SUMMARY
The mouse whipworm Trichuris muris has long been used as a tractable model of human Trichuriasis. Here we look back at the history of T. muris research; from the definition of the species and determination of its life cycle, through to the complex immune responses that we study today. We highlight the key research papers that have developed our understanding of immune responses to this parasite, and reflect on how original concepts have been transformed, as our knowledge of immunology has grown. Although we have a good understanding of host–parasite interactions in the context of the underlying cellular immunology, there are still many aspects of the biology of the Trichuris parasite that remain undefined. We predict that advances in parasite biology will be key in the future development of new and improved treatments for Trichuriasis.
Key words: Trichuris muris, helminth, nematode, parasite, mouse
THE ORIGIN OF THE SPECIES
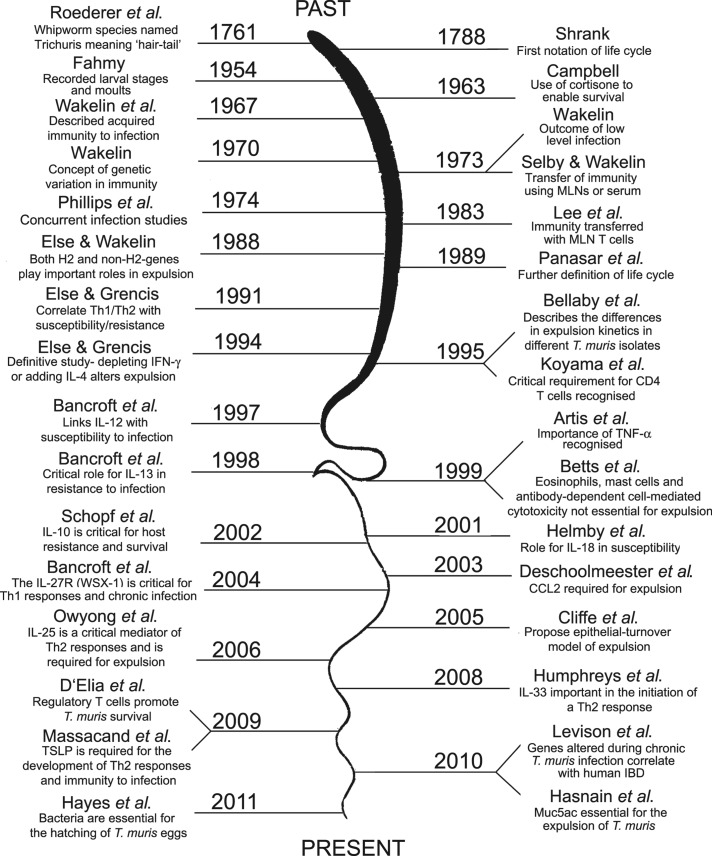
Whipworms are intestinal dwelling nematode parasites of mammals. With a distinctive morphology comprising a long thin anterior end – the stichosome – which embeds in the gut epithelial cells of its host, and a bulbous posterior end, the generic name for all whipworm species is Trichuris or ‘hair tail’. The name Trichuris was applied by Roederer in 1761, mistakenly identifying the thread-like head end of the parasite as the tail. There are over 70 species of Trichuris recognized, including parasites of medical, veterinary and scientific interest. The human whipworm is one of man's ‘heirloom parasites’ with man and worm co-evolving together. Evidence of an association between whipworm and ancient man dates back over 6000 years with whipworm eggs found in ancient archaeological sites of both the Old and New World (Araujo et al. 2008). We focus here on over half a century of research using Trichuris muris in the mouse. We reflect on the early pioneering studies and interesting interpretations of datasets made in the absence of a clear understanding of the cellular immune responses to infection. We define milestones in Trichuris research (summarized in Fig. 1), as our understanding of immunology was revolutionized by the concept of T helper cell subsets.
Fig. 1.
Milestones in T. muris research. The timeline shows an overview of some of the key research papers that have been published on T. muris throughout the years.
The early studies on Trichuris infections in mice were conducted by parasitologists interested in studying the parasite, its life cycle and parasite biology. Later, T. muris infections in the laboratory mouse were used by parasite immunologists to understand host–parasite interactions at an immunological level, with a long-term vision of developing vaccines to promote resistance to infection. Basic immunologists also recognized the usefulness of gut-dwelling worm infections as tools to probe the immune system, exploring more fundamental immunological questions. Recently, there has been a return to understanding parasite biology and the functions of parasite molecules. This resurgence reflects both the lack of lead antigens to include in vaccines, and an interest in identifying parasite immunomodulatory molecules that may have therapeutic potential for inflammatory diseases of the developed world.
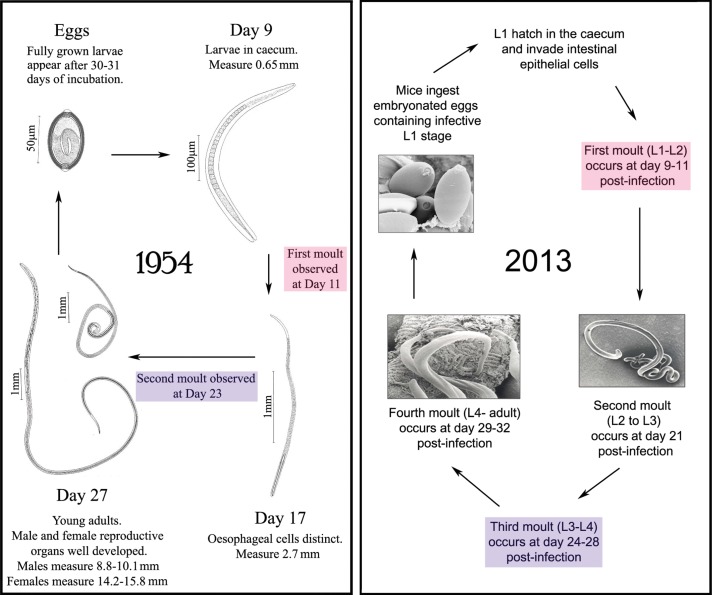
From a parasitological point of view, the life cycle of the Trichuris species of parasite took many years to describe accurately. An understanding of embryonation of Trichuris spp. eggs dates back to Davaine (1858) with Fahmy (1954) including a definition of the conditions required for successful development of the first larval stage within the egg (Davaine, 1858; Fahmy, 1954). These observations have relevance today, with laboratories around the world that maintain the Trichuris life cycle discovering by chance that slightly acidic water impairs embryonation and results in failed egg cultures. There were early misconceptions about the life cycle, including descriptions of migratory phases, before Fahmy (1954) detailed a more precise direct life cycle including two larval moults and fecund adult parasites emerging around day 34 (Fahmy, 1954). The full series of larval moults from L1–L4 – adult were not finally defined until the 1980s (Panesar, 1989) (Fig. 2).
Fig. 2.
Development of the T. muris life cycle; from its initial documentation to our modern understanding. The left panel shows drawings taken from Fahmy et al. (1954) where the life cycle of T. muris was initially described. Originally, only two moults were thought to occur, however, the right panel shows our current understanding, based on the life cycle defined by Panesar (1989). Thus T. muris goes through the four classical larval moults of nematodes to reach adulthood. The similarities between the two life cycles are highlighted. Scanning electron microscope images were taken at the University of Manchester.
GROWING CONCEPTS: FROM ‘non-responders’ to t cell driven susceptibility and resistance
Trichuris muris in the mouse represents a powerful tool with which to explore host–parasite interactions and the immune response to infection. Over the years it has also become an important model for probing the immune system as the parasitic infection delivers antigen to the mucosa in a physiologically relevant manner. Thus T. muris in the mouse has contributed significantly to our understanding of basic immunological principles. One of the strengths of the model system lies in the variation in outcome of infection seen when different strains of mouse are infected. Shikhobalova (1937), Fahmy (1954) and Pike (1965) noted that patent adult worm infections could be established in albino mice (Shikhobalova, 1937; Fahmy, 1954; Pike, 1965). In contrast, Keeling (1961), Worley et al. (1962) and Campbell and Collette (1962) reported individual variation in the outcome of infection in that parasites were carried to patency in only some individuals and could not develop to the adult stage in others (Keeling, 1961; Campbell and Collette, 1962; Worley et al. 1962). At this stage whether the inability to recover parasites from an infected host represented resistance or ‘insusceptibility’ i.e. an incompatibility between the parasite and host environment was unclear. However, Campbell (1963) added clarity to this issue by conducting experiments where the loss of worms from the Sharp and Dohme albino mouse strain could be prevented by administration of the immunosuppressant cortisone (Campbell, 1963). Thus, the inability of worms to mature was not due to insusceptibility, but rather represented an active elimination of the parasites by the host prior to the development of the adult stage: the role of the immune response in resistance to infection was established. Building on these observations, Wakelin (1967) described acquired immunity to infection in the outbred Schofield strain of mouse (Wakelin, 1967). In these experiments most individual mice expelled the infection and were thereafter immune to subsequent infections. However, up to 30% of mice failed to expel the primary infection and were susceptible to further infections. Importantly, as observed by Campbell (1963), administration of cortisone suppressed the development of immunity. Wakelin (1970a, b ) went on to describe acquired immunity to infection in an number of different strains of mouse, the outbred Schofield, CFW, TO, TF1 and VS/MD and the inbred mouse strain A (Wakelin, 1970a, b ). Whilst all the outbred mouse strains had similar proportions of individuals unable to eliminate the infection, only the inbred mouse strain was uniformly resistant to infection. As before, the use of cortisone enabled patent infections to establish in all mice. At this stage it was troubling to understand the mechanism by which cortisone mediated its effects: did treatment inactivate immunity at a key stage in the parasite's life cycle? Was immunity delayed enabling larval stages of the parasite to develop which were not susceptible to immune attack? Did cortisone treatment during infection induce a state of immunological unresponsiveness to the parasite? The hypothesis of unresponsiveness was taken forward, given the fit with the additional observation that not only was there no effective primary immune response to infection after cortisone treatment, but there was also a failure to eliminate second infections.
