Abstract
Reduced production of nitric oxide (NO) is one of the first indications of endothelial dysfunction and precedes overt cardiovascular disease. Increased expression of Arginase has been proposed as a mechanism to account for diminished NO production. Arginases consume l-arginine, the substrate for endothelial nitric oxide synthase (eNOS), and l-arginine depletion is thought to competitively reduce eNOS-derived NO. However, this simple relationship is complicated by the paradox that l-arginine concentrations in endothelial cells remain sufficiently high to support NO synthesis. One mechanism proposed to explain this is compartmentalization of intracellular l-arginine into distinct, poorly interchangeable pools. In the current study, we investigated this concept by targeting eNOS and Arginase to different intracellular locations within COS-7 cells and also BAEC. We found that supplemental l-arginine and l-citrulline dose-dependently increased NO production in a manner independent of the intracellular location of eNOS. Cytosolic arginase I and mitochondrial arginase II reduced eNOS activity equally regardless of where in the cell eNOS was expressed. Similarly, targeting arginase I to disparate regions of the cell did not differentially modify eNOS activity. Arginase-dependent suppression of eNOS activity was reversed by pharmacological inhibitors and absent in a catalytically inactive mutant. Arginase did not directly interact with eNOS, and the metabolic products of arginase or downstream enzymes did not contribute to eNOS inhibition. Cells expressing arginase had significantly lower levels of intracellular l-arginine and higher levels of ornithine. These results suggest that arginases inhibit eNOS activity by depletion of substrate and that the compartmentalization of l-arginine does not play a major role.
Keywords: nitric oxide, endothelial nitric oxide synthase, arginase, l-arginine, urea
nitric oxide (NO) is a vasoactive molecule that is vital for the maintenance of proper vascular function (8, 20, 25). In blood vessels, NO is produced by endothelial nitric oxide synthase (eNOS) and potently relaxes vascular smooth muscle to regulate blood flow as well as prevent leukocyte adhesion, cytokine formation, and platelet aggregation (10, 35, 51). Reduced levels of NO have been shown to play a prominent role in the negative effects of age on vascular function as well as diseases such as atherosclerosis, hypertension, and diabetes (12, 37).
eNOS is expressed predominantly within the vascular endothelial cell and catalyzes the conversion of l-arginine to l-citrulline to generate NO. The purification and genetic identification of eNOS in the early 1990s revealed an enzyme requiring tetrahydrobiopterin (BH4), nicotinamide adenine dinucleotide phosphate, FAD, flavin mononucleotide (FMN), and oxygen and whose activity is acutely controlled by calcium/calmodulin binding (16, 36, 42, 62). However, subsequent studies rapidly expanded the mechanisms controlling its activity, including additional protein-protein interactions, phosphorylation, and subcellular localization (19, 20, 42, 59). Following translation, eNOS traffics to the perinuclear Golgi and also to plasma membrane caveolae (17). eNOS has also been reported in the mitochondria, the nucleus, and the cytoskeleton (1, 14, 66). The activity of eNOS varies depending on its location within the cell, and the highest activity is observed at the plasma membrane, followed by outer membranes of the cis-Golgi and very low activity in the nucleus and mitochondria (17, 23, 30, 61, 65). In cells replete with extracellular l-arginine, we have shown previously that these location-dependent changes in eNOS activity are most prominently influenced by the availability of calcium/calmodulin. Calcium-insensitive forms of eNOS (CIeNOS) and other calcium-independent nitric oxide synthases (iNOS) display equivalent activity regardless of their intracellular location (6, 30).
l-Arginine is regarded as a semiessential amino acid, meaning that in healthy individuals it is nonessential, but in states of development, poor nutrition, or disease, de novo production is insufficient to meet cellular demand, which necessitates exogenous intake (5, 8, 26, 27, 54, 55). l-Arginine is metabolized by a variety of enzymes, including arginine/glycine aminotransferase, arginine decarboxylase, nitric oxide synthases, and Arginase I and II.
Arginase I (Arg I) and arginase II (Arg II) are encoded by two different genes on different chromosomes yet retain 60% amino acid homology and are catalytically similar enzymes that convert l-arginine into urea and ornithine (44). A major difference between Arg I and Arg II is that they reside in separate intracellular compartments, which has led to the proposal that they have different cellular functions. Arg I is located in the cytosol and Arg II within the mitochondria (22, 31, 45, 49, 70). Arg I has been implicated in diabetic retinopathy, asthma, coronary artery dysfunction during renovascular hypertension, and sickle cell disease (22, 43, 46, 53, 73, 75), and Arg II has been shown to be increased specifically in retinopathy of prematurity, human pulmonary arterial endothelial cells during hypertension, atherosclerosis, and diabetic renal injury (46, 48, 56, 71). A clear distinction between the vascular effects of Arg I vs. Arg II has not been established due to the lack of specific isoform inhibitors and also because the Arg I-knockout mouse is not viable beyond postnatal days 10–14. Many publications have broadly investigated increases in Arginase protein expression or activity and established correlations to deficits in vascular function observed in diseases such as hypertension, ischemia-reperfusion, and diabetes, aging, and vascular remodeling to name but a few (2, 24, 28, 32, 33, 39, 52, 68, 73).
The arginases have been proposed as endogenous regulators of eNOS activity via competition for their common substrate l-arginine. It has been reported that increases in Arginase expression and activity track with impaired NO synthesis in cardiovascular disease and also that inhibition of Arginase has been shown to improve NO production (11, 34). However, this relationship between eNOS and the arginases is more complex than it initially appears and has been linked to the “arginine paradox.” The arginine paradox describes a phenomenon where increased extracellular l-arginine is required to elicit maximal NO release from cells despite cytosolic concentrations of l-arginine (100–800 μM) well above that required to support maximum eNOS activity (Km = 3 μM) (70). Based on the high level of intracellular l-arginine, it remains unclear how excess extracellular l-arginine can increase eNOS activity. One mechanism that has been proposed is the colocalization of eNOS with enzymes that consume l-arginine, such as the arginases, and thus l-arginine can be depleted locally, which constrains nearby eNOS activity. Whereas the affinity of NOS enzymes for l-arginine is ∼1,000-fold greater than that of the arginases (Km = 2 mM), the Vmax of the Arginases is ∼1,000-fold greater than that of the NOS enzymes (16, 70). Therefore, it has been postulated that these enzymes can compete equally for available free l-arginine (11, 58, 70). Furthermore, it is theorized that the intracellular compartmentalization of Arg I and Arg II could enable the depletion of poorly interchangeable pools of l-arginine to levels below the threshold required for NO synthesis without robustly decreasing total cellular l-arginine (7, 64). Indeed, noninterchangeable l-arginine pools have been proposed in endothelial cells (63, 64), and therefore, compartmentalization could account for changes in eNOS activity driven by l-arginine. However, this concept has never been rigorously tested.
Therefore, the goal of the current study was to test this hypothesis using a molecular approach to target both eNOS and arginase to the same and different locations within the cell and to determine whether intracellular location can impact the sensitivity of eNOS to l-arginine or l-citrulline. We found that regardless of the location of either enzyme, there was no evidence to support the existence of restricted pools of l-arginine as determined by changes in the production of NO. We also investigated additional mechanisms underlying the ability of arginase to reduce eNOS activity.
MATERIALS AND METHODS
Cell culture and transfection.
COS-7 (ATCC) and bovine aortic endothelial cells (BAEC; VEC Technologies) were grown in Dulbecco's modified Eagle's medium (DMEM) containing penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% (vol/vol) fetal bovine serum and transfected with Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). COS-7 cells were cotransfected with pcDNA3 encoding human eNOS or a CIeNOS (see below) and a control plasmid expressing red florescent protein (RFP). Alternatively, COS-7 cells were cotransfected with eNOS constructs targeting subcellular domains and Arg I (rat; NM 017134) or Arg II (mouse; NM 009705). BAEC were transduced with eNOS and Arginase adenovirus, as described previously (17). l-Arginine- and l-lysine-free DMEM was supplemented with l-lysine and dialyzed serum (Thermo Scientific).
Cell lysis and isolation of cytosolic and mitochondrial fractions.
COS-7 cells (5.5 × 106) expressing Arg I and Arg II constructs were harvested by scraping and washed three times in Ca2+- and Mg2+-free phosphate-buffered saline (PBS). Next, cells were concentrated by centrifuging at ∼600 g, and the resulting cell pellet was suspended in chilled in isotonic MSE buffer (10 mM Tris·HCl, pH 7.5, 220 mM mannitol, 70 mM sucrose, 1 mM EGTA, 0.025% bovine serum albumin, 2 mM taurine, 1.6 mM carnitine, and 5 μg/ml each of leupeptin, aprotinin, phenylmethylsulfonylfluoride, and soybean trypsin inhibitor). Dounce homogenization was then conducted, and the cell lysates were centrifuged twice at 600 g to pellet nuclei and cell debris. The resulting supernatants were then centrifugated at 12,000 g. The final supernatants were used as the cytosolic fraction, and the 12,000-g pellet was used as the mitochondrial fraction in Western blot analyses using cytochrome oxidase IV and β-tubulin (Invitrogen).
Subcellular targeting constructs.
The eNOS subcellular targeting constructs have been described in previous publications (7, 17, 18, 74). Using the same targeting methodology, the various subcellular targeting sequences were fused onto the open reading frame of the NH2 terminus of FLAG-Arg I and subcloned into an expression vector (pcDNA3). The catalytically inactive mutant of arginase, D128G (67), was generated by PCR-driven site-directed mutagenesis using the following primers and confirmed by bidirectional sequencing: forward 5′-CTCACACTggcATCAACACTCCGCTGACAAC-3′; reverse 5′-GTTGATgccAGTGTGAGCATCCACCCAAATG-3′.
