Abstract
Melanocortin receptors (MCRs) are present in the intermediolateral cell column of the spinal cord (IML). We tested the hypothesis that activation of MCRs in the IML elicits cardioacceleratory responses and the source of melanocortins in the IML may be the melanocortin-containing neurons in the hypothalamic arcuate nucleus (ARCN). Experiments were done in urethane-anesthetized, artificially ventilated adult male Wistar rats. Microinjections (50 nl) of α-melanocyte stimulating hormone (α-MSH) (0.4–2 mM) and adrenocorticotropic hormone (ACTH) (0.5–2 mM) into the right IML elicited increases in heart rate (HR). These tachycardic responses were blocked by microinjections of melanocortin receptor 4 (MC4R) antagonists [SHU9119 (0.25 mM) or agouti-related protein (AGRP, 0.1 mM)] into the right IML. Stimulation of right ARCN by microinjections (30 nl) of N-methyl-d-aspartic acid (NMDA, 10 mM) elicited increases in HR. Blockade of MC4Rs in the ipsilateral IML at T1–T3 using SHU9119 (0.25 mM) attenuated the tachycardic responses elicited by subsequent microinjections of NMDA into the ipsilateral ARCN. ARCN neurons retrogradely labeled by microinjections of Fluoro-Gold into the right IML showed immunoreactivity for proopiomelanocortin (POMC), α-MSH, and ACTH. Fibers immunoreactive for POMC, α-MSH, and ACTH were present in the IML at T1-T3. These results indicated that activation of MC4Rs in the right IML elicited tachycardia and one of the sources of melanocortins in the IML is the ARCN. Melanocortin levels are elevated in stress and ARCN neurons are activated during stress. Our results allude to the possibility that cardiac effects of stress may be mediated via melanocortin containing ARCN neurons that project to the IML.
Keywords: arcuate nucleus, heart rate, melanocortin receptors, tachycardia
the melanocortins consist of a family of peptides derived from proopiomelanocortin (POMC) (1, 7, 28). Endogenous peptides that belong to this family include α-melanocyte stimulating hormone (MSH), β-MSH, γ-MSH, adrenocorticotropic hormone (ACTH), and β-endorphin (7). Neuronal networks immunoreactive for POMC or the peptides derived from it have been identified in the medullo-spinal neuronal groups that are involved in the regulation of cardiovascular function (22). POMC gene is expressed in neurons of the hypothalamic arcuate nucleus (ARCN) and the nucleus tractus solitarius (NTS).
The effects of melanocortins are mediated via G-protein coupled melanocortin receptors (MCRs) (35). Five MCRs (MCR1–MCR5) have been identified (1, 7, 11). Of these MCRs, only MCR4 and to a lesser extent MCR3 have been identified in the central nervous system (CNS). The CNS structures where MCR3 and MCR4 are present include cortex, limbic system, thalamus, hypothalamus, midbrain, brain stem, and spinal cord (1, 34).
We have previously reported that activation of sympathetic preganglionic neurons (SPGNs) located in the intermediolateral cell column of the spinal cord (IML) at T1–T3 elicited tachycardia and maximum responses were elicited at T2 level (30–32). Presence of MC4Rs has been reported in the IML (17, 18). Although it is known that an excitatory amino acid mediates cardiac actions elicited from the IML at T1–T3 (33), other neurotransmitters may also play a role in mediating cardiac actions in some situations.
Melanocortin levels have been reported to be elevated in stressful situations such as cardiac arrest (36). Stress is one of the risk factors for cardiovascular disease. It is possible that increases in heart rate (HR) in stressful situations may be mediated via MCRs in the IML. Activation of neurons in the ARCN has been implicated in stressful situations (21). The presence of POMC containing neurons has been demonstrated in the ARCN (9, 16, 20, 24). Based on this information, first we tested the hypothesis that activation of MCRs in the IML elicits cardioacceleratory responses. There are no reports in the literature on the role of MCRs in the IML in mediating cardiac responses. α-MSH and ACTH were selected for activation of MCRs because the levels of these peptides are elevated in stress (36), and we have previously reported responses elicited by these two melanocortins in other cardiovascular regulatory areas (5, 6, 14). There are no reports in the literature regarding the cardiovascular effects of ACTH and α-MSH in the IML. We also tested the hypothesis that cardioacceleratory responses elicited from the ARCN are attenuated by blockade of MCRs in the IML. The two hypotheses were tested in our rat model in which microinjections of l-glutamate (l-Glu) or other excitatory agents (e.g., cholinergic receptor agonists) into the right IML at T1–T3 level elicited tachycardic responses accompanied by no changes in blood pressure (BP).
