Abstract
The purpose of this study was to identify and explain changes in ventricular and cellular function that contribute to aging-associated cardiovascular disease in aging F344 rats. Three groups of female F344 rats, aged 6, 18, and 22 mo, were studied. Echocardiographic measurements in isoflurane-anesthetized animals showed an increase in peak left ventricular torsion between the 6- and the 18-mo-old groups that was partially reversed in the 22-mo-old animals (P < 0.05). Epicardial, midmyocardial, and endocardial myocytes were subsequently isolated from the left ventricles of each group of rats. Unloaded sarcomere shortening and Ca2+ transients were then measured in these cells (n = >75 cells for each of the nine age-region groups). The decay time of the Ca2+ transient and the time required for 50% length relaxation both increased with age but not uniformly across the three regions (P < 0.02). Further analysis revealed a significant shift in the transmural distribution of these properties between 18 and 22 mo of age, with the largest changes occurring in epicardial myocytes. Computational modeling suggested that these changes were due in part to slower Ca2+ dissociation from troponin in aging epicardial myocytes. Subsequent biochemical assays revealed a >50% reduction in troponin I phosphoprotein content in 22-mo-old epicardium relative to the other regions. These data suggest that between 18 and 22 mo of age (before the onset of heart failure), F344 rats display epicardial-specific myofilament-level modifications that 1) break from the progression observed between 6 and 18 mo and 2) coincide with aberrant patterns of cardiac torsion.
Keywords: aging, heart failure, transmural heterogeneity, left ventricular torsion
heart failure and diastolic dysfunction are more common in the elderly than in other age groups (12, 38). Efforts to understand the relationship between aging and heart failure have uncovered many age-dependent changes in various properties of the heart (17, 19). Some of these may actually be beneficial adaptations that compensate for other age-related cardiovascular changes (18). For example, the duration of cardiac contraction and ejection increases with age (13, 39), which probably helps to maintain cardiac output in the face of increasing vascular stiffness (20). Beyond a certain point, however, adaptations may cease to be useful and start to hinder cardiovascular function. To use the same example, systolic contractions that are excessively long compromise diastolic function.
Changes in single myocytes may also reach adaptive limits. In F344 rats, a commonly used model of aging-associated heart failure (3, 25), prolonged cellular contractions in older animals are thought to be caused by slower decay of the intracellular Ca2+ transients and increased expression of the slower β-isoform of myosin heavy chain molecules (β-MHC) (8, 23, 24). These modifications may be useful at first but, if taken to excess, will adversely affect other aspects of cell function, such as electrical activity and contractile power output.
This study was designed to detect the presence of adaptive limits in myocardial aging in F344 rats. Animals were studied at three ages: 6, 18, and 22 mo. Differences in experimental parameters between 6 and 18 mo of age were attributed to normal, “healthy” aging because F344 rats at these ages are largely free of systemic disease. At 24 mo of age, however, F344 rats are known to exhibit marked systolic and diastolic dysfunction (3, 25) and high rates of mortality (36). The differences between the 18- and 22-mo-old animals observed in this study are therefore likely to be signs of degenerative myocardial aging. One of the effects that we measured (ventricular torsion) can be assessed non-invasively in humans and might, in the future, be a useful clinical predictor of aging-associated dysfunction.
METHODS
Animals.
Female Fischer 344 rats were obtained from a National Institute on Aging colony maintained by Harlan (Indianapolis, IN). Rats were purchased at 6, 18, and 22 mo of age and studied 7–14 days after arrival. All animal procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and conformed to standards of the Guide for the Care and Use of Laboratory Animals.
Echocardiography.
Echocardiographic assessment of left ventricular (LV) function was performed using a digital imaging system (Vevo 2100, MS-250 transducer, VisualSonics, Ontario, Canada). Before imaging, each rat was sedated with isoflurane and placed on a heated platform (37°C) in the supine position. Heart rate (HR) and respiration were monitored during the procedure, and the level of anesthesia was adjusted to maintain HR around 300 beats/min. Chest hair in the area of probe placement was removed using clippers, followed by brief treatment with chemical hair remover (Nair, Church and Dwight, Princeton, NJ).
M-mode measurements were performed at the level of papillary muscle insertion and used to determine diastolic and systolic values for the thickness of the septum (IVSd and IVSs, respectively), the thickness of the left ventricular posterior wall (LVPWd and LVPWs, respectively), and the left ventricular inner diameter (LVIDd and LVIDs, respectively). Fractional shortening (FS; %) was computed as FS = 100 × (LVIDd − LVIDs)/LVIDd.
Pulsed-wave Doppler measurements of mitral valve flow and tissue Doppler measurements of mitral valve septal annulus motion were performed using apical four-chamber views. These data were analyzed to determine the ratio of peak early mitral valve flow to peak early mitral valve septal annulus velocity (E/E′).
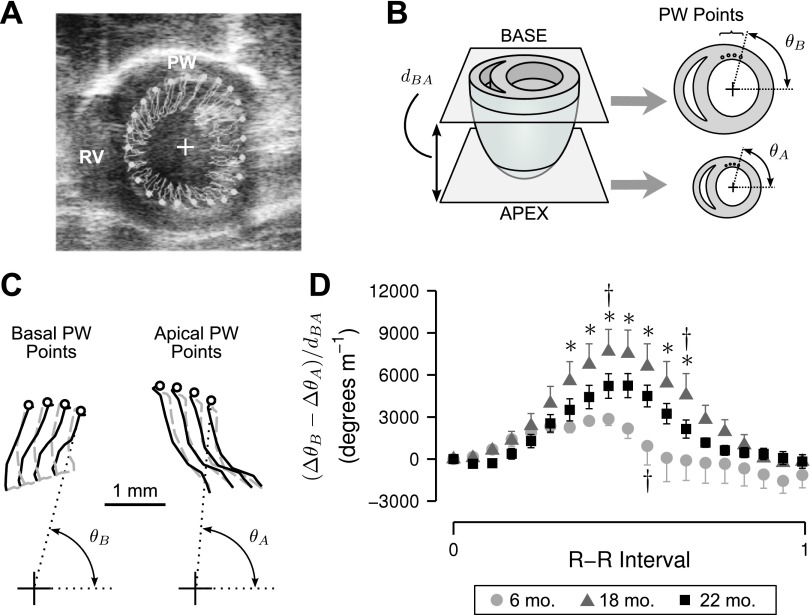
Parasternal short-axis B-mode acquisitions (≥19 frames/beat) were obtained at the apex and the base (immediately below the mitral valve) of the heart. These data were analyzed offline to determine left ventricular torsion. This was accomplished by digitally superposing 48 markers (spaced evenly around the endocardium) on frames acquired for the base and the apex views (Fig. 1A). The positions of these markers were then tracked through a complete cardiac cycle using VisualSonics's VevoStrain speckle tracking software. A routine custom-written in MATLAB (The MathWorks, Natick, MA) was next used to calculate the angular displacement (Δθ) of each marker relative to the centroid of the ventricular chamber during diastole. Positive angular displacements were defined as those which produced a counter-clockwise rotation when viewed from the base of the heart. Finally, torsion values (T) were calculated for each base/apex point pairing as T = (ΔθB − ΔθA)/dBA where ΔθB and ΔθA define the angular displacements for base and apical points, respectively, and dBA is the normal distance between the imaging planes (Fig. 1B).
Fig. 1.
Left ventricular (LV) torsion assessed using echocardiography. A: representative short axis B-mode echocardiographic image taken at the basal plane of an 18-mo-old F344 rat showing the right ventricle (RV), posterior wall (PW), and centroid (+) of manually traced sub-endocardial points (white markers). B: depiction of base and apex image planes with normal distance (dBA). Angular displacements of basal (θB) and apical (θA) PW points (four in each plane) were used to calculate an average torsion time course. C: representative paths of tracked PW points during systole (solid lines) and diastole (shaded dashed lines). Circles indicate PW points at end-diastole. D: mean LV torsion values during a complete cardiac cycle for 6- (n = 3), 18- (n = 6), and 22-mo-old (n = 6) animals. Error bars show ± SE at each time point; for clarity, positive and negative SE have been omitted from 6- and 18-mo traces, respectively. *Signficant difference vs. 6-mo-old animals (P < 0.05). †Significant difference vs. 22-mo-old animals (P < 0.05).
