Abstract
Protein 3-nitrotyrosine (3-NT) formation is frequently regarded as a simple biomarker of disease, an irreversible posttranslational modification that can disrupt protein structure and function. Nevertheless, evidence that protein 3-NT modifications may be site selective and reversible, thus allowing for physiological regulation of protein activity, has begun to emerge. We have previously reported that cyclooxygenase (COX)-1 undergoes heme-dependent nitration of Tyr385, an internal and catalytically essential residue. In the present study, we demonstrate that nitrated COX-1 undergoes a rapid reversal of nitration by substrate-selective and biologically regulated denitrase activity. Using nitrated COX-1 as a substrate, denitrase activity was validated and quantified by analytic HPLC with electrochemical detection and determined to be constitutively active in murine and human endothelial cells, macrophages, and a variety of tissue samples. Smooth muscle cells, however, contained little denitrase activity. Further characterizing this denitrase activity, we found that it was inhibited by free 3-NT and may be enhanced by endogenous nitric oxide and exogenously administered carbon monoxide. Finally, we describe a purification protocol that results in significant enrichment of a discrete denitrase-containing fraction, which maintains activity throughout the purification process. These findings reveal that nitrated COX-1 is a substrate for a denitrase in cells and tissues, implying that the reciprocal processes of nitration and denitration may modulate bioactive lipid synthesis in the setting of inflammation. In addition, our data reveal that denitration is a controlled process that may have broad importance for regulating cell signaling events in nitric oxide-generating systems during oxidative/nitrosative stress.
Keywords: cyclooxygenase-1, 3-nitrotyrosine, denitration, reactive nitrogen species, peroxynitrite, HPLC with electrochemical detection, liquid chromatograph-tandem mass spectrometry
nitric oxide (NO) is a ubiquitous biosignaling molecule that participates in diverse physiological processes such as smooth muscle relaxation, platelet aggregation, neurotransmission, and host defense. The actions of NO are not universally direct (3, 25), as they may be propagated by the transformation of NO into other species, including nitrite (NO2−), nitrate (NO3−), nitrosonium ion (NO+), nitrogen dioxide (˙NO2), peroxynitrite (ONOO−), and nitrosoperoxocarbonate (ONO2CO2−), depending on the cellular redox environment (42, 45, 51). Notably, NO and its derivatives modify physiological processes via posttranslational modifications of proteins (22, 24, 39). For example, elevated levels of superoxide (O2·−) and NO during inflammation will react at a near diffusion-limited rate to form ONOO−, a significant contributor to the nitration of protein tyrosine residues to produce 3-nitrotyrosine (3-NT). 3-NT formation is generally considered to be a pathophysiological biomarker of nitrosative stress, and tyrosine nitration has been associated with irreversible loss of protein function in many cases (23, 31). Characterization of 3-NT as a nonselective and irreversible protein modification has contributed to the widely held view that tyrosine nitration is irrelevant for physiological cell signaling, a concept in sharp contrast to protein S-nitrosylation (the addition of NO to protein cysteine-sulfur), which is well recognized as a regulator of protein activity and cellular function, due in large part to the specificity and reversibility of this modification (14, 24, 39). Observations that 3-NT formation may be analogously reversed, in this case by denitrase activity, were first reported by Murad and colleagues (29). Coincubation of spleen homogenates from LPS-treated rats with chemically nitrated BSA triggered a progressive loss of 3-NT, as visualized by Western blot analysis with a selective anti-3-NT antibody (29). This putative denitrase activity has been further characterized as an endotoxin-induced, O2-regulated, and tissue-specific process that can be detected in the brain, lung, and spleen of LPS-treated rats (21, 29), in the untreated rat brain and heart (36), in rat liver mitochondria (32), and in LPS-activated RAW 264.7 cells in culture (26, 30, 49). In addition to nitrated BSA, a limited number of nitrated substrates for denitrase activity have been identified, including histone H1.2 (26), histone III-S (36), the L-type Ca2+ channel (30), calmodulin (49), and glutamine synthetase (21). These biochemical reports have suggested that denitration may serve as an adaptive mechanism capable of repairing proteins damaged during diseases where levels of reactive nitrogen species are elevated. In addition, they support the hypothesis that protein nitration and denitration may function as a biologically relevant regulatory pathway. Unfortunately, further validation of this denitrase activity has proven difficult. Notably, observation of 3-NT formation in vivo is challenging as it does not accumulate in sufficient amounts for facile detection and quantification by commonly used analytic methods. This has generated concern regarding the authenticity of 3-NT signal loss, the fate of 3-NT, and the feasibility of studies aimed at elucidating the mechanism and role of protein tyrosine denitration in biological systems. These reservations would be partially diminished by the identification of the preferred substrate(s) and the utilization of analytic tools for confident quantification of protein 3-NT denitrase activity.
We (12, 13) have previously shown that selective and targeted cyclooxygenase (COX)-1 nitration occurs by a heme-directed and substrate-dependent mechanism. While heme plays a role in catalyzing internal Tyr385 nitration by ONOO− and the demise of COX-1, active site occupancy with substrate protects against Tyr385 nitration and redirects nitration to alternative and external tyrosine residues on COX-1, preserving catalytic activity (12, 13). Here, we establish COX-1 as a quantifiable target of protein tyrosine denitration by constitutively expressed denitrase activity in cells and tissues. In the present study, we additionally describe an enrichment protocol for denitrase activity from lung tissue that results in a discrete activity-containing fraction. Furthermore, we identify a group of previously known enzymes that all exhibit: 1) denitrase activity, defined by their ability to catalyze the denitration of NO2COX-1; 2) antioxidant defense and detoxification activities; and 3) redox chemistries that underlie their recognized mechanisms of action. These findings, along with the evidence of COX-1 denitration reported here, suggest an intricate relationship between COX-1 and cellular nitration/denitration that may significantly contribute to cellular adaptation to oxidative stress. Protein tyrosine nitration and its reversibility may have significant translational impact, given the widespread occurrence of protein tyrosine nitration in diverse pathophysiological settings.
MATERIALS AND METHODS
Reagents.
All chemicals and reagents were purchased from Sigma-Aldrich (minimum 95% purity, HPLC grade, St. Louis, MO) unless otherwise noted. Ram seminal vesicles and rat organs (brain, heart, lung, liver, kidney, and spleen) were obtained from Pel-Freez Biologicals (Rogers, AR). Purified COX-1 was prepared as previously described (13, 53). N-Octyl-β-d-glucopyranoside (βOG) was from Anatrace (Maumee, OH). ONOO− (100–200 mM in 4.7% sodium hydroxide) was obtained from Calbiochem (La Jolla, CA), aliquoted, stored at −80°C, and used freshly. Tricarbonylchloro(glycinate) ruthenium II, known as carbon monoxide (CO)-releasing molecule (CORM)-3, was synthesized and provided by Dr. J. R. Falck (Dallas, TX). CORM-3 was solubilized in distilled water (7 mM stock), aliquoted, stored at −20°C, and used freshly. Trypsin was obtained from Promega (no. V511A, Madison, WI). Heme-bound FePPCOX-1 was generated by reconstituting apoCOX-1 (heme free) with hemin (from a 500 μM solution) to a 1:1 molar ratio and measuring absorbance at 412 nm (ε = 1.42 × 105 M−1·cm−1) using a Perkin-Elmer Lambda 20 spectrophotometer as previously described (13). Custom-made nitrated peptides were from Genscript (Piscataway, NJ). Peroxiredoxin-6 and Cu,Zn-SOD were purchased from Novus Biologicals (Littleton, CO). Monoclonal COX-1, monoclonal BSA, and polyclonal MnSOD antibodies were purchased from Cayman Chemicals (Ann Arbor, MI). Monoclonal 3-NT antibody was obtained from Upstate Biotechnology (Lake Placid, NY). Materials for SDS-PAGE, Western blot analysis, and anti-rabbit horseradish peroxidase-conjugated IgG were obtained from Bio-Rad Laboratories (Hercules, CA). Anti-mouse horseradish peroxidase-conjugated IgG, enzyme immunoassay kits to measure PGE2, ECL Plus reagents, and diethylaminoethanol (DEAE)-Sepharose beads were obtained from GE Healthcare (Piscataway, NJ).
Cell culture and treatment protocol.
