Abstract
Large increases in muscle sympathetic nerve activity (MSNA) can decrease the diameter of a conduit artery even in the presence of elevated blood pressure, suggesting that MSNA acts to regulate conduit artery tone. Whether this influence can be extrapolated to spontaneously occurring MSNA bursts has not been examined. Therefore, we tested the hypothesis that MSNA bursts decrease conduit artery diameter on a beat-by-beat basis during rest. Conduit artery responses were assessed in the brachial (BA), common femoral (CFA) and popliteal (PA) arteries to account for regional differences in vascular function. In 20 young men, MSNA, mean arterial pressure (MAP), conduit artery diameter, and shear rate (SR) were continuously measured during 20-min periods of supine rest. Spike-triggered averaging was used to characterize beat-by-beat changes in each variable for 15 cardiac cycles following all MSNA bursts, and a peak response was calculated. Diameter increased to a similar peak among the BA (+0.14 ± 0.02%), CFA (+0.17 ± 0.03%), and PA (+0.18 ± 0.03%) following MSNA bursts (all P < 0.05 vs. control). The diameter rise was positively associated with an increase in MAP in relation to increasing amplitude and consecutive numbers of MSNA bursts (P < 0.05). Such relationships were similar between arteries. SR changes following MSNA bursts were heterogeneous between arteries and did not appear to systematically alter diameter responses. Thus, in contrast to our hypothesis, spontaneously occurring MSNA bursts do not directly influence conduit arteries with local vasoconstriction or changes in shear, but rather induce a systemic pressor response that appears to passively increase conduit artery diameter.
Keywords: muscle sympathetic nerve activity, blood pressure, large artery vasoconstriction
sympathetic vasoconstriction is most effective in altering blood flow and blood pressure (BP) by influencing small arteries and arterioles (16, 18). However, conduit arteries also exhibit sympathetic perivascular nerve innervations (11, 15, 28, 33), express α-adrenergic receptors (11, 29, 33), and significantly vasoconstrict in response to α-adrenergic agonists (3, 40). Indeed, the majority of evidence (2, 4, 6, 23, 25, 26) suggests that large reflex-mediated increases in muscle sympathetic nerve activity (MSNA) decrease conduit artery diameter, although a few reports indicate only minimal changes (6, 30). Importantly, sympathoexcitatory maneuvers that evoke substantial elevations in arterial BP also cause a reduction in conduit artery diameter (4, 6, 23, 26). This indicates that the direct vasoconstrictor effects of MSNA prevail over the passive distending influence of arterial BP during large increases in sympathetic activity and suggests a primary role of MSNA in altering conduit artery diameter. Moreover, reflex-mediated increases in MSNA attenuate brachial artery shear-induced dilation via an α-adrenergic mechanism (10), further suggesting that sympathetic activity affects the overall regulation of conduit artery diameter. Together, these data support the view that conduit arteries are influenced by MSNA, but whether such interpretations can be extrapolated to rest and the naturally occurring oscillations in MSNA is unknown. To our knowledge, the potential beat-by-beat influence of spontaneous MSNA on conduit artery diameter has not been explored.
Our laboratory has recently advanced the use of spike-triggered averaging to understand the effects of spontaneously occurring MSNA bursts during rest (7, 8, 36). Collectively we have determined that MSNA bursts produce robust and dynamic beat-by-beat increases in arterial BP, primarily via decreases in limb vascular conductance through an α-adrenergic mechanism. Importantly, these effects were graded to the natural variations in the amount of spontaneous MSNA (i.e., number of consecutive bursts and burst amplitude). Given prior work demonstrating the capacity of large reflex-mediated increases in MSNA to promote decreases in conduit artery diameter, the aim of the present investigation was to examine the influence of spontaneously occurring MSNA bursts on beat-by-beat changes in conduit artery diameter during rest. We reasoned that MSNA may influence conduit artery diameter in three ways: 1) direct contraction of conduit artery smooth muscle, 2) reduction of the dilatory influence of shear stress due to vasoconstriction of downstream resistance vessels and the ensuing decrease in limb blood flow, and 3) elevation of arterial BP to passively distend the conduit artery. We hypothesized that spontaneously occurring MSNA bursts would overcome the passive distension of the artery induced by an increase in arterial BP and result in a decrease in conduit artery diameter. To account for limb differences in α-adrenergic sensitivity (13, 24) and endothelial function (20, 34), conduit artery diameter assessments were made in the arm (brachial artery) and the leg (common femoral and popliteal arteries).