The use of a variety of different mouse strains in these experiments also enabled Wakelin (1970a) to introduce the fundamentally important concept of genetic variation in immunity to infection which was later developed through the use of H-2 congenic and H-2 recombinant mouse strains (Else and Wakelin, 1988, 1989, 1990; Else et al. 1990). Both major histocompatibility complex linked genes and background genes were shown to contribute to immunity to infection, with the strongest influences denoted by the latter. Although host genetics clearly imparts a significant influence over resistance or susceptibility to infection, a parasite-induced influence is also clear, evidenced by the curious phenomenon of differential responsiveness within a genetically uniform strain of mouse (Lee and Wakelin, 1982). Differential responsiveness was thought to be a parasite-induced property with the later larval stages of the parasite hypothesized to be immunosuppressive. Using drug abbreviation and challenge infections, the ideas of Lee and Wakelin (1982) were further developed (Else et al. 1989), although the mechanism of parasite-induced immunomodulation remains unclear even today. Moving through the 1970s and 1980s, the idea that different inbred strains of mouse could be described as ‘responders’ or ‘non-responders’ gained popularity, and the underlying components of immune responses to infection studied in detail.
Using passive transfer and adoptive transfer methodologies Selby and Wakelin (1973) showed that both lymphoid cells and serum from immunized mice conferred recipient mice with an enhanced resistance to infection. Serum from immunized mice transferred varying degrees of protection but only if given at the time of infection (Selby and Wakelin, 1973). Interestingly, if the recipient mouse was immunosuppressed, serum transfer had no effect, implying the need for a cellular component within the recipient to interact with the transferred antibody to mediate expulsion (Wakelin, 1975). Surprisingly, serum from cortisone treated mice was also shown to transfer immunity at levels equivalent to immune sera (Lee and Wakelin, 1982). Lee et al. (1983) focused attention specifically on T cells, using the methodologies of the time (nylon wool columns) to enrich mesenteric lymph node cells into non-adherent T cells or adherent B cells. These experiments implicated T cells as being central in mediating worm expulsion: T cell-enriched fractions transferred immunity whilst B cell-enriched populations did not (Lee et al. 1983). Ito, in 1991, furthered this understanding describing the failure of athymic nude mice to expel T. muris and Koyama et al. (1995) showed a failure of mice to expel after CD4+ T cell depletion (Ito, 1991; Koyama et al. 1995). If the T cell dependency of immunity to infection was now established, exactly how worms were expelled from the gut remained unclear. Lee et al. (1983) described the lack of any gross inflammation accompanying worm expulsion and suggested that the intimate association T. muris has with its host meant that the parasite would be vulnerable to direct effects of intraepithelial lymphocytes, as opposed to pro-inflammatory effector cells such as the mast cell. It was over two decades later before examples of contributing effector mechanisms were more precisely described.
THE CYTOKINE ERA
The mid-90s saw the beginning of investigations into the key cytokine responses underlying immunity to T. muris infection. The discovery of T helper cell subsets by (initially Th1 and Th2) by Mosman and Coffman (1989) revolutionized our understanding of immunity to infection and provided a framework within which to explore resistance and susceptibility to T. muris (Mosman and Coffman, 1989). Resistance to T. muris infection was strongly linked to the generation of a Th2 immune response, whilst the development of a dominant Th1 response led to host susceptibility and chronic infection (Else et al. 1994). Thus, the early concepts of the 1970s and 1980s, suggesting that strains of mouse that fail to clear their infection are ‘not responding’ was revised with the discovery that ‘non-responder’ mouse strains actually make very profound immune responses, but of the wrong type to expel the parasite (Else and Grencis, 1991). With the cellular framework of Th1 and Th2 in place, the cortisone studies of the 1960s were also reinterpreted. A single dose of cortisone did not facilitate the development of immunological unresponsiveness, but rather cortisone treatment in a primary infection enabled the parasite to survive to a stage where it facilitated the development of a Th1 response. Thus, susceptibility to secondary infections would not be surprising if Th1 memory is maintained upon a challenge infection.
Initially, research focused on the key Th2 cytokine, IL-4; using anti-IL-4 receptor monoclonal antibodies (Else et al. 1994) and later, IL-4 knockout mice (Bancroft et al. 1998) this cytokine was shown to be essential for the development of a Th2 response and thus, resistance to T. muris. Furthermore, it was discovered that exogenous IL-4 could be used to induce resistance in susceptible mice, even if given after establishment of the parasite (Else et al. 1994). However, the fact that female BALB/c IL-4 knockout mice were still able to expel the parasite suggested that resistance does not completely depend on IL-4 (Bancroft et al. 2000). Bancroft et al. (2000) ascertained that expulsion in these mice was driven by IL-13, as in vivo blocking of this cytokine induced susceptibility. Moreover, the administration of exogenous recombinant IL-13 to male IL-4 knockout BALB/c mice reversed their susceptible phenotype (Bancroft et al. 2000). IL-13 null mice were also susceptible to infection with T. muris, despite developing a strong Th2 response, whereas IL-4 receptor-α null mice (mice lacking the common signalling receptor for both IL-4 and IL-13) generated a Th1 response and were susceptible (Bancroft et al. 1998). These findings suggest that IL-4, not IL-13, is required for the development of a parasite-specific Th2 response, with IL-13 being fundamental to the resolution of a T. muris infection and functioning downstream of the Th2 response to induce expulsion.
Early work also investigated the opposing Th1 cytokines IFN-γ and IL-12. Primarily, the key Th1 cytokine, IFN-γ, was shown to be strongly associated with susceptibility (Else et al. 1992), and depletion of this cytokine induced resistance in susceptible mice (Else et al. 1994). Administration of the Th1-promoting cytokine, IL-12 (D'Andrea et al. 1992), was able to switch a normally resistance phenotype to a susceptible one (Bancroft et al. 1997), however, concurrently depleting IFN-γ in these mice prevented the induction of susceptibility. These findings suggested that IL-12 induced chronicity is IFN-γ-dependent (Bancroft et al. 1997).
As more cytokines were discovered, and transgenic mice developed to explore the impact of each, the simple paradigm revolving around IL-4/IL-13/Th2 and resistance, and IFN-γ/Th1 and susceptibility was challenged and new models evolved integrating multiple cytokine networks. For example, in vivo blocking of the pro-inflammatory cytokine TNF-α in resistant C57BL/6 mice resulted in failure to expel the parasite, even in the presence of an on-going Th2 immune response. Additionally, the same treatment in female BALB/c IL-4 knockout mice, which require IL-13 for resistance, also induced susceptibility. These findings lead to the conclusion that TNF-α driven IL-13 is essential for expulsion of T. muris (Artis et al. 1999a). Knocking out TNF-α receptors, p55 and p57, produced a susceptible phenotype which further highlights a critical role for TNF-α (Hayes et al. 2007a). This work helped to evolve the model of resistance by incorporating IL-4, IL-13 and TNF-α. Indeed, Artis et al. (1999a) suggested that IL-4 and IL-13 regulate TNFR expression locally in the gut (Artis et al. 1999a). However, more recent work by Hayes et al. (2007a, b ) has presented a more general role for TNF-α, as exogenous application of a recombinant form of this cytokine was able to augment both Th1 and Th2 parasitic-specific immune responses (Hayes et al. 2007b).
IL-10, originally thought of as a Th2 cytokine, but now identified as an anti-inflammatory cytokine made by multiple cell types, including T regulatory cells, Th1 cells, B cells and macrophages, has also been implicated in resistance to T. muris infection. Both IL-10 and IL-10/IL-4 knockout mice suffer from a severe IFN-γ-driven colitis-like pathology, develop Th1 immune responses and are susceptible to T. muris infection. In contrast, however, double IL-10/IL-12-deficient mice develop Th2 immune responses and are completely resistant to T. muris, suggesting that the profound intestinal inflammation that develops in the absence of IL-10 is dependent on IL-12. Moreover, the administration of a broad-spectrum antibiotic in T. muris-infected IL-10/IL-4-deficient mice prevented pathology and enhanced survival of the mice, suggesting that an unregulated Th1 response to commensal bacteria was responsible (Schopf et al. 2002). Taking the work of Schopf et al. further, the importance of IL-13 in the severe phenotype of the T. muris-infected IL-10-deficient mouse was explored. Thus Wilson et al. (2011) identified a critical role for the IL-13 decoy receptor (IL-13Ra2) in permitting the severe gut inflammation observed post T. muris infection, via its ability to attenuate IL-13 activity, enabling the heightened IFN-γ driven inflammation (Wilson et al. 2011).
IL-27 has clear structural homology to IL-12, and, in common with this cytokine, is produced by APCs and is able to induce IFN-γ production from naïve T cells (Pflanz et al. 2002). Knocking out the major IL-27 signalling complex (WSX-1), which is mainly found on CD4+ T cells, demonstrated a role for IL-27 in the generation of Th1 responses during T. muris infection. Thus, WSX-1 null mice did not mount a Th1 response to T. muris, and instead developed Th2 driven immunity associated with increased numbers of CD4+IL-4+ T cells and reduced numbers of antigen-stimulated IFN-γ-producing T cells (Bancroft et al. 2004). Furthermore, it appeared that the inhibition of Th1 development is upstream of IL-12, as the administration of exogenous recombinant IL-12 to the same mice failed to restore the susceptible phenotype of wild-type mice (Bancroft et al. 2004). Interestingly, IL-27 has also been shown to regulate Th1 responses and the production of IFN-γ, as WSX-1 knockout mice infected with the potent inducer of Th1 responses, Toxoplasma gondii, display unregulated IFN-γ production and the development of a lethal, T-cell driven inflammatory disease (Villarino et al. 2003). Thus, IL-27 signalling through WSX-1 is able to both regulate and initiate Th1 and Th2 responses.
