Abstract
Pigment epithelium-derived factor (PEDF) is a multifunctional protein with antiangiogenic, antioxidative, and anti-inflammatory properties. PEDF is involved in the pathogenesis of diabetic retinopathy, but its direct role in the kidneys remains unclear. We hypothesize that a PEDF fragment (P78-PEDF) confers kidney protection in diabetic nephropathy (DN). The localization of the full-length PEDF protein were determined in DBA mice following multiple low doses of streptozotocin. Using immunohistochemistry, PEDF was localized in the kidney vasculature, interstitial space, glomeruli, tubules, and renal medulla. Kidney PEDF protein and mRNA expression were significantly reduced in diabetic mice. Continuous infusion of P78-PEDF for 6 wk resulted in protection from diabetic neuropathy as indicated by reduced albuminuria and blood urea nitrogen, increased nephrin expression, decreased kidney macrophage recruitment and inflammatory cytokines, and reduced histological changes compared with vehicle-treated diabetic mice. In vitro, P78-PEDF blocked the increase in podocyte permeability to albumin and disruption of the actin cytoskeleton induced by puromycin aminonucleoside treatment. These findings highlight the importance of P78-PEDF peptide as a potential therapeutic modality in early phase diabetic renal injury.
Keywords: pigment epithelium-derived factor, diabetic nephropathy, podocytes
diabetes mellitus is a leading cause of morbidity and mortality in the United States. Diabetes mellitus is often complicated by micro- and macrovascular involvement that contributes to damage to one or more target organs. Diabetic nephropathy (DN) is a well-known microvascular complication of diabetes mellitus and is responsible for 40–50% of all cases of end-stage renal disease (ESRD) in the U.S. adult population (13, 35). The rate of progression of DN toward ESRD is influenced by complex interactions between genetic predisposition, dietary and lifestyle factors, and therapeutic interventions. Available therapeutic options directed at delaying the progression of DN include intensive blood glucose control, improved blood pressure control, interruption of the renin-angiotensin-aldosterone system, dietary modification, and cholesterol-lowering agents [for review see (1)]. Despite aggressive multifactorial interventions, DN remains the single leading cause of ESRD in the United States. Therefore, more effective approaches are urgently needed.
The pathogenesis of DN involves multiple processes, including inflammation, angiogenesis, oxidative injury, and podocyte structural and functional abnormalities. Pigment epithelium-derived factor (PEDF) is a multifunctional, pleiotropic secretory glycoprotein with antiangiogenic, antioxidative, and anti-inflammatory properties (31, 33). The PEDF protein was first identified in the eye (34) but is also present in other tissues, including the kidney (26). The human PEDF gene encodes a 418–amino acid protein (9) and shows strong conservation across phyla (32). PEDF acts via multiple high-affinity ligands and cell receptors, although the mechanisms are not clear. Previous reports demonstrated that PEDF is reduced in diabetic retinopathy and that systemic or local delivery of recombinant PEDF protein or viral vector-mediated PEDF gene therapy successfully inhibited retinal neovascularization and reduced retinal vascular permeability in diabetic animals (21). However, the direct role of PEDF in diabetic kidneys is not completely clear. Because PEDF is a 50-kDa protein, this may limit its utility as a therapeutic target. Recent evidence has suggested that fragments of PEDF are bioactive. In particular, a 44–amino acid (AA 78–121; P78-PEDF) peptide shows excellent bioactivity in several reports (15, 21). Thus it is important to identify the direct role of PEDF or P78-PEDF peptide, or both in the setting of DN.
The current study tested the hypothesis that a PEDF fragment (P78-PEDF) confers kidney protection in DN. We found that mice with streptozotocin (STZ)-induced type 1 diabetes are protected from albuminuria and display reduced histopathological changes associated with DN and reduced macrophage infiltration into the kidney when treated with P78-PEDF peptide. In addition, P78-PEDF peptide had a direct effect in restoring podocyte structural and functional integrity. These results provide evidence for P78-PEDF peptide as a novel therapeutic intervention in the treatment of early phase DN.
MATERIALS AND METHODS
Diabetic mouse model.
Experiments were conducted in male 6-wk-old DBA/2J mice (no. 000671; The Jackson Laboratory, Bar Harbor, ME) and approved by the Penn State University College of Medicine Institutional Animal Care and Use Committee. DBA/2J mice are recommended by the Animal Models of Diabetes Complications Consortium as a nephropathy-susceptible model of DN (10, 11). Type 1 diabetes mellitus was induced using multiple low doses of STZ (50 mg/kg body wt dissolved in lactated Ringer solution; Sigma, St. Louis, MO) via intraperitoneal injection for 5 consecutive days. Establishment of diabetes mellitus was confirmed 5 days after the last dose of STZ injection by measuring random blood glucose levels (Accu-Chek glucometer; Boehringer-Mannheim, Indianapolis, IN). All analyses were performed on the left kidney except fluorescence-activated cell sorting (FACS) analysis, which was performed on the right kidney.
Drug delivery.
P78-PEDF, a small PEDF peptide (15, 18, 21) (0.1 μg·g−1·day−1) or vehicle (phosphate-buffered saline; PBS) were administered by continuous subcutaneous infusion beginning immediately after confirming the elevated blood glucose level via an osmotic minipump (no. 2006; Alzet, Durect, Palo Alto, CA), which delivered drug for 6 wk after a single pump placement as described previously (5, 24, 27). The condition of mice and body weight were monitored daily following the pump implantation. All end point data were collected after 6 wk of P78-PEDF treatment (13 wk of age).
Blood pressure measurement.
Mean arterial pressure was measured using the Coda blood pressure system (Kent Scientific, Torrington, CT) (5, 14, 24). Mice were allowed to rest quietly for 10 min at 26°C. All measurements were performed at the same time for all groups to prevent any diurnal variations.
Renal histopathology.
Kidneys were fixed in 4% paraformaldehyde, embedded in paraffin, and 3-μm sections were cut. Sections were stained with periodic acid-Schiff (PAS) stain, all glomeruli were examined individually at 400× in a blinded manner, and scores were averaged. All images were obtained with an Olympus BX51 microscope and DP71 digital camera using cellSens Standard 1.6 imaging software. Images were taken with the 100× (oil) objective (total magnification, 1,000×). Semiquantitative scores (0–4) were assigned on the basis of masked reading as previously described (5, 24, 41). Briefly, each glomerulus on a kidney single section was graded from 0 to 4, where 0 represents no lesion; and 1, 2, 3, and 4 represent mesangial matrix expansion or sclerosis, involving ≤25, 25 to 50, 50 to 75, or >75% of the glomerular tuft area, respectively.
Glomerular macrophage staining.
Immunohistochemistry for macrophages was performed using rat anti-mouse Mac-2 antibody (clone M3/38; Cedarlane, Burlington, NC) on paraffin sections as described previously (5, 24).
PEDF immunocytochemistry.
Kidney samples were fixed in 4% (vol/vol) paraformaldehyde, cryopreserved in 30% sucrose, embedded in optimal cutting temperature compound, and 10-μm cryosections obtained. Nonspecific binding sites on the samples were blocked for 30 min with 0.5% BSA. The polyclonal antibodies PEDF (1:50 dilution) and β-actin (1:50 dilution; Millipore, Temecula, CA) were diluted in PBS containing 0.5% BSA. Cy3- or FITC-conjugated goat anti-rabbit IgG diluted 1:1,000 in 5% BSA/PBS was used as a secondary antibody. DAPI (4,6-diamidino-2-phenylindole; 1:5,000, 5 mg/ml solution) was applied to immunolabeled tissues for 10 min, then samples were mounted on glass slides using Slowfade gold antifade reagent (Invitrogen, Carlsbad, CA). Fluorescence labeling was evaluated via confocal microscopy (Fluoview 1000; Olympus).