To facilitate statistical analyses, the T-value time-series calculated for each heart were interpolated to the appropriate R-R interval. Single TPW records were then calculated for each animal by averaging the T-value time-series for the four marker points nearest the posterior wall where the speckle tracking was most accurate. The final TPW record quantifies how the heart twists near the posterior wall as a function of time with positive TPW values corresponding to clockwise twisting of the apex as viewed from the base.
Myocyte isolation and functional measurements.
Ca2+ transients and unloaded shortening contractions were measured using electrically excitable cardiomyocytes isolated from the endocardial, midmyocardial, and epicardial regions of hearts from the 6-, 18-, and 22-mo-old rats.
The solutions used for isolating the cells were based on a modified Krebs-Henseleit solution (KH) and contained (in mM) 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 10 HEPES, 30 taurine, and 5.6 glucose. Each solution was bubbled with a 95% O2/5% CO2 gas mixture. The pH was adjusted to 7.25 at the appropriate temperature.
Rats were first injected with heparin (800 U IP) and then anesthetized with pentobarbital (50 mg/kg). Complete anesthesia was confirmed by absence of the tail pinch response and paw withdrawal reflex. The heart was then rapidly removed from the chest and cannulated to allow perfusion of the coronary arteries with ice-cold KH containing 10 mM butanedione monoxime (BDM). After the coronary circulation of blood was cleared, the heart was hung from a Langendorf perfusion apparatus and enzymatically digested for 10–12 min at 37°C with digestion solution (KH containing 10 mM BDM, 20 μM CaCl2, and 46 μg/ml Liberase TH, Roche Applied Science, Indianapolis, IN). After the initial digestion step, the LV free wall was removed and sectioned transmurally into three portions of equal thickness (endocardium, midmyocardium, and epicardium). The tissue pieces from each region were placed into separate beakers containing 1 ml of digestion solution and further incubated with gentle agitation. After 5 min, tissue pieces were gently shaken to disperse cells and moved to beakers of fresh digestion solution for two additional 5-min incubation steps. After each incubation period, the solution containing dispersed cells was added to 1 ml of 11% (vol/vol) fetal bovine serum (FBS, Sigma, St. Louis, MO) in KH (13 μM CaCl2) and allowed to settle until a cell pellet became visible (10–15 min). The supernatant was then removed, and the pellet was resuspended in 0.5 ml of KH solution supplemented with 5% (vol/vol) FBS and 13 μM CaCl2. The Ca2+ concentration of the cell-containing solutions was then increased to the following values in 5-min increments: 50, 100, 200, 500, and 1,000 μM. Myocytes were then allowed to equilibrate at room temperature for at least 1 h before use.
During dye loading and functional measurements, cells were placed in Tyrode's solution containing (in mM) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose. Dye loading was performed by adding a 30-μl aliquot of cell-laden solution to 230 μl of loading solution (Tyrodes supplemented with 2.2 μM fura-2/AM and 0.02% wt/vol pluronic, both from Life Technologies, Carlsbad, CA) in a micro-centrifuge tube. After 10 min of loading at room temperature, cells from the bottom of the tube were transferred to 150 μl of fresh Tyrode's solution for 5 min. Myocytes were then placed in a temperature-controlled chamber (Cell MicroControls, Norfolk, VA) and superfused with Tyrode's solution at 25°C. The cell chamber was mounted on a computer-controlled stage (Siskiyou, Grants Pass, OR) positioned over an inverted microscope.
Myocytes were field-stimulated at 0.5 Hz. Fura-2 fluorescence in response to alternating 340- to 380-nm excitation wavelengths was measured using a RatioMaster fluorescence system (PTI, Birmingham, NJ) at a rate of one ratio every 10 ms. Brightfield imaging of cells was conducted simultaneously using low-pass filtered illumination (>610 nm). Fura-2 emission and the brightfield image were split at the microscope output port using a dichroic mirror (620 nm). Myocyte sarcomere length was measured from brightfield images using a real-time system (HVSL, Aurora Scientific, Ontario, Canada). Voltage signals for fura-2 fluorescence and sarcomere length were recorded using a 16-bit data acquisition system (DAP5216a, Microstar Laboratories, Bellevue, WA) and processed using custom software written in MATLAB.
Rod-shaped myocytes with clear striation patterns of >1.7 μm at rest were selected for study. An average of 15 cells were measured per myocardial region per animal. After pooling measurements from 6-mo-old animals (n = 8), 18-mo-old animals (n = 6), and 22-mo-old animals (n = 7), the number of cells measured in each age/region group ranged from 75 (18-mo endocardium) to 176 (6-mo epicardium) for a grand total of 1,087 cells. Fura-2 fluorescence and sarcomere length time courses for each myocyte were determined by averaging signals from 10 consecutive beats.
Biochemical assays.
The relative phosphorylation of selected sarcomeric proteins [myosin light chain 2 (MLC2), Troponin I (TnI), Troponin T (TnT), and Tropomyosin] was examined using gel electrophoresis (n = 3 animals/age group). Samples were prepared by homogenizing small pieces of ventricular tissue in a sample buffer containing 8 M urea, 2 M Thiourea, 3% SDS wt/vol, 75 mM DTT, and 0.05 M Tris·Cl, pH 6.8. The protein concentration in each sample was measured using a Lowry protein assay (RC-DC kit, Bio-Rad, Hercules, CA). Each sample was then modified by adding 0.03% Bromophenol blue and 30% glycerol before being boiled at 95°C for 3 min and subsequently cooled on ice. Precast polyacrylamide gels (Mini-Protean TGX 10%, 15-well combs, Bio-Rad) were then loaded with 3 μl of each sample (protein concentration 1 μg/μl) and run at 200 V for 30 min. Each gel was stained with Pro-Q Diamond phosphoprotein gel stain (Invitrogen, Carlsbad, CA), scanned using a Typhoon Trio+ imager (GE Healthcare, Piscataway, NJ), restained with SYPRO-Ruby (Invitrogen), and finally scanned again. The phosphorylation levels were quantified by integrating the densitometry profile (ImageQuant TL software, GE Healthcare) of each band in the Pro-Q Diamond image and dividing that result by the corresponding integral calculated for the SYPRO-Ruby stained image. Relative phosphorylation levels for the different samples were calculated by normalizing to data obtained using an external control myocardial sample that was run on each gel and subsequently expressed relative to the data for a single 6-mo-old epicardial sample.
The relative content of the α- and β-MHC isoforms in the tissue homogenates were determined using gel electrophoresis. As described by Tikunov (35), myosin was extracted from the tissue homogenates using a buffer containing 100 mM KCl, 100 mM KH2PO4, 50 mM K2HPO4, 10 mM EDTA, 10 mM Na4P2O7·10H2O, 4 mM β-Mercaptoethanol and 5% (vol/vol) Triton X-100 (pH 6.5, 4–8°C, 100-μl volume, 24 h). Laemmli buffer (80 μl) were then added to each sample, after which it was heated to 95°C for 4 min. The treated samples were then resolved using gels that were 60 × 80 × 0.75 mm and contained 7% acrylamide (50:1 with bis-Acrylamide) and 35% glycerol. These gels were run at a constant current of 3.0 mA for ∼18 h at 4°C and subsequently silver stained with a commercial kit (SilverStain Plus, Bio-Rad). Scanned images were then obtained using a conventional office scanner (V500 Photo, Epson, Long Beach, CA). The relative content of each isoform was determined by fitting the densitometry profiles with asymmetrical Gaussian functions using GelBandFitter software previously developed by our laboratory (22).