Arginase activity assay.
Cell lysates were prepared as described previously (6) and centrifuged at 12,000 g for 10 min. Arginase activity in lysates was determined as the conversion of [14C]guanidino-l-arginine (American Radiolabeled Chemicals) to [14C]urea, which was then converted to 14CO2 by urease and trapped as Na214CO3 and counted on a scintillation counter, as described previously (47). Protein concentrations were determined via a DC Lowry assay (Bio-Rad) and used to calculate arginase-specific activities, which are expressed in nanomoles per minute per milligram.
Adenoviral vectors.
eNOS adenoviruses have been described previously (4, 6, 59). Arg I and mutants were subcloned into pAD-CMV-DEST (Invitrogen) and confirmed by restriction enzyme digestion. Recombinant plasmids were then transfected into HEK-293 cells. Viral production was monitored over 7–10 days by visualization of the cytopathic effect or fluorescent protein expression. The virus was then harvested and purified by banding on a CsCl gradient. The purified virus was dialyzed and stored at −80°C.
NO release from cells.
Twenty-four hours after transfection of COS-7 cells, the medium was replaced with fresh DMEM. After another 24 h the medium was collected for NO analysis, and the cells were collected for analysis of protein expression. The medium was diluted 1:2 with 100% ethanol and spun down at 14,000 rpm for 10 min to remove suspended proteins. The level of NO in the medium was then measured by NO-specific chemiluminescence in a Sievers Nitric Oxide Analyzer. The background level of NOx was always subtracted by first measuring the levels in the medium from cells transfected with an empty vector (RFP), as COS-7 cells do not endogenously express any NOS.
Protein analysis by immunoblot.
Cells were washed twice with PBS and lysed in a homogenization buffer containing 50 mM Tris·HCl, 0.1 mM EGTA, 0.1 mM EDTA, 0.1% SDS, 1% Nonidet P-40, and 0.1% deoxycholic acid. Lysates were then further homogenized by three 1-s bursts with a sonic dismembrator (Fisher Model 100, setting 3). Lysates were boiled for 5 min at 100°C and spun down, and the resulting supernatant was used in the Western blot analysis. Membranes were probed with primary antibodies for human eNOS (BD Bioscience), Arg I (BD Bioscience), and Arg II (Santa Cruz Biotechnology). Heat shock protein (Hsp90; BD Bioscience) was used as the protein loading control. For immunoprecipitation, cells were washed twice with cold PBS and then collected in an immunoprecipitation (IP) buffer consisting of 20 mM Tris·HCl, 137 mM NaCl, 10% glycerol, and 1% NP-40 (pH 7.5). Cell extracts were then rotated for 45 min at 4°C and centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was then incubated with eNOS primary antibody overnight at 4°C. Forty micoliters of 2× protein A/G beads (Santa Cruz Biotechnology) was then added to the buffer and rotated at 4°C for an additional hour. Immune complexes were then spun down at 1,000 rpm at 4°C. The supernatant was then aspirated, and the beads were washed twice in IP buffer. Remaining beads were then washed in TBS, centrifuged (1,000 rpm, 4°C, 5 min), resuspended in 2× Laemmli buffer, and boiled for 5 min. The extracted proteins were size fractionated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with appropriate antibodies. In some experiments, immunoblots for Arg I were incubated with chicken anti-Arg I as primary antibody and with IRDye 800CW anti-chicken IgG as secondary antibody in 1% nonfat milk-1% BSA-TBS-T [20 mM Tris (pH 7.6)-137 mM NaCl-5 mM MgCl2-0.1% Tween 20]. Immunoblots for GAPDH were incubated with rabbit anti-GAPDH (Santa Cruz Biotechnology) as primary antibody and with IRDye 680LT anti-rabbit IgG (cat. no. 827-08367) in 5% nonfat milk-TBS-T.
Immunohistochemistry and confocal images.
In brief, BAEC were cultured in Lab-Tek chamber slides (Thermo Fisher Scientific) until 60% confluence. The cells were then transduced with adenovirus for PM Arg I, as described previously. After 24 h, chambers were washed with PBS, fixed with 4% paraformaldehyde (overnight at 4°C), permeabilized with 0.1% Triton-X for 30 min, washed in PBS, and blocked in 10% BSA. Chambers were incubated overnight in primary antibody at 4°C and then incubated in fluorescein-conjugated secondary antibody (Molecular Probes), washed with PBS, and mounted with Vectashield with DAPI (Vector Laboratories). Prepared slides were imaged with a Zeiss confocal microscope (LSM 510 Meta/Axiovert 200M Imaging and Zen Software).
Cellular amino acid quantification analysis.
Transfected cells were cultured in DMEM for 24 h and then switched to l-arginine-free medium supplemented with the variable concentrations of l-arginine for 24 h. Cells were washed twice in 1× PBS, collected, precipitated with sulfosalicylic acid to remove any intact proteins, and centrifuged, and the supernatant was mixed with Li-diluent spiked with AE-Cys final dilution. Samples were analyzed using a Hitachi 8900 amino acid analyzer ith an ion exchange Hitachi column, using Li-based buffers from Hitachi and a postcolumn reaction with ninhydrin. Observations were made at 570 (Vis1) and at 440 nm (Vis2) and calibrated to an amino acid standard (Sigma) and analyzed with a method developed by MSF. Analysis was performed by John Schulze and Jack Presley at the University of California-Davis.
Statistics.
Normally distributed data were analyzed by ANOVA (GraphPad Prism), using the Bonferroni method for multiple comparisons. For data sets with less than n = 5, we used Kruskal-Wallis nonparametric ANOVA with Dunn's method for multiple comparisons. Data are reported as means ± SE.
RESULTS
Demonstration of the arginine paradox in COS-7 cells.
The primary tenet of the arginine paradox is the ability of excess extracellular l-arginine to activate eNOS or stimulate NO production despite the presence of intracellular l-arginine in concentrations predicted to support maximal eNOS activity based on Km. In these experiments, COS-7 cells were chosen because they do not express eNOS endogenously, and exogenously expressed eNOS exhibits a similar subcellular localization to that observed in endothelial cells (18). In COS-7 cells transfected with wild-type eNOS (WT eNOS), NO release was measured in the presence of increasing l-arginine concentrations (0–3,000 μM). l-Arginine induced a concentration-dependent increase in NO production (Fig. 1A). Notably, 100 μM l-arginine, a concentration found in normal human plasma and a value at the Km of the system y+ transporters of l-arginine and well above the Km of eNOS for l-arginine, elicited less than maximal eNOS activity vs. higher l-arginine concentrations. These results support the existence of the arginine paradox in COS-7 cells. In cells expressing CIeNOS constructs targeted to distinct intracellular locations [Golgi and plasma membrane (wild type), cytosol, Golgi (S17), plasma membrane, and the mitochondria], we also compared the relative ability to produce NO in the presence of increasing concentrations of l-arginine and l-citrulline (Fig. 1, B–F). eNOS was targeted to discrete areas of the cells, as described previously (6, 74). In brief, the point mutation of glycine 2 of eNOS to alanine, which prevents both N-myristoylation and subsequent palmitoylation required for targeting the Golgi and plasma membrane, results in a catalytically competent enzyme that resides in the cytosol (G2A) (21, 60). This cytosolic eNOS construct was then fused to a number of targeting sequences that then direct it to the Golgi, the inner leaflet of the mitochondria, the plasma membrane, and the aforementioned cytosolic eNOS. Calcium-activated calmodulin is necessary for eNOS activation (41), and the difference in eNOS activity when targeted to other intracellular locations is critically dependent on calcium/calmodulin availability (30). Previously, a CIeNOS was characterized by our laboratory and allows for constitutive and equal eNOS activity regardless of its intracellular location (4, 6). We found that the ability of l-arginine to activate CIeNOS in different compartments was equivalent. Supplemental l-citrulline, which must first be converted by argininosuccinate synthase (ASS)/argininosuccinate lyase (ASL) to l-arginine to enable NO production, had no effect on NO production at concentrations <1,000 μM. Even at higher concentrations, l-citrulline was less potent on a molar basis than l-arginine at stimulating NO production, and it did not preferentially activate eNOS in the different intracellular locations.
Fig. 1.
The intracellular location of endothelial nitric oxide synthase (eNOS) does not modify the ability of l-arginine (l-Arg) or l-citrulline to stimulate nitric oxide (NO) production. A: COS-7 cells were cotransfected with cDNAs encoding eNOS and control plasmid of the red fluorescent protein (RFP). Cells were washed in l-Arg-free medium for 30 min and incubated in the indicated concentrations of l-Arg for 24 h. NO levels were assessed using a NO analyzer and are reported as %NO release in the presence of DMEM. The relative expression of eNOS and heat shock protein 90 (Hsp90; loading control) was obtained by immunoblotting with anti-eNOS and anti-Hsp90. Results are presented as %control of 3 mM l-Arg. Results are presented as means ± SE; n = 6 experiments. B–F: COS-7 cells were transfected with a calcium-insensitive eNOS (CIeNOS) targeted per wild-type eNOS or to the cytosol, Golgi, plasma membrane, or mitochondria. Cells were incubated in l-Arg-free DMEM in the presence of the indicated concentrations of l-Arg or l-citrulline for 24 h, and NO release was determined by NO-specific chemiluminescence. Results are presented as means ± SE; n = 4. Nonparametric 1-way ANOVA; Kruskal-Wallis test with a Dunns multiple comparison of all columns within each substrate addition. *P < 0.05 vs. 0 μM l-Arg; #P < 0.05 vs. 0 μM l-citrulline.