Other cytokines have also been linked with susceptibility to T. muris infection. IL-18 shares many similarities with IL-12 and can also increase IFN-γ production (Ahn et al. 1997). However, in the context of T. muris infection, recombinant IL-18 induced susceptibility in resistant mice via the downregulation of IL-4 and IL-13, while IFN-γ levels remained unaltered. These findings were reproduced in IFN-γ knockout mice, providing compelling evidence that IL-18 is not an inducer of IFN-γ, but rather a regulator of Th2 responses (Helmby et al. 2001).
Chemokines, or chemoattractive cytokines, are responsible for guiding the migration of activated effector cells to sites of infection or inflammation, and thus deficiencies in chemokines might predict differences in resistance to T. muris. Indeed mice deficient in CCL2, a monocyte chemoattractant fail to expel the parasite (deSchoolmeester et al. 2003). However, given the ability of CCL2 to influence the quality of the T helper cell response, there remains a lack of clarity around whether the susceptibility of the CCL2 knockout mouse reflects an impaired leukocyte recruitment to the worm niche, or the development of a dominant Th1 response.
The homing of lymphocytes to mucosal sites is thought to mainly depend on the interaction of α4β7-integrin on T cells with mucosal adhesion molecule 1 (MAdCAM-1). A more specific targeting to either the small or large intestine has been linked to the expression of CCR9 on T cells and CCL25 on epithelial cells, or mucosal epithelial chemokine (CCL28), respectively (Else, 2002). Surprisingly, the number of T cells infiltrating the gut of infected β7-null mice is not significantly different to that seen in wild-type controls, with β7-null mice remaining resistant to T. muris, perhaps due to compensation by other integrins (Artis et al. 2000). Moreover, using antibodies to block the α4β7-MAdCAM-1 interaction also had no effect on resistance of mice (Bell and Else, 2008). In the absence of available α4β7 and α4β1, however, mice that are usually resistant became chronically infected with T. muris and displayed a Th1-dominated immune response with decreased numbers of infiltrating eosinophils in the caecum (Bell and Else, 2008). These results suggest that when α4β7 is not available, α4β1 is able to aid the recruitment of leukocytes, particularly eosinophils, to the large intestine.
INVESTIGATING THE IMPORTANCE OF INFECTION LEVEL
The clear associations between Th1-mediated susceptibility and Th2-mediated resistance were discovered in the context of a single high-dose infection. However, work from the 1970s had already established important differences in the outcome of infection according to the number of eggs given. Wakelin (1973) showed that patent infections develop from less than 15 eggs, but that repeated trickle infection of 10 or 5 eggs given daily for around 2 weeks elicited protective immunity (Wakelin, 1973). Behnke and Wakelin (1973) developed this work further, reporting survival of low-level infections in both outbred laboratory CFLP mice and wild field mice (Behnke and Wakelin, 1973). Building on this basis, the cytokine profiles associated with a low-dose infection were defined (Bancroft et al. 1994) and describe strong Th1 responses in the absence of worm expulsion. The use of low-level T. muris infections to establish chronic infections in otherwise resistant hosts has become an increasingly important tool in recent years, with the availability of a wide range of transgenic mice on a C57BL6 background. Normally resistant to infection, C57BL6 mice can be made susceptible by the delivery of low-dose infections, thus enabling a dissection of factors important in resistance (high dose) and susceptibility (low dose) in the same mouse strain. For example, using the Th1-associated low-dose protocol in IL-12 knockout mice further confirmed the role of IL-12 in a Th1 response, as these mice expelled T. muris unlike wild-type controls (Helmby et al. 2001).
THE ISOLATES OF T. MURIS
Exploring worm infections in wild mice has given the scientific community insights into how parasites build up in their host and has also yielded new T. muris isolates. Wild mice accumulate worms through repeated low-level ‘trickle’ infections, generating a typically over-dispersed distribution that is also seen in Trichuris trichiura-infected humans (Behnke and Wakelin, 1973). Thus, most mice carry a low-level infection of fecund adults with heavy infections confined to a few individuals. Through work conducted in wild mice different isolates of T. muris were discovered (Johnston et al. 2005). Without doubt, the best characterized isolate is the Edinburgh or E isolate of T. muris. This isolate was recovered from a mouse in Edinburgh zoo in 1964. Many labs maintain the parasite by passaging the worm through immune-deficient or immune-suppressed hosts in order to ensure recovery of fecund adults. Interestingly, such a passage protocol removes any selection pressure on the worm to maintain its own mechanisms of immune-suppression. The discovery of isolates of Trichuris brought our understanding of these concepts forward and specifically the Sobreda – or S isolate – (Bellaby et al. 1995) was shown to be survive in mouse strains which readily expel the E isolate. The survival mechanisms employed by the S isolate were later shown to involve T regulatory cells, with the in vivo depletion of T regulatory cells enabling worm expulsion, but at the cost of an exaggerated gut pathology (D'Elia et al. 2009a).
CONCURRENT INFECTIONS: THE IMPACT OF POLYPARASITISM ON IMMUNITY TO INFECTION
In the field, polyparasitism is the norm. Despite this knowledge, the majority of studies in laboratory models of parasitic infection focus on single species infections. The concept of concurrent infections, and the impact that one parasite infection might have on another, has however attracted interest. The early work of Phillips et al. (1974) and Phillips and Wakelin (1976) showed that the ability of mice to expel T. muris was significantly compromised if they were also infected with Plasmodium berghei, Trypanosoma brucei or Babesia (Phillips et al. 1974; Phillips and Wakelin, 1976). Jenkins and Behnke (1977) and Behnke et al. (1984) explored this further in the context of the impact that Heligmosomoides polygyrus (formally Nematospiroides dubius) infection had on T. muris infections revealing that T. muris worms were more likely to survive to patency in mice which also had a H. polygyrus infection (Jenkins and Behnke, 1977; Behnke et al. 1984). Later, and in the context of a growing understanding of the importance of T helper cell polarization, dual infections were also explored in the context of Schistosomes and Trichuris (Curry et al. 1995). Here, mice normally susceptible to T. muris infection were found to expel the parasite if co-infected with Schistosoma mansoni, owing to increased Th2 responses (Curry et al. 1995). Thus, this later work built upon the observational studies of the 1970s, demonstrating the remarkable influence one parasite can have on the outcome of infection with another, even when they occupy completely different niches within their host.
UNDERSTANDING WORM EXPULSION MECHANISMS
Mouse strains that are resistant to T. muris usually expel their worm burdens between day 12 and 21 post infection (Blackwell and Else, 2002) and the ability to expel this parasite is known to be directly related to developing a Th2 immune response (Else et al. 1994). Following polarization in the local draining lymph nodes (the mesenteric lymph nodes), T cells home to the infected gut where they re-see parasite antigen and secrete the profiles of cytokines that they have been programmed to make at the time of first antigen presentation. Upon re-stimulation with parasite antigen in the gut, Th2 cells secrete cytokines that control local effector cells which enable expulsion of the parasite. IL-4 and IL-13, which act through shared and distinct pathways that are dependent on the type 2 cytokine-associated transcription factor ‘signal transducer and activator of transcription 6’ (STAT6), appear to be the most critical cytokines for immunity (Madden et al. 2004). IL-4 stimulates fluid secretion in the gut, IL-13 promotes goblet cell responses and both cytokines induce smooth muscle contraction (McKenzie et al. 1998; Urban et al. 1998). However, the effector cells and molecular mechanisms involved in expulsion of the parasite are yet to be fully defined and multiple effector mechanisms likely exist. Given the niche exploited by Trichuris parasites and hence their close relationship with the host intestinal epithelium, it is not surprising that work has focused on the cells, tissues and molecules that are present in this location in the search for, and understanding of, how the parasite is physically expelled from the host.
Effector mechanisms
The contraction of the smooth muscle that lines the gastrointestinal tract is one possible mechanism that aids expulsion of the T. muris parasite (reviewed in Khan and Collins, 2006). It has been demonstrated that the immune system can, in fact, mediate muscle contraction. Thus, mice that lack CD4 and MHC II have decreased muscle hypercontractility in response to Trichinella spiralis infection (Vallance et al. 1999). Furthermore, muscle tissue incubated with IL-4 or IL-13 resulted in a 33% increase in muscle hypercontractility (Akiho et al. 2002). Investigations into muscle hypercontractility in T. muris infection have also revealed an increase with infection (Khan et al. 2003). Moreover, blocking IL-9 resulted in the attenuation of muscle hypercontractility and, further, inhibited expulsion of the parasite (Khan et al. 2003). Therefore, muscle hypercontractility may be an important effector mechanism for expelling T. muris.
In 2005, a novel explanation for the expulsion of T. muris in resistant animals was proposed. Cliffe et al. (2005), motivated by the fact that chronic intestinal nematode infections cause altered gut architecture, suggested that increased turnover of epithelial cells in the gut may prevent the parasite from remaining embedded within the host. Specifically, T. muris infection is associated with enhanced epithelial stem cell proliferation within the crypts of Lieberkühn in the large intestine, which is mediated by IFN-γ, and results in a massive crypt hyperplasia (Potten et al. 1997). The IFN-γ-driven increase in proliferation cannot continue in an unregulated manner, and so T. muris infection is also associated with an elevation in apoptosis of epithelial cells in the large intestine. Further complexity in the fine regulation of gut homeostasis is provided by the chemokine CXCL10 which reduces the rate of epithelial cell turnover in a mouse model of colitis (Sasaki et al. 2002).
