Abstract
Ventricular fibrillation (VF) in the globally ischemic heart is characterized by a progressive electrical depression manifested as a decline in the VF excitation rate (VFR) and loss of excitability, which occur first in the subepicardium (Epi) and spread to the subendocardium (Endo). Early electrical failure is detrimental to successful defibrillation and resuscitation during cardiac arrest. Hyperkalemia and/or the activation of ATP-sensitive K+ (KATP) channels have been implicated in electrical failure, but the role of these factors in ischemic VF is poorly understood. We determined the VFR-extracellular K+ concentration ([K+]o) relationship in the Endo and Epi of the left ventricle during VF in globally ischemic hearts (Isch group) and normoxic hearts subjected to hyperkalemia (HighK group) or a combination of hyperkalemia and the KATP channel opener cromakalim (HighK-Crom group). In the Isch group, Endo and Epi values of [K+]o and VFR were compared in the early (0–6 min), middle (7–13 min), and late (14–20 min) phases of ischemic VF. A significant transmural gradient in VFR (Endo > Epi) was observed in all three phases, whereas a significant transmural gradient in [K+]o (Epi > Endo) occurred only in the late phase of ischemic VF. In the Isch group, the VFR decrease and inexcitability started to occur at much lower [K+]o than in the HighK group, especially in the Epi. Combining KATP activation with hyperkalemia only shifted the VFR-[K+]o curve upward (an effect opposite to real ischemia) without changing the [K+]o threshold for asystole. We conclude that hyperkalemia and/or KATP activation cannot adequately explain the heterogeneous electrical depression and electrical failure during ischemic VF.
Keywords: extracellular potassium accumulation, myocardial ischemia, ventricular fibrillation, ATP-sensitive potassium channel, asystole
heterogeneous electrical depression and the eventual loss of excitability occurring in the course of cardiac arrest are major adverse outcomes determining the incidence of reperfusion arrhythmias (1) and asystole, with the latter usually being the point of ultimate failure in resuscitation practice (10). In the ex vivo blood-perfused canine heart, simulated cardiac arrest produced by a combination of ventricular fibrillation (VF) and global ischemia causes a prominent transmural gradient in the VF rate (VFR) followed by the emergence of inexcitable areas in the epicardium (Epi), which spread across the ventricular wall and culminate in global asystole within a clinically relevant timeframe (32, 34). The transmural VFR gradient is also present in explanted human hearts subjected to global ischemia and VF (9). Given the similarity between the canine heart and human heart, understanding the mechanisms of heterogeneous electrical depression in the canine model may help to improve the outcomes of resuscitation from cardiac arrest in patients, based on mechanistic knowledge.
Electrical depression during ischemia without VF has been studied for many years, and the classical teaching posits that the main factor determining excitability during ischemia is the elevation of the extracellular K+ concentration ([K+]o; hyperkalemia), which leads to depolarization of the resting potential and a subsequent reduction in the availability of Na+ channels for activation (30). Moreover, because Na+ channel inactivation occurs at more negative potentials in the Epi than in the endocardium (Endo), even a transmurally uniform increase in [K+]o can bring about a selective depression of Epi action potentials and transmural frequency-dependent conduction block (6). Thus, assuming that the primary sources of high-frequency activation during ischemic VF are harbored by Endo Purkinje fibers (8), global uniform hyperkalemia may be sufficient to explain the VFR gradient during ischemic VF. However, this possibility has not been tested in experiments. The second factor implicated in the loss of excitability during ischemia is activation of ATP-sensitive K+ (KATP) channels, which, in theory, can oppose sarcolemmal depolarization caused by the opening of Na+ channels to the degree at which spread of excitation becomes impossible, leading to so-called “sink block” (1). Moreover, the two factors (hyperkalemia and KATP activation) in theory can be synergistic, such that in the presence of KATP activation, conduction block would occur at lower [K+]o than hyperkalemia alone (30). To the best of our knowledge, however, such synergism has not been tested in experimental studies.
Cardiac arrest secondary to VF presents a more complex context than ischemia alone because the high excitation rate of VF aggravates metabolic challenge by increasing energy demand (16) and can potentially contribute to ionic imbalances that develop in the course of ischemia. For example, it was shown that the high excitation rate present during atrial fibrillation causes a moderate but detectable elevation of [K+]o even in the presence of coronary circulation (19). It is thus possible that VF in globally ischemic hearts would aggravate hyperkalemia, but the time course of [K+]o elevation in ischemic VF has not yet been reported.
The relationship between ionic alterations caused by ischemia and the excitation rate of VF is complex and remains controversial. The VF cycle length, the inverse of VFR, is correlated with the duration of the refractory period, which, in turn, is determined by the sum of the action potential duration and the duration of the period after repolarization during which Na+ channels recover from inactivation. The latter period, termed postrepolarization refractoriness, is extremely short under normal conditions but becomes a prominent component of the refractory period and a major determinant of VFR during ischemia (15, 34). Hyperkalemia causes a decrease in VFR (4, 17, 26), most likely due to an increase in postrepolarization refractoriness (26). In the rabbit heart, a moderate increase in [K+]o (to 8 mM) appeared to be sufficient to reproduce the decrease in VFR caused by low-flow ischemia (4); however, the actual [K+]o during ischemia was not measured, and the case of no-flow ischemia was not tested in that study.
If, indeed, hyperkalemia were sufficient for determining VFR during ischemic VF, this would leave no role for the direct effect of increased KATP current. However, blockade of KATP channels has been shown to modulate VFR during ischemic VF in the canine heart (32) and failing human heart (9). Of interest, the effects of the KATP blocker glybenclamide on ischemic VFR were somewhat opposite in the two abovementioned studies. In explanted failing human hearts, glybenclamide eliminated the transmural VFR gradient and facilitated the termination of ischemic VF (9). In contrast, in the ex vivo canine heart, glybenclamide had little effect on the transmural VFR gradient but postponed the termination of ischemic VF (32). The disparate outcomes of KATP blockade in these studies could be related to differences in the (unknown) ischemic level of [K+]o, if the assumption of synergism between KATP activation and hyperkalemia with regard to the reduction of excitability (30) is correct. It also cannot be excluded that the outcomes were confounded by the off-target effects of glybenclamide, although many of the possible nonspecific effects were ruled out in the canine model (32). Thus, the role of KATP activation in VFR dynamics and loss of excitability during ischemic VF remains controversial.
This study was designed to test the hypothesis that hyperkalemia and/or KATP activation can explain the transmural VFR gradient and loss of excitability observed during VF and no-flow ischemia in the ex vivo blood-perfused canine heart. The results of our study showed that even though a transmural [K+]o gradient is present during ischemic VF, hyperkalemia alone cannot reproduce the VFR suppression observed during ischemic VF, especially in the Epi. Moreover, pharmacological KATP activation alone or in combination with hyperkalemia produced an effect opposite to that of real ischemia: a VFR increase instead of a VFR decrease. These results suggest the presence of yet unknown but powerful ionic mechanisms of electrical depression during ischemic VF not mediated by hyperkalemia or KATP activation. Possible additional/alternative mechanisms are discussed.
METHODS
This investigation conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and was approved by the Institutional Animal Care and Use Committee of the University of Utah (Protocol No. 10–09005).
Experimental protocol.
