Abstract
Egg activation is the series of events that transition a mature oocyte to an egg capable of supporting embryogenesis. Increasing evidence points toward phosphorylation as a critical regulator of these events. We used Drosophila melanogaster to investigate the relationship between known egg activation genes and phosphorylation changes that occur upon egg activation. Using the phosphorylation states of four proteins—Giant Nuclei, Young Arrest, Spindly, and Vap-33-1—as molecular markers, we showed that the egg activation genes sarah, CanB2, and cortex are required for the phospho-regulation of multiple proteins. We show that an additional egg activation gene, prage, regulates the phosphorylation state of a subset of these proteins. Finally, we show that Sarah and calcineurin are required for the Anaphase Promoting Complex/Cyclosome (APC/C)-dependent degradation of Cortex following egg activation. From these data, we present a model in which Sarah, through the activation of calcineurin, positively regulates the APC/C at the time of egg activation, which leads to a change in phosphorylation state of numerous downstream proteins.
Keywords: Drosophila, egg activation, phosphorylation, calcineurin, Anaphase Promoting Complex/Cyclosome (APC/C)
THE cellular events of the oocyte-to-embryo transition are collectively referred to as egg activation. These events include the resumption and completion of meiosis, polyadenylation and translation of some maternal mRNAs, and the degradation of other maternal transcripts and proteins (reviewed in Horner and Wolfner 2008; Krauchunas and Wolfner 2013). Recently we showed that, in Drosophila melanogaster, egg activation is also accompanied by a change in phosphorylation state of hundreds of proteins (Krauchunas et al. 2012). Proteomic screens in sea urchin have similarly observed large-scale changes of the phosphoproteome during fertilization/egg activation (Roux et al. 2006, 2008).
Several lines of evidence suggest the importance of these phosphorylation changes in egg activation. First, the phosphatase calcineurin and the kinase Ca2+/calmodulin-dependent kinase II (CamKII) are required for egg activation in multiple species (Tatone et al. 1999; Markoulaki et al. 2003, 2004; Liu and Maller 2005; Madgwick et al. 2005; Hansen et al. 2006; Knott et al. 2006; Mochida and Hunt 2007; Nishiyama et al. 2007; Chang et al. 2009; Backs et al. 2010; Takeo et al. 2010). Second, levels of phospho-MAPKs decrease upon egg activation in Drosophila, Xenopus, mice, and some marine invertebrates (Ferrell et al. 1991; Sanghera et al. 1991; Shibuya et al. 1992; Kubiak et al. 1993; Sackton et al. 2007). This dephosphorylation inactivates the MAPKs and may thus affect phosphorylation levels of MAPK protein targets present in the egg. Third, Young Arrest (YA) and Giant Nuclei (GNU), two Drosophila phosphoproteins known to function immediately after the oocyte-to-embryo transition, are dephosphorylated upon egg activation (Yu et al. 1999; Renault et al. 2003). However, much work remains to fully understand how these phosphorylation changes relate to the events of egg activation. We are just beginning to identify the repertoire of proteins that are phospho-modulated, and in most cases we do not know which upstream proteins regulate these phosphorylation changes.
In Drosophila, a small number of genes have been identified whose action is required for the events of egg activation. The sarah (sra) gene encodes the Drosophila calcipressin (or RCAN1), a regulator of calcineurin (Horner et al. 2006; Takeo et al. 2006, 2010). Calcineurin is a protein phosphatase composed of a catalytic A subunit and a regulatory B subunit (Rusnak and Mertz 2000). Calcineurin and its regulation by calcipressins are conserved across multiple species (Rusnak and Mertz 2000; Mehta et al. 2009). A role for calcineurin during egg activation has been shown in both Drosophila and Xenopus (Mochida and Hunt 2007; Nishiyama et al. 2007; Takeo et al. 2010). At egg activation in Drosophila, glycogen synthase kinase 3β (GSK-3β) phosphorylates sra, which in turn is hypothesized to activate calcineurin (Takeo et al. 2012). Embryos laid by sra-deficient mothers progress from the metaphase I arrest of stage 14 mature oocytes, but rearrest in anaphase of meiosis I. In addition, they have defects in maternal mRNA polyadenylation and translation (Horner et al. 2006; Takeo et al. 2006). An identical meiotic arrest is seen in germline clones lacking calcineurin or GSK-3β (Takeo et al. 2010, 2012).
Another gene that encodes a regulator of Drosophila egg activation is cortex (cort). Cort encodes a meiosis-specific Cdc20; Cdc20 is a conserved regulatory component of the Anaphase Promoting Complex/Cyclosome (APC/C) (Chu et al. 2001; Pesin and Orr-Weaver 2007). The APC/C is an E3 ubiquitin ligase that is responsible for degrading a number of maternal proteins during the oocyte-to-embryo transition (Pesin and Orr-Weaver 2008). A role for the APC/C in meiotic progression (and egg activation) is seen in Caenorhabditis elegans, where mutations in Cdc20 or other APC/C subunits cause fertilized eggs to remain arrested at metaphase of meiosis I (Furuta et al. 2000; Golden et al. 2000; Davis et al. 2002; Shakes et al. 2003, 2011; Yang et al. 2003; Dong et al. 2007; Kops et al. 2010). APC/C mutations in C. elegans are also associated with incomplete hardening of the egg shell, defects in cytoplasmic streaming, and failure to establish polarity (Furuta et al. 2000; Golden et al. 2000; Davis et al. 2002; Shakes et al. 2003; Yang et al. 2003; Dong et al. 2007). In Drosophila, embryos laid by cort mutant mothers arrest at metaphase of meiosis II (Page and Orr-Weaver 1996). Similar to sra mutants, embryos laid by cort mutant mothers have defects in maternal mRNA polyadenylation and translation, as well as defects in mRNA degradation (Lieberfarb et al. 1996; Tadros et al. 2003).
Two other genes that are required for egg activation in Drosophila are prage and wispy. The prage gene is also required for mRNA translation and degradation at the time of egg activation (Tadros et al. 2003). While the exact cell cycle arrest point of embryos laid by prage mutant mothers has not been determined, it has been reported that they do not complete meiosis (Tadros et al. 2003). Finally, the wispy gene encodes a cytoplasmic poly(A) polymerase that is required for polyadenylation and translation of proteins in the oocyte and embryo (Benoit et al. 2008; Cui et al. 2008). Meiosis is abnormal in eggs produced by wispy mutants, and embryos laid by wispy mutant mothers arrest during, or immediately after, meiosis (Benoit et al. 2008; Cui et al. 2008).
In this study we asked whether the activity of sra, cort, calcineurin, and/or prage is needed for the phosphorylation state changes of maternal proteins. Since wispy has a significant role in oocyte maturation (Cui et al. 2008) in addition to its role during egg activation, we did not include it in this analysis. Although mutations in sra, cort, and prage affect multiple aspects of egg activation, one event that is independent of their function is the dephosphorylation of MAPKs (Sackton et al. 2007). This observation led us to investigate whether other phosphorylation changes rely on these egg activation genes. We used the changes in phosphorylation state of four proteins for which we can observe different phosphorylation states on gels (GNU, YA, Spindly, and Vap-33-1) as markers of the phospho-regulation that takes place during egg activation.