To assess the expression of sarco-/endoplasmic reticulum Ca2+ ATPase (SERCA2a), 4 μl of each sample (Urea-Thiourea sample buffer; 1 μg/μl protein concentration) were loaded onto precast polyacrylamide gels (Mini-Protean TGX 4–15%, 15-well combs, Bio-Rad). Each gel was run at 200 V for 30 min and subsequently transferred onto a polyvinylidene difluoride membrane using a semi-dry transfer cell. The membrane was then probed with an antibody (sc-8094, Santa Cruz Biotechnology) to SERCA2a at a 1:1,000 dilution (34). The membrane was then incubated with a fluorescent secondary antibody (Alexa Fluor 680 donkey anti-goat IgG, Life Technologies) at a 1:7,500 dilution. Immunoblotted bands were visualized using a LI-COR Odyssey imaging system (LI-COR Biosciences, Lincoln, NE) and analyzed using Odyssey version 3.0 software. As described above for the analysis of myofibrillar proteins, relative SERCA contents were calculated by normalizing to data from an external control sample that was run on each gel (31) and subsequently expressed relative to the data for a single 6-mo epicardial sample. This facilitated statistical analysis of data from multiple western blots.
Computational modeling.
A computational model (see appendix) was created to predict how the sarcomere length of an unloaded myocyte would change in response to an intracellular Ca2+ transient. The parameters defining the model's behavior correspond to the kinetic and biophysical properties of selected sarcomeric proteins.
Statistical analysis.
Statistical analyses were performed using JMP (SAS Institute, Cary, NC) using a threshold for significance of P < 0.05 in all cases. Echocardiographic parameters were examined for age-related effects using one-way ANOVA. For parameters with significant age-related effects, pairwise comparisons of age group means were conducted using a Tukey Honestly Significant Difference (HSD) procedure.
Torsion time courses were analyzed by fitting data with a linear mixed-effect (LME) model, with main effect terms for animal age and time point (% R-R interval), and a random effect term for individual animals. Planned pairwise comparisons were subsequently conducted between the three age groups at each time point using mean squared errors from the initial LME model fit.
Properties of Ca2+ transients, unloaded sarcomere shortening dynamics, and results from biochemiocal assays were also analyzed using an LME model. Animal age and myocardial region were designated as main effects, with individual animals being used as a random effect term. Mean squared errors from the LME analysis were subsequently used for planned pairwise comparisons between each age/region group and 1) other regions at the same age and 2) the same region at other ages. LME models have more statistical power than ANOVA for this type of design because they nest data obtained from each myocardial cell within a random variable describing the animal. This increases the efficiency of experiments that obtain data from more than one preparation per animal.
Analysis of covariance was used to test for differences in the slopes of τCa-RT50 relations among age-region groups, followed by a Tukey's HSD procedure to compare slopes of specific groups.
RESULTS
Echocardiography and torsion.
Data describing animal weights and echocardiographic characteristics for the 6-, 18-, and 22-mo-old F344 rats are shown in Table 1. The mean body weights of the 18- and 22-mo-old animals were 51% and 61% greater than those of the 6-mo-old animals. Ventricular wall thicknesses, FS, and dBA did not differ between age groups. LVIDd values were ∼25% greater for 18- and 22-mo-old rats than for 6-mo-old animals. LVIDs values also tended to be larger in the older age groups, although this difference was only statistically significant for comparisons between the 6- and 22-mo-old animals. There were no statistically significant differences in diastolic function as assessed by the ratio of peak mitral valve flow to peak mitral valve septal annulus velocity during early filling (E/E′).
Table 1.
Body weight and echocardiographic characteristics of female F344 rats
| 6 Mo (n = 7) | 18 Mo (n = 9) | 22 Mo (n = 8) | P Value (One-Way ANOVA) | |
|---|---|---|---|---|
| Body weight, g | 203.4 ± 8.7 | 307.1 ± 7.7* | 327.6 ± 8.7* | <0.0001 |
| HR, beats/min | 326.4 ± 12.6 | 321.4 ± 11.1 | 294.9 ± 12.6 | 0.185 |
| FS, % | 52.2 ± 3.1 | 51.4 ± 2.8 | 47.2 ± 2.9 | 0.453 |
| IVSd, mm | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.553 |
| IVSs, mm | 2.8 ± 0.2 | 3.0 ± 0.1 | 2.9 ± 0.1 | 0.592 |
| LVIDd, mm | 5.3 ± 0.2 | 6.7 ± 0.2* | 6.6 ± 0.2* | <0.0001 |
| LVIDs, mm | 2.5 ± 0.2 | 3.2 ± 0.2 | 3.5 ± 0.2* | 0.025 |
| LVPWd, mm | 1.8 ± 0.1 | 1.9 ± 0.1 | 1.8 ± 0.1 | 0.775 |
| LVPWs, mm | 2.7 ± 0.2 | 3.0 ± 0.1 | 2.7 ± 0.1 | 0.282 |
| dBA, mm | 4.6 ± 0.4 | 4.5 ± 0.3 | 5.1 ± 0.3 | 0.473 |
| E/E' | 29.0 ± 6.3 | 41.4 ± 6.3 | 37.3 ± 6.8 | 0.379 |
Values are means ± SE. HR, heart rate; FS, fractional shortening; IVSd, diastolic thickness of the septum; IVSs, systolic thickness of the septum; LVIDd, diastolic left ventricular inner diameter; LVIDs, systolic left ventricular inner diameter; LVPWd, diastolic thickness of the left ventricular posterior wall; LVPWs, systolic thickness of the left ventricular posterior wall; dBA, normal distance between the imaging planes; E/E′,ratio of peak early mitral valve flow to peak early mitral valve septal annulus velocity.
Signficant difference vs. 6-mo values (Tukey's honestly significant difference; P < 0.05).
The mean torsions at the ventricular wall (TPW) for the different aged animals are shown in Fig. 1D. Statistical analysis showed that the TPW time courses were significantly different (P < 0.001). Planned pairwise comparisons showed that the peak torsion value in the 18-mo-old animals (7,700 ± 1,600°/m) was significantly greater (P < 0.05) than the corresponding values in the 6-mo-old animals (2,900 ± 700°/m) and the 22-mo-old animals (5,200 ± 900°/cm). These data suggest that peak TPW values increase as the animals age from 6 to 18 mo and then decline over the next 4 mo.
Myocyte function.
Ventricular torsion is thought to be sensitive to transmural variation in myocyte contractile dynamics (11, 30). We therefore hypothesized that the age-dependent torsion effects (Fig. 1D) might be due to differences in the transmural distributions of myocyte properties. This was tested by measuring Ca2+ transients and unloaded sarcomere shortening (Fig. 2, A and B) using cells isolated from the endocardial, midmyocardial, and epicardial regions of rat hearts from each of the three age groups (n = 1,087 total cells). Statistical analysis showed that some cellular properties exhibited significant interactions between age and region. These interactions imply that the biophysical properties of myocytes from different regions of the heart change with age in different ways.
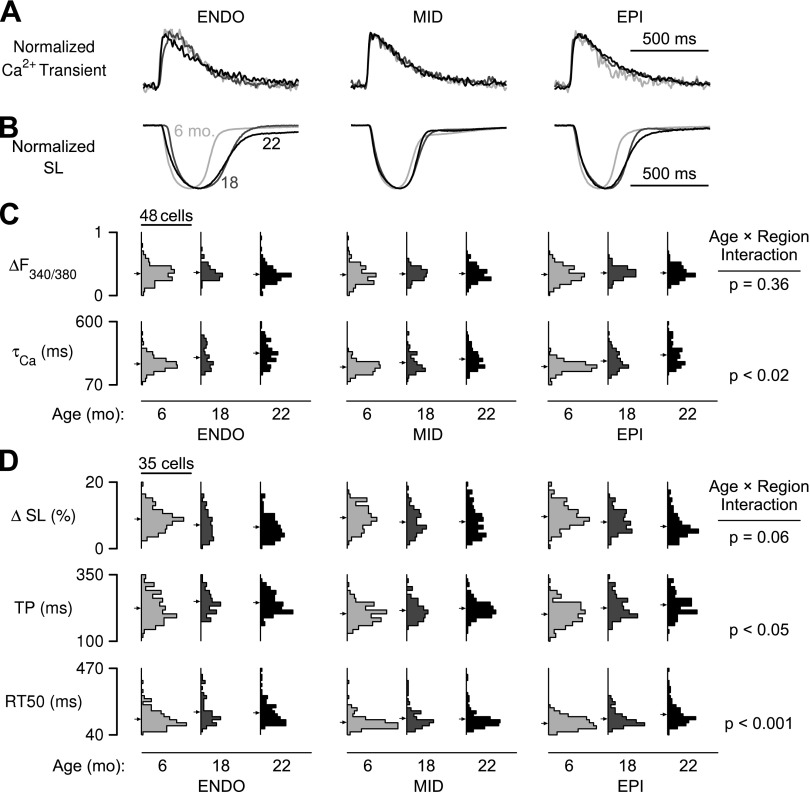
Fig. 2.