In susceptible animals, e.g. AKR mice, T. muris is thought to promote its own survival by inducing the production of IFN-γ, which acts to drive epithelial cell proliferation (Artis et al. 1999b) and also locally induces CXCL10. CXCL10 slows the epithelial turnover, resulting in crypt-cell hyperplasia and an increase in the epithelial niche for the parasite. Further investigations have shown that expression of the enzyme indoleamine 2,3-dioxygenase (IDO) by goblet cells is upregulated during chronic T. muris infection, and also by T. muris E/S antigens, with IDO slowing epithelial cell turnover and thus promoting worm survival (Bell and Else, 2011). In contrast, resistant mice, e.g. BALB/c mice, counter the nematode by elevating the rate of epithelial cell turnover and displacing the parasite. Increased epithelial cell turnover occurs in an IL-13-dependent manner, as IL-13 knock-out mice on a usually resistant BALB/c genetic background have a slow rate of turnover which resembles susceptible AKR mice (Cliffe et al. 2005). The epithelial cell turnover mechanism (coined the ‘epithelial escalator’) of nematode expulsion has been widely accepted as an effector mechanism in resistance to T. muris.
The mucus barrier is the primary defence against invading intestinal pathogens and is composed of products produced by goblet cells in the epithelial lining of the gut. Goblet cell hyperplasia has been linked to the development of a protective Th2 immune response in parasite infections (Bancroft and Grencis, 1998; Else and Finkelman, 1998; Yamauchi et al. 2006). Because of this, the roles of goblet cell products, including RELM-β, interlectins and mucins, have been extensively investigated in T. muris infection. Through microarray analysis of epithelial genes, resistin-like molecule-β (RELMβ) and intelectin-1 and 2 have been found to be upregulated in T. muris infection (Datta et al. 2005; Artis, 2006). RELMβ is a small cysteine-rich protein expressed exclusively by goblet cells in the lung and gastro-intestinal tract (Artis et al. 2004; Zimmermann et al. 2004). RELMβ interactions with parasites have been proposed to involve binding to chemosensory structures in the nematode cuticle and thus potentially impairing their ability to optimally sense their surroundings, as well as inhibit nematode chemotaxis (Artis et al. 2004).
Intelectin-1 is a 120 kDa disulfide-linked homotrimer expressed in the thymus, heart and intestine, although human intelectin-2 is only in the small intestine (Artis, 2006). Both intelectin-1 and intelectin-2 are upregulated in mice genetically resistant to T. muris around the time of expulsion. In contrast in susceptible AKR mice no change in their expression has been noted (Datta et al. 2005). As intelectins bind galactofuranosyl-containing residues in bacterial cell walls, it is postulated that they may also bind similar carbohydrates in parasitic nematodes, thus impairing worm fitness (Artis, 2006). Additional theories for the role of intelectins in parasite resistance include indirect effects on other mucin glycoproteins secreted by intestinal goblet cells; a cross-linking function which results in the formation of a ‘glycoprotein cement’ around the nematodes and aids their expulsion; broader effects during type 2 inflammation including immune regulation or tissue remodelling (Artis, 2006).
Goblet cell-derived angiogenin 4 (Ang4) is another antimicrobial molecule with potential antihelmintic properties (D'Elia et al. 2009b). Part of the RNase superfamily, Ang4 and members of the Ang4 family have been linked to toxicity towards gastrointestinal parasites (Hamann et al. 1987). The expression of Ang4 is elevated in mice infected with the Edinburgh (E) isolate of T. muris compared with those infected with the Sobreda (S) isolate (D'Elia et al. 2009b). Since the E isolate can be expelled by C57BL/6 mice, whereas the S isolate persists to a chronic infection, Ang4 was associated with expulsion of T. muris (D'Elia et al. 2009b). Further research has shown that Ang4 is also more highly expressed in strains of mice such as BALB/c that are resistant to T. muris infection, compared with those that are susceptible to infection, such as AKR, and has been linked to IL-13 production (Forman et al. 2012). Despite correlations between goblet cell-derived antimicrobials, such as the intelectins and Ang4, and the timing of worm expulsion, a definitive role for these molecules in the protective immune response to infection, rather than simply representing Th2 correlates, has not been shown and awaits the development of transgenic mice which lack these molecules.
In contrast, the availability of mucin-deficient mice has enabled clear statements to be made around the importance of mucus in worm expulsion. Mucins are gel-forming proteins that form the major component of the mucous barrier and are responsible for its visco-elastic properties (Thornton et al. 2008). Firstly it was shown that the Muc2 mucin was upregulated only in mice resistant to T. muris infection, and that this correlated with worm expulsion (Hasnain et al. 2010). Disappointingly however, Muc2-deficient mice were still able to expel the parasite and displayed unaltered Th2 responses (Hasnain et al. 2010). These investigators observed that de novo expression in the large intestine of the Muc5ac mucin, which is normally expressed in the airway, stomach and eye mucosa, occurred only in resistant mouse models of T. muris infection and not in susceptible mice (Hasnain et al. 2010). Subsequently, Muc5ac-deficient mice were infected with T. muris and found to be incapable of expelling the parasite, despite generating strong Th2 responses (Hasnain et al. 2011). Furthermore, Muc5ac was able to directly decrease the ATP levels of T. muris in vitro, thus reducing parasite vitality. Remarkably therefore, the expulsion of T. muris seems to be critically dependent on the expression of a single type of mucin, Muc5ac, in the gut, possibly induced by Th2-type cytokines.
Effector cells
Th2-driven antiparasite immunity is associated with a caecal mastocytosis, eosinophilia and an elevated parasite-specific IgG1 and IgE. However, a central role in acquired immunity to infection for any of these classic features of helminth infection has been hard to show in studies which focus on the outcome of infection in the absence of each of these responses singularly. Following the ablation of IL-5, the cytokine responsible for the immunological control of eosinophilia, mice usually resistant to T. muris remained so, despite a significant decrease in gut eosinophilia (Betts and Else, 1999). Although mast cells play key roles in the expulsion of some intestinal nematodes, such as T. spiralis, blocking the stem cell receptor, c-kit, which prevents the development of an intestinal mastocytosis, had no effect on the development of resistance in T. muris-infected mice. Furthermore, mice from a resistant background and lacking the Fcγ receptor, and thus deficient in any effector function requiring high-affinity IgG or IgE binding, were able to expel the parasite before it reached patency. Therefore, expulsion of T. muris does not critically depend on eosinophils, mast cells or mechanisms requiring antibody binding to cells via Fcγ receptors (Betts and Else, 1999) although studies which deplete multiple candidate effector cells have not been performed. The availability of mice genetically deficient in eosinophils added rigour to the early studies. Thus, despite the presence of eosinophils in the MLN correlating with resistance to infection, (Svensson et al. 2011), mice deficient in eosinophils (ΔdblGATA-1 mice) were still able to expel T. muris (Svensson et al. 2011).
Macrophages are pleiotropic cells which are capable of converting between many different phenotypes, and therefore have diverse biological functions, including phagocytosis, the release of cytokines, antigen presentation and tissue repair (Noel et al. 2004). The best characterized cells are the potently antimicrobial classically activated macrophages (CAM), induced by lipopolysaccharide (LPS) and the Th1 cytokine, IFN-γ, and which secrete nitric oxide (NO) (Zhao et al. 2008). In contrast, alternatively activated macrophages (AAM) are thought to have a role in type 2 immune responses, such as those triggered by extracellular helminth infections including T. muris, as they are induced by IL-4 and IL-13 (Gordon, 2003). AAMs produce factors involved in tissue remodelling, as well as increasing the fibrinogenic activity of fibroblasts (Song et al. 2000) and promoting angiogenesis and wound repair in acute and chronic inflammation (Kodelja et al. 1997). AAMs are characterized by their expression of arginase-1 (which out-competes inducible NO synthase, responsible for metabolizing arginine in CAMs), FIZZ-1/RELM-α and Ym-1 (Kreider et al. 2007). Zhao et al. (2008) have recently shown that AAMs are involved in infection-induced hypercontractility of smooth muscle, and contribute to expulsion of some intestinal nematode infections (Zhao et al. 2008). Implicated in immunity to Nippostrongylus brasiliensis and H. polygyrus, AAMs have yet to have a functional role defined in T. muris infection and this may relate to the unique niche within large intestinal epithelial cells occupied by this parasite. Indeed, Bowcutt et al. (2011) using a cell-specific targeting strategy, showed that arginase-1 deficiency in macrophages had no effect on resistance to T. muris infection (Bowcutt et al. 2011). Interestingly however, large numbers of macrophages do infiltrate the gut following an infection with T. muris, and distinctly more accumulate in the lamina propria of resistant mice compared with susceptible mice (Little et al. 2005). Taken together, this evidence suggests a possible arginase-independent role for macrophages, in the immune response to T. muris: a role which is not necessarily focused on immunity but also embraces mucosal healing.