The sEND.1 murine endothelial cell line, rat aortic smooth muscle cell (RASMC) line, and RAW264.7 murine macrophage cell line (American Type Culture Collection, Manassa, VA) were cultured in appropriately sized culture plates in DMEM (GIBCO Life Technologies) supplemented with 10% FBS at 37°C in 5% CO2. The sEND cell line was provided by Dr. Patrick Vallance (University College, London, UK) and originally established from a mouse skin capillary endothelioma (54). RASMCs were isolated as previously described (37). At ∼85% confluency, cells (passages 4–8) were washed with ice-cold PBS followed by cell lysis and preparation for denitration experiments (as described below). To induce an inflammatory response where indicated, cells were exposed to the proinflammatory stimulus LPS (2 μg/ml, Calbiochem) for 12 h. Where noted, cells were pretreated for 12 h with the pharmacological inhibitor of NO synthase (NOS) NG-monomethyl-l-arginine (l-NMMA; 3 mmol/l, Sigma). After treatment protocols, cell culture media were removed, and PBS-washed cells were extracted in preparation for denitration experiments (as described below). Thioglycolate-elicited peritoneal macrophages were provided by Dr. Domenick Falcone (Weill Cornell Medical College) and obtained as previously described (16, 17). Briefly, after cell harvest by lavage, peritoneal cells were centrifuged and allowed to adhere for 4 h on tissue culture plates. Nonadherent cells were washed away, and only adherent peritoneal macrophage cells were used in denitration experiments. This is a standard protocol for obtaining high-purity (>90%) macrophages (55). Human umbilical vascular endothelial cells (HUVECs) were provided by Dr. Timothy Hla (Weill Cornell Medical College).
Animal tissue.
Mouse organs (brain, heart, lung, liver, kidney, and spleen) were harvested from 3-mo-old C57BL/6J mice on a regular diet. Briefly, mice were killed by CO2 asphyxiation, tissues were rinsed with 70% ethanol, and the thorax was opened to expose the heart and lung. The animal procedure described here was approved by the Weill Medical College of Cornell University Care and Use Committee.
Preparation of cell lysates and tissue extracts for denitration experiments.
For most experiments, washed cells were lysed with lysis buffer [20 mM Tris·HCl (pH 7.4), 2% CHAPs, 1 mM PMSF, and 1 mM protease inhibitor cocktail]. Cells were scraped, collected in Eppendorf tubes, sonicated on ice, and centrifuged (13,400 g, 10 min) for clarification. Where noted, cells and tissue samples were homogenized and sonicated in either the lysis buffer described above or detergent-free buffer [10 mM Tris·HCl (pH 8), 10 mM potassium phosphate, 0.5 mM EDTA, 1 mM PMSF, and 1 mM DTT] and centrifuged at various speeds and times to achieve fractions with the desired enrichment of cellular components. Centrifugation pellets were solubilized in detergent-containing buffer [20 mM Tris·HCl (pH 7.4) and 2% CHAPs] before analysis. Total protein in cell lysates and tissue homogenates was determined using the Bio-Rad protein assay.
Chromatographic resolution of denitrase activity from rat lungs.
Frozen lungs (1.5 g) were homogenized in detergent-free buffer [10 mM Tris·HCl (pH 8), 10 mM potassium phosphate, 0.5 mM EDTA, 1 mM fresh PMSF, and 1 mM fresh DTT] and centrifuged at low speed (3,000 g) for 20 min to remove cell debris and nuclei. The resulting supernatant was centrifuged at high speed (120,000 g) for 1 h, and the supernatant was concentrated by filtration on a 10-kDa cutoff membrane (YM10 Centricon). The concentrate was loaded on a fast-flow DEAE-Sepharose column (15 × 1.5 cm) preequilibrated with detergent-free buffer. The column was washed with two column volumes (V0 = 40 ml, 2 ml/fraction) of equilibration buffer followed by elution with a linear salt gradient [(40 ml) 10 mM Tris·HCl (pH 8), 10 mM potassium phosphate, 0.5 mM EDTA, 1 mM fresh PMSF, and 1 mM fresh DTT to (40 ml) 10 mM Tris·HCl (pH 8), 150 mM potassium phosphate, 0.5 mM EDTA, 1 mM fresh PMSF, and 1 mM fresh DTT]. Note that during the column wash stage, a protein band absorbing at 280 nm (F13-F20), which has no denitrase activity, eluted off the column (see Fig. 5B). Fractions with strong denitrase activity (F55-F57) eluted from the column with a salt gradient (starting at ∼ 60 ml potassium phosphate). Fractions with denitrase activity (F55-F57) were combined, desalted, and concentrated on a YM10 to ∼150 μl (containing ∼2 mg protein). The concentrate was confirmed for its ability to denitrate NO2COX-1 by Western blot analysis and by HPLC with electrochemical detection (HPLC-ECD). The concentrate was also screened by liquid chromotography-tandem mass spectroscopy (LC-MS/MS; as described below) for putative denitrase candidates.
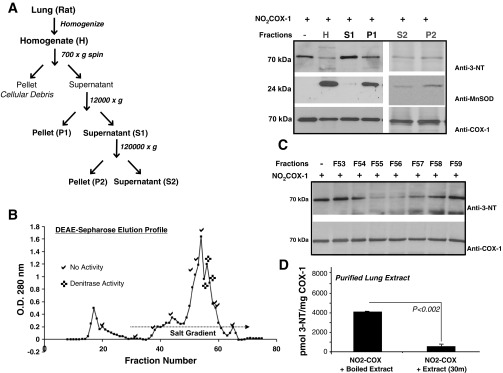
Fig. 5.
Differential centrifugation reveals the intracellular distribution of denitrase activity: chromatographic resolution from the rat lung. A: rat lung homogenate in detergent-free buffer was subjected to differential centrifugation to obtain cellular fractions that are mitochondria rich and mitochondria depleted (as shown in the schematic). Denitration reactions of NO2FePPCOX-1 were carried out by the lung homogenate (H), mitochondria-rich pellet (P1), microsomal pellet (P2), and mitochondria-deplete supernatants (S1 and S2) at 37°C and 1:15 (wt/wt) ratio and monitored by Western blot analysis. Blots were probed with monoclonal anti-3-NT (top), monoclonal anti-MnSOD (middle), and monoclonal anti-COX-1 (bottom) antibodies. B: chromatographic resolution of denitrase activity from the frozen rat lung (1.5 g) was achieved on a diethylaminoethanol (DEAE)-Sepharose anion exchange column with a linear potassium phosphate (KPi) gradient (10–150 mM). Discrete denitrase-containing fractions (F55, F56, and F57) that absorbed at 280 nm and eluted at ∼60 mM KPi are indicated. C: denitrase activity was confirmed by Western blot analysis of reactions that compared the denitration of NO2COX-1 (0.5 μg) by fractions (20 μl each) eluted from the DEAE-Sepharose column relative to undenitrated NO2COX-1. Blots were probed with monoclonal anti-3-NT antibody (top) and with monoclonal anti-COX-1 antibody (bottom). D: total 3-NT content was measured by HPLC-ECD for the denitration reactions of NO2COX-1 after incubation with purified lung extract at 37°C for 30 min. 3-NT was also quantified in control reactions, i.e., NO2COX-1 coincubated with preboiled cell lysates, as well as lysate-free NO2COX-1. Data are averages ± SE (n ≥ 3). P < 0.05 was considered statistically significant.
Treatment of purified proteins with ONOO−.
COX-1 and BSA reactions with ONOO− (500 μM) were conducted in buffer (100 mM Tris, 0.3 mM diethyldithiocarbamate, 5 mM EDTA, and 0.1% Tween or 0.3% βOG) at pH 8 using a concentrated ONOO− stock solution (110 mM). The concentration of ONOO− used for each reaction was spectroscopically determined (ε302 nm: 1,670 M−1·cm−1). Notably, we and others (6, 15) have previously shown by spectroscopic techniques that up to only three tyrosine residues per COX-1 subunit can be nitrated by ONOO− and other nitrating agents at the concentrations described here. Nevertheless, the selectivity of 3-NT detection by immunoblot analysis and its quantification by analytical HPLC (as described below) allow the discrimination of NO2COX-1 from a COX-1 mixture that contains unmodified COX-1 or other oxidized forms. Here, for reactions where the heme-bound FePPCOX-1 form is used, COX-1 is referred to as NO2COX-1 and heme-free COX-1 is referred to as NO2apoCOX-1.
