Abstract
Patients with idiopathic hypercalciuria (IH) have decreased renal calcium reabsorption, most marked in the postprandial state, but the mechanisms are unknown. We compared 29 subjects with IH and 17 normal subjects (N) each fed meals providing identical amounts of calcium. Urine and blood samples were collected fasting and after meals. Levels of three candidate signalers, serum calcium (SCa), insulin (I), and plasma parathyroid hormone (PTH), did not differ between IH and N either fasting or fed, but all changed with feeding, and the change in SCa was greater in IH than in N. Regression analysis of fractional excretion of calcium (FECa) was significant for PTH and SCa in IH but not N. With the use of multivariable analysis, Sca entered the model while PTH and I did not. To avoid internal correlation we decomposed FECa into its independent terms: adjusted urine calcium (UCa) and UFCa molarity. Analyses using adjusted Uca and unadjusted Uca parallel those using FECa, showing a dominant effect of SCa with no effect of PTH or I. The effect of SCa may be mediated via vitamin D receptor-stimulated increased abundance of basolateral Ca receptor, which is supported by the fact PTH levels also seem more responsive to serum Ca in IH than in N. Although our data support an effect of SCa on FECa and UCa, which is more marked in IH than in N, it can account for only a modest fraction of the meal effect, perhaps 10–20%, suggesting additional mediators are also responsible for the exaggerated postprandial hypercalciuria seen in IH.
Keywords: hypercalciuria, kidney stones, parathyroid hormone, serum calcium
compared to normal people (N), many otherwise healthy patients who form calcium (Ca) renal stones are hypercalciuric (25). Their higher urine Ca (UCa) is familial and not associated with systemic disease, so called idiopathic hypercalciuria (IH; Ref. 6). Most researchers have found evidence for increased vitamin D activity in patients with IH, such as high intestinal Ca absorption, high serum 1,25 vitamin D levels, and increased monocyte vitamin D receptor (VDR; Ref. 24). The proximate mechanism for hypercalciuria is not increase of filtered load but rather a higher fractional excretion of filtered calcium (FECa) arising from reduced renal Ca reabsorption (27). Our prior work suggests that at least the proximal tubule (PT) is involved in this decreased Ca reabsorption, but other nephron segments may be abnormal as well (26). The altered renal Ca reabsorption is most marked with eating. Fasting, FECa of IH exceeds N. With meals, the difference between IH and N is exaggerated, FECa and UCa rise in IH and N alike but increases in IH exceed those of N.
Contemporary evidence suggests at least three candidate signalers that could mediate the fall in tubule reabsorption and especially the accentuated fall with meals in IH. PTH increases renal Ca reabsorption, and its levels fall with eating (27). Serum Ca rises with meals and could reduce Ca reabsorption via the basolateral Ca receptor (CaSR) in thick ascending limbs (TAL; Ref. 19), a site of reduced reabsorption in at least one rat model of IH (23). Insulin rises with meals and has been shown to reduce Ca reabsorption in human subjects (10).
These signalers could reduce renal Ca reabsorption in IH more than N in three ways: 1) Blood concentrations of PTH might be lower or Ca or insulin concentrations higher in IH than N. 2) Changes in PTH, Ca, or insulin with meals might be greater in IH than N leading to correspondingly greater increases in FECa. 3) Tubule responses to one or more of these signalers might be greater in IH than N, likewise leading to differences in FECa or increased changes in FECa with meals even though the signaler concentrations or changes of concentration do not themselves differ between IH and N.
We have measured concentrations of these three potential signalers in IH and N eating identical diets in a General Clinical Research Center (GCRC) setting and have asked if any of the three might be a promising candidate to explain differences in FECa between IH and N via any of the three mechanisms detailed above. Whereas the most ideal experiment might be deliberate variation of each signaler separately, such difficult work is not reasonable if direct observations make a positive result unlikely. Further, these signalers may work in concert, which could not be detected except by simultaneous measurements in IH and N, fasting and fed, as we have done.
METHODS
Subjects
We studied 29 subjects (17 male) with IH and 17 (7 male) age- and sex-matched control subjects (N). Twenty seven of the twenty nine IH subjects formed Ca stones (Table 1), and the other two had bone disease. Ages, weights, and heights were well matched between IH and N (Table 1). Some subjects have appeared in other reports from our laboratory, as noted in Table 1. This study was approved by the Institutional Review Board at the University of Chicago (Protocol 12881A).
Table 1.