Intracellular Ca2+ transients and contractile dynamics vary by myocardial region and animal age. A and B: representative normalized Ca2+ transients (A) and sarcomere length records (B) for endocardial (ENDO), midmyocardial (MID), and epicardial (EPI) cells from rats of 6, 18, and 22 mo of age. C: histograms showing the distributions of the Ca2+ transient amplitude (ΔF340/380) and decay constant (τCa) for each age-region group (n = 75 to 176 cells/group). Arrows indicate the population mean. D: same as C but showing distributions of contractile parameters maximum shortening (ΔSL), time to peak shortening (TP), and time from peak to 50% re-lengthening (RT50).
Intracellular Ca2+ transients were characterized by their amplitude (ΔF340/380) and the time constant of transient decay (τCa). Ca2+ transient amplitudes for all groups were similar (Fig. 2C, top). In contrast, τCa exhibited a significant interaction (P < 0.02; Fig. 2C, bottom). The parameter increased with age in all three cell subtypes, but the amplitude of the age-related change was larger for epicardial cells than it was for cells from the endocardial and midmyocardial regions (Fig. 3A).
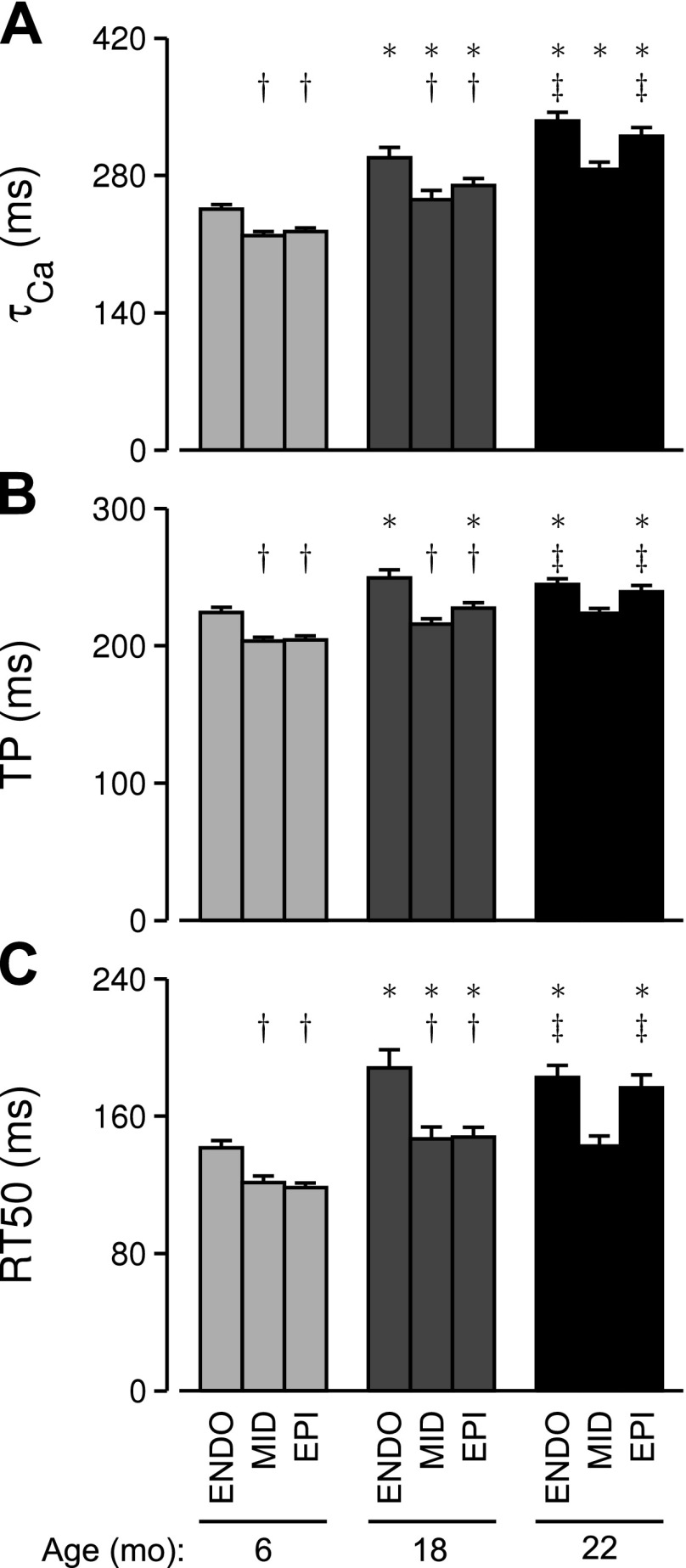
Fig. 3.
Transmural patterns of Ca2+ handling and contraction dynamics are altered with age. A: means ± SE of the time constant of F340/380 decay after peak (τCa) for each age-region group. B and C: same as A but showing unloaded sarcomere TP (B) and RT50 (C). *Significant difference vs. cells from the same region at 6 mo of age (P < 0.05). †Significant difference vs. endocardial cells at the same age (P < 0.05). ‡Significant difference vs. midmyocardial cells at the same age (P < 0.05).
Contraction time courses were characterized by the amount of shortening as a percentage of diastolic sarcomere length (ΔSL; %), the time elapsed between stimulus and peak shortening (TP), and the time elapsed between peak shortening and 50% relengthening (RT50) (Fig. 2D). Statistical analysis showed that ΔSL decreased significantly between 6 and 18 mo but was not different for the 18- and 22-mo-old age groups. This parameter did not exhibit a statistical interaction, indicating that cells from each transmural region underwent similar age-dependent changes. In contrast, TP and RT50 did exhibit significant age-by-region interactions (Fig. 2D). The trends for both variables (Fig. 3, B and C) mimicked those described above for τCa; i.e., the largest age-dependent effects were observed in the epicardial cells.
The net effect of these interactions was an age-dependent change in the transmural pattern of myocyte function (Fig. 3). Between 6 and 18 mo of age, τCa increased for cells from each transmural region, but values for endocardial cells were always larger than those measured with midmyocardial and epicardial cells (Fig. 3A). At 22 mo, this pattern had changed. The endocardial and epicardial τCa values could not be statistically distinguished, and both were significantly larger than the midmyocardial value. Transmural patterns in TP (contraction time) and RT50 (relaxation time) changed in essentially the same way as τCa (Fig. 3, B and C).
One interpretation of these data is that the prolonged contractions reflect slower reuptake of Ca2+ by SERCA. However, RT50 in midmyocardial cells at 22 mo of age did not change significantly from the baseline value at 6 mo of age despite a 31% increase in τCa (Fig. 3, A and C). This result suggested that there must be additional factors in these experiments that alter the relationship between the intracellular Ca2+ concentration and sarcomere length during relaxation. We tested this hypothesis by using analysis of covariance to compare the linear relationships between RT50 and τCa for the different cell populations. The results (Fig. 4 and Table 2) showed that the slopes of the τCa-RT50 relationships for the nine age-region groups were significantly different (P < 0.001). In particular, post hoc tests showed that the slope of the τCa-RT50 relationship for 6-mo epicardial cells was approximately four times smaller (P < 0.05) than the slopes for the aged epicardial cells (Table 2).