Analysis of kidney macrophage content by FACS.
Kidney macrophage (CD11b+F4/80low) content was determined by flow cytometry at the end of experiments as described previously (5, 6, 8). In brief, kidneys were extracted, minced, digested, and then passed through a filter and a cotton wool column. Fresh kidney suspensions were incubated with anti-mouse CD45-FITC (30-F11; eBioscience, San Diego, CA) for 30 min on ice. Kidney macrophages were then identified using allophycocyanin-labeled rat anti-mouse F4/80 (BM8; eBioscience) and phycoerythrin-labeled rat anti-mouse CD11b (M1/70; eBioscience). All samples were treated with anti-mouse CD16/CD32 (2.4G2) to block nonspecific Fc receptor binding and 7-aminoactinomycin D to eliminate dead cells (Invitrogen). Counting beads (Caltag, Carlsbad, CA) were used to determine the total number of CD45+ cells per gram of kidney tissue. Subsequent flow cytometry data acquisition was performed with an LSR2 (Becton Dickinson, San Jose, CA). Data were analyzed with Flowjo software 8.7 (Tree Star, Ashland, OR). All the antibodies were purchased from eBioscience.
Podocyte BSA filter assay.
Collagen-coated Transwell-Col polytetrafluorothylene filters (3 μm pore; Corning, New York, NY) were seeded with 1 × 104 podocytes per filter and cultured under differentiating conditions for 14 days. Cells were then incubated with vehicle or 100 μg/ml puromycin aminonucleoside (PAN, Sigma) with or without P78-PEDF peptide (100 nM) for 24 h at 37°C. The upper compartment was then refilled with 0.5 ml of RPMI 1640, and the lower compartment with 1 ml of BSA medium (RPMI 1640 supplemented with 40 mg/ml BSA). Total protein concentration in the upper compartment was determined at 6 h using a Bio-Rad (Hercules, CA) protein assay as described previously (7).
Immunofluorescence and confocal microscopy.
Differentiated podocytes grown on collagen-coated glass coverslips were incubated with FITC-conjugated phalloidin (Sigma) as described previously (7). The fields of all specimens were selected randomly and examined using a Zeiss LSM 510-UV confocal microscope.
Quantitative real-time RT-PCR.
Total RNA was extracted from kidneys and podocytes using an RNeasy Mini Kit (Qiagen, Hilden, Germany). Single-strand cDNA was synthesized using a Superscript First Strand cDNA Synthesis Kit (Invitrogen) for two-step real-time polymerase chain reaction (RT-PCR). Gene-specific primers for nephrin, PEDF, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed using Beacon Designer Probe/Primer Design Software (OligoPerfect Designer; Invitrogen). Quantitative RT-PCR was performed using the iQ5 system (Bio-Rad). Reactions were performed in duplicate, and threshold cycle numbers were averaged. Results were normalized to GAPDH measured in parallel (4).
Western blot.
Samples were homogenized in lysis buffer and soluble protein was extracted by centrifugation. Thirty-microgram aliquots of extracted proteins were separated in 10% SDS polyacrylamide gels and electro-transferred for 2 h at 0.3 A onto nitrocellulose membranes (Bio-Rad). Nonspecific antigen binding sites were blocked with 5% nonfat dried milk and transblots were incubated overnight at 4°C with 1:500 PEDF polyclonal antibody. PEDF antibody specificity was previously confirmed (21). Membranes were then washed, exposed for 1 h to horseradish peroxidase (HRP)-conjugated affinity-purified goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), and HRP activity was assessed using a Pierce enhanced chemiluminescent Western blotting substrate (Thermo Scientific, Rockford, IL) followed by exposure to Kodak Scientific Imaging X-OMAT LS film (Carestream Health, Rochester, NY). Blots were stripped and reprobed for β-actin. PEDF results were normalized to β-actin.
Glomerular endothelial cell lines.
Mouse glomerular endothelial cells (kindly provided by Dr. Michael Madaio, Medical College of Georgia) were cultured as described previously (3). Briefly, frozen cells were grown under permissive conditions to propagate [in the presence of 50 U/ml gamma-interferon (INF-γ) at 33°C] in DMEM/F12 media containing 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (2 mmol/L) in collagen-coated plates. Cells then were grown under restrictive conditions for 14 days (absence of INF-γ at 37°C in 95% air/5% CO2) and allowed to differentiate in the presence of normal glucose (11 mM) or high-glucose (33 mM) media. After 14 days, cells were subjected to Western blot analysis as described above.
Analytical methods.
Urinary albumin excretion was measured by ELISA using Albuwell M (Exocell, Philadelphia, PA) as described previously (4, 5, 24). Urine creatinine was determined using a Creatinine Liquid Reagens Assay kit (Diazyme Laboratories, Poway, CA) as described previously (5, 24). Blood urea nitrogen (BUN) was measured using VITROS DT60II chemistry slides (Ortho-Clinical Diagnostics, Rochester, NY) as described previously (5, 24). Urine tumor necrosis factor alpha (TNF-α) was measured by ELISA (eBioscience) as described previously (5). Vascular endothelial growth factor (VEGF) level was determined using an Affymetrix Procarta Cytokine assay (Panomics, Fremont, CA) in conjunction with the Bio-Plex instrument (Bio-Plex 200; Bio-Rad) as described previously (21). Body composition was determined using an LF90 Minispec Time Domain Nuclear Magnetic Resonance Spectrometer (Bruker Optics, Billerica MA) as described previously (5, 24).
Statistical analysis.
Comparisons between groups were examined by using the SPSS version 19.0 software (SPSS, Chicago, IL) program. Data are expressed as means ± SE. One-way ANOVA was used when more than two groups were compared, and significance of observed differences among the groups was evaluated with a least significant difference post hoc test. Statistical significance was identified at P < 0.05.
RESULTS
Localization of PEDF in mouse kidneys.