Patients and normal subjects
| Subject Number | Status | Age, yr | Sex | Weight, kg | Height, cm | Stone Type |
|---|---|---|---|---|---|---|
| 1 | Normal*†‡ | 47 | F | 86 | 160 | None |
| 2 | Normal*‡ | 44 | F | 55 | 158 | None |
| 3 | Normal*†‡ | 26 | F | 68 | 168 | None |
| 4 | Normal*†‡ | 55 | F | 67 | 169 | None |
| 5 | Normal*†‡ | 28 | M | 66 | 163 | None |
| 6 | Normal†‡ | 48 | M | 80 | 175 | None |
| 7 | Normal*†‡ | 55 | M | 88 | 181 | None |
| 8 | Normal*†‡ | 44 | M | 85 | 178 | None |
| 9 | Normal† | 38 | F | 71 | 167 | None |
| 10 | Normal† | 36 | M | 77 | 170 | None |
| 11 | Normal† | 52 | F | 62 | 169 | None |
| 12 | Normal*† | 24 | F | 71 | 178 | None |
| 13 | Normal*† | 50 | M | 76 | 183 | None |
| 14 | Normal*† | 29 | F | 62 | 161 | None |
| 15 | Normal*† | 37 | M | 90 | 189 | None |
| 16 | Normal | 38 | F | 78 | 150 | None |
| 17 | Normal | 25 | F | 65 | 164 | None |
| Means | 39 ± 3 | 72 ± 3 | 170 ± 2 | |||
| 18 | IHSF‡ | 41 | M | 80 | 168 | Ca |
| 19 | IHSF*†‡ | 48 | M | 102 | 185 | Caox |
| 20 | IHSF‡ | 59 | M | 85 | 177 | Cap |
| 21 | IHSF*‡ | 64 | M | 89 | 173 | Caox |
| 22 | IHSF‡ | 66 | M | 88 | 185 | Ca |
| 23 | IHSF‡ | 56 | F | 62 | 162 | Caox |
| 24 | IHSF‡ | 55 | M | 87 | 168 | Caox |
| 25 | IHSF‡ | 58 | F | 58 | 158 | Caox |
| 26 | IH‡ | 30 | M | 75 | 179 | None |
| 27 | IHSF*†‡ | 44 | M | 91 | 181 | Caox |
| 28 | IHSF*†‡ | 45 | F | 57 | 164 | Ca |
| 29 | IHSF# | 40 | F | 82 | 164 | Cap |
| 30 | IHSF*†‡ | 27 | M | 77 | 174 | Cap |
| 31 | IHSF*†‡ | 28 | M | 75 | 182 | Caox |
| 32 | IHSF‡ | 22 | M | 93 | 182 | Caox |
| 33 | IHSF*†‡ | 52 | F | 61 | 158 | Caox |
| 34 | IHSF‡ | 42 | F | 68 | 168 | Ca |
| 35 | IHSF‡ | 44 | M | 85 | 182 | Caox |
| 36 | IH‡ | 30 | F | 103 | 160 | None |
| 37 | IHSF† | 41 | F | 49 | 147 | Cap |
| 38 | IHSF† | 43 | M | 80 | 185 | Ca |
| 39 | IHSF*‡ | 29 | M | 89 | 185 | Caox |
| 40 | IHSF† | 45 | M | 87 | 174 | Caox |
| 41 | IHSF*‡ | 47 | F | 58 | 167 | Cap |
| 42 | IHSF*‡ | 47 | F | 79 | 158 | Ca |
| 43 | IHSF‡ | 45 | M | 83 | 179 | Cap |
| 44 | IHSF*‡ | 56 | M | 95 | 178 | Cap |
| 45 | IHSF | 38 | F | 67 | 164 | Cap |
| 46 | IHSF | 63 | F | 52 | 148 | Caox |
| Means | 45 ± 2 | 77 ± 3 | 171 ± 2 |
Values are means are ± SE. M, male, F, female; IH, idiopathic hypercalciuria; IHSF, idiopathic hypercalciuria with stone formation; the 2 IH without stones had bone disease. Means did not differ significantly between IH, IHSF, normals in any category.
Subject previously reported in Worcester et al. (26);
Subject previously reported in Bergsland et al. (Am J Physiol Renal Physiol 297:F1017–F1023, 2009);
Subject previously reported in Bergsland et al. (Am J Physiol Renal Physiol 300:F311–F318, 2011).
Protocol
Subjects were studied in the GCRC at the University of Chicago over 14 h as previously described (26, 27). Briefly, upon admission between 6:00 and 7:00 AM two 1-h fasting urine samples were collected. Subsequently, subjects ate three study meals, with hourly urine collections until 3 h after the last meal (14 urine samples total). Matching blood samples were collected hourly, and also every half hour in the 2 h following meals, for a total of 21 samples.
Diet
The study diet consisting of three isocaloric meals was designed to contain Ca, phosphorus, and sodium evenly distributed among the meals. The diet was planned by a nutritionist in the GCRC using Nutritionist IV, version 4.1 software (N-Squared Computing, San Bruno, CA). The 1,800-kcal base diet provided 1,160 mg calcium and 1,240 mg phosphorus daily, as determined by laboratory analysis of homogenates of the three meals (Covance Laboratories, Madison, WI; Ref. 27). The base diet provided 2,141 mg sodium, 2,427 mg potassium, and 218 mg magnesium per day. Subjects were stratified to one of three caloric levels (1,800, 2,100, or 2,400 kcal/day) according to an estimate of individual energy needs using the Schofield equation (20).
Laboratory Measurements
UCa and creatinine were measured in our laboratory on a Beckman DxC 600 analyzer (2). Serum samples were ultrafiltered using Amicon Ultra-4 filter tubes with a 10-kDa molecular mass cutoff membrane (Millipore, Bedford, MA); creatinine and Ca, were measured in the ultrafiltrate using the same methods as for urine. Serum Ca was measured by atomic absorption on a Perkin Elmer AAnalyst 100 spectrometer. Intact PTH was measured by LabCorp using an electrochemiluminescence immunoassay (Roche, Elecsys, Indianapolis, IN). Insulin was measured by the laboratory of the Diabetes Research and Training Center at University of Chicago using a chemiluminescent assay on an Immulite 2000 (Siemens Healthcare Diagnostics).
Analysis of Data
We propose three mechanisms by which serum Ca, PTH, or insulin might differentially reduce tubule Ca reabsorption in IH vs. N both fasting and with meals: Differences in blood levels within fasting or fed periods; differences in the changes of each with meals between IH and N; differences in the response of tubule Ca reabsorption between IH and N. For the first two, we used conventional ANOVA. The third is complex in the case of Ca because it is not only a potential signaler but also affects filtered load of Ca and is related to the calculation of fractional Ca absorption or excretion via its linear relation to ultrafilterable (UF) Ca.
Spontaneous variation of serum Ca concentration or increases caused by meals may well affect filtered load and therefore Ca excretion, but FECa will reflect both filtered load and × tubule reabsorption: if the increase in blood Ca with meals signals a reduction of tubule Ca reabsorption, then we should find that FECa (Eq. 1) rises with increases in the level of serum Ca:
However, UFCa molarity is in the denominator of the FECa term so that FECa and blood Ca have an inherent mathematical relationship: If total Ca reabsorption remains constant or falls, FECa will rise as UFca (which is strongly correlated with blood Ca) rises. Therefore, a rise of FECa per se cannot be a test of whether Ca signaling from blood is affecting Ca reabsorption. On the other hand, if blood signaling is less prominent in N than in IH, we should expect a stronger correlation between increases in blood Ca and FECa in IH than in N. Given this, we contrast the regression of FECa on blood Ca concentration between IH and N asking if there is a difference in regression behavior.
