Abstract
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that directs many of the cellular responses to hypoxia. In these studies, we have used a mouse model containing a cardiac-specific, oxygen-stabilized, doxycycline (Dox)-off regulated HIF-1α transgene to probe the role of HIF-1α in cardioprotection. Hearts used in these studies were derived from wild-type (WT), noninduced (Non-I), and 2 day (2D) and 6 day (6D) Dox-deprived mice. Whereas HIF-1α protein is undetectable in WT mice, it is present in heart tissue of “noninduced” transgenic mice, presumably because of leakiness of the promoter construct. In mice denied Dox for 2 or 6 days, HIF-1α is overexpressed to a much greater extent than Non-I or WT animals, as expected. WT and HIF-1α-expressing hearts (Non-I, 2D and 6D induced) were subjected to 30 min of ischemia, and functional recovery was measured upon reperfusion. Recovery of preischemic left ventricular developed pressure was 14% for WT, 67% for Non-I hearts, 64% for 2D-induced, and 62% for 6D-induced hearts. 6D-induced HIF hearts have increased preischemic glycogen reserves, higher glycogen synthase protein levels, and significantly higher lactic acid release during ischemia. 6D-induced HIF hearts were also better able to maintain ATP levels during ischemia compared with WT and Non-I hearts. Interestingly, Non-I hearts showed no significant increase in glycogen reserves, glycolytic flux, or greater ATP preservation during ischemia and yet were protected to a similar extent as the 6D-induced hearts. Finally, the mitochondrial membrane potential of isolated adult myocytes was monitored during anoxia or treatments with cyanide and 2-deoxyglucose. HIF-1α expression was shown to protect mitochondrial polarization during both stress treatments. Taken together these data indicate that, while HIF-1α expression in heart does induce increases in compensatory glycolytic capacity, these changes are not necessarily required for cardioprotection, at least in this model of ischemic stress.
Keywords: cardioprotection, hibernation, mitochondrial and metabolism
hypoxia-inducible factor-1α (HIF-1α) is a master regulatory transcription factor that directs the cellular response to hypoxia. When in vivo oxygen tension is sufficient, HIF-1α is hydroxylated at proline residues 402 and 564 by a family of prolyl hydroxylase domain-containing proteins (PHDs1–3) (2, 25). Hydroxylation of HIF-1α results in its recognition by von Hippel-Lindau factor and its subsequent degradation via proteasomal pathways. Hydroxylation of asparagine residue 803 (ASN803) by factor-inhibiting HIF provides an additional regulatory mechanism for HIF-1α (10, 13). The hydroxylation of ASN803 blocks the binding of coactivators CBP/p300 to the COOH-terminal-activating domain of HIF-1α, thereby limiting its transcriptional activity. Thus, HIF-1α is both stabilized and its transcription activity increased through these mechanisms when oxygen tension drops. The present studies are designed to establish if HIF-1α is sufficient to confer protection to heart against ischemia-reperfusion injury and to begin to explore the mechanisms through which this protection is affected. In pursuit of these aims, we used a transgenic mouse model containing the HIF-1α cDNA with alanine substitutions at Pro402, Pro564, and Asn803 (1). These substitutions result in a HIF-1α protein, termed HIF-1α-PPN, that is stable and displays full transcriptional activity in normoxic conditions. A tetracycline-regulated construct was used to obtain regulation of transgene expression. In the presence of doxycyline, transgene transcriptional expression is suppressed and induced when doxycycline is excluded from the diet. The expression of tetracycline transactivator protein is driven by a myosin heavy chain promoter, thus limiting HIF-1α transgene expression to the cardiac myocytes in these animals.
HIF-1α was identified in kidney cells as the transcriptional inducer of the erythropoietin gene (26, 27, 30). Subsequently, HIF-1α has been found to be ubiquitously expressed and to regulate hundreds of genes involved in metabolism, angiogenesis, and stress survival. One can view the changes directed by HIF-1α as compensatory adaptations to an oxygen-deficient environment. For instance, HIF-1α induces the expression of multiple glycolytic enzymes, including glucose transporter 1, aldolase A, enolase 1, lactate dehydrogenase, phosphofructokinase, and phosphoglycerate kinase (24). This upregulation of glycolysis decreases the cell's reliance on oxidative phosphorylation for ATP. Most studies on HIF-1α have been performed in cancer models, either cell lines or tumor tissue. Here HIF-1α has been found to be a potent promoter of angiogenesis via its upregulation of vascular endothelial growth factor and other angiogenic factors (23). More recently HIF-1α has been ascribed the role as the primary driver of the Warburg effect, or the high rates of aerobic glycolysis displayed by cancer cells (15). The close linkage between tumor growth and metastatic potential and the ability to attract vascularization explains the intense interest in HIF signaling as it relates to cancer.
In heart, much less is known about the role of HIF-1α in pathophysiology. Recently, prolonged overexpression of HIF-1α was shown to induce a cardiomyopathy that was fully reversible upon cessation of HIF-1α expression. In the aforementioned study, reduced sarco(endo)plasmic reticulum Ca2+-ATPase expression was linked to the contractile dysfunction that was observed (1). In another study, cardiomyocytes treated with prolyl hydroxylase inhibiters to induce HIF-1α reduce ATP turnover by 85%, with the majority of these energy savings derived from attenuation of calcium handling/contractile activities (29). These findings indicate that HIF-1α may be an important driver of the dysfunction observed in ischemic heart disease. On the other hand, evidence has begun to emerge that HIF-1α plays a central role in cardioprotection. Ischemic preconditioning is a phenomenon where exposure of hearts to short sublethal durations of ischemia-reperfusion provides protection from further damage upon exposure to subsequent longer and normally lethal episodes of ischemic stress. In a mouse model with a null allele of the HIF-1α locus, resulting in less expression of HIF-1α, a complete loss of preconditioning protection in heart was noted (3). In another recent study, small-interfering RNA (siRNA) repression of HIF-1α was found to attenuate preconditioning protection (6). Several reports have also shown that the use of pharmacological prolyl hydroxylase inhibitors to induce HIF-1α levels confers cardioprotection in several disparate cardiac model systems (19, 22, 28, 31). Similarly, administration of siRNA, which target PHD2 (16) or genetic models where PHD2 is ablated, proves cardioprotective (17). Because PHDs have targets other than HIF-1α, such as Iκkinase-1 (5), studies where PHD is inhibited do not necessarily prove HIF-1α involvement. Nonetheless, taken together, these recent findings strongly indicate a prominent role for HIF-1α in preconditioning and cardioprotection. Less clear are the HIF-1α-induced mechanisms that are important in providing protection from ischemia-reperfusion injury in heart. In these studies, we compare the ischemic stress tolerance of hearts that are induced to express HIF-1α-PPN in varying amounts and durations to begin to probe the aforementioned questions.