GNU, YA, and Spindly are all dephosphorylated upon egg activation. GNU is a Drosophila-specific protein that is required for the assembly of the Pan Gu kinase complex (Lee et al. 2003), which is essential for chromosome condensation and the coupling of S phase and mitosis during early embryo cell cycles (Renault et al. 2003; Zhang et al. 2004). The Pan Gu kinase complex is also required for translation of Smaug, a protein that regulates maternal mRNA degradation during egg activation (Tadros et al. 2007). YA is another Drosophila-specific protein; it is necessary for progression through the first embryonic mitosis (Lin and Wolfner 1991; Sackton et al. 2009). YA’s dephosphorylation is hypothesized to allow it to disassociate from cytosolic binding partners, permitting it to enter the nucleus where it can then function (Yu et al. 2002). Spindly is a conserved cell cycle regulator that was previously shown to act during mitosis and meiosis (Griffis et al. 2007; Barisic et al. 2010; Zhang et al. 2010). In mitotic cells, Spindly associates with the Rod-Zw10-Zwilch complex and is necessary for the metaphase-to-anaphase transition (Griffis et al. 2007; Chan et al. 2009; Barisic et al. 2010). We hypothesize that Spindly may play the same role during meiosis and that dephosphorylation of Spindly may be important for the release from the metaphase arrest of mature oocytes. In contrast to the other three proteins, Vap-33-1 is phosphorylated during egg activation (Krauchunas et al. 2012). While Vap-33-1 is a conserved protein, its function in the oocyte is currently unknown in any species.
We find that during egg activation sra and cort contribute to the phospho-modulation of all four proteins that we tested. We also find that degradation of Cort, a process that is dependent on the APC/C (Pesin and Orr-Weaver 2007), fails to occur in sra mutants. In contrast, prage is required for the dephosphorylation of YA and Spindly, but not for the phosphorylation changes of GNU and Vap-33-1. Thus, we propose that sra and cort work in a common pathway that ultimately regulates the phosphorylation state of many proteins during egg activation, while prage acts downstream or in a parallel pathway to regulate a smaller subset of proteins.
Materials and Methods
Flies
Drosophila melanogaster stocks were raised on standard yeast–glucose–agar medium at room temperature on a 12:12-hr light:dark cycle. To make sarah hemizygotes (Horner et al. 2006), sra687/TM3 (Fbal0175443) were crossed to Df(3R)sbd45, mwh1 e1/TM6 (FBst0003678) or sraA426/TM3 (FBal0194825) were crossed to Df(3R)sbd45, mwh1 e1/TM3. To make cortex hemizygotes (Chu et al. 2001), cortQW55 cn1bw1/CyO, l(2)DTS5131 (Fbst0004974) were crossed to Df(2L)BSC9, w+mC/SM6a (FBst0006454). Prage mutants (Tadros et al. 2003) were prage32/prage32 homozygotes obtained from the prage32/FM6 stock. CanB2 germline clones (Takeo et al. 2010) were produced by crossing w; P{FRT}2R-G13CanB2EP(2)0774/CyO Cy females with P{hsFLP}12, y w/Y; P{FRT}2R-G13P{ovoD1}2R/CyO Cy males. Progeny from this cross were heat-shocked at 37° for 2 hr when they reached approximately the third-instar larval stage. The effects of constitutively active calcineurin (CnAact) in the germline were tested by expressing UASp-Pp2B-14Dact driven by nanos-GAL4 (Takeo et al. 2006). In all cases, balancer siblings were used as controls.
Oocytes, embryos, and protein extraction:
Ovaries or stage 14 mature oocytes were obtained from 3- to 5-day-old wild-type virgin females that had been reared on heavily yeasted food. Dissection was performed in isolation buffer, a hypertonic solution that does not activate eggs (Page and Orr-Weaver 1997). Oocytes were dissected in 1-hr blocks of time and then flash frozen in liquid nitrogen. Ovarian protein was used for Western blots in which we examined the germline-specific proteins YA and GNU, because the majority of the oocytes in ovaries of aged and yeast-fed virgin females are at stage 14 (mature oocytes), and ovarian somatic cells did not contain YA or GNU that would interfere with our analysis. Since Vap-33-1 and Spindly are expressed in somatic cells as well as in germ cells, for analysis of these proteins we used extracts of pure hand-dissected oocytes, so that our Western blots would detect only germline-expressed Vap-33-1 and Spindly. To obtain embryos, newly eclosed virgin females were aged on yeasted vials for 3–8 days and then mated to wild-type males [OregonR P2 (Allis et al. 1977)]. Mated females were allowed to deposit eggs onto petri plates containing grape juice–agar for 0- to 30-min, 0- to 1-hr, or 0- to 2-hr periods. Eggs were washed off the plates in egg wash (Karr and Alberts 1986), dechorionated in a 50% sodium hypochlorite solution for 2 min, and flash frozen in liquid nitrogen. No differences were seen in Western blot results between 0- to 30-min, 0- to 1-hr, and 0- to 2-hr samples. All phosphorylation changes were examined at least once with 0- to 30-min samples. Due to the technical difficulties of collecting enough material during 0- to 30-min collections, additional biological replicates were sometimes run with 0- to 1-hr or 0- to 2-hr samples, as noted. Degradation of Cort was examined in either 0- to 1-hr or 0- to 2-hr samples.
Samples of 40–100 mature oocytes or activated eggs were homogenized in protease-inhibiting homogenization buffer (PIHB) (Monsma and Wolfner 1988) with the addition of two phosphatase inhibitors (20 mM NaF and 10 mM β-glycerophosphate) or in extraction buffer (10 mM Tris, pH 7.5, 20 mM NaF, 2 mM EGTA, 10 mM DTT, 400 nM okadaic acid, and 2% SDS), followed by the addition of an equal amount of SDS sample buffer. Samples were boiled for 5 min and stored at –80° until use.
Immunoblotting
Proteins were separated on SDS polyacrylamide gels and subjected to Western blotting analysis as previously described (Sackton et al. 2007). Acrylamide percentage was adjusted based on the protein of interest: 7.5% for YA and Spindly, 12% for GNU, 10.6% for Vap-33-1 and Cortex. Gels for Vap-33-1 also contained 25 µM Phos-tag contained 3.5 µM Phos-tag (Wako Pure Chemical Industries, Richmond, VA) (Kinoshita et al. 2006). Primary antibodies were used at the following dilutions in 1% milk: rabbit anti-YA, 1:1000 (Liu et al. 1995); guinea pig anti-GNU, 1:5000 [gift of T. Orr-Weaver (Lee et al. 2003)]; guinea pig anti-Vap-33-1, 1:10,000 [gift of H. Bellen (Pennetta et al. 2002)]; rabbit anti-Spindly, 1:1000 [gift of R. Vale (Griffis et al. 2007)]; guinea pig anti-Cort, 1:2000 [gift of T. Orr-Weaver (Pesin and Orr-Weaver 2007)]. HRP-conjugated secondary antibodies were used at a 1:2000 dilution and visualized with the ECL Plus Western Blotting Detection system (GE Healthcare, Piscataway, NJ).
Results and Discussion
sarah regulates the phosphorylation state of multiple proteins during egg activation
We took advantage of the fact that different phosphorylation states can cause different electrophoretic mobilities on polyacrylamide gels; a more highly phosphorylated form of a protein will often have a slower mobility than a less phosphorylated form of the same protein. GNU, YA, Vap-33-1, and Spindly all have different electrophoretic mobilities when derived from wild-type embryos (or unfertilized activated eggs) relative to their mobilities when derived from wild-type mature (stage 14) oocytes. These gel mobility differences are abolished when samples are treated with a phosphatase, showing that the slower-migrating forms of these proteins are phosphorylated (Yu et al. 1999; Renault et al. 2003; Krauchunas et al. 2012). Thus, we compared the electrophoretic mobilities of these four proteins in mature oocytes and embryos from sra mutants to determine whether they underwent the typical changes that are observed upon fertilization.
