Abstract
The classic role of the Na-K-ATPase is that of a primary active transporter that utilizes cell energy to establish and maintain transmembrane Na+ and K+ gradients to preserve cell osmotic stability, support cell excitability, and drive secondary active transport. Recent studies have revealed that Na-K-ATPase located within cholesterol-containing lipid rafts serves as a receptor for cardiotonic steroids, including ouabain. Traditionally, ouabain was viewed as a toxin produced only in plants, and it was used in relatively high concentrations to experimentally block the pumping action of the Na-K-ATPase. However, the new and unexpected role of the Na-K-ATPase as a signal transducer revealed a novel facet for ouabain in the regulation of a myriad of cell functions, including cell proliferation, hypertrophy, apoptosis, mobility, and metabolism. The seminal discovery that ouabain is endogenously produced in mammals and circulates in plasma has fueled the interest in this endogenous molecule as a potentially important hormone in normal physiology and disease. In this article, we review the role of the Na-K-ATPase as an ion transporter in the kidney, the experimental evidence for ouabain as a circulating hormone, the function of the Na-K-ATPase as a signal transducer that mediates ouabain's effects, and novel results for ouabain-induced Na-K-ATPase signaling in cystogenesis of autosomal dominant polycystic kidney disease.
Keywords: cardiotonic steroids, Na-K-ATPase signalosome, polycystic kidney disease
the na-k-atpase plays an essential role in cell biology not only through its classic action as an ion pump but also as a signaling molecule that transduces the effects of ouabain and other cardiotonic steroids into cell responses. The discovery of this signaling function for the Na-K-ATPase took place long after its ion transporter properties were described and initiated a fascinating field of investigation that is rapidly expanding. The finding of a “non-pumping” function for the Na-K-ATPase also changed the traditional view of ouabain, from a compound that, at “toxic” concentrations, served to inhibit and define Na-K-ATPase activity, to a molecule that, at relatively low concentrations, has cell-regulatory properties. Results from a number of laboratories now show that ouabain activation of Na-K-ATPase-mediated intracellular signaling represents a novel mechanism for the modulation of a variety of cell processes involved in both normal cell physiology and disease. Ouabain has been shown to influence growth, apoptosis, and function (i.e., Na+ absorption) of the normal kidney epithelium. More recently, ouabain was found to stimulate cell proliferation and transepithelial fluid secretion by renal cyst epithelial cells cultured from patients with autosomal dominant polycystic kidney disease (ADPKD). This article reviews new advances in the field of ouabain-induced signaling function of the Na-K-ATPase, emphasizing the potential cystogenic effects of circulating levels of ouabain in the pathogenesis of ADPKD.
The Na-K-ATPase
Structure and classic ion transporter function.
The Na-K-ATPase or “Na pump” is an ion transporter within the plasma membrane of most animal cells that utilizes the energy from the hydrolysis of ATP to catalyze the movement of Na+ out of the cell in exchange for K+ in a 3 Na+:2 K+ stoichiometry (27, 89, 186). Na-K-ATPase establishes transmembrane chemical gradients for Na+ and K+ that are essential for maintaining cell osmolarity, volume, and pH, resting membrane potential, and the electrochemical energy for secondary transport of ions and nutrients across the cell membrane (17, 41, 58, 72, 135, 199, 246). In the kidney, the Na-K-ATPase is expressed within the basolateral membranes of tubuIe epithelial cells, where it has a central role in the reabsorption and secretion of a variety of solutes. The differential expression and function of the Na-K-ATPase and its particular regulation along the nephron is key to the formation of urine with the appropriate volume and composition and for the maintenance of body salt and water homeostasis (58).
The Na-K-ATPase is composed of a heterodimer of two main polypeptides, the α- and β-subunits (Fig. 1A). The α-polypeptide constitutes the catalytic subunit of the enzyme, containing the binding sites for ATP, Na+, K+, and several regulators of the Na-K-ATPase (142). The α-polypeptide is a 10-transmembrane-spanning protein, with intracytoplasmic N and C termini, a large intracellular region, and 5 small extracellular loops (141). The ATP-dependent translocation of ions takes place through a reaction cycle characterized by Na+- and K+-induced conformational changes of the α-subunit, which oscillates between two main states, designated E1 and E2 (10). These conformational changes are driven by the alternating covalent phosphorylation and dephosphorylation of an aspartate residue located between transmembrane helixes 4 and 5 of the protein and involve the sequential movement of three portions of the molecule, the actuator (A), nucleotide binding (N), and phosphorylating (P) domains (32, 201). While ATP hydrolysis and ion translocation primarily occur through the α-subunit, the Na-K-ATPase also requires the β-subunit for its activity. The β-polypeptide is a type II glycosylated protein that has an N-terminal cytoplasmic tail, a single transmembrane domain, and most of its mass facing the extracellular medium (64). The β-subunit acts as a chaperone protein that directs the correct folding and targeting of the α-polypeptide during its synthesis and delivery to the plasma membrane (63). In addition, the β-subunit serves as an adhesion molecule. In the kidney, the interaction between β-subunits from neighboring cells helps to maintain the structural integrity and apical-to-basal polarity of the tubule epithelium (37, 173, 211).
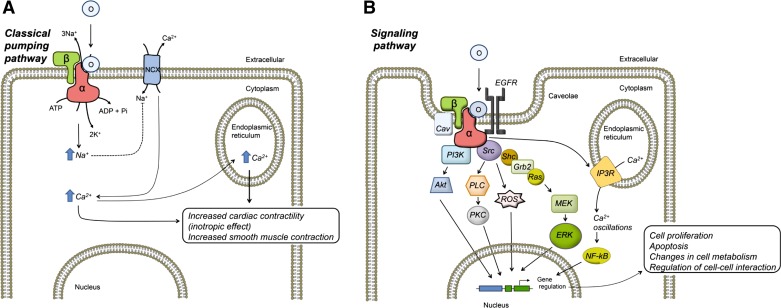
Fig. 1.
Diagram showing Na-K-ATPase control of cell function by ouabain via its ion transport and signaling functions. A: by inhibiting the Na-K-ATPase pumping mode, ouabain can directly modify intracellular Na+ levels and secondarily cytosolic Ca2+ via the Na/Ca exchanger (NCX). This represents the well-described mechanism of action of the cardiotonic steroids that lead to cardiac- and smooth muscle-enhanced contraction. B: by acting through a subpopulation of Na-K-ATPase in caveolae, ouabain activates the Na-K-ATPase signaling pathway, which involves a series of intracellular intermediates that ultimately modulate gene expression and cause a variety of cell type-specific effects, such as those listed in the figure. O, ouabain, (α and β) subunits of the Na-K-ATPase. See the text for additional definitions.
In mammals, four α-polypeptides (α1, α2, α3, and α4) and three β-subunits (β1, β2, and β3) are expressed in different combinations and in a cell type-specific and developmentally regulated manner (20, 116, 138, 196). Distinct association of α- and β-isoforms results in multiple αβ dimers or isozymes of the Na-K-ATPase. The α1- and β1-isoforms are widely expressed and present in most cells, including renal epithelial cells. In contrast, the other α- and β-isoforms have a more limited pattern of expression. For example, α2 predominates in adipocytes, muscle, the heart, and the brain, α3 is abundant in nervous tissues, and α4 is confined to the male germ cells in the testis. Similarly, the β-isoforms are distributed in a tissue-dependent manner. The β2-polypeptide is found in skeletal muscle, the pineal gland, and nervous tissues, and β3 is expressed in the testis, retina, liver, and lung (21, 138). Interestingly, the pattern of Na-K-ATPase isozyme expression is altered in some pathological states, including several cardiovascular and neurological diseases (8, 28, 39, 65, 145). Examination of tissues enriched with a specific Na-K-ATPase isozyme and analysis of various α- and β-isoform combinations in heterologous expression systems have shown that each Na-K-ATPase isozyme has unique enzymatic properties (21, 146, 181). The α-isoform is responsible for most of the functional dissimilarities between the Na-K-ATPase isozymes, with the β-subunit only modestly influencing the affinity of the α-subunit to Na+ (52, 84, 168). One of the greatest kinetic differences among α-isoforms is their unique sensitivity to ouabain. In rodents, α1 activity is highly resistant to ouabain inhibition, whereas α2, α3, and α4 demonstrate progressively higher sensitivity (20, 157, 195). This distinct response to ouabain has been commonly used as a tool to identify the contribution of each α-isoform to the total Na-K-ATPase activity in a sample. In contrast, in other mammals, including humans, the α1-isoform has a higher ouabain affinity, making it difficult to distinguish α1 from the other isoforms (43, 144, 220). The finding that Na-K-ATPase isozymes have singular enzymatic properties suggests that they play specific roles (20). The advent of transgenic technologies and studies in various pathological states suggests that the α1β1 complex is the ubiquitous Na-K-ATPase that maintains Na+ and K+ transmembrane gradients. In contrast, other Na-K-ATPase isozymes are responsible for cell-specific tasks. In this manner, Na-K-ATPase isozyme heterogeneity appears to have evolved as a mechanism to serve particular cell physiological needs (45, 49, 81, 87, 118, 143, 245). In addition, alterations of the function of Na-K-ATPase isozymes may play critical roles in disease (19, 40, 97, 176, 213).
The Cardenolide Ouabain
General structure and properties.
Ouabain belongs to a group of compounds, the cardiotonic steroids, which are composed of a steroidal backbone, the aglycone, or genin; a five-membered unsaturated lactone ring; and a sugar moiety, which varies depending on the type of cardenolide (15). Ouabain binds to the extracellular side of the Na-K-ATPase α-subunit, preferentially docking to the E2 phosphorylated conformation of the transporter. Ouabain interferes with the reaction cycle, inhibiting the ion transport and enzymatic activity of the Na-K-ATPase (117, 216). Because of this property, relatively high doses of ouabain have been conventionally used to specifically define Na-K-ATPase-dependent ion transport (10). Ouabain is classified as a cardenolide, one of two subfamilies of cardiotonic steroids, which include several plant derivatives (3). Ouabain is found in the African plants Strophantus gratus and Acokanthera ouabaio. Other cardenolides are digitalis, present in the foxglove Digitalis purpurea, and digoxin, found in Digitalis lanata. The other subfamily of cardenolides comprises the bufadienolides, which include bufalin and marinobufagenin. These cardenolides are produced in the skin of the toad, Bufo marinus.
The practical use of cardenolides and bufadienolides can be traced back centuries, much before their effects on the Na-K-ATPase were known. The toxic effects of cardiotonic steroids made them useful as poisons. In addition, utilized in limited and controlled amounts, these substances were found to have therapeutic benefits, which opened their application as herbal remedies (113). It was not until ∼200 years ago that cardiotonic steroids were formally used in the clinical setting. The positive inotropic effects of nontoxic doses of cardiotonic steroids made these compounds useful for the treatment of cardiac insufficiency and conferred them their general designation as “cardiotonics” (55, 80). Because digitalis and digoxin cause positive chronotropism, they have also proven useful for the treatment of atrial fibrillation (132). Despite their application in the treatment of heart failure and arrhythmias, the use of cardiotonic steroids remained mostly empirical for quite a long time (18, 214); only recently have insights into their mode of action been obtained. Simultaneous measurements of intracellular Na+ concentrations and contractile force in cardiac fibers, along with studies of the effect of extracellular K+, showed that the “cardiotonic” effect was caused by inhibition of the Na-K-ATPase (90, 107). Subsequent experiments established a correlation between cytosolic Na+ and Ca2+ levels and the functional association of Na-K-ATPase with the Na/Ca exchanger (NCX) in cardiac cells (107, 135, 187, 219, 221, 227). A model developed for the mechanism of action of cardiotonic steroids, by which inhibition of Na-K-ATPase increases intracellular Na+ and produces a secondary rise in Ca2+ by reducing the inward movement of Na+ through the sarcolemmal NCX. The higher intracellular Ca2+ is taken up by the sarcoplasmic reticulum via the Ca-ATPase, allowing stronger contractility of the heart muscle when Ca2+ is released back into the cytoplasm (Fig. 1A) (24, 25). Later evidence indicated that the cardiotonic effects of ouabain are mediated by the Na-K-ATPase α2-isoform in mouse hearts (45, 48). Through mechanisms similar to those of the heart, ouabain can enhance contraction of vascular smooth muscle cells to augment vascular tone and produce arterial constriction in rodents (26, 49).