MATERIALS AND METHODS
Reagents.
Sterile oxyrase was purchased from Oxyrase (Mansfield, OH). Tetramethylrhodamine (TMRM) was purchased from Life Technologies (Grand Island, NY). 2,3,5-Triphenyl-2H-tetrazolium chloride (TTC) was obtained from Tokyo Kasei Kogyo (Tokyo, Japan). Rabbit polyclonal primary antibody against HIF-1α was obtained from Novus Biologicals (Littleton, CO), whereas rabbit monoclonal primary antibody against glycogen synthase was obtained from Cell Signaling (Danvers, MA). Goat anti-rabbit secondary antibodies were purchased from Millipore (Billerica, MA) and Cell Signaling. Doxycycline hydrochloride was purchased from RPI (Mount Prospect, IL).
Animal model.
B6C3F1 mice containing the HIF-1α-PPN transgene have been previously described (1). All mice used were males between 2 and 3.5 mo of age and were routinely maintained on a 625 mg/kg doxycycline-replete diet (Harlan Research Laboratories, Madison, WI). In experiments requiring 2 days of HIF-1α expression (2D), mice were switched from doxycycline food to doxycycline-replete drinking water containing 73 mM sucrose (Mallinckrodt Baker, Phillsburg, NJ) and 0.416 mM doxycycline hydrochloride for 2 days followed by maintenance of mice on regular food and water for two additional days. In experiments requiring 6 days of HIF-1α expression, mice were maintained on doxycycline-free food and water for 5–7 days before experimentation. Animals were handled in accordance to a protocol reviewed and approved by the East Tennessee State University Committee on Animal Care.
Isolation of cardiomyocytes from adult mice.
Myocytes from adult mice hearts were isolated according to the procedure described by O'Connell et al. (18). Isolated myocytes were suspended in 10 ml of plating medium with 25 μM blebbistatin and incubated on plates that were precoated with matrigel (BD Biosciences, Rockville, MD) diluted 1:40 in DMEM-F-12 (GIBCO, Grand Island, NY) for 1 h in 5% CO2. After incubation in plating medium, myocytes were maintained on culture medium with 25 μM blebbistatin. Experiments using adult mice cardiomyocytes were performed on the same day as cell isolation.
Preparation of mice heart homogenates.
Hearts from wild-type (WT), noninduced (Non-I), 2D, and 6D mice were excised and washed briefly in PBS to remove excess blood. Hearts were then immediately clamped with a set of tongs that were prechilled in liquid nitrogen. They were then ground into a fine powder using a mortar and pestle under liquid N2. The powdered heart samples were homogenized in RIPA buffer composed of 50 mM Tris·HCl, pH 7.4 (Calbiochem, Darmstadt, Germany), 1% vol/vol Triton X-100 (Fisher, Pittsburgh, PA), 1% wt/vol sodium deoxycholate (Fisher), 0.1% wt/vol SDS (EMD, Billerica, MA), and 1 mM EDTA (Fisher) with 1:40 vol/vol protease inhibitor cocktail mix (Sigma, St. Louis, MO). The homogenates were incubated on ice for 1 h and then centrifuged at 12,000 g at 4°C for 10 min. The supernatant was collected. Protein concentration for mice heart homogenates was determined using the Pierce BCA protein assay kit from Thermoscientific (Rockford, IL) according to the manufacturer's protocol.
SDS-PAGE and western blot.
HIF-1α and glycogen synthase expression were evaluated using standard SDS-PAGE and Western blotting techniques. Protein samples were separated using SDS-PAGE in Pierce Tris-HEPES-SDS 4–20% precast polyacrylamide gels (Thermoscientific). Proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad, Richmond, CA) at 75 volts for 2 h. After transfer, Ponceau S (Sigma) staining was used to ensure complete transfer and equal protein loading. Membranes were blocked in 5% nonfat dry milk in TBS with 1% Tween 20 (TBS-T) for 1 h at room temperature. HIF-1α expression was probed using a rabbit polyclonal primary antibody diluted 1:500 in TBS-T, and glycogen synthase expression was probed with a rabbit monoclonal primary antibody at 1:1,000 dilution in TBS-T. Both membranes were incubated at 4°C overnight and washed for 5 min in TBS-T (5×) before incubation with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody. Protein bands were detected using the Pierce supersignal west pico chemiluminescence substrate (Thermoscientific) in the G-Box fluorescence and chemiluminenscence imaging system.
Langendorff perfusion.
Hearts were retrograde perfused through the aorta with Krebs buffer containing (in mM): 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 24.8 NaCHO3, 2.5 CaCl2, and 10.6 glucose. The buffer was equilibrated with 95% O2 and 5% CO2 and maintained at 37°C. A fluid-filled silicon balloon was inserted in the left ventricle through the mitral valve for left ventricular developed pressure (LVDP) measurement with a pressure transducer (AD Instruments, Dunedin, New Zealand). Balloons were fabricated using methods previously published (14). Hearts were allowed to stabilize during a 25-min baseline period after which, function, tissue viability, lactate accumulation, ATP, and ADP levels were evaluated after hearts had been subjected to various ischemia-reperfusion protocols. For measurement of lactate production during ischemia, the perfusate during the first 5 min of reperfusion was collected. Lactate content was measured colorimetrically (450 nm) in a 96-well plate format following instructions from the Biovision lactate assay kit (Biovision, Milpitas, CA).
Evaluation of tissue viability after ischemia-reperfusion.
After the initial stabilization period, hearts were subjected to 30 min of ischemia and reperfusion for 60 min. At the end of the protocol, the hearts were perfused with 1% TTC and then taken off the cannula to incubate for 15 min at 37°C. Afterward, the hearts were sliced transversely, and images of the transverse slices were taken using a Microtek film scanner (Microtek International, Hsinchu, Taiwan). The viable (stained dark orange) and nonviable (unstained yellow) sections were analyzed using Adobe Photoshop (Adobe Systems, San Jose, CA).
Measurement of preischemic glycogen reserves.
For glycogen content, frozen powdered heart tissue was transferred to an Eppendorf tube, and 500 μl of ddH2O were added. The samples were immediately boiled for 5 min and centrifuged at 13,000 revolutions/min. Protein concentration was determined using the Pierce BCA assay kit (Thermoscientific). Glycogen content was measured colorimetrically at 570 nm on a 96-well plate format following instructions provided with the Biovision glycogen assay kit (Biovision).
Measurement of nucleotides.
