experimental data collected from intravenous injections and serial bleeds, a correction factor for the rapid phase of the decay curve was determined, which allowed the GFR measurements during flight, rest, and feeding to be recalculated using Eq. 4 as described above.
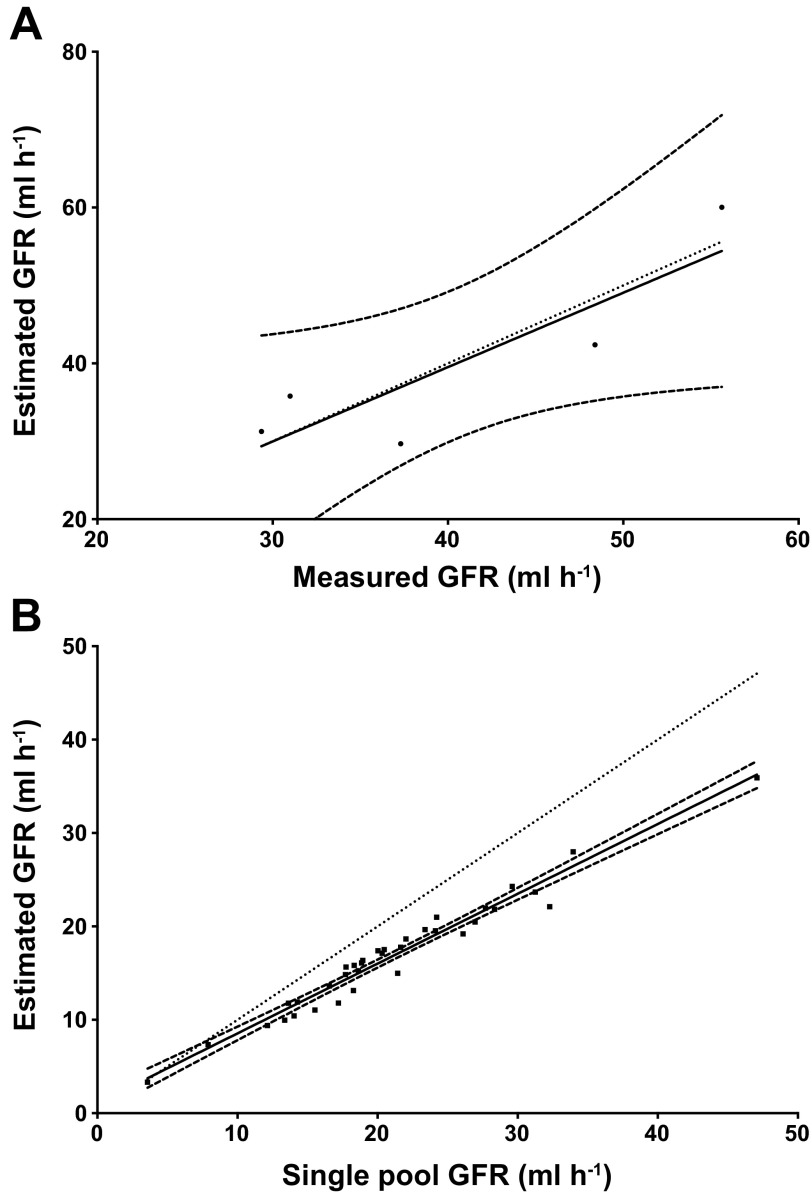
To determine this correction factor, GFR was calculated using Eq. 4 for all intravenously injected birds. The derived values for all parameters in Eq. 4 for each individual were used to calculate “Measured GFR” (Fig. 2A), which was used as a benchmark by which estimated GFR could be compared. For these same birds, a correction factor was substituted for the [A1(0)/k1] term, and the estimated GFR was compared with the measured GFR. It was determined that the mean value for the rapid phase of the decay curve [A1(0)/k1; from intravenously injected birds] provided the most accurate correction factor. The two values of GFR (measured GFR and estimated GFR) were compared using linear regression where inclusion of unity within the confidence intervals of the slope of this relationship as well as r2 values were used to determine the accuracy of the estimated parameter. The experimentally derived term for the rapid phase of the curve was then used to correct experimental values of GFR for fed, resting and flying birds to more accurately determine GFR.