A total of 36 dogs (12 mongrel dogs and 24 purpose-bred dogs, weight: 24.6 ± 0.7 kg, 20 male and 16 female) were euthanized for this study. Animals were premedicated with acepromazine (0.4 mg/kg), propofol (8 mg/kg), or Telazol (9 mg/kg), and a deep plane of anesthesia was maintained with pentobarbital in all dogs (initial dose: 50 mg/kg, additional doses as needed to maintain deep anesthesia for the duration of surgery, <1 h). Hearts were exposed via a midline sternotomy and rapidly excised for perfusion on a Langendorff apparatus with a mixture of heparinized blood and Tyrode solution as previously described (12, 37). After isolation, hearts were placed in either a vapor chamber or immersed in Tyrode superfusion to maintain an Epi temperature of 37 ± 1°C. Twenty-six hearts were subjected to 20 min of ischemic VF by inducing VF with a 9-V battery 10 s before the onset of global ischemia initiated by the interruption of aortic perfusion (Isch group). In these hearts, [K+]o increased naturally during ischemia and was measured using K+-sensitive electrodes (see below). In the remaining 10 hearts, VF was induced the same way, and normal oxygenated perfusion was maintained in the fibrillating heart while [K+]o was varied between 4 and 15 mM by altering [K+] in the perfusate. In five of these hearts, hyperkalemia was the only factor tested (HighK group). In the remaining five hearts, 20–34 μM cromakalim (a KATP channel opener) was administered before [K+]o was increased (HighK-Crom group). In the HighK and HighK-Crom groups, [K+] was increased stepwise by adding 1–3 ml of 600 mM KCl solution at a time to the recirculating blood-Tyrode mixture while the resulting [K+] in the perfusate was measured every 2–3 min using a NOVA 8 ion analyzer (Nova Biomedical, Waltham, MA). A VFR measurement was accepted for analysis if it was bracketed by two venous [K+]o measurements within 0.5 mM of each other, and the average of the two [K+]o values was used for analysis.
K+-sensitive electrodes and [K+]o measurements.
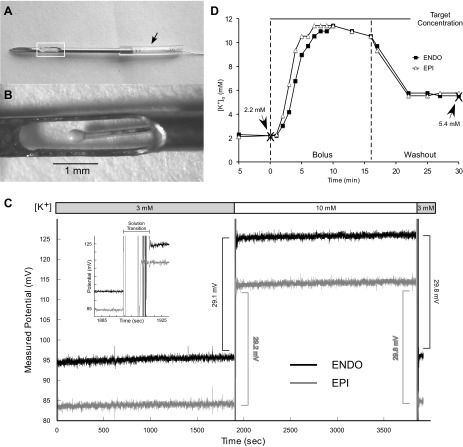
K+-sensitive electrodes were prepared following as close as possible the process described by Johnson et al. (13). However, the electrodes manufactured in this fashion turned out to be extremely fragile, with many not surviving the experimental protocol. To improve our success rate, the prepared electrodes were then housed in a 20-gauge hypodermic needle with an aperture exposing the sensing part of the K+-sensitive electrode to the interstitial fluid (Fig. 1, A and B). The depth of the K+-sensitive electrode could be adjusted by sliding the thin polymer tubing (arrow in Fig. 1A) along the shaft of the needle. The assembled K+-sensitive electrode and housing are referred to as “K+ needle” below.
Fig. 1.
Custom-made K+-sensitive electrodes. A: K+-sensitive electrodes were enclosed in a 20-gauge hypodermic needle that contained a window (white box) that exposed the K+ sensor to the myocardial tissue. The polymer tubing (arrow) could be moved along the shaft of the needle to adjust the position of the K+ sensor at the desired distance from the epicardial (Epi) surface. B: close up of the sensing part of the K+ needle indicated by the white box in A. This design provided both adjustable depth and protection for the delicate sensor. C: example of calibration of endocardial (Endo) and Epi K+ needles. Note that both electrodes showed stable measurements over 30-min periods in both 3 and 10 mM KCl solution with an ∼30-mV shift between solutions. The inset shows the rapid (in the order of seconds) response of the K+ needles to the transition from 3 to 10 mM solution. D: response of Endo (squares) and Epi (triangles) K+ needles placed in a heart to a bolus of 600 mM KCl solution added to the recirculating blood-Tyrode mixture. The indicated target concentration was calculated based on the estimated dilution factor. The two vertical dashed lines indicate the timing of bolus injection and (partial) washout. Note that during steady-state conditions both before and after the KCl bolus, there was a close match between extracellular K+ concentration ([K+]o) values obtained by K+ needles and those obtained using the blood ion analyzer (large Xs).
K+ needles were calibrated both before and after the experiment using 3 and 10 mM KCl solution; any electrode that showed a voltage shift outside the range of 30 ± 4 mV before or after the experiment was excluded as damaged, and the data collected was discarded. Figure 1C shows an example calibration of Endo and Epi K+ needles. Note that both electrodes showed stable measurements over 30-min periods in both 3 and 10 mM KCl solution with an ∼30-mV shift between solutions. The inset in Fig. 1C shows the rapid (in the order of seconds) response of the K+ needles to the transition from 3 to 10 mM solution. Millivolt values were converted to millimolar values using the Nernst equation [for details of the calibration, see Johnson et al. (13)]. Since the calibration constant could be slightly different pre- and postexperiment, we used the average of the pre- and postexperimental calibration values for conversion purposes. In addition, the response of K+ needles placed in hearts was tested by adding a bolus of 600 mM KCl solution to the recirculating blood-Tyrode mixture. Figure 1D shows an example of recordings from Endo and Epi K+ needles (squares and triangles, respectively) verified by measurements using a blood ion analyzer (large Xs). The initial value of [K+]o in the perfusate was 2.2 mM. The indicated target concentration was calculated based on the estimated dilution factor. The two vertical dashed lines indicate the timing of bolus injection and washout. Washout was performed by replacing ∼700 ml of the K+-enriched perfusate with an equivalent amount of a surplus blood-Tyrode mixture. Since all of the blood with elevated [K+] could not be removed (due to the limited volume of the blood-Tyrode mixture), the final [K+] remained elevated (5.4 mM) compared with before the K+ bolus (2.2 mM). Note, however, that during steady-state conditions both before and after the K+ bolus there was a close match between [K+] values obtained by K+ needles and the blood ion analyzer.
K+ needles were placed in the anterior left ventricular (LV) free wall. Epi K+ needles were manufactured to have the sensor reliably submerged into the tissue, and the actual depth of the Epi [K+]o measurement was ∼3 mm from the epicardial surface. The depth of the Endo [K+]o measurement could not be determined at the moment of the needle insertion due to the trabeculated structure of the LV Endo (in our estimates, the thickness of the LV wall varied between 5 mm at the thinnest part and 20 mm at locations where the papillary muscles were attached to the LV free wall). Thus, it was not possible to have a fixed depth of the sensing aperture in Endo K+ needles. To address the irregular thickness of the LV free wall and to make sure that the sensing portion of the K+ needle was located close to the Endo surface, we used two complementary approaches. First, we performed “pacing threshold mapping” using one of the multicontact plunge needle electrodes used for recording unipolar electrogams across the LV wall (see below). In essence, we applied pacing stimuli to consecutive pairs of adjacent leads (starting from the most Endo leads: 1 and 2, 2 and 3, etc.) and measured the end-diastolic threshold for excitation. Based on the observation that Endo pacing thresholds were typically the lowest across the LV wall and very high in the LV cavity (not shown), the pacing cathode location yielding the lowest excitation threshold between 5 and 16 mm from the Epi surface was considered to be the best estimate for the Endo location. With this estimate, the depth of the sensing window on the Endo K+ needle was adjusted by sliding the Epi cuff (see arrow in Fig. 1A) along the shaft of the needle, and the needle was inserted within 2 mm of the multicontact electrode used for the pacing mapping. Second, to obtain the actual position of sensors in Endo K+ needles, we exposed the LV Endo after finishing our experimental protocol and visually determined the distance of the sensing portion of the K+ needles from the Endo. The accepted Endo [K+]o measurements were collected at a distance of 1–6 mm from the Endo surface. One to three Epi K+ needles and one to three Endo K+ needles were used in each experiment, with the goal of having at least one measurement from both the Endo and Epi after discarding any electrodes not meeting our requirements for calibration and the intramural position of the sensor. In those experiments in which two or (rarely) three acceptable K+ recordings were obtained either from the Epi or Endo, the data from these individual measurements were averaged to represent the respective layer in that particular experiment.