ATP and ADP measurements in hearts subjected to perfusion protocols on the Langendorff apparatus were done using high-performance liquid chromatography (HPLC) following a method established by Giannattasio et al. (7). At the end of the respective perfusion protocol (preischemic; 5, 10, 20, or 30 min of ischemia; or 30 min of ischemia/30 min of reperfusion), hearts were freeze-clamped and then pulverized to a fine powder using a mortar and pestle. Next, the frozen heart tissue was homogenized in 400 μl of 4% perchloric acid (Alfa Aesar, Ward Hill, MA) to extract nucleotides. The homogenate was incubated on ice for 20 min. Following incubation, the homogenate was centrifuged at 15,000 g for 15 min at 4°C. The supernatant containing total nucleotides was stored in −80°C until further processing. The remaining tissue pellet was lyophilized to obtain the dry tissue weight.
Before measurement of ATP and ADP on HPLC, the samples containing total heart nucleotides were neutralized in a solution consisting of 4/5 volume 2 M KOH (Fisher) and 1/5 volume 1 M KH2PO4 (MP Biomedical, Solon, OH). After neutralization, the samples were incubated on ice for 10 min and centrifuged for 15 min at 15,000 g in 4°C. The resulting supernatant was collected and filtered through a 0.22-μm syringe filter (Millipore).
HPLC runs were performed using a binary gradient with increasing organic strength. The binary gradient was programmed into the LCsolutions data acquisition software (Shimadzu Scientific Instruments, Columbia, MD). The mobile phases were buffer A, which consists of 8 mM tetrabutylammonium hydrogen sulfate (Acros Organic, Morris Plains, NJ) and 0.1 M KH2PO4 (MP Biomedical). Buffer B consists of 8 mM tetrabutylammonium hydrogen sulfate (Acros Organic) and 0.1 M KH2PO4 (MP Biomedical) with the addition of 30% CH3CN (Fisher). Both mobile phase buffers were pH to 6.0. Sample (100 μl) was injected into a 20-μl loop, and peak detection was done using the SPD-M20A diode array detector at 254 nm (Shimadzu Scientific Instruments) and recorded with the LCsolutions data acquisition software. The areas of the peaks corresponding to ATP and ADP were integrated using the LCsolutions postrun analysis program (Shimadzu Scientific Instruments). Total ATP and ADP content expressed in units of micromole was calculated from the respective calibration curves and normalized against the amount of dry tissue collected (grams).
Measurement of mitochondrial membrane potential during anoxia and metabolic inhibition.
Adult mice cardiomyocytes were plated on 50-mm MatTek dishes (MatTek, Ashland, MA) and incubated in 1.5 ml of culture medium as described by O'Connell et al. (18) with 200 nM TMRM at 37°C and 5% CO2. After 30 min of incubation in 200 nM TMRM, the culture medium was substituted with 50 nM TMRM in PBS plus 5 mM glucose and 10 mM succinate. Before imaging, a cover slip was placed over the cells, and 15 μl sterile oxyrase were added. Oxyrase selectively removes oxygen, creating depletion of oxygen in the cardiomyocyte's immediate surroundings. Fluorescent imaging was performed on the Zeiss Axio Observer Z1 inverted fluorescent microscope (Zeiss, Gottingen, Germany). Images for cardiomyocytes from all mice groups were captured using the Zeiss AxioCam MRm monochrome digital camera at t0, the time point immediately after culture medium was substituted with PBS plus 5 mM glucose, 10 mM succinate, and 50 nM TMRM (Zeiss). Subsequent images were captured at 0.5, 1, 1.5, and 2 h of incubation with oxyrase. Images were analyzed with the Zeiss AxioVision 4.8.2 software. The number of polarized cells (i.e., those exhibiting TMRM fluorescence) was counted at each time point of oxyrase incubation and was expressed as a percent of total cells imaged using phase-contrast light microscopy. For measurement of mitochondrial membrane potential during inhibition of oxidative phosphorylation and glycolysis, isolated myocytes were incubated with 200 nM TMRM in culture medium for 30 min. After this period, culture medium was replaced with PBS plus 50 nM TMRM, 2 mM sodium cyanide (Mallinckrodt, Paris, KY), and 5 mM 2-deoxyglucose (2-DG; Acros), and a series of fluorescent images were taken at 0, 15, 30, 45, and 60 min of incubation. The number of polarized rod-shaped cells at each incubation time point (i.e., those displaying TMRM fluorescence) were counted.