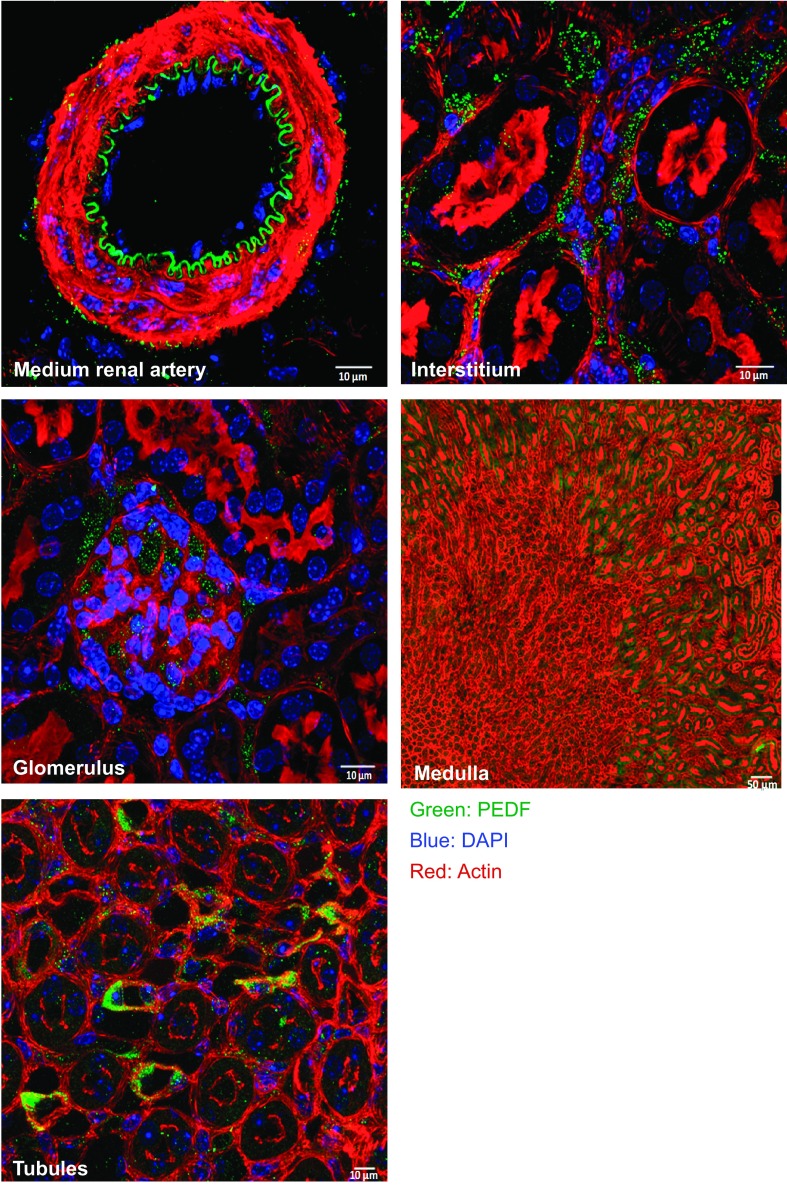
The precise localization of PEDF in the kidney is not clear. Therefore, we first assessed the localization of PEDF in the kidneys under normal conditions. Using a PEDF-specific antibody (21), we identified PEDF expression in mouse kidneys (Fig. 1) mainly in the vasculature, interstitial spaces, glomeruli, medulla, and tubular epithelial cells.
Fig. 1.
Localization of pigment epithelium-derived factor (PEDF) in the mouse kidney. Immunofluorescence staining for PEDF on a representative kidney section in normal mice.
PEDF protein and mRNA expression is reduced in diabetic kidneys.
Next, we determined PEDF expression in diabetic kidneys. Our data show that PEDF protein (Fig. 2A) and mRNA (Fig. 2B) expressions are markedly reduced in diabetic kidneys after 6 wk of STZ-induced diabetes mellitus. We also confirmed the expression of PEDF in glomerular endothelial cells in vitro and noted that PEDF expression was significantly reduced after growth in high-glucose media for 14 days (Fig. 2C). Although total PEDF expression was reduced in diabetic mice, the localization within the kidney was similar to that of control mice (data not shown).
Fig. 2.
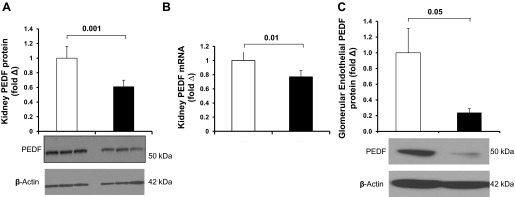
PEDF expression in vivo and in vitro. Western-blot and RT-PCR were performed to detect PEDF expression in kidney lysates from indicated groups of mice. A: Western blot analysis of total kidney PEDF expression (n = 6 each group). Semiquantification of PEDF expression was performed by densitometry followed by normalization to β-actin. B: quantitative real-time PCR of total kidney PEDF expression (n = 5 each group). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Open bar, normal; black-filled bar, diabetes. Results are means ± SE. C: Western blot analysis of PEDF in glomerular endothelial cells grown in normal or high glucose media (n = 6 each group). Semiquantification of PEDF expression was performed by densitometry followed by normalization to β-actin. Open bar, normal glucose; black bar, high glucose. Results are means ± SE.
P78-PEDF peptide administration reduces characteristics of diabetic nephropathy.
Because PEDF is reduced in diabetic kidneys, we questioned whether restoring PEDF using P78-PEDF peptide would ameliorate diabetic renal injury. Toward this goal, we continuously infused the P78-PEDF peptide or vehicle into diabetic mice for 6 wk. As shown in Table 1, vehicle-treated diabetic mice had increased blood glucose levels, decreased body weight, decreased kidney weight, increased kidney weight/body weight ratio, and reduced fluid composition compared with normal mice. P78-PEDF peptide administration to diabetic mice significantly increased body weight and reduced the kidney weight/body weight ratio without affecting other measurements compared with the vehicle-treated diabetic group. Importantly, treatment with P78-PEDF peptide did not affect blood pressure, blood glucose, or fluid composition compared with vehicle-treated diabetic mice.
Table 1.
Effects of P78-PEDF peptide administration on diabetic mice
| Group | Nondiabetic | Diabetes + Vehicle | Diabetes + P78-PEDF |
|---|---|---|---|
| Animal No. | 13 | 14 | 12 |
| Blood glucose (mg/dl) | 135 ± 5 | 471 ± 9* | 458 ± 21* |
| Body weight (g) | 27 ± 0.7 | 20 ± 0.5* | 23 ± 0.5*‡ |
| SBP (mmHg) | 126 ± 3 | 123 ± 5 | 124 ± 5 |
| Kidney weight (mg) | 281 ± 8 | 245 ± 4† | 236 ± 6* |
| Kidney weight/body weight (mg/g) | 10 ± 0.2 | 12 ± 0.2* | 10 ± 0.2§ |
| Fluid (%) | 7.6 ± 0.09 | 6.1 ± 0.12* | 6.1 ± 0.21* |
| Plasma BUN (mg/dl) | 16 ± 1 | 34 ± 2* | 21 ± 2§ |
SBP, systolic blood pressure. Data are mean ± SE.
P < 0.0001;
P < 0.001 compared with the nondiabetic group;
P < 0.005;
P < 0.0001 compared with the diabetes + vehicle group (n = 12–14 mice per group).
P78-PEDF peptide administration reduces albuminuria and BUN in diabetic mice.