METHODS
General procedures.
Experiments were done in 67 adult male Wistar rats (Charles River Laboratories, Wilmington, MA), weighing 300–350 g. All animals were housed under controlled conditions with a 12:12-h light/dark cycle. Food and water were allowed to the animals ad libitum. The experimental procedures were performed in accordance with the National Institutes of Health Guidelines for Research Involving Animals. All protocols were approved by the Institutional Animal Care and Use Committee at Rutgers, New Jersey Medical School, Newark, NJ. The number of animals used was the minimum required for statistical analyses of the data.
Anesthesia was induced by administration of isoflurane (3% in 100% oxygen), the trachea was cannulated with polyethylene tubing (PE 240), the rats were artificially ventilated using a rodent ventilator (model 683, Harvard Instruments, Holliston, MA, USA) and the end-tidal CO2 was maintained at 3.5–4.5%. Both femoral veins were cannulated with polyethylene tubing (PE 50). Urethane (1.2–1.4 g/kg) was injected into one of the femoral veins in eight to nine aliquots at 2-min intervals. The tracheal administration of isoflurane was discontinued as soon as the administration of urethane was completed. To maintain the BP at or near normal levels, normal saline (0.9%) was continuously infused through another femoral vein (2.3 ml/h) using a syringe pump (model 341, SAGE Instruments, Cambridge, MA). The animal remained artificially ventilated with room air throughout the experiment. For monitoring the BP, a cannula (PE 50) was placed in one of the femoral arteries and connected to a pressure transducer (P23 XL-1, Argon Medical Devices, Clearwater, FL). The pulsatile arterial pressure (PAP) signal was amplified (model 1902, Cambridge Electronic Design, Cambridge, UK) (CED), digitized (model micro-1401, CED), and processed using Spike 2 software (CED). Mean arterial pressure (MAP) and HR were derived from PAP using Spike 2 software. The absence of a pressor response and the lack of withdrawal of the hindlimb in response to a strong pinch indicated that the rats were properly anesthetized. Rectal temperature was maintained at 37 ± 0.5°C using an infrared lamp connected to a temperature controller (model TCAT-2AC, Physitemp Instruments, Clifton, NJ). All tracings were stored on a computer hard drive.
Microinjections into the IML.
The rats were placed in a prone position in a stereotaxic instrument (model 1430, David Kopf Instruments, Tajunga, CA) with a rat spinal unit attachment (model 980, David Kopf Instruments), and the dorsal surface of the spinal cord from eighth cervical to third thoracic level was exposed (31). Microinjections into the right IML were made using four barreled glass micropipettes (tip size 20–40 μm) connected to a picospritzer (General Valve, Fairfield, NJ). The right IML site was identified by microinjections of l-Glu (5 mM). The volume of all microinjections into the right IML was 50 nl. The duration of microinjection was 10 s. The coordinates for the right IML at T1–T3 were as follows: 0.8–1 mm lateral to the midline and 0.7–0.9 mm deep from the dorsal surface of the spinal cord. Controls for microinjections consisted of artificial cerebrospinal fluid (aCSF). The micropipettes always included one barrel for l-Glu, one for aCSF, and one for green retrobeads IX. The contents of other barrels varied according to the requirements of the experiment being conducted. All of the solutions for the microinjections were freshly prepared in aCSF.
Microinjections into the ARCN.