Statistical analysis.
Data reported here are expressed as means ± SE. Statistical analysis was performed using one-way ANOVA followed by Student-Newman-Keuls post hoc testing using GraphPad Prism 5 (La Jolla, CA).
RESULTS
HIF-1α-expressing hearts better tolerate ischemia.
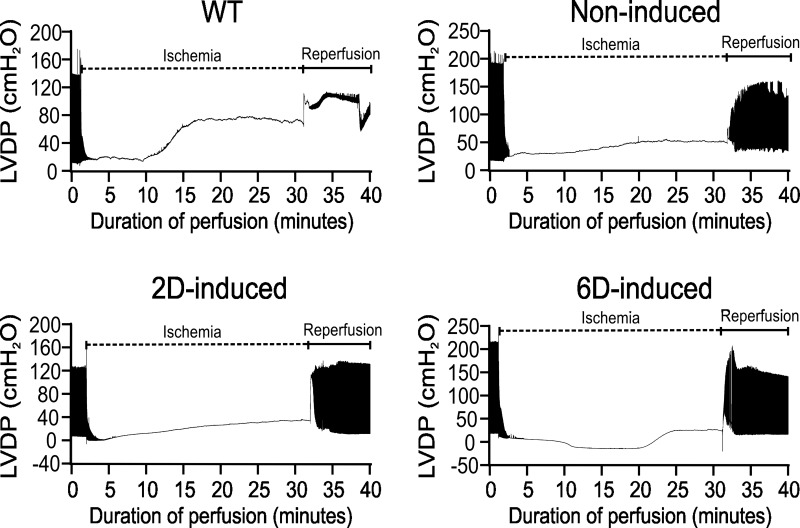
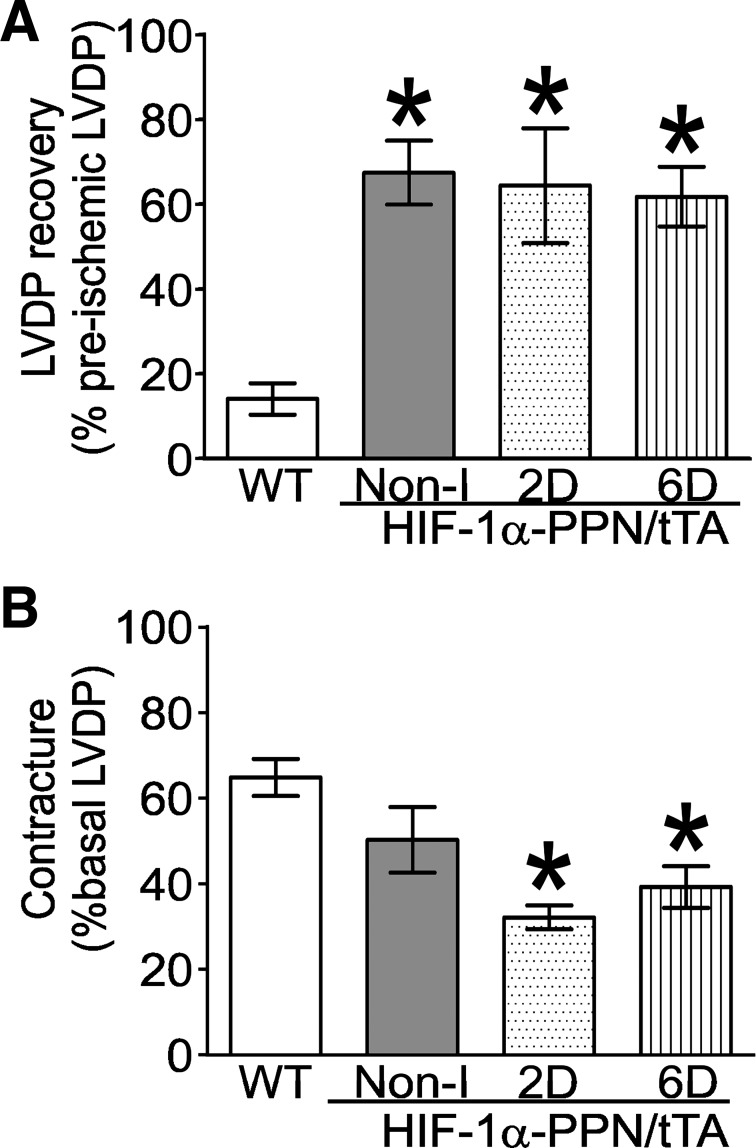
Hearts derived from WT and transgenic animals induced to express HIF-1α for the indicated times were retrograde perfused and subjected to 30 min of ischemia followed by reperfusion to allow recovery. No differences in the spontaneous beating rate or LVDP were noted between HIF-1α-expressing and control hearts before ischemia. Upon cessation of perfusion, the ischemic contracture was found to be more slowly developing and of a lower ultimate magnitude in 2D- and 6D-induced HIF-1α hearts (Fig. 1). Recovery of function following ischemia was remarkably higher in Non-I, 2D, and 6D HIF-1α-induced hearts compared with WT hearts (Fig. 2A). The magnitude of ischemic contracture was significantly lower for 2D-induced and 6D-induced hearts, but not Non-I hearts, compared with the WT hearts (Fig. 2B).
Fig. 1.
Response of murine hearts with varying degrees of hypoxia-inducible factor-1α (HIF-1α) expression to 30 min of ischemia, followed by reperfusion. Shown are exemplary condensed pressure tracings from Langendorf-perfused hearts derived from wild-type (WT) and HIF-1α-PPN mice that were not induced (Non-I) and induced to express HIF-1α for 2 days (2D induced) or 6 days (6D induced). Hearts were subjected to 30 min of ischemia following preequilibration for at least 20 min, whereupon they were allowed to recover. In some hearts, the left ventricular balloon was deflated during ischemia and reperfusion. In the example shown here, the balloon was allowed to remain inflated so that ischemic contraction could be measured. LVDP, left ventricular developed pressure.
Fig. 2.
Quantification of cardiac functional recovery after ischemia of the experiment shown in Fig. 1. A: recovery of LVDP after 30 min of ischemia. B: ischemic contracture expressed as a percent of basal LVDP. *P < 0.05 vs. WT; n = 6–8 hearts.
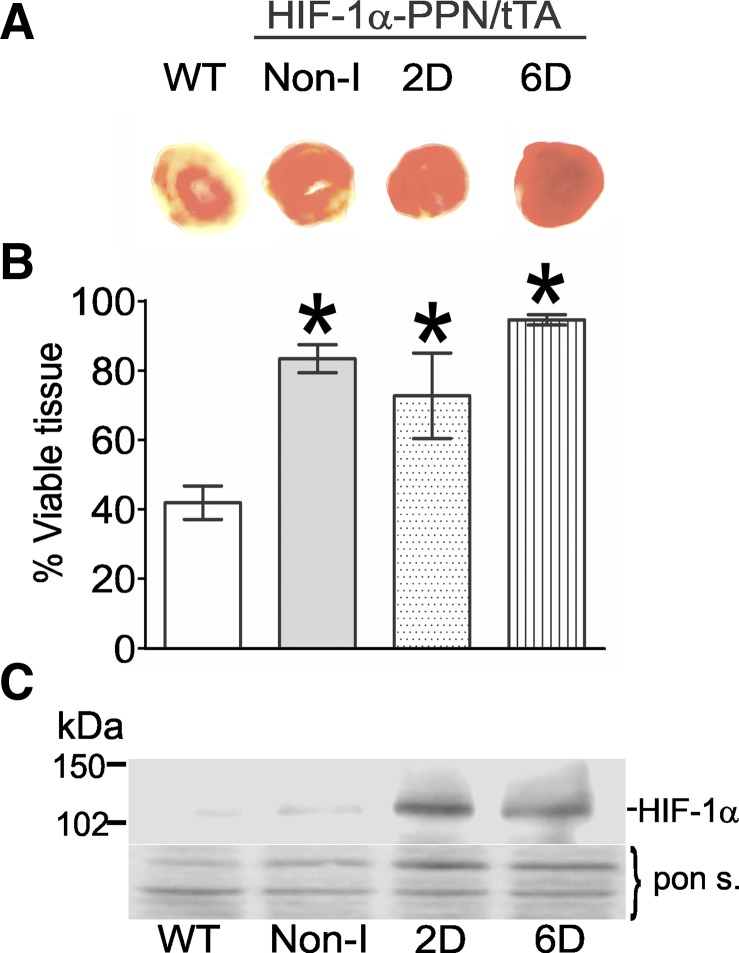
Next, tissue viability after the ischemic challenge was assessed using TTC staining. Tissue viability was found to correlate well with the enhanced functional recovery of the hearts (Fig. 3, A and B). Somewhat surprisingly, the recovery of preischemic LVDP and tissue viability of HIF-1α-PPN Non-I hearts was equivalent to the 2D-induced and 6D-induced hearts (Figs. 2 and 3). This finding caused us to examine the possibility of HIF-1α-PPN “leakage” in the hearts of animals maintained on a doxycycline-replete diet. Indeed, we find that, while Non-I heart extracts contain far less HIF-1α protein than their induced counterparts, they do have higher levels than WT hearts (Fig. 3C). Perhaps this should not have been unexpected given that HIF-1α-PPN has been modified to be stable under normoxic conditions via the substitution of critical amino acids within its degradation domain. Thus, any small expression leakage will result in some accumulation of the stable HIF variant. The results indicate that, while HIF-1α-PPN expression is much lower in the Non-I hearts, it is sufficient to protect to a similar extent, at least against this stress protocol. In contrast, the moderation of ischemic contracture appeared to require the higher, induced levels of HIF-1α (Fig. 2B).
