Abstract
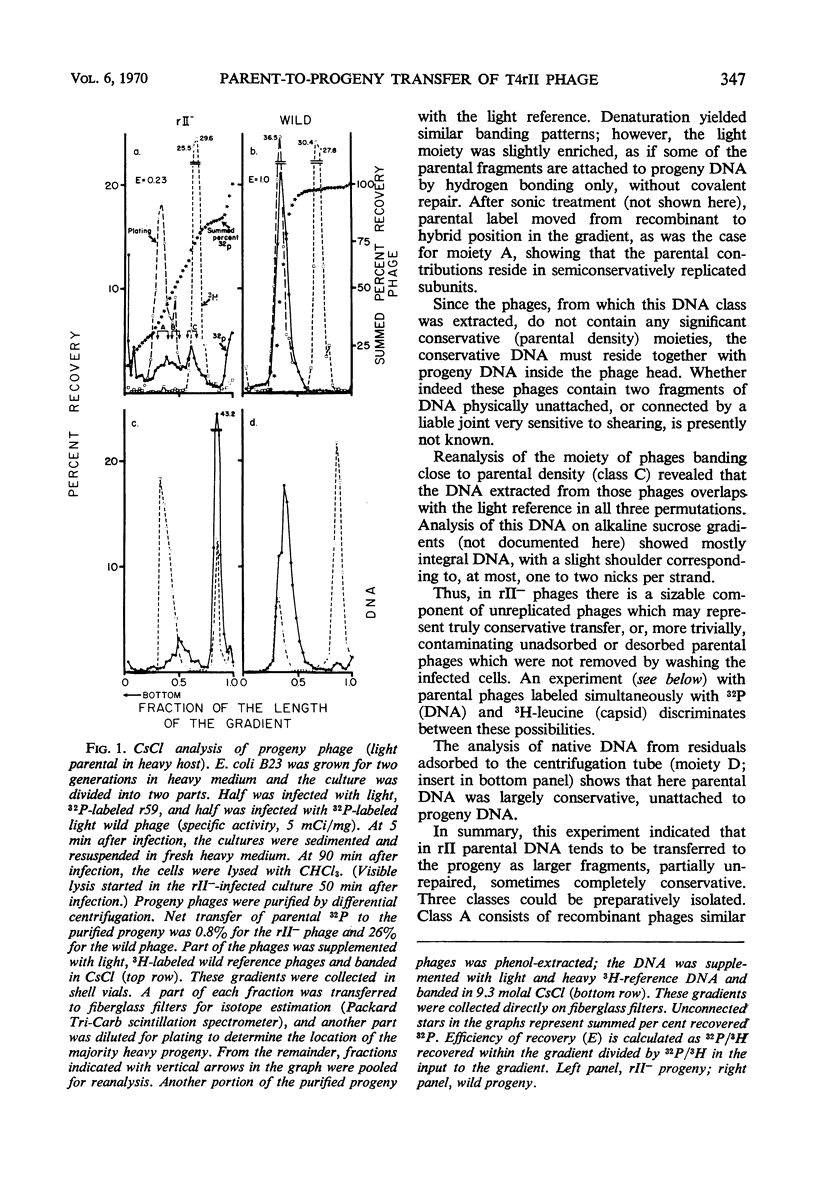
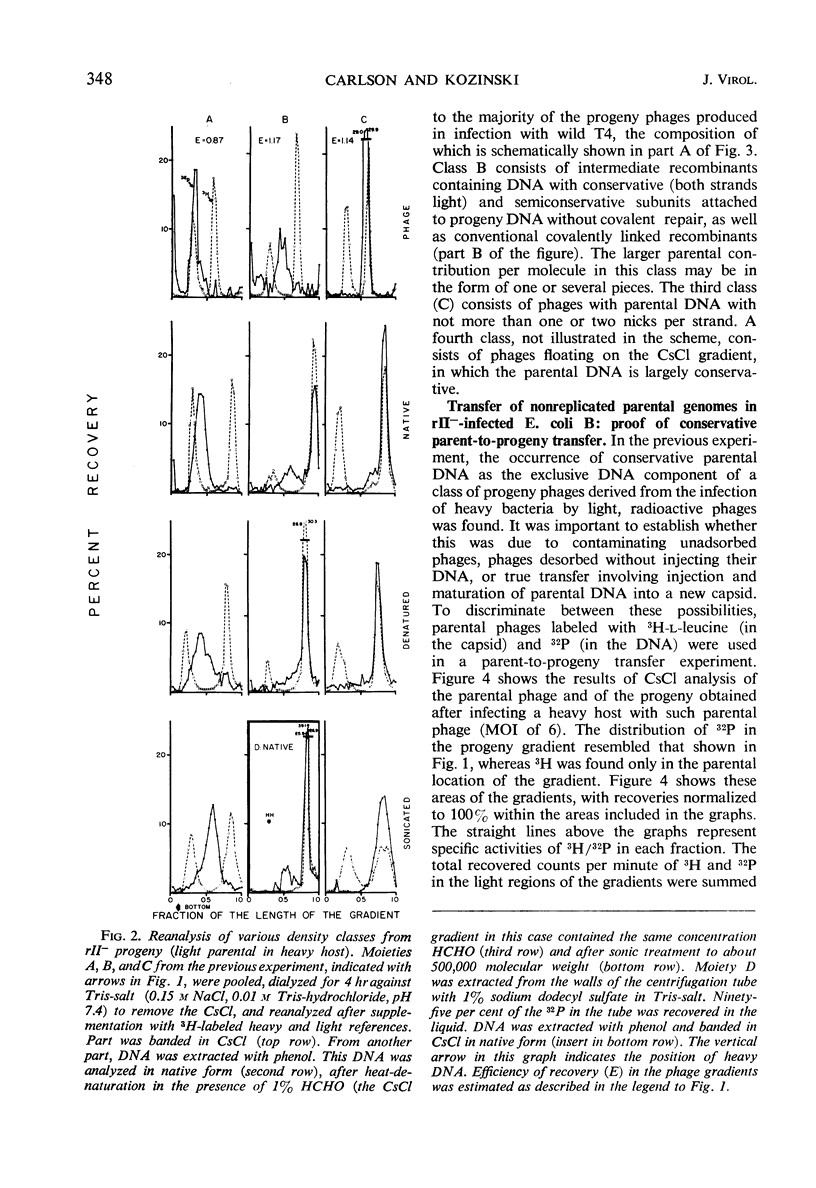
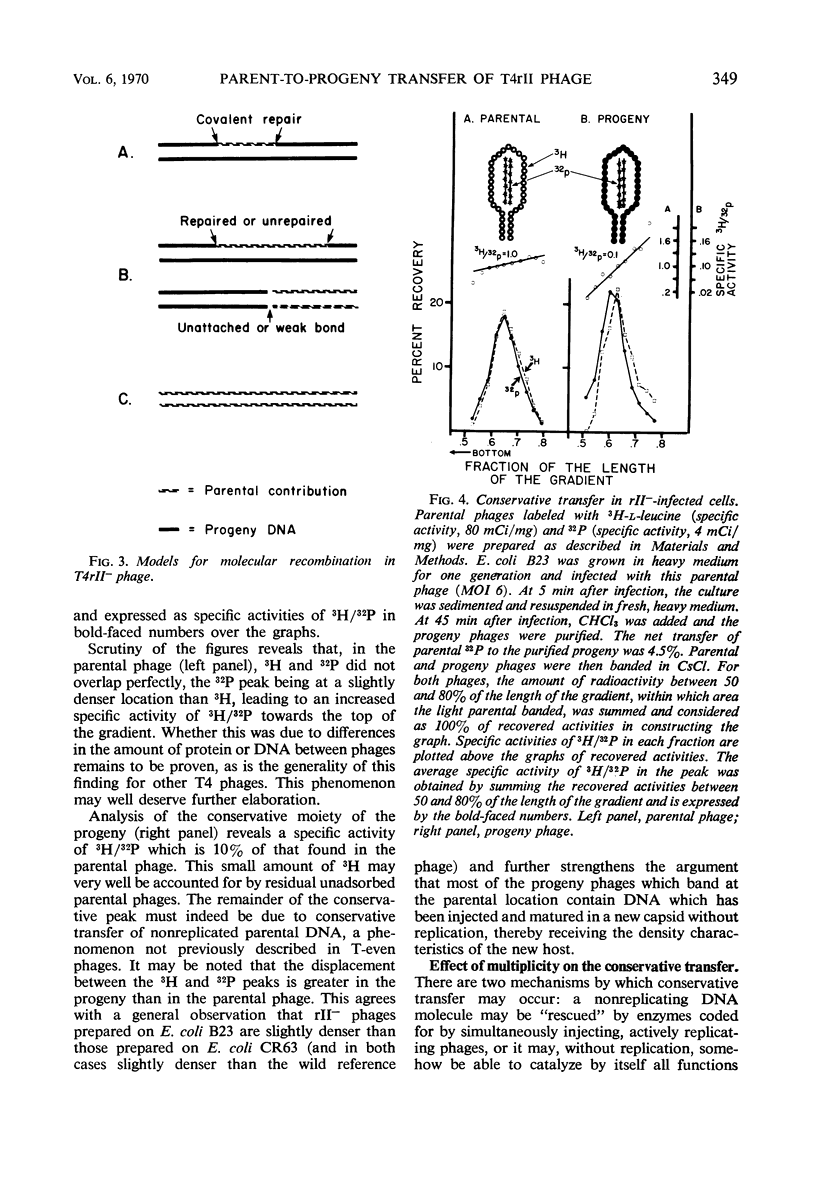
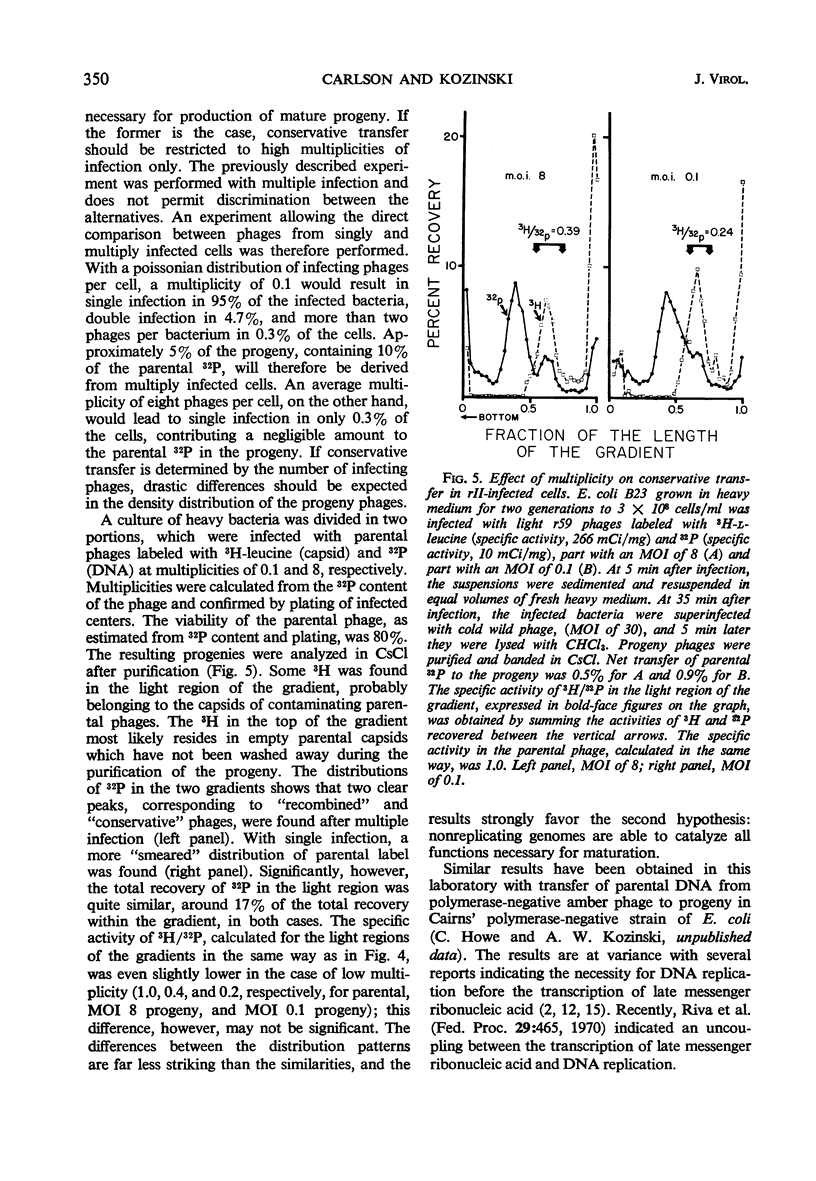
Transfer of parental, light (not substituted with 5-bromodeoxyuridine) 32P-deoxyribonucleic acid (DNA) from rII− mutants of T4 bacteriophage to heavy (5-bromodeoxyuridine-substituted) progeny in Escherichia coli B was less homogeneous than in wild phages. The net transfer was 5 to 20% of the value for wild T4 phage, and the parental contribution per progeny DNA molecule amounted to 7 to 100% of the genome. Three classes could be distinguished, based on the density distribution of parental label in CsCl analysis of the progeny phages. “Far recombined” phages contain parental material only in semiconservatively replicated subunits covalently attached to progeny DNA, amounting to 5 to 10% parental contribution per genome. “Intermediate recombinants” contain, aside from conventional recombinant DNA, parental DNA banding at the original, light density. This DNA may be unattached to heavy progeny DNA or attached by weak bonds which are very sensitive to shearing during the extraction procedure. The parental contribution is 10 to 50% per