In some experiments, the right ARCN was stimulated by microinjections of N-methyl-d-aspartic acid (NMDA, 10 mM), and an MCR antagonist (SHU9119, 0.25 mM) was microinjected into the right IML at T1–T3 in the same animal. The volume of all microinjections into the ARCN was 30 nl, and the duration of microinjection was 10 s. The coordinates for the ARCN were as follows: 3 mm caudal to the bregma, 0.2–0.4 mm lateral to the midline, and 9.4–9.8 mm deep from the dura.
Histological identification of microinjection sites.
The microinjection sites in the right IML and ARCN were marked by a microinjection of diluted (1:50) green retrobeads IX (Lumafluor, Durham, NC). The animals were perfused with arterial administration of heparinized normal saline followed by 2% formalin, and the spinal cord and brain were removed and fixed in 2% formalin for 72 h. The tissues were fixed in 30% sucrose for another 72 h. After the fixation procedure was completed, sections were cut (30 μm) in a cryostat (Leica CM1900, Leica Microsystems, Bannockburn, IL). The microinjection sites were identified under a microscope (model AX70, Olympus Provis, Middlebush, NJ). The sections were photographed (Neurolucida software, version 7.5, MicroBrightField, Williston, VT) and compared with a standard atlas (23).
Retrograde tracing of ARCN projection to the IML and immunohistochemistry.
The rats were anesthetized with pentobarbital sodium (50 mg/kg ip), and 4% diluted Fluoro-Gold (50 nl) was microinjected into the right IML at T1–T3. The wound was closed and the rats were kept alive for a total of 9 days. The rats were again anesthetized with pentobarbital on the seventh day after the surgery, and colchicine (120 μg, 10 μl) was microinjected into the lateral ventricle unilaterally (the coordinates for the lateral ventricle: 0.8–0.9 mm caudal to the bregma, 1.7–1.8 mm lateral to the midline, and 3.8–4.0 mm deep from the dura). An antibiotic (cefazolin, 30 mg/kg, twice a day for 3 days) and an analgesic (one dose of slow-release buprenorphine, 1 mg/kg) were administered subcutaneously. On the ninth day, the femoral vein and artery on one side were cannulated. The animals were then deeply anesthetized with intravenous injections of urethane and perfused with heparinized normal saline which was followed by 2% paraformaldehyde solution containing 0.2% picric acid. The brains and spinal cord were removed and placed in 2% paraformaldehyde containing 0.2% picric acid for 72 h. On completion of the fixation procedure, serial sections of the hypothalamic area were cut (30 μm) in a cryostat (Leica CM1900, Leica Microsystems, Bannockburn, IL). The microinjection site of Fluoro-Gold in the spinal cord and the retrogradely labeled cells in the ARCN were visualized under a microscope (model AX70, Olympus Provis, Middlebush, NJ). The sections were photographed (Neurolucida software, version 7.5, MicroBrightField) and compared with a standard atlas (23).
Some sections containing the ARCN were used for immunostaining of POMC, α-MSH, and ACTH. The sections for immunohistochemistry were rinsed with 0.1 M phosphate-buffered saline (PBS) and blocked for 60 min at room temperature with 10% normal goat serum (NGS) in 0.1 M PBS containing 0.3% Triton X-100 (TPBS). The sections were incubated for 24 h at 4°C with one of the following primary antibodies (each diluted with TPBS containing 3% NGS): rabbit anti-POMC antibody (1:5,000; Phoenix Pharmaceuticals; Burlingame, CA), rabbit anti-ACTH antibody (1:200; Phoenix Pharmaceuticals), or with rabbit anti-α-MSH antibody (1:1,000; Immunostar, Hudson, WI). After rinsing with PBS, the sections were incubated for 2 h at 4°C with Cy3-goat anti-rabbit IgG (1:200, Amax = 550 nm, Emax = 570 nm, Jackson Immuno-Research Laboratories, West Grove, PA; diluted with PBS containing 3% NGS). The sections were rinsed with PBS and incubated for 2 h at 4°C with the same secondary antibody (Cy3-goat anti-rabbit IgG; 1:200). After the completion of incubation with the primary and secondary antibodies in each of these procedures, the sections were rinsed in PBS, mounted on subbed slides, covered with Citifluor mountant medium (Ted Pella, Redding, CA), and coverslipped. The images of the sections were captured, 1 μm apart, using a laser scanning confocal microscope (AIR confocal microscope, Nikon Instruments, Melville, NY).