Fig. 3.
HIF-1α expression limits myocardial ischemic damage. A: typical triphenyltetrazolium chloride (TTC) staining pattern in WT, noninduced, 2D-induced, and 6D-induced mouse hearts postischemic reperfusion insult; note that viable tissue stains a darker orange color. B: viable tissue was determined by TTC staining in perfused hearts following 30 min of ischemia and 60 min of reperfusion. The percent viable tissue was quantified using images of tranverse section of heart ventricle and Adobe Photoshop software. *P < 0.05 vs. WT. C: representative Western blot showing HIF-1α protein levels in protein extracts from hearts in the indicated treatment groups.
HIF-1α causes glycogen synthase induction, glycogen accumulation, and increased glycolytic flux during ischemia.
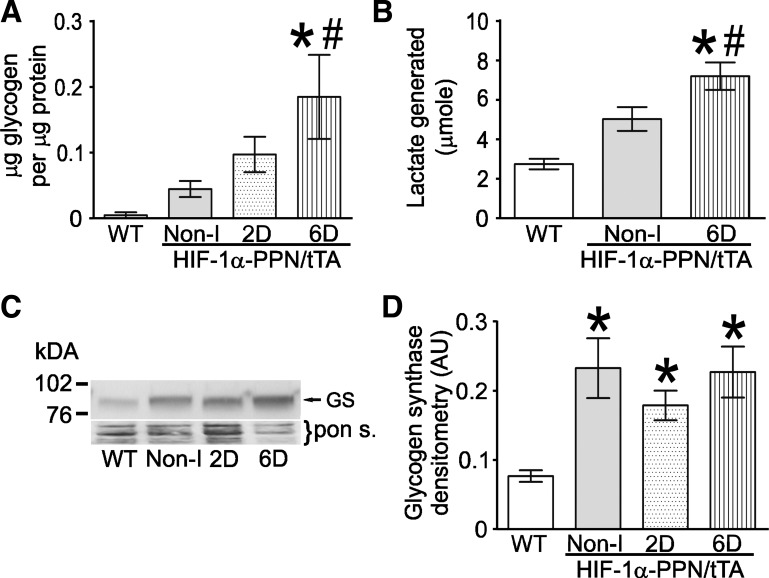
Because ischemic contracture has been reported to be delayed and reduced in magnitude by higher glycogen reserves, we next examined the effects of HIF-1α expression on glycogen levels in the HIF-expressing and WT hearts. Although a trend was observed for higher glycogen reserves in all the hearts expressing HIF-1α-PPN, only the 6D HIF-induced mouse hearts had significantly higher levels of glycogen stores compared with WT and Non-I hearts. Given the higher glycogen stores at the initiation of ischemia, we sought to estimate the glycolytic flux during the 30 min of no-flow ischemia. Accordingly, the next experiment measured the lactic acid accumulated during the 30 min of ischemia that is released during the first 5 min of reperfusion (Fig. 4B). As with glycogen stores, lactic acid release was significantly elevated in the 6D HIF-induced hearts compared with hearts from WT and the Non-I HIF-1α-PPN mice. Recent reports indicating that glycogen synthase is a HIF-1α-responsive gene might explain the higher levels of glycogen stores in the HIF-expressing hearts. Consistent with this, we find significantly elevated glycogen synthase protein levels in HIF-1α-PPN transgenic mice hearts (Fig. 4C). Interestingly, glycogen synthase protein levels are elevated to a similar extent in Non-I hearts as those induced for 2 and 6 days. The discrepancy between glycogen synthase and glycogen levels among the experimental groups probably reflects the complex array of factors that ultimately determine tissue glycogen levels. Nonetheless, these results showing that HIF-1α directs induction of glycogen synthase and glycogen accumulation in adult heart are important findings. Given that glycogen granule accumulation is a hallmark of the hibernating myocardium (9), it indicates that HIF-1α may be a driver of this pathophysiological phenotype.
Fig. 4.
Glycogen reserves are increased in HIF-1α-expressing hearts. A: measurement of glycogen content in WT, noninduced, 2D-induced, and 6D-induced mouse hearts (n = 4 hearts) B: accumulated lactate was measured in the initial 5 min of perfusion buffer effluent following 30 min of ischemia. C: Western blot showing glycogen synthase protein levels in WT, noninduced, 2D-induced, and 6D-induced mouse heart extracts. D: densitometry analysis of the glycogen synthase protein band intensity from the Western blot experiment shown in C (n = 4). *P < 0.05 vs. WT. #P < 0.05 vs. noninduced.
The upregulation of glycolytic metabolism does not explain the protection afforded by HIF-1α.