Arg I and Arg II are targeted to distinct subcellular domains but have similar, nonadditive inhibitory effects on eNOS-derived NO.
To determine whether the Arg I and Arg II constructs target their reported subcellular locations, COS-7 cells were transfected with Arg I and Arg II and then subjected to subcellular fractionation. The mitochondrial fraction was separated from the cytosolic fraction using serial centrifugal fractionation, and fractions were then probed for mitochondrial markers [cytochrome oxidase subunit 4 (CO4); Mitosciences] and cytosolic markers (β-tubulin; Cell Signaling Technology) as well as Arg I (BD Biosciences) and Arg II (Santa Cruz Biotechnology) via Western blot. The cytosolic fraction was identified by β-tubulin immunoreactivity and was positive for Arg I but not Arg II. The mitochondrial fraction was identified by the presence of CO4 and the absence of β-tubulin and was positive for Arg II (Fig. 2A).
Fig. 2.
Arginase (Arg) I and Arg II target different subcellular compartments but do not have an additive effect on NO release. A: COS-7 cells were cotransfected with plasmids encoding Arg I and Arg II. Cells were fractionated into cytosolic (Cyto) or mitochondrial (Mito) fractions by differential centrifugation. β-Tubulin was used as a marker for the Cyto compartment and cytochrome oxidase subunit 4 (CO4) for mitochondria. The relative expression of Arg I and Arg II was confirmed by immunoblotting using anti-Arg I and anti-Arg II antibodies. Results are representative of 4 independent experiments. B and C: COS-7 cells were cotransfected with plasmids encoding wild-type eNOS or a CIeNOS construct and either a control gene (RFP) or Arg I/Arg II. NO production was measured by NO analyzer and is reported as %control (eNOS + RFP or CIeNOS + RFP). The expression of relevant proteins was determined by immunoblotting. Hsp90 was used as a loading control. D: titration of Arg I expression against a fixed concentration of eNOS. Numbers indicate that the ratio of transfected DNA and the expression of relevant proteins are shown below. Results are presented as means ± SE; n = 6 experiments. *P < 0.05 vs. control.
To assess the effect of Arg I and Arg II on eNOS-derived NO, COS-7 cells were cotransfected with eNOS and RFP (DNA control), eNOS and Arg I, and eNOS and Arg II. The control group of eNOS and RFP was assigned as 100% NO production. As shown in Fig. 2B, both Arg I and Arg II reduced eNOS-derived NO despite the abundance of l-arginine in the media (DMEM, 400 μM l-arginine). Although the amount of NO produced in cells expressing the CIeNOS was greater than in cells expressing the calcium-sensitive wild-type eNOS, both Arg I and Arg II maintained the ability to significantly reduce eNOS-derived NO (Fig. 2C). To determine the importance of the intracellular location of arginase in its ability to impact eNOS activity, we also investigated whether the combination of Arg I (cytosol) and Arg II (mitochondria) was more effective than either Arg I or Arg II alone. We found that there was no additive inhibition with the combined expression of Arg I and Arg II vs. single expression of either arginase isoform (Fig. 2C). Densitometric analysis of eNOS expression revealed no significant difference in expression in the presence of Arg I and Arg II. To establish a dose relationship between the level of Arg I expression and eNOS inhibition, we cotransfected increasing concentrations of Arg I together along with a fixed concentration of CIeNOS. We found that the ability of Arg I to reduce CIeNOS-derived NO was apparent at low amounts of Arg I expression and then rapidly plateaued despite higher concentrations (Fig. 2D).
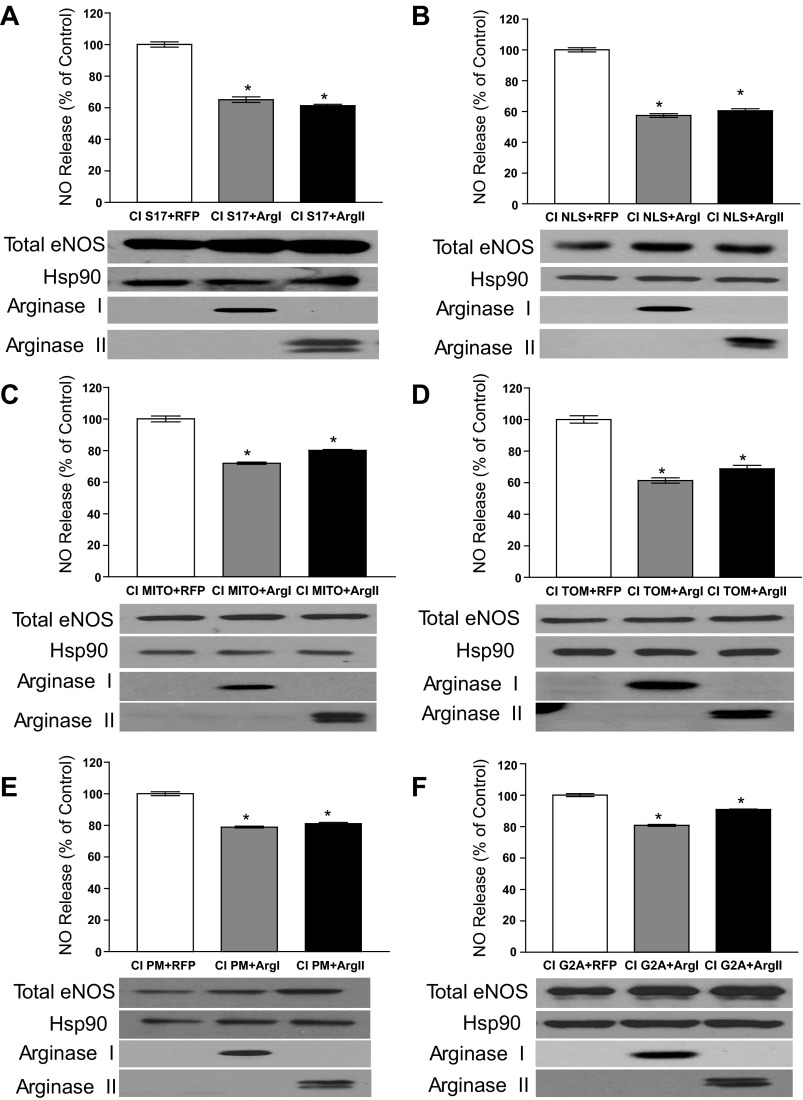
Arg I and Arg II reduce eNOS activity equally regardless of the subcellular location of eNOS.
To directly address the concept of intracellular pools of l-arginine, the targeted CIeNOS constructs were cotransfected with Arg I and Arg II to determine whether the ability of Arg I and Arg II to suppress eNOS activity is due to the presence of restricted pools of l-arginine. However, we found that regardless of where eNOS was targeted, the cotransfection of either Arg I or Arg II resulted in equivalent reduction in eNOS activity in the S17 (Fig. 3A) or nucleus (NLS; Fig. 3B), inner mitochondria (MITO; Fig. 3C), outer mitochondria (TOM; Fig. 3D), plasma membrane (PM; Fig. 3E), and G2A (Fig. 3F). Given the unavoidable interexperiment variability inherent with multiple cotransfection experiments, the goal of Fig. 3 was to compare the respective inhibitory effects of cotransfected Arg I vs. Arg II on the activity of a single localized eNOS within the same experiment. Comparisons between the effect of Arg I or Arg II on the different localized eNOS constructs cannot be made since they were assessed independently in different experiments. Regardless of the location of eNOS, the differential effect of Arg I and Arg II was equivalent. In the absence of arginase, the production of NO from CIeNOS was found to be similar despite changes in location, and these data are consistent with Fig. 1 and previous publications (50). There was no significant difference between eNOS expression as determined by densitometry relative to Hsp90.
Fig. 3.
The location of eNOS does not affect the relative inhibition by Arg I vs. Arg II. COS-7 cells were cotransfected with different eNOS constructs targeted to different intracellular locations and either RFP (transfection control), Arg I, or Arg II. CI S17, CIeNOS localized to the Golgi (A); CI NLS, CIeNOS localized to the nucleus (B); CI Mito, CIeNOS localized to the inner mitochondrial membrane (C); CI TOM, CIeNOS localized to the outer mitochondrial membrane (D); CI PM, CIeNOS localized to the plasma membrane (E); CI G2A, CIeNOS localized to the cytosol (F). NO release was measured by NO analyzer and is presented as %RFP cotransfection control (top of graphs). The expression levels of relevant proteins were determined by immunoblotting (bottom). Hsp90 was used as a loading control. Results are represented as means ± SE; n = 6 experiments. *P < 0.05 vs. control.
Irrespective of its intracellular location, ArgI reduces eNOS-derived NO equally.