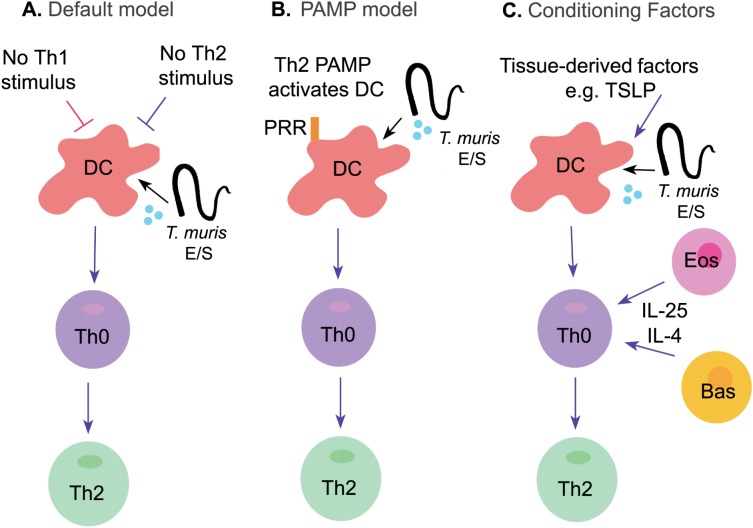
THE QUEST TO FIND THE TH2 DRIVING FORCE
The importance of a Th2 immune response in resistance to T. muris is unequivocal, however, it remains unclear how Th2 responses develop within the host. The upstream events in the induction of Th2 responses have been debated for many years (Fig. 3). Originally it was suggested that Th2 responses were a default response in the absence of Th1 inducing conditions, such as those provided by dendritic cell-derived IL-12. Following this, pathogen-associated molecular patterns (PAMPs) were thought to drive Th cell differentiation by binding to pattern recognition receptors (PRRs) on the dendritic cell. However, although, PAMPS which ligate antigen-presenting cells to induce Th1 responses are well established, any nematode PAMP that promotes Th2 responses remain elusive. The emerging field of innate cytokines and innate helper cells opened up a new framework of Th2 induction, with cytokines such as TSLP, IL-25 and IL-33 in the spotlight as factors conditioning antigen-presenting cells to drive Th2 responses. In the context of T. muris infection, however, mechanisms underlying Th2 induction remain unclear and likely involve multiple redundant roles for innate cells and the cytokines they secrete in promoting protective Th2 responses, as detailed below. Classically, dendritic cells are key players in determining the generation of Th1 or Th2 immune responses and therefore should be central in the immune response to T. muris infection. Surprisingly therefore, an essential role for the dendritic cell has not been established and indeed other innate cells have been put forward as key antigen presenting cells. Cruickshank et al. (2009) showed that dendritic cells infiltrate the large intestine significantly earlier in mice resistant to T. muris infection compared with susceptible mice, with this infiltration correlating with an increase in the epithelial-derived chemokines CCL5 and CCL20 (Cruickshank et al. 2009). In contrast, recent data has demonstrated that CD103+ cells are not required for the expulsion of T. muris, as CD103−/− mice are still resistant to infection (Mullaly et al. 2011). Therefore, DCs may not be critical in the development of the anti-Trichuris Th2 response, although the role, if any, of CD103− DCs remains to be investigated.
Fig. 3.
Developing models for the induction of Th2 responses following T. muris infection. (A) Originally, Th2 cell differentiation was thought to occur by ‘default’, in the absence of a Th1-inducing stimulus; (B) Moving forward, it was hypothesized that a Th2 pathogen associated molecular pattern (PAMPs) might drive Th2 cell differentiation by binding to pattern recognition receptors (PRRs), however, any nematode PAMP that promotes Th2 responses is yet to be defined; (C) Modern understanding suggests that endogenous factors, such as alarmins, tissue factors (e.g. TSLP) and cytokines act on DCs to drive Th2 priming. Furthermore, other cells such as eosinophils (EOS) and basophils (BAS) may also help to drive Th2 differentiation through secreting Th2-driving signals e.g. IL-25.
Basophil numbers are increased during helminth infection and, using eGFP/IL-4 reporter mice, they have been shown to secrete IL-4 which could promote the generation of protective Th2 responses (Min et al. 2004). Perrigoue et al. (2009) demonstrate that basophils expressing MHC II are important accessory cells in the generation of Th2 responses to T. muris through direct stimulation of CD4+ T cells. These investigators show that the depletion of basophils during T. muris infection resulted in reduced Th2 cytokine responses but no significant effect on worm expulsion (Perrigoue et al. 2009). Therefore, basophils could play a role in the protective responses to T. muris infection by contributing to the development of Th2 responses. NK cells have also been shown to have a potential supporting role in IL-13 production, as depleting this cell type causes a delay in parasite expulsion (Hepworth and Grencis, 2009), however whether they are able to initiate Th2 responses is yet to be investigated, and is perhaps unlikely given IL-13 is not a polarizing cytokine.
The recent identification of ‘alarmin’ cytokines (IL-25, IL-33 and TSLP) produced by intestinal epithelial cells, as well as by immune cells such as macrophages, mast cells, eosinophils and basophils, has triggered studies exploring their role in Th2 responses to helminth infections. IL-25 is a member of the IL-17 family of cytokines and is known to induce IL-5 and IL-13 by an accessory cell population (Fort et al. 2001). In 2006, experiments were carried out to show that exogenous IL-25 can induce resistance to T. muris in usually susceptible AKR mice, and also that this cytokine was important for reducing pathology during infection (Owyang et al. 2006).
Recent work on the IL-1-like cytokine, IL-33, has demonstrated a role in IgE-independent induction of IL-13 from human and mouse mast cells, as well as the optimization of Th2 cytokine and chemokine production in these same cells (Ho et al. 2007; Iikura et al. 2007). Additionally, IL-33 has been described as a chemo-attractant and potent inducer of Th2 cells, and can cause IL-13 dependent pathological changes in mucosal tissue (Schmitz et al. 2005; Komai-Koma et al. 2007). In the context of T. muris infection, IL-33 has been shown to be produced very early and have a dual role in providing the initial impetus for Th2 polarization and acting at the infection site in a T-cell independent manner to influence the gut tissue response to parasite invasion (Humphreys et al. 2008).
Thymic stromal lymphopoietin (TSLP) is also implicated in resistance to Trichuris (Taylor et al. 2009). Blocking TSLP signalling with anti-TSLP monoclonal antibodies or deletion of TSLP-R in normally resistant mice, resulted in decreased Th2 cytokine expression and a delayed T. muris expulsion (Taylor et al. 2009). Furthermore, disruption of TSLP signalling caused increased levels of IFN-γ, IL-17A and IL-12/23p40 accompanied by exacerbated intestinal inflammation. TSLP, however, was not thought to be critical in the induction of parasite-specific Th2 immune responses as Th2 responses were still present in TSLP−/− mice treated with anti-IFN-γ antibodies (Taylor et al. 2009), but rather acted by limiting IL-12p40 production (Massacand et al. 2009). Interestingly, in the latter study Massacand et al. highlight the differences between T. muris and other intestinal nematode infections, showing that H. polygyrus and N. brasiliensis differ from T. muris as they can directly inhibit host IL-12p40 through their excretory/secretory antigens and so bypass the need for TSLP (Massacand et al. 2009).
Until recently NK cells were the only innate immune cell known to be derived from a lymphoid progenitor. The last 3 years, however, has seen the discovery of a family of innate lymphoid cells (ILCs), with some of these family members thought to be important in potentiating Th2 responses. Type 2 ILCs, also named nuocytes (Neill et al. 2010), natural helper cells (Moro et al. 2009) or innate type 2 helper cells (Price et al. 2010) by their respective discoverers, are lineage negative, respond to IL-25 or IL-33 and produce Th2 cytokines. Original studies showed that type 2 ILCs are important for expulsion of N. brasiliensis, as mice lacking the IL-25 or IL-33 receptor had very few type 2 ILCs and could not effectively clear the parasite infection (Neill et al. 2010). Further, adoptive transfer of the type 2 ILCs restored immunity to N. brasiliensis infection in an IL-13 dependent manner (Neill et al. 2010). Whether the type 2 ILC plays a role in T. muris infection is yet to be determined. A similar lineage negative innate cell type, the multi-potent progenitor type 2 cell (MPPtype2), which responds to IL-25 and IL-33, has also recently been discovered (Saenz et al. 2010). Transfer of these cells to IL-25−/− mice infected with T. muris was able to reverse the susceptible phenotype caused by lack of IL-25 (Saenz et al. 2010). Therefore, type 2 cytokines released from MPPtype2 cells may be important in promoting protective Th2 responses, likely acting by elevating levels of IL-13 locally in the gut. However, this area of research requires further exploration as the physiological relevance of transferring relatively large numbers of ILCs to mice where they exist as a rare lineage, even post infection, is unclear.
T. MURIS AND ITS RELATIONSHIP WITH GUT FLORA
Around 1013 bacteria line the mammalian gastrointestinal tract and the large intestine is especially well colonized. Since this region of the gut is also the niche of the T. muris parasite it is perhaps not surprising that links between the parasite and host flora have been established. A recent study has shown that bacteria are critical for the hatching of Trichuris eggs, in a mechanism involving the interaction of bacterial type 1 fimbriae with the opercula poles of Trichuris eggs (Hayes et al. 2010). Further, antibiotic treatment of susceptible AKR mice resulted in a dramatic reduction in the number of established worms at day 21 p.i., and these mice displayed increased Th2 responses (Hayes et al. 2010). Colonization of the intestinal epithelium by the T. muris parasite is thought to damage the mucosal barrier. Such damage may potentially lead to leakage of low levels of bacterial products including LPS into the tissue (Helmby and Grencis, 2003), thus generating a Th1 permissive environment via LPS-induced IFN-γ and hence influencing the development of resistance to the parasite. Such a hypothesis is attractive, as it explains the essential requirement for TLR4 in the development of a chronic T. muris infection (Helmby and Grencis, 2003). Therefore, host flora may be a contributing factor not only in the parasite egg-hatching mechanism, but also in influencing the ability of the host to expel T. muris. Recently, newly obtained isolates of T. muris have been difficult to establish in laboratory mice (J. Behnke, personal communication). One explanation for this could be the difference in gut flora between wild and laboratory mice, with the flora of laboratory mice being less conducive to triggering the hatching of eggs of parasites freshly isolated from the wild.
WHAT LIES AHEAD …
Over the years, many important discoveries, concerning both immunity to gut-dwelling nematode parasites and our understanding of the immune system, have been made using the T. muris laboratory model. Figure 1 shows a summary timeline of the key milestones in T. muris research. The knowledge and understanding gained from the animal model have translated to human Trichuriasis (Faulkner et al. 2002), and other intestinal nematode parasite infections (Turner et al. 2003). Indeed, human studies show correlations between Th2 responses (parasite-specific IgE; cytokines) and reduced worm burdens with increasing host age, supporting the mouse studies which describe a Th2-mediated resistance to infection. Further, there is no clear evidence in man linking susceptibility to infection with Th1 responses as seen in the mouse models, although high worm burdens correlate with reduced Th2 responses. In addition, the recent demonstration that the pathological, cellular and molecular changes associated with chronic T. muris infection correlate with those seen in human IBD (Levison et al. 2010) may open up the use of this experimental system to understand IBD.