METHODS
Twenty men with a mean age of 25 ± 1 yr, height of 177 ± 1 cm, and weight of 77 ± 1 kg (means ± SE) were recruited for voluntary participation in this study. A portion of these data were generated from previous studies published by our laboratory (7, 8), applying additional analyses to address the novel hypothesis of this study. Exclusion criteria included hypertension (resting BP > 140/90 mmHg), obesity (body mass index > 30 kg/m2), cigarette smoking, and any known neurological, pulmonary, metabolic, or cardiovascular disease. Each subject provided written consent after being informed of the risks and benefits associated with the investigation. All experimental measurements and study objectives were explained in detail prior to participation. Experimental measurements and protocols were approved by the University of Missouri Health Sciences Review Board and were performed in accordance with the Declaration of Helsinki. On study days, subjects were instructed to report to the laboratory at least 3 h following a meal and to refrain from alcohol and caffeine consumption and strenuous physical activity for 12 h.
Experimental measures.
Cardiac cycles were detected using a lead II electrocardiogram (ECG; Quinton Q710, Bothell, WA). Beat-by-beat arterial BP was measured noninvasively with a servo-controlled finger photoplethysmograph (Finometer; Finapres Medical Systems, Amsterdam, Netherlands) placed on the middle finger of the hand. In addition, an automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY) verified absolute brachial artery BP values. Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace; UFI, Morro Bay, CA). Multiunit postganglionic MSNA was recorded using standard microneurographic techniques, as described in detail previously (23, 35, 42). Briefly, a unipolar tungsten microelectrode was inserted into muscle fascicles of the peroneal nerve near the fibular head of the left leg. Neural signals were amplified, filtered (bandwidth; 700–2,000 Hz), rectified, and integrated (0.1 s time constant) to obtain mean voltage neurograms. MSNA recordings were identified by the presence of spontaneously occurring bursts with characteristic pulse synchronicity, responsiveness to an end-expiratory breath hold, and lack of response to arousal or skin stimulation. Resting MSNA burst frequency is demonstrated to be equivalent between contralateral limbs (37) as well as between the arm and leg (27). Time-averaged values for MSNA were quantified over the 20-min recording period as burst frequency (bursts/min) and burst incidence (bursts/100 cardiac cycles).
Duplex Doppler ultrasonography was performed using a linear array transducer (P5 and Logiq 7, GE Medical Systems, Milwaukee, WI). Conduit arteries were studied in standard locations, with the brachial artery imaged in the distal one-third of the upper arm, the common femoral artery 2–3 cm proximal to the bifurcation into the superficial and deep femoral arteries, and the popliteal artery within or just distal to popliteal fossa. The brachial and common femoral arteries were imaged at 12 MHz, while the popliteal artery was imaged at 4–10 MHz to optimize image quality at varying vessel depths. Blood velocity was obtained simultaneously with diameter in pulsed-wave mode at an insonation angle of 60°, operating at a linear frequency of 4–5 MHz. The transducer for each artery was stabilized with a custom-designed clamp and was confirmed by its maintained proximity to markings on the skin.
Experimental protocol.
The influence of spontaneously occurring MSNA on arterial hemodynamics and conduit artery diameter was examined in the three most commonly studied human conduit arteries. This was performed to account for known differences in α-adrenergic responsiveness (13, 24) and vasodilator responses between limbs (20) as well as differences associated with artery size (34, 41). The order and ultrasound unit (P5 vs. Logiq 7) used to study each artery was randomized, and in most cases two arteries were measured simultaneously. A total of 19 brachial arteries, 11 common femoral arteries, and 8 popliteal arteries were assessed. For all measurements, subjects were positioned comfortably supine in a quiet, dimly lit, climate-controlled room (23 ± 1°C). Subjects were instrumented for simultaneous measurements of heart rate, BP, respiration, MSNA, and duplex Doppler ultrasound. Before data collection, signals were acquired for 10 min to verify stability of the MSNA recording. Subsequently, all variables were continuously recorded for 20 min while the subject lay resting quietly and awake.
Data analyses.
All experimental measurements were acquired into a custom LabVIEW program interfaced with video output of the Doppler ultrasound machine, as previously described (7, 8, 22, 31). Briefly, the ECG, BP, and MSNA signals were embedded into an AVI file containing video images output at 30 Hz from the ultrasound machine. Offline edge detection analysis determined the diameter (mm) and weighted mean velocity (cm/s) from the captured video output. Synchronized beat-by-beat data were generated using the integral of each recorded variable between R waves in the ECG. Blood flow (ml/min) was calculated beat-by-beat using the following equation: Vmean·π·(Dmean/2)2·60 s/min, where Vmean is mean velocity (in cm/s) and Dmean is arterial diameter (in cm). Shear rate (SR) was calculated as 4·Vmean/Dmean to estimate shear stress, as supported by Ade et al. (1). Stroke volume measurements were estimated from the Finapres BP waveform using Modelflow software, as previously described in detail (5, 7, 8, 36). These values were aligned with LabVIEW program output via changes in cardiac interval and allowed for the estimation of beat-by-beat total vascular conductance.