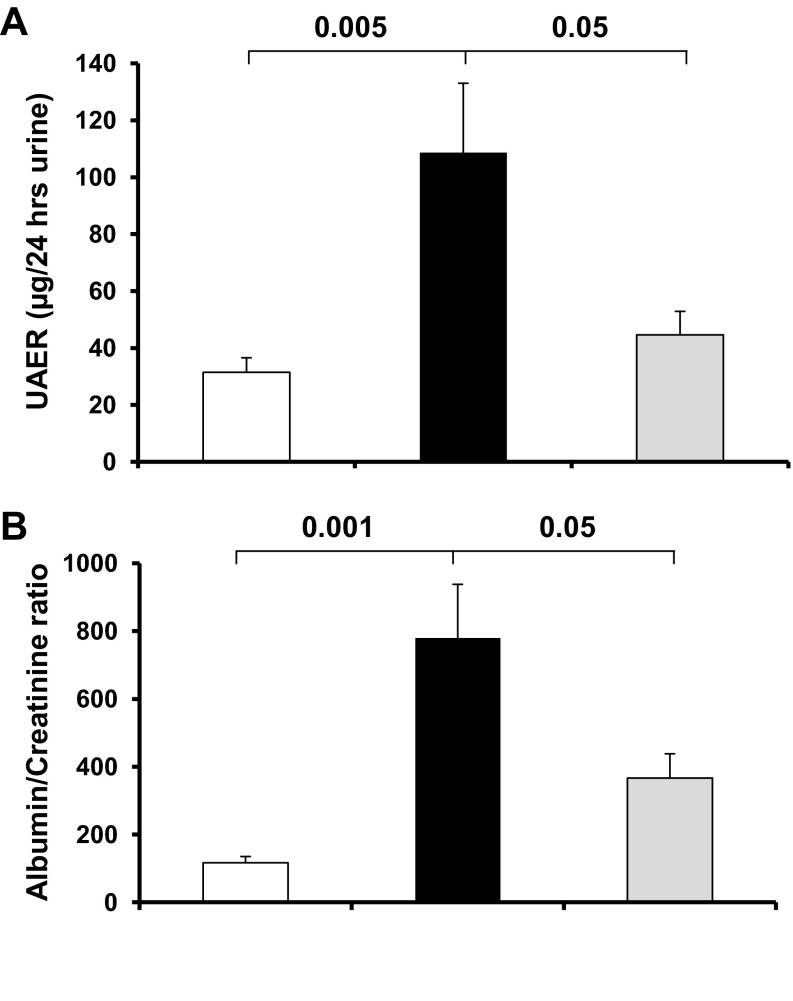
To determine whether PEDF deficiency is associated with diabetic renal injury, we measured 24-h urine albumin excretion, albumin/creatinine ratio, and BUN as indicators of renal injury in diabetic mice treated with vehicle or P78-PEDF peptide. Vehicle-treated diabetic mice had a significant increase in albuminuria (Fig. 3A), albumin/creatinine ratio (Fig. 3B), and BUN (Table 1) compared with normal mice after 6 wk of diabetes mellitus. These effects were significantly reduced in diabetic mice treated with P78-PEDF peptide for 6 wk.
Fig. 3.
P78-PEDF peptide attenuates diabetic renal injury. Diabetic mice were treated with P78-PEDF peptide or vehicle via osmotic minipump for 6 wk. Urine was collected for measurement of urine albumin excretion (UAE) (A) or albumin/creatinine ratio (B). Open bar, normal group; black bar, vehicle-treated diabetic groups; gray bar, P78 PEDF-treated groups. Results are means ± SE.
P78-PEDF peptide administration decreases macrophage recruitment in STZ-induced diabetic mice.
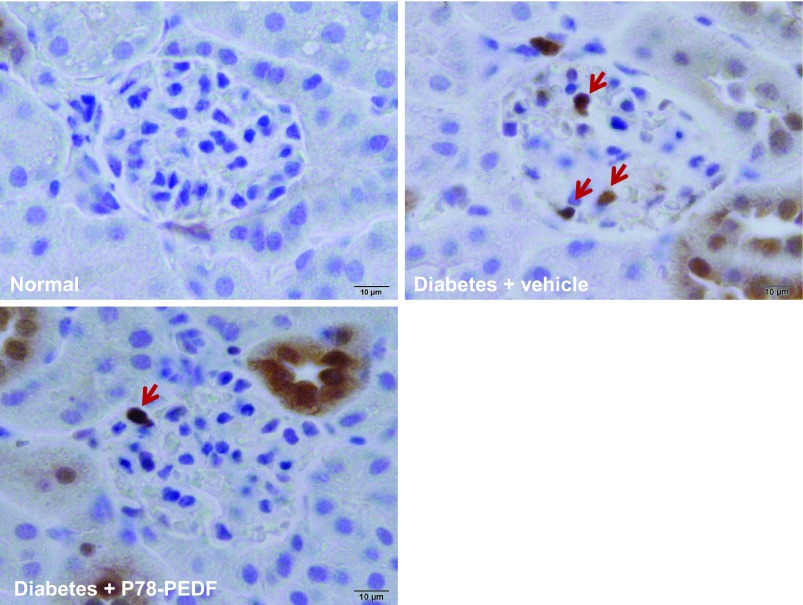
To determine whether the reduction in PEDF is critical for macrophage infiltration in DN, we show the distribution and quantitation of macrophages in kidneys by immunohistochemistry (Mac-2-positive macrophages) (Fig. 4). Vehicle-treated diabetic mice showed increases in glomerular macrophages compared with normal mice. In contrast, P78-PEDF peptide-treated diabetic mice had reduced glomerular macrophage recruitment. Similar results were obtained when total kidney macrophages (identified as CD11b+F4/80low) were measured by FACS. Kidneys of vehicle-treated diabetic mice had a significantly greater number of macrophages compared with normal mice (24.1 ± 2.7 × 104 vs. 5.2 ± 0.8 × 104 macrophages/g kidney tissue, P < 0.0001). In contrast, kidneys of P78-PEDF peptide-treated diabetic mice had significantly fewer macrophages (16.7 ± 2.4 × 104 macrophages/g kidney tissue, P < 0.05) compared with vehicle-treated diabetic mice at 6 wk of STZ-induced diabetes mellitus.
Fig. 4.
P78-PEDF peptide reduces macrophage infiltration in diabetic mice. Mac-2-positive macrophages in glomeruli were identified by immunohistochemical staining after 6 wk in normal, vehicle-treated diabetic, and P78-PEDF-treated mice. Images are representative of 40 fields from 12–14 mice in each group. Red arrow indicates stained macrophages.
P78-PEDF peptide administration decreases kidney and urinary cytokines in diabetic mice.