Despite a good and constantly evolving understanding of the host immune response to Trichuris infection, antihelminthic drugs are still the only treatment available in the field. However, drug treatment is less than ideal, as re-infection with the parasite is all too easy. Crude subcutaneous vaccination studies carried out in the 1970s, 1980s and 1990s using worm homogenate or excretory/secretory antigens revealed that vaccination can enhance resistance in resistant strains of mouse (Wakelin and Selby, 1973; Jenkins and Wakelin, 1977, 1983) and can protect otherwise susceptible strains (Else and Wakelin, 1990). Robinson et al. (1995) explored this further considering the optimal route of vaccination to elicit protection and identified oral vaccination as a route via which to enhance resistance in some strains of mouse (Robinson et al. 1995). Although the immune responses which accompany vaccine-induced resistance have now been described in detail (Dixon et al. 2010), disappointing progress has been made towards the identification of target antigens to include in any vaccine of the future. Therefore, there is an urgent unmet need for research in this area.
We still understand little about the biology of the parasite. Apart from defining the conditions required for successful embryonation (Fahmy, 1954) and the definition of the gut flora required for optimal egg hatching 56 years later (Hayes et al. 2010), factors impacting on the lifestyle of Trichuris are not well understood. For example, we do not know how the parasite forms its syncytial tunnels and we know little about T. muris antigens and their roles in worm survival.
The significant investment in sequencing the T. muris genome (a current collaboration between Richard Grencis (University of Manchester) and the Wellcome Trust (Sanger Institute) using second-generation sequencing technologies should enable some of these questions to be answered, opening up new avenues of research, including the identification of antigens as possible vaccination targets.
ACKNOWLEDGEMENTS
We would like to thank Professor Richard Grencis for his comments on this manuscript.
FINANCIAL SUPPORT
RMJH is supported by the Wellcome trust (Grant number: 091815).
REFERENCES
- Ahn H. J., Maruo S., Tomura M., Mu J., Hamaoka T., Nakanishi K., Clark S., Kurimoto M., Okamura H. and Fujiwara H. (1997). A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. Journal of Immunology 159, 2125–2131 [PubMed] [Google Scholar]
- Akiho H., Blennerhassett P., Deng Y. and Collins S. M. (2002). Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. American Journal of Physiology – Gastrointestinal and Liver Physiology 282, G226–G232 [DOI] [PubMed] [Google Scholar]
- Araujo A., Reinhard K. J., Ferreira L. F. and Gardner S. L. (2008). Parasites as probes for prehistoric human migrations? Trends in Parasitology 24, 112–115 [DOI] [PubMed] [Google Scholar]
- Artis D. (2006). New weapons in the war on worms: identification of putative mechanisms of immune-mediated expulsion of gastrointestinal nematodes. International Journal of Parasitology 36, 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D., Humphreys N. E., Bancroft A. J., Rothwell N. J., Potten C. S. and Grencis R. K. (1999a). Tumor necrosis factor alpha is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. Journal of Experimental Medicine 190, 953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D., Potten C. S., Else K. J., Finkelman F. D. and Grencis R. K. (1999b). Trichuris muris: host intestinal epithelial cell hyperproliferation during chronic infection is regulated by interferon-gamma. Experimental Parasitology 92, 144–153 [DOI] [PubMed] [Google Scholar]
- Artis D., Humphreys N. E., Potten C. S., Wagner N., Muller W., McDermott J. R., Grencis R. K. and Else K. J. (2000). Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. European Journal of Immunology 30, 1656–1664 [DOI] [PubMed] [Google Scholar]
- Artis D., Wang M. L., Keilbaugh S. A., He W., Brenes M., Swain G. P., Knight P. A., Donaldson D. D., Lazar M. A., Miller H. R., Schad G. A., Scott P. and Wu G. D. (2004). RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences USA 101, 13596–13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft A. J. and Grencis R. K. (1998). Th1 and Th2 cells and immunity to intestinal helminths. Chemical Immunology 71, 192–208 [DOI] [PubMed] [Google Scholar]
- Bancroft A. J., Else K. J. and Grencis R. K. (1994). Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. European Journal of Immunology 24, 3113–3118 [DOI] [PubMed] [Google Scholar]
- Bancroft A. J., Else K. J., Sypek J. P. and Grencis R. K. (1997). Interleukin-12 promotes a chronic intestinal nematode infection. European Journal of Immunology 27, 866–870 [DOI] [PubMed] [Google Scholar]
- Bancroft A. J., Mckenzie A. N. and Grencis R. K. (1998). A critical role for IL-13 in resistance to intestinal nematode infection. Journal of Immunology 160, 3453–3461 [PubMed] [Google Scholar]
- Bancroft A. J., Artis D., Donaldson D. D., Sypek J. P. and Grencis R. K. (2000). Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. European Journal of Immunology 30, 2083–2091 [DOI] [PubMed] [Google Scholar]
- Bancroft A. J., Humphreys N. E., Worthington J. J., Yoshida H. and Grencis R. K. (2004). WSX-1: a key role in induction of chronic intestinal nematode infection. Journal of Immunology 172, 7635–7641 [DOI] [PubMed] [Google Scholar]
- Behnke J. M. and Wakelin D. (1973). The survival of Trichuris muris in wild populations of its natural hosts. Parasitology 67, 157–164 [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Ali N. M. and Jenkins S. N. (1984). Survival to patency of low level infections with Trichuris muris in mice concurrently infected with Nematospiroides dubius. Annals of Tropical Medicine and Parasitology 78, 509–517 [DOI] [PubMed] [Google Scholar]
- Bell L. V. and Else K. J. (2008). Mechanisms of leucocyte recruitment to the inflamed large intestine: redundancy in integrin and addressin usage. Parasite Immunology 30, 163–170 [DOI] [PubMed] [Google Scholar]
- Bell L. V. and Else K. J. (2011). Regulation of colonic epithelial cell turnover by IDO contributes to the innate susceptibility of SCID mice to Trichuris muris infection. Parasite Immunology 33, 244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaby T., Robinson K., Wakelin D. and Behnke J. M. (1995). Isolates of Trichuris muris vary in their ability to elicit protective immune responses to infection in mice. Parasitology 111, 353–357 [DOI] [PubMed] [Google Scholar]
- Betts C. J. and Else K. J. (1999). Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunology 21, 45–52 [DOI] [PubMed] [Google Scholar]
- Blackwell N. M. and Else K. J. (2002). A comparison of local and peripheral parasite-specific antibody production in different strains of mice infected with Trichuris muris. Parasite Immunology 24, 203–211 [DOI] [PubMed] [Google Scholar]
- Bowcutt R., Bell L. V., Little M., Wilson J., Booth C., Murray P. J., Else K. J. and Cruickshank S. M. (2011). Arginase-1-expressing macrophages are dispensable for resistance to infection with the gastrointestinal helminth Trichuris muris. Parasite Immunology 33, 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. C. (1963). Spontaneous cure in Trichuris muris infections in albino mice and its suppression by cortisone. Journal of Parasitology 49, 628–632 [PubMed] [Google Scholar]
- Campbell W. C. and Collette J. V. (1962). Effect of cortisone upon infection with Trichuris muris in albino mice. Journal of Parasitology 48, 933–934 [Google Scholar]
- Cliffe L. J., Humphreys N. E., Lane T. E., Potten C. S., Booth C. and Grencis R. K. (2005). Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308, 1463–1465 [DOI] [PubMed] [Google Scholar]
- Cruickshank S. M., Deschoolmeester M. L., Svensson M., Howell G., Bazakou A., Logunova L., Little M. C., English N., Mack M., Grencis R. K., Else K. J. and Carding S. R. (2009). Rapid dendritic cell mobilization to the large intestinal epithelium is associated with resistance to Trichuris muris infection. Journal of Immunology 182, 3055–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry A. J., Else K. J., Jones F., Bancroft A., Grencis R. K. and Dunne D. W. (1995). Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. Journal of Experimental Medicine 181, 769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E., Chizzonite R., Wolf S. F. and Trinchieri G. (1992). Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. Journal of Experimental Medicine 176, 1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R., Deschoolmeester M. L., Hedeler C., Paton N. W., Brass A. M. and Else K. J. (2005). Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infection and Immunity 73, 4025–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davaine C. J. (1858). Recherches sur le developpement et la propagation du Trichocephale de l'homme et de l'Ascaride lombricoide. Comptes rendus de l'academie des sciences 46, 1217–1219 [Google Scholar]
- D'elia R., Behnke J. M., Bradley J. E. and Else K. J. (2009a). Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. Journal of Immunology 182, 2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'elia R., Deschoolmeester M. L., Zeef L. A., Wright S. H., Pemberton A. D. and Else K. J. (2009b). Expulsion of Trichuris muris is associated with increased expression of angiogenin 4 in the gut and increased acidity of mucins within the goblet cell. BMC Genomics 10, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschoolmeester M. L., Little M. C., Rollins B. J. and Else K. J. (2003). Absence of CC chemokine ligand 2 results in an altered Th1/Th2 cytokine balance and failure to expel Trichuris muris infection. Journal of Immunology 170, 4693–4700 [DOI] [PubMed] [Google Scholar]