Spike-triggered averaging was used to characterize the systematic influence of spontaneously occurring MSNA bursts on beat-by-beat changes in cardiovascular variables, as previously described (7, 8, 36). Briefly, the percent change in Dmean, MAP, and SR was calculated over the 15 heartbeats following each cardiac cycle containing a MSNA burst. The percent changes following all MSNA bursts during the 20-min period were averaged for each subject and a group mean was determined. The rationale for selecting 15 cardiac cycles originated with the work of Wallin and Nerhed (38) and follows previous work from our laboratory (7, 8, 36). This 15-heartbeat duration successfully captures the period of dynamic change for each variable. In addition, characterization of the entire 15-heartbeat period beat-by-beat provided a detailed evaluation of the time course and magnitude of the responses, while the peak or nadir of the mean served as an overall estimate of the responses. Percent changes were also averaged following nonbursting cardiac cycles to assess the systematic changes in Dmean, MAP, and SR without the influence of MSNA. Because spontaneous MSNA bursts vary in amplitude and may occur in isolation or in consecutive sequence with other MSNA bursts, we examined the influence of these varying intensities of sympathetic outflow on Dmean and MAP. For this analysis, all MSNA bursts were segregated into clusters of activity according to the sum of amplitudes of consecutive MSNA bursts, as described in detail previously (7, 8). Briefly, burst clusters were defined as any bursting activity separated on each side by ≥1 cardiac cycle(s) lacking MSNA. The sum of amplitudes for each burst cluster was then ranked and divided equally into quartiles among defined burst patterns according to the number of consecutive bursts, including singlet clusters (1 isolated burst), couplet clusters (two consecutive bursts), triplet clusters (3 consecutive bursts), and quadruplet clusters (≥4 consecutive bursts). This generated 16 cluster assignments (4 patterns × 4 quartiles). Each MSNA burst cluster was considered as a single event, and therefore, percent changes in Dmean and MAP were calculated following only the first heartbeat of the cluster. The average percent change following each MSNA burst cluster was calculated for each subject, and the group mean was determined. Last, to assess the specificity of responses to MSNA bursts, a white-noise procedure was used wherein randomly selected cardiac cycles were followed for 15 heartbeats to remove any systematic relationship with MSNA occurrence. The number of randomly selected cardiac cycles was matched for each subject's MSNA burst count to keep the number of observations consistent for within-subject comparisons.
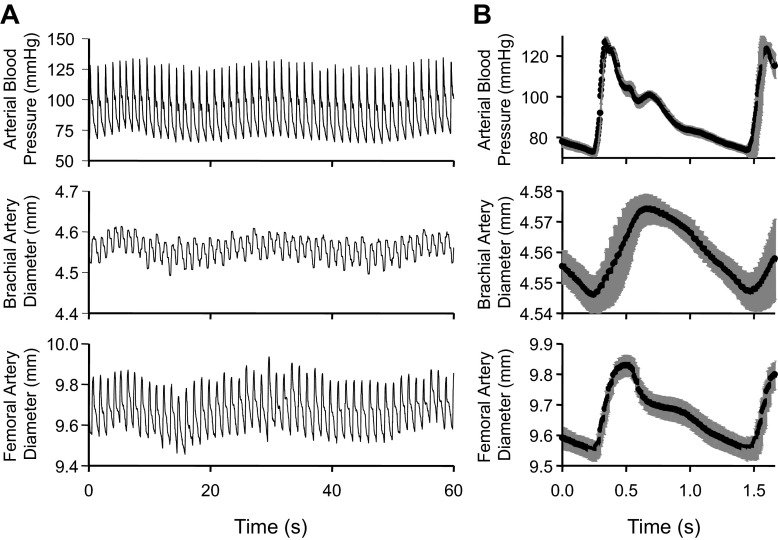
To accurately report small changes in conduit artery diameter, the axial resolution of our ultrasound machines and our LabVIEW analysis system was thoroughly assessed. Using manufacturer-provided values, the theoretical axial resolution of our GE systems were estimated to be 0.139–0.556 mm according to the equation n/2·cs·ƒ (39), wherein the number of cycles per ultrasonic pulse (n) is 1.5, the speed of sound in soft tissue (cs) is 1,540 m/s, and the transducer frequencies (ƒ) ranged between 4 and 12 MHz. The spatial limit of captured ultrasound images is represented by the length of each pixel, 0.11–0.15 mm. Our LabVIEW analysis program incorporates ∼1,500 independent diameter measures in each heartbeat (∼50 pixels/frame, 30 frames/s, ∼1 s/beat), yielding a very small measurement error (SD/√n) of ∼0.9 μm. As a result of this high precision, arterial BP waveforms within each cardiac cycle were nearly perfectly replicated in conduit artery diameter measurements (Fig. 1).
Fig. 1.
Example of Doppler ultrasound resolution using our custom LabVIEW software. A: 1-min period of simultaneously acquired measurements of arterial blood pressure and brachial and common femoral artery diameter from one subject. B: the overlaid average change within each heartbeat from the same 1- min time period, aligned on the initiating ECG R wave. Black symbols represent means; gray bars represent SE.