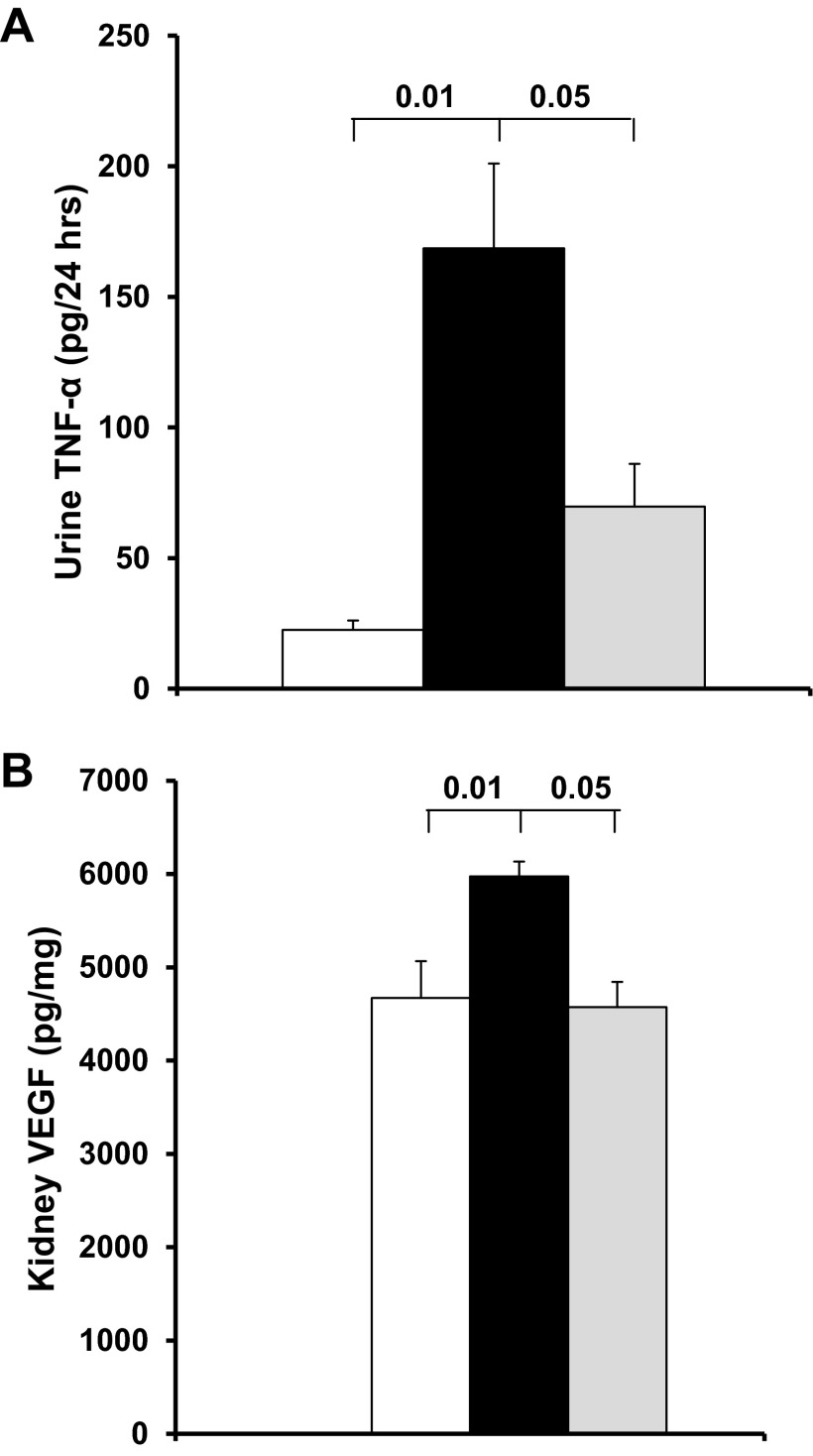
Increased inflammatory cytokines is a major feature of DN and an important predictor of it (4, 16). Therefore, we further assessed the anti-inflammatory effect of P78-PEDF peptide treatment in diabetic mice (Fig. 5). Urinary TNF-α excretion (Fig. 5A) and kidney VEGF (Fig. 5B) were significantly increased in the vehicle-treated diabetic mice at 6 wk of diabetes mellitus (P < 0.01) compared with normal mice. In contrast, P78-PEDF peptide significantly decreased urinary TNF-α excretion and kidney VEGF in diabetic mice (P < 0.05 vs. vehicle-treated mice).
Fig. 5.
P78-PEDF peptide reduces inflammatory cytokines in diabetic mice. Kidney lysate and urine were collected for measurements of vascular endothelial growth factor (VEGF) and tumor necrosis factor-alpha (TNF-α) after 6 wk of diabetes mellitus. Open bar, normal group; black bar, vehicle-treated diabetic groups; gray bar, P78-PEDF-treated groups. Results are means ± SE.
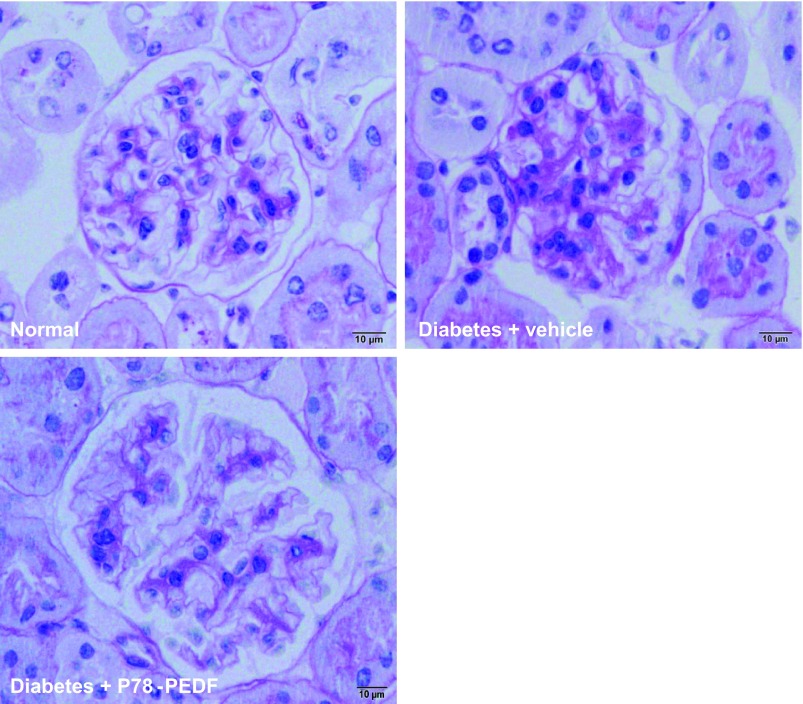
P78-PEDF peptide administration decreases renal histopathological changes in STZ-induced diabetic mice.
PAS staining of kidney sections (Fig. 6) showed increased glomerular cellularity and mesangial expansion (score, 0.9 ± 0.03 vs. 0.3 ± 0.01; P < 0.0001 in vehicle-treated STZ-diabetic mice vs. normal mice; respectively). P78-PEDF peptide treatment resulted in significantly reduced glomerular changes (score, 0.4 ± 0.06; P < 0.005) compared with vehicle-treated diabetic mice.
Fig. 6.
P78-PEDF peptide reduces histological changes in diabetic mice. Sections were stained with periodic acid-Schiff (PAS) and all glomeruli were graded individually at 400× magnification. Normal mice have normal amounts of delicate mesangium supporting capillaries. In vehicle-treated diabetic mice, PAS-positive matrix expands the glomerular tuft by less than 25%. Mice treated with P78-PEDF have glomeruli indistinguishable from normal mice. Images were taken with the 100× (oil) objective with a total magnification of 1,000×. Images are representative of 12–14 mice in each group.
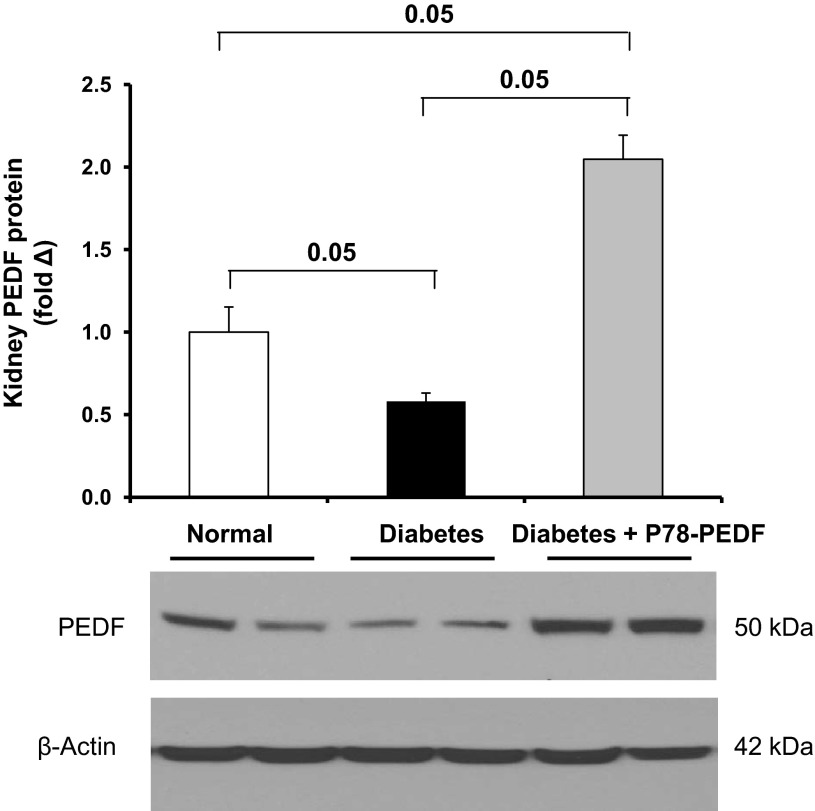
P78-PEDF peptide administration increases renal PEDF expression in STZ-induced diabetic mice.