Electrical recordings and analysis.
Plunge needle electrodes with 10 evenly spaced unipolar leads (interlead distance: 1.6 mm) were manufactured in house following the design developed by Rogers et al. (27). Ten needles were placed across the anterior surface of the heart, and three needles in close proximity to the K+ needles were chosen for analysis. The distance between needles was 10–15 mm. Unipolar electrograms from all contacts of the needle electrodes were recorded continuously during the first 20 min of ischemic VF at a sampling rate of 1 kHz using a custom-made multichannel data-acquisition system (31). Unipolar electrograms were analyzed using custom software developed in the Matlab (Mathworks, Natick, MA) framework. Electrograms were first filtered using a 60-Hz notch filter including several shorter harmonics. We applied the Hilbert transform to unipolar electrograms as previously described (18, 23, 32). Briefly, the Hilbert transform converts a fluctuating voltage signal into its corresponding phase so that consecutive cycles of activation are represented by changes in phase from −2π to 2π. Thus the number of phase transitions from 2π to −2π gives an estimate of frequency. To decrease the influence of noise, activation cycles with a length of <33 ms and amplitude of the waveform between two consecutive phase transitions of <10% of the electrogram amplitude measured at 0 min of ischemic VF were excluded from the total cycle counts. After that, VFR was calculated as the average number of activations per second over 4-s intervals taken at 4 s after ischemic VF induction and at minutes 1–20 of ischemic VF. For simplicity, the first time point is referred to as 0 min of ischemic VF. Local tissue was considered inexcitable if VFR was 0 Hz (no detectable activations). The percentage of Endo or Epi leads that were inexcitable was determined at each time point of ischemia.
Whereas the length of the plunge needle electrodes was constant and designed to span the largest thickness observed in the LV of the canine heart, as previously mentioned the actual thickness of the ventricular wall is highly nonuniform. Thus, in some cases, plunge needle electrodes were longer than the wall thickness at the site of insertion. In the beginning of this study, we used our elaborate set of criteria to exclude the contacts protruding from the ventricular wall based on the analysis of the electrograms that we previously described (34). However, in the vast majority of experiments, we determined the relationship between plunge needle length and LV wall thickness by visual inspection and wall thickness measurements after opening the LV cavity at the end of the experiments, simultaneous with determining the depth of sensor location in Endo K+ needles.
Plots of VFR and percentage of inexcitable sites versus [K+]o.
VFR and the percentage of inexcitable sites in the Endo and Epi were plotted against measured [K+]o for all three experimental groups (Isch, HighK, and HighK-Crom groups). Data were grouped into increments of 1 mM [K+]o for comparison. In some experiments from the HighK and HighK-Crom groups, [K+]o measurements were sparse and were apart by >1 mM. In these cases, VFR and the percentage of inexcitable sites for intermediate [K+]o values were linearly interpolated. In addition, in some early experiments from the HighK and HighK-Crom groups when full asystole was observed at [K+]o between 10 and 15 mM, higher levels of [K+]o were not tested. In those cases, we assumed that VFR would remain at 0 Hz and that the percentage of inexcitable sites would remain at 100%, for all [K+]o above the highest [K+]o for which asystole was actually observed.
Statistical analysis.
All curves were compared using two-way ANOVA. The time course of VFR and [K+]o during ischemic VF was compared separately for three phases: early (0–6 min), middle (7–13 min), and late (14–20 min) ischemia. Data are given as means ± SE. Differences of P < 0.05 were considered statistically significant.
RESULTS
Time course of [K+]o, VFR, and the occurrence of inexcitability during ischemic VF.
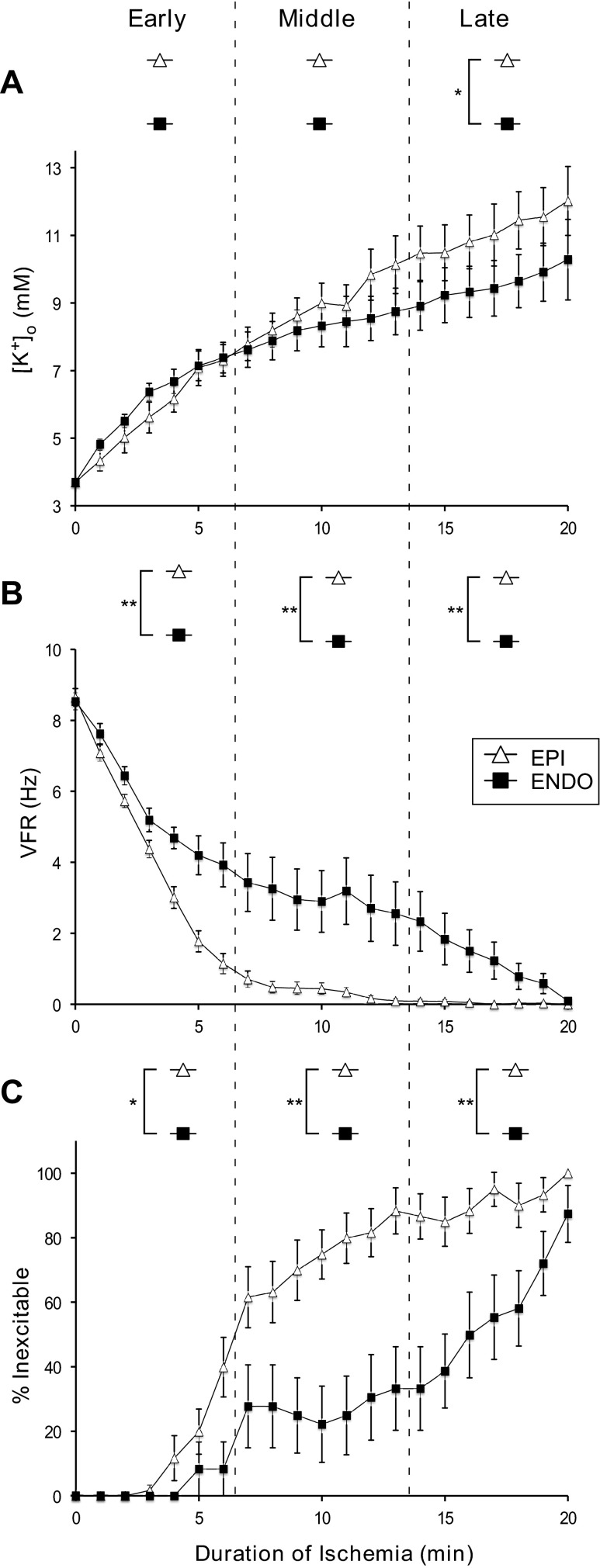
Figure 2A shows the time course of [K+]o elevation in the Endo and Epi during ischemic VF. Extracellular K+ accumulation started immediately upon the onset of ischemic VF and monotonically increased over the entire 20-min ischemic interval. The total increase (Δ[K+]o) was greater in the Epi than in the Endo (8.3 vs. 6.6 mM). However, the Epi-to-Endo difference was statistically significant only during the late phase of ischemic VF (14–20 min). This relationship persisted when the Endo [K+]o measurements used for analysis were limited only to those collected within 0–3 mm from the Endo surface (not shown). The transmural difference in VFR (Fig. 2B) was much more pronounced than the difference in [K+]o and was statistically significant throughout all three phases of ischemic VF, consistent with previous studies (8, 34, 36). When measurements with VFR = 0 were excluded from analysis, the transmural VFR difference was overall similar and remained statistically significant throughout all three phases of ischemic VF (not shown). Figure 2C shows the percentage of electrodes with undetectable electrical activity (thus deemed inexcitable according to the formal criterion; see methods) as a function of ischemia duration. As shown in Fig. 2C, the prevalence of inexcitable sites steadily increased during ischemic VF in both the Endo and Epi, but at a much higher rate in the Epi than in the Endo. The transmural difference in this parameter was statistically significant during all three phases of ischemic VF. Note that the largest Endo-to-Epi difference in VFR and excitability was observed during the middle phase of ischemic VF (7–13 min), after which time point these parameters converged, indicating nearly complete electrical suppression by 20 min of ischemia.