Statistical analyses.
Statistical analyses were performed using Sigmastat version 3.0. Comparisons of changes in Dmean, MAP, and SR following all bursts, nonbursts, and white noise was considered using two-way repeated-measures ANOVA to test for differences between cardiac cycles and between categories of MSNA burst occurrence. One-way ANOVA tested for differences in peak changes among burst amplitudes and patterns. The beat-by-beat change in any variable was considered significant when results indicated within-cardiac cycle differences from the corresponding change in white noise control, which was determined using the Student-Newman-Keuls post hoc test. Significance was set at P < 0.05, and data are expressed as means ± SE.
RESULTS
The average values of individual conduit arteries, systemic cardiovascular measurements, and MSNA are presented in Table 1.
Table 1.
Average resting cardiovascular variables and MSNA
| Common |
|||
|---|---|---|---|
| Brachial | Femoral | Popliteal | |
| Diameter, mm | 4.1 ± 0.2 | 9.4 ± 0.3*† | 5.5 ± 0.1* |
| Mean velocity, cm/s | 14.4 ± 1.3 | 7.4 ± 0.9* | 3.2 ± 0.4* |
| Blood flow, ml/min | 109 ± 10 | 300 ± 34*† | 45 ± 5* |
| Shear rate, s−1 | 146 ± 15 | 33 ± 5* | 23 ± 3* |
| Systolic blood pressure, mmHg | 125 ± 3 | 124 ± 3 | 128 ± 4 |
| Diastolic blood pressure, mmHg | 71 ± 2 | 70 ± 2 | 70 ± 3 |
| Mean arterial pressure, mmHg | 90 ± 2 | 90 ± 2 | 90 ± 3 |
| Total vascular conductance, ml·min−1·mmHg−1 | 75 ± 4 | 76 ± 6 | 79 ± 5 |
| Burst frequency, bursts/min | 18 ± 2 | 17 ± 2 | 16 ± 2 |
| Burst incidence, bursts/100 heartbeats | 30 ± 3 | 29 ± 3 | 26 ± 4 |
Values are means ± SE. MSNA, muscle sympathetic nerve activity.
P < 0.05 vs. brachial artery,
P < 0.05 vs. popliteal artery.
Effect of spontaneous MSNA on the brachial artery.
Beat-by-beat changes in brachial artery diameter, MAP, and brachial SR following spontaneously occurring MSNA bursts are shown in Fig. 2. Following all MSNA bursts, brachial artery diameter increased, reaching a peak of +0.14 ± 0.02% (+5.4 ± 0.1 μm; P < 0.05). MAP also increased with a very similar profile of change, reaching a peak of +3.1 ± 0.4% (+2.7 ± 0.4 mmHg; P < 0.05). Brachial SR exhibited a biphasic change following all MSNA bursts, wherein SR increased for the first three cardiac cycles (+2.5 ± 0.4%, +4.3 ± 0.8 s−1 peak; P < 0.05), and then decreased to a sustained value of −2.6 ± 0.6% (−3.5 ± 1.1 s−1; P < 0.05). Following cardiac cycles without MSNA bursts (i.e., nonbursts), brachial artery diameter and MAP significantly decreased to −0.05 ± 0.01% and −1.0 ± 0.1%, respectively (P < 0.05). No statistically significant changes in SR were observed following nonbursts. Additionally, no changes in any variable were observed following white noise controls.
Fig. 2.
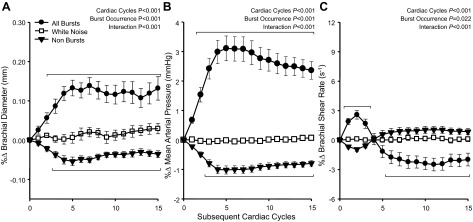
Summary data showing beat-by-beat percent changes in brachial artery diameter (A), mean arterial pressure (B), and brachial artery shear rate (C) following all spontaneous muscle sympathetic nerve activity (MSNA) bursts, following nonbursts, and a white noise control. Brackets denote significant differences from percent changes in white noise (P < 0.05). Values are means ± SE.
Effect of spontaneous MSNA on the common femoral artery.
Beat-by-beat changes in common femoral artery diameter, MAP, and common femoral artery SR following spontaneously occurring MSNA bursts are shown in Fig. 3. Similar to the brachial artery, common femoral artery diameter increased following MSNA bursts, reaching a peak of +0.17 ± 0.03% (+15.0 ± 0.2 μm; P < 0.05). MAP also increased, reaching a peak of +3.2 ± 0.5% (+2.8 ± 0.5 mmHg; P < 0.05). Common femoral artery SR exhibited a very different pattern of change compared with the brachial artery, decreasing to a value of −5.6 ± 0.7% (−1.6 ± 0.3 s−1; P < 0.05). Common femoral artery diameter and MAP decreased following nonbursts to −0.06 ± 0.01% and −1.1 ± 0.1%, respectively (P < 0.05). Following nonbursts, common femoral artery SR significantly increased, reaching a peak of +2.2 ± 0.5% (0.61 ± 0.14 s−1; P < 0.05). No changes in any variable were observed following white noise controls.