We also determined the effect of P78-PEDF peptide treatment on PEDF expression in diabetic kidneys. Our data show that PEDF protein expression is markedly reduced in diabetic kidneys after 6 wk of STZ-induced diabetes mellitus. Interestingly, P78-PEDF peptide treatment significantly increased PEDF protein expression in diabetic mice (Fig. 7). Immunohistochemistry for PEDF did not reveal any changes in PEDF cellular localization in peptide-treated mice (data not shown).
Fig. 7.
P78-PEDF peptide increases PEDF expression in diabetic mice. Western blot was performed to detect PEDF expression in kidney lysates from indicated groups of mice. Semiquantification of PEDF expression was performed by densitometry followed by normalization to β-actin. Open bar, normal group; black bar, vehicle-treated diabetic groups; gray bar, P78-PEDF-treated groups. Results are means ± SE.
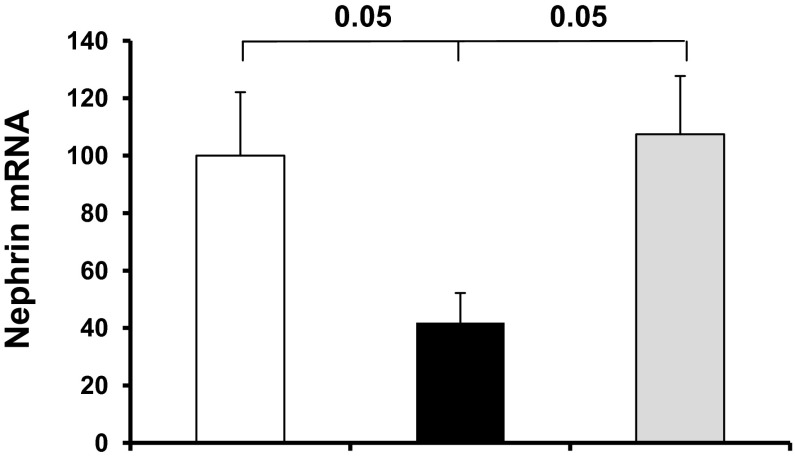
P78-PEDF peptide administration regulates podocyte-specific proteins such as nephrin.
To determine the possible mechanisms by which P78-PEDF peptide reduced albuminuria and mediates renal tissue protection, we examined its effect on podocytes. At 6 wk of diabetes mellitus, nephrin (a podocyte-specific protein) mRNA was reduced in kidneys from vehicle-treated diabetic mice, as expected. P78-PEDF-treated diabetic mice had markedly increased expression of nephrin compared with vehicle-treated controls (P < 0.05), but was unchanged compared with the normal group (Fig. 8).
Fig. 8.
P78-PEDF peptide regulates podocyte-specific nephrin expression in diabetic mice. Quantitative real-time RT-PCR was performed on whole kidney total RNA isolated after 6 wk of diabetes. Expression of nephrin mRNA was normalized to GAPDH and data were calculated as expression relative to control. Open bar, normal group; black bar, vehicle-treated diabetic groups; gray bar, P78-PEDF-treated groups (n = 11–12 mice per group). Results are means ± SE.
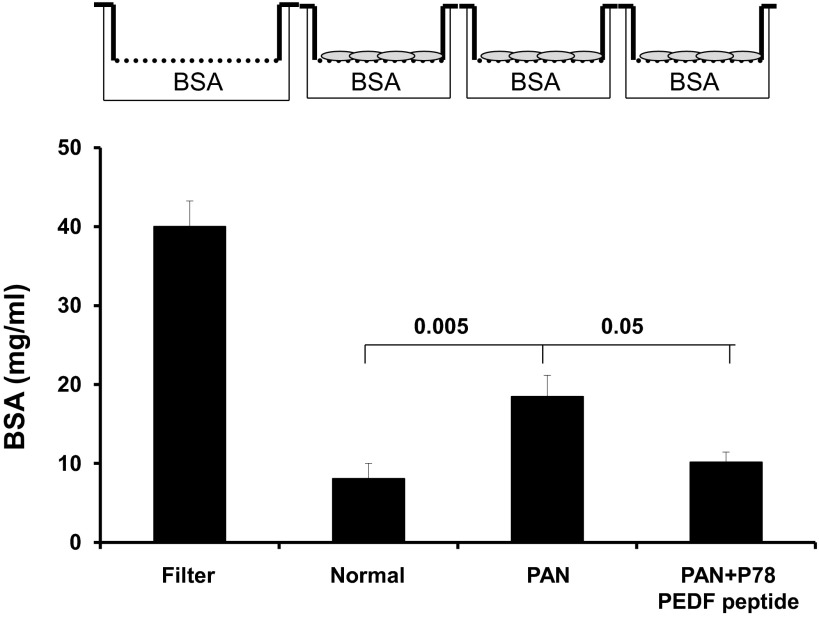
P78-PEDF peptide attenuates the PAN-induced increase in podocyte permeability in vitro.
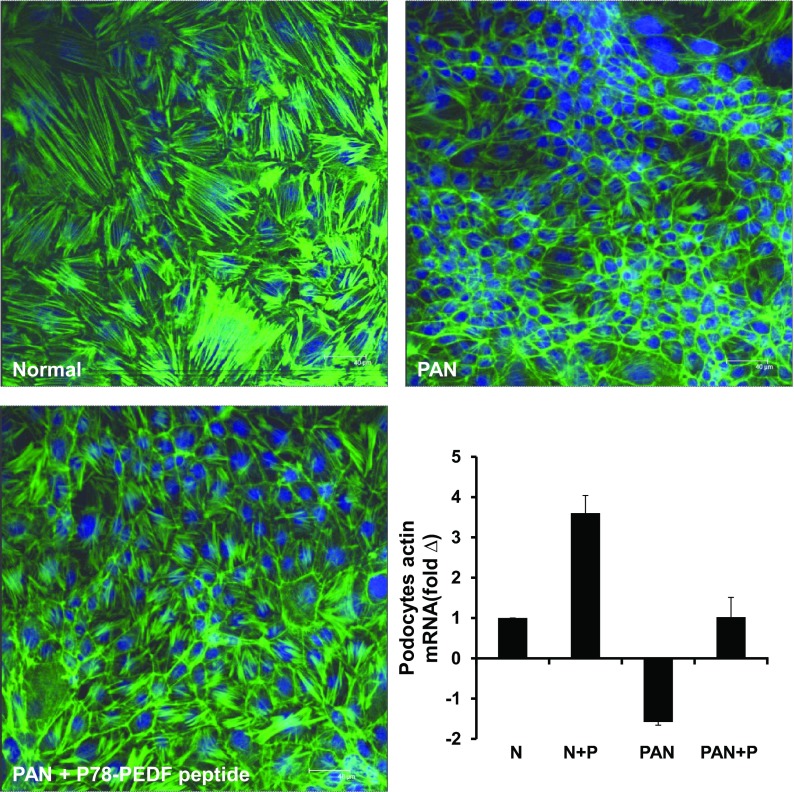
To confirm the direct functional effect of P78-PEDF peptide on podocytes, we determined its effect on podocyte permeability by measuring the transepithelial passage of BSA across differentiated podocytes grown on Transwell chambers as described previously (7). As shown in Fig. 9, differentiated podocytes treated with PAN significantly increased albumin passage across podocytes (P < 0.005 compared with normal). P78-PEDF peptide treatment completely blocked the effect of PAN on podocyte permeability to albumin (P < 0.05). P78-PEDF peptide treatments also resulted in preservation of podocyte structural integrity and prevention of the marked disruption and organization of the actin cytoskeleton produced by treatment with PAN and preservation of podocyte actin mRNA expression (Fig. 10).