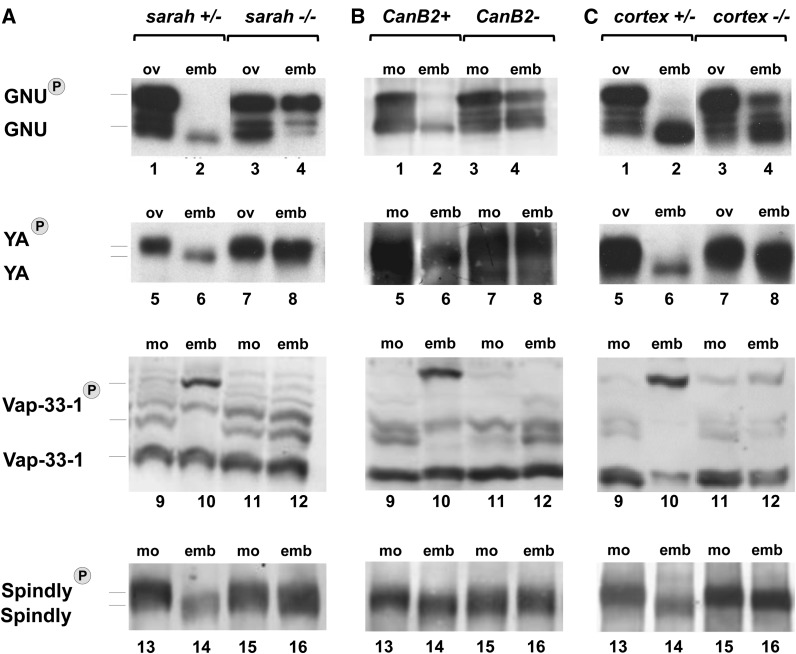
GNU, YA, Vap-33-1, and Spindly all show normal gel mobility in oocytes of sra mutants (Figure 1A, compare lanes 1 and 3, 5 and 7, 9 and 11, and 13 and 15), but in all cases lack of sra activity prevents the normal shift in gel mobility upon egg activation (Figure 1A, compare lanes 2 and 4, 6 and 8, 10 and 12, and 14 and 16). GNU mobility in embryos laid by sra mutant mothers (hereafter referred to as “sra embryos”) is comparable to that seen for mature oocytes, rather than showing the faster gel mobility observed for control embryos (Figure 1A, compare lanes 2 and 4). Similarly, the gel mobility of Vap-33-1 from sra embryos (Figure 1A, lane 12) is indistinguishable from its mobility when isolated from mature oocytes (Figure 1A, lanes 9 and 11). This is in contrast to the mobility of Vap-33-1 in heterozygous control embryos where the majority of the protein is present in the slowest-mobility band and the band of intermediate mobility is no longer observed (Figure 1A, lane 10). The gel mobilities of YA and Spindly proteins in sra embryos are intermediate between their mobilities in mature oocytes and their mobilities in control embryos (Figure 1A, compare lane 8 with lanes 6 and 7 and lane 16 with lanes 14 and 15). This intermediate mobility is consistent with these proteins being phosphorylated at multiple sites (Yu et al. 1999, 2002; Griffis et al. 2007; Barisic et al. 2010) and suggests that dephosphorylation of some, but not all, of those sites require sra. Thus, YA and Spindly are able to be partially, but not fully, dephosphorylated in the absence of sra. We also observe reduced levels of YA in control embryos, but not in sra embryos, which suggests that the dephosphorylated form of YA may be less stable than the phosphorylated form of the protein. Thus the phosphorylation state of YA may not only regulate the localization of the protein, but also target it for destruction once it has performed its function.
Figure 1.
sra, calcineurin, and cort function in the phospho-modulation of GNU, YA, Vap-33-1, and Spindly. Shown are Western blots of GNU (lanes 1–4), YA (lanes 5–8), Vap-33-1 (lanes 9–12), and Spindly (lanes 13–16) in ovaries (ov) or mature oocytes (mo) and embryos (emb) from heterozygotes (controls) and hemizygous mutants. Lane numbers are indicated at the bottom of each gel. For each protein examined, equal amounts of protein were loaded into each lane based on the number of oocytes and embryos or micrograms of protein. (A) GNU, YA, Spindly, and Vap-33-1 have normal mobilities in oocytes of sra mutants compared to controls. In sra embryos, all four proteins fail to shift to the mobilities observed in control embryos. The mobilities of GNU and Vap-33-1 are indistinguishable from those in oocytes, while YA and Spindly run at intermediate mobilities between those in control oocytes and embryos. (B) Lack of calcineurin activity in oocytes and embryos causes the same mobility phenotypes for GNU, YA, Vap-33-1, and Spindly as those seen in sra mutants. (C) GNU, YA, Spindly, and Vap-33-1 have normal mobilities in oocytes of cort mutants compared to controls. In cort embryos, all four proteins run at mobilities that are more similar to those in oocytes than in control embryos. A significant fraction of GNU protein remains phosphorylated in cort embryos and YA and Spindly run at intermediate mobilities between those in control oocytes and embryos. The oocyte-specific band of Vap-33-1 can be seen in cort embryos, although there may also be a slight increase in the fully phosphorylated form of the protein. Western blots shown are representative of three or more independent replicates.
Calcineurin is required to regulate the phosphorylation state of multiple proteins in the same manner as sarah
It has been proposed that the primary role of sra is to regulate calcineurin (Horner et al. 2006; Takeo et al. 2010, 2012). Consistent with this, loss of calcineurin function in the oocyte leads to the same meiotic arrest phenotype as in sra mutants (Takeo et al. 2010). Here we show that GNU, YA, Spindly, and Vap-33-1 have the same phosphorylation defects in embryos produced from germline clones lacking calcineurin as they do in sra embryos (Figure 1B). Without calcineurin activity, these proteins fail to change in phosphorylation state upon egg activation.
Since calcineurin activity is required for the phosphorylation changes of GNU, YA, Spindly, and Vap-33-1, we tested whether expression of constitutively active calcineurin (CnAact) was sufficient to induce any of these phosphorylation changes in the mature oocyte. Expression of CnAact in the female germline leads to female sterility and meiotic defects (Takeo et al. 2006). Instead of maintaining a metaphase I arrest, the majority of the stage 14 oocytes from these females have abnormal nuclei with dispersed chromatin (Takeo et al. 2006). YA is either partially or fully dephosphorylated in mature oocytes of female flies that express CnAact in their germlines (Supporting Information, Figure S1A, compare lanes 3 and 7 to lanes 1 and 5). This suggests that either the activity of calcineurin is sufficient to dephosphorylate YA or misexpression of calcineurin during oogenesis prevents YA from being properly phosphorylated. In addition, we are unable to detect YA in embryos laid by CnAact-expressing females, supporting the hypothesis that the dephosphorylated form of YA is less stable than the phosphorylated form of the protein. We also find that GNU can be partially dephosphorylated in the ovaries of females expressing CnAact in the female germline (Figure S1A, compare lanes 9 and 11).