Fig. 2.
A: linear regression of estimated glomerular filtration rate (GFR) against calculated GFR. Estimated GFR was determined using second order exponential decay parameters and Eq. 4 where the rapid phase of the decay curve was estimated from experimentally derived parameters from intravenous injections. Measured GFR represents GFR calculated using Eq. 4 and all experimentally derived parameters. B: linear regression of estimated GFR and single pool GFR for experimental birds (fed, flown, and rest). Estimated GFR utilizes Eq. 4 and the correction factor determined from (A; see text). Relationship between estimated GFR and single pool GFR can be described by the equation: Y = 0.747 (x) + 1.072. In both A and B, the dotted line represents the line of unity, and curved dashed lines represent 95% confidence interval.
The mean value for the rapid phase [A1(0)/k1] of the two-phase exponential decay model (equation 4) determined during the serial determination of GFR was 7.43 F·h−1·μl−1 (± 2.63 SD). Applying this value as an estimation of the rapid decay term in equation 4 yielded an accurate estimation of GFR (Fig. 2A). When regressed against the measured values of GFR, the slope of this relationship was 0.95 ± 0.299 with an R2 value of 0.77. As the confidence intervals include the line of unity, the corrected values closely approximate the measured values. Although the sample size was small due to the difficulty of intravenous injections in a small bird, this provided a satisfactory correction factor to be applied to experimental data. This correction factor was then applied to the experimental data from flown, resting, and fed birds. The relationship of single pool uncorrected GFR values to the corrected ones are shown in Fig. 2B and is described by the equation: corrected GFR = 0.747 (x) + 1.072, where × is the single pool calculation of GFR. Units are milliliters per hour. The mean coefficient of variation between these values was 15.3%, and the uncorrected single-pool determination of GFR overestimated GFR by an average of 19.3%. GFR values calculated using two-phase exponential decay were compared with allometrically predicted values using the equation presented in Bennett and Hughes (6).
Statistics.
To increase statistical power and control for individual variation in fuel mixture in flight, as seen in Gerson and Guglielmo (13), we attempted to measure GFR in flight in the same individual under both HEWL and LEWL conditions. However, many birds would not repeat a flight within the experimental timeframe. Thus the dataset contains unbalanced repeated measures and general linear mixed models were used with individual as a random factor for all analysis. All statistics used a critical significance value of 0.05. Models were determined using backward stepwise selection where nonsignificant terms were dropped sequentially until only significant factors remained. At the outset all analyses included initial body mass as a covariate, but it was not significant and was dropped from all models. Statistical comparisons of GFR were made using the values calculated using first order exponential decay and Eq. 1 because even though these are relative measures of GFR, they do not require estimates of decay curve parameters [A1(0)/k1]. Although these values may not be directly comparable to other studies, this GFR determination makes no assumptions and therefore comparisons within this study are most accurate.
To investigate the overall effect of state (fed, resting, or flying) on GFR and FWR, the HEWL and LEWL treatment groups were pooled. Thus only state (fed, resting, and flying) were factors in this analysis. To investigate the effect of humidity on GFR and FWR, the fed group was not included in this analysis as this group was not exposed to humidity treatments. Thus state (rest or flight) and humidity (HEWL or LEWL) were both factors in the model. Flight duration was initially included as a covariate but was not significant and was removed. All statistical analyses were performed using SPSS 19.0.
RESULTS
Feeding, resting, and flying.
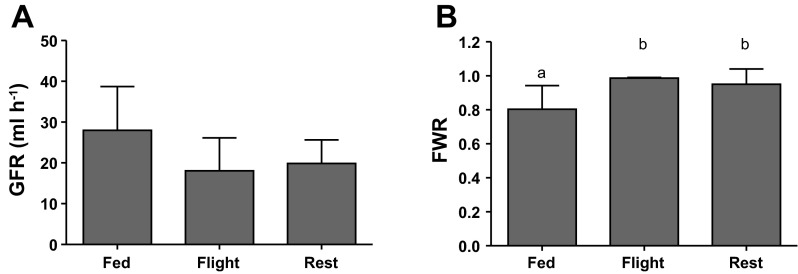
No significant differences in single pool GFR were evident among fed, resting, and flying birds [F2,29.245 = 2.163, P = 0.133; Fig. 3A], but there were significant differences among these groups in FWR (F2,9.975 = 9.659, P = 0.005; Fig. 3B). Post hoc analysis indicated that fed birds had a significantly lower FWR compared with both flight birds (P = 0.003), and rest birds (P = 0.003). However, flight and rest birds did not differ in FWR (P = 0.932).