A second approach we use is to decompose FECa into two terms: Uca × (pCr/Ucr), and UFCa. The first term embodies the “urine” component of FECa, which is the UCa concentration at the end of the nephron corrected for water reabsorption; we call this “adjusted UCa.” The second term, UFCa, is the concentration of Ca delivered into the nephron. One expects adjusted UCa to move in parallel with UFCa, but if serum Ca were itself reducing Ca reabsorption more in IH than in N, one would expect an increased regression slope of the former on the latter in IH compared with N.
Our third approach is to ask if Ca excretion adjusted for filtered load will increase with serum Ca and, moreover, do so more in IH than N. We analyzed this using multivariable linear regression modeling.
We calculate filtered loads and FECa using UFCa, as is appropriate. However, for blood signaling we have preferred to use total serum Ca because our measurement system, atomic absorption spectroscopy, is superior in precision to the usual automated measurements we use for UFCa. We cannot measure UFCa on our AA instrument because of technical issues having to do with viscosity and dispersion in the flame. We did however, calculate regressions with both UFCa and total serum Ca and find they give similar conclusions (not shown).
For PTH and insulin, regression analysis was not complicated by either being part of the calculation of FECa; therefore, the analyses are conventional in nature.
Statistical Analysis
Multiple measurements were made on study subjects throughout the day, resulting in within-subject correlated measurement. Accordingly, statistical methods for correlated data were used to analyze the mean difference in each laboratory measurement of interest between IH and N during fasting and fed time periods. Generalized estimating equations (GEE; Ref. 11) utilizing an identity link function and independence working covariance structures were used to compare laboratory values between IH and N (Table 2). To correct inference for within-subject correlation, robust variance estimates clustering on subject were used (11). To determine whether the mean difference in each laboratory value comparing IH and N changed with respect to fasting state, multiplicative interactions between the indicator of IH status and fasting state were modeled. Simultaneous tests of regression model coefficients were conducted using multivariate Wald tests (Table 3).
Table 2.
Estimated mean chemistries by fast-fed N and IH standard errors and confidence intervals based on robust variance estimates to account for within subject correlation
|
Fast |
Fed |
Fed–Fast |
||||
|---|---|---|---|---|---|---|
| N | IH | N | IH | N | IH | |
| FECa, % | 1.63 ± 0.14 | 2.77 ± 0.17 | 2.9 ± 0.22 | 5.31 ± 0.32 | 1.26 (0.83, 1.69)* | 2.54 (2.04, 3.04)* |
| PTH | 34.9 ± 3 | 38.7 ± 2.7 | 28.7 ± 1.6 | 29 ± 1.6 | −6.26 (−10.91, −1.6)* | −9.65 (−12.91, −6.4)* |
| SCa, mmol | 2.34 ± 0.016 | 2.31 ± 0.014 | 2.37 ± 0.014 | 2.38 ± 0.017 | 0.032 (0.018, 0.046)* | 0.068 (0.049, 0.086)* |
| Serum insulin | 39.8 ± 4.9 | 48.2 ± 5.6 | 206.1 ± 15.4 | 259.9 ± 25.7 | 166.3 (142.2, 190.4)* | 211.7 (169.5, 253.9)* |
| Urine Ca, mmol/h | 0.14 ± 0.01 | 0.23 ± 0.02 | 0.26 ± 0.02 | 0.48 ± 0.03 | 0.115 (0.078, 0.152)* | 0.25 (0.198, 0.301)* |
| Filtered Ca, mmol/h | 9.5 ± 0.8 | 8.7 ± 0.5 | 9.1 ± 0.4 | 9.2 ± 0.4 | −0.45 (−1.95, 1.05) | 0.44 (−0.4, 1.28) |
Values are means ± SE; 95% confidence interval (CI) are in parentheses. FECa, fractional excretion of calcium; SCa, serum Ca; PTH, parathyroid hormone; N, normal; IH, idiopathic hypercalciuria. Italics: differs from fast, same group; underline: differs from N, same meal period.
Change differs from 0.
Table 3.
Generalized estimating equations estimates of percent FECa on PTH, SCa, and insulin by meal period
| Fast |
Fed |
Both |
||||
|---|---|---|---|---|---|---|
| N | IH | N | IH | N | IH | |
| PTH (per 5) | 0.01 (−0.11, 0.14) | −0.14*(−0.21, −0.06) | −0.02 (−0.27, 0.23) | −0.21 (−0.42, 0.00) | 0.03 (−0.16, 0.21) | −0.21*(−0.36, −0.05) |
| SCa (per 0.05 mMol) | −0.01 (−0.14, 0.13) | 0.20 (0.03, 0.36) | 0.10 (−0.12, 0.31) | 0.38(0.06, 0.70) | 0.05 (−0.13, 0.24) | 0.37 (0.09, 0.66) |
| Serum insulin (per 100) | −1.17 (−2.43, 0.09) | 0.27 (−0.85, 1.39) | 0.01 (−0.17, 0.19) | 0.06 (−0.07, 0.19) | −0.08 (−0.25, 0.09) | 0.08 (−0.04, 0.20) |
Values are percent change in FECa relative to the change specified for the independent variable (PTH, SCa, serum insulin) with 95% CI in parentheses. Analysis over both fasting and fed periods adjust for feeding. Underline: slope in IH is significant and that the 95% CI for the effect in IH does not contain the estimated value in N. Italics: P < 0.05 vs. 0.