- Dixon H., Little M. C. and Else K. J. (2010). Characterisation of the protective immune response following subcutaneous vaccination of susceptible mice against Trichuris muris. International Journal of Parasitology 40, 683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else K. J. (2002). Chemokines and leucocyte migration in parasitic disease. Parasite Immunology 24, 281–283 [DOI] [PubMed] [Google Scholar]
- Else K. J. and Finkelman F. D. (1998). Intestinal nematode parasites, cytokines and effector mechanisms. International Journal of Parasitology 28, 1145–1158 [DOI] [PubMed] [Google Scholar]
- Else K. J. and Grencis R. K. (1991). Helper T-cell subsets in mouse trichuriasis. Parasitology Today (Personal ed) 7, 313–316 [DOI] [PubMed] [Google Scholar]
- Else K. and Wakelin D. (1988). The effects of H-2 and non-H-2 genes on the expulsion of the nematode Trichuris muris from inbred and congenic mice. Parasitology 96, 543–550 [DOI] [PubMed] [Google Scholar]
- Else K. and Wakelin D. (1989). Genetic variation in the humoral immune responses of mice to the nematode Trichuris muris. Parasite Immunology 11, 77–90 [DOI] [PubMed] [Google Scholar]
- Else K. J. and Wakelin D. (1990). Genetically-determined influences on the ability of poor responder mice to respond to immunization against Trichuris muris. Parasitology 100, 479–489 [DOI] [PubMed] [Google Scholar]
- Else K. J., Wakelin D. and Roach T. I. (1989). Host predisposition to trichuriasis: the mouse–T. muris model. Parasitology 98(Pt 2), 275–282 [DOI] [PubMed] [Google Scholar]
- Else K. J., Wakelin D., Wassom D. L. and Hauda K. M. (1990). The influence of genes mapping within the major histocompatibility complex on resistance to Trichuris muris infections in mice. Parasitology 101, 61–67 [DOI] [PubMed] [Google Scholar]
- Else K. J., Hultner L. and Grencis R. K. (1992). Modulation of cytokine production and response phenotypes in murine trichuriasis. Parasite Immunology 14, 441–449 [DOI] [PubMed] [Google Scholar]
- Else K. J., Finkelman F. D., Maliszewski C. R. and Grencis R. K. (1994). Cytokine-mediated regulation of chronic intestinal helminth infection. Journal of Experimental Medicine 179, 347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy M. A. (1954). An investigation on the life cycle of Trichuris muris. Parasitology 44, 50–57 [DOI] [PubMed] [Google Scholar]
- Faulkner H., Turner J., Kamgno J., Pion S. D., Boussinesq M. and Bradley J. E. (2002). Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. Journal of Infectious Diseases 185, 665–672 [DOI] [PubMed] [Google Scholar]
- Forman R. A., Deschoolmeester M. L., Hurst R. J., Wright S. H., Pemberton A. D. and Else K. J. (2012). The goblet cell is the cellular source of the anti-microbial angiogenin 4 in the large intestine post Trichuris muris infection. PLoS ONE 7, e42248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort M. M., Cheung J., Yen D., Li J., Zurawski S. M., Lo S., Menon S., Clifford T., Hunte B., Lesley R., Muchamuel T., Hurst S. D., Zurawski G., Leach M. W., Gorman D. M. and Rennick D. M. (2001). IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 [DOI] [PubMed] [Google Scholar]
- Gordon S. (2003). Alternative activation of macrophages. Nature Reviews Immunology 3, 23–35 [DOI] [PubMed] [Google Scholar]
- Hamann K. J., Barker R. L., Loegering D. A. and Gleich G. J. (1987). Comparative toxicity of purified human eosinophil granule proteins for newborn larvae of Trichinella spiralis. Journal of Parasitology 73, 523–529 [PubMed] [Google Scholar]
- Hasnain S. Z., Wang H., Ghia J. E., Haq N., Deng Y., Velcich A., Grencis R. K., Thornton D. J. and Khan W. I. (2010). Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138, 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain S. Z., Evans C. M., Roy M., Gallagher A. L., Kindrachuk K. N., Barron L., Dickey B. F., Wilson M. S., Wynn T. A., Grencis R. K. and Thornton D. J. (2011). Muc5ac: a critical component mediating the rejection of enteric nematodes. Journal of Experimental Medicine 208, 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K. S., Bancroft A. J. and Grencis R. K. (2007a). The role of TNF-alpha in Trichuris muris infection I: influence of TNF-alpha receptor usage, gender and IL-13. Parasite Immunology 29, 575–582 [DOI] [PubMed] [Google Scholar]
- Hayes K. S., Bancroft A. J. and Grencis R. K. (2007b). The role of TNF-alpha in Trichuris muris infection II: global enhancement of ongoing Th1 or Th2 responses. Parasite Immunology 29, 583–594 [DOI] [PubMed] [Google Scholar]
- Hayes K. S., Bancroft A. J., Goldrick M., Portsmouth C., Roberts I. S. and Grencis R. K. (2010). Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 328, 1391–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmby H. and Grencis R. K. (2003). Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. European Journal of Immunology 33, 2974–2979 [DOI] [PubMed] [Google Scholar]
- Helmby H., Takeda K., Akira S. and Grencis R. K. (2001). Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. Journal of Experimental Medicine 194, 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth M. R. and Grencis R. K. (2009). Disruption of Th2 immunity results in a gender-specific expansion of IL-13 producing accessory NK cells during helminth infection. Journal of Immunology 183, 3906–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L. H., Ohno T., Oboki K., Kajiwara N., Suto H., Iikura M., Okayama Y., Akira S., Saito H., Galli S. J. and Nakae S. (2007). IL-33 induces IL-13 production by mouse mast cells independently of IgE-F cepsilonRI signals. Journal of Leukocyte Biology 82, 1481–1490 [DOI] [PubMed] [Google Scholar]
- Humphreys N. E., Xu D., Hepworth M. R., Liew F. Y. and Grencis R. K. (2008). IL-33, a potent inducer of adaptive immunity to intestinal nematodes. Journal of Immunology 180, 2443–2449 [DOI] [PubMed] [Google Scholar]
- Iikura M., Suto H., Kajiwara N., Oboki K., Ohno T., Okayama Y., Saito H., Galli S. J. and Nakae S. (2007). IL-33 can promote survival, adhesion and cytokine production in human mast cells. Laboratory Investigation; a Journal of Technical Methods and Pathology 87, 971–978 [DOI] [PubMed] [Google Scholar]
- Ito Y. (1991). The absence of resistance in congenitally athymic nude mice toward infection with the intestinal nematode, Trichuris muris: resistance restored by lymphoid cell transfer. International Journal of Parasitology 21, 65–69 [DOI] [PubMed] [Google Scholar]
- Jenkins S. N. and Behnke J. M. (1977). Impairment of primary expulsion of Trichuris muris in mice concurrently infected with Nematospiroides dubius. Parasitology 75, 71–78 [DOI] [PubMed] [Google Scholar]
- Jenkins S. N. and Wakelin D. (1977). The source and nature of some functional antigens of Trichuris muris. Parasitology 74, 153–161 [DOI] [PubMed] [Google Scholar]
- Jenkins S. N. and Wakelin D. (1983). Functional antigens of Trichuris muris released during in vitro maintenance: their immunogenicity and partial purification. Parasitology 86, 73–82 [DOI] [PubMed] [Google Scholar]
- Johnston C. E., Bradley J. E., Behnke J. M., Matthews K. R. and Else K. J. (2005). Isolates of Trichuris muris elicit different adaptive immune responses in their murine host. Parasite Immunology 27, 69–78 [DOI] [PubMed] [Google Scholar]
- Keeling J. E. (1961). Experimental trichuriasis. I. Antagonism between Trichuris muris and Aspiculuris tetraptera in the albino mouse. Journal of Parasitology 47, 641–646 [PubMed] [Google Scholar]
- Khan W. I. and Collins S. M. (2006). Gut motor function: immunological control in enteric infection and inflammation. Clinical and Experimental Immunology 143, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W. I., Richard M., Akiho H., Blennerhasset P. A., Humphreys N. E., Grencis R. K., Van Snick J. and Collins S. M. (2003). Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infection and Immunity 71, 2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodelja V., Muller C., Tenorio S., Schebesch C., Orfanos C. E. and Goerdt S. (1997). Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology 197, 478–493 [DOI] [PubMed] [Google Scholar]
- Komai-Koma M., Xu D., Li Y., McKenzie A. N., McInnes I. B. and Liew F. Y. (2007). IL-33 is a chemoattractant for human Th2 cells. European Journal of Immunology 37, 2779–2786 [DOI] [PubMed] [Google Scholar]
- Koyama K., Tamauchi H. and Ito Y. (1995). The role of CD4+ and CD8+ T cells in protective immunity to the murine nematode parasite Trichuris muris. Parasite Immunology 17, 161–165 [DOI] [PubMed] [Google Scholar]
- Kreider T., Anthony R. M., Urban J. F. Jr. and Gause W. C. (2007). Alternatively activated macrophages in helminth infections. Current Opinion in Immunology 19, 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. D. and Wakelin D. (1982). Cortisone-induced immunotolerance to nematode infection in CBA/Ca mice. I. Investigation of the defect in the protective response. Immunology 47, 227–232 [PMC free article] [PubMed] [Google Scholar]
- Lee T. D., Wakelin D. and Grencis R. K. (1983). Cellular mechanisms of immunity to the nematode Trichuris muris. International Journal of Parasitology 13, 349–353 [DOI] [PubMed] [Google Scholar]
- Levison S. E., McLaughlin J. T., Zeef L. A., Fisher P., Grencis R. K. and Pennock J. L. (2010). Colonic transcriptional profiling in resistance and susceptibility to trichuriasis: phenotyping a chronic colitis and lessons for iatrogenic helminthosis. Inflammatory Bowel Diseases 16, 2065–2079 [DOI] [PubMed] [Google Scholar]
- Little M. C., Bell L. V., Cliffe L. J. and Else K. J. (2005). The characterization of intraepithelial lymphocytes, lamina propria leukocytes, and isolated lymphoid follicles in the large intestine of mice infected with the intestinal nematode parasite Trichuris muris. Journal of Immunology 175, 6713–6722 [DOI] [PubMed] [Google Scholar]
- Madden K. B., Yeung K. A., Zhao A., Gause W. C., Finkelman F. D., Katona I. M., Urban J. F. Jr. and Shea-Donohue T. (2004). Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. Journal of Immunology 172, 5616–5621 [DOI] [PubMed] [Google Scholar]