Fig. 3.
A: no differences in single pool GFR were evident among fed (n = 7), flight (n = 8 from 5 birds), and rest (n = 24 from 13 birds) groups (P > 0.05). B: fractional water reabsorption (FWR) was significantly reduced in fed birds. Different letters indicate significant differences at the P < 0.05 level. Values are means ± SD.
Humidity.
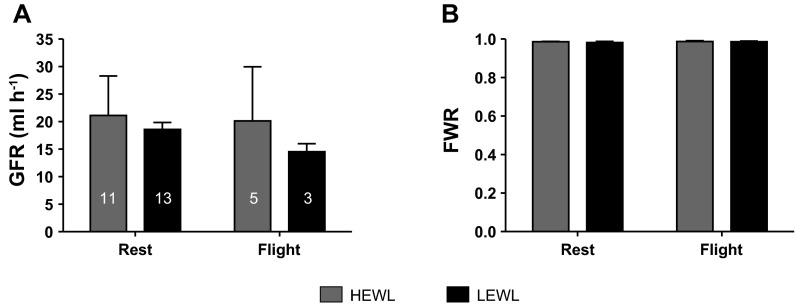
There were no significant differences in single pool GFR between humidity treatments or between flying and resting birds (state: F1,21.791 = 0.002, P = 0.966; humidity: F1,12.44 = 0.851, P = 0.374; Fig. 4A). No significant differences were evident in FWR between flying and resting or between humidity regimes (state: F1,10 = 0.159, P = 0.698; humidity: F1,10 = 0.417, P = 0.533; Fig. 4B).
Fig. 4.
No differences in single pool GFR (A) or fractional water reabsorption (FWR; B) were evident between flight and rest or between high evaporative water loss (HEWL) and low evaporative water loss (LEWL) treatments (P > 0.05). Samples sizes indicated on bars in A. Values are means ± SD.
GFR determination using two-phase exponential decay and comparisons to other species.
Estimating the rapid phase of the second order exponential decay of inulin yielded GFR measurements that were 26% lower than what was estimated using first order kinetics (Fig. 2B). GFR calculated using first and second order equations were, respectively, 54 and 27% higher than allometrically predicted values (Table 1).
Table 1.
Comparison of the methods for the determination of GFR in birds
| Method | GFR, ml/h |
|---|---|
| Slope intercept method assuming first order kinetics | 21.08 (8.07) |
| Corrected two-pool determination | 16.82 (6.15) |
| Allometrically predicted | 11.01 (1.13) |
Values are means (SD). GFR, glomerular filtration rate. Single pool determination utilizes the slope intercept method and Eq. 1. Two-pool determination utilizes the slope intercept method and Eq. 4, where the rapid phase of the decay curve is estimated. Allometrically predicted values us the equation GFR = 0.013 Mb0.76 from Ref. 6.
DISCUSSION
Many studies of the renal function of birds have demonstrated dynamic control of GFR in response to water loading or restriction (10, 18, 21, 30, 31, 38, 45). The renal function of Swainson's thrushes does not seem to respond dramatically to water restriction, flight, or feeding, but the trends identified in this study do agree with previously published studies. Other studies investigating GFR in flight have shown reductions in the GFR of flown birds compared with resting birds, but these differences were small (15). On the other hand, it seems that FWR, rather than GFR, is regulated initially to minimize water losses during resting and flying, while increasing the amount of water voided during feeding.
Feeding, resting, and flying.