Pretreatment of cell extracts with oxidants and ligands.
Before incubation with substrate, cellular extracts in 20 mM Tris·HCl (pH 7.4), 2% CHAPs, 1 mM PMSF, and 1 mM protease inhibitor cocktail were pretreated with N2 (Tech Air) gas in a sealed chamber (60 s), CO (T.W. Smith) gas in a sealed chamber (60 s), 100 μM CORM-3 (20 min), or varying concentrations of 3-NT (0–50 μmol/l, 60 min).
Denitration of nitrated substrates by cell and tissue extracts.
Nitrated substrates (0.5 μg) were incubated with clarified supernatants from cells or tissues at 37°C in a concentration- and time-dependent manner. The ratio of nitrated substrate to cell or tissue extract ranged from 1:5 (wt/wt) to 1:40 (wt/wt), and reactions were monitored over a period of 2 h. As a control, denitrase-containing extracts were either heated at 100°C for 5 min or proteolyzed with trypsin at a 20:1 (wt/wt) ratio (16 h, 37°C), and the digestion was neutralized by adding a 10:1 (wt/wt) ratio of PMSF to trypsin before incubation with nitrated substrate. Nitrated substrate in the absence of denitrase-containing extract was also used as a control.
Detection of 3-NT by immunoblot analysis.
Loss of 3-NT content in NO2COX-1 and NO2BSA was monitored by Western blot analysis. Nitrated protein/sample (0.5 μg) was treated with SDS-β-mercaptoethanol or SDS-Tris(2-carboxyethyl)phosphine (TCEP), separated on a 10% acrylamide gel, and transferred onto a nitrocellulose membrane (Bio-Rad). After separation and protein transfer, membranes were blocked (in 5% nonfat milk, 1 h) followed by an incubation with either mouse monoclonal 3-NT antibody (1:1,000 dilution with 1% nonfat milk, overnight), mouse monoclonal COX-1 antibody (1:1,000 dilution with 1% nonfat milk, 1 h), mouse monoclonal BSA antibody (1:1,000 dilution with 1% nonfat milk, 1 h), or rabbit polyclonal MnSOD antibody (1:2,000 dilution with 1% nonfat milk, overnight). Bands were revealed using enhanced chemiluminescence (ECL plus) and visualized on film or by Typhoon Trio+ (GE Healthcare).
Quantification of 3-NT using HPLC-ECD.
3-NT quantification by HPLC-ECD was performed as previously described (43) with modifications. A denitration reaction sample containing 12 μg nitrated protein or peptide was digested with proteinase K (150 U/mg, 8 h at 55°C). The cooled digest was precipitated with 3 volumes of ice-cold buffer (0.1 M phosphoric acid and 0.23 M trichloroacetic acid), vortexed, incubated on ice for 5 min, and centrifuged at 12,000 g for 15 min at 4°C. The resulting supernatant was concentrated at room temperature (SpeedVac) followed by extraction with 2 volumes of chloroform. The aqueous fraction containing 3-NT was dried (SpeedVac) and then reconstituted with vacuum-filtered (0.2-μm nylon membrane) and degassed HPLC mobile-phase buffer containing 90 mM sodium acetate, 35 mM citric acid, 130 μM EDTA, and 460 μM sodium octane sulfonate (pH 4.35) prepared in 18-MΩ resistance water. An isocratic HPLC system with a multichannel electrochemical CoulArray detector and electrochemical cell (ESA, Chelmsford, MA) was used to resolve 3-NT (+700 mV, room temperature, ≈15 min) and 3-aminotyrosine (3-AT, +350 mV, room temperature, ≈4.5 min) from other species using a 100-mm C18 column (Microsorb-MV, Varian, Agilent Technologies, Santa Clara, CA) and a flow rate of 0.75 ml/min. Results for total 3-NT are represented as picomoles of 3-NT per milligram of protein.
LC-MS/MS analysis and database search.
Nanoflow LC-MS/MS was used to map peptides from denitrase-containing fractions that were enriched on a DEAE-Sepharose anion exchange column. Analyses were performed using a 6520 accurate-mass quadrupole-time of flight mass spectrometer with a chip cube and C18 column on-chip (Agilent). The mobile phases were 0.1% formic acid in water (solvent A) and 0.1% formic acid in 90% acetonitrile (solvent B). The protein digest (8 μl; preparation described in the Supplemental Material)1 was injected onto a 4-mm 40-nl Zorbax 300SB-C18 enrichment column at a flow rate of 5 μl/min, and peptides were resolved on a 0.075 × 43-mm Zorbax 300SB-C18 analytic column (3.5-μm particle size) at a flow rate of 0.3 μl/min with a gradient of 3–50% solvent B for 20 min and 50–90% solvent B for 2 min. Mass spectra were acquired in the automated MS/MS mode, in which MS/MS scans were performed on the four most intense ions from each MS scan. Peptides were identified by a database search using SpectrumMill software (Agilent). Searching parameters were as follows: minimum matched peak intensity of 50%, precursor mass tolerance of 20 ppm, and product mass tolerance of 50 ppm. Protein identifications were validated using a false discovery rate of 1% or less.
Statistical analysis.
All experiments were reproduced at least three times. Where appropriate, results are presented as averages ± SE with significant differences determined by an unpaired t-test for two group comparisons. Figures that present P values include the number of experimental replicates. P values of <0.05 were considered statistically significant. ImageJ (version 1.36b, National Institutes of Health) was used to quantify Western blot band densities in Figs. 1 and 2.
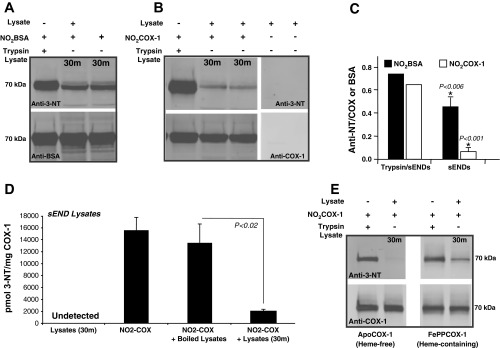
Fig. 1.
Endothelial cells exhibit cyclooxygenase (COX)-1 3-nitrotyrosine (3-NT) denitrase activity that is authentic, substrate selective, and site specific. In the reactions described, 0.5 μg of NO2COX-1 or NO2BSA were denitrated with 7.5 μg of sEND.1 cellular lysates [sENDs; 1:15 (wt/wt) ratio] at 37°C for 30 min. A and B: Western blots comparing the denitration of NO2FePPCOX-1 (A) and NO2BSA (B). Blots were probed with monoclonal anti-3-NT antibody (top) or with monoclonal anti-COX-1 or monoclonal anti-BSA (bottom) antibodies. C: bar graph showing relative nitration obtained by quantifying band density ratios between NO2FePPCOX-1 and total COX-1 (n = 5) as well as NO2BSA and total BSA (n = 7). D: denitration of NO2FePPCOX was quantified by HPLC with electrochemical detection (HPLC-ECD). Total 3-NT content was measured for the denitration reaction of NO2FePPCOX-1 after incubation with sEND.1 cell lysates at 37°C for 30 min. 3-NT was also quantified in control reactions, i.e., cell lysates incubated alone at 37°C for 30 min, NO2FePPCOX-1 incubated alone at 37°C for 30 min, and NO2FePPCOX-1 coincubated with preboiled cell lysates. E: Western blots comparing the denitration of heme-free NO2apoCOX-1 (0.5 μg/lane) and NO2FePPCOX-1 by endothelial cell lysates [sEND.1 lysates, 1:15 (wt/wt) ratio] at 37°C for 30 min. Blots were probed with monoclonal anti-3-NT antibody (top) and with monoclonal anti-COX-1 antibody (bottom). Where applicable, data are shown as averages ± SE (n ≥ 3). P < 0.05 was considered statistically significant (*).
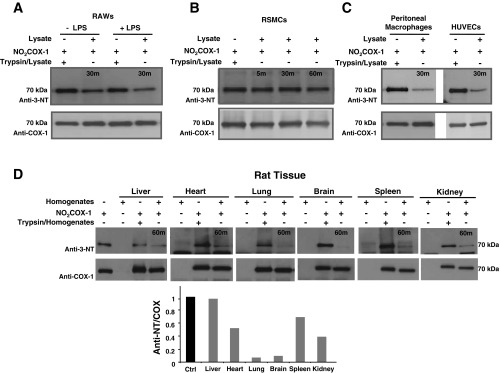
Fig. 2.