progeny DNA molecule in this class. “Conservative” phages band close to the parental, light density in CsCl; their DNA is purely light. When the parental phage is labeled with both 3H-leucine (capsid) and 32P (DNA), the specific activity of 3H/32P in the “conservative progeny” is 10 to 40% of that in the parental, showing that at least some of the 32P in this area belongs to phages with parental DNA as the sole DNA component inside an unlabeled capsid, i.e., parental DNA which has been injected into the host and matured in a new capsid without replication or recombination. This phenomenon occurs to about the same extent in both single and multiple infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H., Kozinski A. W. Suppression of T4D ligase mutations by rIIa and rIIb mutations. Proc Natl Acad Sci U S A. 1969 Nov;64(3):897–904. doi: 10.1073/pnas.64.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Carlson K. Intracellular fate of deoxyribonucleic acid from T7 bacteriophages. J Virol. 1968 Oct;2(10):1230–1233. doi: 10.1128/jvi.2.10.1230-1233.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Szybalski W. Fractionation of the complementary strands of coliphage T4 DNA based on the asymmetric distribution of the poly U and poly U,G binding sites. Virology. 1968 Apr;34(4):608–616. doi: 10.1016/0042-6822(68)90082-2. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. Fragmentary transfer of P32-labeled parental DNA to progeny phage. II. The average size of the transferred parental fragment. Two-cycletransfer. Repair of the polynucleotide chain after fragmentation. Virology. 1963 Jun;20:213–229. doi: 10.1016/0042-6822(63)90109-0. [DOI] [PubMed] [Google Scholar]
- Karam J. D. DNA replication of phage T4 rII mutants without polynucleotide ligase (gene 30). Biochem Biophys Res Commun. 1969 Oct 22;37(3):416–422. doi: 10.1016/0006-291x(69)90931-0. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., Shannon P. REPLICATIVE FRAGMENTATION IN T4 PHAGE: INHIBITION BY CHLORAMPHENICOL. Proc Natl Acad Sci U S A. 1963 Oct;50(4):746–753. doi: 10.1073/pnas.50.4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Mitchell M. Restoration by Chloramphenicol of Bacteriophage Production in Escherichia coli B Infected with a Ligase-Deficient Amber Mutant. J Virol. 1969 Dec;4(6):823–836. doi: 10.1128/jvi.4.6.823-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W. Molecular recombination in the ligase negative T4 amber mutant. Cold Spring Harb Symp Quant Biol. 1968;33:375–391. doi: 10.1101/sqb.1968.033.01.044. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Kuninaka A., Buchanan J. M. The relationship of DNA replication to the control of protein synthesis in protoplasts of T4-infected Escherichia coli B. Proc Natl Acad Sci U S A. 1969 Feb;62(2):446–453. doi: 10.1073/pnas.62.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Jr, Buckley P. Early intracellular events in the replication of bacteriophage T4 deoxyribonucleic acid. VI. Newly synthesized proteins in the T4 protein-deoxyribonucleic acid complex. J Virol. 1970 Apr;5(4):502–506. doi: 10.1128/jvi.5.4.502-506.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI M., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. II. SYNTHESIS OF PHAGE-INDUCED RNA AND SEQUENTIAL ENZYME PRODUCTION. J Mol Biol. 1964 May;8:638–659. doi: 10.1016/s0022-2836(64)80114-5. [DOI] [PubMed] [Google Scholar]
- Shahn E., Kozinski A. Fragmentary transfer of P32 labeled parental DNA to progeny phage. 3. Incorporation of a single parental fragment to the progeny molecule. Virology. 1966 Nov;30(3):455–470. doi: 10.1016/0042-6822(66)90122-x. [DOI] [PubMed] [Google Scholar]