Ouabain as an endogenous compound in mammals.
Originally, ouabain and other cardiotonic steroids were thought to only be the products of plants and amphibians; however, it was later discovered that they are also endogenously expressed in mammals. This finding resulted from the search for a circulating substance that could explain Na+-dependent changes in blood pressure. The factor that was discovered to be increased in plasma after salt-induced volume expansion had the ability to partially inhibit Na-K-ATPase activity and induced natriuresis. This natriuretic factor also raised blood pressure, an effect that was later found to be dependent on the ability of the substance to block Na-K-ATPase activity within the smooth muscle of the arterial walls (60). Several different endogenous chemical scaffolds with the ability to inhibit the Na-K-ATPase were subsequently reported, and cardiotonic steroids appeared as principal candidates that fulfilled the observed properties. However, there was concern that cardiotonic steroids found in the circulation were simply contaminants from ingesting plant products in the diet. In addition, the scarce amounts and the technical difficulties in both purification and identification of these substances represented major obstacles before the existence of cardiotonic steroids as endogenous products of higher animals could be validated. Ouabain was purified from extracts of bovine adrenal gland, hypothalamus, hypophysis, and adrenal gland tumors, demonstrating the presence of this cardenolide in mammalian tissues (14, 33, 46, 88, 150, 178, 185). Ouabain was also isolated from conditioned media of PC12 and rat adrenal gland cell cultures, confirming that mammalian cells produce ouabain (100).
Hamlyn and coworkers (74) were the first to identify ouabain as a circulating substance in human plasma. Endogenous ouabain has also been found in the plasma of other species where concentrations vary in the picomolar-to-nanomolar range. Several lines of evidence suggest that ouabain functions as a natriuretic factor. Administration of anti-ouabain antibodies to rats reduced Na+ excretion by the kidney (151), and circulating levels of ouabain increased in rats after salt loading (233). In Dahl salt-sensitive rats, an intraperitoneal Na+ load stimulated the release of a ouabain-like compound into the circulation and caused an increase in renal Na+ excretion, suggesting the role of ouabain as a regulatory mechanism for the maintenance of Na+ homeostasis (57). In cultured LLC-PK1 cells, a renal epithelial cell line, ouabain inhibited apical-to-basolateral transepithelial flux of 22Na+ across the cell monolayers by decreasing the expression of Na-K-ATPase and Na/H exchanger 3 (NHE3) at the plasma membrane. This effect was mediated by a combination of endocytosis and reduced gene transcription of the ion transporters (121, 239). More recently, transgenic mice that express a mutated renal Na-K-ATPase α1-isoform that has a greater sensitivity to ouabain exhibited a greater natriuretic response to Na+ load than wild-type mice (126).
While research has mainly advanced our understanding of the endogenous nature and effects of ouabain in different cells and organs, relatively little is known regarding the endocrine aspects of this hormone. The site of ouabain synthesis has been mapped to the zona glomerulosa and fasciculata of the adrenal gland cortex (104, 133). The biosynthetic pathway for ouabain requires hydroxycholesterol, pregnenolone, and progesterone as precursors and follows metabolic reactions that are, in part, shared with those of other adrenal steroidal hormones (75, 179). Also, there is evidence that, once synthesized, ouabain is released into the circulation in a regulated fashion. Several conditions are associated with an increase in ouabain, including chronic renal insufficiency (191), experimental uremia (78), congestive heart failure (129, 130), hypertension (79), primary hyperaldosteronism (175), physical exercise (9), hypoxia (174), and chronic salt intake (23). Ouabain secretion is also stimulated by ACTH, angiotensin II (ANG II), and α1-adrenergic receptor agonists (179). Data on other characteristics of endogenous ouabain, such as its half-life in plasma, metabolism, and elimination, are scarce and mostly limited to the pharmacokinetic and pharmacodynamic information reported for exogenously administered ouabain (92, 169, 182, 183).
Ouabain-Na-K-ATPase Signaling
The Na-K-ATPase is a receptor and signal transducer.
The detection of low levels of ouabain in the circulation encouraged studies of the nontoxic effects of cardenolides. Ouabain, in concentrations, which do not completely inhibit the Na-K-ATPase, is capable of eliciting a wide variety of effects, including cell proliferation, hypertrophy, apoptosis, and changes in cell motility and metabolism. These effects are cell type specific, resulting in different functional outcomes at the tissue and organ level (14, 180, 185). A breakthrough in the mechanisms of action of ouabain was the discovery that ouabain not only affected ion transport activity of the Na-K-ATPase but also activated a series of intracellular messengers and signaling events in cells. This revealed an unforeseen novel receptor and signal transduction function for the Na-K-ATPase, which upon binding ouabain, at endogenous concentrations, mediates cellular effects (165, 229, 230). Pioneering research by Askari (99) and Xie (120) in myocardial and kidney epithelial cells showed that ouabain induces the interaction of the Na-K-ATPase with signaling proteins, leading to activation of the tyrosine kinase Src and the phosphorylation of downstream effector proteins. Allen (2, 13) also contributed to the original findings for a role of the Na-K-ATPase as a transducer of ouabain's effects, providing the first evidence of Na-K-ATPase-mediated intracellular signaling in vascular smooth muscle cells. Additional studies extended these observations to other cell types (4, 31, 47, 53, 94, 101, 102, 140, 147, 210, 232). It is now clear that Na-K-ATPase functions as a molecular scaffold that assembles a multiprotein signaling complex known as the Na-K-ATPase signalosome (230) (Fig. 1B). Proteins found to be associated to the ouabain-bound Na-K-ATPase complex include adducin phosphoinositide-3-kinase, ankyrin, annexin 2, E-cadherin, and caveolin (113, 123). The observation that caveolin 1 also interacts with the Na-K-ATPase suggests that the Na-K-ATPase signalosome resides in caveolae (119, 122), distinctive flask-shaped invaginations of the plasma membrane rich in cholesterol (162). Indeed, cholesterol was required for ouabain-induced Na-K-ATPase signaling, and the absence of caveolin 1 abolished ouabain's effects, confirming a role of caveolae in this signaling complex (119, 124, 172, 218). Using a clever approach, consisting of stepwise downregulation of the Na-K-ATPase in LLC-PK1 cells, Xie and associates (115, 124) showed that not all of the Na-K-ATPase at the cell surface was involved in cell signaling, but rather only the subpopulation of Na-K-ATPase associated with caveolae responded to ouabain stimulation. Therefore, the idea of two pools of Na-K-ATPase, one involved in signaling and another devoted to ion pumping, emerged.
Na-K-ATPase signaling pathway.
A central event in ouabain-Na-K-ATPase signaling is tyrosine phosphorylation of a series of proteins (Fig. 1B). The Na-K-ATPase has no inherent tyrosine kinase or phosphatase activity and therefore depends on the recruitment and activation of Src. In this manner, the Na-K-ATPase serves as the ouabain binding receptor within the signaling apparatus, and Src functions as the transduction element involved in protein phosphorylation and signal amplification (165). In several cell types, including myocardiocytes, ouabain causes a dose-dependent rapid activation of Src, which is dependent on autophosphorylation of tyr 418, leading to subsequent tyrosine phosphorylation of proteins (113). Coimmunolocalization and coimmunoprecipitation experiments and fluorescence resonance energy transfer analysis showed that Na-K-ATPase and Src physically interact (114, 204). This association involves two protein-protein interacting sites, which include the second and third cytoplasmic regions in the Na-K-ATPase α-subunit and two sites in Src, one of which includes the kinase domain of Src. Under basal conditions, Na-K-ATPase-Src association keeps Src in an inactive state; however, ouabain binding disrupts Na-K-ATPase/Src interaction specifically at the Src kinase domain, activating the kinase (243). Interestingly, Na-K-ATPase regulation of Src activity depends on changes in the protein conformational state of the α-subunit. Induction of conformational changes in Na-K-ATPase by chemical modifiers and manipulation of the extracellular K+ concentrations showed that in E2 conformation the Na-K-ATPase releases from the kinase domain of Src, leading to Src activation (243). These experiments concluded that Na-K-ATPase signaling depends on the ability of ouabain to lock the Na-K-ATPase in the E2 conformation, thus providing, for the first time, a mechanistic view of ouabain's action on Na-K-ATPase. Furthermore, it was later found that Na-K-ATPase signaling could be influenced by ion-induced conformational changes in the enzyme and conditions that favor transition to the E2 conformation (243).
Other events in Na-K-ATPase signaling are transactivation and phosphorylation of the epidermal growth factor receptor (EGFR) and Src-dependent assembly of the adaptor proteins Shc and Grb2 (73). This, in turn, stimulates Ras and the mitogen-activated protein kinase ERK, an important pathway involved in cell proliferation (99) (Fig. 1B). Studies in different cell types have expanded the list of pathways activated by ouabain. Additional mediators that operate downstream of the Na-K-ATPase/Src complex include phospholipase C and several isoforms of PKC, phosphatidylinositol 3-kinase (PI-3K) and AKT. Ouabain can also exert effects through the generation of reactive oxygen species from mitochondria (56, 231). Aperia and collaborators (12, 137) showed that, in primary cultures of renal proximal tubular cells, nanomolar amounts of ouabain generated spatial and temporal changes in intracellular Ca2+, which exhibited a characteristic low-frequency oscillatory pattern, involved in cell proliferation. As shown in Fig. 1B, the mechanism responsible for these Ca2+ changes involves the interaction of the N-terminal portions of the Na-K-ATPase α-subunit and the 1,4,5-triphosphate receptor (IP3R) (11). The close proximity between Na-K-ATPase/Src and IP3R constitutes a Ca2+-signaling microdomain that can transduce the effect of ouabain into endoplasmic reticulum Ca2+ release (125, 137, 205). The Ca2+ oscillations are important to enhance the activity of the transcription factor NF-κB, which in turn upregulates early and late response genes (5, 12). Ouabain also increases capacitative Ca2+ entry in kidney proximal tubule cells via store-operated Ca2+ channels, which activates Akt (protein kinase B) (95). In this manner, Na-K-ATPase is able to control intracellular Ca2+ via mechanisms that are independent of the increase in intracellular Na+ and Ca2+ secondary to inhibition of Na-K-ATPase ion transport and reduction in NCX activity. A purposed schematic for the function of the Na-K-ATPase as an ion transporter and a signaling molecule is summarized in Fig. 1B.
Cellular effects of ouabain in kidney cells.
In the kidney, the most prominent consequences of ouabain's effect are stimulation of cell proliferation and inhibition of apoptosis. Ouabain has been shown to have a mitogenic effect on opossum and rat kidney proximal tubular cells (47, 95), it protects renal epithelial cells from apoptosis induced by serum deprivation and rescues renal development from the adverse effects of malnutrition (11, 93, 110, 111). Ouabain has also been shown to have effects on renal function, including the regulation of Na+ reabsorption by tubular epithelial cells (22, 35, 151), modulation of epithelial cell-cell adhesion and attachment (42, 106), epithelial ciliogenesis (105), and changes in the contractile state and resistance of isolated descending vasa recta (36). Altogether, these examples show that the actions of ouabain are complex and highlight the important role that this poorly understood hormone plays in the modulation of renal development and function.
ADPKD
Etiology and characteristics.
ADPKD is the most common, potentially lethal renal disorder, with a prevalence of 1 in 500–1,000 births worldwide (reviewed in Ref. 67). While the prominent feature and major cause of morbidity in ADPKD is the formation and progressive enlargement of innumerous fluid-filled cysts within the kidneys, there are also extrarenal manifestations, such as cysts in the pancreas, liver, and intestines, aortic and intracranial aneurysms, and cardiac valve prolapse (167). It is currently thought that the majority of the cysts form in utero and expand at a rate of ∼12%/yr after birth (70), compressing and distorting the renal parenchyma and enlarging the kidney as much as four- to eightfold. Glomerular filtration rate in these patients remains relatively conserved for many years due to compensation by the unaffected nephrons (69). Eventually, approximately one-half of ADPKD patients progress to chronic renal failure by 50–60 yr of age, requiring kidney replacement therapy. ADPKD is the fourth most frequent cause of end-stage renal disease (ESRD), being responsible for ∼8–10% of all ESRD cases (6).