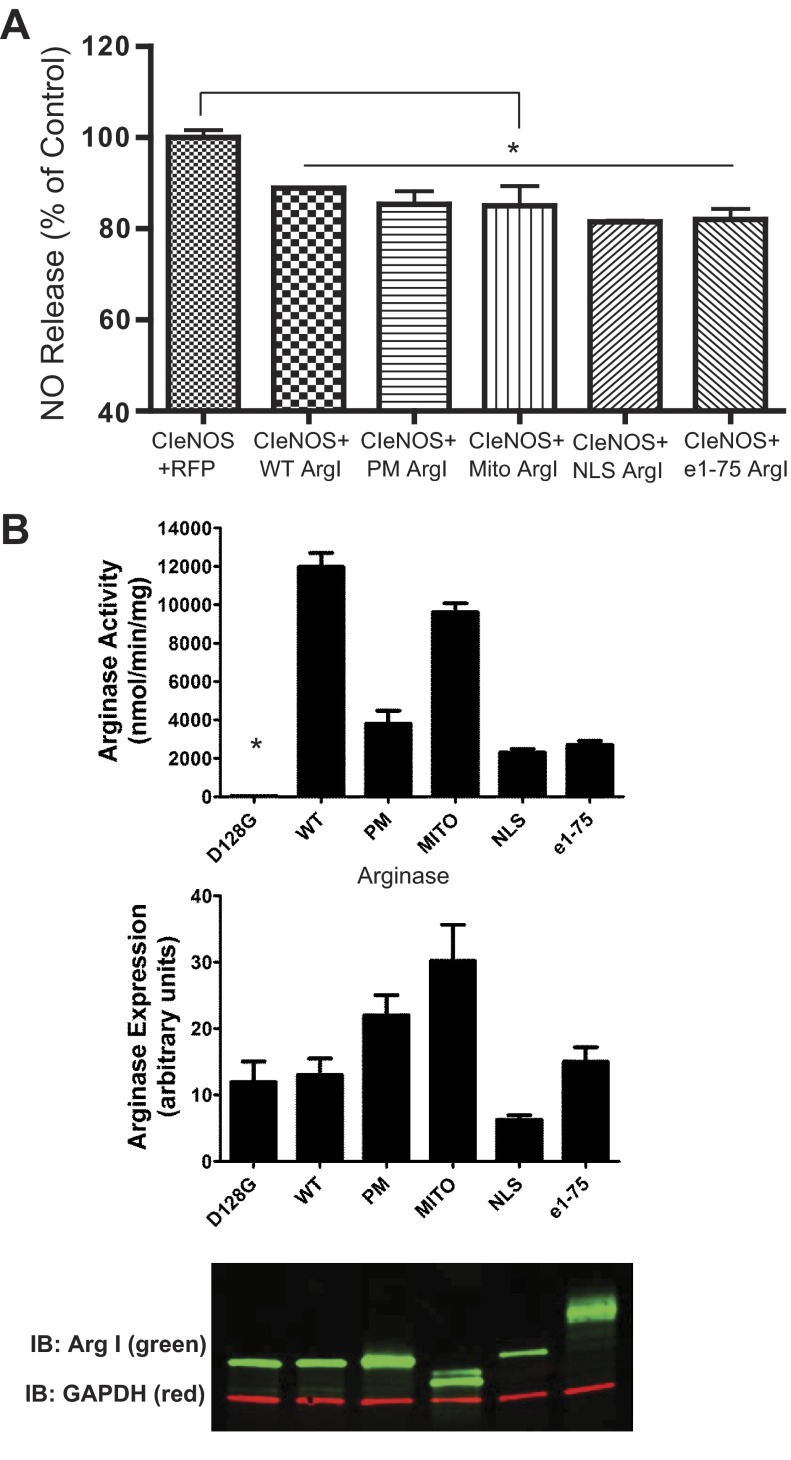
Since there was no additive effect of cotransfection of Arg I and Arg II on eNOS activity, intimating that there was a similar mechanism of action between the two arginases, subsequent studies focused exclusively on Arg I. To further explore the importance of subcellular location to the arginine paradox, we redirected Arg I from the cytosol to the PM, the MITO, and the NLS, using the same targeting sequence as per native eNOS, to the S17 and plasma membrane (e1–e75) using a strategy similar to that described above for CIeNOS and evaluated the effect on eNOS activity. The first 75 amino acids of eNOS (e1–e75), which contain the three sites of fatty acylation, are sufficient to target other proteins, including green fluorescent protein (GFP) and iNOS to the same location as eNOS, namely the S17 and PM (30).
We found that the targeting of Arg I to different intracellular locations did not modify its ability to suppress eNOS activity (Fig. 4A). However, the different Arg I constructs did appear to vary from the wild types in their specific activities in cell lysates (Fig. 4B), although these were not significant, using a nonparametric ANOVA. The highest arginase activity was observed in the Arg I constructs targeted to the cytosol (wild type) and to the mitochondria, but Arg I constructs targeted to the membrane (PM and e1–e75) or the NLS exhibited lower activity. The expression level of Arg I targeted to the NLS was slightly lower (Fig. 4B) than the wild types, but this is unlikely to account for the differences in activity and was not significant. The doublet observed for MITO-Arg I and the smear for e1–e75-Arg I is suggestive of posttranslational modification. Mutation of aspartic acid 128 to glycine (D128G) has been shown to disturb the binding pocket for manganese, a crucial cofactor for arginase activity. When expressed in COS-7 cells, the Arg I D128G mutant was present at the expected molecular weight but exhibited a complete loss of activity.
Fig. 4.
Arg I constructs targeted to different locations reduce eNOS-derived NO equally despite differences in relative activity. A: COS-7 cells were transfected with Arg I constructs that target specific subcellular domains by inframe fusion of targeting sequences. PM, plasma membrane; Mito, inner mitochondria; NLS, nucleus; e1–e75 (the targeting sequence from eNOS) represents the plasma membrane and Golgi. NO levels were measured and presented as %control (RFP). Results are represented as means ± SE; n = 12 experiments. *P < 0.05 vs. RFP. B: arginase activities in cell lysates from COS-7 cells transfected with targeted Arg I constructs. The expression levels of Arg I protein and the loading control GAPDH were determined by immunoblotting (green and red, respectively; bottom). Results are means ± SE; n = 4. *P < 0.05 vs. wild type using nonparametric 1-way ANOVA and Kruskal-Wallis test with Dunn's multiple comparison.
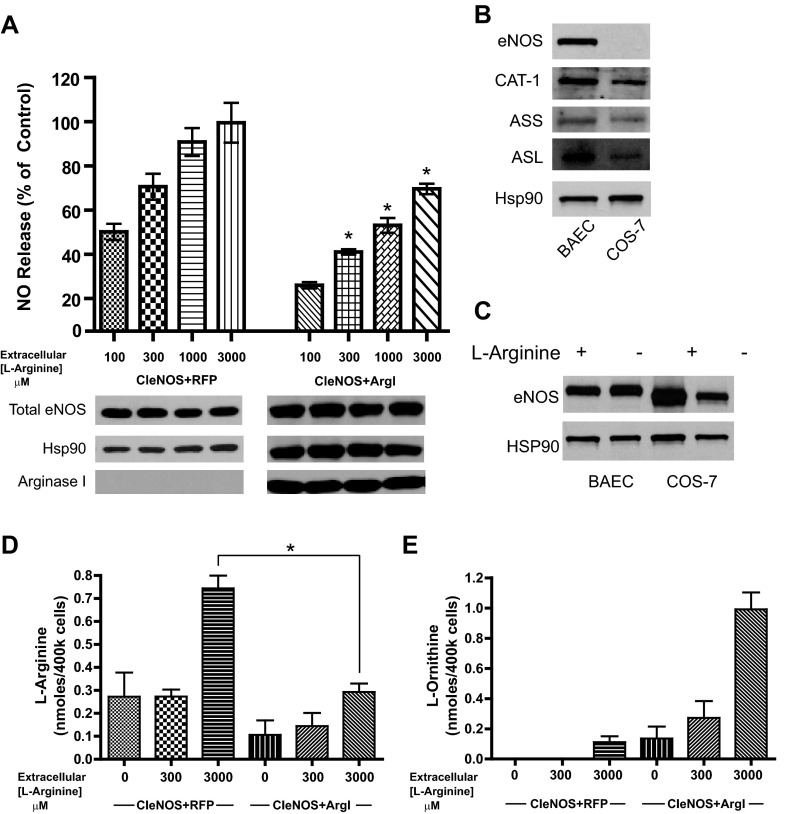
Arginase-dependent inhibition of eNOS activity cannot be fully reversed by supplemental l-arginine.
The goal of these experiments were to determine whether arginase is eliciting its inhibitory effects on eNOS by substrate competition. Two groups of cells were cotransfected with either CIeNOS and RFP or CIeNOS and Arg I and then incubated in medium with increasing amounts of l-arginine (0–3,000 μM). Consistent with previous experiments, l-arginine dose-dependently increased the activity of CIeNOS (Fig. 5A). Also as shown previously, Arg I reduced the activity of CIeNOS, but increasing amounts of l-arginine failed to completely restore NO production to control levels (Fig. 5A). Because we employed COS-7 to investigate the complexity of l-arginine metabolism, it was important to determine the relevance of this system to endothelial cells. Native BAEC and nontransfected COS-7 cells were subjected to Western blot for analysis of endogenous enzymes involved with l-arginine recycling. In Fig. 1, B–F, we demonstrated the ability of COS-7 cells to synthesize NO from l-citrulline. This cannot occur without conversion of l-citrulline to l-arginine through ASS and ASL. In Fig. 5B, eNOS is present in BAEC but is not expressed in untransfected COS-7 cells. As predicted from Fig. 1, B–F, cationic amino acid transporter (CAT-1), ASS, and ASL are expressed in both cell types. We found that omission of l-arginine in the culture medium results in a loss of transfected CIeNOS expression in transfected COS-7 cells. In contrast, BAEC maintain eNOS expression in l-arginine-free conditions (Fig. 5C). CIeNOS has a reduced size on SDS-PAGE due to the deletion of 59 amino acids in the autoinhibitory loops (6).
Fig. 5.