P < 0.05, slopes differ between IH and N in generalized estimating equations analysis.
Data-driven variable selection was used to build parsimonious models to determine those factors that were most predictive of FECa and UCa excretion (Tables 4 and 5, respectively). Model selection for predictors of UCa excretion was performed by maximizing Akaike's Information Criteria (AIC; Ref. 1). We used AIC as a model selection criteria to balance the predictive ability of each proposed statistical model relative to the complexity of the model to avoid overfitting the observed data in our sample. AIC achieves this balance by penalizing each model's goodness of fit by a factor proportional to the number of parameters included in the model. After multiple predictive models for FECa and UCa excretion were assessed, those models that minimized AIC were selected and are reported in Tables 4 and 5. All statistical calculations were performed using R (13) and Systat 12 software (Systat Software, Chicago, IL).
Table 4.
Data-driven selection of covariates associated with FECa. Variables were chosen using Aikiake's information criteria
| Covariate | Derived Value (95% CI) | P Value |
|---|---|---|
| SCa (per 0.05) | Slope = 0.294 (0.051, 0.538) | 0.018 |
| N* | ||
| Food | mmol/h = 1.06 (0.636, 1.484) | <.0001 |
| IH* | ||
| Food | mmol/h = 2.123 (1.592, 2.655) | <.0001 |
Insulin, PTH, subject type × PTH, subject type × insulin, food × PTH, food × SCa, and food × insulin did not enter the regression.
Interaction between N and IH was statistically significant (P = 0.0007).
Table 5.
Data-driven selection of covariates associated with urine calcium excretion. Variables were chosen using Aikiake's information criteria
| Covariate | Derived Value (95% CI) | P Value |
|---|---|---|
| SCa (per 0.05) | 0.022 (0.008, 0.036) | 0.003 |
| N* | ||
| FLCa, mmol/h | 0.020 (0.013, 0.026) | <.0001 |
| Food | 0.109 (0.070, 0.148) | <.0001 |
| IH* | ||
| FLCa, mmol/h | 0.041 (0.032, 0.051) | <.0001 |
| Food | 0.197 (0.153, 0.241) | <.0001 |
Insulin, PTH, subject type × SCa, subject type × PTH, subject type × insulin, food × SCa, food × PTH, and food × insulin did not enter the regression. FLCa, filtered load of Ca.
Interaction between N and IH was statistically significant (P = 0.0007).
RESULTS
Analysis of FECa
Differences between IH and N, fasting and fed.
Even though FECa of IH exceeds N (Table 2), values of PTH, serum Ca, and insulin do not differ between IH and N within either the fasting or the fed periods (Table 2). Therefore, the differences in FECa between IH and N within fasting and fed periods cannot be readily ascribed to differences in concentrations of these signalers. However, FECa and serum Ca both rise with meals more in IH than N (Table 2: fed-fast values), whereas changes in PTH and insulin with meals do not differ between IH and N. Altogether, all three signalers changed with meals and could therefore explain the increase in FECa that occurs in both in IH and N. However, only serum Ca changed more with meals in IH than in N and might therefore explain the bigger change in FECa in IH than occurred in N.
Results for UCa excretion paralleled those for FECa.
UCa excretion of IH exceeded N fasting and fed (Table 2), fed values exceeded fasting in both groups, and the increment of UCa between fasting and fed states in IH exceeded that of N. Filtered loads of Ca did not differ between IH and N fasting or fed nor did filtered load change in either group between fasting and fed states (Table 2). This steadiness of filtered load accounts for the fact that results for FECa parallel those for UCa excretion.
Univariate regression analysis.
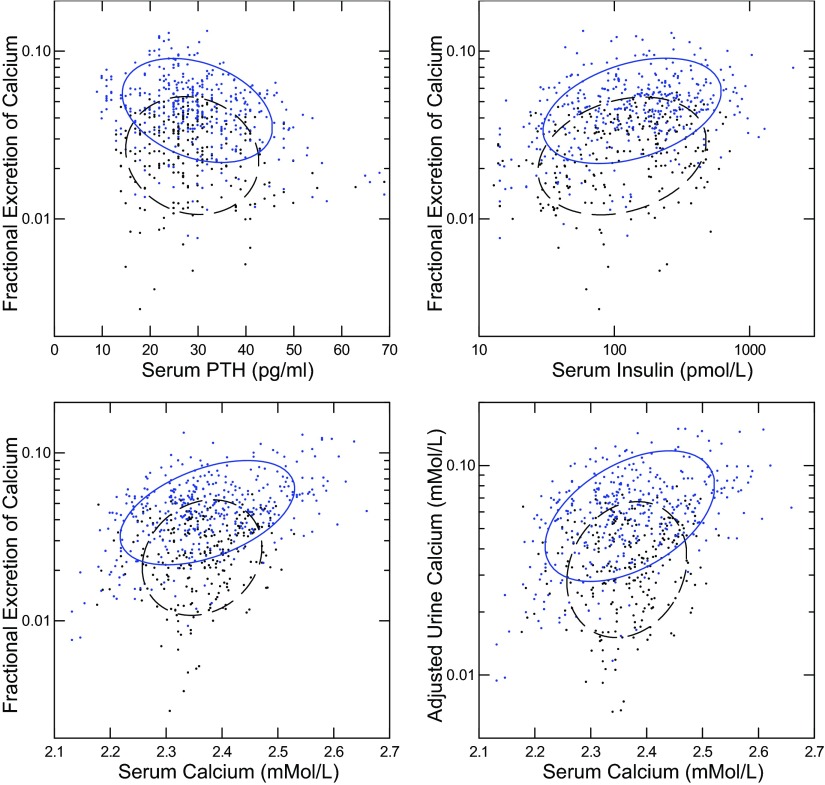
In IH, but not N, FECa rose with falling PTH (Fig. 1, top left; regression coefficients in legend). FECa rose with serum insulin and serum Ca (Fig. 1, top right and bottom left). The regression slope was larger in IH than N for serum Ca but not serum insulin. These differences in correlation between IH and N could reflect differing sensitivity of FECa to PTH and Ca. Because FECa contains UFCa in its denominator, creating an internal correlation, we decomposed FECa into its two components: the adjusted UCa and UFCa (see methods), and plotted the adjusted UCa against serum Ca. The results (Fig. 1, bottom right) correspond well with those for FECa itself: the regression slope was higher in IH than in N.