Fig. 2.
Time course of [K+]o (A), ventricular fibrillation (VF) rate (VFR; B), and the percentage of inexcitable sites (%Inexcitable; C) during 20 min of ischemic VF. The open triangles and filled squares represent the Epi and Endo, respectively. The vertical dashed lines denote the early, middle, and late phases of ischemic VF. A: [K+]o progressively increased in both the Epi and Endo throughout 20 min of ischemic VF. Note that the difference between Endo and Epi [K+]o was statistically significant (P < 0.05) only during the late phase of ischemic VF. B: time course of VFR decline. VFR decreased faster in the Epi than in the Endo, creating a prominent and statistically significant Endo-to-Epi difference during all phases of ischemic VF. The magnitude of the VFR gradient eventually decreased, as both Epi and Endo VFR values converged to zero by 20 min of ischemic VF. C: progressive increase in the percentage of inexcitable sites. The Epi showed a more rapid onset and greater extent of local inexcitability compared with the Endo, but both Endo and Epi locations approached 100% inexcitability by the end of 20-min episodes of ischemic VF. The Endo-to-Epi difference in the percentage of inexcitable sites was statistically significant during all phases of ischemic VF. Note the discrepancy between the dynamics of the [K+]o gradient and the gradients of VFR and excitability: the largest transmural difference in the electrical parameters was observed when there was no transmural difference in [K+]o, and vice versa. *P < 0.05; **P < 0.0001.
Relationship between [K+]o and VFR under various experimental conditions.
To assess the role of hyperkalemia in the progressive VFR decline and eventual inexcitability during ischemic VF, we compared the relationship between VFR and [K+]o during ischemic VF (Isch group), normoxic VF in the presence of hyperkalemia (HighK group), and normoxic VF in the presence of hyperkalemia in combination with the KATP channel opener cromakalim (HighK-Crom group). In normoxic experiments, [K+]o was varied between 4 and 15 mM. Note that in ischemic hearts, the elevated K+ concentration was achieved naturally, whereas in all other experiments it was adjusted by adding boluses of KCl to the perfusate (see methods).
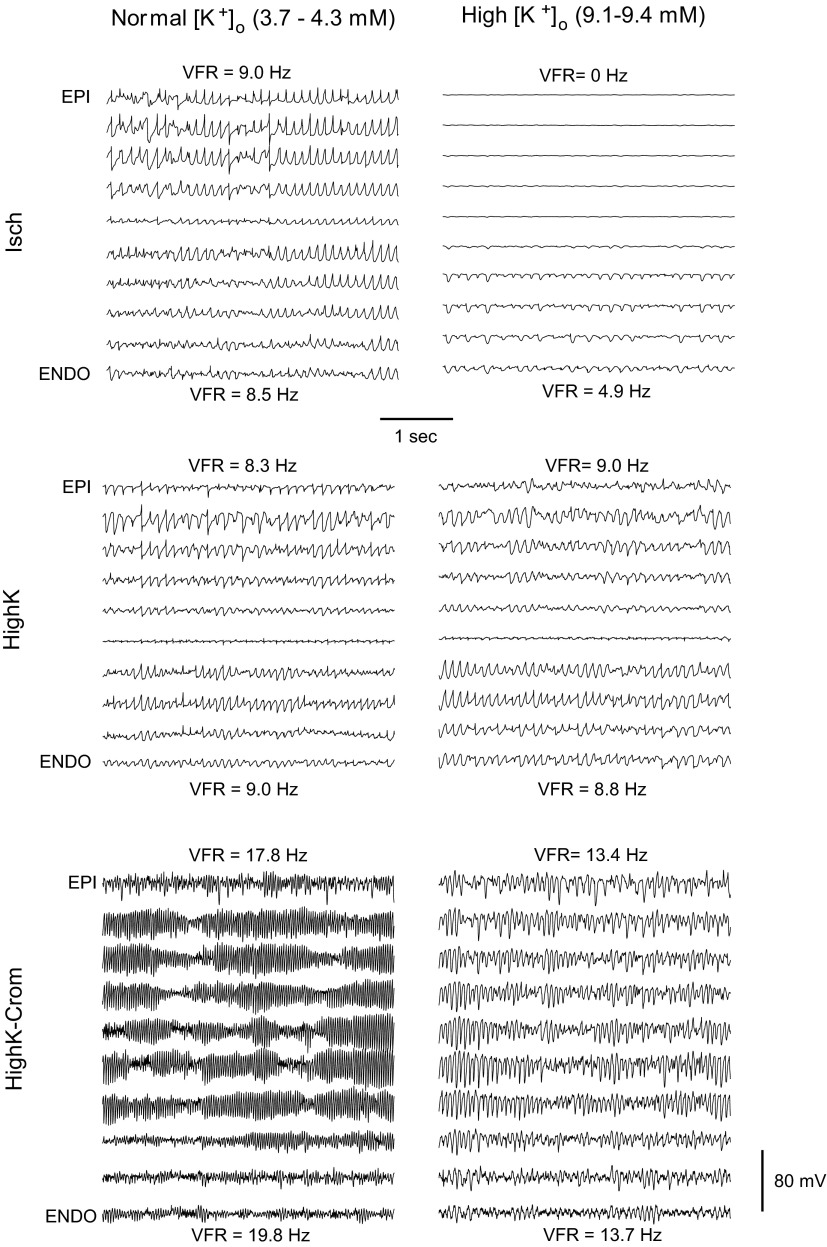
Figure 3 shows examples of transmural unipolar electrograms recorded at approximately normal [K+]o (3.7–4.3 mM; left) and at high [K+]o (9.1–9.4 mM; right) in the three experimental groups (from top to bottom: Isch, HighK, and HighK-Crom groups). Figure 3, top left, shows electrograms obtained at 0 min of ischemic VF when the level of [K+]o was 3.7 mM. Figure 3, top right, shows electrograms obtained at 7 min of ischemic VF when the level of [K+]o was 9.1 mM in the Epi and 9.3 mM in the Endo. As was the typical situation at 0 min and between 5 and 9 min of ischemic VF (see Fig. 2A), in this example, there was no significant difference between [K+]o in the Epi and Endo. As shown in Fig. 3, at 0 min of ischemia and [K+]o = 3.7 mM, there was no perceptible difference in VFR between the Epi and Endo. In contrast, at 7 min of ischemia and [K+]o ∼ 9 mM, there was a drastic difference in VFR between the Epi and Endo, despite the relative uniformity of [K+]o. In fact, the outer half of the LV wall did not have detectable activations at all (VFR = 0).