Extracellular l-arginine promotes greater NO production but cannot overcome the Arg I-mediated reduction in eNOS activity. A: COS-7 cells were transfected with either CIeNOS + RFP (control) or CIeNOS + Arg I. Twenty-four hours after transfection, cells were incubated in medium containing l-arginine (0–3,000 μM). NO release was measured by NO analyzer and presented as %NO release from cells cotransfected with CIeNOS + RFP control at 3,000 μM. Expression levels of eNOS, Arg I, and Hsp90 (loading control) were determined by immunoblotting (bottom). B: expression levels of eNOS, the cationic amino acid transporter (CAT-1), argininosuccinate synthase (ASS), and argininosuccinate lyase (ASL) vs. Hsp90 (loading control) in untreated bovine aortic endothelial cells (BAEC) and COS-7. C: expression levels of eNOS in BAEC and COS-7 grown in l-arginine replete and free DMEM. D and E: COS-7 cells cotransfected with CIeNOS + RFP or CIeNOS + Arg I and incubated for 24 h in the concentration of l-arginine notated below the bar graph. Cells were isolated, and relative amounts (nmol) of l-arginine (D) and ornithine (E) determined. Results are presented as means ± SE; n = 6 experiments. *P < 0.05 vs. control.
To gain further insight into the mechanism by which arginase reduces eNOS activity, we measured amino acid levels in COS-7 cells expressing eNOS and either ArgI or a control gene. Consistent with its ability to increase NO, 3,000 μM extracellular l-arginine resulted in increased intracellular l-arginine (Fig. 5D). However, the ability of l-arginine to achieve similar increases was diminished in cells expressing Arg I (Fig. 5D). The reduced level of intracellular l-arginine was likely due to the catalytic activity of increased levels of arginase, as l-ornithine levels were proportionally increased (Fig. 5E).
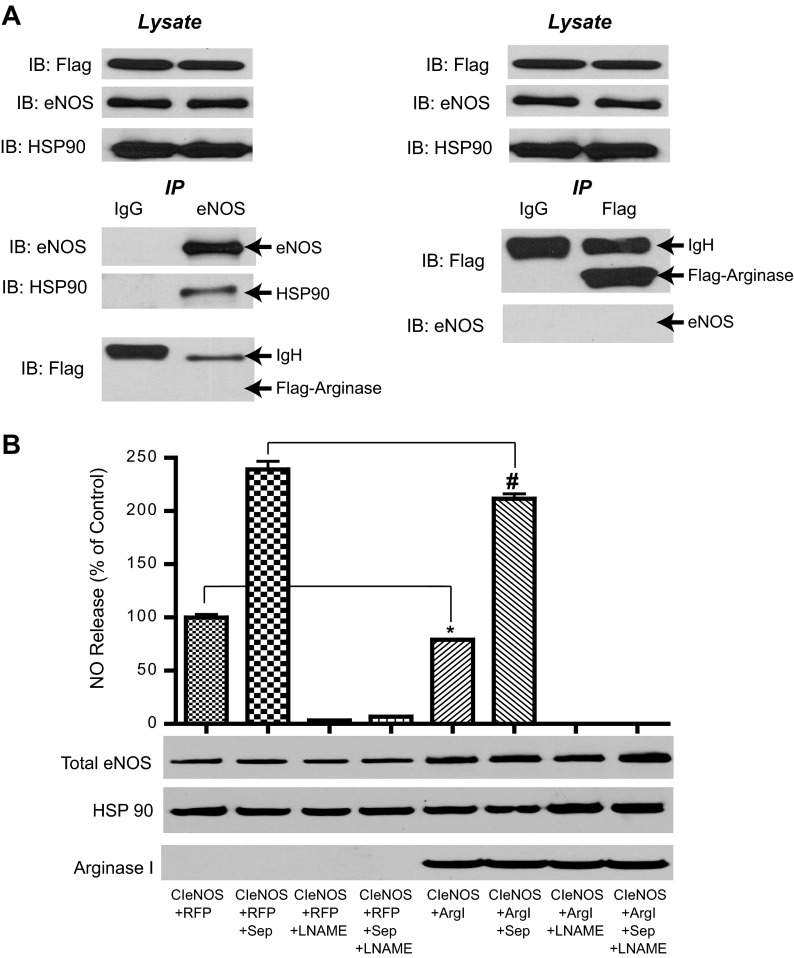
Neither BH4 nor protein-protein interactions between eNOS and Arg I can account for reduced NO production.
To exclude other possible mechanisms mediating the reduced activity of eNOS in the presence of arginase, we next examined whether a direct protein-protein interaction exists between the two proteins. Cells expressing eNOS plus Arg I were lysed, and both eNOS and arginase (Flag) were immunoprecipitated (IP) and immune complexes immunoblotted for Arg I (Flag), eNOS, and Hsp90 (eNOS IP control). Protein-protein interactions were not observed between eNOS and Arg I following immunoprecipitation of arginase or eNOS (Fig. 6A). Reduced BH4 levels are a major mechanism for compromised eNOS activity in cardiovascular diseases. To exclude the possibility that arginases, which can uncouple eNOS (11), impact BH4 levels, the BH4 precursor sepiapterin was added to cells expressing eNOS and arginase. Sepiapterin increased NO production in both groups, but NO levels in the CIeNOS and Arg I groups remained significantly reduced compared with the control (Fig. 6B).
Fig. 6.
Reduced eNOS activity in the presence of arginase is unrelated to cofactor suppression or direct interaction. A: COS-7 cells were transfected with eNOS + Arg I; eNOS and arginase (Flag epitope) were immunoprecipitated (IP) vs. a nonimmune control (IgG), and associated proteins were immunoblotted (IB). B: COS-7 cells transfected with CIeNOS + RFP or CIeNOS + Arg I were treated with or without the tetrahydrobiopterin (BH4) precursor sepiapterin (Sep; 100 μM) and with or without NG-nitro-l-arginine methyl ester (l-NAME; 1 mM) and NO levels determined by NO-specific chemiluminescence. Results are represented as means ± SE; n = 6 experiments. *P < 0.05 vs. CIeNOS + RFP; #P < 0.05 vs. CIeNOS + RFP + Sep.
Arginase catalytic activity is necessary for decreased eNOS-derived NO and can be pharmacologically but not metabolically reversed.
After determining that the ability of arginase to reduce eNOS activity was independent of intracellular location, studies were initiated to investigate whether the catalytic activity of arginase is necessary to account for reduced eNOS activity. The D128G Arg I mutant, which is catalytically inactive (Fig. 4B), had no inhibitory effect on eNOS activity (Fig. 7A). In the process of eNOS oxidizing l-arginine to NO, the intermediate product N-hydroxy-l-arginine (NOHA) is an effective inhibitor of arginase (3, 9). Modification of NOHA to nor-NOHA has yielded a more stable and potent arginase inhibitor. The addition of nor-NOHA to cells expressing eNOS and Arg I resulted in a significant reclamation of NO production (Fig. 7B). To determine whether enzymes downstream of arginase contribute to the inhibition of eNOS activity, we pursued other pathways. Arginase converts l-arginine into ornithine, which can be metabolized by ornithine decarboxylase (ODC) to the polyamines. The ODC inhibitor, difluoromethylornithine (DFMO) significantly inhibited NO production in both the control and experimental groups but did not prevent the arginase-dependent inhibition of eNOS activity (Fig. 7C). To assess whether the enzymatic products of arginase can influence NO production from eNOS, cells were exposed to progressively higher concentrations of urea and ornithine. Neither urea nor ornithine at concentrations as high as 10 mM influenced NO release (Fig. 7, D and E).
Fig. 7.
Catalytic activity is necessary for arginase-mediated inhibition of eNOS-derived NO but not ornithine metabolism. A: COS-7 cells were transfected with CIeNOS and either wild-type Arg I (WT Arg I) or the catalytically inactive Arg I (D128G). Top: NO was measured by NO-specific chemiluminescence and is presented as %NO release from cells transduced with RFP control. Bottom: the expression of relevant proteins was determined by Western blotting. B: NO release from COS-7 cells cotransfected with CIeNOS and either RFP or Arg I in the presence and absence of the arginase inhibitor nor-N-hydroxy-l-arginine (NOHA; 1 mM). C: NO release in the presence and absence of the ornithine decarboxylase (ODC) inhibitor difluoromethylornithine (DFMO; 50 μM). Results are represented as means ± SE; n = 6–12 experiments. *P < 0.05 vs. control (RFP); #P < 0.05, CIeNOS + Arg I vs. CIeNOS + Arg I + Nor-NOHA. D and E: NO release was measured in COS-7 cells expressing eNOS in the presence and absence of urea (0.1–10 mM) and l-ornithine (0.3–10 mM). There is no significant difference between NO release in urea or ornithine addition as determined by nonparametric 1-way ANOVA and Kruskal-Wallis test, with a Dunn's multiple comparison of all columns within urea addition or ornithine addition. Results are represented as means ± SE; n = 4. NS, not significant.
Differences in subcellular localization of arginase in endothelial cells do not reveal the existence of noninterchangeable l-arginine pools.
To determine whether the findings observed in COS-7 cells are consistent with that in endothelial cells, BAEC were transduced with adenoviruses encoding a control (RFP), catalytically inactive D128G Arg I, wild-type Arg I (WT Arg I) and PM-targeted Arg I (PM Arg I). Transduction of the inactive D128G Arg I adenovirus did not impact NO production in BAEC. In contrast, the WT Arg I and PM Arg I showed a 25–30% inhibition of NO compared with the RFP control (Fig. 8A), which was similar to that observed in COS-7 cells. The intracellular location of the PM Arg I was determined in BAEC by confocal microscopy. The Arg I adenovirus includes an inframe FLAG-epitope tag that enables detection of the intracellular location of the introduced arginase transgenes (Arg I) vs. that of the endogenous Arg I. In BAEC, the PM-targeted FLAG-Arg I was found predominantly at the PM (Fig. 8B). To determine the proportion of immunostaining observed within endomembranes vs. at the periphery of the cell, i.e., PM, we assessed Z-stacked confocal images. In Fig. 8B, the four micrographs are top down images of the same endothelial cell. The sidebars are image sections of the cell taken in 1-μm sections and then stacked to create a composite image. The cursor lines in Fig. 8B, top right, coordinate the plane of section stacking on the sidebars, with the x- and y-axis locations within the main image. The sidebar images of the cell appear “hollow,” which shows that the majority of the PM Arg I is located at the external membranes of the cell compared with the cytosol of the cell.