ADPKD is caused by mutations in either Pkd1 and Pkd2, which encode for polycystin 1 and 2 (PC1 and PC2), respectively (1, 77, 96, 164). Approximately 85% of ADPKD cases are due to defects in PC1, while the remaining 15% of cases are caused by mutations in PC2. PC1 is a large glycoprotein of 4,303 amino acids and ∼450 kDa that is expressed in epithelial cells during renal development, but its expression declines to very low levels in the adult kidney. PC1 is composed of a large N-terminal extracellular region that has adhesion and protein-protein interaction domains, 11 transmembrane-spanning domains, and a short C-terminal portion that contains a G protein binding motif (44, 155, 161). PC2 is a 968-amino acid protein of ∼110 kDa that contains 6 membrane-spanning domains and cytosolic N- and C-terminal regions. PC2 shares homology to the transient receptor potential (TRP) family of cation channels and functions as a Ca2+-permeable nonselective cation channel (66, 76, 212). PC1 and PC2 interact with each other through a coiled-coiled domain at their C-terminal region and are thought to form a macromolecular receptor/signaling complex that controls cytoplasmic levels of Ca2+ (170).
While the inherited mutated Pkd allele is present in all cells, cysts develop only in a few nephrons from clonal growth of single cells within the renal tubular epithelium. Therefore, inheritance of a mutated allele from a parent, although necessary, does not appear sufficient to induce cyst formation. The initiation of cyst formation is thought to occur either due to a somatic inactivation of the other allele of the PKD gene, referred to a second-hit hypothesis, or insufficient expression of the normal allele below a critical threshold, referred to as haploinsufficiency (103, 171). There is evidence for both mechanisms in cyst formation in animal models of polycystic kidney disease (PKD) (29, 83, 103, 171, 228). There is a high degree of variability in renal cyst progression even among family members that carry the same PKD mutation, suggesting that nongenetic factors influence the course of the disease. Current research is focused on identifying key factors and downstream signaling pathways that contribute to the relentless growth of renal cysts. Among these are endogenous circulating hormones and exogenous pharmacological agents, which accelerate cyst epithelial cell proliferation and/or stimulate transepithelial fluid secretion. These compounds include caffeine, forskolin, vasopressin, EGF, prostaglandins, IGF, and catecholamines (reviewed in Ref. 215).
Cellular mechanisms of cyst growth.
The development of in vitro and in vivo models of ADPKD and the use of genetic, biochemical, cell biology, and molecular biology approaches have immensely broadened our understanding of ADPKD. While the genetic basis of ADPKD has been identified, the relationship between the lack of polycystin function and the mechanisms leading to cystogenesis remains unclear. ADPKD has a complex and multifactorial pathophysiology, with several mechanisms converging to induce the formation of renal cysts. Cystic epithelial cells are characterized as being incompletely differentiated, and, while the initial cellular event initiating cystogenesis remains uncertain, it is clear that a primary manifestation is abnormal cell proliferation (68). Uncontrolled cell growth causes focal expansions of the tubule epithelium into “blister like” structures that eventually pinch off to form isolated structures that continue to expand in size. Once an isolated cyst is formed, its enlargement is determined by the combined effects of cell proliferation and the accumulation of fluid within the cyst cavity due to Cl−-dependent fluid secretion (71, 202, 215). As cysts expand, there is remodeling of the extracellular matrix (ECM) (50), excessive deposition of ECM molecules (223), inflammatory changes (136, 149), and renal interstitial fibrosis (156). A diagram of the genetic abnormality that causes ADPKD, the pathophysiological mechanisms, and the nongenetic factors that contribute to disease progression are depicted in Fig. 2.
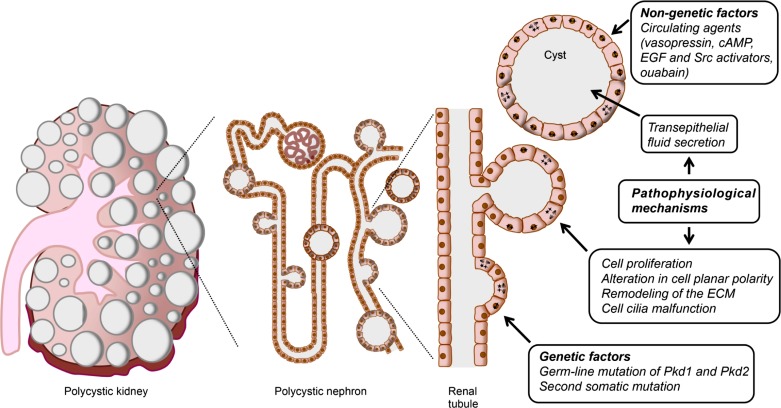
Fig. 2.
Evolution of autosomal dominant polycystic kidney disease (ADPKD) cysts from epithelial cells of the renal tubules. Polycystic kidney (left), result from the growth of multiple fluid-filled cysts that originate in the renal tubules (middle) and continue developing through different pathophysiological mechanisms (right). Cysts evolve from individual cells carrying a germ-line mutation in one of the alleles for the Pkd1 or Pkd2 genes (right). Every cell in the tubule carries a mutated allele, and a somatic mutation or haploinsufficiency of the second allele is believed to trigger the disease (cells marked with stars). Initially, cell proliferation leads to the development of blister-like structures. Several abnormalities, including cell cilia malfunction, alteration in planar cell polarity, and remodeling of the extracellular matrix are also implicated in ADPKD cystogenesis. Once the cysts separate from the nephron that originated them, they continue growing through cell proliferation and transepithelial fluid secretion into the expanding cyst cavity. In addition to the genetic abnormality, nongenetic factors, such as several circulating agents, aid to enhance cyst growth and determine the progression of the disease.
Several signaling pathways have been implicated in PKD pathogenesis; however, cAMP plays a central role in cyst growth by stimulating both cell proliferation and transepithelial fluid secretion (16, 215, 237). Renal levels of cAMP are elevated in several rodent models of PKD, in part, due to elevated circulating levels of AVP and persistent activation of AVP V2 receptors and G protein-coupled receptors that stimulate cAMP synthesis in renal epithelial cells (62, 166, 215, 235). In ADPKD cells, cAMP stimulates PKA, which phosphorylates and activates B-Raf, a key kinase upstream of the MEK/ERK signaling pathway, leading to cell proliferation (236). In striking contrast, B-Raf is repressed in normal renal cells and cAMP does not stimulate ERK and cell growth. This unique phenotypic difference in the mitogenic response to cAMP that occurs when both PKD1 alleles become dysfunctional in normal cells appears to depend on the levels of intracellular Ca2+. In normal renal cells, B-Raf is maintained in an inactivated state (236). ADPKD cells have reduced intracellular Ca2+ levels, compared with normal human kidney cells, leading to derepression of B-Raf and cAMP activation of B-Raf/MEK/ERK signaling and cell proliferation (234).
Na-K-ATPase in ADPKD
Role of Na-K-ATPase ion transport in the physiopathology of renal cysts.
The extraordinary appearance of an end-stage ADPKD kidney is due to the accumulation of fluid within the hundreds or thousands of cysts that grossly expand the total kidney volume. Accumulation of fluid within the cysts of ADPKD kidneys is driven by cAMP-dependent transcellular Cl− secretion (194). As with other secretory epithelia, Cl−-dependent fluid secretion is dependent on the expression of key ion transporters within the apical and basolateral membranes. The Na-K-ATPase, acting in concert with K+ channels in the basolateral membrane, establishes and maintains the chemical and electrical gradients for secondary active transport. Chloride enters the cell through basolateral NKCC1, an electrically neutral Na+-K+-2Cl− cotransporter, that brings Na+, K+, and Cl− into the cell using the Na+ gradient. Thus intracellular Cl− is maintained above its electrochemical gradient and is poised for rapid Cl− efflux across the luminal membrane upon cAMP activation of CFTR. High concentrations of ouabain that completely inhibit Na-K-ATPase activity block cAMP-dependent net fluid secretion by intact cysts and anion secretion by polarized ADPKD cell monolayers. Because of the essential role of the Na-K-ATPase in salt and water movement across the renal epithelium, it is not surprising that this ion transporter had been the focus of early research on fluid secretion by the ADPKD cysts. These studies examined expression levels, localization, and activity of the Na-K-ATPase in normal and cystic kidneys of human tissues and various animal models. Normally, the Na pump displays a highly polarized distribution in renal epithelial cells, being confined to the basolateral membrane of renal epithelial tubular cells, where it is held in place by its anchoring to the cytoskeletal proteins ankyrin and fodrin (51). Due to its basolateral localization, the Na-K-ATPase pumps Na+ from the cells into the blood side of the epithelium, creating the Na+ transmembrane gradient, responsible for Na+ reabsorption through Na+ channels and transporters in the apical aspect of the cells (10). Wilson and associates (224) reported that the Na-K-ATPase was mislocalized to the apical plasma membrane of cystic epithelial cells and proposed that the active transport of Na+ by the Na-K-ATPase was responsible for the vectorial transport of NaCl and water into the lumen of the cysts.
The Na-K-ATPase mislocalization model to account for fluid secretion by ADPKD cysts was provocative, and several research laboratories set out to confirm this novel observation. However, studies in human tissues and animal models of PKD, confirmed an orthodox localization of the Na-K-ATPase in the basolateral membrane (30, 54, 86, 91, 177, 200, 203). Forskolin, a cAMP agonist, stimulated fluid secretion into intact, excised cysts from ADPKD kidneys (242). Forskolin also stimulated a lumen-negative transepithelial voltage and a positive short-circuit current in ADPKD cell monolayers mounted in Ussing chambers, consistent with active anion secretion (128). These effects of forskolin were sensitive to concentrations of ouabain that completely inhibit the Na-K-ATPase when applied exclusively to the basolateral, but not apical surface of the cell monolayer, confirming normal localization of the Na pump (192). Further experiments exploring the mechanism for fluid secretion, revealed that Cl− was the anion secreted by the ADPKD cells and that Cl− secretion involved the Na-K-2Cl cotransporter NKCC1, K+ channels, and the Na-K-ATPase in the basolateral membrane; and the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel in the apical membrane (194). Based on these studies, a model was developed for Cl−-dependent fluid secretion by renal cysts, in which basolateral Na-K-ATPase generates the Na+ gradient which causes Cl− entry across the basolateral membrane via NKCC1. Na+ and K+ that enter the cell are transported back out of the cell by the Na-K-ATPase and K+ channels, respectively. These basolateral transporters and channels maintain Cl− above its electrochemical gradient. In the presence of cAMP, Cl− exits the cell into the cyst lumen via the apical CFTR Cl− channels. The efflux of Cl− depolarizes the apical membrane, while the efflux of K+ hyperpolarizes the basal membrane. These effects generate a lumen negative transepithelial potential that drives passive movement of Na+ into the cyst lumen via the paracellular pathway (193). The net addition of NaCl to the lumen drives the osmotic movement of water into the cyst.
Na-K-ATPase signaling in ouabain-induced ADPKD cell proliferation.