Fig. 9.
P78-PEDF peptide regulates podocyte permeability in vitro. Transepithelial permeability of differentiated podocytes to BSA was measured as described previously. After 14 days of culture on Transwell filter chambers (3-μm pore) at 37°C, podocytes were pretreated with vehicle or P78-PEDF peptide (100 nM) for 1 h and then exposed to PAN (100 μg/ml) for 24 h. The lower chambers were then filled with BSA-containing medium, and the upper chambers were sampled at 6 h (n = 6 each group). Data are means ± SE.
Fig. 10.
P78-PEDF peptide regulates podocyte actin cytoskeleton in vitro. After 14 days of culture at 37°C, podocytes were pretreated with vehicle or P78-PEDF peptide (100 nM) for 1 h and then exposed to PAN (100 μg/ml) for 24 h. Confocal images of normal differentiated murine podocytes, podocytes treated with PAN alone, or podocytes treated with PAN + P78-PEDF peptide. Bar graph: quantitative real-time RT-PCR was performed on differentiated podocytes. Expression of actin mRNA was normalized to GAPDH and data were calculated as expression relative to control. Results are means ± SE. N, normal podocytes; N+P, normal podocytes treated with P78-PEDF peptide; PAN, podocytes treated with puromycin aminonucleoside; PAN+P, podocytes treated with puromycin aminonucleoside and P78-PEDF peptide (n = 4 each group).
DISCUSSION
PEDF has well-established antiangiogenic, antioxidative, and anti-inflammatory actions in the eye (31, 33), yet its role in diabetic kidney injury is not completely clear. This study shows that a small bioactive peptide fragment of PEDF (P78-PEDF) mediates renal tissue protection as evidenced by a reduction in albuminuria and BUN, histopathological changes, kidney macrophage recruitment, and inflammatory cytokines during diabetes mellitus. Furthermore, P78-PEDF preserved expression of podocyte structural protein (nephrin) during diabetes mellitus in vivo and directly preserved podocyte structural and functional integrity in vitro. These findings reveal an important role for P78-PEDF peptide and/or other PEDF peptides in the pathogenesis of early phase DN. P78-PEDF could provide a new therapeutic modality for treating patients with diabetes.
PEDF protein is expressed in several tissues and cells including the postnatal kidney (26). However, the precise localization of PEDF in the kidney is not known. In our experiment, we used a specific antibody to clearly identify PEDF expression in the mouse kidney. Our data show that PEDF is expressed mainly in the kidney vasculature, interstitial spaces, glomeruli, medulla, and tubular epithelial cells. We also were able to detect the expression of PEDF in glomerular endothelial cells in vitro in normal glucose media, an effect that was significantly reduced when using high-glucose media. We further questioned whether PEDF is altered in diabetes mellitus. Our data show that both PEDF protein and mRNA expression are markedly reduced in diabetic kidneys. These data are consistent with a previous report of decreased PEDF protein and mRNA expression in diabetic rat kidney (38).
To examine the role of PEDF in DN, we continuously infused a small bioactive fragment of PEDF, P78-PEDF peptide, in this mouse model of type 1 diabetes mellitus. P78-PEDF peptide has been recently characterized (21) and shown to have excellent bioactivity. Unlike P78-PEDF peptide, other approaches have mainly focused on PEDF delivery using viral vectors (39, 40). These approaches have problems such as inflammation associated with viral components, difficulty regulating the level of PEDF produced, and concerns over the rapidity of onset and longevity of PEDF expression (9). For example, adenoviral delivery of PEDF failed to maintain the reduction in albuminuria in diabetic rats after 5 wk of diabetes mellitus (40). Given these concerns, we investigated the effects of P78-PEDF peptide on diabetic kidney dysfunction, specifically glomerular histopathological changes and macrophage recruitment. P78-PEDF peptide significantly ameliorated diabetic albuminuria and was associated with reduced kidney macrophage infiltration and glomerular pathology. Taken together, our results provide support for P78-PEDF peptide as a therapeutic modality for the treatment of early phase DN. Additional study is needed to explore the effect of P78-PEDF peptide treatment in late-stage DN.
The renal protective effect of P78-PEDF peptide correlates with a significant reduction in kidney macrophage infiltration. Whether the reduction in macrophage recruitment is mediated directly by P78-PEDF peptide or indirectly by reducing diabetic renal injury will require additional further studies. Infiltrating macrophages release lysosomal enzymes, nitric oxide, reactive oxygen species, transforming growth factor-beta, and VEGF; and cytokines such as TNF-α, interleukin-1, and IFN-γ (30) which could play a pivotal role in the development and progression of DN. We have shown previously that urinary TNF-α is increased in a mouse and rat model of type 1 diabetes mellitus (4, 5). The current study confirms these results and shows that P78-PEDF peptide significantly reduced the increase in urinary TNF-α. TNF-α is produced mainly by monocytes/macrophages and has been associated with increasing vascular endothelial permeability in diabetes mellitus (25). Our data also show reduced kidney VEGF with P78-PEDF peptide treatment, confirming the observation that PEDF counteracts the effects of VEGF (20). The role of VEGF in renal physiology and physiopathology is controversial. Under physiological conditions, VEGF maintains glomerular endothelial integrity. In certain conditions such as hypertension, inhibition of VEGF is associated with severe glomerulosclerosis, mesangial expansion, and albuminuria (2). In contrast, increased VEGF can lead to glomerular hypertrophy and proteinuria (19). Indeed, overexpression of VEGF in podocytes of transgenic mice is associated with thickened glomerular basement membrane and proteinuria (36, 37); a cardinal feature of DN. In animal models of DN, VEGF levels are elevated (12) and inhibition of VEGF using a small molecule inhibitor of VEGF receptor (23) significantly ameliorated albuminuria (29). Likewise, ruboxistaurin has been shown to attenuate the effect of VEGF and progression in animal studies of DN (17). Additional study is needed to explore the direct role of P78-PEDF peptide on kidney VEGF following diabetes mellitus. Interestingly, P78 PEDF peptide reduced retinal IFN-γ and TNF-α, led to a loss of the retinal ganglion cell layer, and increased the thickness of the inner plexiform layer in our diabetic mice compared with the vehicle-treated group (data not shown). P78-PEDF peptide also resulted in less mesangial expansion and glomerular hypercellularity in diabetes mellitus, indicating a possible contribution of PEDF reduction to the initiation and/or progression of diabetic renal fibrosis.
Renal PEDF expression was reduced in STZ diabetic mice; however, our data show that P78-PEDF peptide treatment increased endogenous PEDF expression in diabetic mice. It is possible that some of the beneficial actions of P78-PEDF we observed in DN could be mediated by the increase in endogenous PEDF. Studies in PEDF-deficient mice will be needed to address this possibility. Another possible interpretation is that the reduction in endogenous PEDF expression is a result of DN. These two possibilities are not mutually exclusive, because a reduction in PEDF could contribute to the pathogenesis of DN and, in turn, DN could decrease PEDF, leading to further disease progression. Additional studies are needed to confirm this hypothesis.