Feeding resulted in a significant reduction in FWR relative to both resting and flying, indicating increased water reabsorption is primarily relied upon during periods of water restriction (resting and flying treatments). The diet our birds ate in captivity had a water content of ∼45%, and the efficient elimination of water would be paramount during feeding. There was also a trend toward decreased GFR with water restriction. The mean GFR of fed birds was ∼60% higher than that of the flying and resting birds, yet no significant differences were evident. Typically, an increase in GFR is associated with decreased FWR (21, 31, 38, 47, 54). However, low plasma levels of arginine vasotocin affect tubular water reabsorption more so than GFR in domestic fowl (47). It is possible that the conditions experienced by the bird in the present study did not result in high enough water loss rates to reduce GFR as well as increase tubular water reabsorption.
It is clear from our findings that the magnitude of the response of FWR is much greater than that of GFR, as FWR increased significantly from 0.8 to 0.98 within an hour of removing water. The conditions experienced during flight and rest were not extreme, and were designed to mimic those experienced by wild migrants as in Gerson and Guglielmo (13). Thus it is possible that flight under hotter, drier conditions would elicit a more dramatic reduction in GFR, although it does appear that the upper limit of FWR was reached.
Humidity.
There were no significant differences in GFR or FWR between flying and resting birds nor were any differences evident between humidity treatments. Previous to flight and resting measurements, birds were food and water restricted for 3 h. This was done so that all birds were in a postabsorptive state, allowing for more accurate determination of changes in body mass during flight, while also ensuring no exogenous water input through digestion or absorption of water through the gastrointestinal tract. It seems that this period of water restriction was sufficient for birds to respond by increasing FWR >0.98.
GFR determination using two-phase exponential decay.
It has been previously shown that the calculation of GFR from first order decay parameters can result in overestimation of GFR by as much as 50% (42, 48). The determination of GFR using a two-pool model has been shown to approximate constant infusion within 10% (42). The application of a correction factor to a first order decay model is legitimate if the parameters of the entire second order decay curve have been experimentally determined in the species in question. The rapid phase of the decay curve represents the distribution of the marker throughout the body and the distribution in the interstitial fluid, both of which are independent of the experimental manipulation, and likely scale with body mass and/or metabolic rate. Small animals quickly distribute the marker, and the total contribution of the rapid distribution phase of the decay curve would contribute less to the overall calculation of GFR. The overestimation of GFR by the slope intercept method and assuming first order kinetics likely increases with body mass.
Most studies utilizing the slope intercept method and first order exponential decay for the determination of GFR in hummingbirds, small bats, or other small animals (1–10 g) report a reasonable marker distribution space ∼20% Mb for inulin (30, 31, 38). Interestingly, the dilution space calculated in the current study from A1(0) of the intravenously injected birds was 18.4% of Mb, but the dilution space calculated from Ai(0) using first order was in excess of 100% of Mb. We recommend that the marker dilution space be reported in future studies as a diagnostic for the appropriate use of first order kinetics and to determine whether a correction factor should be determined for the experimental species.
Comparisons to other species.
The GFRs we measured are higher than allometrically predicted values for this species (Table 1). However, the list of species used by Bennett and Hughes (6) to determine this predictive equation does not include many passerines of similar size or diet to the Swainson's thrush. Due to interest in the renal function of desert and marine animals, these types of birds are overrepresented in the dataset used to derive the allometric equation. Desert and marine birds may have a greater ability to reduce GFR compared with migratory species. Thus it is possible that the allometrically predicted values may underestimate true GFR for many species, increasing the likelihood that the GFR values presented in Table 1 are accurate.
Role of kidneys in osmoregulation of migratory birds.
Overall, it does not seem that reduction of GFR is the primary mechanism for the reduction of excretory water losses in flight. In both our study and Giladi et. al. (14), a modest reduction in GFR was observed in flight, while FWR was not different from resting values. During flight, cardiac output is increased fivefold (9), which, without reflexive action in the renal arterioles, would lead to an increase in glomerular filtration pressure. A lack of increase in GFR indicates sufficient autoregulation of mean afferent arteriole pressure in response to exercise (19), indicating very strict regulation GFR during flight, perhaps indicating the necessity for the filtration of metabolites generated during flight, such as uric acid which is found in high concentrations in the plasma of flying birds (13, 34). This results in heavy reliance on tubular water reabsorption and postrenal modification to maintain water balance.
Conclusions.