In contrast to the findings with YA and GNU, the phosphorylation states of Spindly and Vap-33-1 were unaffected by the expression of CnAact in mature oocytes and embryos (Figure S1A). The mobilities of these proteins are the same in mature oocytes expressing CnAact and control oocytes (Figure S1A, compare lanes 13 and 15, and 17 and 19). The phosphorylation changes of GNU, Spindly, and Vap-33-1 that take place at egg activation were also unaffected by expression of CnAact (Figure S1A, compare lanes 10 and 12, 14 and 16, and 18 and 20). One explanation for why active calcineurin does not induce phosphorylation changes for Spindly and Vap-33-1 is that other factors, active in the embryo, are not yet active in the mature oocyte. If calcineurin requires other proteins to bind or recruit its targets, then these additional factors must also be activated for calcineurin to exert its function. If these proteins are held in an inactive state in the mature oocyte, then we may fail to see an effect of constitutively active calcineurin in the oocyte. sra is phosphorylated upon egg activation, and this phosphorylation is required for its activity (Takeo et al. 2010, 2012). Thus, if sra (or additional unidentified regulators) is required for calcineurin to recognize or bind its targets, and sra is not active in the mature oocyte, then even constitutively active calcineurin may not be able to dephosphorylate its targets until egg activation.
cortex is required for the phosphorylation state changes of GNU, YA, Spindly, and Vap-33-1 upon egg activation
Cortex (cort) mutants show defects in multiple egg activation events (Lieberfarb et al. 1996; Page and Orr-Weaver 1996; Tadros et al. 2003; Pesin and Orr-Weaver 2008). We tested whether cortex is also required for any of the phosphorylation changes that take place at egg activation. We found that embryos laid by cort mutant mothers (“cort embryos”) have defects in the dephosphorylation of GNU, YA, and Spindly, as well as in the phosphorylation of Vap-33-1 (Figure 1C). Similar to what is seen in sra embryos, YA and Spindly from cort embryos have mobilities intermediate between the mobilities of the proteins from either oocytes or control embryos, indicating that partial dephosphorylation takes place (Figure 1C, compare lane 8 with lanes 6 and 7, and lane 16 with lanes 14 and 15). Likewise, cort embryos retain a proportion of their GNU in its phosphorylated form. But unlike in sra embryos, a significant fraction of GNU protein is still dephosphorylated in the absence of Cort (Figure 1C, lane 4). In addition, cort embryos retain the mature oocyte specific phospho-state of Vap-33-1, but also show a small increase in the slowest-mobility form of the protein (Figure 1C, lane 12). Thus, the phosphorylation defects in cort embryos are less severe than those seen in sra or calcineurin mutants.
The finding that GNU, YA, Spindly, and Vap-33-1 all require both sra and cort for their phosphorylation changes upon egg activation suggests that sra and cort might work in a common pathway to regulate the phosphorylation of these, and possibly other, proteins. That the defects in cort embryos are not as complete as in sra embryos led us to propose that cort acts downstream of sra. However, it is important to note that the cort mutation is a single point mutation and though it has been proposed to be a functional null, it is possible that this allele retains some function (Chu et al. 2001; Pesin and Orr-Weaver 2007). In addition, the canonical Cdc20, fizzy, is also present in the Drosophila oocyte and embryo (Swan and Schüpbach 2007). Both of these factors could contribute to our finding that the defects in phosphorylation changes are less severe in cort embryos than in sra embryos.
sarah is required for Cort degradation after egg activation
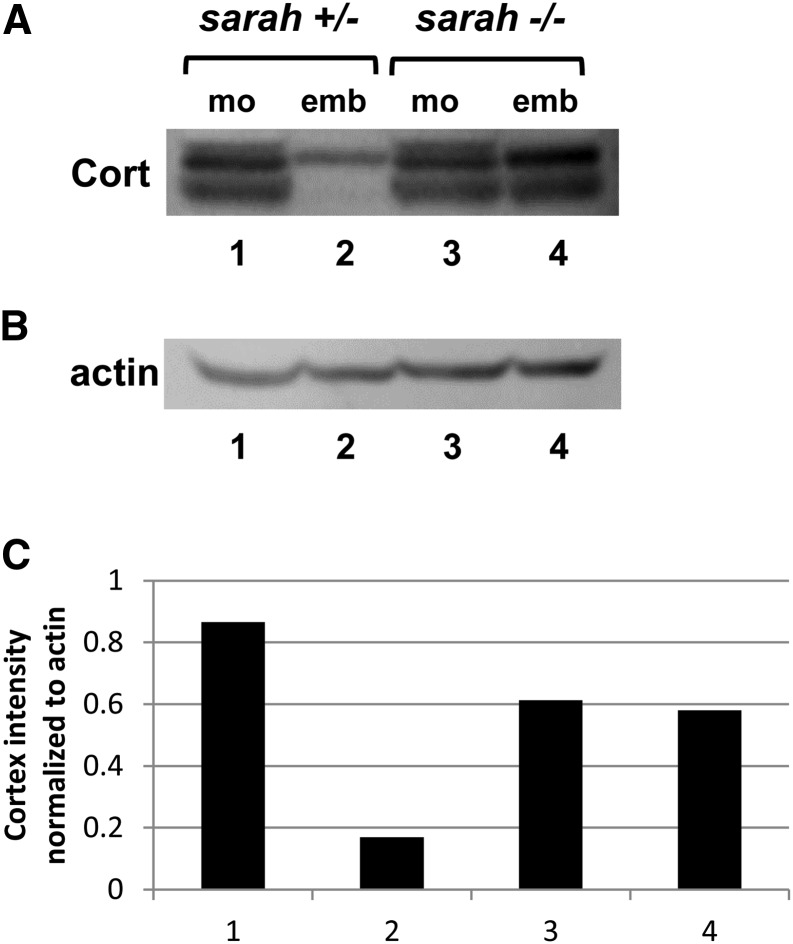
Our hypothesis that sra acts upstream of cort is supported by the fact that in Xenopus, Cdc20 is dephosphorylated during egg activation and this dephosphorylation requires calcineurin (Mochida and Hunt 2007). Drosophila Cort, a meiosis-specific Cdc20, is degraded by the APC/C upon egg activation (Pesin and Orr-Weaver 2007). We looked at whether Cort is degraded in sra embryos. We found that sra is required for complete Cort degradation after egg activation (Figure 2). In control embryos, Cort is present at <45% of the levels observed in control mature oocytes and the lower band of the doublet is completely absent. In contrast, in sra embryos the amount of Cort protein is >70% of the amount observed in sra mature oocytes and the lower band of the doublet is still present (Figure 2A). We also found that Cort degradation is incomplete in the absence of calcineurin (Figure S2), supporting the model that sra regulates Cort degradation through the activation of calcineurin. Additionally, we found that when calcineurin is constitutively active in the female germline, Cort is nearly undetectable in the ovary or mature oocytes (Figure S1B).
Figure 2.
Cortex is not degraded in sra embryos. (A and B) Western blot showing Cort (A, doublet) and actin loading control (B) in mature oocytes (mo) and embryos (emb) from sra heterozygotes (controls) and sra hemizygous mutants. Equal amounts of protein were loaded into each lane based on the number of oocytes and embryos. Levels of Cort appear similar in control and sra oocytes, but remain high in sra embryos compared to controls. (C) Levels of Cort for the Western blot shown in A were quantified by normalizing to actin. Numbers on the x-axis correspond to the lane numbers in A. Results shown are representative of three independent biological replicates. While the high levels of Cortex in sra embryos relative to control embryos were repeatedly seen in all three independent replicates, the decreased levels of Cortex in sra oocytes relative to control oocytes were unique to the replicate shown.