Statistical analyses.
The means and standard error of mean (SE) were calculated for maximum changes in BP and HR in response to microinjections into the ARCN or IML. One-way analysis of variance (ANOVA) followed by Tukey-Kramer's multiple comparison test was used for comparisons of maximum increase in HR in different groups of rats in dose-response and tachyphylaxis studies. Comparisons of the maximum increases in HR elicited by microinjections of α-MSH, ACTH, and l-Glu into the right IML, before and after the microinjections of SHU9119 or agouti-related protein (AGRP) at the same site, were made by using the Student's paired t-test. Comparisons of the maximum increases in HR elicited by microinjections of NMDA into the ARCN before and after the microinjections of SHU9119 into the ipsilateral IML at T1–T3 were made by using the Student's paired t-test. In all cases, the differences were considered significant at P < 0.05.
Drugs and chemicals used.
α-MSH amide, ACTH (1–39) (guinea pig), SHU9119 [Ac-Nle-cyclo(-Asp-His-d-2-Nal-Arg-Trp-Lys)-NH2, a potent MC3/4 receptor antagonist] (12), AGRP (83–132)-amide (human) (endogenous MC4 receptor antagonist) (25), l-glutamic acid monosodium salt (l-Glu), NMDA, green retrobeads IX, Fluoro-Gold, isoflurane, urethane, pentobarbital sodium, cefazolin and buprenorphine hydrochloride were used. Where applicable, the concentrations of drugs injected into the right IML or ARCN refer to their salts. Sources of the drugs were as follows: α-MSH and ACTH (American Peptide, Sunnyvale, CA); SHU9119 (Tocris Bioscience, Ellisville, MO); AGRP (Phoenix Pharmaceuticals, Belmont, CA); isoflurane (Piramal Critical Care, Bethlehem, PA); pentobarbital (Ovation Pharmaceuticals, Deerfield, IL); cefazolin (West-ward Pharmaceutical, Eatontown, NJ); buprenorphine SR (Hospira, Lake Forest, IL); green retrobeads IX (Lumafluor, Durham, NC); and Fluoro-Gold (Fluorochrome, Denver, CO). All other drugs and chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
RESULTS
In urethane-anesthetized rats, baseline values for MAP and HR were 97.6 ± 2.2 mmHg and 373.2 ± 5.4 beats/min, respectively (n = 60).
l-Glu microinjections into the IML.
Increases in HR elicited by microinjections of l-Glu (5 mM) at T1 and T2 were 21.1 ± 1.6 (n = 25) and 20.8 ± 1.4 (n = 30) beats/min, respectively. There was no significant difference between tachycardic responses to l-Glu elicited at T1 and T2. Therefore, in this and other experiments, the data obtained at T1 and T2 were pooled together. Thus, increase in HR elicited by microinjections of l-Glu at T1-T2 was 20.9 ± 1 beats/min (n = 55). The onset and duration of these tachycardic responses were 1–3 s and 3–6 min, respectively. Tachycardic responses elicited at T3 level were smaller (10.6 ± 1.2 beats/min, n = 4). No BP responses were elicited by microinjections of l-Glu into the right IML at T1–T3.
Concentration response of α-MSH and ACTH.
α-MSH in different concentrations (0.4, 0.8, 1.6, and 2 mM) was microinjected into the right IML at T1–T2 (n = 5 for each concentration). The increases in HR elicited by the aforementioned concentrations of α-MSH at T1–T2 were 6.9 ± 0.5, 13.0 ± 1.2, 13.9 ± 1.1, and 17.0 ± 0.8 beats/min, respectively (Fig. 1A). No changes in BP were elicited by microinjections of α-MSH at T1–T2. Since the responses to 2 mM concentration of α-MSH were greater than other concentrations of α-MSH, this concentration was selected for further studies in other groups of rats.