Denitrase activity is constitutive and present in a variety of cell types and tissues. In all reactions described, 0.5 μg of NO2FePPCOX-1 were denitrated with 7.5 μg of cellular lysates [RAW 264.7 (RAWs), rat aortic smooth muscle cells (RSMCs), or sEND.1 cells] or rat tissue homogenates [1:15 (wt/wt) ratio] at 37°C for 30 or 60 min. A: Western blots comparing the denitration of NO2COX-1 by lysates from LPS-treated RAW 264.7 cells (overnight treatment, 2 μg/ml) relative to untreated control cells. B: Western blots comparing the denitration of NO2COX-1 by lysates from RSMCs. C: Western blots comparing the denitration of NO2COX-1 by lysates from peritoneal macrophages and human umbilical vein endothelial cells (HUVECs). D: Western blots comparing the denitration of NO2COX-1 by homogenates from the rat liver, heart, lung, brain, spleen, and kidney. Blots were probed with monoclonal anti-3-NT antibody (top) and with monoclonal anti-COX-1 antibody (bottom). The bar graph represents the relative nitration obtained by quantifying band density ratios between NO2COX-1 and total COX-1.
RESULTS
Endothelial cells exhibit COX-1 3-NT denitrase activity that is authentic, substrate selective, and site specific.
Numerous reports have used NO2BSA as a substrate to assess protein tyrosine denitrase activity in mammalian cell and tissue extracts (29, 36). In pilot studies, we found that the extent of BSA denitration by sEND.1 and RAW 264.7 cell lysates was significant, yet did not approach complete denitration. We have previously shown a biologically relevant role for nitration in COX-1. Hence, we sought to determine the extent to which denitration may occur in NO2COX-1. We predicted that enzymatic protein denitrase activity would exhibit substrate selectivity. Thus, we compared the susceptibility of NO2BSA (Fig. 1A) and NO2COX-1 (Fig. 1B) to denitration by lysates prepared from a murine skin endothelial cell line (sEND.1 cells). Denitration of substrates was apparent after incubation with sEND.1 lysates at a 1:15 (wt/wt) ratio at 37°C. Western blot results with anti-3-NT, anti-COX-1, and anti-BSA antibodies further indicated that denitrase activity was constitutive in sEND.1 cells and more effective with NO2COX-1 as a substrate than with NO2BSA (Fig. 1C). Probing blots with antibodies that recognize nonnitrated BSA or COX-1, respectively, confirmed that the 3-NT signal loss was not a consequence of proteolysis or protein loading errors (Fig. 1, A and B, bottom).
With the exception of calmodulin denitration, where protein MS was used to detect nitrated proteins (49), all previous attempts to identify denitrase activity have used Western blot analysis with an anti-3-NT antibody. While Western blot analysis is a widely accepted detection method, the sole reliance on immunodetection to observe protein denitration leaves open the possibility that the loss of 3-NT signal could be due to the loss of antigenicity toward the anti-3-NT antibody, which could arise from 1) inadvertent chemical reduction of the nitro (NO2) group to an amine (NH2) during sample preparation [e.g., which can occur upon boiling of hemoprotein-rich lysates in the presence of β-mercaptoethanol-containing SDS-PAGE loading buffer (4)] or 2) unanticipated protease activity (although this would have to cleave nitrated peptides and be resistant to protease inhibitors present in the cell lysis buffer and denitration reaction mixture). To confirm the authenticity of denitration observed by Western blot analysis, COX-1 denitrase activity in sEND.1 cell lysates was evaluated using HPLC-ECD, an analytic method for absolute quantification of 3-NT levels (Fig. 1D). Incubation with sEND.1 cell lysates for 30 min at 37°C resulted in an 85% loss in 3-NT content from purified NO2COX-1 (Fig. 1D). As negative controls, coincubation of NO2COX-1 with preboiled lysates failed to diminish the 3-NT content of NO2COX-1, and 3-NT was not detected in cell lysates without added NO2COX-1. Since these samples were digested with proteinase K to the single amino acid level before analysis, identification of 3-NT loss by HPLC-ECD negates the possibility that protease activity was responsible for the observed denitration. Importantly, HPLC-ECD also detects the formation of 3-AT, as this species is electrochemically active, separated from 3-NT by C18 chromatography (3-NT: 17 min; 3-AT: 4.5 min), and unchanged under conditions where 3-NT denitration is observed (Supplemental Fig. S1). Indeed, 3-AT formation was undetected in these samples, indicating that reductive chemistry cannot explain the observed 3-NT signal loss in NO2COX-1 after incubation with cell lysates. This conclusion is further supported by the fact that NO2COX-1 denitration with sEND.1 cell lysates for 30 min at 37°C was also observed by Western blot analysis when a thiol-free reducing agent, TCEP, was used in the SDS-PAGE loading buffer in place of β-mercaptoethanol (data not shown). Additionally, NO2BSA (a heme-free protein) was equally denitrated in both the presence and absence of native heme-bound FePPCOX-1 (data not shown), indicating that the contribution of heme from a hemoprotein does not contribute to reductive chemistry on 3-NT in our experimental setting.
Given that differential denitration by endothelial cells was observed with NO2COX-1 and NO2BSA under identical experimental conditions (Fig. 1, A and B), we hypothesized that protein 3-NT denitration may be site selective and dependent on molecular determinants that confer substrate conformation. This hypothesis predicts that for a protein with multiple 3-NT sites, denitration patterns would be differential and site selective. To test this, we compared the denitration rates for nitrated heme-bound (FePPCOX-1) versus nitrated heme-free (apoCOX-1) forms of COX-1. We have previously demonstrated that apoCOX-1 and FePPCOX-1 are differentially and site selectively nitrated due to a heme-catalyzed mechanism that preferentially targets nitration to Tyr385. Furthermore, while ONOO−-induced COX-1 nitration still occurs in the absence of Fe3+-heme, we showed that internal Tyr385 (the primary target of FePPCOX-1 nitration) remains unmodified after apoCOX-1 nitration (13). As shown in Fig. 1E using endothelial sEND.1 cell lysates, NO2apoCOX-1, but not NO2FePPCOX-1, denitrated to completion under identical experimental conditions, suggesting that internal Tyr385 may not be a denitration target. Denitration of NO2FePPCOX-1 did not restore enzymatic activity, as determined by an assay for PGE2 formation or by an arachidonic acid-coupled COX-peroxidase assay (13). These results are consistent with a site-specific denitration mechanism that depends, at least in part, on protein conformation and solvent accessibility.
It is possible that 3-NT placement in a peptide sequence contributes to substrate susceptibility for 3-NT denitration. Thus, we compared the extent to which sEND.1 cell lysates mediate denitration of free (nonprotein-bound) 3-NT as well as three synthetic 3-NT-containing peptides that were designed based on previously identified protein tyrosine nitration in two distinct proteins, namely, COX (12) and histone H1.2 (26). Notably, we chose to study two different COX-1 peptides because the peptide sequence containing COX-1 Tyr254 has previously been shown to be the primary target of apoCOX-1 nitration, whereas the peptide sequence containing COX-1 Tyr385 is the primary target of nitration in FePPCOX-1 (12, 13). As determined by HPLC-ECD, the extent of denitration in putative protein and peptide substrates varied markedly under identical assay conditions (Supplemental Fig. S2). Together, these results indicate that the extent of tyrosine denitration is substrate dependent and likely to be determined by protein/peptide conformation and 3-NT accessibility, since nitrated peptides lacking secondary structure were unable to replicate the denitration observed in their counterpart proteins.
Denitrase activity is constitutive in a variety of cell types and tissues.
Using NO2COX-1 as a probe, we screened for denitrase activity in primary cell culture and cell lines as well as diverse rat and murine organ tissues (Fig. 2). Denitration of NO2COX-1 was observed after treatment with murine macrophage RAW 264.7 cell lysates at 37°C [1:15 (wt/wt) ratio], and activity was slightly enhanced in lysates from LPS-pretreated versus naïve cells (Fig. 2A), an observation that has previously been demonstrated by Irie et al. (26). Under our denitration test conditions, activity was not detected in RASMCs (Fig. 2B) but was found to be constitutively expressed in HUVECs and murine peritoneal macrophages, as shown in Fig. 2C. Additionally, denitrase activity was identified in a variety of rat (Fig. 2D) and murine (data not shown) tissues.