Fig. 4.
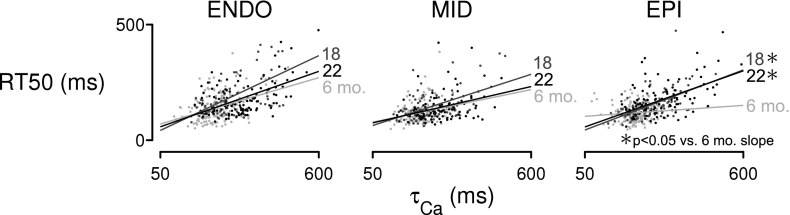
Aging alters Ca2+-relaxation coupling in epicardial myocytes. Plotted points show RT50 vs. τCa for each cell in the study, grouped according to region and animal age. Analysis of covariance on τCa-RT50 relations indicates that the slope depends significantly on the age-region group (P < 0.001). RT50 in epicardial myocytes from 6-mo-old rats was significantly less sensitive to τCa than in 18- and 22-mo-old cells from the same region.
Table 2.
Linear regression values for TCa-RT50 relations from each age-region group
| Region | Age, mo | r2 | Slope, ms/ms | Intercept, ms |
|---|---|---|---|---|
| Endo | 6 | 0.40 | 0.36 | 52 |
| 18 | 0.57 | 0.59 | 13 | |
| 22 | 0.55 | 0.44 | 36 | |
| Mid | 6 | 0.28 | 0.26 | 65 |
| 18 | 0.53 | 0.40 | 44 | |
| 22 | 0.37 | 0.28 | 61 | |
| Epi | 6 | 0.11 | 0.09* | 99 |
| 18 | 0.59 | 0.47 | 20 | |
| 22 | 0.52 | 0.44 | 35 |
Endo, endocardial; Mid, midmyocardial; Epi, epicardial.
Significant difference vs. 18 and 22 mo Epi cells (P < 0.05).
Computational modeling.
The next stage of the study used a mathematical model of myocyte Ca2+-contraction coupling to identify molecular mechanisms that can alter the slope of the τCa-RT50 relationship. We started this process by calculating a set of parameter values that allowed our model (see appendix) to reproduce the mean shortening behavior of 6-mo-old midmyocardial myocytes when driven by an appropriate Ca2+ transient (Fig. 5A). We then generated a baseline τCa-RT50 relationship by analyzing the shortening profiles computed by the model for simulated Ca2+ transients with different decay times. Once this had been accomplished, we tested potential molecular mechanisms by running additional simulations using parameter values that had been adjusted to mimic specific molecular effects.
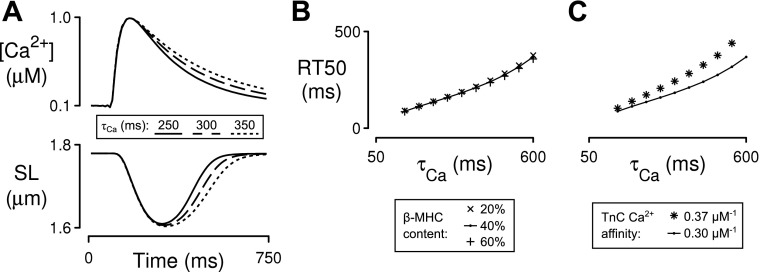
Fig. 5.
Computer simulations of unloaded sarcomere shortening. Ca2+ transients with various rates of decay (τCa) were used as inputs to predict relationships between τCa and RT50 under different conditions. A: example input Ca2+ transients of increasing τCa and corresponding SL outputs for the baseline model (40% β-MHC, 0.3 μM TnC Ca2+ affinity). B: sensitivity of the τCa-RT50 relation to doubling and halving relative β-MHC content. C: sensitivity of the τCa-RT50 relation to a 20% increase in TnC Ca2+ affinity.
The most important findings from these calculations are summarized in Fig. 5, B and C. First, changing the relative content of the slower cycling β-MHC by ±50% did not change the slope of the τCa-RT50 relationship (Fig. 5B). This suggests that, even though the relative content of the β-isoform has been shown to increase with age in F344 rats (8, 23, 24), this molecular effect does not change the time course of relaxation. Second, a modest 20% reduction in the rate at which Ca2+ ions unbind from troponin C mimicked the age-dependent slope changes observed in the epicardial cells (Fig. 5C). This computational result implies that the age- and region-dependent effects summarized in Fig. 4 may reflect changes in the rate at which Ca2+ dissociates from the troponin complex in the different types of cell.
Biochemical analyses.
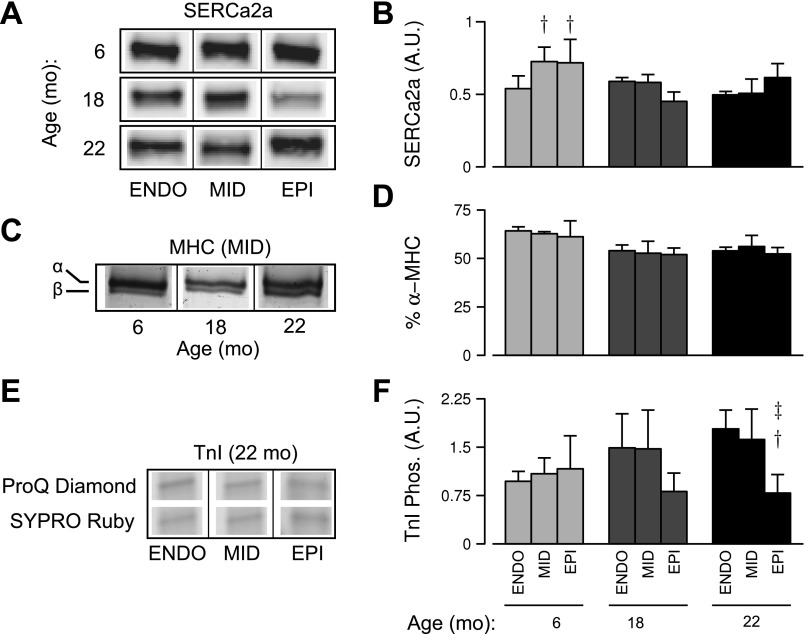
We subsequently studied expression and phosphoprotein content in tissue homogenates from the nine age-regions groups (Fig. 6), focusing on proteins implicated by our functional measurements. SERCA2a protein expression, which could affect Ca2+ transient decay rates (Fig. 3A), was assessed by Western blot and densitometry analysis (Fig. 6, A and B). There was a trend toward an age-region interaction, but the effect was not statistically significant with three animals per group (interaction, P = 0.06). However, at 6 mo of age, epicardial and midmyocardial regions expressed significantly more SERCA2a than the endocardium (P < 0.05; Fig. 6B) in a pattern that fits qualitatively with the observed transmural variation in τCa (Fig. 3A). Relative α- and β-MHC isoform expression was also measured in tissue homogenates (Fig. 6, C and D). The relative content of α-MHC trended downward with age, but the effect was not statistically significant.
Fig. 6.
Biochemical analyses of tissue homogenates obtained from each age-region group (n = 3 animals per age group). Representative Western blot images (A) and summary data from densitometry (B) showing expression of SERCA2a (arbitrary units). Representative bands from midmyocardial samples of various ages showing separated α- and β-MHC isoforms (C) and the mean α-MHC isoform percentages across groups (D). E: representative TnI bands from gels stained for phosphoprotein (Pro-Q Diamond) and total protein content (Sypro Ruby). F: mean values of phosphoprotein-to-total protein ratios from densitometric analysis. †Significant difference vs. endocardial cells at the same age (P < 0.05). ‡Significant difference vs. midmyocardial cells at the same age (P < 0.05).