Fig. 1.
Cardioacceleratory effects of melanocortins in right intermediolateral cell column of the spinal cord (IML) at T1–T2. A: microinjections of 0.4, 0.8, 1.6, and 2.0 mM concentrations of α-melanocyte stimulating hormone (α-MSH) elicited increases in heart rate (HR) (n = 5 for each concentration). *P < 0.05 compared with 0.4 mM α-MSH. B: microinjections of 0.5, 1.0, and 2.0 mM concentrations of adrenocorticotropic hormone (ACTH) elicited increases in HR (n = 5 for each concentration). *P < 0.05 compared with 0.5 mM ACTH. Bars represent means ± SE. The volume of all microinjections into the right IML was 50 nl. bpm, beats/min.
ACTH in different concentrations (0.5, 1, and 2 mM) was microinjected into the right IML at T1–T2 (n = 5 for each concentration). The increases in HR elicited by the aforementioned concentrations of ACTH were 10.2 ± 3.0, 22.5 ± 1.9 and 26.5 ± 3.3 beats/min, respectively (Fig. 1B). No changes in BP were elicited by microinjections of ACTH into the right IML at T1–T2. Since the responses to 1 mM concentration of ACTH were significantly (P < 0.05) greater than 0.5 mM concentration of ACTH and not different from 2 mM concentration of ACTH (P > 0.05), 1 mM concentration of ACTH was selected for other experiments.
No more than three concentrations of α-MSH and ACTH were microinjected randomly into the right IML at T1 and T2 in each rat, and the interval between these microinjections was at least 50 min.
The onset and duration of HR responses to 2 mM concentration of α-MSH were 2–30 s and 16–22 min, respectively. The onset and duration of HR responses to 1 mM concentration of ACTH were 2–12 s and 16–36 min, respectively.
Site specificity of α MSH and ACTH responses.
At T1–T2, microinjections of either l-Glu (5 mM), α-MSH, or ACTH outside IML (e.g., 0.5–0.6 mm lateral to the midline and 1.6–1.8 mm deep from the dorsal spinal surface) did not elicit any cardiovascular response.
Reproducibility of responses.
The increases in HR in response to three microinjections of α-MSH (2 mM each) into the right IML at T1–T2, at 50-min intervals, were 17.1 ± 2.8, 17.9 ± 3.2, and 18.9 ± 2.7 beats/min, respectively (n = 5). In another group of rats (n = 5), the increases in HR in response to three consecutive microinjections of ACTH (1 mM) were 21.2 ± 2.9, 23.6 ± 4.8, and 17.2 ± 1 beats/min, respectively. The differences between the tachycardic responses to repeated microinjections of either α-MSH or ACTH were not statistically significant (P > 0.05).
SHU9119 abolished α-MSH- and ACTH-induced responses.