Since the degradation of Cort is dependent on the function of the APC/C, this could mean that the APC/C is not fully functional in the absence of sra or calcineurin. sra could regulate APC/C activity through Cort or other subunits of the complex. In such a model, regulation of the APC/C by both sra and Cort could explain the overlapping requirements of sra and cort for the phospho-regulation of GNU, YA, Spindly, and Vap-33-1. Alternatively, sra could regulate Cort stability by targeting it for degradation. Our finding that Cort is not present in CnAact oocytes suggests that calcineurin activity is sufficient to activate the APC/C and leads to our model that calcineurin positively regulates APC/C activity at egg activation.
If our hypothesis is correct that cort acts downstream of sra, the defects observed in sra embryos might then be due to misregulation of the APC/C and at least one other protein/pathway to result in the complete sra phenotype. This scenario would also imply none of the four proteins that we analyzed (GNU, YA, Spindly, and Vap-33-1) are direct targets of calcineurin. Alternatively, it is possible that calcineurin directly dephosphorylates GNU, YA, or Spindly and that cort (through the APC/C) is necessary to inhibit the kinase(s) that phosphorylate these proteins in the oocyte.
prage is required only for a subset of phosphorylation changes at egg activation
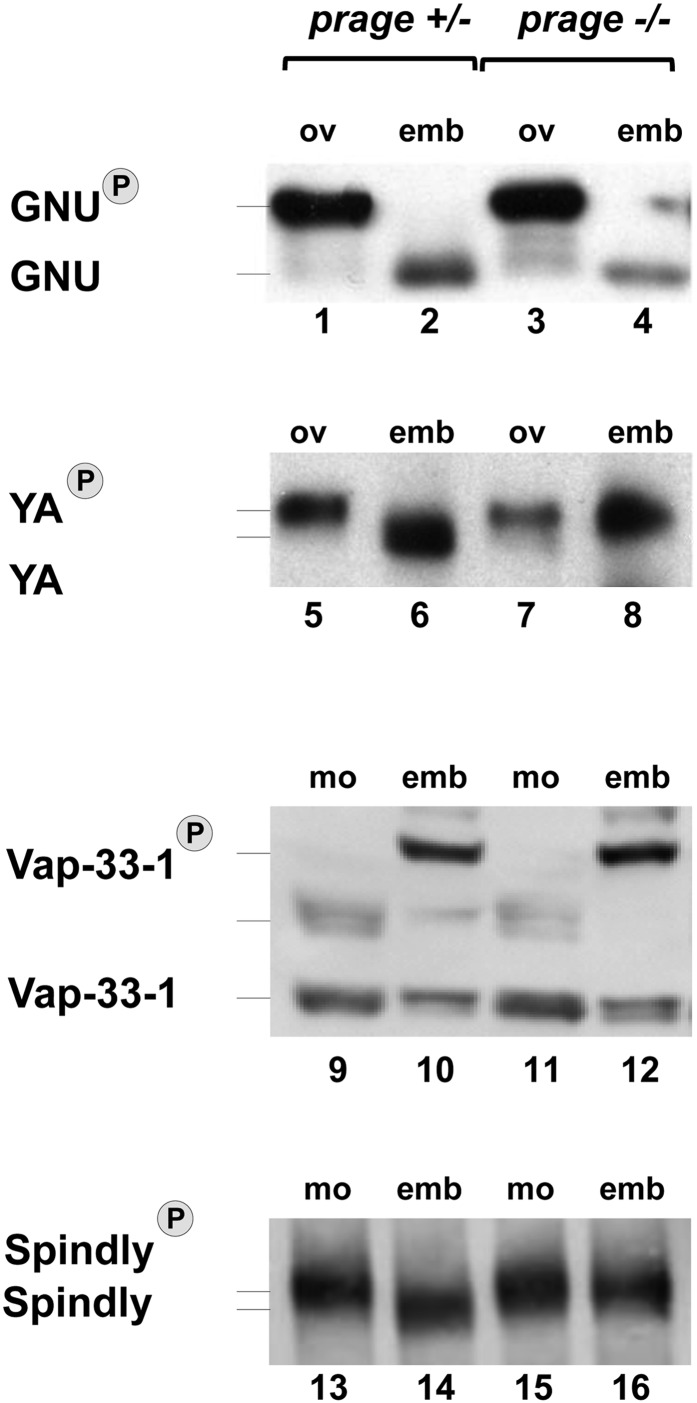
We next asked whether prage acts in the same pathway as sra and cort to regulate the phosphorylation states of GNU, YA, Spindly, and Vap-33-1 during egg activation. We find that YA and Spindly have the same mobilities in embryos laid by prage mutant mothers (“prage embryos”), as is observed for prage mature oocytes (Figure 3, compare lanes 7 and 8, and 15 and 16), although for YA this is a slightly lower mobility than seen for YA from control oocytes (Figure 3, compare lanes 15 and 16 with lane 13). Thus, like sra and cort, prage is required for dephosphorylation of YA and Spindly during egg activation. In addition, the failure to dephosphorylate both proteins is more complete than in either sra or cort embryos where intermediate mobilities were observed.
Figure 3.
prage is required for YA and Spindly dephosphorylation, but not for phospho-modulation of GNU or Vap-33-1. For each protein examined equivalent amounts of protein were loaded into each lane. No differences are seen in GNU (lanes 1–4) or Vap-33-1 (lanes 9–12) mobilities between prage mutant and control mature oocytes (mo) or between prage embryos and control embryos (emb). The mobilities of YA (lanes 5–8) and Spindly (lanes 13–16) in prage embryos are the same as those seen in prage oocytes. These proteins fail to shift to the mobilities seen in control embryos. Western blots shown are representative of three or more independent replicates. Lane numbers are indicated at the bottom of each gel. The grey lines mark the mobilities of the different protein phospho-states.
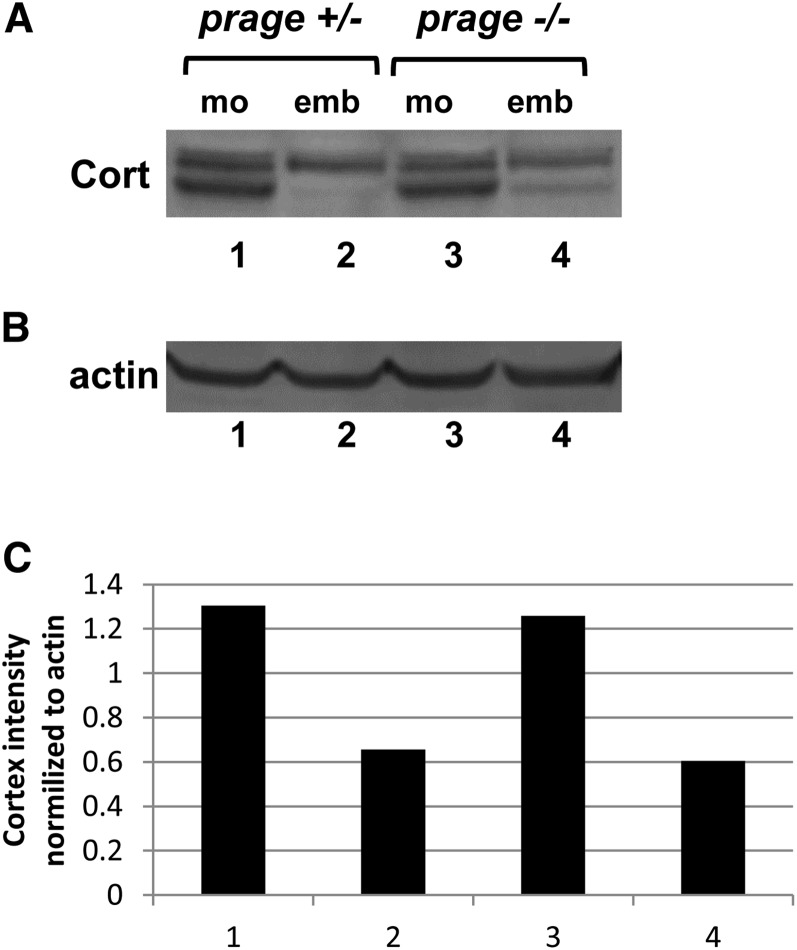
In contrast, prage does not regulate the phosphorylation states of GNU and Vap-33-1, as the mobilities of GNU and Vap-33-1 from prage embryos are indistinguishable from those observed for these proteins from control embryos (Figure 3, compare lanes 2 and 4, and 10 and 12). These results show that there are at least two subsets of phosphorylation changes that are regulated during egg activation, prage-dependent and prage-independent. These findings also suggest that prage works downstream of, or in parallel to, the sra-cort pathway to regulate the prage-dependent subset of phosphorylated proteins. If prage were upstream of sra and cortex, we would expect that prage would also be required for the phosphorylation changes of GNU and Vap-33-1 that take place at egg activation. Consistent with prage being downstream of or parallel with sra and cort, we find that Cort is degraded in prage embryos (Figure 4). In control embryos, Cort is present at ∼50% of the levels observed in mature oocytes and in prage embryos we also observed 50% of the levels observed in mature oocytes (Figure 4B). However, the lower band of the Cort doublet is still observed (although highly reduced) in prage embryos, whereas it is absent from control embryos. Thus, we cannot rule out the possibility that prage plays a role in the regulation of the APC/C.