Fig. 8.

Transduction of endothelial cells with plasma membrane-targeted Arg I elicits equal eNOS inhibition compared with WT Arg I. BAEC were transduced with 20 MOI of RFP, catalytically inactive Arg I (D128G), WT Arg I, and PM-targeted arginase (PM Arg I) adenoviruses. A, top: NO release from BAEC was measured by NO-specific chemiluminescence and presented as %NO release from cells transduced with RFP control. A, bottom: expression of relevant proteins was determined by immunoblotting. Hsp90 was used as a loading control. Nonparametric 1-way ANOVA and Kruskal-Wallis test with Dunn's multiple comparison. Results are represented as means ± SE; n = 6 experiments. *P < 0.05 vs. RFP. B: confocal microscopy of BAEC transduced with PM Arg I shows the predominant PM location of targeted arginase. Green indicates the FLAG tag (PM Arg I), and blue indicates DAPI (nucleus). Results are representative of more than 3 independent experiments.
Arginase-dependent inhibition of eNOS activity in BAEC cannot be fully reversed with supplemental l-arginine.
The goal of this experiment was to determine whether the inhibitory effects of arginase on eNOS activity in endothelial cells are achieved by substrate depletion and whether this is consistent with results observed in COS-7 cells (Fig. 5A). BAEC were transduced with adenovirus expressing GFP, the catalytically inactive arginase (D128G), or the WT Arg I and incubated in medium with increasing amounts of l-arginine (0, 300, and 3,000 μM). Consistent with previous experiments in COS-7 cells, l-arginine dose-dependently increased NO production. NO production was reduced significantly in BAEC transduced with the Arg I adenovirus, and supplemental l-arginine failed to completely restore NO production to control levels (Fig. 9). The relative expression of endogenous, wild-type, and D128G Arg I adenoviruses was determined by blotting for FLAG and Arg I (Fig. 9, bottom).
Fig. 9.
Extracellular l-arginine stimulates NO production in BAEC but does not fully reverse the effect of Arg I. BAEC were transduced with adenovirus encoding green florescent protein (GFP; control), the catalytically inactive arginase (D128G), or WT Arg I at 20 MOI. Twenty-four hours after transduction, cells were incubated in medium containing l-arginine (0, 300, or 3,000 μM). NO release was measured via NO analyzer and presented as %NO release from control (GFP; 3,000 μM) cells. Expression levels of eNOS, Arg I, FLAG (D128G and Arg I virus tag), and Hsp90 (loading control) were determined by immunoblotting (bottom). Results are represented as means ± SE; n = 8 experiments. *P < 0.05 vs. D128G and GFP controls at 3,000 μM.
DISCUSSION
The relationship between eNOS, l-arginine, and arginase has been well studied, and yet some facets remain enigmatic. First and foremost, the mechanism by which the addition of excess l-arginine promotes greater production of NO (i.e., the arginine paradox), when the enzyme theoretically should be saturated with substrate, remains controversial. The best explanation for this posits that l-arginine does not exist in a single homogenously distributed pool inside the cell that is readily available to NOS enzymes. Discrete, poorly interchangeable pools of intracellular l-arginine would allow for the selective depletion of l-arginine in the vicinity of eNOS and be more dependent on replenishment from the extracellular environment or l-arginine recycling pathways. McDonald et al. (40) explored this relationship when they observed the colocalization of CAT-1, which imports l-arginine and eNOS in caveolae of the plasma membrane of porcine pulmonary endothelial cells. A nonhomogenous distribution of CAT-1/caveolin/eNOS complexes was discovered at the plasma membrane and was proposed to mediate l-arginine-stimulated transport into the cell and the selective utilization of extracellular l-arginine by eNOS at the plasma membrane. A series of elegant articles has exploited the ability of the CAT-1 transporters to bind l-lysine and prevent l-arginine import to characterize the intracellular pools of l-arginine (7, 63, 64). Collectively, these studies showed that incubation of endothelial cells with l-lysine (which cannot be used by eNOS for NO production) does not diminish the capacity of cells to continually produce NO. An explanation for these findings was that there must be unique intracellular pools of l-arginine within endothelial cells that are not freely accessible to l-arginine-metabolizing enzymes or freely interchangeable with the extracellular l-arginine supply and that these pools are more reliant on l-citrulline recycling or l-arginine obtained from the breakdown of proteins. However, the nature and importance of these pools of l-arginine have not been rigorously defined.
The importance of arginase to this relationship is underscored by a large number of studies showing that arginase expression and activity are increased in many cardiovascular disease states, such as atherosclerosis and diabetes, and that this occurs contemporaneously with reduced NO levels (2, 11, 32, 46, 53, 56, 57). One explanation provided for the inhibitory effect of increased arginase expression is that arginase can compete with eNOS for l-arginine, and the reduction in local l-arginine levels compromises the ability of eNOS to produce NO or promotes the uncoupling of eNOS so that it produces superoxide rather than NO. Therefore, the goal of the present study was to use a molecular approach to determine whether putative pools of l-arginine exist, whether they are functionally relevant, and also whether they can explain the arginine paradox.
In the initial experiments, COS-7 cells were used to determine the functional relationship between arginase, eNOS, and l-arginine. COS-7 cells possess numerous properties that make them suitable for these studies. First, they are amenable to transfection and have the metabolic enzymes required to support the synthesis of NO, such as GTP cyclohydrolase. They lack eNOS and have minimal arginase expression, which enables the expression of eNOS and arginase constructs targeted to various intracellular locations without confounding interference from the endogenous enzymes. COS-7 cells also contain amino acid transporters and metabolic enzymes (CAT-1, ASS, and ASL) that allow for l-arginine uptake and the conversion of l-citrulline to l-arginine. We confirmed this functionally using l-arginine and l-citrulline to drive NO production and more directly by Western blotting. A recent study by Erez et al. (13) revealed the importance of the arginine-recycling enzyme ASL to the eNOS regulatory complex and NO production, and its presence in COS-7 cells further supports the applicability of our approach. We found that in eNOS-transfected COS-7 cells the extracellular addition of l-arginine increased intracellular l-arginine concentrations and stimulated NO production, which is consistent with the arginine paradox.
To determine the importance of the intracellular location of eNOS to this relationship, we expressed a CIeNOS targeted to different intracellular locations. In both endothelial cells and transfected COS-7 cells, eNOS can be localized to the perinuclear Golgi and also to cholesterol-rich domains of the plasma membrane. In previous experiments using the wild-type eNOS, we found that the location of eNOS within the cell has a major influence on its ability to produce NO. The highest activity is observed from eNOS tethered to the plasma membrane vs. progressively reduced amounts of NO released from eNOS bound to endomembranes (Golgi) and from eNOS residing in the cytosol or within intracellular organelles (6, 17, 30, 74). In contrast to the wild-type enzyme, the CIeNOS produces equal amounts of NO, regardless of its location in the cell (6, 30), and thus this construct was used to explore the relationship between the intracellular location of eNOS and its substrate availability. We found that l-arginine dose-dependently stimulated NO production from wild-type (plasma membrane and Golgi), Golgi, plasma membrane, cytosolic, and mitochondria-targeted CIeNOS with approximately equal efficacy. l-Citrulline, which must first be converted to l-arginine by the enzymes ASS and ASL, which are present in plasmalemmal caveolae of endothelial cells (15), was less effective than l-arginine in stimulating NO production, possibly indicating less efficient cellular uptake. Consistent with the data obtained for l-arginine, l-citrulline did not show a preference for the activation of eNOS localized to a specific intracellular compartment. These data suggest that the compartmentalization of eNOS is not a major influence on l-arginine- or l-citrulline-stimulated NO release.
We next established whether exogenously expressed Arg I and Arg II reside in distinct intracellular locations, and our findings were consistent with previous reports showing expression of Arg I in the cytosol and Arg II in the mitochondria (31, 45). To determine whether preferential utilization of l-arginine takes place in different parts of the cell, we coexpressed Arg I and Arg II and measured the relative inhibition of NO production. We found that none of the intracellular locations reported previously for eNOS (Golgi, plasma membrane, cytosol, nucleus, and inner or outer mitochondria) showed differential inhibition with Arg I (cytosol) vs. Arg II (mitochondria), thus establishing that arginase-dependent inhibition of eNOS-derived NO is also independent of the location of eNOS.
To assess the relative importance of arginase localization to reduced eNOS activity, we first assessed whether the combination of Arg I and Arg II localized to the cytosol and mitochondria, respectively, had a greater level of inhibition when combined (depleting 2 pools) vs. that of either Arg I or Arg II expressed separately (depleting 1 distinct pool). We found that the combination of Arg I and Arg II did not provide additive inhibition over that of Arg I or II alone. These data suggest that Arg I and Arg II share a common mechanism to inhibit eNOS activity, and accordingly we focused on one isoform (Arg I) for the remainder of our study. To determine the relationship between arginase expression level and reduced eNOS activity, we expressed arginase at varying levels and measured NO release. The ability of arginase to maximally reduce eNOS activity was observed at low levels of expression, and further increases did not lead to additional decreases in NO release. This finding suggests that the catalytic properties of arginase do not allow for precipitous decreases in l-arginine beyond that mediating the ∼20–30% inhibition of eNOS activity that was observed. Although arginase has a relatively low affinity for arginine (∼2 mM), it has been proposed that it can compete with eNOS for l-arginine due to its 1,000-fold higher Vmax. Our results argue against the linearity of this relationship and suggest that there is a low point of intracellular l-arginine that arginase cannot drive lower, regardless of its expression level.