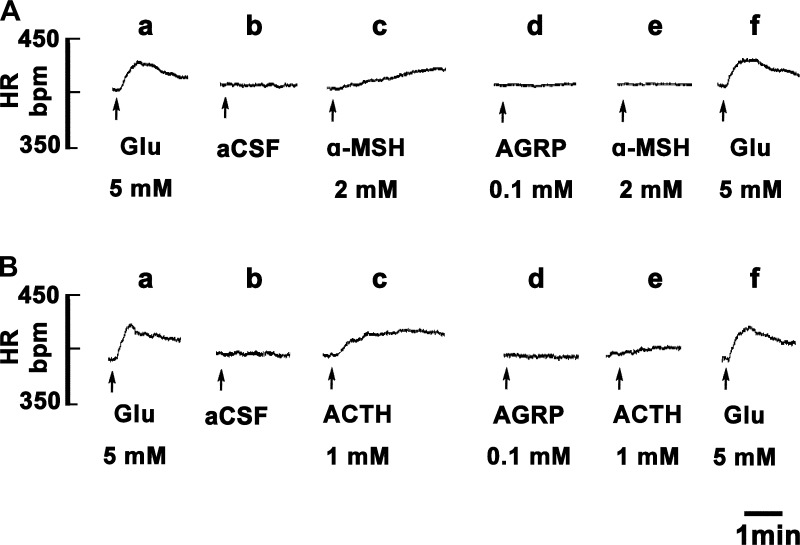
Tracings, obtained in two different rats, showing the effects of an MCR antagonist on responses elicited by α-MSH and ACTH are presented in Fig. 2. The right IML site at T2 was identified by a microinjection of l-Glu (5 mM, Fig. 2, Aa and Ba). Five minutes later, microinjection of aCSF (50 nl) at the same site elicited no responses (Fig. 2, Ab and Bb). After an interval of 2 min, microinjection of α-MSH (2 mM) and ACTH (1 mM) at the same site elicited increases in HR with no accompanying changes in BP (Fig. 2, Ac and Bc). SHU9119 (0.25 mM) was microinjected into the right IML 50 min after the microinjection of either α-MSH (2 mM) or ACTH (1 mM); microinjections of SHU9119 alone did not elicit appreciable changes in HR (Fig. 2, Ad and Bd). Subsequent microinjections (within 2 min) of α-MSH (2 mM) or ACTH (1 mM) failed to elicit any response (Fig. 2, Ae and Be). The lack of responses to α-MSH and ACTH was not due to tachyphylaxis because repeated microinjections of ACTH at 50-min intervals did not exhibit tachyphylaxis as described earlier. SHU9119 (0.25 mM) did not alter the tachycardic responses to microinjections of an unrelated agonist (l-Glu, 5 mM) injected 2 min after α-MSH and ACTH (Fig. 2, Af and Bf).
Fig. 2.
Effect of microinjections of SHU9119 into the IML on melanocortin-induced responses at the same site. A: tracings showing the effects of α-MSH in one rat. Right IML at T2 was identified by microinjection of l-Glu (5 mM); an increase in HR was elicited (a). Five minutes later, microinjection of artificial cerebrospinal fluid (aCSF) elicited no response (b). After 2 min, microinjection of α-MSH (2 mM) elicited a slow increase in HR (c). Fifty minutes later, microinjection of SHU9119 (MC4R antagonist; 0.25 mM) elicited no response (d). α-MSH (2 mM) microinjected within 2 min failed to elicit a response (e). Two minutes later, microinjection of l-Glu (5 mM) continued to elicit tachycardia (f). B: tracings showing the effects of ACTH in another rat. Right IML at T2 was identified by microinjection of l-Glu (a). Five minutes later, microinjection of aCSF elicited no response (b). After 2 min, microinjection of ACTH (1 mM) elicited an increase in HR (c). Fifty minutes later, microinjection of SHU9119 elicited no response (d). ACTH (1 mM) microinjected within 2 min failed to elicit a response (e). Two minutes later, microinjection of l-Glu continued to elicit tachycardia (f). The volume of all microinjections into the right IML was 50 nl. Glu, l-glutamate; SHU, SHU9119.