Previous work by Zhang et al. (41) has shown that decreasing the phosphorylation of TnI slows cardiac relaxation by reducing the rate at which Ca2+ unbinds from the troponin complex. Since this effect could explain the altered τCa-RT50 relations observed in our work (Fig. 4), we used ProQ Diamond staining to measure the relative phosphorylation of TnI in our experimental samples. Figure 6, E and F, shows that the TnI phosphoprotein content did not differ across regions in 6- and 18-mo-old samples but fell by >50% in 22-mo epicardial samples (P < 0.05; Fig. 6F). Similar tests were performed for other myofilament proteins (TnT, MLC2, and tropomyosin) but did not reveal significant differences among the age-region groups (data not shown).
DISCUSSION
This study identifies novel molecular-, cellular-, and tissue-level events that precede aging-associated heart failure in female F344 rats. Several previous studies have noted transmural variation in myocyte contractile properties in healthy rat hearts (1, 9, 10), whereas other studies have shown that increasing age slows the rates at which individual myocytes contract and relax (7). In this study, we show for the first time that the degree of slowing is transmurally uniform in early aging (between 6 and 18 mo of age) but becomes nonuniform between 18 and 22 mo of age (Figs. 2 and 3). The shift in transmural properties is caused by age- and region-dependent changes in Ca2+ transient morphology and altered Ca2+-relaxation coupling at the level of the myofilaments (Fig. 4). We also present evidence that decreased phosphorylation of TnI contributes to the myofilament level effect (Fig. 6F).
Our results reveal other non-monotonic changes as the heart ages. Close examination of Fig. 4 shows that the slopes of the τCa-RT50 relationships measured in isolated cells increase with age from 6 to 18 mo of age but decrease slightly between 18 and 22 mo. Similarly, ventricular torsion (quantified as TPW; Fig. 1D) increases during early aging but decreases over the next 4 mo. Since F344 rats exhibit age-dependent heart failure at ∼24 mo of age (25, 36), the partial reversals in cellular and organ level function may indicate the onset of degenerative or high-risk aging.
Features of early aging.
Our study shows that in early aging (6–18 mo of age), myocyte contraction, and Ca2+ transient parameters slowed proportionally in all three myocardial regions so that transmural patterns were preserved (Fig. 3). These functional effects probably reflect at least three molecular mechanisms.
The first of these mechanisms is an age-dependent decrease in the content (32, 33) and/or activity (40) of SERCA. Both of these effects would slow the decay of the Ca2+ transient and thus reduce the rate of sarcomere relaxation. As an example, Belke et al. (2) have shown that mice overexpressing SERCA2a have smaller τCa values. Our own measurements of SERCA2a expression (Fig. 6, A and B) revealed a transmural pattern in 6-mo-old myocardium (Fig. 6B) that agrees qualitatively with the transmural pattern of τCa (Fig. 3A). In these data, higher SERCA2a expression in midmyocardium and epicardium corresponded with smaller τCa values (faster Ca2+ transient decay). However, at 18 and 22 mo of age, the relationship between SERCA2a expression and τCa was not as straightforward, suggesting the presence of an additional modulating factor. We therefore think that the activity of SERCA, as well as its content, may be differentially regulated in our study. Phospholamban phosphorylation is one possible mediator. Xu and Narayan (40) have shown that Ca2+-/calmodulin-dependent kinase activity is reduced in aged rat cells, which reduces the relative phosphorylation of phospholamban. Their study did not measure transmural effects.
The second potential mechanism is an age-dependent change in myosin isoform expression. Previous studies have shown that the relative content of the β-MHC isoform increases with age in rodents (8, 23, 24); our experiments confirmed this trend, although the effect was not statistically significant with three animals per group (Fig. 6, C and D). Since the β-isoform cycles more slowly than its α counterpart, altered expression of MHC is likely to contribute to slowed contraction.
Changes in MHC expression do not, however, seem to influence the τCa-RT50 relationship (Fig. 5B). Indeed, in our simulations, the only way of increasing the slope of the relationship in a comparable way to that observed in early aging (Fig. 4) was to reduce the rate at which Ca2+ dissociated from the troponin complex. This led us to examine the relative phosphorylation levels of TnI in our samples, since others have shown similar kinetic effects produced by dephosphorylation of TnI (28, 41). Our data (Fig. 6F) show that the aged epicardial cells had lower relative levels of TnI phosphorylation than the endocardial and midmocardial samples. Since the aged epicardial cells also relaxed more slowly (Fig. 3), we conclude that a third mechanism that contributes to age-dependent slowing is a reduced rate of Ca2+-troponin dissociation following dephosphorylation of TnI.
An isolated reduction in TnI phosphorylation would also increase myofilament Ca2+ sensitivity (41). Indeed, in a previous study using permeabilized myocardial preparations, we reported a slight leftward shift (∼0.04 pCa units) in the tension-pCa curve during the early aging period for F344 rats (23). However, we stop short of concluding that intact, aging epicardial cells display increased Ca2+ sensitivity. This is because we cannot rule out the presence of additional myofilament modifications affecting this parameter and because we could not directly measure Ca2+ sensitivity in the intact myocytes used in this study.
Features of pre-heart failure aging.
Several previous studies have shown that F344 rats typically exhibit systolic and diastolic dysfunction and subsequent heart failure at ∼24 mo of age (3, 25, 36). The oldest animals in our study (22 mo) had not yet developed severe organ-level dysfunction (Table 1), but they did exhibit lower peak ventricular torsions than the 18-mo-old animals (Fig. 1D). This suggests that reduced “twisting” of the heart may be a characteristic feature of pre-heart failure aging.
Torsion is complicated and reflects factors including myocyte fiber orientation, ventricular morphology, and transmural distributions of contractile properties (29). Since the 18- and 22-mo-old hearts were essentially the same size (Table 1), altered transmural distributions seem to be the mostly likely mechanism driving the torsional changes. The data in Fig. 3 show that cells aged differently between 18 and 22 mo of age than they did between 6 and 18 mo. Instead of cells in all three regions slowing uniformly as they did before 18 mo of age, epicardial cells experienced larger changes between 18 and 22 mo than the endocardial and midmyocardial cells. The net result of this complex process was a shift in the transmural pattern of dynamic properties so that cells in the midmyocardium in 22-mo-old animals contracted and relaxed faster than the cells in the other layers. Advanced finite element modeling (6) as well as isolated cell data collected at more physiological pacing rates will be required to investigate the full consequences of this arrangement. Still, it is interesting to speculate that the altered transmural distribution hinders ventricular function and predisposes the aging heart to failure.
Relevance to human disease.
Twenty-two-month-old F344 rats could serve as a useful model for studying early markers of high-risk myocardial aging in humans, provided that proper attention is given to the known differences between rodents and humans. Age-related shifts in β-MHC isoform expression have been shown in some studies to contribute to slower active contraction in aging rats (4, 8, 23); but because humans express nearly 100% β-MHC as adults (21), this mechanism is unlikely to impact human aging. However, the age-associated shifts in Ca2+-relaxation dynamics we report here (Fig. 4) do not appear to depend on MHC isoform regulation. Instead, the modeling and biochemical evidence suggests that these shifts are due to altered TnI phosphorylation, which is known to be regulated by kinase activity in both rat (15) and human myocardium (37). Reduced SERCA2a content and/or activity has also been demonstrated in the aging myocardium of rats (32, 33, 40) and humans (5) alike.
Other important similarities can be seen at the ventricular level. Changes in ventricular morphology and torsion that we observe in early aging occur in the absence of discernable heart failure symptoms and are consistent with measurements in healthy aging humans. Hees et al. (14) have reported that the LV becomes more spherical in aging humans, which resembles the increased LVIDd at constant base-apex distance we see in F344 rats during early aging (Table 1). The elevated peak torsion we observed between the 6- and 18-mo-old groups (Fig. 1D) is also consistent with a recent report showing that the torsion-to-shortening ratio increases with age in healthy humans (16).
More work is required to quantitatively relate age-associated shifts in transmural contractile properties to the observed changes in LV torsion, and approaches must be sought to confirm the key results of this study in humans. Nevertheless, our findings raise the possibility that non-invasive clinical imaging could be used to identify patients that, by virtue of abnormal aging, are at greater risk of developing severe heart failure.