Given the available data, it seems that the modulation of kidney function is not as central to the conservation of water in flight as originally proposed. Reductions in GFR are modest, and FWR is higher after flight, but only marginally so. Also, urine osmolality does not seem to be increased postflight (14). Urine flow rate is reduced in flight (14), but no information exists on the role of postrenal urine modification in water conservation during flight, which may play a substantial role in water conservation in these birds. Many species rely on postrenal modification of urine to reabsorb water, a process that is hindered by urine of high osmolarity. In this process, urine is refluxed in the lower intestine, where water, ions and protein can be reabsorbed (22). However, migratory birds show dramatic reductions in the sizes of many organs after long duration flights (2–5, 7, 35). Of particular interest are the large reductions in both the kidney and intestine. Garden warblers crossing the Sahara have kidneys that are reduced in size by up to 40%, and small intestines are typically reduced by as much as 50% (4, 5). Given these reductions in the kidney, it would be interesting to investigate renal function immediately after a long duration flight. If urine concentrating ability and water reabsorbtion are compromised after flight, there may be increased reliance on postrenal modification. Few investigations have documented the plasticity of the lower intestine (colon) after migratory flight, but evidence suggests it too is substantially catabolized (5) indicating migratory birds may face severe osmoregulatory challenges during stopover.
GRANTS
A. R. Gerson was supported by a National Sciences and Engineering Research Council (NSERC) Alexander Graham Bell Canada Graduate Scholarship. This study was funded by an NSERC Discovery Grant and a Discovery Accelerator Supplement (311901-2010 to C. G. Guglielmo) and by grants from the Canada Foundation for Innovation and Ontario Research Fund (to C.G. Guglielmo and other colleagues).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.R.G. and C.G.G. conception and design of research; A.R.G. performed experiments; A.R.G. analyzed data; A.R.G. and C.G.G. interpreted results of experiments; A.R.G. prepared figures; A.R.G. drafted manuscript; A.R.G. and C.G.G. edited and revised manuscript; A.R.G. and C.G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Liam McGuire for statistical advice, Kim Schmidt, and Dr. Haruka Wada for help with animal procedures and Wayne Bezner-Kerr for technical assistance with many aspects of this project.
REFERENCES
- 1.Alerstam T, Linström A. Optimal bird migration: the relative importance of time, energy, and safety. In: Bird Migration: the Physiology and Ecophysiology, edited by Gwinner E. Berlin, Germany: Heidelberg, 1990, p. 331–351 [Google Scholar]
- 2.Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K. Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc Biol Sci 267: 191–195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauchinger U, Biebach H. Differential catabolism of muscle protein in garden warblers (Sylvia borin): flight and leg muscle act as a protein source during long distance migration. J Comp Phyiol B 171: 293–301, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bauchinger U, McWilliams SR. Carbon turnover in tissues of a passerine bird: allometry, isotopic clocks and phenotypic flexibility in organ size. Phys Biochem Zool 82: 787–797, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bauchinger U, Wohlmann A, Biebach H. Flexible remodeling of organ size during spring migration of the garden warbler (Sylvia borin). Zoology 108: 97–106, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bennett DC, Hughes MR. Comparison of renal and salt gland function in three species of wild ducks. J Exp Biol 206: 3273–3284, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Biebach H. Phenotypic flexibility in garden warblers Sylvia borin during long-distance migration. J Avian Biol 29: 529–535, 1998 [Google Scholar]
- 8.Biebach H. Strategies of trans-Sahara migrants. In: Bird Migration: Physiology and Ecophysiology, edited by Gwinner E. Berlin, Germany: Springer-Verlag, 1990, p. 352–367 [Google Scholar]
- 9.Butler PJ, West NH, Jones DR. Respiratory and cardiovascular responses of the Pigeon to sustained, level flight in a wind-tunnel. J Exp Biol 71: 7–26, 1977 [Google Scholar]