Fig. 1.
Relationships between fractional excretion of calcium (FECa) and parathyroid hormone (PTH), insulin, and serum Ca. Idiopathic hypercalciuria (IH; solid ellipses) and normal subjects (N; dashed ellipses) are plotted together in each panel. Nonparametric ellipses include 68% of points. Regression coefficients are shown with 95% confidence interval (CI) expressed in units to avoid excessive trailing 0s. Significant regressions are bolded; italics denotes that the 95% CI and mean value for N are outside that of IH; values are presented as IH vs. N, respectively. Regression of FECa (%) on PTH/5 pg/ml (top left): −0.32 (−0.41, −0.22) vs. −0.09 (−0.18, 0.08). Regression of FECa (%) on insulin/100 pmol/l (top right): 0.20 (0.10, 0.29) vs. 0.15 (0.04, 0.25). Regression of FECa on serum Ca (bottom left): 0.45 (0.36, 0.54) vs. 0.14 (0.036, 0.244). Regression of adjusted urine Ca (see methods) on serum Ca (bottom right): 0.73 (0.60, 0.86) vs. 0.21 (0.072, 0.35).
The visual impressions and simple regressions in Fig. 1 and its legend are partly confirmed by GEE regression analysis (Table 3). During the fasting and fed periods, and for fasting and fed periods combined, the regression of FECa is significant (bolded) for PTH and serum Ca in IH but, by contrast with simple regression, neither is significant in N. The values of the slope in N are outside the 95% confidence interval (CI) of the slope for IH fasting, and for fasting and fed combined (denoted by italics). The interaction between IH and N for the PTH effect is significant by GEE analysis (*) but that for serum Ca is not significant because of the high variability of the regression in the current sample.
Multivariable analysis.
The sensitivity of FECa to each of the three signalers is perhaps best not tested one at a time, because PTH varies with serum Ca and insulin might interact with either in affecting FECa. For this reason we built a data-driven model for all three potential signalers, fasting and fed, asking which if any were statistically independent covariates of FECa as gauged by AIC (see methods) and of these which if any differed in their covariance between IH and N (Table 4). Serum Ca, food (fed vs. fast), type (IH vs. N), and food × type interaction entered the model. The values for change of FECa by food (fed-fast) adjusted for serum Ca differed (P = 0.007) between IH and N as shown by lack of overlap of their 95% CI, and both were significant (P values in the right column). Serum Ca entered the model as a main effect but the effect of serum calcium was not observed to differ by food status or subject type. Neither PTH nor insulin entered at all. These results indicate that serum Ca is the main independent covariate of FECa in our subjects. That PTH did not enter the regression suggests that its effects in our univariate regression analysis (Table 3) may arise from its correlation with serum Ca. Reanalysis using log serum insulin did not alter these conclusions (not shown).
The values in Table 4 might not themselves be immediately interpretable and therefore warrant a comment. That for serum Ca is merely the overall (fed and fast, IH and N) slope term of FECa on serum Ca, like the corresponding individual IH and N, fed and fast values for slope of FECa on serum Ca in Table 3 and the simple regression slopes in the legend to Fig. 1. Those in Table 4 for “Food” are the changes (fed-fast) in FECa from Table 2 adjusted for serum Ca and shown for IH vs. N separately. For example, in N adjusted FECa change from fast to fed is 1.06 mmol/h vs. the unadjusted value of 1.26 (Table 2). The 95% CI for unadjusted and adjusted values overlap, and this is also true for IH. Serum Ca affects FECa but the effects of food are accounted for only modestly by serum Ca; the food and serum Ca effects are therefore independent.
FECa contains UFCa in its denominator, and UFCa is strongly correlated with serum Ca, so any regression analysis of FECa against serum Ca has an organic internal correlation. To avoid this problem, as in the univariate analysis, we decomposed FECa into its two independent terms: adjusted UCa and UFCa molarity (see methods). As in the analysis of FECa, serum Ca entered as a main effect on adjusted UCa (P = 0.011) but did not have a significant interaction with type (P = 0.08; not shown in tables). Serum PTH and insulin did not factor in but food and type and their interaction did enter with significance. Because these results parallel those using FECa itself, we conclude that the confounding effect of UFCa in the denominator of FECa does not lead to significant effects on our conclusions. Given this, we omit further details of this special regression analysis.
Analysis of UCa
Univariate regression.