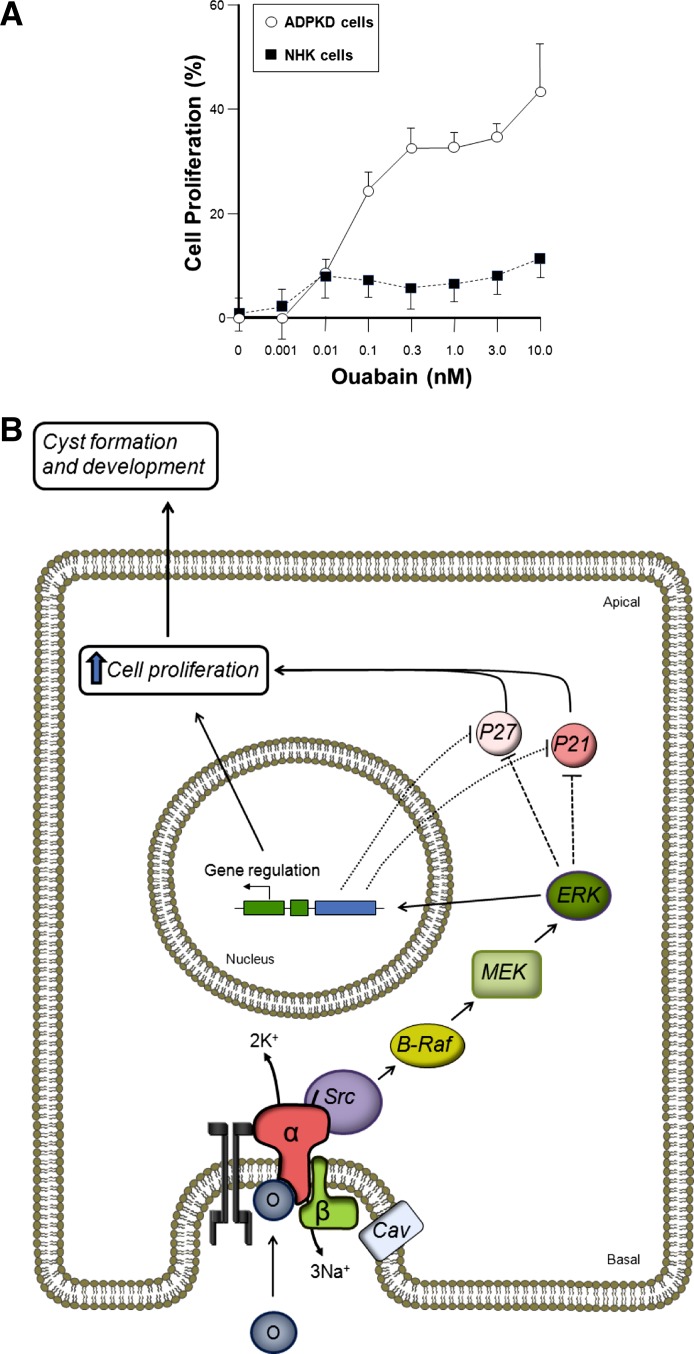
In ADPKD, ouabain at relatively high (millimolar) concentrations had been used experimentally to inhibit Na-K-ATPase activity to investigate its involvement in ion transport; however, the effects of physiological (nanomolar) concentrations remained unknown. Using primary cultures of cells isolated from renal cysts of ADPKD patients, we found that ouabain at concentrations as low as 0.1 nM enhanced cyst epithelial cell proliferation (Fig. 3A). Higher concentrations of ouabain, 0.1 μM and above, inhibited proliferation of both normal and cystic cells, likely due to the toxic effects of Na-K-ATPase inhibition (154). The proliferative response to nanomolar concentrations of ouabain in ADPKD cells agrees with the mitogenic effect of this cardiotonic steroid in other cell types, including normal rat and opossum kidney proximal tubule cells (47, 94). In contrast to ADPKD cells, nanomolar concentrations of ouabain had little effect on the proliferation of normal human kidney (NHK) cells (Fig. 3A). It remains unclear why NHK cells are unresponsive to ouabain. This discrepancy may be due to species differences or differences between immortalized and primary cells. Regardless, these studies uncovered a novel mitogenic response of ADPKD cells to physiological concentrations of ouabain. These results were one of the first reports to demonstrate that ouabain enhances cell proliferation in a hyperplastic disorder.
Fig. 3.
Ouabain stimulates proliferation of ADPKD cells. A: physiological concentrations of ouabain stimulate the growth of human renal epithelial ADPKD cells but has only a slight effect on normal human kidney epithelial cells (NHK). Values are expressed as percentage of untreated controls, without ouabain. Modified from Ref. 152. B: pathway by which ouabain enhances cell proliferation and fluid secretion in ADPKD cells. Levels of ouabain far below those needed to completely block Na-K-ATPase pumping, bind to the basolaterally located Na-K-ATPase of ADPKD cells and triggers the Na-K-ATPase signaling machinery in the cell caveolae. Ouabain binding induces association of the Na-K-ATPase to EGFR and the kinase Src. Activation of Src initiates the phosphorylation of the B-Raf-MEK and ERK cascade. This, by actions that may be direct or indirect, leads to downregulation of the cyclin kinase inhibitors p21 and p27, relieving inhibition of the cell cycle to favor the increase in cell growth. O, ouabain, (α and β) subunits of the Na-K-ATPase; Cav, caveolin.
The effect of ouabain in ADPKD cell proliferation only takes place when the cardenolide is applied to the basolateral aspect of the cells. This agrees with our immunocytochemical studies which show normal localization of the Na-K-ATPase in ADPKD cells. In addition, we did not detect expression of the Na-K-ATPase β2-isoform in ADPKD cells (154), an event that was suggested to be responsible for the mistargeting of the Na-K-ATPase complex to the apical membrane of ADPKD cells (222). Therefore, ouabain-induced Na-K-ATPase signaling takes place through the orthodox distribution of the Na-K-ATPase in the epithelium, which agrees with the presence of circulating endogenous ouabain at the interstitial side of the cells.
To determine ouabain's mechanisms of action, the signaling events triggered by physiological concentrations of this cardenolide in ADPKD cells were studied (153). Ouabain-induced proliferation of cystic cells was found to be dependent on the presence and normal function of the microdomains of cholesterol-containing lipid rafts of the plasma membrane caveolae. Experimental approaches commonly used to disrupt the caveolae, such as cholesterol depletion (methyl-β-CD) and the subsequent recovery of caveolae by cholesterol replenishment, clearly modulated ouabain signaling. Moreover, the involvement of caveolae in Na-K-ATPase signaling was confirmed by the capacity of ouabain to induce, in a methyl-β-CD-sensitive manner, the phosphorylation of caveolin-1 (153), a major protein present in caveolae (124). Another early event in the ouabain-dependent Na-K-ATPase signaling in ADPKD cells is the activation of the kinase Src, through phosphorylation of Tyr 418. The activation of Src by ouabain was confirmed through specific inhibition by 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), or (PP2), a Src inhibitor. Moreover, ouabain activation apparently does not extend to other kinases of the Src family, since phosphorylation of Fyn kinase was unchanged by ouabain. Also, ouabain increased the phosphorylation of EGFR in ADPKD cells and the association of EGFR with the Na-K-ATPase α1-subunit (153). Ouabain increased the phosphorylation and activity of B-Raf and elevated the levels of phosphorylated ERK in ADPKD cells (153). Therefore, it is possible that the exacerbated effects of ouabain in ADPKD cells may depend on stimulation of the B-Raf/MEK/ERK pathway independently of cAMP. Interestingly, both ouabain and cAMP impinge on B-Raf, leading to ADPKD cell proliferation. The ouabain-dependent phosphorylation of ERK was susceptible to tyrphostin AG1478 and PP2, supporting the involvement of EGFR and Src as upstream mediators of the ouabain response in ADPKD cells. Phosphorylated ERK translocates to the nuclei of ADPKD cells, leading to transcription of genes involved in cell proliferation. There is a reduction in the expression levels of two fundamental cyclin kinase inhibitors, p21 and p27, which normally suppress cyclin-dependent kinases and the G1-to-M transition of the cell cycle. A scheme of the cellular intermediates involved in ouabain activation of cell proliferation is shown in Fig. 3B.
Na-K-ATPase signaling in ouabain-enhanced cAMP-dependent Cl− secretion in ADPKD cells.
In addition to cell growth, the effect of physiological concentrations of ouabain was explored on the transepithelial fluid secretion by polarized ADPKD cell monolayers grown on permeable supports. Ouabain had no effect when applied alone; however, it significantly enhanced cAMP-induced fluid secretion (Fig. 4A). By contrast, ouabain had no effect on fluid secretion by NHK cell monolayers. These results suggest that ouabain acts as a cofactor enhancing the effects of cAMP on fluid secretion by ADPKD cystic epithelial cells (85). Similar to the proliferative response to ouabain by ADPKD cells, ouabain only enhanced fluid secretion when applied to the basolateral side of the cells. The effect of ouabain in ADPKD cell fluid secretion was prevented by inhibitors of EGFR, Src and MEK, suggesting that these messengers are involved in the response (Fig. 4B).
Fig. 4.
Ouabain stimulates cAMP-induced fluid secretion in ADPKD monolayers. A: physiological levels of ouabain (3 nM) enhanced the movement of transepithelial basolateral to apical fluid in the presence of 5 μM forskolin but had no effect when used alone. Ouabain, either alone or in the presence of forskolin, did not affect fluid secretion of NHK cells. *Significant differences (P < 0.05). (Modified from reference 85). B: pathway involved in the activation of cAMP-dependent fluid secretion in ADPKD cells. Physiological amounts of ouabain, acting on the Na-K-ATPase signalosome, stimulates Src-dependent phosphorylation and the downstream activation of the B-Raf-MEK and ERK cascade. This, by actions that may be direct or indirect, produces activation of the CFTR, enhancing Cl− movement across the apical membrane of ADPKD cells by a mechanism that has not yet been determined. Also, low physiological concentrations of ouabain partially inhibit Na-K-ATPase activity, which may slightly reduce Na+ and water reabsorption by the epithelium. Both of these actions of ouabain may shift the reabsorption/secretion balance of the epithelium to favor fluid secretion and help cystogenesis.
To find mechanisms underlying the effects of ouabain on fluid secretion, short-circuit current (Isc) was measured across ADPKD cell monolayers mounted in Ussing chambers. Ouabain enhanced the positive Isc response of ADPKD monolayers to forskolin, and this current was inhibited by the CFTR inhibitor CFTR(inh)-172, consistent with an increase in cAMP-dependent Cl− secretion (166). While the cellular mechanism by which ouabain enhances Cl− secretion by ADPKD cells remains unclear, it is possible that the increase is due to stimulation of CFTR channel activity and not dependent on changes in the expression of either CFTR or NKCC1 (85). The capacity for ouabain to influence apical ion transport in renal cells has previously been demonstrated. In LLC-PK1 cells, ouabain was shown to regulate the function of the Na+/H+ exchanger (NHE3) via internalization and degradation of the transporter (35, 240). Additional studies are needed to delineate the effect of ouabain on the Cl− secretory pathway of ADPKD cells. Figure 4B depicts the proposed mechanism for the effect of ouabain to enhance cAMP-dependent Cl− secretion in ADPKD cells. In addition to ouabain's effect on Cl− secretion, it is also possible that physiological concentrations of ouabain partially inhibit the activity of the Na-K-ATPase in ADPKD cells, thus decreasing both the electrical and chemical driving force for Na+ entry via apical ENaC. Considering that the entry mechanism for Cl− via the basolateral NKCC1 is electroneutral, we speculate that the secretory mechanism may be less sensitive to a small degree of inhibition in pump activity. Therefore, ouabain may decrease Na+-dependent fluid absorption and increase Cl−-dependent fluid secretion, both of which would favor net fluid secretion and exacerbate the ADPKD cystic phenotype (Fig. 4B). Undoubtedly, additional studies will be required to ascertain this possibility.
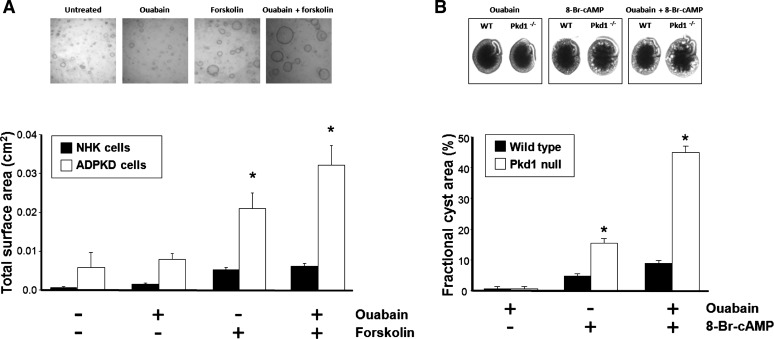
Fig. 5.
Ouabain stimulates ADPKD cystogenesis. A: ouabain alone (3 nM) had no effect but enhanced the increase in size of microcysts caused by forskolin in ADPKD cells grown in a 3-dimensional collagen matrix. In contrast, ouabain did not significantly influence the growth of NHK cells. Top: microphotographs of representative ADPKD microcysts in the absence and presence of ouabain with or without added forskolin. Bottom: average total surface area of the formed microcysts. B: ouabain induces tubular dilations in metanephric organ cultures from Pkd1m1Bei mice (Pkd1−/−), an established model of ADPKD. Ouabain (30 nM) enhanced the effect of the cell-permeable cAMP analog 8-Br-cAMP to increase cyst area (volume) in intact embryonic kidneys from Pkd1m1Bei mice, but had little effect on metanephroi from wild-type mice. Ouabain by itself did not induce cystic dilations in either wild-type or Pkd1m1Bei mice organ cultures. Top: representative images of metanephroi. Bottom: average fractional cyst area for the different experimental conditions. *Significant differences (P < 0.05). Modified from reference 85.