- 10.Dawson TJ, Herd R, Skadhauge E. Osmotic and ionic regulation during dehydration in a large bird, the Emu (Dromaius novaehollandiae): an important role for the cloaca-rectum. Q J Exp Physiol 70: 423–436, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Engel S, Suthers RA, Biebach H, Visser GH. Respiratory water loss during rest and flight in European starlings (Sturnus vulgaris). Comp Biochem Physiol A Mol Integr Physiol 145: 423–432, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Florijn K, Barendregt J, Lentjes E, Van Dam W, Prodjosudjadi W, Van Saase J, van Es L, Chang P. Glomerular filtration rate measurement by a “single-shot” injection of inulin. Kidney Int 46: 252–259, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Gerson AR, Guglielmo CG. Flight at low ambient humidity increases protein catabolism in migratory birds. Science 333: 1434–1436, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Giladi I, Goldstein DL, Pinshow B, Gerstberger R. Renal function and plasma levels of arginine vasotocin during free flight in pigeons. J Exp Biol 200: 3203, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Giladi I, Pinshow B. Evaporative and excretory water loss during free flight in pigeons. J Comp Physiol B 169: 311–318, 1999 [Google Scholar]
- 16.Gill RE, Tibbitts TL, Douglas DC, Handel CM, Mulcahy DM, Gottschalck JC, Warnock N, McCaffery BJ, Battley P, Piersma T. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc Biol Sci 276: 447–457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goecke C, Goldstein DL. Renal glomerular and tubular effects of antidiuretic hormone and two antidiuretic hormone analogues in house sparrows (Passer domesticus). Physiol Zool 70: 283–291, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein D, Braun E. Contributions of the kidneys and intestines to water conservation, and plasma levels of antidiuretic hormone, during dehydration in house sparrows (Passer domesticus). J Comp Physiol B 158: 353–361, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein D, Skadhauge E. Renal and extrarenal regulation of body fluid composition. In: Sturkie's Avian Physiology, edited by Whittow London, UK: Academic, 2000, p. 265–298 [Google Scholar]
- 20.Goldstein DL. Regulation of the avian kidney by arginine vasotocin. Gen Comp Endo 147: 78–84, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DL, Bradshaw S. Renal function in red wattlebirds in response to varying fluid intake. J Comp Phyiol B 168: 265–272, 1998 [Google Scholar]
- 22.Goldstein DL, Braun E. Lower intestinal modification of ureteral urine in hydrated house sparrows. Am J Physiol Regul Integr Comp Physiol 250: R89–R95, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DL, Braun E. Structure and concentrating ability in the avian kidney. Am J Physiol Regul Integr Comp Physiol 256: R501–R509, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DL, Rothschild E. Daily rhythms in rates of glomerular-filtration and cloacal excretion in captive and wild song sparrows (Melospiza-Melodia). Physiol Zool 66: 708–719, 1993 [Google Scholar]
- 25.Guglielmo C, McGuire LP, Gerson AR, Seewagen CL. Simple, rapid, and non-invasive measurement of fat, lean, and total water masses of live birds using quantitative magnetic resonance. J Ornithol 152: 75–85, 2011 [Google Scholar]
- 26.Guglielmo C, Piersma T, Williams TD. A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. J Exp Biol 204: 2683–2690, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Guglielmo CG. Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr Comp Biol 50: 336–345, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Guglielmo CG, Cerasale DJ, Eldermire C. A field validation of plasma metabolite profiling to assess refueling performance of migratory birds. Phys Biochem Zool 78: 116–125, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Hartman Bakken B. Gastrointestinal and renal responses to water intake in the green-backed firecrown (Sephanoides sephanoides), a South American hummingbird. Am J Physiol Regul Integr Comp Physiol 291: R830, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Hartman Bakken B, Herrera ML, Carroll RM, Ayala-Berdon J, Schondube JE, Martinez del Rio C. A nectar-feeding mammal avoids body fluid disturbances by varying renal function. Am J Physiol Renal Physiol 295: F1855–F1863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman Bakken B, McWhorter TJ, Tsahar E, del Rio C. Hummingbirds arrest their kidneys at night: diel variation in glomerular filtration rate in Selasphorus platycercus. J Exp Biol 207: 4383, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Hartman Bakken B, Sabat P. The mechanisms and ecology of water balance in hummingbirds. Ornitol Neotrop 19: 501–509, 2008 [Google Scholar]