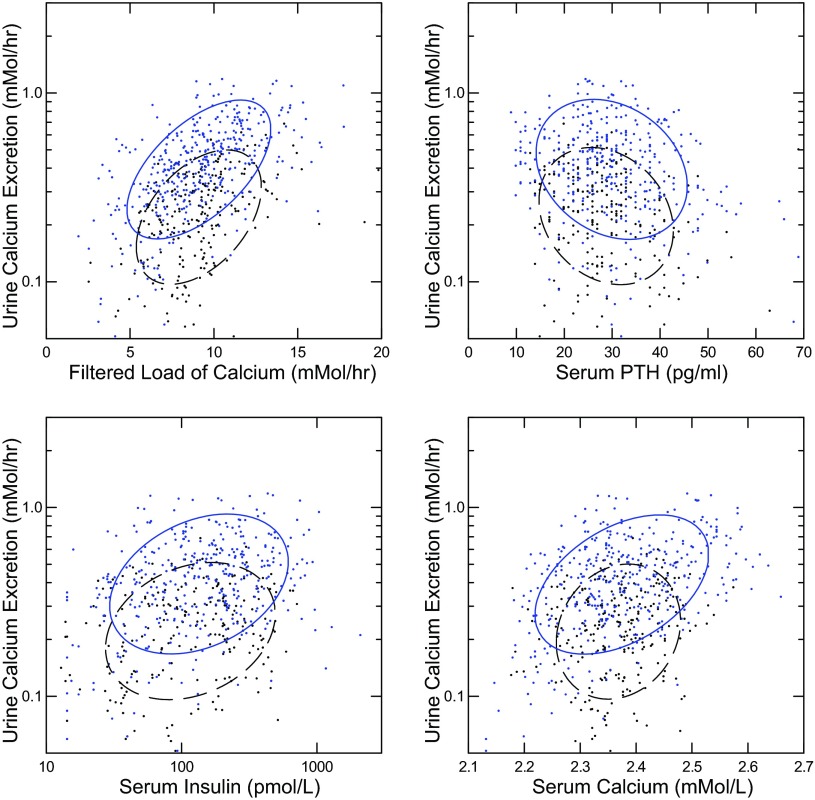
UCa excretion was correlated with filtered load of Ca in IH and N (Fig. 2, top left); the regression coefficient was larger in IH than in N. Likewise for serum Ca (Fig. 2, bottom right). UCa was inverse to serum PTH (Fig. 2, top right); the regressions did not differ between IH and N. Finally, UCa rose with serum insulin significantly in IH and N (Fig. 2, bottom left); the regressions did not differ between IH and N.
Fig. 2.
Relationships between urine Ca excretion and Ca filtered load, PTH, insulin, and serum Ca. IH (solid ellipses) and N (dashed ellipses) are plotted together in each panel. Nonparametric ellipses include 68% of points. Regression coefficients are shown with 95% CI expressed in units to avoid excessive trailing 0s. All regressions are significant; italics denote that the 95% CI and mean value for N are outside that of IH; values are presented as IH vs. N, respectively. Regression of Ca excretion on filtered load/ml/h (top left): 0.045 (0.038, 0.051) vs. 0.019 (0.015, 0.024). Regression of Ca excretion on PTH/5 pg/ml (top right): −0.25 (−0.35, −0.15) vs. −0.15 (−0.24, −0.06). Regression of Ca excretion on insulin/100 pmol/l (bottom left): 0.14 (0.043, 0.24) vs. 0.16 (0.047, 0.28). Regression of Ca excretion on serum Ca (bottom right): 0.87 (0.66, 1.06) vs. 0.27 (0.054, 0.49).
Multivariable analysis.
To assess the independent covariance of UCa excretion with filtered load, PTH, serum Ca, and insulin, we developed a data-driven model (Table 5) exactly as we did for FECa (Table 4). Serum Ca entered as a main effect along with filtered load of Ca, food, and subject type. As in the analysis of Table 4, the interaction of serum Ca and subject type was not significant. The effects of filtered load (change of UCa per 1 mmol/h) and the difference in Ca excretion between fasting and fed both differed between IH and N adjusted for serum Ca (P = 0.002 and P = 0.0001, respectively). These results parallel those for FECa in showing a dominant effect of serum Ca with no effects of PTH or insulin. The concordance of this analysis with that using FECa as the dependent variable confirms that the dominant effects of serum Ca are not arising from an artifact of UFCa being part of FECa or filtered load but rather represent a true physiological mechanism.
The effects of the adjustments in Table 5 were very modest. For example, the unadjusted regression coefficient for UCa on filtered load (Fig. 2 legend) was 0.019 in N vs. the adjusted value of 0.02 (Table 5); the 95% CI overlap. The unadjusted change in UCa with food (Table 2) was 0.115 for N vs. 0.109 (Table 5) fully adjusted for both serum Ca and filtered load; 95% CI overlap. Filtered load has a large effect on UCa of which only a modest fraction can be accounted for by changes in serum Ca. Likewise, food has a large effect on UCa that filtered load and serum Ca taken together as independent factors account for to only a small extent. Serum Ca, filtered load, and food have, therefore, independent effects on UCa the magnitudes of which are perhaps best judged from Table 5 and the comparisons we have made between the adjusted and unadjusted regressions.
Relationship of PTH to Serum Ca in IH and N, Fasting and Fed
The strong and independent covariance of FECa and UCa excretion with serum Ca suggests a possible link via the CaSR that is perhaps different in IH vs. N. Parathyroid cells regulate PTH secretion via their CaSR, and therefore covariance of PTH with serum Ca, in IH and N, fasting and fed, is a possible window into whether the CaSR signaling pathway is altered from normal in IH. Since levels of PTH and serum Ca do not differ between IH and N, fasting or fed (Table 2) they give no evidence to support a difference in signaling. Likewise, the fall in PTH in IH with meals is not different from N (Table 2).