Fig. 3.
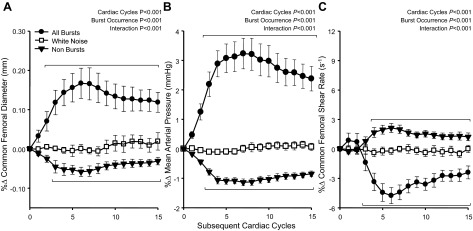
Summary data showing beat-by-beat percent changes in common femoral artery diameter (A), mean arterial pressure (B), and common femoral artery shear rate (C) following all spontaneous MSNA bursts, following nonbursts, and a white noise control. Brackets denote significant differences from percent changes in white noise (P < 0.05). Values are means ± SE.
Effect of spontaneous MSNA on the popliteal artery.
Beat-by-beat changes in popliteal artery diameter, MAP, and popliteal artery SR following spontaneously occurring MSNA bursts are shown in Fig. 4. Analogous to the brachial and common femoral artery, popliteal artery diameter increased following MSNA bursts and reached a peak of +0.18 ± 0.03% (+10.1 ± 0.2 μm; P < 0.05). MAP also increased to a peak of +3.5 ± 0.5% (+3.0 ± 0.5 mmHg; P < 0.05). In contrast to responses observed in the brachial and common femoral arteries, popliteal artery SR increased for the first six heartbeats following all MSNA bursts, reaching a peak of +7.0 ± 1.3% (+1.5 ± 0.2 s−1; P < 0.05), and then returned to baseline. Following nonbursts, popliteal artery diameter and MAP decreased to −0.07 ± 0.02% and −1.2 ± 0.2%, respectively (P < 0.05), while popliteal artery SR decreased but did not reach statistical significance. As with the brachial and common femoral arteries, no changes in any variable were observed following white noise controls.
Fig. 4.
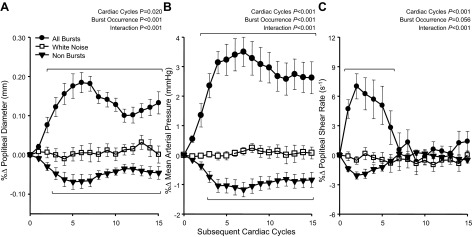
Summary data showing beat-by-beat percent changes in popliteal artery diameter (A), mean arterial pressure (B), and popliteal artery shear rate (C) following all spontaneous MSNA bursts, following nonbursts, and a white noise control. Brackets denote significant differences from percent changes in white noise (P < 0.05). Values are means ± SE.
Effect of MSNA burst clustering on changes in conduit artery diameter and MAP.
In each artery, the peak increases in conduit artery diameter were positively related to the total amount of MSNA in each of the 16 burst clusters (Fig. 5), which also appeared to be associated with the magnitude of the sympathetically mediated systemic pressor response. Indeed, the increase in MAP and conduit artery diameter for each artery was graded with the sum of amplitude of the 16 MSNA burst clusters with no differences observed between arteries. Moreover, all arteries exhibited a strong relationship between the peak increase in MAP and conduit artery diameter in response to the spontaneous MSNA burst clusters (Fig. 6).
Fig. 5.
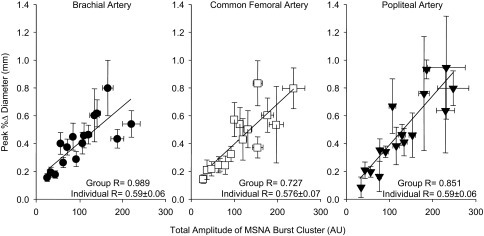
Correlations demonstrating relationships between the peak percent increase in conduit artery diameter and the total amplitude of the 16 MSNA burst clusters. The correlation derived from group means is displayed along with the mean for individual correlations. Values are means ± SE.
Fig. 6.
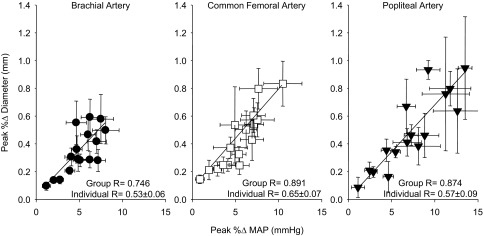
Correlations demonstrating relationships between the peak percent increase in mean arterial pressure (MAP) and the peak percent increase in conduit artery diameter following 16 MSNA burst clusters of varying total amplitude. The correlation derived from group means is displayed along with the mean for individual correlations. Values are means ± SE.