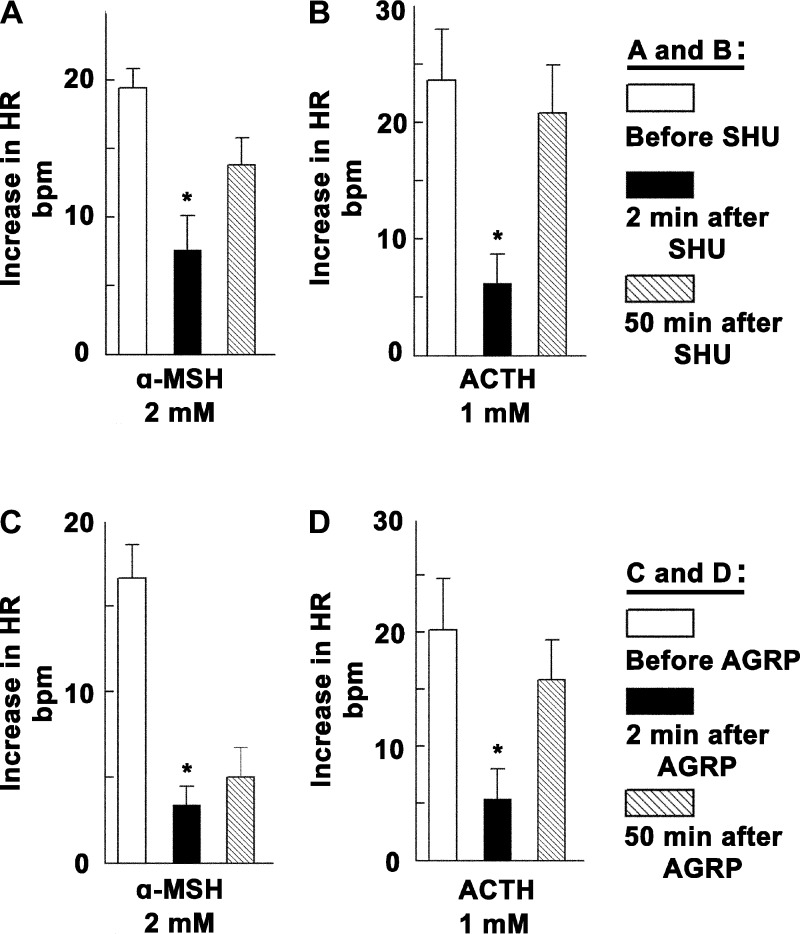
Group data (n = 5) for the blocking effects of SHU9119 (0.25 mM) on α-MSH (2 mM) and ACTH (1 mM) responses are shown in Fig. 3, A and B, respectively. Increases in HR to microinjections of α-MSH (2 mM) before, 2 min, and 50 min after the microinjection of SHU9119 (0.25 mM) were 19.4 ± 1.4, 7.6 ± 2.5, and 13.8 ± 2 beats/min, respectively (n = 5). Increases in HR to microinjections of ACTH (1 mM) before, 2 min, and 50 min after the microinjection of SHU9119 (0.25 mM) were 23.6 ± 4.4, 6.2 ± 2.5, and 20.8 ± 4.1 beats/min, respectively (n = 5). SHU9119 (0.25 mM) significantly (P < 0.05) attenuated tachycardic responses induced by α-MSH (2 mM) and ACTH (1 mM). Microinjections of SHU9119 alone into the right IML did not elicit appreciable changes in HR (0.1 ± 1.1 beats/min, n = 10).
Fig. 3.
Group data showing changes in tachycardic responses to melanocortins after MCR blockade. A: HR responses to microinjections of α-MSH (2 mM) into the right IML at T1–T2 before, 2 min after blockade of MC4Rs at the same site by SHU9119 (SHU; 0.25 mM), and 50 min after blockade of MC4Rs at the same site by SHU9119 (n = 5). B: HR responses to microinjections of ACTH (1 mM) into the right IML at T1–T2 before, 2 min after blockade of MC4Rs at the same site by SHU9119 (0.25 mM), and 50 min after blockade of MC4Rs at the same site by SHU9119 (n = 5). C: HR responses to microinjections of α-MSH (2 mM) into the right IML at T1–T2 before, 2 min after blockade of MC4Rs at the same site by agouti-related protein (AGRP) (0.1 mM), and 50 min after blockade of MC4Rs at the same site by AGRP (n = 5). D: HR responses to microinjections of ACTH (1 mM) into the right IML at T1–T2 before, 2 min after blockade of MC4Rs at the same site by AGRP (0.1 mM), and 50 min after blockade of MC4Rs at the same site by AGRP (n = 5). Open bar = before; black bar = 2 min after either SHU9119 (A and B) or AGRP (C and D); hatched bar = 50 min after either SHU9119 or AGRP. *P < 0.05.
AGRP abolished α-MSH- and ACTH-induced responses.