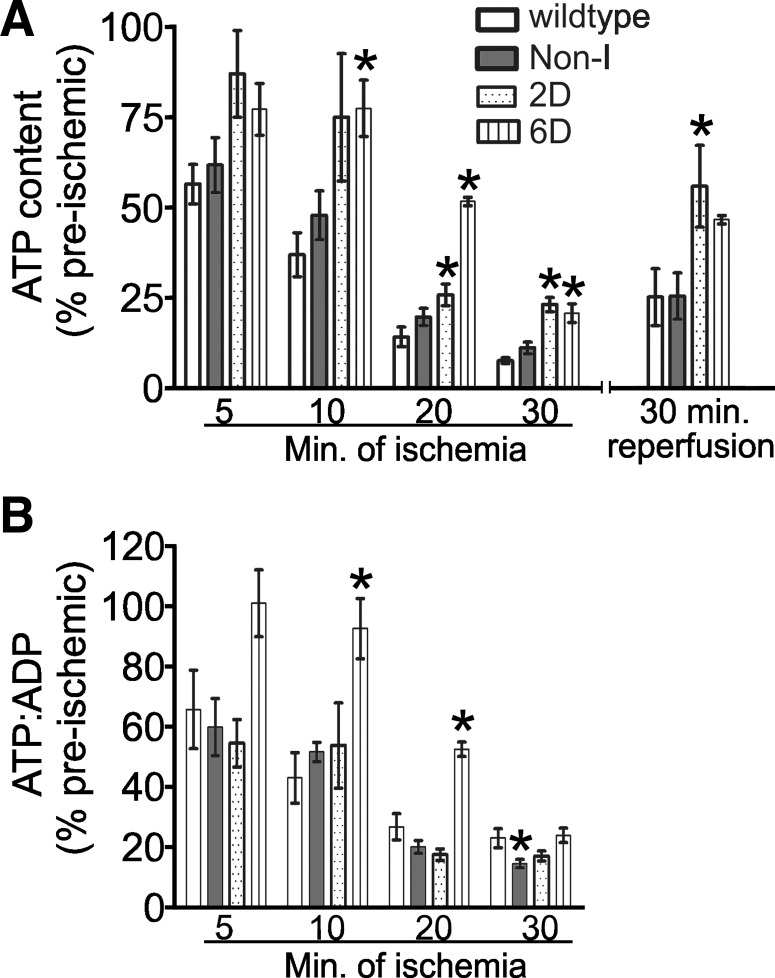
The well-known ability of HIF-1α to induce many of the enzymes that comprise the glycolytic pathway when coupled to elevated glycogen stores suggests an obvious avenue to achieve the cardioprotection observed in the HIF-1α-PPN hearts. Mobilization of the glucose stores, and enhanced glycolysis and its attendant ATP production during stoppage of perfusion, is consistent with the moderation of ischemic contracture and increased lactic acid release that we observe in HIF-expressing hearts. To address the issue of glycolytically produced ATP moderating the contracture and the damage sustained during ischemia, hearts were flash-frozen at intervals during ischemia and extracted, and nucleotide contents were measured with HPLC (Fig. 5A). Hearts derived from 2D- and 6D-induced HIF-1α-PPN hearts maintained significantly elevated levels of ATP at 20 and 30 min of ischemia compared with WT control hearts. Among the experimental groups, only 6D-induced HIF-1α-PPN hearts maintained significantly elevated ATP-to-ADP ratios at any time of ischemia (Fig. 5B). The comparison of ATP depletion between Non-I HIF-1α-PPN and WT hearts showing no differences in the degree of ATP depletion is most interesting given the high degree of ischemic tolerance the Non-I hearts display. These findings call into question the relative importance of the compensatory increases in glucose stores and glycolytic enzymes in the protective phenotype of the HIF-1α-PPN hearts. Other mechanisms besides increasing glycolytic flux during ischemia are suggested to be employed by HIF-1α to protect the myocardium. The next series of experiments were designed to begin to probe alternative mechanisms of protection.
Fig. 5.
ATP levels are better maintained during ischemia in hearts where HIF-1α expression has been induced. Hearts were freeze-clamped and extracted at the indicated time points of ischemia. ATP and ADP levels were measured by reverse-phase HPLC (see materials and methods). A: ATP content in hearts subjected to increasing durations of ischemia. ATP levels are expressed as a percentage of preischemic values within all experimental groups. Preischemic ATP values were 26.4 ± 3.7, 27.7 ± 8.7, 19.4 ± 11.2, and 21.4 ± 2.4 μmol/g dry tissue in WT, noninduced, 2D-induced, and 6D-induced mouse hearts, respectively. B: the ATP-to-ADP ratio expressed as a percentage of the preischemic values, which are 3.3 ± 0.6, 4.5 ± 1.0, 4.8 ± 0.3, and 3.1 ± 0.6 for WT, noninduced, 2D-induced, and 6D-induced mouse hearts, respectively. The data represent means ± SE (n = 3–9). *P < 0.05 vs. WT.
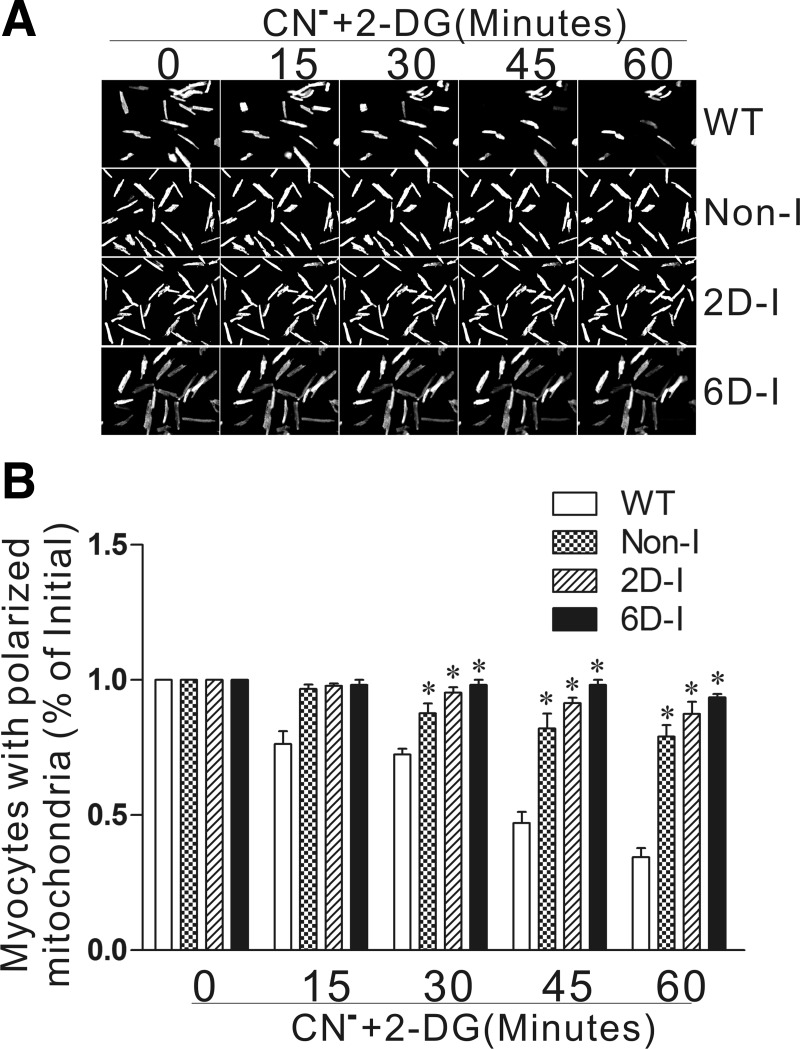
Previously, we found that the ability of mitochondria to maintain polarization during simulated ischemia is strongly associated with protection in the heart cell (28). Thus we tested whether isolated adult cardiomyocytes from HIF-1α-PPN hearts also display improved mitochondrial function under similar conditions. Adult cardiomyocytes were isolated from WT, Non-I, 2D- and 6D-induced HIF-1α-PPN hearts and subjected to anoxia. Mitochondrial polarization was monitored with TMRM potentiometric dye for 2 h under these conditions. We find that HIF-1α-PPN myocytes maintained mitochondrial polarization in significantly greater numbers than WT cells, with 6D-induced cardiomyocytes performing best, being little affected over the 2-h period (Fig. 6). In the next experiment, the dependency of protection on enhanced glycolytic flux was directly addressed. Myocytes derived from WT, Non-I, 2D- and 6D-induced HIF-1α-PPN hearts were treated with cyanide and 2-DG to block both oxidative phosphorylation and glycolysis, and mitochondrial polarization was followed for 1 h. We find that HIF-1α-expressing myocytes are able to maintain mitochondrial membrane potential under these conditions to a significantly greater extent than WT control myocytes (Fig. 7). This finding causes us to conclude that the well-known compensatory increase in glycolytic capacity that is induced by HIF-1α is not necessary for the ischemic tolerance conferred by HIF-1α and points to the existence of additional mechanisms that center on the mitochondrion.