In accordance with its effect on cell mitosis and fluid secretion, ouabain enhanced forskolin-dependent expansion of microscopic cysts of ADPKD cells cultured within a three-dimensional collagen matrix (Fig. 5A). This effect of ouabain was not limited to cultured cells since ouabain also accelerated cAMP-dependent enlargement of cyst-like dilations in metanephric organs from the Pkd1 null kidneys of Pkd1m1Bei mice (Fig. 5B), a well-established model of ADPKD (127). In contrast, ouabain had no effect on cyst formation of NHK cells grown in collagen gels and caused only slight cystic dilations in wild-type mouse kidneys (Fig. 5, A and B, respectively). Interestingly, the effect of ouabain was more pronounced in the kidneys from homo- than heterozygous mice, suggesting that the influence of ouabain correlates with PKD gene dosing (85). The action of ouabain in ADPKD cells and Pkd1m1Bei metanephroi was prevented by inhibitors of EGFR, Src, and ERK (85). Together, these data show that physiological concentrations of ouabain act as a mitogenic agent and have synergistic effects with forskolin to increase ADPKD fluid secretion via the EGFR-Src-MEK pathway. These observations suggest a novel role for ouabain as a cystogenic factor in ADPKD.
Ouabain affinity in ADPKD and normal human kidney cells.
The molecular basis for the difference in the ouabain response between ADPKD and normal cells remains unclear; however, it may be due to a higher affinity of the Na-K-ATPase to ouabain in ADPKD cells compared with NHK cells. In kinetic assays for Na-K-ATPase activity, we found that ADPKD cells had a heterogeneous response to ouabain, consistent with two Na-K-ATPase populations with different affinities for ouabain. Approximately 20% of the total enzyme of ADPKD cells had sensitivity to ouabain that was three orders of magnitude higher than that of NHK cells (154). This characteristic property of cystic cells suggests that they have a greater capacity to bind the steroid, making them more susceptible to endogenous circulating levels of ouabain (154). The unique ouabain sensitivity found in ADPKD cells could depend on the aberrant expression of Na-K-ATPase isoforms. However, we determined that this is not the case, since only the α1β1-isozyme could be detected in both ADPKD and NHK cells (154). Alternatively, ouabain affinity may depend on an interaction between Na-K-ATPase and other proteins. The large intracellular region of the Na-K-ATPase located between transmembrane domains 4 and 5 has been shown to interact with the C terminus of polycystin 1 (159, 244). Interestingly, we found that, in insect cells exogenous expression of a construct containing the transmembrane and C-terminal domains of polycystin 1 increased the sensitivity of the Na-K-ATPase to ouabain, with an inhibition constant that was similar to that found in ADPKD cells (21). However, the same change in ouabain affinity was not found in Cos cells that had been engineered to overexpress PC1 (244). This disparity in results is unclear and may depend on differences in the expression levels for PC1 or proteins that bind PC1, including PC2. The required integrity of caveolae for ouabain-mediated effects in ADPKD cells supports the notion that only a subpopulation of caveolae-associated Na-K-ATPase is involved in signaling (115). It is possible that this Na-K-ATPase fraction in ADPKD cells is more sensitive to ouabain. At present, whether the affinity of the Na-K-ATPase for ouabain is influenced by the localization of the enzyme in caveolae or plasmalemma remains unclear. A subpopulation of Na-K-ATPase with high affinity for ouabain was reported in caveolae isolated from normal rat kidneys (61). By contrast, the kinetics for ouabain inhibition of Na-K-ATPase activity was similar between caveolar and noncaveolar membrane preparations from normal pig kidneys (123). The dissimilarities in results cannot be attributed to different expression of Na-K-ATPase isoforms, since both reports showed the presence of the α1-isoform. However, it is possible that differences in species, or the protocols used for caveolar extraction, may account for the disparity in results. Studies in human ADPKD cells are needed to determine whether specific association of the Na-K-ATPase with components of the caveolae is responsible for the high sensitivity to ouabain that the enzyme has in these cells. If the difference in the ouabain effect between ADPKD cells and normal renal cells is due to differences in ouabain affinity, then it might be expected that higher concentrations of ouabain would have the same effect on NHK cell proliferation and ion transport. However, this does not appear to be the case. It is possible that the concentrations of ouabain required to activate these pathways in normal human renal cells also inhibit pump activity, masking the non-pumping effects of the Na-K-ATPase.
In summary, ouabain accelerates two key pathways in cystogenesis: cell proliferation and Cl−-dependent fluid secretion, through classic intermediates of the Na-K-ATPase signalosome, including EGFR, Src, and the B-Raf/MEK/ERK signaling pathways. This pathway appears to be a common pathway for ADPKD cyst growth, which can also be activated by arginine vasopressin, prostaglandins, and other cAMP agonists (152, 194), epidermal growth factor (EGF) (225), and Src activators (198). We propose that ADPKD cells express a pool of the Na-K-ATPase with an abnormally high affinity for ouabain that causes the cells to respond to endogenous ouabain levels. It is also possible that circulating ouabain levels are elevated in ADPKD patients, which will exacerbate the initial phase of cyst formation and/or promote the continuous growth of the cysts. Interestingly, endogenous cardiotonic steroids have been reported to be elevated in ESRD caused by a variety of kidney pathologies, including PKD (98). Undoubtedly, determination of the endogenous levels of ouabain in patients with ADPKD may shed light on this possibility.
Potential Therapies for ADPKD
Our observations suggest that both endogenous ouabain, as well as ouabain ingested in the diet, may be an important factor in accelerating cyst growth by increasing epithelial cell proliferation and fluid secretion, two key processes in ADPKD pathophysiology. Until a cure to halt cyst formation is available, identification of factors that favor cyst progression provides opportunities to control the advancement and morbidity of the disease. The progress made in understanding the pathogenesis of ADPKD has provided important information on the mechanisms involved in cystogenesis that can be targeted. In particular, efforts have been directed toward interfering with the intracellular pathways governing cyst growth. A more comprehensive review of this topic can be found elsewhere (38, 139, 160, 163, 190, 206, 209, 215). Among the approaches to slowing cyst growth is the vasopressin V2 receptor antagonist tolvaptan, a drug that blocks the renal effect of AVP on cAMP production. (207). Along the same line, increased water intake to suppress AVP levels has been shown to decrease cystic disease in PKD rats (148), and water has been suggested as a potential therapy for ADPKD (208). Somatostatin analogs, such as octreotide, through inhibition of the activity of adenylyl cyclase and reduction of intracellular cAMP levels, have also been shown to slow total kidney volume increase in ADPKD (82). Inhibition of the mTOR pathway, which is abnormally stimulated in ADPKD, with sirolimus has shown beneficial effects in animal models of ADPKD (189); however, it remains unclear whether this is a therapy for patients (184, 188, 217). Targeting of other signaling pathways has also been explored in different animal models of PKD, including EGFR and Src inhibitors EKI-785 and bosutinib (197, 198), and the B-Raf and MEK inhibitors sorafenib and PD184352 (158, 238). The restoration of the abnormal cytosolic Ca2+ levels in ADPKD with calcimimetics or triptolide has shown promising results in mice with PKD (108). A different approach consisting of therapies directed to reduce Cl−-driven fluid secretion with small-molecule CFTR inhibitors, AMPK activation with metformin, or KCa3.1 potassium channel blockers has been explored (7, 109, 134, 241). Finally, other approaches have been directed to stop the hyperproliferative phenotype of the cystic cells using chemotherapeutic agents, such as paclitaxel (131), taxol (226), or cyclin-dependent kinases (34). Due to the complex pathophysiology of ADPKD, it is likely that best treatment will require a combination of therapies. Based on the cystogenic effects of ouabain in ADPKD, it is relevant to propose the development of small-molecule inhibitors to prevent ouabain binding to Na-K-ATPase on cystic cells (59, 112), or inhibiting intracellular pathways activated by ouabain to reduce the progression of the disease. The advantage of targeting the ouabain-Na-K-ATPase signaling pathway is that both cell proliferation and fluid secretion will be affected.
Concluding Remarks
In classic renal physiology, the Na-K-ATPase holds an exalted position as a primary active transporter that is required to translocate Na+ and K+ across the renal tubular epithelium. As if being responsible for life was not enough, more recent evidence indicates that the Na-K-ATPase is also a cell surface receptor for both endogenous and exogenous ouabain and other cardiotonic steroids. In this manner, the Na-K-ATPase is a multifaceted protein involved in regulating several physiological events, once again illustrating that nature is conservative and builds elaborate networks around stable underlying core mechanisms. Understanding the many roles of the Na-K-ATPase is essential to delineating the function of normal kidney physiology and renal pathophysiological states. Studies in ADPKD have given insight into the ion transport and non-pumping functions of the Na-K-ATPase and have revealed a potentially important role for endogenous ouabain in cyst growth. Further investigation of the molecular mechanisms involved in ouabain-Na-K-ATPase signaling on cell proliferation, and cAMP-dependent Cl− and fluid secretion in ADPKD is still required. The synthesis of novel compounds with the capacity to modulate Na-K-ATPase signaling is in progress, and this may provide new therapeutic approaches that will help ameliorate ADPKD and other renal pathological conditions in which there is aberrant Na-K-ATPase-mediated signaling.
GRANTS
This work was supported by National Institutes of Health Grants DK081431 and HD043044 to G. Blanco and DK081579 to D. P. Wallace.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.B. and D.P.W. interpreted results of experiments; G.B. and D.P.W. prepared figures; G.B. and D.P.W. drafted manuscript; G.B. and D.P.W. edited and revised manuscript; G.B. and D.P.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jared Grantham (University of Kansas Medical Center) for insightful comments on the manuscript. The authors apologize in the event that relevant publications were inadvertently omitted in the review.