Effect of MSNA burst frequency on changes in conduit artery diameter and MAP.
To assess the influence of resting MSNA burst frequency on the main outcome variables in our cohort, subjects were divided into low and high [10 ± 1 (range 3–14) vs. 21 ± 1 (range 16–25) bursts/min, respectively; P < 0.001] MSNA groups based on the median burst frequency. The peak change in MAP following MSNA bursts was not different between the low and high MSNA groups (3.33 ± 0.56% vs. 3.46 ± 0.70%, respectively; P = 0.547). Similarly, the peak change in brachial diameter did not differ between groups (0.19 ± 0.03% low vs. 0.13 ± 0.02% high; P = 0.149). Corresponding analyses were performed for femoral and popliteal arteries, which also demonstrated no influence of resting burst frequency.
DISCUSSION
This study was the first to explore the influence of spontaneously occurring MSNA bursts on conduit artery diameter, and several novel findings emerged. In contrast to our hypothesis, the diameter of conduit arteries increased following MSNA bursts rather than decreased. This effect was consistently observed with parallel pressor responses following MSNA bursts. Indeed, variations in the total amount of preceding MSNA (i.e., burst clusters) were positively associated with the magnitude of the pressor response as well as the subsequent increase in conduit artery diameter in all vessels. In contrast, although significant changes in shear rate followed spontaneously occurring MSNA bursts, these changes were heterogeneous between arteries and did not appear to systematically alter diameter responses. Together, these results suggest that the influence of sympathetic conduit artery smooth muscle contraction and changes in limb shear rate induced by spontaneously occurring MSNA bursts are inadequate to decrease the diameter of conduit arteries during rest. Rather, the increase in systemic pressure following spontaneously occurring MSNA bursts appears to elicit a passive distension of conduit arteries that is consistent among limbs (brachial vs. common femoral artery) and vessels of varying size (common femoral vs. popliteal).
A primary role of sympathetic nerve activity is to bring about vasoconstriction, which increases vascular resistance and blood pressure. All muscular arteries appear to exhibit the necessary machinery (i.e., sympathetic perivascular nerves, α-adrenergic receptors, etc.) to participate in sympathetic vasoconstriction (11, 16, 18, 28, 29, 33). Nevertheless, an overwhelming amount of evidence demonstrates that small arteries and arterioles actively respond to all levels of sympathetic activity, whereas it appears that only large reflex-mediated increases in MSNA and infusion of adrenergic agonists raise conduit artery tone (3, 23, 25, 40). In a series of studies (7, 8, 36), we have recently established that spontaneous MSNA bursts are capable of decreasing limb and total vascular conductance and consequently, raising arterial blood pressure. To capitalize on the high resolution of our ultrasound system and the sensitivity of spike-triggered averaging for detecting systematic dynamic changes, we tested whether conduit artery diameter also decreases in response to spontaneously occurring MSNA bursts. In contrast to our hypothesis, we found that all three conduit arteries examined (brachial, common femoral, and popliteal) consistently increased in diameter following MSNA bursts, strongly suggesting that resting MSNA does not decrease conduit artery diameter and that downstream vasoconstriction is insufficient to consistently bring about shear-induced changes in diameter. Rather, increases in systemic blood pressure following MSNA bursts appear to cause an increase in conduit artery diameter.
The observation that spontaneously occurring MSNA bursts induce vasoconstriction of limb resistance arteries (7, 8), but not of conduit arteries, is intriguing, particularly since conduit arteries can have a greater density of perivascular innervations than skeletal muscle feed arteries (15). It is possible that a sympathetic vasoconstrictor threshold exists for conduit arteries but not for limb resistance arteries. A number of plausible factors may underlie this discrepancy in sympathetic vascular transduction along the arterial tree. For example, a greater relative number of smooth muscle cells and eNOS protein in larger arteries compared with smaller arteries (14) potentially reduces the amount of norepinephrine available per contractile unit as well as provides a greater dilatory opposition to sympathetic vasoconstriction. Adrenergic receptor density is inversely related with arterial size (33), possibly reducing the ability of conduit artery smooth muscle to respond to spontaneous sympathetic signals. Notably, all of the aforementioned evidence is derived from animal experiments; thus it is uncertain to what extent these findings apply to humans. Nevertheless, while large and small arteries have the capacity to constrict in response to large increases in MSNA (16, 18, 25, 40), our current data indicate that spontaneously occurring MSNA bursts during rest are insufficient to cause vasoconstriction in conduit arteries. In contrast, our previous work consistently demonstrated α-adrenergic mediated vasoconstriction of limb resistance arteries in response to resting MSNA (7, 8) as indicated by vascular conductance decreases within both the arm and leg.