Figure 4.
Cortex degradation is independent of prage. (A and B) Western blot showing Cort (A, doublet) and actin loading control (B) in mature oocytes and embryos from prage heterozygotes (controls) and prage hemizygous mutants. Equal amounts of protein were loaded into each lane based on the number of oocytes and embryos. Total levels of Cort appear similar between control and prage oocytes and between prage and control embryos. However, the lower band of the Cortex doublet can still be observed, although at highly reduced levels, in prage embryos while it is completely absent from control embryos. (C) Levels of Cort for the Western blot shown in A were quantified by normalizing to actin. Numbers on the x-axis correspond to the lane numbers in A. Results shown are representative of three independent biological replicates.
Conclusions
Large-scale phosphorylation changes are observed at the time of egg activation/fertilization in Drosophila (Krauchunas et al. 2012) and in sea urchins (Roux et al. 2006, 2008). In Xenopus and mouse, phosphorylation regulators such as CaMKII and calcineurin are critical effectors of egg activation events (Tatone et al. 1999; Markoulaki et al. 2004; Liu and Maller 2005; Madgwick et al. 2005; Hansen et al. 2006; Knott et al. 2006; Mochida and Hunt 2007; Nishiyama et al. 2007; Chang et al. 2009; Backs et al. 2010). However, we still have a limited understanding of which genes and enzymes regulate the phosphorylation changes at egg activation.
We examined the role of three Drosophila egg activation genes, sra, cort, and prage, in regulating the phosphorylation state of four proteins: GNU, YA, Spindly, and Vap-33-1. We find that sra is required for all four of these proteins to change in phosphorylation state upon egg activation. Since we observe an identical phenotype in calcineurin-deficient germline clones, the effects we observed are most likely due to the misregulation of calcineurin in the absence of sra function.
The fact that GNU fails to be dephosphorylated in sra mutants begins to link phosphorylation changes with functional consequences. GNU acts within the PNG kinase complex (Lee et al. 2003), which is necessary for the translation of Smaug upon egg activation (Tadros et al. 2007). It has been shown previously that Smaug is not translated in sra embryos (Cui et al. 2008); however, no mechanism for how sra affects Smaug translation had been proposed. If GNU dephosphorylation is required for its activity, our finding that GNU is not dephosphorylated in sra embryos provides one possible explanation for why Smaug translation requires sra function.
We find that cort also regulates the phosphorylation state of GNU, YA, Spindly, and Vap-33-1, although cort mutants show a less severe phenotype in this trait than do sra embryos. We also show that the degradation of Cort requires sra. From these data we propose a model (Figure 5) in which sra and calcineurin act upstream of cort (and the APC/C), as well as additional unknown factors, to regulate the phosphorylation state of multiple proteins. In contrast, prage regulates only a subset of these phosphorylation changes; YA and Spindly fail to be dephosphorylated in prage embryos, but GNU and Vap-33-1 are unaffected by a lack of prage. In addition, we find that Cort is still degraded in the absence of prage. Therefore, we hypothesize that prage acts downstream of, or in parallel to, sra and cort as an additional regulator of only a subset of the sra-cort targets. Additional work will be necessary to clarify where prage acts in relation to this pathway.
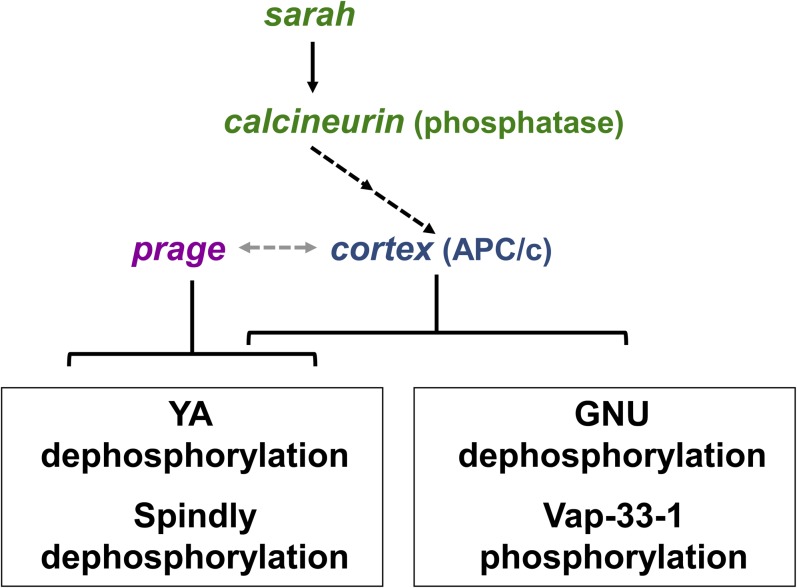
Figure 5.
Summary of regulatory relationships among genes controlling protein phospho-modulation at egg activation. The relationships between gene activities and protein phosphorylation observed in our study are summarized; dashed arrows represent genetic interactions but do not necessarily imply a direct effect. The arrow between prage and cortex is fainter to indicate less certainty about their interaction, although data in this article suggest such an interaction. Our results show that sarah (a calcineurin regulator) and calcineurin act in a single pathway with cortex; prage acts downstream or in a parallel pathway. The subsets of proteins whose phosphorylation state is regulated by each of these genes/pathways are indicated in boxes.
Thus, we have shown a link between known egg activation genes and phospho-regulation during egg activation and have begun to place these genes into a common pathway. It is possible that the interactions that we report act to regulate critical kinases or phosphatases that act on YA, GNU, Vap-33-1, and Spindly. Some of these target enzymes are suggested by sequences within the proteins we assayed. For example, both GNU and YA contain predicted MAPK sites (Yu et al. 1999, 2002; Renault et al. 2003; Zhang et al. 2004). YA is also phosphorylated at a predicted GSK-3 site (K. L. Sackton and M. F. Wolfner, unpublished data) and Spindly contains seven consensus Cdk1 sites (Griffis et al. 2007). Since MAPK activity decreases normally in sra and cort embryos (Sackton et al. 2007), either there are additional kinases that phosphorylate GNU or activation of at least one phosphatase is required for the dephosphorylation of GNU.