The mechanism by which P78-PEDF peptide mediates protection in STZ-induced DN is not known; however, we speculate that P78-PEDF peptide may have a direct effect on podocytes. This hypothesis is based on two important findings. First, we show that P78-PEDF peptide restored nephrin expression in diabetic mice. Second, our previous publication (26) indicates a colocalization of PEDF with synaptopodin in the kidney. Podocytes play a key role in the maintenance of the glomerular filtration barrier (28), and normal podocyte function is intimately linked to its complex cytoskeletal architecture. Because the expression of nephrin is limited to podocytes, we questioned whether the protective effect of P78-PEDF peptide in reducing proteinuria may, in part, be due to direct effects on podocytes. Toward this goal, we examined the direct effect of P78-PEDF peptide on the maintenance of podocyte functional and structural integrity in vitro. In the current study, we used puromycin as a nonspecific insult because high glucose insults do not induce podocyte injury in vitro. Our data show that PAN disrupts the highly organized actin cytoskeleton and increases the transepithelial passage of BSA. Treatment of podocytes with P78-PEDF peptide restored both the architecture of the podocyte cytoskeleton and its functional integrity, suggesting that the observed effect of P78-PEDF peptide in vivo may be due to direct effects on podocytes. However, additional experiments are needed to demonstrate the direct effect of P78-PEDF peptide in podocytes in vivo.
In conclusion, our study demonstrates that a PEDF fragment (P78-PEDF) confers kidney protection in early phase DN. This conclusion is based on two novel observations: first, we demonstrated for the first time a beneficial effect of P78-PEDF peptide in animal models of DN; second, we showed that P78-PEDF peptide has a direct effect in maintaining podocyte functional and structural integrity. Results of this study may ultimately result in novel therapeutic interventions using P78-PEDF peptide in the treatment of diabetic kidney disease.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-077444 and DK-094930, and by the Pennsylvania Department of Health using Tobacco CURE Funds.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S.A. and J.T.-T. conception and design of research; A.S.A., T.G., A.G., H.Y., and Y.L. performed experiments; A.S.A., A.G., H.Y., T.K.C., W.B.R., and J.T.-T. analyzed data; A.S.A. and J.T.-T. interpreted results of experiments; A.S.A., A.G., H.Y., and J.T.-T. prepared figures; A.S.A. drafted manuscript; A.S.A., T.K.C., W.B.R., and J.T.-T. edited and revised manuscript; A.S.A., T.G., A.G., H.Y., Y.L., T.K.C., W.B.R., and J.T.-T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Colin J. Barnstable for helpful discussion and Wade Edris for technical assistance.
REFERENCES
- 1.Abdel-Rahman EM, Saadulla L, Reeves WB, Awad AS. Therapeutic modalities in diabetic nephropathy: standard and emerging approaches. J Gen Intern Med 27: 458–468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani A, Kelly DJ, Advani SL, Cox AJ, Thai K, Zhang Y, White KE, Gow RM, Marshall SM, Steer BM, Marsden PA, Rakoczy PE, Gilbert RE. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A 104: 14448–14453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akis N, Madaio MP. Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int 65: 2223–2227, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol 290: F828–F837, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Awad AS, Kinsey GR, Khutsishvili K, Gao T, Bolton WK, Okusa MD. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am J Physiol Renal Physiol 301: F1358–F1366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awad AS, Rouse M, Liu L, Vergis AL, Rosin DL, Linden J, Sedor JR, Okusa MD. Activation of adenosine 2A receptors preserves structure and function of podocytes. J Am Soc Nephrol 19: 59–68, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res 23: 561–577, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Breyer MD, Bottinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Brosius FC, 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T. Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 48: 2229–2239, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, Steffes MW, Toto R. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis 42: 617–622, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21: 1288–1291, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Gvritishvili AG, Leung KW, Tombran-Tink J. Codon preference optimization increases heterologous PEDF expression. PLoS One 5: e15056, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int 64: 1208–1213, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kelly DJ, Buck D, Cox AJ, Zhang Y, Gilbert RE. Effects on protein kinase C-beta inhibition on glomerular vascular endothelial growth factor expression and endothelial cells in advanced experimental diabetic nephropathy. Am J Physiol Renal Physiol 293: F565–F574, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Li H, Tran VV, Hu Y, Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp Eye Res 83: 824–833, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Liu E, Morimoto M, Kitajima S, Koike T, Yu Y, Shiiki H, Nagata M, Watanabe T, Fan J. Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J Am Soc Nephrol 18: 2094–2104, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Ren JG, Cooper WL, Hawkins CE, Cowan MR, Tong PY. Identification of the antivasopermeability effect of pigment epithelium-derived factor and its active site. Proc Natl Acad Sci U S A 101: 6605–6610, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Leo LF, McGregor C, Grivitishvili A, Barnstable CJ, Tombran-Tink J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2 (Akita) mice. Mol Med 18: 1387–1401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK, Cherrington JM. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des 15: 29–41, 2000 [PubMed] [Google Scholar]
- 24.Morris SM, Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes 60: 3015–3022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Ebihara I, Fukui M, Osada S, Nagaoka I, Horikoshi S, Tomino Y, Koide H. Messenger RNA expression for growth factors in glomeruli from focal glomerular sclerosis. Clin Immunol Immunopathol 66: 33–42, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Pina AL, Kubitza M, Brawanski A, Tombran-Tink J, Kloth S. Expression of pigment-epithelium-derived factor during kidney development and aging. Cell Tissue Res 329: 329–338, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res 102: 923–932, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol 17: 3093–3104, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Tesch GH. Role of macrophages in complications of type 2 diabetes. Clin Exp Pharmacol Physiol 34: 1016–1019, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Tombran-Tink J. PEDF in angiogenic eye diseases. Curr Mol Med 10: 267–278, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Tombran-Tink J, Aparicio S, Xu X, Tink AR, Lara N, Sawant S, Barnstable CJ, Zhang SS. PEDF and the serpins: phylogeny, sequence conservation, and functional domains. J Struct Biol 151: 130–150, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Tombran-Tink J, Barnstable CJ. Therapeutic prospects for PEDF: more than a promising angiogenesis inhibitor. Trends Mol Med 9: 244–250, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res 53: 411–414, 1991 [DOI] [PubMed] [Google Scholar]
- 35.United States Renal Data System USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Diseases in the United States. Bethesda, MD: National Institutes of Diabetes and Digestive and Kidney Diseases, 2005 [Google Scholar]
- 36.Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, Jimenez J, Velazquez H, Kashgarian M, Moeckel GW, Tufro A. Podocyte vascular endothelial growth factor (Vegf(1)(6)(4)) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia 54: 1227–1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989–999, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Wang JJ, Zhang SX, Lu K, Chen Y, Mott R, Sato S, Ma JX. Decreased expression of pigment epithelium-derived factor is involved in the pathogenesis of diabetic nephropathy. Diabetes 54: 243–250, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Wang JJ, Zhang SX, Mott R, Chen Y, Knapp RR, Cao W, Ma JX. Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol 294: F1166–F1173, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wang JJ, Zhang SX, Mott R, Knapp RR, Cao W, Lau K, Ma JX. Salutary effect of pigment epithelium-derived factor in diabetic nephropathy: evidence for antifibrogenic activities. Diabetes 55: 1678–1685, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]