REFERENCES
- 1.Anonymous. Polycystic kidney disease: the complete structure of the PKD1 gene, and its protein. The International Polycystic Kidney Disease Consortium. Cell 81: 289–298, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Abramowitz J, Dai C, Hirschi KK, Dmitrieva RI, Doris PA, Liu L, Allen JC. Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell line, A7r5. Circulation 108: 3048–3053, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S. Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol 194: 28–45, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Aizman O, Aperia A. Na,K-ATPase as a signal transducer. Ann NY Acad Sci 986: 489–496, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci USA 98: 13420–13424, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam A, Perrone RD. Management of ESRD in patients with autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 164–172, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Albaqumi M, Srivastava S, Li Z, Zhdnova O, Wulff H, Itani O, Wallace DP, Skolnik EY. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int 74: 740–749, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Allen PD, Schmidt TA, Marsh JD, Kjeldsen K. Na,K-ATPase expression in normal and failing human left ventricle. Basic Res Cardiol 87, Suppl 1: 87–94, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Antolovic R, Bauer N, Mohadjerani M, Kost H, Neu H, Kirch U, Grunbaum EG, Schoner W. Endogenous ouabain and its binding globulin: effects of physical exercise and study on the globulin's tissue distribution. Hypertens Res 23, Suppl: S93–S98, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Apell HJ, Schneeberger A, Sokolov VS. Partial reactions of the Na,K-ATPase: kinetic analysis and transport properties. Acta Physiol Scand Suppl 643: 235–245, 1998 [PubMed] [Google Scholar]
- 11.Aperia A. 2011 Homer Smith Award: to serve and protect: classic and novel roles for Na+, K+ -adenosine triphosphatase. J Am Soc Nephrol 23: 1283–1290, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Aperia A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J Intern Med 261: 44–52, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Aydemir-Koksoy A, Abramowitz J, Allen JC. Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem 276: 46605–46611, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev 61: 9–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrueto F, Jr, Kirrane BM, Cotter BW, Hoffman RS, Nelson LS. Cardioactive steroid poisoning: a comparison of plant- and animal-derived compounds. J Med Toxicol 2: 152–155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM, Jr, Grantham JJ. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 66: 964–973, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Benarroch EE. Na+, K+-ATPase: functions in the nervous system and involvement in neurologic disease. Neurology 76: 287–293, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Bessen HA. Therapeutic and toxic effects of digitalis: William Withering, 1785. J Emerg Med 4: 243–248, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 45: 440–444, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol 25: 292–303, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Blanco G. The NA/K-ATPase and its isozymes: what we have learned using the baculovirus expression system. Front Biosci 10: 2397–2411, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Blaustein MP, Hamlyn JM. Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na+ pump, the Na+/Ca2+ exchanger and TRPC proteins. Biochim Biophys Acta 1802: 1219–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaustein MP, Juhaszova M, Golovina VA. The cellular mechanism of action of cardiotonic steroids: a new hypothesis. Clin Exp Hypertens 20: 691–703, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 302: H1031–H1049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boldyrev AA. Na+,K+-ATPase: 40 years of investigations. Membr Cell Biol 13: 715–719, 2000 [PubMed] [Google Scholar]
- 28.Book CB, Moore RL, Semanchik A, Ng YC. Cardiac hypertrophy alters expression of Na+,K+-ATPase subunit isoforms at mRNA and protein levels in rat myocardium. J Mol Cell Cardiol 26: 591–600, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Brasier JL, Henske EP. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest 99: 194–199, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brill SR, Ross KE, Davidow CJ, Ye M, Grantham JJ, Caplan MJ. Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc Natl Acad Sci USA 93: 10206–10211, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodie C, Tordai A, Saloga J, Domenico J, Gelfand EW. Ouabain induces inhibition of the progression phase in human T-cell proliferation. J Cell Physiol 165: 246–253, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Bublitz M, Poulsen H, Morth JP, Nissen P. In and out of the cation pumps: P-type ATPase structure revisited. Curr Opini Struct Biol 20: 431–439, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Buckalew VM. Endogenous digitalis-like factors. An historical overview. Front Biosci 10: 2325–2334, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444: 949–952, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Cai H, Wu L, Qu W, Malhotra D, Xie Z, Shapiro JI, Liu J. Regulation of apical NHE3 trafficking by ouabain-induced activation of the basolateral Na,K-ATPase receptor complex. Am J Physiol Cell Physiol 294: C555–C563, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Cao C, Payne K, Lee-Kwon W, Zhang Z, Lim SW, Hamlyn J, Blaustein MP, Kwon HM, Pallone TL. Chronic ouabain treatment induces vasa recta endothelial dysfunction in the rat. Am J Physiol Renal Physiol 296: F98–F106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cereijido M, Contreras RG, Shoshani L, Larre I. The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am J Physiol Cell Physiol 302: C473–C481, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Chang MY, Ong AC. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract 120: c25–e34, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Chu Y, Parada I, Prince DA. Temporal and topographic alterations in expression of the alpha3 isoform of Na+, K+-ATPase in the rat freeze lesion model of microgyria and epileptogenesis. Neuroscience 162: 339–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clapcote SJ, Duffy S, Xie G, Kirshenbaum G, Bechard AR, Rodacker Schack V, Petersen J, Sinai L, Saab BJ, Lerch JP, Minassian BA, Ackerley CA, Sled JG, Cortez MA, Henderson JT, Vilsen B, Roder JC. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc Natl Acad Sci USA 106: 14085–14090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke RJ, Fan X. Pumping ions. Clin Exp Pharmacol Physiol 38: 726–733, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Contreras RG, Flores-Maldonado C, Lazaro A, Shoshani L, Flores-Benitez D, Larre I, Cereijido M. Ouabain binding to Na+,K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J Membr Biol 198: 147–158, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem 275: 1976–1986, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Delmas P. Polycystins: from mechanosensation to gene regulation. Cell 118: 145–148, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Despa S, Lingrel JB, Bers DM. (Na+/K+)-ATPase alpha2-isoform preferentially modulates Ca2+ transients and sarcoplasmic reticulum Ca2+ release in cardiac myocytes. Cardiovasc Res 95: 480–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dmitrieva RI, Doris PA. Cardiotonic steroids: potential endogenous sodium pump ligands with diverse function. Exp Biol Med (Maywood) 227: 561–569, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem 278: 28160–28166, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB. The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 278: 53026–53034, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The α2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Drummond IA. Polycystins, focal adhesions and extracellular matrix interactions. Biochim Biophys Acta 1812: 1322–1326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunbar LA, Caplan MJ. The cell biology of ion pumps: sorting and regulation. Eur J Cell Biol 79: 557–563, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Duran MJ, Pierre SV, Carr DL, Pressley TA. The isoform-specific region of the Na,K-ATPase catalytic subunit: role in enzyme kinetics and regulation by protein kinase C. Biochemistry 43: 16174–16183, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Dvela M, Rosen H, Ben-Ami HC, Lichtstein D. Endogenous ouabain regulates cell viability. Am J Physiol Cell Physiol 302: C442–C452, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Eaton KA, Biller DS, DiBartola SP, Radin MJ, Wellman ML. Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Vet Pathol 34: 117–126, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Eichhorn EJ, Gheorghiade M. Digoxin. Progr Cardiovasc Dis 44: 251–266, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Eva A, Kirch U, Scheiner-Bobis G. Signaling pathways involving the sodium pump stimulate NO production in endothelial cells. Biochim Biophys Acta 1758: 1809–1814, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation 102: 3009–3014, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Ferrandi M, Barassi P, Molinari I, Torielli L, Tripodi G, Minotti E, Bianchi G, Ferrari P. Ouabain antagonists as antihypertensive agents. Curr Pharm Des 11: 3301–3305, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Ferrandi M, Manunta P. Ouabain-like factor: is this the natriuretic hormone? Curr Opin Nephrol Hypertens 9: 165–171, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem 279: 33306–33314, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr 33: 425–438, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens 17: 526–532, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Gidh-Jain M, Huang B, Jain P, Gick G, El-Sherif N. Alterations in cardiac gene expression during ventricular remodeling following experimental myocardial infarction. J Mol Cell Cardiol 30: 627–637, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grantham JJ. 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol 3: 1841–1857, 1993 [DOI] [PubMed] [Google Scholar]
- 68.Grantham JJ, Calvet JP. Polycystic kidney disease: in danger of being X-rated? Proc Natl Acad Sci USA 98: 790–792, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol 1: 148–157, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clini J Am Soc Nephrol: 889–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Green HJ. Membrane excitability, weakness, and fatigue. Can J Appl Physiol 29: 291–307, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 277: 18694–18702, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA 88: 6259–6263, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamlyn JM, Lu ZR, Manunta P, Ludens JH, Kimura K, Shah JR, Laredo J, Hamilton JP, Hamilton MJ, Hamilton BP. Observations on the nature, biosynthesis, secretion and significance of endogenous ouabain. Clin Exp Hypertens 20: 523–533, 1998 [DOI] [PubMed] [Google Scholar]
- 76.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Harris PC. 2008 Homer W. Smith Award: insights into the pathogenesis of polycystic kidney disease from gene discovery. J Am Soc Nephrol 20: 1188–1198, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Harwood S, Mullen AM, McMahon AC, Dawnay A. Plasma OLC is elevated in mild experimental uremia but is not associated with hypertension. Am J Hypertens 14: 1112–1115, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Hauck C, Frishman WH. Systemic hypertension: the roles of salt, vascular Na+/K+ ATPase and the endogenous glycosides, ouabain and marinobufagenin. Cardiol Rev 20: 130–138, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Hauptman PJ, Kelly RA. Digitalis. Circulation 99: 1265–1270, 1999 [DOI] [PubMed] [Google Scholar]
- 81.He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, Lingrel JB. The α1- and α2-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol 281: R917–R925, 2001 [DOI] [PubMed] [Google Scholar]
- 82.Hogan MC, Masyuk TV, Page L, Holmes DR, 3rd, Li X, Bergstralh EJ, Irazabal MV, Kim B, King BF, Glockner JF, Larusso NF, Torres VE. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant 27: 3532–3539, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaisser F, Jaunin P, Geering K, Rossier BC, Horisberger JD. Modulation of the Na,K-pump function by beta subunit isoforms. J Gen Physiol 103: 605–623, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jansson K, Nguyen AN, Magenheimer BS, Reif GA, Aramadhaka LR, Bello-Reuss E, Wallace DP, Calvet JP, Blanco G. Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. Am J Physiol Renal Physiol 303: F982–F990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang ST, Chiou YY, Wang E, Lin HK, Lin YT, Chi YC, Wang CK, Tang MJ, Li H. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol 168: 205–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jimenez T, McDermott JP, Sanchez G, Blanco G. Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc Natl Acad Sci USA 108: 644–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jortani SA, Valdes R., Jr Digoxin and its related endogenous factors. Crit Rev Clin Lab Sci 34: 225–274, 1997 [DOI] [PubMed] [Google Scholar]