Fig. 6.
Mitochondrial membrane polarization is better maintained in HIF-1α-expressing adult cardiomyocytes during anoxia. A: typical fluorescent image of WT, noninduced (Non-I), 2 day-induced (2D-I), and 6 day-induced (6D-I) mouse heart cardiomyocytes after 0, 0.5, 1, 1.5, and 2 h of anoxia. B: cells with polarized mitochondria relative to the amount before anoxia expressed as means ± SE (n = 3–8). *P < 0.05.
Fig. 7.
HIF-1α-expressing hearts maintain polarized mitochondria when both oxidative phosphorylation and glycolysis is blocked. A: representative fluorescent images of cardiomyocytes obtained from WT, Non-I, 2D-induced, and 6D-induced mice after 0, 15, 30, 45, and 60 min of cyanide (CN−) and 2-deoxyglucose (2-DG) treatment to block oxidative phosphorylation and glycolysis. B: no. of rod-shaped cells with polarized mitochondria expressed as means ± SE (n = 3). *P < 0.01 vs. WT. No significance differences were found between the HIF-expressing groups.
DISCUSSION
Twenty years after its discovery, HIF-1α is well established to play a central role in the cellular response to hypoxia; however, much less is known about its cardiospecific effects. In these studies, we show that forced expression of a stabilized mutant version of HIF-1α confers robust protection to adult heart in a common ex vivo model of ischemia-reperfusion injury. Given the prominence of ischemic stress in the constellation of pathologies grouped under the umbrella of heart disease, it is of great interest to understand the mechanisms the cardiomyocyte employs to protect itself against hypoxia stress. Certainly HIF's actions in protecting the myocyte from irreversible damage during ischemia can be regarded as beneficial. On the other hand, evidence also exists that ascribes a pathological role to HIF-1α in the context of deceased myocardial function during chronic hypoxia such as occurs in ischemic heart disease. Indeed HIF's pathological and protective mechanisms are likely to overlap, in that decreased function and lower ATP turnover represent powerful protective maneuvers when oxygen becomes limited (11, 12). In this vein, we should note that HIF-1α-PPN induction for up to 6 days did not change the basal contractile characteristics (i.e., LVDP when paced, or spontaneous heart rate, data not shown) of mice hearts when they were examined ex vivo with Langendorf perfusion. Thus, the robust ischemic protection afforded by HIF-1α expression was not accompanied by any indication of pathological response in the time frames examined in this study.
The most prominent effect of HIF-1α-PPN expression on the response of the perfused heart to 30 min of ischemia is an attenuated ischemic contracture. Sometimes referred to as “stone heart,” the underlying mechanisms governing contracture are not completely understood (8). Initiation of contracture is closely tied to the rate of ATP depletion and exhaustion of internal glycogen stores (8). It is important to note, however, that the severity of ischemic contracture does not correlate with myocardial protection or recovery of function in heart. This is best illustrated by the fact that ischemic preconditioning, which is highly protective, shortens the time until onset of contracture probably through depletion of glycogen stores. This has led to the suggestion that glycogen and high rates of glycolysis and acidification during ischemia are detrimental to cellular recovery. The degree of severity and length of the ischemic insult probably determine whether mobilization of glycogen stores and glycolytic ATP production are effectively beneficial or detrimental to tissue viability (4). For instance, with chronic low-flow ischemia, enhanced glycolytic ATP generation greatly improves functional outcomes.
Recently, muscle glycogen synthase was shown to be a HIF-1α-inducible gene in C2C12 skeletal muscle myotubes and several cell lines (21). Our results show that HIF-1α elevates glycogen synthase in adult heart cells and leads to glycogen accumulation, especially in 6D HIF-1α-induced hearts, where significant elevations of glycogen synthase, glycogen, and lactic acid production during ischemia were noted. Of importance, the enhanced glycolysis was reflected in a significant diminishment of ATP depletion in the 6D-induced hearts during ischemia. The finding that HIF-1α is sufficient to cause glycogen accumulation in the adult myocardium is also of significance. Glycogen accumulation is a hallmark of hibernating myocardium. Given previous findings that HIF-1α suppresses oxidative phosphorylation (20), ATP turnover, and contractile function (29), a strong circumstantial argument can be made that HIF-1α may play a large role in directing the hibernating phenotype in heart. As mentioned above, however, we were unable to detect any changes in contractile function or oxidative respiration in 6D HIF-induced hearts. Specifically, LVDP, dP/dt, and O2 consumption were measured in WT, Non-I, and 2D- and 6D-induced hearts, whereas pacing was increased in 200-beat/min step increments from 300 to 1,300 beats/min. No significant changes in these parameters were found between the hearts in any of the experimental groups (data not shown). We are presently uncertain why HIF-1α-expressing hearts fail to show any evidence of reduced oxidative phosphorylation (29) or contractile function (1) noted in other model systems. Several possible explanations are currently being tested but are beyond the scope of these studies, which have focused upon the protective effects of HIF-1α-directed changes.
In these studies, we have exploited our inducible HIF-1α-PPN transgenic system to provide something akin to the dose-response curve, where the influence of HIF-1α is absent in the wild type: < in Non-I << 2D-induced < 6D-induced derived hearts. This pseudo dose-response yielded interesting insights into the biology of the compensatory responses elicited by HIF-1α. We examined preischemic glycogen reserve, glycolytic activity, and ATP depletion kinetics during ischemia in WT, Non-I, and 2D- and 6D-induced mice hearts. We found that the Non-I HIF-1α-PPN hearts did not show significant increases in preischemic glycogen store, glycolytic flux during 30 min of ischemia, or increased ability to maintain ATP levels during various times of ischemia compared with WT hearts. Nonetheless, Non-I hearts were protected to the same extent as hearts induced to express HIF-1α for 2 and 6 days. This suggests that, while increasing glycogen reserves before, and glycolytic flux during, ischemia clearly represent a compensatory response; it may not comprise the primary mechanism of protection to the total global ischemia employed in these studies.