GRANTS
This work was supported by the University of Kentucky Research Challenge Trust Fund; grants from the National Institutes of Health [HL-090749 to K. S. Campbell, and UL 1TR-000117, Center for Clinical and Translational Sciences]; and a Postdoctoral Fellowship Award from the American Heart Association Great Rivers Affiliate (11POST7360038 to S. G. Campbell). The content is solely the responsibility of the authors and does not necessarily represent the views of the funding agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.G.C., W.K.S., and K.S.C. conception and design of research; S.G.C., P.H., W.K.S., and K.E.N. performed experiments; S.G.C., P.H., W.K.S., and K.E.N. analyzed data; S.G.C., P.H., W.K.S., and K.S.C. interpreted results of experiments; S.G.C. and P.H. prepared figures; S.G.C. and P.H. drafted manuscript; S.G.C., P.H., W.K.S., K.E.N., and K.S.C. edited and revised manuscript; S.G.C., P.H., W.K.S., and K.S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Heather Bush (University of Kentucky, College of Public Health) for invaluable advice on statistical analysis and Dr. Gregory Frolenkov (University of Kentucky, Department of Physiology) and Charles Chung (Campbell laboratory) for performing pilot experiments and providing comments on the manuscript.
Appendix
Computational Modeling
Description of the Model.
We constructed a mathematical model of a free-floating myocyte by linking a nonlinear spring and a viscous element in parallel with a previously published model of myofilament force production (30).
The three components of the model generate forces TE, TV, and Tact, respectively, and their sum is constrained to zero (since the free-floating myocytes are assumed to contract against zero load). Thus
| (A1) |
We used the convention that positive forces were those producing sarcomere shortening. TE represents the mechanical effects of titin-based passive stiffness according to the formulation of Rice et al. (27) and is given by
Here, SLslack is the slack sarcomere length, whereas kp and τp are linear and nonlinear scaling coefficients for passive stiffness, respectively. The negative coefficient implies that if SL < SLslack, titin produces a negative force that restores sarcomeres to their slack length.
Tv represents the viscous drag of the myofilaments and is given by
| (A2) |
where η is a coefficient representing passive viscous properties of the cell.
Tact is given by
| (A3) |
where P{Mpr} and P{Mpo} are the fraction of myofilament regulatory units having bound cross bridges in the pre- and postpower-stroke states (30), xpr and xpo are the mean cross-bridge distortions of the respective populations (26), ε is the stiffness of a single cross bridge, Nxb is the number of cross bridges per unit cross-sectional area, and λ(SL) is the fraction of thin-thick filament overlap as a function of sarcomere length (SL) computed using the formulation and constants of Rice et al. (27).
The myofilament model itself was extended from its published form (30) by including length dependence (see Eq. A3 above) and strain-dependent cross-bridge kinetics. Strain dependence was implemented using equations developed by Razumova et al. (26) and Rice et al. (27). The detachment rate of cross bridges from the postpower-stroke state (gxb) varied with the mean distortion of the postpower-stroke state (xpo) according to
where gxb0 is the detachment rate under isometric conditions, xpo0 is the mean postpower-stroke cross-bridge distortion under isometric conditions, and σ is a scaling variable. As in the original work of Razumova et al. (26), the value of σ depended on whether the postpower-stroke distortion was greater than or less than xpo0:
The forward power-stroke rate (hf) was also strain dependent. As previously described by Rice et al. (27)
where hxb0 is the forward rate under isometric conditions and σh is another parameter scaling strain dependence.
Simulations
Sarcomere length was calculated as a function of time by solving the following equation in conjunction with the 387 additional coupled differential equations (30) required to calculate Tact:
| (A4) |
Equation A4 was itself derived by solving Eq. A1 for TV, substituting the result into Eq. A2, and finally solving for dSL/dt. Dynamic twitch contractions were generated by calculating the response of the system to a Ca2+ transient parameterized [as previously described by Rice et al. (27)] as
where
Calculations
All simulations were performed using MATLAB (The Mathworks, Natick, MA). Camin2+ and Camax2+ values were set to nominal values of 0.1 and 1.0 μM for all conditions. τ1 and τ2 were obtained by fitting the Ca2+ transients to mean experimental data for midmyocardial cells from 6-mo-old rats. The resulting values are shown in Table A1.
Table A1.
Ca2+ transient Parameters simulating midmyocardial cells from 6-mo-old rats
| Cell Subtype | τ1, ms | τ2, ms |
|---|---|---|
| Midmyocardial | 38 | 178 |
Model parameters
Numerical values for the parameters defining the behavior of the model were obtained by fitting simulated twitch contractions to the mean experimental data from 6-mo-old midmyocardial cells. This was accomplished by using particle swarm optimization to search for a parameter set Φ that minimized the objective function
where TP is time to peak shortening, RT50 is the time from peak shortening to 50% relengthening, ΔSL is the change in sarcomere length from rest to maximum shortening, and the superscripts sim and meas indicate data from simulations and experiments, respectively. The resulting values are reported in Supplemental Table A2.
Table A2.
Cell shortening model parameters fit to 6-mo-old midmyocardial cell measurements
| Value | Units | Description | |
|---|---|---|---|
| Markov model parameters | |||
| kCa+ | 350 | μM−1 · s−1 | TnC-Ca binding rate |
| kCa− | 1.15e3 | s−1 | TnC-Ca dissociation rate |
| kB+ | 3.00e5 | s−1 | Tropomyosin nonpermissive to permissive transition rate |
| kB− | 330 | s−1 | Tropomyosin permissive to nonpermissive transition rate |
| γB | 350 | Interregulatory unit cooperativity coefficient | |
| q | 0.5 | Forward-reverse weighting coefficient for kinetic effects of cooperativity | |
| pf | 0.25 | RLC phosphorylation effect coefficient for cross-bridge binding | |
| ϵBS | 2.00e-3 | N/m | Baseline myosin stiffness |
| ϵRLCP | 2.20e-3 | N/m | Myosin stiffness under RLC phosphorylation |
| T | 298 | K | Temperature |
| f | 200 | s−1 | Cross-bridge binding rate |
| g | 70.0 | s−1 | Cross-bridge dissociation rate (prepower-stroke) |
| hf | 2.38e3 | s−1 | Cross-bridge forward power-stroke rate |
| hb | 306 | s−1 | Cross-bridge reverse power-stroke rate |
| gxb | 225 | s−1 | Cross-bridge detachment rate (postpower-stroke) |
| ξ | 2.84 | Universal cross-bridge kinetic scaling coefficient | |
| Qp | 0.54 | Fractional phosphorylation of RLC at baseline | |
| Cross-bridge distortion and strain dependence parameters | |||
| xpo0 | 7.00e-9 | m | Cross-bridge distortion induced by power-stroke |
| σ+ | 8.03 | Cross-bridge detachment strain modifier for negative distortion values | |
| σ− | 3.90 | Cross-bridge detachment strain modifier for positive distortion values | |
| σh | 6.00 | Cross-bridge forward power-stroke strain-dependent modifier | |
| Cell mechanics parameters | |||
| η | 1.92e9 | N · s · m−1 | Myocyte viscous constant |
| kp | 1.07e5 | N/m2 | Scaling coefficient for nonlinear myocyte stiffness |
| τp | 5.22 | Exponential coefficient for nonlinear myocyte stiffness | |
| SLslack | 1.8 | μm | Slack sarcomere length |
| Nxb | 1e17 | m−2 | Cross bridges per unit cross-sectional area |
The model parameter ξ [called xbmodsp in the original work of Rice et al. (27)] scales all cross-bridge kinetic rates equally. It was adjusted in this work to mimic the age-dependent slowing of cross-bridge cycling that reflects altered relative content of β-MHC molecules (Fβ-MHC). The adjustments were implemented by assuming that ξ is the product of two scaling factors, one ξcond, which reflects the difference between chemically permeabilized and intact cells, and the other ξiso, which scales linearly with Fβ-MHC. Thus
| (A5) |
ξiso was estimated using experimental data. Previous work from our group (23) reported a significant correlation between Fβ-MHC and the rate of force redevelopment after slack-restretch (ktr) in skinned Fischer 344 rat myocardium. The best-fit line for these data has the equation
| (A6) |
Meanwhile, simulations of ktr in the myofilament model showed a linear relationship between ξ and ktr
Substituting this into Eq. A6 and solving for ξ gives a final relationship of
| (A7) |
The fitted value of ξmid6 was 2.84. Carnes et al. (8) reported a mean value of Fβ-MHC for midmyocardium at 5 mo of age, which gives ξiso by Eq. A7. Solving Eq. A5 for ξcond yields
Numerical experiments
The mathematical model predicted the twitch response of an unloaded midmyocardial cell for a given Ca2+ transient. We first used the model to calculate the relationship between τCa and RT50 for 6-mo-old midmyocardial cells by inputting Ca2+ transients with different decay times and observing the RT50 values of simulated contractions.