- 89.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem 71: 511–535, 2002 [DOI] [PubMed] [Google Scholar]
- 90.Katz AM. Effects of digitalis on cell biochemistry: sodium pump inhibition. J Am Coll Cardiol 5: 16A–21A, 1985 [DOI] [PubMed] [Google Scholar]
- 91.Kawa G, Nagao S, Yamamoto A, Omori K, Komatz Y, Takahashi H, Tashiro Y. Sodium pump distribution is not reversed in the DBA/2FG-pcy, polycystic kidney disease model mouse. J Am Soc Nephrol 4: 2040–2049, 1994 [DOI] [PubMed] [Google Scholar]
- 92.Kelman AW, Sumner DJ, Lonsdale M, Lawrence JR, Whiting B. Comparative pharmacokinetics and pharmacodynamics of cardiac glycosides. Br J Clin Pharmacol 10: 135–143, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khodus GR, Kruusmagi M, Li J, Liu XL, Aperia A. Calcium signaling triggered by ouabain protects the embryonic kidney from adverse developmental programming. Pediatr Nephrol 26: 1479–1482, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Khundmiri SJ, Amin V, Henson J, Lewis J, Ameen M, Rane MJ, Delamere NA. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol 293: C1171–C1180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 291: C1247–C1257, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Kimberling WJ, Kumar S, Gabow PA, Kenyon JB, Connolly CJ, Somlo S. Autosomal dominant polycystic kidney disease: localization of the second gene to chromosome 4q13–q23. Genomics 18: 467–472, 1993 [DOI] [PubMed] [Google Scholar]
- 97.Kirshenbaum GS, Clapcote SJ, Duffy S, Burgess CR, Petersen J, Jarowek KJ, Yucel YH, Cortez MA, Snead OC, 3rd, Vilsen B, Peever JH, Ralph MR, Roder JC. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase alpha3 sodium pump. Proc Natl Acad Sci USA 108: 18144–18149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kolmakova EV, Haller ST, Kennedy DJ, Isachkina AN, Budny GV, Frolova EV, Piecha G, Nikitina ER, Malhotra D, Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant 26: 2912–2919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 273: 15249–15256, 1998 [DOI] [PubMed] [Google Scholar]
- 100.Komiyama Y, Nishimura N, Munakata M, Mori T, Okuda K, Nishino N, Hirose S, Kosaka C, Masuda M, Takahashi H. Identification of endogenous ouabain in culture supernatant of PC12 cells. J Hypertens 19: 229–236, 2001 [DOI] [PubMed] [Google Scholar]
- 101.Kotova O, Galuska D, Essen-Gustavsson B, Chibalin AV. Metabolic and signaling events mediated by cardiotonic steroid ouabain in rat skeletal muscle. Cell Mol Biol 52: 48–57, 2006 [PubMed] [Google Scholar]
- 102.Kulikov A, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim Biophys Acta 1768: 1691–1702, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, Peters DJ. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet 13: 3069–3077, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Laredo J, Hamilton BP, Hamlyn JM. Secretion of endogenous ouabain from bovine adrenocortical cells: role of the zona glomerulosa and zona fasciculata. Biochem Biophys Res Commun 212: 487–493, 1995 [DOI] [PubMed] [Google Scholar]
- 105.Larre I, Castillo A, Flores-Maldonado C, Contreras RG, Galvan I, Munoz-Estrada J, Cereijido M. Ouabain modulates ciliogenesis in epithelial cells. Proc Natl Acad Sci USA 108: 20591–20596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larre I, Contreras RG, Cereijido M. Ouabain modulates cell contacts as well as functions that depend on cell adhesion. Methods Mol Biol 763: 155–168, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Lee CO. 200 years of digitalis: the emerging central role of the sodium ion in the control of cardiac force. Am J Physiol Cell Physiol 249: C367–C378, 1985 [DOI] [PubMed] [Google Scholar]
- 108.Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol 19: 1659–1662, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li H, Sheppard DN. Therapeutic potential of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors in polycystic kidney disease. BioDrugs 23: 203–216, 2009 [DOI] [PubMed] [Google Scholar]
- 110.Li J, Khodus GR, Kruusmagi M, Kamali-Zare P, Liu XL, Eklof AC, Zelenin S, Brismar H, Aperia A. Ouabain protects against adverse developmental programming of the kidney. Nat Commun 1: 42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J, Zelenin S, Aperia A, Aizman O. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-kappaB. J Am Soc Nephrol 17: 1848–1857, 2006 [DOI] [PubMed] [Google Scholar]
- 112.Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem 284: 21066–21076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Z, Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflügers Arch 457: 635–644, 2009 [DOI] [PubMed] [Google Scholar]
- 114.Liang M, Cai T, Tian J, Qu W, Xie ZJ. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Biol Chem 281: 19709–19719, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282: 10585–10593, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Lingrel J, Moseley A, Dostanic I, Cougnon M, He S, James P, Woo A, O'Connor K, Neumann J. Functional roles of the alpha isoforms of the Na,K-ATPase. Ann NY Acad Sci 986: 354–359, 2003 [DOI] [PubMed] [Google Scholar]
- 117.Lingrel JB, Croyle ML, Woo AL, Arguello JM. Ligand binding sites of Na,K-ATPase. Acta Physiol Scand Suppl 643: 69–77, 1998 [PubMed] [Google Scholar]
- 118.Lingrel JB, Williams MT, Vorhees CV, Moseley AE. Na,K-ATPase and the role of alpha isoforms in behavior. J Bioenerg Biomembr 39: 385–389, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int 67: 1844–1854, 2005 [DOI] [PubMed] [Google Scholar]
- 120.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem 275: 27838–27844, 2000 [DOI] [PubMed] [Google Scholar]
- 121.Liu J, Xie ZJ. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim Biophys Acta 1802: 1237–1245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu L, Abramowitz J, Askari A, Allen JC. Role of caveolae in ouabain-induced proliferation of cultured vascular smooth muscle cells of the synthetic phenotype. Am J Physiol Heart Circ Physiol 287: H2173–H2182, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A. Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry 50: 8664–8673, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol 284: C1550–C1560, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Liu X, Spicarova Z, Rydholm S, Li J, Brismar H, Aperia A. Ankyrin B modulates the function of Na,K-ATPase/inositol 1,4,5-trisphosphate receptor signaling microdomain. J Biol Chem 283: 11461–11468, 2008 [DOI] [PubMed] [Google Scholar]
- 126.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-Sensitive alpha1 Na,K-ATPase enhances natriuretic response to saline load. J Am Soc Nephrol 19: 1947–1954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Magenheimer BS, St, John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na+,K+,2Cl− co-transporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006 [DOI] [PubMed] [Google Scholar]
- 128.Mangoo-Karim R, Ye M, Wallace DP, Grantham JJ, Sullivan LP. Anion secretion drives fluid secretion by monolayers of cultured human polycystic cells. Am J Physiol Renal Fluid Electrolyte Physiol 269: F381–F388, 1995 [DOI] [PubMed] [Google Scholar]
- 129.Manunta P, Ferrandi M, Bianchi G, Hamlyn JM. Endogenous ouabain in cardiovascular function and disease. J Hypertens 27: 9–18, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Manunta P, Messaggio E, Casamassima N, Gatti G, Carpini SD, Zagato L, Hamlyn JM. Endogenous ouabain in renal Na+ handling and related diseases. Biochim Biophys Acta 1802: 1214–1218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martinez JR, Cowley BD, Gattone VH, 2nd, Nagao S, Yamaguchi T, Kaneta S, Takahashi H, Grantham JJ. The effect of paclitaxel on the progression of polycystic kidney disease in rodents. Am J Kidney Dis 29: 435–444, 1997 [DOI] [PubMed] [Google Scholar]
- 132.Mashour NH, Lin GI, Frishman WH. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Intern Med 158: 2225–2234, 1998 [DOI] [PubMed] [Google Scholar]
- 133.Masugi F, Ogihara T, Hasegawa T, Sakaguchi K, Kumahara Y. Normalization of high plasma level of ouabain-like immunoreactivity in primary aldosteronism after removal of adenoma. J Hum Hypertens 2: 17–20, 1988 [PubMed] [Google Scholar]
- 134.McCarty MF, Barroso-Aranda J, Contreras F. Activation of AMP-activated kinase as a strategy for managing autosomal dominant polycystic kidney disease. Med Hypotheses 73: 1008–1010, 2009 [DOI] [PubMed] [Google Scholar]
- 135.McDonough AA, Velotta JB, Schwinger RH, Philipson KD, Farley RA. The cardiac sodium pump: structure and function. Basic Res Cardiol 97, Suppl 1: I19–I24, 2002 [DOI] [PubMed] [Google Scholar]
- 136.Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M. Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol 6: 7–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H, Zelenin S, Aperia A. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem 278: 50355–50361, 2003 [DOI] [PubMed] [Google Scholar]
- 138.Mobasheri A, Avila J, Cozar-Castellano I, Brownleader MD, Trevan M, Francis MJ, Lamb JF, Martin-Vasallo P. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep 20: 51–91, 2000 [DOI] [PubMed] [Google Scholar]
- 139.Mochizuki T, Tsuchiya K, Nitta K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol 17: 317–326, 2013 [DOI] [PubMed] [Google Scholar]
- 140.Montero A, Rodriguez-Barbero A, Ricote M, Sancho J, Lopez-Novoa JM. Effect of ouabain and hypothalamic, hypophysary inhibitory factor on rat mesangial cell proliferation. J Cardiovasc Pharmacol 22, Suppl 2: S35–S37, 1993 [DOI] [PubMed] [Google Scholar]
- 141.Morth JP, Pedersen BP, Buch-Pedersen MJ, Andersen JP, Vilsen B, Palmgren MG, Nissen P. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol 12: 60–70, 2011 [DOI] [PubMed] [Google Scholar]
- 142.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature 450: 1043–1049, 2007 [DOI] [PubMed] [Google Scholar]
- 143.Moseley AE, Cougnon MH, Grupp IL, El Schultz J, Lingrel JB. Attenuation of cardiac contractility in Na,K-ATPase alpha1 isoform-deficient hearts under reduced calcium conditions. J Mol Cell Cardiol 37: 913–919, 2004 [DOI] [PubMed] [Google Scholar]
- 144.Muller-Ehmsen J, Juvvadi P, Thompson CB, Tumyan L, Croyle M, Lingrel JB, Schwinger RH, McDonough AA, Farley RA. Ouabain and substrate affinities of human Na+-K+-ATPase α1β1, α2β1, and α3β1 when expressed separately in yeast cells. Am J Physiol Cell Physiol 281: C1355–C1364, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Muller-Ehmsen J, McDonough AA, Farley RA, Schwinger RH. Sodium pump isoform expression in heart failure: implication for treatment. Basic Res Cardiol 97, Suppl 1: I25–I30, 2002 [DOI] [PubMed] [Google Scholar]
- 146.Munzer JS, Daly SE, Jewell-Motz EA, Lingrel JB, Blostein R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J Biol Chem 269: 16668–16676, 1994 [PubMed] [Google Scholar]
- 147.Murata Y, Matsuda T, Tamada K, Hosoi R, Asano S, Takuma K, Tanaka K, Baba A. Ouabain-induced cell proliferation in cultured rat astrocytes. Jpn J Pharmacol 72: 347–353, 1996 [DOI] [PubMed] [Google Scholar]
- 148.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17: 2220–2227, 2006 [DOI] [PubMed] [Google Scholar]
- 149.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Yamada S, Ueda Y, Koide H. Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. Am J Med Sci 343: 46–51, 2012 [DOI] [PubMed] [Google Scholar]
- 150.Nakanishi K, Berova N, Lo LC, Zhao N, Ludens JH, Tymiak AA, Warrack B, Haupert GT., Jr Search for an endogenous mammalian cardiotonic factor. Adv Exp Med Biol 404: 219–224, 1996 [DOI] [PubMed] [Google Scholar]
- 151.Nesher M, Dvela M, Igbokwe VU, Rosen H, Lichtstein D. Physiological roles of endogenous ouabain in normal rats. Am J Physiol Heart Circ Physiol 297: H2026–H2034, 2009 [DOI] [PubMed] [Google Scholar]
- 152.Nguyen AN, Sanchez G, Wallace DP, Blanco G. Abnormal ouabain sensitivity of the Na,K-ATPase in polycystic kidney disease (Abstract). J Am Soc Nephrol: 315A, 2003 [Google Scholar]
- 153.Nguyen AN, Jansson K, Sanchez G, Sharma M, Reif GA, Wallace DP, Blanco G. Ouabain activates the Na-K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. Am J Physiol Renal Physiol 301: F897–F906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol 18: 46–57, 2007 [DOI] [PubMed] [Google Scholar]
- 155.Nims N, Vassmer D, Maser RL. Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene: evidence for 11 membrane-spanning domains. Biochemistry 42: 13035–13048, 2003 [DOI] [PubMed] [Google Scholar]
- 156.Norman J. Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim Biophys Acta 1812: 1327–1336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O'Brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch Biochem Biophys 310: 32–39, 1994 [DOI] [PubMed] [Google Scholar]
- 158.Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol 17: 1604–1614, 2006 [DOI] [PubMed] [Google Scholar]
- 159.Pagel P, Zatti A, Kimura T, Duffield A, Chauvet V, Rajendran V, Caplan MJ. Ion pump-interacting proteins: promising new partners. Ann NY Acad Sci 986: 360–368, 2003 [DOI] [PubMed] [Google Scholar]
- 160.Park EY, Woo YM, Park JH. Polycystic kidney disease and therapeutic approaches. BMB Rep 44: 359–368, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 251: 625–631, 1998 [DOI] [PubMed] [Google Scholar]
- 162.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14: 98–112, 2013 [DOI] [PubMed] [Google Scholar]