Our data are also consistent with an alternative interpretation: that the phosphorylation changes of GNU, YA, Spindly, and Vap-33-1 might require progression past a particular stage of the meiotic cell cycle. As losses of sra, CanB2, prage, and cort all result in cell cycle arrest prior to the completion of meiosis (Page and Orr-Weaver 1996; Tadros et al. 2003; Horner et al. 2006; Takeo et al. 2006, 2010), the eggs laid by these mutants could arrest prior to the stage at which the phosphorylation changes are triggered. The earlier arrest of sra mutants compared to cort mutants may also explain why the phosphorylation defects seen in sra mutants are more severe than those seen in cort mutants. However, the earlier arrest of sra mutant embryos still suggests that sra and calcineurin act prior to cort. In addition, the requirement of both sra and cort for the phospho-regulation of all four proteins examined indicates that these genes are working in the same regulatory network.
Changes in the phosphorylation state of many proteins at once can rapidly alter molecular and cellular properties and evidence suggests that phosphorylation changes are a key aspect of egg activation (reviewed in Krauchunas and Wolfner 2013). Therefore, it is important that we figure out the pathways that lead to the global phosphorylation changes taking place at this time. We have shown that the conserved proteins, calcineurin and Cdc20, are upstream of multiple phosphorylation events during egg activation. The changes in phosphorylation could potentially affect the activity or localization of the target proteins. They could also affect the stability of those proteins, marking them for degradation, or regulating the APC/C and/or the proteasome. Our finding that mutation of the APC/C subunit Cort affects these phosphorylation changes, in addition to the defects in protein degradation observed by others (Pesin and Orr-Weaver 2007; Swan and Schupbach 2007), suggests a potential link between phosphorylation and protein degradation in cell cycle progression and other egg activation events. By understanding how proteins work together to alter the phosphoproteome of the oocyte, we will gain important insight into how the events of egg activation are coordinated to achieve the oocyte-to-embryo transition.
Supplementary Material
Acknowledgments
We thank T. Aigaki, S. Takeo, R. S. Hawley, H. Lipshitz, and the Bloomington Stock Center for fly lines. Antibodies were kindly provided by T. Orr-Weaver, H. Bellen, R. Vale, and J. Raff. We thank M. Goldberg, A. Clark, M. Roberson, W. L. Kraus, and anonymous reviewers for helpful comments on the manuscript. National Institutes of Health (NIH) grant R01-GM044659 (to M.F.W.) provided support along with NIH training grant T32-GM07273, which provided partial support (to A.R.K. and K.L.S.).
Footnotes
Communicating editor: L. Cooley
Literature Cited
- Allis C. D., Waring G. L., Mahowald A. P., 1977. Mass isolation of pole cells from Drosophila melanogaster. Dev. Biol. 56: 372–381. [DOI] [PubMed] [Google Scholar]
- Backs J., Stein P., Backs T., Duncan F. E., Grueter C. E., et al. , 2010. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc. Natl. Acad. Sci. USA 107: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisic M., Sohm B., Mikolcevic P., Wandke C., Rauch V., et al. , 2010. Spindly/CCDC99 is required for efficient chromosome congression and mitotic checkpoint regulation. Mol. Biol. Cell 21: 1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Papin C., Kwak J. E., Wickens M., Simonelig M., 2008. PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila. Development 135: 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. W., Fava L. L., Uldschmid A., Schmitz M. H., Gerlich D. W., et al. , 2009. Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J. Cell Biol. 185: 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. Y., Minahan K., Merriman J. A., Jones K. T., 2009. Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development 136: 4077–4081. [DOI] [PubMed] [Google Scholar]
- Chu T., Henrion G., Haegeli V., Strickland S., 2001. Cortex, a Drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/fizzy protein family. Genesis 29: 141–152. [DOI] [PubMed] [Google Scholar]
- Cui J., Sackton K. L., Horner V. L., Kumar K. E., Wolfner M. F., 2008. Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation. Genetics 178: 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. S., Wille L., Chestnut B. A., Sadler P. L., Shakes D. C., et al. , 2002. Multiple subunits of the Caenorhabditis elegans anaphase-promoting complex are required for chromosome segregation during meiosis I. Genetics 160: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Bogdanova A., Habermann B., Zachariae W., Ahringer J., 2007. Identification of the C. elegans anaphase promoting complex subunit Cdc26 by phenotypic profiling and functional rescue in yeast. BMC Dev. Biol. 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S., 1991. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol. Cell. Biol. 11: 1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T., Tuck S., Kirchner J., Koch B., Auty R., et al. , 2000. EMB-30: an APC4 homologue required for metaphase-to-anaphase transitions during meiosis and mitosis in Caenorhabditis elegans. Mol. Biol. Cell 11: 1401–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Sadler P. L., Wallenfang M. R., Schumacher J. M., Hamill D. R., et al. , 2000. Metaphase to anaphase (mat) transition-defective mutants in Caenorhabditis elegans. J. Cell Biol. 151: 1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E. R., Stuurman N., Vale R. D., 2007. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J. Cell Biol. 177: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. V., Tung J. J., Jackson P. K., 2006. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc. Natl. Acad. Sci. USA 103: 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner V. L., Wolfner M. F., 2008. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev. Dyn. 237: 527–544. [DOI] [PubMed] [Google Scholar]
- Horner V. L., Czank A., Jang J. K., Singh N., Williams B. C., et al. , 2006. The Drosophila calcipressin sarah is required for several aspects of egg activation. Curr. Biol. 16: 1441–1446. [DOI] [PubMed] [Google Scholar]
- Huang J., Raff J. W., 1999. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 18: 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr T. L., Alberts B. M., 1986. Organization of the cytoskeleton in early Drosophila embryos. J. Cell Biol. 102: 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T., 2006. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5: 749–757. [DOI] [PubMed] [Google Scholar]
- Knott J. G., Gardner A. J., Madgwick S., Jones K. T., Williams C. J., et al. , 2006. Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev. Biol. 296: 388–395. [DOI] [PubMed] [Google Scholar]
- Kops G. J., van der Voet M., Manak M. S., van Osch M. H., Naini S. M., et al. , 2010. APC16 is a conserved subunit of the anaphase-promoting complex/cyclosome. J. Cell Sci. 123: 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas A. R., Wolfner M. F., 2013. Molecular events of egg activation. Curr. Top. Dev. Biol. 102: 267–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas A. R., Horner V. L., Wolfner M. F., 2012. Protein phosphorylation changes reveal new candidates in the regulation of egg activation and early embryogenesis in D. melanogaster. Dev. Biol. 370: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak J. Z., Weber M., de Pennart H., Winston N. J., Maro B., 1993. The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J. 12: 3773–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. A., Van Hoewyk D., Orr-Weaver T. L., 2003. The Drosophila cell cycle kinase PAN GU forms an active complex with PLUTONIUM and GNU to regulate embryonic divisions. Genes Dev. 17: 2979–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberfarb M. E., Chu T., Wreden C., Theurkauf W., Gergen J. P., et al. , 1996. Mutations that perturb poly(A)-dependent maternal mRNA activation block the initiation of development. Development 122: 579–588. [DOI] [PubMed] [Google Scholar]