Another potential factor in the modulation of conduit artery diameter in response to spontaneously occurring MSNA bursts is the change in shear stress stimulus. Under resting conditions, removal of the resting shear stimulus by cuff occlusion causes a reduction in conduit artery diameter, demonstrating that shear stress is an important determinant of baseline conduit artery diameter in humans (9, 32). Because elevated MSNA also reduces conduit artery shear rate, partly through increases in retrograde velocity (23), we hypothesized that a reduction in shear rate following spontaneously occurring MSNA bursts would contribute to reductions in conduit artery diameter. Although shear rate changed in each artery following MSNA bursts, the pattern of change was heterogeneous between arteries and did not appear to consistently influence conduit artery diameter. Indeed, shear rate decreased in the common femoral artery, increased and then decreased in the brachial artery, and robustly increased in the popliteal artery. These findings do not appear to be explained by either limb differences (brachial vs. common femoral) or differences in artery size (common femoral vs. popliteal). Although the reason for this heterogeneity in shear responses remains unclear, these data clearly suggest that changes in shear rate following spontaneous bursts of MSNA are not sufficient to modulate conduit artery diameter.
The most consistent finding from this investigation was the close matching of arterial blood pressure and conduit artery diameter. The systemic pressor effect of spontaneous MSNA bursts has been consistently observed (8, 36, 38). Recently, we identified that the pressor response is primarily due to decreases in total vascular conductance (36) and the associated decrease in limb vascular conductance (7, 8). Because the elevation in blood pressure following MSNA bursts was accompanied by remarkably consistent increases in diameter across the conduit arteries examined, our data strongly suggest that changes in conduit artery diameter can be attributed to the distension of the artery produced by the systemic pressor response. Indeed, the increases in blood pressure due to MSNA burst clusters were closely associated with the resulting increases in conduit artery diameter. Additionally, a similar proportional decrease in MAP and conduit artery diameter occurred during periods lacking MSNA (i.e., nonbursts). However, of note, from our data we cannot dismiss the possibility that the observed increases in conduit artery diameter were restrained by sympathetic vasoconstrictor influences and/or sympathetically mediated reductions in the dilatory influence of shear rate, but we can conclude that their influences were inadequate to prevent arterial distension resulting from the rise in systemic pressure. Likewise, it is possible that sympathetically mediated smooth muscle contraction of conduit arteries, unable to decrease diameter, elicited small reductions in arterial compliance, which limited the observed rise in conduit artery diameter. Nevertheless, these findings underscore the importance of spontaneous MSNA in the regulation of resting arterial blood pressure, and suggest that sympathetic reductions in conduit artery diameter may only occur during periods of intense stress wherein MSNA is markedly elevated.
Perspectives.
In healthy young men, spontaneously occurring MSNA bursts appear to have a minimal effect to constrict upstream conduit arteries, while the same stimulus efficiently reduces downstream limb vascular conductance (7, 8). These findings strengthen the primary role for resistance arteries in the sympathetic regulation of blood pressure and potentially in the development of hypertension. Furthermore, combined with previous work demonstrating decreases in conduit artery diameter during marked sympathoexcitation (4, 23, 26), a potential threshold may exist such that the amount of norepinephrine released by spontaneous MSNA is insufficient to constrict conduit arteries. In this regard, in our study of healthy subjects with ≤25 MSNA bursts/min, resting MSNA burst frequency did not influence the diameter or MAP changes following MSNA bursts. However, we speculate that substantially elevated resting MSNA burst frequency, such as observed in aging (12, 19) or heart failure (17, 21), may supply a neurotransmitter stimulus which overcomes the threshold for smooth muscle contraction in conduit arteries to demonstrate a decrease in compliance relative to healthy subjects, or outright vasoconstriction, depending on the degree of conduit artery smooth muscle contraction. Accordingly, it is possible that spontaneously occurring MSNA bursts may evoke vasoconstriction throughout the arterial tree in pathological overactive sympathetic states, instead of mediating an effect exclusively in resistance arteries as observed in young healthy men. Such concepts warrant future studies.
In summary, contrary to our hypothesis, spontaneously occurring MSNA bursts were followed by an increase, and not a decrease, in conduit artery diameter. Our data suggest that the increase in conduit artery diameter following MSNA bursts is positively associated with the magnitude of the rise in systemic pressure, suggesting the sympathetically mediated pressor response induces distension of large arteries. These findings reveal important functional discrepancies in the responsiveness of conduit arteries to resting vs. evoked MSNA, as well as demonstrate that overt vasoconstriction in response to spontaneous MSNA in humans is conferred in resistance, but not conduit, arteries.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant RO1-HL-093167 (P. J. Fadel). J. Padilla and M. J. Davis were supported by American Heart Association Grant AHA-11-POST5080002 and NHLBI Grant P01-HL-095486, respectively.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.T.F. and P.J.F. conception and design of research; S.T.F., J.P., L.C.V., S.W.H., and P.J.F. performed experiments; S.T.F. analyzed data; S.T.F., J.P., M.J.D., and P.J.F. interpreted results of experiments; S.T.F. prepared figures; S.T.F. drafted manuscript; S.T.F., J.P., L.C.V., M.J.D., and P.J.F. edited and revised manuscript; S.T.F., J.P., L.C.V., S.W.H., M.J.D., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
This research was submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy for S. T. Fairfax.