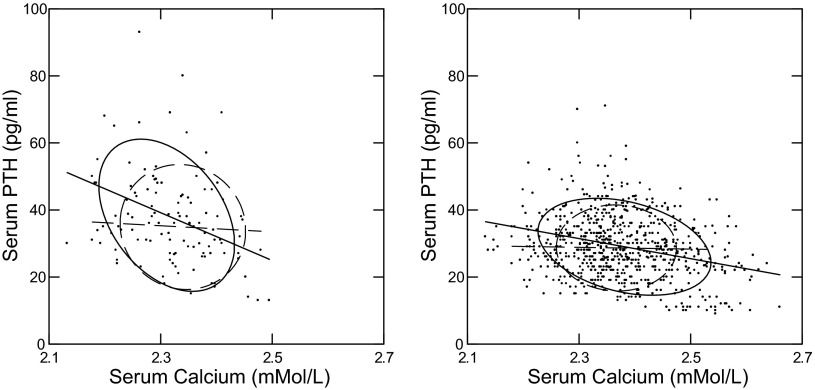
Simple inspection of PTH vs. serum Ca, however, reveals an inverse slope of PTH on serum Ca among IH but not N, fasting and fed (Fig. 3). Regression coefficients are significant for IH but not N both fasting and fed (Fig. 3 legend). To estimate the effect of serum Ca on the changes in PTH with meals, we constructed a general linear model with PTH as dependent and serum Ca, subject type, and food status as independent. The interaction between food status and subject type was not significant, meaning that meal changes did not differ between IH and N when adjusted for serum Ca. The adjusted values for PTH fed-fast did not differ between IH and N: −5.42 (−8.48, −2.37) vs. −5.38 (−9.52, and −1.23) IH vs. N, respectively, P = NS. The 95% CI for the unadjusted change (Table 2) overlap with these adjusted values meaning that the effect of the adjustment was modest. The actual differences (4.23 and 0.88, IH and N) between the unadjusted and adjusted values give a kind of intuitive metric for the Ca effect. This means that the effect of food is not entirely or even mainly that of serum Ca even though serum Ca certainly does have an effect. What there is about eating that lowers serum PTH remains uncertain. Amino acids or other nutrients are possible explanations.
Fig. 3.
Relationships between plasma PTH and serum Ca. IH (solid ellipses and linear regression lines) and N (dashed ellipses and linear regression lines) are plotted together in each panel. Nonparametric ellipses include 68% of points. Regression coefficients are shown with 95% CI expressed in units to avoid excessive trailing 0s. Significant regressions are bolded; italics denote that the 95% CI and mean value for N are outside that of IH; values are presented as IH vs. N, respectively. Fasting (left) and fed (right) regression of PTH on serum Ca/1 mmol/l is significant for IH but not N: −72 (−111, −31) vs. −9 (−63, 45) and −30 (−38, −22) vs. −2.6 (−17, 11.8), fasting and fed, respectively.
DISCUSSION
We began with the notion that PTH, serum Ca, and insulin could differentially regulate FECa via three different mechanisms: differences in blood levels between IH and N within fasting or fed states; differences in the change of each between IH and N in going from fasting to fed state; or a difference in tubule response between IH and N within the fasting or fed state reflected by regression analysis. Concentration differences between IH and N are not present for PTH, serum Ca, or insulin (Table 2). PTH, serum Ca, and insulin all change significantly between fasting and fed, but changes differ between IH and N only for serum Ca. This latter finding supports serum Ca as a possible candidate signaler via differences in meal related changes in concentration but do not support PTH or insulin in that role.
Regression analysis (Table 3), however, supports both PTH and serum Ca as potential regulators via differential sensitivity of FECa in that regressions of FECa on PTH and serum Ca are significant in IH both fasting and fed but are not significant in N. When meal periods are combined, the regressions of FECa on PTH and serum Ca are significant for only IH as well.
When all three factors are considered together, serum Ca appears the main regulator (Table 4); insulin and PTH do not enter into a full multivariable regression. This latter analysis is crucial because serum Ca affects PTH and the reverse. The main confounder, that serum Ca is closely related to UFCa which is part of the FECa calculation, is offset by the fact that conclusions remain the same when only UCa excretion rate (Table 5) or adjusted UCa (not shown) are considered.
The most straightforward assumption about how serum Ca might differentially affect FECa and UCa in IH vs. N is via the CaSR. The basolateral CaSR regulates TAL Ca reabsorption through modulation of both paracellular and transcellular Ca movement (19). CaSR stimulation inhibits the renal outer medullary potassium (ROMK) channel, which decreases activity of the Na-K-2Cl (NKCC2) cotransporter, dissipating the lumen positive electrochemical driving force for passive reabsorption of Ca (19). CaSR stimulation also leads to translocation of Claudin 16 into the lysosomal degradation pathway, which decreases paracellular movement of Mg and Ca (14). The CaSR exerts control over both apical and basolateral components of active Ca transport in TAL, which is decreased by CaSR activation (18). Overall, CaSR stimulation can decrease TAL Ca reabsorption through several mechanisms. Reduction of PTH itself with a resulting loss of the Ca conserving effects of PTH seems less plausible given the loss of PTH effects in our multivariable analysis. Effects of CaSR in the later nephron are less clear. In the distal convoluted tubule, CaSR has been shown to colocalize with TRPV5 and increase TRPV5 activity (22), although not all investigators agree (16, 21). Some investigators have suggested that the CaSR may also regulate basolateral Ca efflux from the distal convoluted tubule cell (12). It is difficult to infer the final effects of the distal tubule CaSR on UCa, but increased delivery out of TAL is likely to result in hypercalciuria (3).
One way that CaSR signaling might be higher in IH vs. N is increased CaSR abundance driven by the well described increase of vitamin D activity in IH subjects (4, 24). Serum levels of 1,25 vitamin D have generally been increased in IH vs. N, as have measurements of intestinal Ca absorption. In a strain of rats inbred for hypercalciuria over 70 generations, renal VDR and CaSR abundance levels are increased (5, 28), and TAL Ca reabsorption is reduced (23). Data on the responsiveness of Ca excretion to serum Ca levels in these rats is not available. The mechanism for vitamin D-mediated increase of CaSR is direct stimulation of gene expression; the promotor region of the CaSR gene has a VDR recognition site (19). It has been proposed that increased CaSR signaling and consequent rise in FECa might be a biological protection against hypercalcemia in states of increased vitamin D activity (15).
The assumption of a VDR-mediated increased abundance of CaSR is strengthened by our observations that PTH levels also seem more responsive to serum Ca in IH than in N. Regression coefficients for PTH on serum Ca are significant both fasting and fed in IH but not at all in N. PTH secretion is well known to be regulated by serum Ca via the parathyroid CaSR.