- Lin H. F., Wolfner M. F., 1991. The Drosophila maternal-effect gene fs(1)Ya encodes a cell cycle-dependent nuclear envelope component required for embryonic mitosis. Cell 64: 49–62. [DOI] [PubMed] [Google Scholar]
- Liu J., Maller J. L., 2005. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 15: 1458–1468. [DOI] [PubMed] [Google Scholar]
- Liu J., Song K., Wolfner M. F., 1995. Mutational analyses of fs(1)Ya, an essential, developmentally regulated, nuclear envelope protein in Drosophila. Genetics 141: 1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S., Levasseur M., Jones K. T., 2005. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J. Cell Sci. 118: 3849–3859. [DOI] [PubMed] [Google Scholar]
- Markoulaki S., Matson S., Abbott A. L., Ducibella T., 2003. Oscillatory CaMKII activity in mouse egg activation. Dev. Biol. 258: 464–474. [DOI] [PubMed] [Google Scholar]
- Markoulaki S., Matson S., Ducibella T., 2004. Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev. Biol. 272: 15–25. [DOI] [PubMed] [Google Scholar]
- Mehta S., Li H., Hogan P. G., Cunningham K. W., 2009. Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol. Cell. Biol. 29: 2777–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Hunt T., 2007. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449: 336–340. [DOI] [PubMed] [Google Scholar]
- Monsma S. A., Wolfner M. F., 1988. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 2: 1063–1073. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Yoshizaki N., Kishimoto T., Ohsumi K., 2007. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature 449: 341–345. [DOI] [PubMed] [Google Scholar]
- Page A. W., Orr-Weaver T. L., 1996. The Drosophila genes grauzone and cortex are necessary for proper female meiosis. J. Cell Sci. 109(Pt 7): 1707–1715. [DOI] [PubMed] [Google Scholar]
- Page A. W., Orr-Weaver T. L., 1997. Activation of the meiotic divisions in Drosophila oocytes. Dev. Biol. 183: 195–207. [DOI] [PubMed] [Google Scholar]
- Pennetta G., Hiesinger P. R., Fabian-Fine R., Meinertzhagen I. A., Bellen H. J., 2002. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron 35: 291–306. [DOI] [PubMed] [Google Scholar]
- Pesin J. A., Orr-Weaver T. L., 2007. Developmental role and regulation of cortex, a meiosis-specific anaphase-promoting complex/cyclosome activator. PLoS Genet. 3: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesin J. A., Orr-Weaver T. L., 2008. Regulation of APC/C activators in mitosis and meiosis. Annu. Rev. Cell Dev. Biol. 24: 475–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault A. D., Zhang X. H., Alphey L. S., Frenz L. M., Glover D. M., et al. , 2003. giant nuclei is essential in the cell cycle transition from meiosis to mitosis. Development 130: 2997–3005. [DOI] [PubMed] [Google Scholar]
- Roux M., Townley I., Raisch M., Reade A., Bradham C., et al. , 2006. A functional genomic and proteomic perspective of sea urchin calcium signaling and egg activation. Dev. Biol. 300: 416–433. [DOI] [PubMed] [Google Scholar]
- Roux M. M., Radeke M. J., Goel M., Mushegian A., Foltz K. R., 2008. 2DE identification of proteins exhibiting turnover and phosphorylation dynamics during sea urchin egg activation. Dev. Biol. 313: 630–647. [DOI] [PubMed] [Google Scholar]
- Rusnak F., Mertz P., 2000. Calcineurin: form and function. Physiol. Rev. 80: 1483–1521. [DOI] [PubMed] [Google Scholar]
- Sackton K. L., Buehner N. A., Wolfner M. F., 2007. Modulation of MAPK activities during egg activation in Drosophila. Fly 1: 222–227. [DOI] [PubMed] [Google Scholar]
- Sackton K. L., Lopez J. M., Berman C. L., Wolfner M. F., 2009. YA is needed for proper nuclear organization to transition between meiosis and mitosis in Drosophila. BMC Dev. Biol. 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Pelech S. L., 1991. Role of protein phosphorylation in the maturation-induced activation of a myelin basic protein kinase from sea star oocytes. J. Biol. Chem. 266: 6700–6707. [PubMed] [Google Scholar]
- Shakes D. C., Sadler P. L., Schumacher J. M., Abdolrasulnia M., Golden A., 2003. Developmental defects observed in hypomorphic anaphase-promoting complex mutants are linked to cell cycle abnormalities. Development 130: 1605–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakes D. C., Allen A. K., Albert K. M., Golden A., 2011. emb-1 encodes the APC16 subunit of the Caenorhabditis elegans anaphase-promoting complex. Genetics 189: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya E. K., Boulton T. G., Cobb M. H., Ruderman J. V., 1992. Activation of p42 MAP kinase and the release of oocytes from cell cycle arrest. EMBO J. 11: 3963–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A., Schupbach T., 2007. The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila. Development 134: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W., Houston S. A., Bashirullah A., Cooperstock R. L., Semotok J. L., et al. , 2003. Regulation of maternal transcript destabilization during egg activation in Drosophila. Genetics 164: 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W., Goldman A. L., Babak T., Menzies F., Vardy L., et al. , 2007. SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev. Cell 12: 143–155. [DOI] [PubMed] [Google Scholar]
- Takeo S., Tsuda M., Akahori S., Matsuo T., Aigaki T., 2006. The calcineurin regulator sra plays an essential role in female meiosis in Drosophila. Curr. Biol. 16: 1435–1440. [DOI] [PubMed] [Google Scholar]
- Takeo S., Hawley R. S., Aigaki T., 2010. Calcineurin and its regulation by sra/RCAN is required for completion of meiosis in Drosophila. Dev. Biol. 344: 957–967. [DOI] [PubMed] [Google Scholar]
- Takeo S., Swanson S. K., Nandanan K., Nakai Y., Aigaki T., et al. , 2012. Shaggy/glycogen synthase kinase 3beta and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc. Natl. Acad. Sci. USA 109: 6382–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatone C., Iorio R., Francione A., Gioia L., Colonna R., 1999. Biochemical and biological effects of KN-93, an inhibitor of calmodulin-dependent protein kinase II, on the initial events of mouse egg activation induced by ethanol. J. Reprod. Fertil. 115: 151–157. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., McNally K., McNally F. J., 2003. MEI-1/katanin is required for translocation of the meiosis I spindle to the oocyte cortex in C. elegans. Dev. Biol. 260: 245–259. [DOI] [PubMed] [Google Scholar]
- Yu J., Liu J., Song K., Turner S. G., Wolfner M. F., 1999. Nuclear entry of the Drosophila melanogaster nuclear lamina protein YA correlates with developmentally regulated changes in its phosphorylation state. Dev. Biol. 210: 124–134. [DOI] [PubMed] [Google Scholar]
- Yu J., Garfinkel A. B., Wolfner M. F., 2002. Interaction of the essential Drosophila nuclear protein YA with P0/AP3 in the cytoplasm and in vitro: implications for developmental regulation of YA’s subcellular location. Dev. Biol. 244: 429–441. [DOI] [PubMed] [Google Scholar]
- Zhang Q. H., Wei L., Tong J. S., Qi S. T., Li S., et al. , 2010. Localization and function of mSpindly during mouse oocyte meiotic maturation. Cell Cycle 9: 2230–2236. [DOI] [PubMed] [Google Scholar]
- Zhang X. H., Axton J. M., Drinjakovic J., Lorenz L., White-Cooper H., et al. , 2004. Spatial and temporal control of mitotic cyclins by the Gnu regulator of embryonic mitosis in Drosophila. J. Cell Sci. 117: 3571–3578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.