- 163.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens 18: 99–106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract 118: c19–e30, 2011 [DOI] [PubMed] [Google Scholar]
- 165.Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys 46: 303–316, 2006 [DOI] [PubMed] [Google Scholar]
- 166.Pinto CS, Reif GA, Nivens E, White C, Wallace DP. Calmodulin-sensitive adenylyl cyclases mediate AVP-dependent cAMP production and Cl− secretion by human autosomal dominant polycystic kidney cells. Am J Physiol Renal Physiol 303: F1412–F1424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 173–180, 2010 [DOI] [PubMed] [Google Scholar]
- 168.Pressley TA, Duran MJ, Pierre SV. Regions conferring isoform-specific function in the catalytic subunit of the Na,K-pump. Front Biosci 10: 2018–2026, 2005 [DOI] [PubMed] [Google Scholar]
- 169.Proppe D. Pharmacokinetics of tritiated ouabain, digoxin and digitoxin in guinea-pigs. Clin Exp Pharmacol Physiol 2: 489–502, 1975 [DOI] [PubMed] [Google Scholar]
- 170.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997 [DOI] [PubMed] [Google Scholar]
- 171.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87: 979–987, 1996 [DOI] [PubMed] [Google Scholar]
- 172.Quintas LE, Pierre SV, Liu L, Bai Y, Liu X, Xie ZJ. Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts. J Mol Cell Cardiol 49: 525–531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rajasekaran SA, Rajasekaran AK. Na,K-ATPase and epithelial tight junctions. Front Biosci 14: 2130–2148, 2009 [DOI] [PubMed] [Google Scholar]
- 174.Riganti C, Campia I, Kopecka J, Gazzano E, Doublier S, Aldieri E, Bosia A, Ghigo D. Pleiotropic effects of cardioactive glycosides. Curr Med Chem 18: 872–885, 2011 [DOI] [PubMed] [Google Scholar]
- 175.Rossi G, Manunta P, Hamlyn JM, Pavan E, De Toni R, Semplicini A, Pessina AC. Immunoreactive endogenous ouabain in primary aldosteronism and essential hypertension: relationship with plasma renin, aldosterone and blood pressure levels. J Hypertens 13: 1181–1191, 1995 [DOI] [PubMed] [Google Scholar]
- 176.Schack VR, Holm R, Vilsen B. Inhibition of phosphorylation of Na+,K+-ATPase by mutations causing familial hemiplegic migraine. J Biol Chem 287: 2191–2202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schafer K, Gretz N, Bader M, Oberbaumer I, Eckardt KU, Kriz W, Bachmann S. Characterization of the Han:SPRD rat model for hereditary polycystic kidney disease. Kidney Int 46: 134–152, 1994 [DOI] [PubMed] [Google Scholar]
- 178.Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem 269: 2440–2448, 2002 [DOI] [PubMed] [Google Scholar]
- 179.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs 7: 173–189, 2007 [DOI] [PubMed] [Google Scholar]
- 180.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol 293: C509–C536, 2007 [DOI] [PubMed] [Google Scholar]
- 181.Segall L, Daly SE, Blostein R. Mechanistic basis for kinetic differences between the rat alpha 1, alpha 2, and alpha 3 isoforms of the Na,K-ATPase. J Biol Chem 276: 31535–31541, 2001 [DOI] [PubMed] [Google Scholar]
- 182.Selden R, Haynie G. Ouabain plasma level kinetics and removal by dialysis in chronic renal failure. A study in fourteen patients. Ann Intern Med 83: 15–19, 1975 [DOI] [PubMed] [Google Scholar]
- 183.Selden R, Smith TW. Ouabain pharmacokinetics in dog and man. Determination by radioimmunoassay. Circulation 45: 1176–1182, 1972 [DOI] [PubMed] [Google Scholar]
- 184.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 185.Silva E, Soares-da-Silva P. New insights into the regulation of Na+,K+-ATPase by ouabain. Int Rev Cell Mol Biol 294: 99–132, 2012 [DOI] [PubMed] [Google Scholar]
- 186.Skou JC. The identification of the sodium pump. Biosci Rep 24: 436–451, 2004 [DOI] [PubMed] [Google Scholar]
- 187.Smith TW. The basic mechanism of inotropic action of digitalis glycosides. J Pharmacol 15, Suppl 1: 35–51, 1984 [PubMed] [Google Scholar]
- 188.Soliman A, Zamil S, Lotfy A, Ismail E. Sirolimus produced s-shaped effect on adult polycystic kidneys after 2-year treatment. Transplant Proc 44: 2936–2939, 2012 [DOI] [PubMed] [Google Scholar]
- 189.Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, Bruno F, Montemurno E, Schirinzi A, Sabbatini M, Pisani A, Tataranni T, Schena FP, Gesualdo L. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol Dial Transplant 27: 3560–3567, 2012 [DOI] [PubMed] [Google Scholar]
- 190.Steinman TI. Polycystic kidney disease: a 2011 update. Curr Opin Nephrol Hypertens 21: 189–194, 2012 [DOI] [PubMed] [Google Scholar]
- 191.Stella P, Manunta P, Mallamaci F, Melandri M, Spotti D, Tripepi G, Hamlyn JM, Malatino LS, Bianchi G, Zoccali C. Endogenous ouabain and cardiomyopathy in dialysis patients. J Intern Med 263: 274–280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sullivan LP, Grantham JJ. Mechanisms of fluid secretion by polycystic epithelia. Kidney Int 49: 1586–1591, 1996 [DOI] [PubMed] [Google Scholar]
- 193.Sullivan LP, Wallace DP, Grantham JJ. Chloride and fluid secretion in polycystic kidney disease. J Am Soc Nephrol 9: 903–916, 1998 [DOI] [PubMed] [Google Scholar]
- 194.Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev 78: 1165–1191, 1998 [DOI] [PubMed] [Google Scholar]
- 195.Sweadner KJ. Multiple digitalis receptors. A molecular perspective. Trends Cardiovasc Med 3: 2–6, 1993 [DOI] [PubMed] [Google Scholar]
- 196.Sweadner KJ. Overview: subunit diversity in the Na,K-ATPase. Soc Gen Physiol Ser 46: 63–76, 1991 [PubMed] [Google Scholar]
- 197.Sweeney WE, Chen Y, Nakanishi K, Frost P, Avner ED. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int 57: 33–40, 2000 [DOI] [PubMed] [Google Scholar]
- 198.Sweeney WE, Jr, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol 19: 1331–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na+-K+-ATPase. J Appl Physiol 93: 1860–1866, 2002 [DOI] [PubMed] [Google Scholar]
- 200.Takahashi M, Tsuchiya K, Komatsu Y, Nihei H. A role for Na/K adenosine triphosphatase in the pathogenesis of cyst formation in experimental polycystic kidney disease. J Lab Clin Med 129: 517–526, 1997 [DOI] [PubMed] [Google Scholar]
- 201.Takeuchi A, Reyes N, Artigas P, Gadsby DC. Visualizing the mapped ion pathway through the Na,K-ATPase pump. Channels (Austin) 3: 383–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1812: 1314–1321, 2011 [DOI] [PubMed] [Google Scholar]
- 203.Thomson RB, Mentone S, Kim R, Earle K, Delpire E, Somlo S, Aronson PS. Histopathological analysis of renal cystic epithelia in the Pkd2WS25/- mouse model of ADPKD. Am J Physiol Renal Physiol 285: F870–F880, 2003 [DOI] [PubMed] [Google Scholar]
- 204.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell 17: 317–326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology (Bethesda) 23: 205–211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Torres VE. Treatment strategies and clinical trial design in ADPKD. Adv Chronic Kidney Dis 17: 190–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Torres VE. Vasopressin antagonists in polycystic kidney disease. Semin Nephrol 28: 306–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol 4: 1140–1150, 2009 [DOI] [PubMed] [Google Scholar]
- 209.Torres VE, Harris PC. Polycystic kidney disease in 2011: connecting the dots toward a polycystic kidney disease therapy. Nat Rev Nephrol 8: 66–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Trevisi L, Pighin I, Bazzan S, Luciani S. Inhibition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) endocytosis by ouabain in human endothelial cells. FEBS Lett 580: 2769–2773, 2006 [DOI] [PubMed] [Google Scholar]
- 211.Vagin O, Dada LA, Tokhtaeva E, Sachs G. The Na-K-ATPase α1β1 heterodimer as a cell adhesion molecule in epithelia. Am J Physiol Cell Physiol 302: C1271–C1281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vandorpe DH, Chernova MN, Jiang L, Sellin LK, Wilhelm S, Stuart-Tilley AK, Walz G, Alper SL. The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+-permeable cation channel. J Biol Chem 276: 4093–4101, 2001 [DOI] [PubMed] [Google Scholar]
- 213.Vanmolkot KR, Kors EE, Hottenga JJ, Terwindt GM, Haan J, Hoefnagels WA, Black DF, Sandkuijl LA, Frants RR, Ferrari MD, van den Maagdenberg AM. Novel mutations in the Na+, K+-ATPase pump gene ATP1A2 associated with familial hemiplegic migraine and benign familial infantile convulsions. Ann Neurol 54: 360–366, 2003 [DOI] [PubMed] [Google Scholar]
- 214.Wade OL. Digoxin 1785–1985. I. Two hundred years of digitalis: J Clin Hosp Pharm 11: 3–9, 1986 [DOI] [PubMed] [Google Scholar]
- 215.Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta 1812: 1291–1300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wallick ET, Lindenmayer GE, Lane LK, Allen JC, Pitts BJ, Schwartz A. Recent advances in cardiac glycoside-Na+,K+-ATPase interaction. Fed Proc 36: 2214–2218, 1977 [PubMed] [Google Scholar]
- 217.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 218.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 279: 17250–17259, 2004 [DOI] [PubMed] [Google Scholar]
- 219.Wang J, Schwinger RH, Frank K, Muller-Ehmsen J, Martin-Vasallo P, Pressley TA, Xiang A, Erdmann E, McDonough AA. Regional expression of sodium pump subunits isoforms and Na+-Ca++ exchanger in the human heart. J Clin Invest 98: 1650–1658, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang J, Velotta JB, McDonough AA, Farley RA. All human Na+-K+-ATPase α-subunit isoforms have a similar affinity for cardiac glycosides. Am J Physiol Cell Physiol 281: C1336–C1343, 2001 [DOI] [PubMed] [Google Scholar]
- 221.Warren JV. William Withering revisited: 200 years of the foxglove. Am J Cardiol 58: 189–190, 1986 [DOI] [PubMed] [Google Scholar]
- 222.Wilson PD, Devuyst O, Li X, Gatti L, Falkenstein D, Robinson S, Fambrough D, Burrow CR. Apical plasma membrane mispolarization of NaK-ATPase in polycystic kidney disease epithelia is associated with aberrant expression of the beta2 isoform. Am J Pathol 156: 253–268, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wilson PD, Hreniuk D, Gabow PA. Abnormal extracellular matrix and excessive growth of human adult polycystic kidney disease epithelia. J Cell Physiol 150: 360–369, 1992 [DOI] [PubMed] [Google Scholar]
- 224.Wilson PD, Sherwood AC, Palla K, Du J, Watson R, Norman JT. Reversed polarity of Na+-K+-ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol Renal Fluid Electrolyte Physiol 260: F420–F430, 1991 [DOI] [PubMed] [Google Scholar]
- 225.Wilson SJ, Amsler K, Hyink DP, Li X, Lu W, Zhou J, Burrow CR, Wilson PD. Inhibition of HER-2(neu/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim Biophys Acta 1762: 647–655, 2006 [DOI] [PubMed] [Google Scholar]
- 226.Woo DD, Miao SY, Pelayo JC, Woolf AS. Taxol inhibits progression of congenital polycystic kidney disease. Nature 368: 750–753, 1994 [DOI] [PubMed] [Google Scholar]
- 227.Wray S, Eisner DA, Allen DG. Two hundred years of the foxglove. Med Hist Suppl: 132–150, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 229.Xie Z, Askari A. Na+/K+-ATPase as a signal transducer. Eur J Biochem 269: 2434–2439, 2002 [DOI] [PubMed] [Google Scholar]
- 230.Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv 3: 157–168, 2003 [DOI] [PubMed] [Google Scholar]
- 231.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem 274: 19323–19328, 1999 [DOI] [PubMed] [Google Scholar]
- 232.Xu JW, Jin RM, Li EQ, Wang YR, Bai Y. Signal pathways in ouabain-induced proliferation of leukemia cells. World J Pediatr 5: 140–145, 2009 [DOI] [PubMed] [Google Scholar]
- 233.Yamada K, Goto A, Nagoshi H, Terano Y, Omata M. Elevation of ouabainlike compound levels with hypertonic sodium chloride load in rat plasma and tissues. Hypertension 30: 94–98, 1997 [DOI] [PubMed] [Google Scholar]
- 234.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006 [DOI] [PubMed] [Google Scholar]
- 235.Yamaguchi T, Nagao S, Kasahara M, Takahashi H, Grantham JJ. Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis 30: 703–709, 1997 [DOI] [PubMed] [Google Scholar]
- 236.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 237.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 238.Yamaguchi T, Reif GA, Calvet JP, Wallace DP. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol 299: F944–F951, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yan Y, Haller S, Shapiro A, Malhotra N, Tian J, Xie Z, Malhotra D, Shapiro JI, Liu J. Ouabain-stimulated trafficking regulation of the Na,K-ATPase and NHE3 in renal proximal tubule cells. Mol Cell Biochem 367: 175–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 1300–1310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ye M, Grantham JJ. The secretion of fluid by renal cysts from patients with autosomal dominant polycystic kidney disease. N Engl J Med 329: 310–313, 1993 [DOI] [PubMed] [Google Scholar]
- 243.Ye Q, Li Z, Tian J, Xie JX, Liu L, Xie Z. Identification of a potential receptor that couples ion transport to protein kinase activity. J Biol Chem 286: 6225–6232, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zatti A, Chauvet V, Rajendran V, Kimura T, Pagel P, Caplan MJ. The C-terminal tail of the polycystin-1 protein interacts with the Na,K-ATPase alpha-subunit. Mol Biol Cell 16: 5087–5093, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhou G, Dada LA, Sznajder JI. Regulation of alveolar epithelial function by hypoxia. Eur Respir J 31: 1107–1113, 2008 [DOI] [PubMed] [Google Scholar]