To further explore the importance of the intracellular location of arginase to eNOS activity, we generated Arg I constructs that target the cytosol, plasma membrane, mitochondria, nucleus, and even the same location as eNOS itself, the plasma membrane and Golgi, and assessed the effect on NO production. The targeting of Arg I to different intracellular locations had no major effect on the ability of arginase to reduce eNOS activity. However, we did observe significant changes in arginase activity with maximal activity evident in the cytosol, followed closely by the mitochondria and lower levels of activity when attached to intracellular membranes (e1–e75) and the nucleus. The mechanisms underlying the altered activity of Arg I targeted to different locations are unclear at the moment and might relate to the presence of the targeting sequence, which may directly affect enzymatic activity or result in posttranslational modifications that might affect activity. Evidence of the latter was observed in Western blots of the mitochondria-targeted Arg I. In contrast to the other Arg I construct, mitochondria-targeted Arg I was detected as a doublet, possibly reflecting intramolecular cleavage or other modifications. An important caveat of these studies is that the measurements of arginase activity were obtained in cell lysates and not in intact cells, and thus it remains possible that activity in vivo might be different. Collectively, these data support a mechanism of reduced eNOS activity that is independent of the subcellular location of Arg I. Furthermore, the lack of a relationship between arginase activity and the degree of eNOS inhibition suggests a mechanism that may not be due entirely to substrate depletion.
Therefore, to assess whether l-arginine can reverse the arginase-dependent inhibition of eNOS activity, we exposed cells expressing eNOS and arginase to progressive and even supraphysiological concentrations of l-arginine. We found that excess l-arginine could not reverse the ability of arginase to suppress eNOS activity, and these data suggest an alternative mechanism of reduced eNOS activity. However, measurement of intracellular amino acids revealed that l-arginine concentrations are significantly lower in cells expressing arginase. A reciprocal relationship was observed with l-ornithine levels that were higher in cells expressing arginase and indicative of increases in arginase catalytic activity. The intracellular concentration of l-citrulline was also measured and was found to be below the limit of detection (low pmol range), suggesting rapid recycling by metabolic enzymes.
We also investigated whether substrate-independent mechanisms are responsible for the ability of arginase to inhibit eNOS activity. To determine whether Arg I can bind directly and allosterically suppress eNOS activity, eNOS was immunoprecipitated from cells expressing eNOS and Arg I. In immune complexes containing eNOS, there were significant amounts of bound Hsp90 but not Arg I. This suggests that the direct binding of Arg I to eNOS is not the source of reduced eNOS activity and is consistent with NO release data showing that arginase targeting distinct or even the very same (e1–e75) locations as eNOS has equal potency in suppressing eNOS-derived NO. To determine whether the catalytic activity of arginase is necessary for reduced eNOS activity, we generated a catalytically inactive mutant of Arg I (D128G). The D128G Arg I did not reduce eNOS-derived NO, indicating that arginase activity is essential for the inhibition of eNOS activity. These data were supported by the ability of a pharmacological inhibitor of arginase activity, nor-NOHA, to significantly reverse Arg I-dependent inhibition of eNOS-derived NO. Because the catalytic activity of Arg I was essential for reduced eNOS activity, we next investigated whether downstream metabolites of the arginases may constitute a novel mechanism of reduced eNOS activity. The primary arginase metabolite l-ornithine is further metabolized to the polyamines by ODC. Therefore we investigated whether an inhibitor of ODC (DFMO) can reverse the ability of arginase to inhibit eNOS activity. DFMO directly reduced eNOS-derived NO and did not reverse the arginase-mediated inhibition of eNOS activity. Previously, DFMO has been shown to improve or stimulate blood vessel relaxation (29, 72). However, the mechanisms involved are contentious, and currently, no studies have examined directly the ability of DFMO to modify eNOS-derived NO. Because higher levels of l-ornithine were seen in cells transfected with arginase, we next examined whether progressively higher concentrations of the arginase metabolites urea and l-ornithine would influence eNOS activity directly. Both urea and l-ornithine failed to modify eNOS-derived NO. Because cardiovascular diseases are associated with increased arginase activity and reduced BH4 levels and uncoupled eNOS, we also investigated whether arginase could influence eNOS activity via an effect on BH4 levels. Exposure of cells to sepiapterin, which increases BH4 levels, stimulated NO release but did not reverse the degree of eNOS inhibition by arginase, and collectively, these data suggest that l-arginine depletion is the major mechanism.
To establish physiological relevance, it was important to show that the reciprocal relationship we have observed between eNOS and arginase in COS-7 cells can be replicated in endothelial cells. To achieve this, we generated arginase-expressing adenoviruses to manipulate arginase activity in BAEC. Transduction of BAEC with wild-type Arg I resulted in a modest inhibitory effect on NO production that was equivalent to that seen in COS-7 cells. Also consistent with previous results in COS-7 cells, the catalytically inactive D128G Arg I failed to reduce eNOS activity in BAEC. Reversal of arginase-dependent inhibition of NO in BAEC was similar to that observed in COS-7 cells. The catalytically inactive arginase virus D128G was incapable of reducing NO levels in BAEC, also supporting data obtained in COS-7 cells that the catalytic activity of arginase is necessary to inhibit eNOS activity. To assess the importance of the intracellular location of arginase in endothelial cells, we focused on the plasma membrane-targeted Arg I because eNOS activity in endothelial cells is highest at this location (17). If there are exclusive pools of l-arginine residing in close proximity to the plasma membrane, i.e., near caveolar eNOS, CAT-1 transporters, l-arginine, and recycling enzymes, then targeting arginase to the plasma membrane should elicit a more profound inhibition of eNOS activity compared with the WT Arg I located in the cytosol. However, we observed a similar degree of inhibition with the plasma membrane-targeted Arg I compared with WT Arg I, and this result is consistent with data obtained in COS-7 cells. To confirm the plasma membrane targeting of PM Arg I in endothelial cells, we used indirect immunofluorescence and confocal microscopy. The results of these experiments revealed abundant green fluorescence at the plasma membrane, with minimal expression observed in a perinuclear location likely to be either the endoplasic reticulum or the Golgi.
The best explanation for the arginine paradox has been the concept of noninterchangeable intracellular pools of l-arginine. This elegant model has allowed for the high concentrations of l-arginine inside endothelial cells to mesh with the known ability of eNOS to produce more NO with the extracellular addition of additional substrate. However, we found that the intracellular location of eNOS does not afford access to specialized pools of l-arginine and that l-arginine appears to freely diffusible to all locations inside the cell. Furthermore, the targeting of arginase to different locations within the cell with either WT Arg I or Arg II that target the cytosol and mitochondria, respectively, or with a variety of fusion proteins that target other intracellular locations does not influence the relative ability of arginase to inhibit eNOS activity. These data are in agreement with other studies showing that the colocalization of CAT-1 and ASS/ASL with eNOS does not actually influence eNOS activity in an l-arginine-dependent manner. Instead both CAT-1 and ASL are eNOS-binding proteins that allosterically regulate enzyme activity independent of l-arginine transport or metabolism (13, 38).
The catalytic activity of arginase was necessary for reduced eNOS-derived NO, but the metabolic end products of arginase did not impact eNOS activity. An important finding of our study was that the arginase-dependent inhibition of eNOS activity was not fully reversible with l-arginine supplementation in either COS-7 or endothelial cells. The measurement of intracellular amino acids revealed diminished levels of l-arginine even in the presence of high extracellular l-arginine and supports substrate depletion as the primary mechanism by which arginase reduces eNOS activity. However, arginase could only decrease intracellular l-arginine levels to the degree where eNOS activity was compromised by ∼20%, regardless of how high the level of arginase expression was. This is an understandable property, as very low levels of l-arginine would have dire consequences on cellular function beyond the loss of eNOS regulation. Higher levels of extracellular l-arginine drive the production of l-ornithine but do not fully restore NO production. Accordingly, these results suggest that the strategy of using l-arginine supplementation to mitigate the effects of arginase upregulation in cardiovascular diseases may not be as helpful in improving endothelial function as previously thought. Evidence to support this is derived from studies in human subjects where the short-term administration of l-arginine improves endothelial function, but long-term increases in l-arginine levels are paradoxically detrimental. l-Arginine supplementation results in increased levels of ornithine and urea, byproducts of arginase metabolism, but not l-citrulline, the byproduct of eNOS (69).
In summary, our study reveals that intracellular location is not a major factor controlling the accessibility of l-arginine to eNOS and that catalytically active arginase can negatively regulate only a fraction of total eNOS activity but can effectively metabolize supplemental l-arginine to ornithine and other metabolites.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-085827, R01-HL-092446, and P01-HL-101902-01A1 and an established investigator award from the American Heart Association (D. J. R. Fulton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E., F.C., J.Q., R.B.C., and D.J.F. contributed to the conception and design of the research; S.E., F.C., Y.W., J.Q., D.P., Y.Y., and J.A.I. performed the experiments; S.E., F.C., and D.J.F. interpreted the results of the experiments; S.E., F.C., J.Q., and D.J.F. prepared the figures; S.E., F.C., and D.J.F. drafted the manuscript; S.E., F.C., and D.J.F. edited and revised the manuscript; S.E., F.C., Y.W., J.Q., B.A., D.P., J.A.I., R.B.C., and D.J.F. approved the final version of the manuscript; F.C., B.A., and D.J.F. analyzed the data.