Fig. 3.
Representative transmural unipolar electrograms from globally ischemic hearts (Isch group), normoxic hearts subjected to hyperkalemia (HighK group), and normoxic hearts subjected to a combination of hyperkalemia and the ATP-sensitive K+ (KATP) channel opener cromakalim (HighK-Crom group) (top to bottom) at approximately normal [K+]o (left) and elevated [K+]o (right). Epi and Endo VFR values are shown next to the respective electrogram recordings. Note that at [K+]o close to normal (3.7–4.3 mM), there were no appreciable transmural VFR gradients in any group, but KATP activation in the HighK-Crom group caused a dramatic increase in VFR (bottom left). At high [K+]o (9.1–9.4 mM), a prominent VFR gradient (with Epi VFR = 0) was observed only in the Isch group (top right) but not in the HighK or HighK-Crom groups. This suggests that the ischemic VFR gradient and regional inexcitability are not caused by hyperkalemia or KATP activation.
Figure 3, middle, shows a representative example of transmural unipolar electrograms from a HighK heart subjected to [K+]o = 3.7 mM (left) and [K+]o = 9.3 mM (right). As shown in Fig. 3, middle, at both levels of [K+]o, VFR was uniform across the ventricular wall and the actual VFR was virtually unaffected by the increase in [K+]o from 3.7 to 9.3 mM. Figure 3, bottom, shows a representative example of transmural unipolar electrograms from a HighK-Crom heart subjected to [K+]o = 4.3 mM (left) and [K+]o = 9.4 mM (right). As shown in Fig. 3, bottom, activation of KATP channels by cromakalim induced a huge and transmurally uniform increase in VFR compared with the absence of the drug. Note that VFR remained exceedingly high even when [K+]o was raised to 9.4 mM, with no appreciable transmural VFR gradient. Moreover, note the striking contrast between VFR during real ischemia (Fig. 3, top right) and VFR during hyperkalemia in combination with KATP activation (Fig. 3, bottom right), despite the same level of [K+]o.
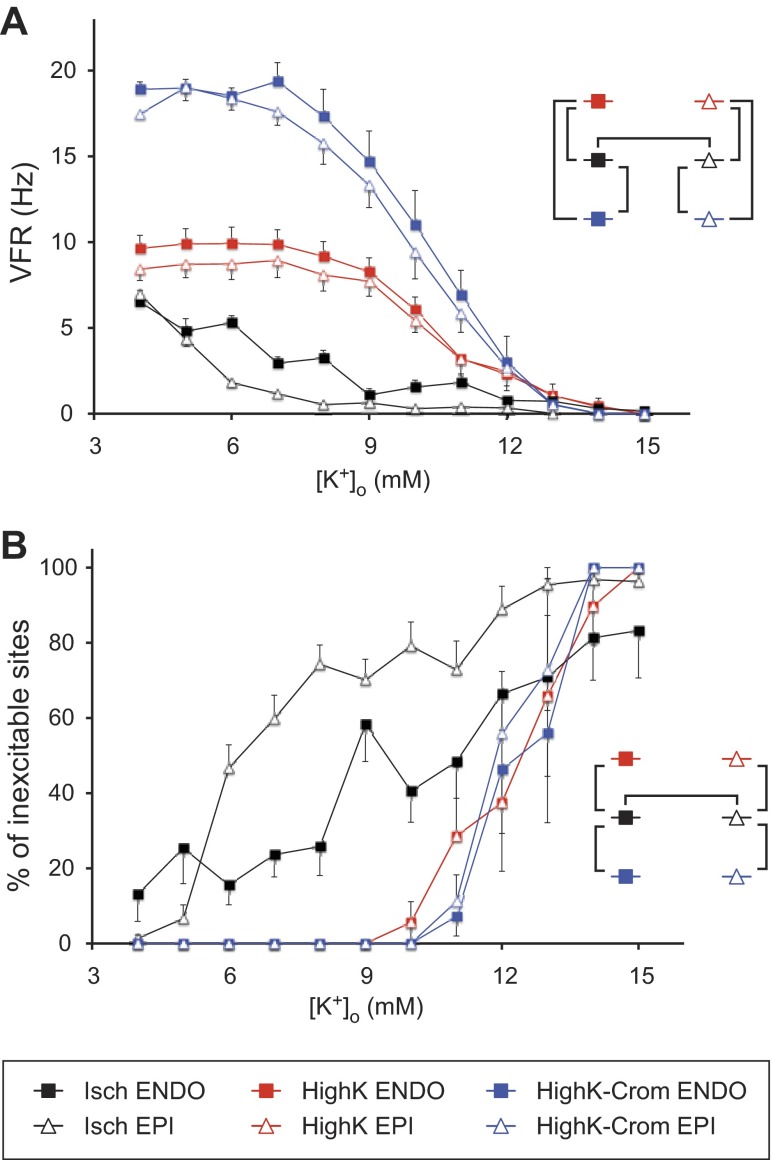
Figure 4A shows the statistical summary of the VFR-[K+]o relationship in all three experimental groups. The different colors represent the different experimental groups; open triangles and solid squares represent Epi VFR and Endo VFR, respectively. The diagram shown in the inset in Fig. 4A shows relevant pairwise comparisons. The vertical brackets indicate statistically significant difference between the same layer in different experimental groups; the horizontal brackets indicate statistically significant difference between the Endo and Epi in the same experimental group. As shown in Fig. 4, in the HighK group (red), an appreciable decrease in VFR in both the Endo and Epi occurred only at [K+]o exceeding 8 mM and VFR approached zero at [K+]o between 12 and 14 mM. Even though Endo VFR appeared to be slightly higher than Epi VFR at each tested [K+]o, the difference was not statistically significant. In the Isch group (black in Fig. 4), the relationship between [K+]o and VFR was profoundly different from that in the HighK group. First, at each [K+]o, VFR in the Isch group was lower than the respective VFR in the HighK group. Second, in the Isch group, there was also a large and significant difference in the sensitivity of VFR to [K+]o between the Epi and Endo. Note that the average Epi VFR was much lower than the Endo VFR in the range of [K+]o between 6 and 9 mM. Also, uniquely among all groups, the average Epi VFR in the Isch group was very slow (<1 Hz) starting from a concentration of [K+]o equal to 8 mM. This made a stark contrast with the HighK group, in which at 8 mM [K+]o, Epi VFR was barely different from that observed at normal [K+]o.
Fig. 4.