Similar to these studies, our work examining the cardioprotective mechanisms of the O2 sensor in neonatal cardiomyocytes called into question the central importance of the induction of glycolytic capacity and glycogen accumulation in protection (28). In previous work, we found that HIF-1α expression, albeit as the result of dimethyloxalyglycine treatment rather than genetic manipulation, led a persistent ability to maintain mitochondrial polarization during anoxia or cyanide poisoning, even when glycolysis was blocked or the reverse mode of ATP synthase was inhibited. After extensive analysis, we ultimately concluded that fumarate was used as an alternate oxygen electron acceptor to allow electron flux to continue through complex I during anoxia or cyanide poisoning (29). In the studies shown in Figs. 6 and 7, isolated adult heart cells were tested in vitro to determine whether the protection observed in HIF-expressing hearts would translate into stabilized mitochondrial polarization during anoxia. In both anoxic conditions permissive for glycolysis, and in conditions where both oxidative phosphorylation and glycolysis are blocked (cyanide and 2-DG present), mitochondrial membrane potential was significantly better maintained in the HIF-1α-expressing cardiomyocytes. Taken together, these results reveal that HIF-1α can provide ischemic cardioprotection via mechanisms independent of compensatory increases in glycolytic flux and ATP preservation. Studies are underway to quantitatively measure the flux through fumarate respiratory pathways that HIF-1α induces.
Clearly HIF-1α is initiating a complex multifactorial compensatory program that equips the adult cardiomyocyte with remarkable tolerance to hypoxic stress. The most well-known HIF-1α-driven response to hypoxia is the induction of glycolytic pathway enzymes (23). Our data confirm that glycolytic capacity is increased by HIF-1α in adult murine cardiomyocytes and that this reserve is tapped during acute ischemia. Somewhat surprising, the findings also call into question the central importance of this increased glycolytic capacity as the central mechanism leading to the powerful protection conferred by HIF-1α in the acute no-flow ischemic protocol that we employ. Rather, isolated cell studies implicate mechanisms that preserve mitochondrial function during ischemia in the powerful cardioprotection that is observed.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-084302 to G. L. Wright.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.W., R.V.S., and G.L.W. conception and design of research; J.W., P.C., Y.L., C.A., T.D., and M.C. performed experiments; J.W. and P.C. analyzed data; J.W., P.C., Y.L., and T.D. prepared figures; J.W. and G.L.W. edited and revised manuscript; J.W., P.C., Y.L., C.A., T.D., R.V.S., and G.L.W. approved final version of manuscript; C.A., M.C., and G.L.W. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Collette Hunt for work to establish the transgenic animal colony at ETSU. Thanks are also extended to Megan Ewing for efforts in maintaining the mouse colony.
REFERENCES
- 1.Bekeredjian R, Walton CB, MacCannell KA, Ecker J, Kruse F, Outten JT, Sutcliffe D, Gerard RD, Bruick RK, Shohet RV. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS One 5: e11693, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res 77: 463–470, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cross HR, Opie LH, Radda GK, Clarke K. Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circ Res 78: 482–491, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IκB kinase-beta, giving insight into hypoxia-induced NFκB activity. Proc Natl Acad Sci USA 103: 18154–18159, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckle T, Kohler D, Lehmann R, El KK, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118: 166–175, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Giannattasio S, Gagliardi S, Samaja M, Marra E. Simultaneous determination of purine nucleotides, their metabolites and beta-nicotinamide adenine dinucleotide in cerebellar granule cells by ion-pair high performance liquid chromatography. Brain Res 10: 168–174, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Hearse DJ, Garlick PB, Humphrey SM. Ischemic contracture of the myocardium: mechanisms and prevention. Am J Cardiol 39: 986–993, 1977 [DOI] [PubMed] [Google Scholar]
- 9.Heusch G, Schulz R. Myocardial hibernation. Ital Heart J 3: 282–284, 2002 [PubMed] [Google Scholar]
- 10.Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, Ratcliffe PJ, Pugh CW, Schofield CJ. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem 277: 26351–26355, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Hochachka PW. Molecular mechanisms of defense against oxygen lack. Undersea Biomed Res 16: 375–379, 1989 [PubMed] [Google Scholar]
- 12.Hochachka PW, Dunn JF. Metabolic arrest: the most effective means of protecting tissues against hypoxia. Prog Clin Biol Res 136: 297–309, 1983 [PubMed] [Google Scholar]
- 13.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 16: 1466–1471, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller A, Wright GL. Fabrication of murine ventricular balloons for the langendorff heart preparation. J Biotechnol Biomater 1: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minchenko A, Leshchinsky I, Opentanova I, Sang N, Srinivas V, Armstead V, Caro J. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem 277: 6183–6187, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA., III Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ Res 98: 133–140, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Natarajan R, Salloum FN, Fisher BJ, Ownby ED, Kukreja RC, Fowler AA., III Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am J Physiol Heart Circ Physiol 293: H1571–H1580, 2007 [DOI] [PubMed] [Google Scholar]
- 18.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol 357: 271–296, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA, III, Kukreja RC. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol 289: H542–H548, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3: 187–197, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Pescador N, Villar D, Cifuentes D, Garcia-Rocha M, Ortiz-Barahona A, Vazquez S, Ordonez A, Cuevas Y, Saez-Morales D, Garcia-Bermejo ML, Landazuri MO, Guinovart J, Del PL. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One 5: e9644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philipp S, Cui L, Ludolph B, Kelm M, Schulz R, Cohen MV, Downey JM. Desferoxamine and ethyl-3,4-dihydroxybenzoate protect myocardium by activating NOS and generating mitochondrial ROS. Am J Physiol Heart Circ Physiol 290: H450–H457, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol 59: 47–53, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med 7: 345–350, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 19: 176–182, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL, Koury ST, Nejfelt MK, Gearhart JD, Antonarakis SE. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci USA 88: 8725–8729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sridharan V, Guichard J, Bailey RM, Kasiganesan H, Beeson C, Wright GL. The prolyl hydroxylase oxygen-sensing pathway is cytoprotective and allows maintenance of mitochondrial membrane potential during metabolic inhibition. Am J Physiol Cell Physiol 292: C719–C728, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sridharan V, Guichard J, Li CY, Muise-Helmericks R, Beeson CC, Wright GL. O2-sensing signal cascade: clamping of O2 respiration, reduced ATP utilization, and inducible fumarate respiration. Am J Physiol Cell Physiol 295: C29–C37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright G, Higgin JJ, Raines RT, Steenbergen C, Murphy E. Activation of the prolyl hydroxylase oxygen-sensor results in induction of GLUT1, heme oxygenase-1, and nitric-oxide synthase proteins and confers protection from metabolic inhibition to cardiomyocytes. J Biol Chem 278: 20235–20239, 2003 [DOI] [PubMed] [Google Scholar]