REFERENCES
- 1.Ade CJ, Broxterman RM, Wong BJ, Barstow TJ. Anterograde and retrograde blood velocity profiles in the intact human cardiovascular system. Exp Physiol 97: 849–860, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation 79: 93–100, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Barrett-O'Keefe Z, Witman MA, McDaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE, Sander M, Richardson RS, Wray DW. Angiotensin II potentiates α-adrenergic vasoconstriction in the elderly. Clin Sci (Lond) 124: 413–422, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol Heart Circ Physiol 267: H1368–H1376, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Dyson KS, Shoemaker JK, Arbeille P, Hughson RL. Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Exp Physiol 95: 561–568, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke IIPC, Wray DW, Davis MJ, Fadel PJ. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51: 1953–1958, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Hirst GD, Choate JK, Cousins HM, Edwards FR, Klemm MF. Transmission by post-ganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience 73: 7–23, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Iwase S, Mano T, Watanabe T, Saito M, Kobayashi F. Age-related changes of sympathetic outflow to muscles in humans. J Gerontol 46: M1–M5, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Jacob G, Costa F, Shannon J, Robertson D, Biaggioni I. Dissociation between neural and vascular responses to sympathetic stimulation: contribution of local adrenergic receptor function. Hypertension 35: 76–81, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol 284: H1307–H1312, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Long JB, Segal SS. Quantifying perivascular sympathetic innervation: regional differences in male C57BL/6 mice at 3 and 20 months. J Neurosci Methods 184: 124–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol 332: 169–186, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maslov PZ, Breskovic T, Brewer DN, Shoemaker JK, Dujic Z. Recruitment pattern of sympathetic muscle neurons during premature ventricular contractions in heart failure patients and controls. Am J Physiol Regul Integr Comp Physiol 303: R1157–R1164, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Mellander S, Johansson B. Control of resistance, exchange, and capacitance functions in the peripheral circulation. Pharmacol Rev 20: 117–196, 1968 [PubMed] [Google Scholar]
- 19.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol 556: 1001–1011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notarius CF, Morris BL, Floras JS. Dissociation between reflex sympathetic and forearm vascular responses to lower body negative pressure in heart failure patients with coronary artery disease. Am J Physiol Heart Circ Physiol 297: H1760–H1766, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol 96: 1019–1027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol 92: 2105–2113, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Pellinger TK, Halliwill JR. Effect of propranolol on sympathetically mediated leg vasoconstriction in humans. J Physiol 583: 797–809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perret F, Mooser V, Waeber B, Yanik T, Jean-Jacques M, Mooser E, Nussberger J, Brunner HR. Effect of cold pressor test on the internal diameter of the radial artery. Am J Hypertens 2: 727–728, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol 66: 2778–2781, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Reddy S, Kumar P, Prasad K. Histomorphometric and sympathetic innervation of the human posterior intercostal artery and its clinical importance. Folia Morphol 70: 161–167, 2011 [PubMed] [Google Scholar]
- 29.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation 100: 2336–2343, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Salzer DA, Medeiros PJ, Craen R, Shoemaker JK. Neurogenic-nitric oxide interactions affecting brachial artery mechanics in humans: roles of vessel distensibility vs. diameter. Am J Physiol Regul Integr Comp Physiol 295: R1181–R1187, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol 110: 389–397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spieker LE, Luscher TF, Noll G. ETA receptors mediate vasoconstriction of large conduit arteries during reduced flow in humans. J Cardiovasc Pharmacol 42: 315–318, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Stassen FR, Maas RG, Schiffers PM, Janssen GM, De Mey JG. A positive and reversible relationship between adrenergic nerves and alpha-1A adrenoceptors in rat arteries. J Pharmacol Exp Ther 284: 399–405, 1998 [PubMed] [Google Scholar]
- 34.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol 295: H1927–H1934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 36.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallin BG, Burke D, Gandevia S. Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol 474: 331–338, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst 6: 293–302, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Wikstrand J. Methodological considerations of ultrasound measurement of carotid artery intima-media thickness and lumen diameter. Clin Physiol Funct Imaging 27: 341–345, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of alpha-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol 555: 545–563, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol 99: 81–86, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Young CN, Deo SH, Kim A, Horiuchi M, Mikus CR, Uptergrove GM, Thyfault JP, Fadel PJ. Influence of endurance training on central sympathetic outflow to skeletal muscle in response to a mixed meal. J Appl Physiol 108: 882–890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]