Statistical analysis of VFR (A) and the percentage of inexcitable sites (B) as functions of [K+]o in the Isch, HighK, and HighK-Crom groups (black, red, and blue, respectively). In each group, the Epi and Endo are represented by open triangles and filled squares, respectively. The insets in both A and B indicate relevant pairwise comparisons that yielded statistically significant difference (P < 0.05). The vertical brackets indicate differences between experimental groups in a given myocardial layer; the horizontal brackets indicate differences between the Endo and Epi in the same experimental group. A: VFR as the function of [K+]o. VFR in the Isch group was lower, whereas VFR in the HighK-Crom was higher, than VFR in the HighK group at all [K+]o between 4 and 11 mM. Transmural differences in VFR were statistically significant only in the Isch group. Note that hyperkalemia combined with KATP activation did not reproduce the [K+]o-VFR relationship observed during ischemia. B: percentage of inexcitable sites as a function of [K+]o. Note the difference between the Isch group and the two normoxic groups (HighK and HighK-Crom groups). In the Isch group, inexcitable sites appeared at relatively low [K+]o, and there was a large difference in the percentage of inexcitable sites between the Epi and Endo at intermediate levels of [K+]o. In contrast, both the HighK and HighK-Crom groups started to exhibit a loss of excitability only at [K+]o above 10 mM, and there was no transmural difference in the percentage of inexcitable sites. Note that the upper limit of excitability in all experimental groups and myocardial layers was at [K+]o ∼14–15 mM, a level consistent with the inactivation of fast Na+ channels, which are necessary to maintain propagation of cardiac impulse (30).
The combination of hyperkalemia and KATP activation by cromakalim (HighK-Crom group; blue in Fig. 4) poorly reproduced the VFR-[K+]o relationship observed during ischemia. KATP activation caused a massive increase in VFR, which was maintained at all [K+]o between 4 and 11 mM, in contrast to VFR slowing observed in the Isch group. Even though in the HighK-Crom group there was a trend of slower VFR in the Epi than in the Endo, especially at [K+]o between 7 and 8 mM, the transmural VFR difference was not statistically significant.
Figure 4B shows the statistical summary of the relationship between [K+]o and the percentage of inexcitable LV locations (VFR = 0) in all tested groups. Note that this information is somewhat related to the relationship between [K+]o and VFR shown in Fig. 4A, especially given the fact that sites with VFR = 0 were included in the average VFR values shown in Fig. 4A. Yet, the results shown in Fig. 4B underscore the uniqueness of the ischemic data, especially in the Epi, in that during ischemic VF loss of excitability occurred at very modest levels of [K+]o. Note that in the Epi, almost 50% of recorded locations were inexcitable at [K+]o as low as 6 mM. In contrast, both HighK and HighK-Crom groups showed a significant loss of excitability only at [K+]o above 12 mM, thus approaching the canonical level of K+-induced inexcitability (30). It is of interest that KATP activation did not enhance the loss of excitability in the presence of hyperkalemia, despite the theoretical possibility of a synergistic relationship between these two factors with respect to electrical depression (30).
DISCUSSION
VF is a frequent cause of sudden cardiac arrest and, in combination with ensuing global myocardial ischemia, determines the context of cardiopulmonary resuscitation and other life-saving procedures. VF evolving in globally ischemic hearts (sometimes termed long-duration VF) is a highly dynamic process featuring progressive transmural dispersion of VFR (8, 34, 36), which, at least in part, reflects the transmurally heterogeneous increase in postrepolarization refractoriness (15, 34). This increase is unbounded and culminates in a complete loss of excitability first in the LV Epi and subsequently in the rest of the ventricles (32, 34). Total loss of excitability (asystole) is in many cases the early point of no return after out-of-hospital cardiac arrest. Regional inexcitability can contribute to postreperfusion lethal arrhythmias (1), which is a major complication of cardiac arrest in the aftermath of successful defibrillation (33).
Despite the obvious significance of progressive electrical depression in the course of ischemic VF, little is known about the major determinants of this phenomenon. Shaw and Rudy (30) used a numeric model to analyze the relative roles of hyperkalemia, acidosis, and KATP activation in conduction failure during ischemia. In this study, elevation of [K+]o to ∼14 mM was fully sufficient to cause conduction block. Acidosis did not have a significant additional effect, but a large degree of KATP activation (20% channel availability assuming an intracellular ATP concentration of 0.5 mM) lowered the threshold [K+]o necessary to cause conduction block from 14 to 10 mM. From this, it would be reasonable to assume that elevated [K+]o and a massive activation of KATP channels are major determinants of electrical failure during ischemic VF.
Hyperkalemia.
To the best of our knowledge, our study is the first to measure the progressive accumulation of [K+]o during ischemic VF. We found a larger K+ efflux in the Epi than in the Endo, but the difference was significant only in the late phase of ischemic VF (14–20 min). We can compare these results only to previous studies performed in nonfibrillating hearts. In an in situ canine model of regional ischemia (20, 21), there was no transmural difference in [K+]o, and the total K+ accumulation in the Epi and Endo during 20 min of left anterior descending coronary artery (LAD) occlusion was similar to that observed in the Endo after 20 min of ischemic VF in our study (∼9–10 mM). In another study (7) using isolated blood-perfused canine hearts, [K+]o was measured in the regionally ischemic myocardium at a depth of 5 mm from the Epi surface during pacing at a normal heart rate. In that study, at the end of an 8-min LAD occlusion period, the level of [K+]o in the ischemic zone reached ∼8 mM, which was very close to both Endo and Epi values of [K+]o observed at the same duration of ischemia in our study (see Fig. 2B). Those authors also measured the incidence of inexcitability as the percentage of unipolar electrograms exhibiting “monophasic” morphology and found that after 8 min of ischemia, only ∼25% of recording sites were inexcitable (see Fig. 5A in Ref. 7). In our case, the degree of inexcitability was drastically different between the Endo and Epi. Whereas in the Endo the percentage of inexcitable sites at 8 min of ischemia (28%) was similar to that observed by Coronel et al. (7) in the midmyocardium, in the Epi at the same time point of ischemia, it was much larger (63%). In stark contrast to ischemic conditions, raising [K+]o to the level of 8 mM during normal perfusion in our study never caused inexcitability in any layer of the LV wall, and even the effect with respect to VFR was minimal (see Fig. 4A). These results disagree with those obtained during ischemic VF in the isolated rabbit heart, where an elevation of [K+]o to 8 mM in normoxemic hearts reproduced the decrease in VFR observed during ischemia (4). Apart from the differences in species, another important difference between the rabbit study and our study is the degree of ischemia, which was complete (no flow) in our experiments and was only partial (remaining flow at 15% of normal) in the study by Caldwell et al. (4).
Because Na+ channel inactivation occurs at more negative potentials in the Epi than in the Endo, even a transmurally uniform increase in [K+]o can cause differential electrical depression in the Epi and Endo, leading, in particular, to the inability of the Epi to follow a rapid rate of excitation waves coming from the Endo (6). If this effect were significant in our study, we would expect that uniform hyperkalemia due to an elevation of [K+]o in the perfusate would induce the transmural difference in VFR and/or the percentage of inexcitable sites. In fact, neither of these occurred in our study. Hyperkalemia in normoxemic hearts caused a transmurally uniform VFR decline at [K+]o above 8 mM, culminating in a transmurally uniform loss of excitability when [K+]o exceeded 12–14 mM. Thus, it is unlikely that the differential properties of Na+ channel inactivation (6) contribute significantly to the transmural differences in VFR and excitability observed during ischemic VF.
KATP channels.