ACKNOWLEDGMENTS
We thank Sidney M. Morris, Jr., and Diane Kepka-Lenhart for determining arginase activities, for quantifying arginase expression in immunoblots, and for helpful discussions. We thank Stephen Haigh for technical support and Dr. Stephen Looney for help with the statistics.
REFERENCES
- 1.Bates TE, Loesch A, Burnstock G, Clark JB. Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochem Biophys Res Commun 213: 896–900, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Boucher JL, Custot J, Vadon S, Delaforge M, Lepoivre M, Tenu JP, Yapo A, Mansuy D. N omega-hydroxyl-l-Arginine, an intermediate in the l-Arginine to nitric oxide pathway, is a strong inhibitor of liver and macrophage Arginase. Biochem Biophys Res Commun 203: 1614–1621, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Chen PF, Wu KK. Structural elements contribute to the calcium/calmodulin dependence on enzyme activation in human endothelial nitric-oxide synthase. J Biol Chem 278: 52392–52400, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chin-Dusting JP, Willems L, Kaye DM. L-Arginine transporters in cardiovascular disease: a novel therapeutic target. Pharmacol Ther 116: 428–436, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem 281: 1477–1488, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Closs EI, Scheld JS, Sharafi M, Forstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol 57: 68–74, 2000 [PubMed] [Google Scholar]
- 8.Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest 88: 1663–1671, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daghigh F, Fukuto JM, Ash DE. Inhibition of rat liver Arginase by an intermediate in NO biosynthesis, NG-hydroxy-l-Arginine: implications for the regulation of nitric oxide biosynthesis by Arginase. Biochem Biophys Res Commun 202: 174–180, 1994 [DOI] [PubMed] [Google Scholar]
- 10.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation 88: 77–81, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK, Black JO, Zeng H, Tang Y, Reddy AK, Summar M, O'Brien WE, Harrison DG, Mitch WE, Marini JC, Aschner JL, Bryan NS, Lee B. Requirement of Arg Ininosuccinate lyase for systemic nitric oxide production. Nat Med 17: 1619–1626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun 256: 192–197, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of Arginine regeneration enzymes, Arg Ininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide 5: 187–197, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23: 1121–1131, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem 279: 30349–30357, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Fontana J, Sowa G, Gratton JP, Lin M, Li KX, Michell B, Kemp BE, Rodman D, Sessa WC. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J Biol Chem 277: 4277–4284, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther 299: 818–824, 2001 [PubMed] [Google Scholar]
- 20.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA 93: 6448–6453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotoh T, Sonoki T, Nagasaki A, Terada K, Takiguchi M, Mori M. Molecular cloning of cDNA for nonhepatic mitochondrial Arginase (Arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett 395: 119–122, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280: F193–F206, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased Arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med 172: 1523–1528, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res 5: 211–224, 1979 [PubMed] [Google Scholar]
- 26.Ha YH, Milner JA, Corbin JE. Arginine requirements in immature dogs. J Nutr 108: 203–210, 1978 [DOI] [PubMed] [Google Scholar]
- 27.Hanssen H, Brunini TM, Conway M, Banning AP, Roberts NB, Mann GE, Ellory JC, Mendes Ribeiro AC. Increased l-Arginine transport in human erythrocytes in chronic heart failure. Clin Sci (Lond) 94: 43–48, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of Arginase. FASEB J 17: 2328–2330, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Huynh NN, Harris EE, Chin-Dusting JF, Andrews KL. The vascular effects of different Arginase inhibitors in rat isolated aorta and mesenteric arteries. Br J Pharmacol 156: 84–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagnandan D, Sessa WC, Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol 289: C1024–C1033, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of Arginases. Comp Biochem Physiol B Biochem Mol Biol 114: 107–132, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 288: R1057–R1062, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kampfer H, Pfeilschifter J, Frank S. Expression and activity of Arginase isoenzymes during normal and diabetes-impaired skin repair. J Invest Dermatol 121: 1544–1551, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Kashyap SR, Lara A, Zhang R, Park YM, DeFronzo RA. Insulin reduces plasma Arginase activity in type 2 diabetic patients. Diabetes Care 31: 134–139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA 89: 6348–6352, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 111: 363–368, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Li C, Huang W, Harris MB, Goolsby JM, Venema RC. Interaction of the endothelial nitric oxide synthase with the CAT-1 Arginine transporter enhances NO release by a mechanism not involving Arginine transport. Biochem J 386: 567–574, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loyaga-Rendon RY, Sakamoto S, Beppu M, Aso T, Ishizaka M, Takahashi R, Azuma H. Accumulated endogenous nitric oxide synthase inhibitors, enhanced Arginase activity, attenuated dimethylArginine dimethylaminohydrolase activity and intimal hyperplasia in premenopausal human uterine arteries. Atherosclerosis 178: 231–239, 2005 [DOI] [PubMed] [Google Scholar]
- 40.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “Arginine paradox”. J Biol Chem 272: 31213–31216, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 272: 15583–15586, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 90: 6252–6256, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated Arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294: 81–90, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris SM., Jr Regulation of enzymes of the urea cycle and Arginine metabolism. Annu Rev Nutr 22: 87–105, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Morris SM, Jr, Bhamidipati D, Kepka-Lenhart D. Human type II Arginase: sequence analysis and tissue-specific expression. Gene 193: 157–161, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Morris SM, Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes 60: 3015–3022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of Arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol Endocrinol Metab 275: E740–E747, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Narayanan SP, Suwanpradid J, Saul A, Xu Z, Still A, Caldwell RW, Caldwell RB. Arginase 2 deletion reduces neuro-glial injury and improves retinal function in a model of retinopathy of prematurity. PloS One 6: e22460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtake A, Takiguchi M, Shigeto Y, Amaya Y, Kawamoto S, Mori M. Structural organization of the gene for rat liver-type Arginase. J Biol Chem 263: 2245–2249, 1988 [PubMed] [Google Scholar]
- 50.Qian J, Zhang Q, Church JE, Stepp DW, Rudic RD, Fulton DJ. Role of local production of endothelium-derived nitric oxide on cGMP signaling and S-nitrosylation. Am J Physiol Heart Circ Physiol 298: H112–H118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2: 1057–1058, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez S, Richert L, Berthelot A. Increased Arginase activity in aorta of mineralocorticoid-salt hypertensive rats. Clin Exp Hypertens 22: 75–85, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, Caldwell RB, Caldwell RW. Diabetes-induced vascular dysfunction involves Arginase I. Am J Physiol Heart Circ Physiol 302: H159–H166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rose WC, Haines WJ, Warner DT. The amino acid requirements of man. V. The rôle of lysine, Arginine, and tryptophan. J Biol Chem 206: 421–430, 1954 [PubMed] [Google Scholar]
- 55.Rossitch E, Jr, Alexander E, 3rd, Black PM, Cooke JP. L-Arginine normalizes endothelial function in cerebral vessels from hypercholesterolemic rabbits. J Clin Invest 87: 1295–1299, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryoo S, Berkowitz DE, Lim HK. Endothelial Arginase II and atherosclerosis. Korean J Anesthesiol 61: 3–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryoo S, Bhunia A, Chang F, Shoukas A, Berkowitz DE, Romer LH. OxLDL-dependent activation of Arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis 214: 279–287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol 105: 1632–1642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scotland RS, Morales-Ruiz M, Chen Y, Yu J, Rudic RD, Fulton D, Gratton JP, Sessa WC. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res 90: 904–910, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Sessa WC, Barber CM, Lynch KR. Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circ Res 72: 921–924, 1993 [DOI] [PubMed] [Google Scholar]
- 61.Sessa WC, Garcia-Cardena G, Liu J, Keh A, Pollock JS, Bradley J, Thiru S, Braverman IM, Desai KM. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem 270: 17641–17644, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D'Angelo DD, Lynch KR, Peach MJ. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem 267: 15274–15276, 1992 [PubMed] [Google Scholar]
- 63.Simon A, Plies L, Habermeier A, Martine U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res 93: 813–820, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial Arginase II modulates nitric-oxide synthesis through nonfreely exchangeable l-Arginine pools in human endothelial cells. J Pharmacol Exp Ther 318: 1368–1374, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Venema RC, Sayegh HS, Arnal JF, Harrison DG. Role of the enzyme calmodulin-binding domain in membrane association and phospholipid inhibition of endothelial nitric oxide synthase. J Biol Chem 270: 14705–14711, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Venema VJ, Marrero MB, Venema RC. Bradykinin-stimulated protein tyrosine phosphorylation promotes endothelial nitric oxide synthase translocation to the cytoskeleton. Biochem Biophys Res Commun 226: 703–710, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Vockley JG, Tabor DE, Kern RM, Goodman BK, Wissmann PB, Kang DS, Grody WW, Cederbaum SD. Identification of mutations (D128G, H141L) in the liver Arginase gene of patients with hyperArgininemia. Hum Mutat 4: 150–154, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Waddington SN, Cattell V. Arginase in glomerulonephritis. Exp Nephrol 8: 128–134, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-Arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation 116: 188–195, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased Arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of Arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J 15: 1264–1266, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular Arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension 44: 935–943, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol 26: 1015–1021, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies Arginase in asthma pathogenesis. J Clin Invest 111: 1863–1874, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]