Changes in serum Ca are very small with meals, but they may not be the only meal-induced signaling mechanism for CaSR in IH. Aromatic l-amino acids sensitize the CaSR to Ca (8), and amino acid levels rise after eating, so that the effect of even small changes in serum Ca might be magnified. This may be one reason for the well-documented rise in UCa after ingestion of protein (7, 9), which is not explained by the acid load imposed by protein ingestion (17). We have not measured amino acid levels in these subjects, and cannot compare levels in IH and N, but all subjects are eating identical amounts of protein during the study. In future experiments, amino acid infusion, or deliberate changes in serum Ca at a constant PTH may be able to test the CaSR hypothesis with greater rigor than possible here.
Although our data support an effect of serum Ca on FECa and UCa which is more marked in IH than in N, it can account for only a modest fraction of the meal effect in our experiment, perhaps 10–20%. This applies to N as well as IH even though the effects of meals and serum Ca are more marked in IH than in N. The food effect on FECa and UCa require considerably more research.
GRANTS
This publication was made possible by National Institutes of Health (NIH) Grants P01-DK-56788 and UL1-RR-024999 to the University of Chicago GCRC from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
DISCLOSURES
F. L. Coe and E. M. Worcester are consultants for Litholink, Labcorp.
AUTHOR CONTRIBUTIONS
Author contributions: E.M.W. and F.L.C. conception and design of research; E.M.W. and K.J.B. performed experiments; E.M.W., K.J.B., D.G., and F.L.C. analyzed data; E.M.W., K.J.B., D.G., and F.L.C. interpreted results of experiments; E.M.W., K.J.B., and F.L.C. drafted manuscript; E.M.W., K.J.B., D.G., and F.L.C. approved final version of manuscript; K.J.B. and F.L.C. prepared figures; D.G. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank the patients for participating and the nursing staff at the University of Chicago GCRC for expert assistance.
REFERENCES
- 1.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 19: 716–723, 1974 [Google Scholar]
- 2.Asplin JR, Parks JH, Coe FL. Dependence of upper limit of metastability on supersaturation in nephrolithiasis. Kidney Int 52: 1602–1608, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bonny O, Edwards A. Calcium reabsorption in the distal tubule: regulation by sodium, pH, and flow. Am J Physiol Renal Physiol 304: F585–F600, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Broadus AE, Insogna KL, Lang R, Ellison AF, Dreyer BE. Evidence for disordered control of 1,25 dihydroxyvitamin D production in absorptive hypercalciuria. N Engl J Med 311: 73–80, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Bushinsky DA, Frick KK, Nehrke K. Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens 15: 403–418, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N Engl J Med 300: 337–340, 1979 [DOI] [PubMed] [Google Scholar]
- 7.Conigrave AD, Brown EM, Rizzoli R. Dietary protein and bone health: roles of amino acid-sensing receptors in the control of calcium metabolism and bone homeostasis. Annu Rev Nutr 28: 131–155, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Conigrave AD, Lok HC. Activation of renal calcium and water excretion by novel physiological and pharmacological activators of the calcium-sensing receptor. Clin Exp Pharmacol Physiol 31: 368–371, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Conigrave AD, Mun HC, Brennan SC. Physiological significance of l-amino acid sensing by extracellular Ca2+-sensing receptors. Biochem Soc Trans 35: 1195–1198, 2007 [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 845–855, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford, UK: Oxford Univ. Press, 2002 [Google Scholar]
- 12.Ferre S, Hoenderop JG, Bindels RJ. Sensing mechanisms involved in Ca2+ and Mg2+ homeostasis. Kidney Int 82: 1157–1166, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat 5: 299–314, 1996 [Google Scholar]
- 14.Ikari A, Okude C, Sawada H, Sasaki Y, Yamazaki Y, Sugatani J, Degawa M, Miwa M. Activation of a polyvalent cation-sensing receptor decreases magnesium transport via claudin-16. Biochim Biophys Acta 1778: 283–290, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kantham L, Quinn SJ, Egbuna OI, Baxi K, Butters R, Pang JL, Pollak MR, Goltzman D, Brown EM. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab 297: E915–E923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122: 3355–3367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maalouf NM, Moe OW, Adams-Huet B, Sakhaee K. Hypercalciuria associated with high dietary protein intake is not due to acid load. J Clin Endocrinol Metab 96: 3733–3740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motoyama HI, Friedman PA. Calcium-sensing receptor regulation of PTH-dependent calcium absorption by mouse cortical ascending limbs. Am J Physiol Renal Physiol 283: F399–F406, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 298: F485–F499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39, Suppl 1: 5–41, 1985 [PubMed] [Google Scholar]
- 21.Toka HR, Al-Romaih K, Koshy JM, DiBartolo S, 3rd, Kos CH, Quinn SJ, Curhan GC, Mount DB, Brown EM, Pollak MR. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol 23: 1879–1890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium 45: 331–339, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Tsuruoka S, Bushinsky DA, Schwartz GJ. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney Int 51: 1540–1547, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol 28: 120–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worcester EM, Coe FL. Clinical practice. Calcium kidney stones. N Engl J Med 363: 954–963, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worcester EM, Coe FL, Evan AP, Bergsland KJ, Parks JH, Willis LR, Clark DL, Gillen DL. Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone-forming patients. Am J Physiol Renal Physiol 295: F1286–F1294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worcester EM, Gillen DL, Evan AP, Parks JH, Wright K, Trumbore L, Nakagawa Y, Coe FL. Evidence that postprandial reduction of renal calcium reabsorption mediates hypercalciuria of patients with calcium nephrolithiasis. Am J Physiol Renal Physiol 292: F66–F75, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Yao JJ, Bai S, Karnauskas AJ, Bushinsky DA, Favus MJ. Regulation of renal calcium receptor gene expression by 1,25-dihydroxyvitamin D3 in genetic hypercalciuric stone-forming rats. J Am Soc Nephrol 16: 1300–1308, 2005 [DOI] [PubMed] [Google Scholar]