The timing, conditions, and electrophysiological consequences of KATP activation during real ischemia in intact hearts remain as controversial now as three decades ago, when the channel was first discovered (25). While the principal role of KATP channels in ischemic action potential duration shortening appears to be undisputable, its potential contribution to decreased membrane excitability and conduction failure during ischemia is far less clear. In their theoretical analysis of factors influencing conduction failure during early ischemia, Shaw and Rudy (30) concluded that the contribution of KATP activation to conduction failure is limited unless the degree of activation of this channel is very large, which would require intracellular ATP concentration to reach very low (submillimolar) levels, much below what is typically observed within the first 10–15 min of ischemia. However, since it is not possible to directly measure activation of KATP channels in intact tissues during ischemia, and because the regulation of this channel is dependent on a number of metabolites besides ATP, the possibility of any degree of KATP activation during early ischemia remains open. Akar et al. (1) asserted the role of KATP channel opening as the primary mechanism of ischemic inexcitability by an experimental demonstration of “metabolic sink block” resulting from KATP activation secondary to mitochondrial depolarization (1). Noteworthy, in isolated ventricular cardiomyocytes, complete loss of excitability can occur without any increase in [K+]o, provided that the KATP channel is sufficiently activated in response to mitochondrial depolarization (1, 22).
Since hyperkalemia alone failed to reproduce the [K+]o-VFR relationship observed during ischemic VF, we tested the possibility that in the presence of a strong activation of KATP channels, the [K+]o-VFR relationship observed during normoxic VF would approach more closely the relationship observed during ischemic VF. However, this was not the case. The KATP opener cromakalim caused a dramatic increase in VFR in the range of [K+]o between 5 and 10 mM, which was opposite to the effect of ischemia. Also, cromakalim did not decrease the critical [K+]o at which excitability is lost (it remained in the range of 12–14 mM). The concentration of cromakalim used in this study is expected to activate ∼50% of the channels (24), which exceeds even what Shaw and Rudy (30) considered to be a result of extreme anoxia (20% of channels available). Yet even such an extreme level of KATP activation failed to cause VFR suppression or loss of excitability up to [K+]o of 12 mM, the concentration at which ∼40% of the Endo locations and ∼80% of the Epi locations exhibited loss of activity during ischemic VF (see Fig. 4B). These observations speak against the principal role of KATP activation in electrical depression during ischemic VF, somewhat contradicting the concept of metabolic sink block (1) and our own previous finding that the blockade of KATP channels by glybenclamide slightly postponed VFR decline and the emergence of local and global inexcitability during ischemic VF (32). To reconcile these apparent discrepancies, we suggest that KATP activation does contribute to electrical depression during ischemic VF, but perhaps only in the presence of other ischemic factors yet to be established.
Possible additional factors.
The Na+-activated K+ (KNa) channel could theoretically contribute to electrical depression in the course of ischemic VF. This channel has conductance even larger than that of KATP channels (35), and, thus, the consequences of its activation during ischemia could be similar to those of KATP activation [i.e., sink block (1)]. The effects of KATP and KNa activation could be synergistic with respect to electrical depression. Even though the available experimental evidence speaks against functional expression of KNa channels in the sarcolemma of canine ventricular myocytes (28), one cannot exclude that the lack of functioning KNa channels in single canine myocytes is a consequence of the cell isolation procedure.
As Shaw and Rudy (29) pointed out, the ability of KATP channels to oppose the excitatory Na+ current depends very much on the driving force for K+ at the foot of the action potential, in other words, on the deviation of the resting potential (Vrest) from the K+ equilibrium potential (EK). Assuming, as they did, that during ischemia Vrest is close to EK (and therefore the magnitude of the KATP current to oppose the Na+ current is small), the effect of KATP activation on excitability should be limited. The difference between Vrest and EK depends on the magnitude of inward leak currents present during diastole. Assuming that the ischemic K+ leak is due to an increased conductance through K+-selective channels (currently the most accepted theory), Carmeliet (5) postulated the necessity of a substantial inward leak to explain the presence of significant rate-independent K+ efflux, observed even after the cessation of electrical activity (14). Note that in our experiments, Epi [K+]o continued to rise throughout 20 min of ischemia, even though the average Epi VFR was already close to zero at 10 min of ischemia (see Fig. 2). Whereas the Endo had a much higher VFR, the level of K+ accumulation was lower in the Endo than in the Epi, clearly indicating that the frequency of excitations did not influence the intensity of K+ efflux.
The extent to which Vrest during ischemia can deviate from EK remains controversial. Kleber (14) showed that these two parameters, separated by 7–8 mV under normal conditions, converge to within 1 mV after 6–7 min of ischemia. However, an intriguing study by Blake et al. (3) suggests that in the ischemic canine heart, the relationship between Vrest and EK is frequency dependent, such that in the presence of an increased excitation rate (180 vs. 90 beats/min), depolarization of Vrest significantly exceeds that predicted by the ischemic level of EK. Clearly, these conditions are relevant to the case of ischemic VF, where the excitation rate is initially in excess of 300–400 beats/min. Those authors showed that a Ca2+ infusion had a similar effect and that the two factors combined enhanced deviation of Vrest from EK during ischemia. This suggests a possibility that the effect of rapid activations on Vrest during ischemia is mediated via an attendant increase in the intracellular Ca2+ concentration. The depolarizing effects of metabolic inhibition on Vrest were observed in spontaneously beating chick embryo myocytes (11) and adult rat ventricular myocytes subjected to cyanide (2) but not in adult mammalian ventricular myocytes subjected to a mitochondrial uncoupler (1, 22). The mechanisms of depolarizing effects of metabolic stress that are not related to hyperkalemia remain largely unknown and warrant further studies.
Conclusions.
This is the first study to measure [K+]o accumulation during ischemic VF. Comparison with published data obtained in the same species suggests that the presence of VF during ischemia has little impact on the rate of K+ efflux compared with ischemia alone. Moreover, the higher level of [K+]o in the sub-Epi than in the sub-Endo, despite the opposite distribution of the VFR, further supports the relative independence of ischemic K+ efflux from excitation rate.
This study tested, and rejected, the hypothesis that the VFR decline and loss of excitability during ischemic VF in the canine heart can be explained in terms of “canonical” factors of electrical depression, hyperkalemia and KATP activation, by showing a great disparity in the relationship between [K+]o and VFR in the absence of ischemia (with or without a KATP agonist), on the one hand, and the presence of ischemia, on the other hand. The alternative mechanisms of electrical depression during ischemic VF remain wide open for speculation but may include enhancement of inward leak currents, which could promote sarcolemmal depolarization beyond the level induced by extracellular K+ accumulation.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants 5-R01-HL-088444 and 1-RO1-HL-103877 (to A. V. Zaitsev) and 1-F32-HL-097576 (to J. Shibayama) and by a Nora Eccles Treadwell Foundation Research Grant (to A. V. Zaitsev).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.G.T., A.B., and A.V.Z. conception and design of research; T.G.T., P.W.V., A.B., V.G., J.S., and A.V.Z. performed experiments; T.G.T., P.W.V., A.B., J.S., and A.V.Z. analyzed data; T.G.T. and A.V.Z. interpreted results of experiments; T.G.T. and A.V.Z. prepared figures; T.G.T. and A.V.Z. drafted manuscript; T.G.T., P.W.V., A.B., V.G., J.S., and A.V.Z. edited and revised manuscript; T.G.T., P.W.V., A.B., V.G., J.S., and A.V.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jayne H. Davis and Nancy C. Allen for the excellent technical support and Curtis Booth for proofreading the manuscript and providing suggestions in English language and style.