- 33.Jenni L, Jenni-Eiermann S. Fuel supply and metabolic constraints in migrating birds. J Avian Biol 29: 521–528, 1998 [Google Scholar]
- 34.Jenni-Eiermann S, Jenni L, Kvist A, Lindstroem A, Piersma T, Visser GH. Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long-distance migrant shorebird. J Exp Biol 205: 2453–2460, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Karasov WH, Pinshow B. Changes in lean mass and in organs of nutrient assimilation in a long-distance passerine migrant at a springtime stopover site. Physiol Zool 71: 435–448, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Klaassen M, Kvist A, Lindstrom A. Flight costs and fuel composition of a bird migrating in a wind tunnel. Condor 102: 444–451, 2000 [Google Scholar]
- 37.McKechnie AE, Wolf BO. Partitioning of evaporative water loss in white-winged doves: plasticity in response to short-term thermal acclimation. J Exp Biol 207: 203–210, 2004 [DOI] [PubMed] [Google Scholar]
- 38.McWhorter TJ, del Rio C, Pinshow B, Roxburgh L. Renal function in Palestine sunbirds: elimination of excess water does not constrain energy intake. J Exp Biol 207: 3391–3398, 2004 [DOI] [PubMed] [Google Scholar]
- 39.McWilliams SR, Guglielmo C, Pierce B, Klaassen M. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol 35: 377–393, 2004 [Google Scholar]
- 40.McWilliams SR, Karasov WH. Phenotypic flexibility in digestive system structure and function in migratory birds and its ecological significance. Comp Biochem Physiol A 128: 579–593, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Owen J, Moore F. Swainson's thrushes in migratory disposition exhibit reduced immune function. J Etholol 26: 383–388, 2008 [Google Scholar]
- 42.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris R, Fogo A, Breyer M. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Schmaljohann H, Liechti F, Bruderer B. Trans-Sahara migrants select flight altitudes to minimize energy costs rather than water loss. Behav Eco Sociobiol 63: 1609–1619, 2009 [Google Scholar]
- 44.Schwilch R, Grattarola A, Spina F, Jenni L. Protein loss during long-distance migratory flight in passerine birds: adaptation and constraint. J Exp Biol 205: 687–695, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Skadhauge E. Osmoregulation in Birds. New York: Springer-Verlag, 1981 [Google Scholar]
- 46.Smith SB. Fruit quality and consumption by songbirds buring autumn migration. Wilson J Ornithol 119: 419–428, 2007 [Google Scholar]
- 47.Stallone JN, Braun EJ. Contributions of glomerular and tubular mechanisms to antidiuresis in conscious domestic fowl. Am J Physiol Renal Fluid Electrolyte Physiol 249: F842–F850, 1985 [DOI] [PubMed] [Google Scholar]
- 48.Sturgeon C, Sam AD, 2nd, Law WR. Rapid determination of glomerular filtration rate by single-bolus inulin: a comparision of estimation analysis. J Appl Physiol 84: 2154–2162, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Stutchbury BJ, Tarof SA, Done T, Gow E, Kramer PM, Tautin J, Fox JW, Afanasyev V. Tracking long-distance songbird migration by using geolocators. Science 323: 896, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Tieleman BI, Williams JB. Cutaneous and respiratory water loss in larks from arid and mesic environments. Phys Biochem Zool 75: 590–599, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Tieleman BI, Williams JB, Michaeli G, Pinshow B. The role of the nasal passages in the water economy of crested larks and desert larks. Phys Biochem Zool 72: 219–226, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Tsurim I, Sapir N, Belmaker J, Shanni I, Izhaki I, Wojciechowshi MS, Karasov WH, Pinshow B. Drinking water boosts food intake rate, body mass increase, and fat accumulation in migratory blackcaps (Sylvia atricapilla). Oecologia 156: 21–30, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Wikelski M, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser GH. Costs of migration in free-flying songbirds. Nature 423: 704, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Williams JB, Pacelli MM, Braun E. The effect of water deprivation on renal function in consious unrestrained Gambel's Quail (Callipepla gambelli). Physiol Zool 64: 1200–1216, 1991 [Google Scholar]
- 55.Wolf BO, Walsberg G. Respiratory and cutaneous evaporative water loss at high environmental temperatures in a small bird. J Exp Biol 199: 451–457, 1996 [DOI] [PubMed] [Google Scholar]