Of the tissues analyzed for activity, lung and brain homogenates were most efficient at denitrating NO2COX-1, whereas liver homogenates possessed little to no detectable activity.
COX-1 denitration by endothelial cell lysates is time and protein dependent.
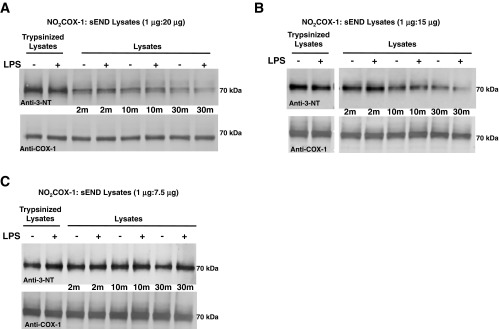
Since NO2COX-1 is extensively denitrated by cell lysates, with endothelial cells proving to be a rich source of activity, we investigated whether denitration displays features of a regulated biochemical process. After coincubation of NO2COX-1 with sEND.1 cell lysates [1:20 (wt/wt) ratio] at 37°C, we observed, by Western blot analysis, a time-dependent decrease in the 3-NT signal that was near completion in 30 min and abolished by trypsin pretreatment of cell lysates (Fig. 3A). Diminishing the ratio of NO2COX-1:lysates from 1:20 (wt/wt) to 1:15 (wt/wt) attenuated the rate and extent of NO2COX-1 denitration (Fig. 3B). Further diminishing the ratio of NO2COX-1:lysates to 1:7.5 (wt/wt) resulted in a marked attenuation of the rate and extent of NO2COX-1 denitration (Fig. 3C). Denitration of NO2COX-1 by sEND.1 cell lysates occurred to a similar extent, irrespective of whether lysates were prepared from naïve or LPS-immunoactivated cells (Fig. 3, A–C). These results establish that the extent of NO2COX-1 denitration by sEND.1 cell lysates was a function of time and lysate protein amount and can be abolished by protease pretreatment of cell lysates. Therefore, protein 3-NT denitration exhibits properties that are consistent with an enzymatic process.
Fig. 3.
Denitration of NO2COX by endothelial cell (sEND.1) lysates is time and protein dependent. A–C: Western blots comparing the denitration of NO2FePPCOX-1 (0.5 μg/lane) by LPS-stimulated sEND.1 lysates (overnight treatment, 2 μg/ml) relative to control at 37°C over a 30-min timecourse. The ratio of NO2COX-1 to cell lysates was 1:20 (wt/wt) in A, 1:15 (wt/wt) in B, and 1:7.5 (wt/wt) in C. Blots were probed with monoclonal anti-3-NT antibody (top) and with monoclonal anti-COX-1 antibody (bottom).
Endothelial cell protein denitrase activity is inhibited by free 3-NT, suppressed after inhibition of NOS, and enhanced by CO.
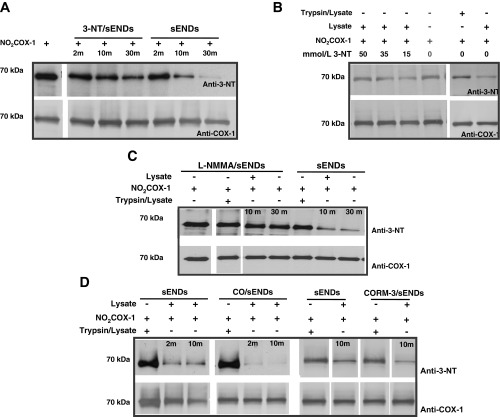
Since nonprotein 3-NT was found to be a substrate for denitrase activity, we tested whether this molecule would effectively compete for denitration of NO2COX-1. As shown in Fig. 4A, coincubation with 3-NT (50 μM) resulted in a dramatic decrease in the extent of NO2COX-1 denitration by sEND.1 lysates relative to control when monitored as a function of time. In fact, 3-NT dose dependently altered the extent of NO2COX-1 denitration at 30 min by sEND.1 lysates (Fig. 4B). As sEND.1 cells are known to constitutively express endothelial NOS and basally synthesize arginine-derived NO (10), we investigated whether endogenous NO production influences the expression of NO2COX-1 denitrase activity. Denitrase activity was assessed in lysates from sEND.1 cells harvested after overnight culture in the presence or absence of l-NMMA, a selective NOS inhibitor (1). As shown in Fig. 4C, l-NMMA treatment resulted in an almost complete loss of denitrase activity relative to control. A major mechanism by which NO modulates protein activities is by covalent addition to transition metals in proteins (7). Since this metal reactivity is shared with another gas, CO (7), we additionally evaluated the effect of CO gas and a water-soluble CO releaser (CORM-3) on denitrase activity in sEND.1 cell lysates (Fig. 4D). As an inert gas control, we tested the effect of N2. The results shown in Fig. 4D demonstrate that bubbling of lysates with pure CO for 60 s or exposure to the CO donor CORM-3 for 20 min markedly enhanced denitrase activity in cell lysates relative to untreated control lysates. Notably, the concentration of CORM-3-released CO in lysates was estimated to be <1 μmol/l based on a previous report (38) demonstrating that 1 μmol/l CO correlates to CO release from 10 to 100 μmol/l CORM-3. On the other hand, vigorous bubbling of CO for 20 min produces saturation at 1 mmol/l (2). Pretreatment of lysates with N2 also enhanced denitrase activity relative to nongassed lysates (data not shown), albeit to a lesser extent than lysates exposed to CO. Since N2 sparging depletes levels of dissolved O2 in sEND.1 cell lysates, the finding of attenuated denitrase activity may be reconciled by an O2-regulated process, as also demonstrated by Koeck et al. (32). These findings suggest that protein 3-NT denitrase activity may be regulated by NO and other metal-binding molecules in vivo. Furthermore, our finding that that relatively low concentrations of free (nonprotein) 3-NT suppress protein 3-NT denitrase activity (presumably via competition for binding of protein 3-NT substrates) offers a potential probe to selectively assess the role of protein denitration in biological systems.
Fig. 4.
Cellular denitrase activity is modulated by ligands. In all reactions described, 0.5 μg of NO2FePPCOX-1 were denitrated with 7.5 μg of sEND.1 cellular lysates [1:15 (wt/wt) ratio] at 37°C over a 30-min timecourse. A: Western blots comparing the denitration of NO2COX-1 by 3-NT-treated lysates (50 μM) relative to untreated control cell lysates. B: Western blots comparing the denitration of NO2COX-1 at 30 min by sEND.1 lysates pretreated with varying concentrations of 3-NT relative to untreated control cell lysates. C: Western blots comparing the denitration of NO2COX-1 by sEND.1 lysates after overnight treatment of whole cells with NG-monomethyl-l-arginine (l-NMMA; 3 mM) relative to untreated control cells. D: Western blots comparing the denitration of NO2COX-1 by sEND.1 lysates after direct application of carbon monoxide (CO) gas (60 s) or CO-releasing molecule (CORM)-3 (100 μM, 20 min) relative to untreated control cell lysates. Blots were probed with monoclonal anti-3-NT antibody (top) and with monoclonal anti-COX-1 antibody (bottom).
The intracellular distribution of denitrase activity and its chromatographic resolution from rat lungs.
Previously, Koeck et al. (32) demonstrated that at least a portion of a cell's denitrase activity is localized to its mitochondria. Thus, we examined the solubility and intracellular distribution associated with denitrase activity. Rat lungs were homogenized in detergent-free buffer and subjected to differential centrifugation enabling for the comparison of NO2COX-1 denitration by mitochondria-enriched [12,000-g pellet (P1)] (19) relative to microsomal [120,000-g pellet (P2)] and soluble cell fractions [supernatants (S1 and S2)], as shown in Fig. 5A. Of note, whereas S1 was depleted from mitochondria but contained microsomal membrane components, S2 was depleted from microsomal debris as well. Subsequently, denitration of NO2COX-1 was compared in the crude lung homogenate versus centrifugation fractions [1:15 (wt/wt) ratio, 37°C] by Western blot analysis with anti-3-NT antibody. Mitochondrial enrichment in P1 was confirmed by probing Western blots for MnSOD, a mitochondrial-specific inner matrix protein. While the results shown in Fig. 5A confirm that the mitochondria-enriched fraction (P1) possessed strong denitrase activity, comparable with that in the unfractionated lung homogenate, incubation of NO2COX-1 with the microsomal fraction (P2) as well as mitochondria-depleted fractions (S1 and S2) also elicited a decrease in the 3-NT signal. These findings suggest that denitrase activity is distributed in both soluble and membrane components of cells and that it is enriched in, but not limited to, the mitochondrial fraction.