REFERENCES
- 1.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115: 3527–3535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baczkó I, Giles WR, Light PE. Resting membrane potential regulates Na+-Ca2+ exchange-mediated Ca2+ overload during hypoxia-reoxygenation in rat ventricular myocytes. J Physiol 550: 889–898, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake K, Clusin WT, Franz MR, Smith NA. Mechanism of depolarization in the ischaemic dog heart: discrepancy between T-Q potentials and potassium accumulation. J Physiol 397: 307–330, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell JANE, Burton FL, Smith GL, Cobbe SM. Heterogeneity of ventricular fibrillation dominant frequency during global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol 18: 854–861, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet E. Cardiac ionic currents and acute ischemia: From channels to arrhythmias. Physiol Rev 79: 917–1017, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Cordeiro JM, Mazza M, Goodrow R, Ulahannan N, Antzelevitch C, Di Diego JM. Functionally distinct sodium channels in ventricular epicardial and endocardial cells contribute to a greater sensitivity of the epicardium to electrical depression. Am J Physiol Heart Circ Physiol 295: H154–H162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronel R, Fiolet JW, Wilms-Schopman JG, Opthof T, Schaapherder AF, Janse MJ. Distribution of extracellular potassium and electrophysiologic changes during two-stage coronary ligation in the isolated, perfused canine heart. Circulation 80: 165–177, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Dosdall DJ, Tabereaux PB, Kim JJ, Walcott GP, Rogers JM, Killingsworth CR, Huang J, Robertson PG, Smith WM, Ideker RE. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol 295: H883–H889, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farid TA, Nair K, Masse S, Azam MA, Maguy A, Lai PF, Umapathy K, Dorian P, Chauhan V, Varro A, Al-Hesayen A, Waxman M, Nattel S, Nanthakumar K. Role of KATP channels in the maintenance of ventricular fibrillation in cardiomyopathic human hearts. Circ Res 109: 1309–1318, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Hallstrom A, Herlitz J, Kajino K, Olasveengen TM. Treatment of asystole and PEA. Resuscitation 80: 975–976, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Hasin Y, Barry WH. Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Physiol Heart Circ Physiol 247: H322–H329, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Huizar JF, Warren MD, Shvedko AG, Kalifa J, Moreno J, Mironov S, Jalife J, Zaitsev AV. Three distinct phases of VF during global ischemia in the isolated blood-perfused pig heart. Am J Physiol Heart Circ Physiol 293: H1617–H1628, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Johnson TA, Engle CL, Kusy RP, Knisley SB, Graebner CA, Gettes LS. Fabrication, evaluation, and use of extracellular K+ and H+ ion-selective electrodes. Am J Physiol Heart Circ Physiol 258: H1224–H1231, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Kléber AG. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res 52: 442–450, 1983 [DOI] [PubMed] [Google Scholar]
- 15.Kong W, Ideker RE, Fast VG. Transmural optical measurements of Vm dynamics during long-duration ventricular fibrillation in canine hearts. Heart Rhythm 6: 796–802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusuoka H, Chacko VP, Marban E. Myocardial energetics during ventricular fibrillation investigated by magnetization transfer nuclear magnetic resonance spectroscopy [Erratum. Circ Res 72(1): 237, 1993]. Circ Res 71: 1111–1122, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Mandapati R, Asano Y, Baxter WT, Gray RA, Davidenko JM, Jalife J. Quantification of effects of global ischemia on dynamics of ventricular fibrillation in isolated rabbit heart. Circulation 98: 1688–1696, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Masse S, Downar E, Chauhan V, Sevaptsidis E, Nanthakumar K. Ventricular fibrillation in myopathic human hearts: mechanistic insights from in vivo global endocardial and epicardial mapping. Am J Physiol Heart Circ Physiol 292: H2589–H2597, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Miyata A, Dowell JD, Zipes DP, Rubart M. Rate-dependent [K+]o accumulation in canine right atria in vivo: electrophysiological consequences. Am J Physiol Heart Circ Physiol 283: H506–H517, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Miyoshi S, Miyazaki T, Moritani K, Ogawa S. Different responses of epicardium and endocardium to KATP channel modulators during regional ischemia. Am J Physiol Heart Circ Physiol 271: H140–H147, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi S, Miyazakt T, Asanagi M, Moritani K, Ogawa S. Differential role of epicardial and endocardial KATP channels in potassium accumulation during regional ischemia induced by embolization of a coronary artery with latex. J Cardiovasc Electrophysiol 9: 292–298, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Morley GE, Anumonwo JM, Delmar M. Effects of 2,4-dinitrophenol or low [ATP]i on cell excitability and action potential propagation in guinea pig ventricular myocytes. Circ Res 71: 821–830, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, Paterson DJ, Taggart P. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 114: 536–542, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Ng B, Kang Y, Xie H, Sun H, Gaisano HY. Syntaxin-1A inhibition of P-1075, cromakalim, and diazoxide actions on mouse cardiac ATP-sensitive potassium channel. Cardiovasc Res 80: 365–374, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983 [DOI] [PubMed] [Google Scholar]
- 26.Pandit SV, Warren M, Mironov S, Tolkacheva EG, Kalifa J, Berenfeld O, Jalife J. Mechanisms underlying the antifibrillatory action of hyperkalemia in guinea pig hearts. Biophys J 98: 2091–2101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers JM, Melnick SB, Huang J. Fiberglass needle electrodes for transmural cardiac mapping. IEEE Trans Biomed Eng 49: 1639–1641, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Saxena NC, Fan JS, Tseng GN. Effects of elevating [Na]i on membrane currents of canine ventricular myocytes: role of intracellular Ca ions. Cardiovasc Res 33: 548–560, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Cardiovasc Res 35: 256–272, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia. A mechanistic investigation of action potential conduction and conduction failure. Circ Res 80: 124–138, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Taccardi B, Punske BB, Macchi E, Macleod RS, Ershler PR. Epicardial and intramural excitation during ventricular pacing: effect of myocardial structure. Am J Physiol Heart Circ Physiol 294: H1753–H1766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor TG, Venable PW, Shibayama J, Warren M, Zaitsev AV. Role of KATP channel in electrical depression and asystole during long-duration ventricular fibrillation in ex vivo canine heart. Am J Physiol Heart Circ Physiol 302: H2396–H2409, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Alem AP, Post J, Koster RW. VF recurrence: characteristics and patient outcome in out-of-hospital cardiac arrest. Resuscitation 59: 181–188, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Venable PW, Taylor TG, Shibayama J, Warren M, Zaitsev AV. Complex structure of electrophysiological gradients emerging during long-duration ventricular fibrillation in the canine heart. Am J Physiol Heart Circ Physiol 299: H1405–H1418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Kimitsuki T, Noma A. Conductance properties of the Na+-activated K+ channel in guinea-pig ventricular cells. J Physiol 433: 241–257, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worley SJ, Swain JL, Colavita PG, Smith WM, Ideker RE. Development of an endocardial-epicardial gradient of activation rate during electrically induced sustained ventricular fibrillation in dogs. Am J Cardiol 1985: 813–820, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Zaitsev AV, Guha PK, Sarmast F, Kolli A, Berenfeld O, Pertsov AM, de Groot JR, Coronel R, Jalife J. Wavebreak formation during ventricular fibrillation in the isolated, regionally ischemic pig heart. Circ Res 92: 546–553, 2003 [DOI] [PubMed] [Google Scholar]