- Massacand J. C., Stettler R. C., Meier R., Humphreys N. E., Grencis R. K., Marsland B. J. and Harris N. L. (2009). Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proceedings of the National Academy of Sciences USA 106, 13968–13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenzie G. J., Bancroft A., Grencis R. K. and McKenzie A. N. (1998). A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Current Biology 8, 339–342 [DOI] [PubMed] [Google Scholar]
- Min B., Prout M., Hu-Li J., Zhu J., Jankovic D., Morgan E. S., Urban J. F. Jr., Dvorak A. M., Finkelman F. D., Legros G. and Paul W. E. (2004). Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. Journal of Experimental Medicine 200, 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J., Ohtani M., Fujii H. and Koyasu S. (2009). Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 [DOI] [PubMed] [Google Scholar]
- Mosman T. R. and Coffman R. L. (1989). TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology 1, 145–173 [DOI] [PubMed] [Google Scholar]
- Mullaly S. C., Burrows K., Antignano F. and Zaph C. (2011). Assessing the role of CD103 in immunity to an intestinal helminth parasite. PLoS ONE 6, e19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill D. R., Wong S. H., Bellosi A., Flynn R. J., Daly M., Langford T. K., Bucks C., Kane C. M., Fallon P. G., Pannell R., Jolin H. E. and McKenzie A. N. (2010). Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel W., Raes G., Hassanzadeh Ghassabeh G., De Baetselier P. and Beschin A. (2004). Alternatively activated macrophages during parasite infections. Trends in Parasitology 20, 126–133 [DOI] [PubMed] [Google Scholar]
- Owyang A. M., Zaph C., Wilson E. H., Guild K. J., McClanahan T., Miller H. R., Cua D. J., Goldschmidt M., Hunter C. A., Kastelein R. A. and Artis D. (2006). Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. Journal of Experimental Medicine 203, 843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panesar T. S. (1989). The moulting pattern in Trichuris muris (Nematoda Trichuroidea). Canadian Journal of Zoology 67, 2340–2343 [Google Scholar]
- Perrigoue J. G., Saenz S. A., Siracusa M. C., Allenspach E. J., Taylor B. C., Giacomin P. R., Nair M. G., Du Y., Zaph C., Van Rooijen N., Comeau M. R., Pearce E. J., Laufer T. M. and Artis D. (2009). MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nature Immunology 10, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S., Timans J. C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., Blumenschein W. M., Mattson J. D., Wagner J. L., To W., Zurawski S., McClanahan T. K., Gorman D. M., Bazan J. F., De Waal Malefyt R., Rennick D. and Kastelein R. A. (2002). IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 16, 779–790 [DOI] [PubMed] [Google Scholar]
- Phillips R. S. and Wakelin D. (1976). Trichuris muris: effect of concurrent infections with rodent piroplasms on immune expulsion from mice. Experimental Parasitology 39, 95–100 [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Selby G. R. and Wakelin D. (1974). The effect of Plasmodium berghei and Trypanosoma brucei infections on the immune expulsion of the nematode Trichuris muris from mice. International Journal of Parasitology 4, 409–415 [DOI] [PubMed] [Google Scholar]
- Pike E. H. (1965). Bionomic, blood, and chromium51 investigations of Trichuris muris and studies with two related species. Dissertation Abstracts 25, 7430–7431 [Google Scholar]
- Potten C. S., Booth C. and Pritchard D. M. (1997). The intestinal epithelial stem cell: the mucosal governor. International Journal of Experimental Pathology 78, 219–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. E., Liang H. E., Sullivan B. M., Reinhardt R. L., Eisley C. J., Erle D. J. and Locksley R. M. (2010). Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences USA 107, 11489–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K., Bellaby T. and Wakelin D. (1995). Efficacy of oral vaccination against the murine intestinal parasite Trichuris muris is dependent upon host genetics. Infection and Immunity 63, 1762–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz S. A., Siracusa M. C., Perrigoue J. G., Spencer S. P., Urban J. F. Jr., Tocker J. E., Budelsky A. L., Kleinschek M. A., Kastelein R. A., Kambayashi T., Bhandoola A. and Artis D. (2010). IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 464, 1362–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Yoneyama H., Suzuki K., Suriki H., Aiba T., Watanabe S., Kawauchi Y., Kawachi H., Shimizu F., Matsushima K., Asakura H. and Narumi S. (2002). Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. European Journal of Immunology 32, 3197–3205 [DOI] [PubMed] [Google Scholar]
- Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F. and Kastelein R. A. (2005). IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 [DOI] [PubMed] [Google Scholar]
- Schopf L. R., Hoffmann K. F., Cheever A. W., Urban J. F. Jr and Wynn T. A. (2002). IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. Journal of Immunology 168, 2383–2392 [DOI] [PubMed] [Google Scholar]
- Selby G. R. and Wakelin D. (1973). Transfer of immunity against Trichuris muris in the mouse by serum and cells. International Journal of Parasitology 3, 717–722 [DOI] [PubMed] [Google Scholar]
- Shikhobalova N. P. (1937). Experimental study of the chemotherapy of trichocephalosis. I. Trichocephalosis of white mice. Medskya Parazit 6, 389–400 [Google Scholar]
- Song E., Ouyang N., Horbelt M., Antus B., Wang M. and Exton M. S. (2000). Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cellular Immunology 204, 19–28 [DOI] [PubMed] [Google Scholar]
- Svensson M., Bell L., Little M. C., Deschoolmeester M., Locksley R. M. and Else K. J. (2011). Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection. Parasite Immunology 33, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. C., Zaph C., Troy A. E., Du Y., Guild K. J., Comeau M. R. and Artis D. (2009). TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. Journal of Experimental Medicine 206, 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J., Rousseau K. and McGuckin M. A. (2008). Structure and function of the polymeric mucins in airways mucus. Annual Review of Physiology 70, 459–486 [DOI] [PubMed] [Google Scholar]
- Turner J. D., Faulkner H., Kamgno J., Cormont F., Van Snick J., Else K. J., Grencis R. K., Behnke J. M., Boussinesq M. and Bradley J. E. (2003). Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. Journal of Infectious Diseases 188, 1768–1775 [DOI] [PubMed] [Google Scholar]
- Urban J. F. Jr, Noben-Trauth N., Donaldson D. D., Madden K. B., Morris S. C., Collins M. and Finkelman F. D. (1998). IL-13, IL-4 Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 [DOI] [PubMed] [Google Scholar]
- Vallance B. A., Galeazzi F., Collins S. M. and Snider D. P. (1999). CD4T cells and major histocompatibility complex class II expression influence worm expulsion and increased intestinal muscle contraction during Trichinella spiralis infection. Infection and Immunity 67, 6090–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino A., Hibbert L., Lieberman L., Wilson E., Mak T., Yoshida H., Kastelein R. A., Saris C. and Hunter C. A. (2003). The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19, 645–655 [DOI] [PubMed] [Google Scholar]
- Wakelin D. (1967). Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology 57, 515–524 [DOI] [PubMed] [Google Scholar]
- Wakelin D. (1970a). The stimulation of immunity and the induction of unresponsiveness to Trichuris muris in various strains of laboratory mice. Zeitschrift für Parasitenkunde 35, 162–168 [DOI] [PubMed] [Google Scholar]
- Wakelin D. (1970b). Studies on the immunity of albino mice to Trichuris muris. Suppression of immunity by cortisone acetate. Parasitology 60, 229–237 [DOI] [PubMed] [Google Scholar]
- Wakelin D. (1973). The stimulation of immunity to Trichuris muris in mice exposed to low-level infections. Parasitology 66, 181–189 [DOI] [PubMed] [Google Scholar]
- Wakelin D. (1975). Immune expulsion of Trichuris muris from mice during a primary infection: analysis of the components involved. Parasitology 70, 397–405 [DOI] [PubMed] [Google Scholar]
- Wakelin D. and Selby G. R. (1973). Functional antigens of Trichuris muris. The stimulation of immunity by vaccination of mice with somatic antigen preparations. International Journal of Parasitology 3, 711–715 [DOI] [PubMed] [Google Scholar]
- Wilson M. S., Ramalingam T. R., Rivollier A., Shenderov K., Mentink-Kane M. M., Madala S. K., Cheever A. W., Artis D., Kelsall B. L. and Wynn T. A. (2011). Colitis and intestinal inflammation in IL10−/− mice results from IL-13Ralpha2-mediated attenuation of IL-13 activity. Gastroenterology 140, 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley D. E., Meisenhelder J. E., Sheffield H. G. and Thompson P. E. (1962). Experimental studies on Trichuris muris in mice with an appraisal of its use for evaluating anthelmintics. Journal of Parasitology 48, 433–437 [PubMed] [Google Scholar]
- Yamauchi J., Kawai Y., Yamada M., Uchikawa R., Tegoshi T. and Arizono N. (2006). Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. Acta Pathologica Microbiologica et Immunologica Scandinavica 114, 270–278 [DOI] [PubMed] [Google Scholar]
- Zhao A., Urban J. F. Jr., Anthony R. M., Sun R., Stiltz J., Van Rooijen N., Wynn T. A., Gause W. C. and Shea-Donohue T. (2008). Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135, 217–225, e211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N., Mishra A., King N. E., Fulkerson P. C., Doepker M. P., Nikolaidis N. M., Kindinger L. E., Moulton E. A., Aronow B. J. and Rothenberg M. E. (2004). Transcript signatures in experimental asthma: identification of STAT6-dependent and -independent pathways. Journal of Immunology 172, 1815–1824 [DOI] [PubMed] [Google Scholar]