Purification will be essential for defining the structural and functional properties of denitrase activity and for developing molecular tools for studying biological functions. Since we identified lungs to be a rich source of denitrase activity (Fig. 2), we tested the feasibility of purification and characterization of denitrase activity from this source. Our enrichment scheme included separation of rat lung homogenates with a 120,000-g spin, concentration on a 10-kDa microcon filter chromatographic resolution on a DEAE-Sepharose anion exchange column with a linear potassium phosphate gradient (10–150 mM), as shown in Fig. 5B, and, finally, a final desalting and concentration step on a 10-kDa microcon filter. Using Western blot analysis (Fig. 5C) and HPLC-ECD (Fig. 5D), we found that the denitration activity was present in only three of the fractions eluted from the DEAE-Sepharose column (F55, F56, and F57), which we then pooled and concentrated. Enrichment of activity was demonstrated by the fact that equal volumes from DEAE fractions (20 μl) were used to test their ability to denitrate NO2COX-1. To this end, while fractions F55, F56, and F57 robustly denitrated NO2COX-1, they contained a reduced protein content relative to other eluting fractions. The enriched denitrase activity using the described scheme 1) does not require detergent for solubilization or chromatographic elution, 2) purifies as a single peak with ion exchange chromatography, and 3) is stable at pH 8 and retains functionality throughout pH variations between 5.5 and 9. This procedure resulted in significant enrichment of a discrete denitrase-containing fraction that absorbed at 280 nm and maintained functionality throughout the purification process. Starting with 1.5 g rat lung protein, a discrete denitrase activity was contained in 2 mg protein. The column-purified denitrase fractions were analyzed for peptide sequence information by nanoflow LC-MS/MS. Since the denitrase-containing homogenate was impure, numerous proteins were likely to be present at levels that limited identification by MS analysis.
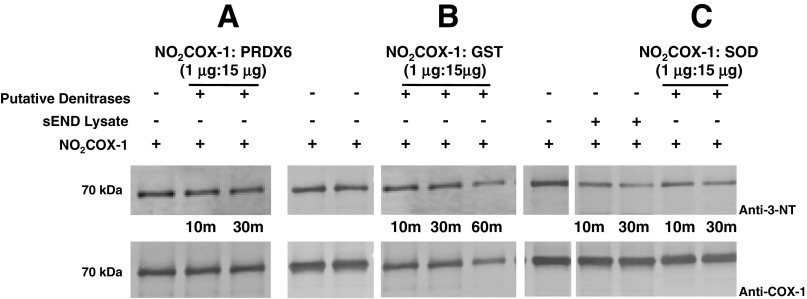
From the 18 proteins that could be identified by LC-MS/MS (Supplemental Table S1) we selected Cu,Zn-SOD, glutathione S-transferase (GST), and peroxiredoxin-6 for further testing as to whether these pure proteins could denitrate NO2COX-1 (Fig. 6, A–C). These three enzymes were evaluated based on their known redox regulatory roles in cells (18, 20, 48). Denitration of NO2COX-1 at 37°C was observed by Western blot analysis after treatment of GST (Fig. 6B) and Cu,Zn-SOD (Fig. 6C) at a 1:15 (wt/wt) ratio over a period of 30 min. Denitration of NO2COX-1 by these candidate enzymes was compared with NO2COX-1 denitration using sEND.1 lysates as a control. Under identical experimental conditions, peroxiredoxin-6 (Fig. 6A) did not elicit NO2COX-1 denitration. These findings suggest that there may be multiple enzymes that possess denitrase activity. Further purification and inhibition studies are required to elucidate the relative contribution of these enzymes to cellular denitrase activity.
Fig. 6.
Proteins identified that possess NO2COX-1 denitrase activity. In all reactions described, 1 μg of NO2COX-1 were denitrated with 15 μg of sEND.1 cellular lysates [1:15 (wt/wt) ratio], serving as a control, or with 15 μg of pure protein identified as a putative denitrase [1:15 (wt/wt) ratio] at 37°C for 10 and 30 min. A–C: Western blots comparing the denitration of NO2COX-1 by peroxiredoxin (PRDX)-6 (A), glutathione S-transferase (GST; B), and Cu,Zn-SOD (C). Blots were probed with monoclonal anti-3-NT antibody (top) and with monoclonal anti-COX-1 antibody (bottom).
DISCUSSION
Since the first report of 3-NT removal in a biological system (29), an increasingly strong rationale to characterize a protein “denitrase” activity has emerged. Here, we identified nitrated COX-1 as a novel substrate for ubiquitously expressed protein 3-NT denitrase activity and described an enrichment method that enables further characterization of 3-NT denitrase activity.
We (12, 13) have previously shown that COX-1 undergoes selective heme-directed nitration that is dependent on active site substrate occupancy. While COX-1 is a membrane-bound hemoprotein that is structurally and functionally distinct from the metal-free serum protein BSA, these proteins possess similar tyrosine content (21 Tyr/BSA vs. 27 Tyr/COX-1 subunits) and monomeric masses (66 kDa for BSA vs. 70 kDa for COX-1). Interestingly, we provided evidence showing that endothelial cell homogenates denitrate NO2COX-1 more effectively than NO2BSA (Fig. 1).
To date, demonstration of protein denitration has relied on Western blot analysis for the visualization of protein 3-NT signal attenuation. Western blot analysis is an accurate and reliable semiquantitative assay for protein modifications, but it is prone to artifacts such as interference with 3-NT antigenicity in a protein substrate and/or loss of anti-3-NT antibody recognition that may arise from secondary chemical modifications, e.g., reduction of 3-NT to 3-AT (vs. removal of an NO2 group). Thus, we used analytic HPLC-ECD to directly quantify 3-NT levels in proteolyzed nitrated substrates (Fig. 1). We confirmed that denitration of NO2COX-1 by denitrase-containing extracts from sEND.1 endothelial cells was associated with an 85% reduction in 3-NT levels and that these findings were not the result of compromised antigenicity. As samples were enzymatically proteolyzed to the single amino acid before HPLC-ECD analysis of free 3-NT, we can eliminate the possibility that the observed decreases in NO2COX-1 levels was due to protein proteolysis. Furthermore, 3-AT formation from NO2COX-1 was undetectable by HPLC-ECD in our denitration assays, indicating that 3-NT loss was not a result of heme-thiol-mediated reduction of the tyrosine-bound NO2 to NH2. This is in agreement with MS analysis of denitration products from NO2-calmodulin where 3-AT formation was not detected (49). With respect to COX-1, studies (6, 15, 34) have shown that only a small percentage of tyrosine residues (∼3 tyrosine residues per COX-1 monomer) are subject to NOx-induced nitration. Although separation and detection of free tyrosine can be achieved by HPLC-ECD, it was difficult to accurately assess whether the 3-NT signal loss was associated with a commensurate formation of new tyrosine residues, owing to the significantly larger signal that was generated from the endogenous tyrosine pool in existing proteins from the lysate/substrate incubation mixture. Since nitration of COX-1 is incomplete, it was problematic to use MS to differentiate between newly de- and non-nitrated COX-1 peptides. Thus, we can only verify that denitration of NO2COX-1 by cell/tissue lysates did not result in 3-AT accumulation. We cannot rule out the possibility that a different tyrosine modification is the actual product, as opposed to the “hypothetical” product of unmodified tyrosine.