We then conducted numerical experiments by perturbing specific model parameters and recomputing the τCa-RT50 relation as before. The parameterization for β-MHC content (Eq. A5) was used to predict τCa-RT50 relations at different isoform expression levels. τCa-RT50 relations were also computed in response to altering troponin C Ca2+ affinity. Troponin C Ca2+ affinity was increased by reducing the dissociation rate kCa− under the assumption that kCa+ is diffusion limited and not likely to change in response to posttranslational modifications or other physiological events. τCa-RT50 relations simulated under altered β-MHC content or TnC Ca2+ affinity were then compared with baseline results.
REFERENCES
- 1.Ait Mou Y, Reboul C, Andre L, Lacampagne A, Cazorla O. Late exercise training improves non-uniformity of transmural myocardial function in rats with ischaemic heart failure. Cardiovasc Res 81: 555–564, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Belke DD, Swanson E, Suarez J, Scott BT, Stenbit AE, Dillmann WH. Increased expression of SERCA in the hearts of transgenic mice results in increased oxidation of glucose. Am J Physiol Heart Circ Physiol 292: H1755–H1763, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol 96: 822–828, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Boluyt MO, Devor ST, Opiteck JA, White TP. Regional variation in cardiac myosin isoforms of female F344 rats during aging. J Gerontol A Biol Sci Med Sci 54: B313–B317, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Cain BS, Meldrum DR, Joo KS, Wang JF, Meng X, Cleveland JC, Jr, Banerjee A, Harken AH. Human SERCA2a levels correlate inversely with age in senescent human myocardium. J Am Coll Cardiol 32: 458–467, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Campbell SG, Howard E, Aguado-Sierra J, Coppola BA, Omens JH, Mulligan LJ, McCulloch AD, Kerckhoffs RCP. Effect of transmurally heterogeneous myocyte excitation-contraction coupling on canine left ventricular electromechanics. Exp Physiol 94: 541–552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capasso JM, Fitzpatrick D, Anversa P. Cellular mechanisms of ventricular failure: myocyte kinetics and geometry with age. Am J Physiol Heart Circ Physiol 262: H1770–H1781, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Carnes CA, Geisbuhler TP, Reiser PJ. Age-dependent changes in contraction and regional myocardial myosin heavy chain isoform expression in rats. J Appl Physiol 97: 446–453, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cazorla O, Le Guennec JY, White E. Length-tension relationships of sub-epicardial and sub-endocardial single ventricular myocytes from rat and ferret hearts. J Mol Cell Cardiol 32: 735–744, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Cazorla O, Szilagyi S, Le Guennec JY, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB J 19: 88–90, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell 107: 631–641, 2001 [DOI] [PubMed] [Google Scholar]
- 12.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 1 13: 1–209, 2007 [PubMed] [Google Scholar]
- 13.Harrison TR, Dixon K, Russell RO, Jr, Bidwai PS, Coleman HN. The relation of age to the duration of contraction, ejection, and relaxation of the normal human heart. Am Heart J 67: 189–199, 1964 [DOI] [PubMed] [Google Scholar]
- 14.Hees PS, Fleg JL, Mirza ZA, Ahmed S, Siu CO, Shapiro EP. Effects of normal aging on left ventricular lusitropic, inotropic, and chronotropic responses to dobutamine. J Am Coll Cardiol 47: 1440–1447, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hofmann PA, Lange JH., 3rd Effects of phosphorylation of troponin I and C protein on isometric tension and velocity of unloaded shortening in skinned single cardiac myocytes from rats. Circ Res 74: 718–726, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth KG, Blamire AM, Keavney BD, Macgowan GA. Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol 302: H885–H892, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73: 413–467, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lakatta EG, Sollott SJ. The “heartbreak” of older age. Mol Interven 2: 431–446, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Mercadier JJ, Bouveret P, Gorza L, Schiaffino S, Clark WA, Zak R, Swynghedauw B, Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res 53: 52–62, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Mitov MI, Greaser ML, Campbell KS. GelBandFitter: a computer program for analysis of closely spaced electrophoretic and immunoblotted bands. Electrophoresis 30: 848–851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitov MI, Holbrook AM, Campbell KS. Myocardial short-range force responses increase with age in F344 rats. J Mol Cell Cardiol 46: 39–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orchard CH, Lakatta EG. Intracellular calcium transients and developed tension in rat heart muscle. A mechanism for the negative interval-strength relationship. J Gen Physiol 86: 637–651, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Haskó G, Kollai M, Szabó C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol 287: H2132–H2137, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razumova MV, Bukatina AE, Campbell KB. Stiffness-distortion sarcomere model for muscle simulation. J Appl Physiol 87: 1861–1876, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Rice J, Wang F, Bers D, De Tombe P. Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary differential equations. Biophys J 95: 2368–2390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem 257: 260–263, 1982 [PubMed] [Google Scholar]
- 29.Russel IK, Gotte MJ, Bronzwaer JG, Knaapen P, Paulus WJ, van Rossum AC. Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. JACC Cardiovasc Imaging 2: 648–655, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Sheikh F, Ouyang K, Campbell SG, Lyon RC, Chuang J, Fitzsimons D, Tangney J, Hidalgo CG, Chung CS, Cheng H, Dalton ND, Gu Y, Kasahara H, Ghassemian M, Omens JH, Peterson KL, Granzier HL, Moss RL, McCulloch AD, Chen J. Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J Clin Invest 122: 1209–1221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stride N, Larsen S, Hey-Mogensen M, Hansen CN, Prats C, Steinbruchel D, Kober L, Dela F. Impaired mitochondrial function in chronically ischemic human heart. Am J Physiol Heart Circ Physiol 304: H1407–H1414, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Taffet GE, Tate CA. CaATPase content is lower in cardiac sarcoplasmic reticulum isolated from old rats. Am J Physiol Heart Circ Physiol 264: H1609–H1614, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Tate CA, Helgason T, Hyek MF, McBride RP, Chen M, Richardson MA, Taffet GE. SERCA2a and mitochondrial cytochrome oxidase expression are increased in hearts of exercise-trained old rats. Am J Physiol Heart Circ Physiol 271: H68–H72, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: mechanism for hereditary arrhythmia. Proc Natl Acad Sci USA 100: 11759–11764, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tikunov BA, Sweeney HL, Rome LC. Quantitative electrophoretic analysis of myosin heavy chains in single muscle fibers. J Appl Physiol 90: 1927–1935, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54: B492–B501, 1999 [DOI] [PubMed] [Google Scholar]
- 37.van der Velden J, de Jong JW, Owen VJ, Burton PB, Stienen GJ. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res 46: 487–495, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 33: 1948–1955, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Wei JY, Spurgeon HA, Lakatta EG. Excitation-contraction in rat myocardium: alterations with adult aging. Am J Physiol Heart Circ Physiol 246: H784–H791, 1984 [DOI] [PubMed] [Google Scholar]
- 40.Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol Heart Circ Physiol 275: H2087–H2094, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res 76: 1028–1035, 1995 [DOI] [PubMed] [Google Scholar]