Ischiropoulos and colleagues (27) have suggested criteria that determine susceptibility of individual protein tyrosine residues to nitration, including that tyrosine residue 1) should preferentially be solvent accessible, 2) present on a peptide loop structure, and 3) adjacent to an acidic amino acid (glutamic or aspartic acid) (27). Using a diverse set of 3-NT-containing proteins, custom-made peptides, and free 3-NT, we clearly demonstrated that tyrosine residues in proteins undergo differential denitration. Specifically, we observed that only the external (i.e., solvent exposed) 3-NT residues in COX-1 were susceptible to denitration, since heme-free NO2apoCOX-1, but not heme-bound NO2FePPCOX-1 (containing internally nitrated Tyr385), experienced complete denitration (Fig. 1). 3-NT residues in proteins that were reported to be most susceptible to nitration, specifically Tyr254 of COX-1 (12) and Tyr71 of histone H1.2 (26), were not found to be denitration substrates when present on synthetic peptides (Fig. 1 and Supplemental Fig. S2). Thus, we predict that protein/peptide conformation and solvent access will prove to be a critical determinant of a 3-NT residue's susceptibility to denitration in vivo, but the relevance of conformation and context remain to be determined. Although nitration of surface tyrosine residues in COX-1 do not impact COX-1 activity, as is the case for nitration of internal catalytic Tyr385, it is possible that this superficial nitration/denitration may play an as yet undiscovered role in COX-1 function during inflammation. In addition to direct effects on enzymatic activity, tyrosine nitration can target proteins for proteosomal degradation (50), inhibit or enhance protein-protein interactions (11, 35), block targets for tyrosine phosphorylation (28, 33), and modify protein association with lipid bilayers (47).
Despite the wide array of potential nitration targets in arachidonic acid metabolism, it is notable that only a very limited number of protein targets and tyrosine nitration sites have been identified to date (44, 52). This may be due to the complexity of NO interactions highlighted by the fact that the impact of tyrosine nitration is markedly varied between structurally similar COX-1 and COX-2. While ONOO− can inactivate COX-1 by inducing nitration of catalytically essential Tyr385 (13), ONOO− has been shown to activate COX-2 by tyrosine nitration in vivo (8, 40). Of note, prostacyclin synthase, a crucial enzyme in maintaining normal endothelial homeostasis, may be one of the first targets for NOx species (56). Therefore, it is possible for nitration and denitration to alter the flux of arachidonic acid and shunt activity from the COX pathway to other pathways in the arachidonic acid cascade, as has been previously described for aspirin-induced COX-2 inhibition (9, 46).
Using NO2COX-1 as a target for denitrase activity, we screened cell lines and rodent organs for the distribution of activity. Although previous reports (29, 32, 36) have individually shown denitrase activity to be localized to specific tissues and cell types, these reports collectively showed that cellular denitration is ubiquitous. Consistent with earlier reports, we observed significant denitrase activity in a broad collection of tissues and cells, including all organs examined, with the liver displaying the least activity (Fig. 2). The observation of weak denitrase activity in liver homogenates is consistent with a previous study (5) that found that rat liver extracts possessed high 3-NT content relative to other organs (spleen and lung) from the same animal. The total complement of protein 3-NT in a given cell or organ may be determined by two opposing kinetic processes, protein 3-NT nitration mediated by locally produced NO-derived species and protein 3-NT denitration, mediated by the denitrase activity described here (Supplemental Fig. S3). We observed denitrase activity in macrophages and endothelial cells, although not in RASMCs. Protein denitration has been suggested to be markedly induced by LPS (26, 30, 49); however,we observed only a slight induction of activity after LPS-induced immunoactivation of macrophages in vitro, and endothelial cells were found to constitutively express equal levels of denitrase activity both with and without cytokine stimulation (Fig. 2). Given the broad expression of protein denitrase activity, more than one protein or enzyme isoform may contribute to the denitrase activity observed in different cell types, organelles, and physiological/pathophysiological settings.
Here, we provide evidence showing that protein denitrase activity displays features of an enzyme, including time and concentration dependence (Fig. 3) as well as susceptibility to inactivation by proteolysis and heating. Indeed, our findings concur with those of Murad and colleagues, who showed that protein denitrase activity was retained by a 30-kDa cutoff filter (26) and abolished by boiling and trypsinolysis (29).
We used NO2COX-1 denitration to determine whether expression of protein denitrase activity in endothelial cells could be modulated by potential ligands (Fig. 4). While nonprotein 3-NT competed for denitration of NO2COX-1 by endothelial cells, we found that endogenous NO, exogenous CO, and, to a lesser extent, N2 were capable of enhancing denitrase activity. One possibility is that a metalloprotein(s) may modulate this activity, as both NO and CO bind directly to certain transition metal centers in proteins (7) and N2-mediated deoxygenation can indirectly affect metalloprotein activities (41).
Although we have found sEND.1 cells and lungs to be rich sources of denitrase activity, the ubiquity of denitrase activity suggests that it may be present in multiple cellular compartments. While fractionation experiments with lung homogenates (Fig. 5) confirmed previous findings that denitrase activity resides in isolated mitochondria (32), our observations that activity was retained in both soluble and microsomal fractions imply that the denitrase is not exclusive to a specific organelle and could be present in both aqueous and membrane phases of cells.
We recognize that the most practical approach for final identification of the denitrase enzyme(s) will be through complete biochemical purification, followed by protein knockdown to demonstrate necessity and the development of molecular tools for studying its biological function. Complete denitrase(s) purification will be facilitated by the development of a high-throughput, rapid, and quantitative denitrase assay. Here, we successfully enriched enzymatic activity from soluble fractions of the rat lung that was capable of denitrating NO2COX-1 (Fig. 5). From MS analysis of proteins identified in the active fraction (Supplemental Table S1), we selected three enzymes with known redox properties. The amount of enzyme used for NO2COX-1 denitration was determined based on the weight needed to accomplish denitration and not specific activity. Of these, CuZn-SOD and GST demonstrated denitrase capabilities (Fig. 6). CuZn-SOD is an important antioxidant defense enzyme that catalyzes the dismutation of O2·− into O2 and H2O2, thus preventing its reaction with NO and yielding nitrated proteins. That CuZn-SOD possesses denitrase activity presents two potential mechanisms for this enzyme to reduce cellular 3-NT levels. Notably, CuZn-SOD contains a metal center necessary for its main catalytic activity. Also, GST has been shown to detoxify 4-hydroxynonenal, a ONOO−-induced lipid peroxidation product (48), and incubation of GST with nitrated histone increases nitrate levels, suggestive of histone denitration (36). Therefore, GST may be a powerful antioxidant enzyme with the capability of reversing multiple ONOO−-induced modifications. Given that multiple enzymes present denitrase activity in vitro, as well as our findings showing that denitrase activity is present in multiple cellular compartments, there could be mutiple denitrase enzymes in cells. Further studies are needed to identify these and assess their relative contributions to denitration in alternative cell settings.
This study provides new information regarding the specificity, localization, characterization, and regulation of cellular denitration and highlights a significant cellular activity that may be capable of repairing proteins damaged during diseases where levels of reactive nitrogen species are elevated. Until the enzyme(s) is pure, it is premature to attempt an elucidation of mechanism, although we infer the substrates to be a proteinacious nitrotyrosine and a yet-to-be defined reductant and the products to be a proteinacious tyrosine and a nitrogenous end product (Supplemental Fig. S3). We anticipate that the molecular basis for protein denitrase activity will have significant translational impact, given the widespread occurrence of protein tyrosine nitration in diverse pathophysiological settings.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants P01-HL-46403 and R01-HL-091101 (to D. P. Hajjar) and P01-HL-46403 and HL-87062 (to S. S. Gross) as well as NIH Predoctoral Fellowship F31-AG-32195 (to T. N. Nuriel), American Heart Association Grant-In-Aid AHA655783T (to R. S. Deeb), and grants (to D. P. Hajjar) from the Julia and Seymour Gross Foundation and by the Abercrombie Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.S.D., T.N., B.D.L., S.S.G., and D.P.H. conception and design of research; R.S.D., T.N., C.C., and B.S. performed experiments; R.S.D., C.C., B.S., and B.D.L. analyzed data; R.S.D., T.N., C.C., B.S., B.D.L., S.S.G., and D.P.H. interpreted results of experiments; R.S.D., C.C., and B.S. prepared figures; R.S.D., S.S.G., and D.P.H. drafted manuscript; R.S.D., T.N., C.C., B.S., B.D.L., S.S.G., and D.P.H. edited and revised manuscript; R.S.D., T.N., B.D.L., S.S.G., and D.P.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Domenick Falcone (Weill Cornell Medical College) for helpful discussions during the course of this study and Dr. John Falck (University of Texas Southwestern Medical Center) for kindly providing the compound CORM-3. The authors thank Jay Patel and Hilal Koyuncu for superb technical assistance and Dr. Yevgeniy Lukyanov for help with harvesting murine organs.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Addabbo F, Ratliff B, Park HC, Kuo MC, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol 174: 34–43, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen JJ, Shafi NI, Durante W, Bryan RM., Jr Effects of carbon monoxide and heme oxygenase inhibitors in cerebral vessels of rats and mice. Am J Physiol Heart Circ Physiol 291: H223–H230, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA 74: 3203–3207, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balabanli B, Kamisaki Y, Martin E, Murad F. Requirements for heme and thiols for the nonenzymatic modification of nitrotyrosine. Proc Natl Acad Sci USA 96: 13136–13141, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian K, Murad F. Diversity of endotoxin-induced nitrotyrosine formation in macrophage-endothelium-rich organs. Free Radic Biol Med 31: 421–429, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Boulos C, Jiang H, Balazy M. Diffusion of peroxynitrite into the human platelet inhibits cyclooxygenase via nitration of tyrosine residues. J Pharmacol Exp Ther 293: 222–229, 2000. [PubMed] [Google Scholar]
- 7.Brune B, Schmidt KU, Ullrich V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur J Biochem 192: 683–688, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Chen YJ, Santos M, Quilley J. Treatment of diabetic rats with a peroxynitrite decomposition catalyst prevents induction of renal COX-2. Am J Physiol Heart Circ Physiol 300: H1125–H1132, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA 92: 9475–9479, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 294: H1530–H1540, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crow JP, Ye YZ, Strong M, Kirk M, Barnes S, Beckman JS. Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilamnet-L. J Neurochem 69: 1945–1953, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Deeb RS, Cheung C, Nuriel T, Lamon BD, Upmacis RK, Gross SS, Hajjar DP. Physical evidence for substrate binding in preventing cyclooxygenase inactivation under nitrative stress. J Am Chem Soc 132: 3914–3922, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeb RS, Hao G, Gross SS, Laine M, Qiu JH, Resnick B, Barbar EJ, Hajjar DP, Upmacis RK. Heme catalyzes tyrosine 385 nitration and inactivation of prostaglandin H-2 synthase-1 by peroxynitrite. J Lipid Res 47: 898–911, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Deeb RS, Nuriel T, Gross SS. Untargeted discovery of NO-modified proteins. In: Nitric Oxide: Biology and Pathobiology (2nd ed.), edited by L. Ignarro. San Diego: Elsevier Inc., 2010, p. 327–390. [Google Scholar]
- 15.Deeb RS, Resnick MJ, Mittar D, McCaffrey T, Hajjar DP, Upmacis RK. Tyrosine nitration in prostaglandin H2 synthase. J Lipid Res 43: 1718–1726, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Edelson PJ, Cohn ZA. Purification and Cultivation of Monocytes and Macrophages. New York: Academic, 1976. [Google Scholar]
- 17.Falcone DJ, Ferenc MJ. Acetyl-LDL stimulates macrophage-dependent plasminogen activation and degradation of extracellular matrix. J Cell Physiol 135: 387–396, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24: 1367–1373, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Vizarra E, Lopez-Perez MJ, Enriquez JA. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods 26: 292–297, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal 15: 831–844, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett 581: 84–90, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Greenacre SAB, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic Res 34: 541–581, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Grune T, Blasig IE, Sitte N, Roloff B, Haseloff R, Davies KJA. Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J Biol Chem 273: 10857–10862, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irie Y, Kamisaki Y, Saeki M, Wada K, Murad F, Miki N. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in protein. J Pharm Sci 91: 278P-–278P., 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun 305: 776–783, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys 356: 1–11, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Kamisaki Y, Wada K, Bian K, Balabanli B, Davis K, Martin E, Behbod F, Lee YC, Murad F. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc Natl Acad Sci USA 95: 11584–11589, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang M, Akbarali HI. Denitration of L-type calcium channel. FEBS Lett 582: 3033–3036, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry 44: 13071–13081, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Koeck T, Fu XM, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem 279: 27257–27262, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Kong SK, Yim MB, Stadtman ER, Chock PB. Peroxynitrite disables the tyrosine phosphorylation regulatory mechanism: lymphocyte-specific tyrosine kinase fails to phosphorylate nitrated cdc2(6-20)NH2 peptide. Proc Natl Acad Sci USA 93: 3377–3382, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulmacz RJ, Ren Y, Tsai AL, Palmer G. Prostaglandin H synthase: spectroscopic studies of the interaction with hydroperoxides and with indomethecin. Biochemistry 29: 8760–8771, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D, Walter J, Pape HC, König S, Roeber S, Jessen F, Klockgether T, Korte M, Heneka MT. Nitration of tyrosine 10 critically enhances amyloid beta aggregation and plaque formation. Neuron 71: 833–844, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Kuo WN, Kanadia RN, Shanbhag VP, Toro R. Denitration of peroxynitrite-treated proteins by “protein nitratases” from rat brain and heart. Mol Cell Biochem 201: 11–16, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Lamon BD, Upmacis RK, Deeb RS, Koyuncu H, Hajjar DP. Inducible nitric oxide synthase gene deletion exaggerates MAPK-mediated cyclooxygenase-2 induction by inflammatory stimuli. Am J Physiol Heart Circ Physiol 299: H613–H623, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A. Dual pathways of carbon monoxide-mediated vasoregulation: modulation by redox mechanisms. Circ Res 105: 775–783, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane P, Hao G, Gross SS. S-nitrosylation is emerging as a specific and fundamental posttranslational protein modification: head-to-head comparison with O-phosphorylation. Sci STKE 2001: RE1, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Qi J, Liu K, Li B, Wang H, Jia J. Peroxynitrite-induced nitration of cyclooxygenase-2 and inducible nitric oxide synthase promotes their binding in diabetic angiopathy. Mol Med 16: 335–342, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T, Li B, Singleton ML, Hall MB, Darensbourg MY. Sulfur oxygenates of biomimetics of the diiron subsite of the [FeFe]-hydrogenase active site: properties and oxygen damage repair possibilities. J Am Chem Soc 131: 8296–8307, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Lymar SV, Jiang Q, Hurst JK. Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry 35: 7855–7861, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Nuriel T, Deeb RS, Hajjar DP, Gross SS. Protein 3-nitrotyrosine in complex biological samples: quantification by high-pressure liquid chromatography/electrochemical detection and emergence of proteomic approaches for unbiased identification of modification sites. Methods Enzymol 441: 1–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radi R, Denicola A, Freeman BA. Peroxynitrite reactions with carbon dioxide-bicarbonate. Methods Enzymol 301: 353–367, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA 81: 5335–5339, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sevcsik E, Trexler AJ, Dunn JM, Rhoades E. Allostery in a disordered protein: oxidative modifications to α-synuclein act distally to regulate membrane binding. J Am Chem Soc 133: 7152–7158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi Y. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 6: 289–300, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Smallwood HS, Lourette NM, Boschek CB, Bigelow DJ, Smith RD, Pasa-Tolic L, Squier TC. Identification of a denitrase activity against calmodulin in activated macrophages using high-field liquid chromatography–FTICR mass spectrometry. Biochemistry 46: 10498–10505, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Smith CD, Carson M, van der Woerd M, Chen J, Ischiropoulos H, Beckman JS. Crystal structure of peroxynitrite-modified bovine Cu,Zn superoxide dismutase. Arch Biochem Biophys 299: 350–355, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science 258: 1898–1902, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Upmacis RK, Deeb RS, Hajjar DP. Oxidative alterations of cyclooxygenase during atherogenesis. Prostagland Other Lipid Mediat 80: 1–14, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Van der Ouderaa FJ, Buytenhek M, Nugteren DH, Van Dorp DA. Purification and characterisation of prostaglandin endoperoxide synthetase from sheep vesicular glands. Biochim Biophys Acta 487: 315–331, 1977. [DOI] [PubMed] [Google Scholar]
- 54.Williams RL, Courtneidge SA, Wagner EF. Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell 52: 121–131, 1988. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Prot Immunol; 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol 154: 1359–1365, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.