Tracings showing the effects of AGRP on α-MSH- and ACTH-induced responses in the right IML at T1–T2 are presented in Fig. 4. The right IML site was identified by a microinjection of l-Glu (5 mM, Fig. 4, Aa and Ba). Five minutes later, microinjection of aCSF (50 nl) did not elicit a response (Fig. 4, Ab and Bb). Microinjection of α-MSH (2 mM) and ACTH (1 mM) 2 min later elicited increases in HR with no accompanying changes in BP (Fig. 4, Ac and Bc). Fifty minutes after the microinjection of α-MSH and ACTH, AGRP (0.1 mM) was microinjected at the same site; microinjection of AGRP alone did not elicit appreciable changes in HR (Fig. 4, Ad and Bd). Subsequent microinjection (within 2 min) of α-MSH (2 mM) and ACTH (1 mM) failed to elicit any response (Fig. 4, Ae and Be). AGRP did not alter the tachycardic responses to microinjections of l-Glu (5 mM) microinjected 2 min after α-MSH and ACTH (Fig. 4, Af and Bf).
Fig. 4.
Effect of blockade of MCRs in the IML by AGRP at T1–T2 on melanocortin-induced responses at the same site. A: tracings showing the effects of α-MSH in one rat. Right IML at T2 was identified by microinjection of l-Glu (5 mM); an increase in HR was elicited (a). Five minutes later, microinjection of aCSF elicited no response (b). Two minutes later, microinjection of α-MSH (2 mM) elicited a slow increase in HR (c). Fifty minutes later, microinjection of AGRP (MC4R antagonist; 0.1 mM) elicited no response (d). α-MSH (2 mM) microinjected within 2 min failed to elicit a response (e). Two minutes later, microinjection of l-Glu (5 mM) continued to elicit tachycardia (f). B: tracings showing the effects of ACTH in another rat. Right IML at T2 was identified by microinjection of l-Glu (a). Five minutes later, microinjection of aCSF elicited no response (b). After two minutes, microinjection of ACTH (1 mM) elicited an increase in HR (c). Fifty minutes later, microinjection of AGRP (0.1 mM) elicited no response (d). ACTH (1 mM) microinjected within 2 min failed to elicit a response (e). Two minutes later, microinjection of l-Glu continued to elicit tachycardia (f). Volume of all microinjections into the right IML was 50 nl.
Group data (n = 5) for the blocking effects of AGRP (0.1 mM) on α-MSH (2 mM) and ACTH (1 mM) responses are shown in Fig. 3, C and D, respectively. Increases in HR to microinjections of α-MSH (2 mM) before, 2 min, and 50 min after the microinjection of AGRP (0.1 mM) were 16.6 ± 1.9, 3.4 ± 1, and 5 ± 1.7 beats/min, respectively (n = 5). Increases in HR to microinjections of ACTH (1 mM) before, 2 min, and 50 min after the microinjection of AGRP (0.1 mM) were 20.2 ± 4.5, 5.4 ± 2.6, and 15.8 ± 3.5 beats/min, respectively (n = 5). AGRP (0.1 mM) significantly (P < 0.05) attenuated HR responses induced by α-MSH (2 mM) and ACTH (1 mM). Microinjections of AGRP (0.1 mM) alone into the right IML did not elicit appreciable changes in HR (1.6 ± 1.2 beats/min, n = 10).
Attenuation of tachycardic responses elicited from the ARCN by blockade of MCRs in the IML.
The right ARCN was identified by a microinjection of NMDA (10 mM). Tachycardic responses, which lasted for 8.2–11.8 min, were observed (Fig. 5Aa). In the same rat, 50 min later, microinjection of SHU9119 (0.25 mM, 30 nl) into the right IML at T1–T3 did not elicit any response (Fig. 5Ab). Two minutes later, microinjection of NMDA (10 mM) into the right ARCN elicited a smaller increase in HR (Fig. 5Ac). Fifty minutes later, HR responses to microinjection of NMDA (10 mM) into the right ARCN recovered to the initial level (Fig. 5Ad).
Fig. 5.
Effect of blockade of MCRs in the IML at T1–T3 on tachycardic responses elicited from the ARCN. A: tracings of HR responses in one rat. Microinjection of N-methyl-d-aspartic acid (NMDA; 10 mM; 30 nl) into the right hypothalamic arcuate nucleus (ARCN) elicited an increase in HR (a). Fifty minutes later, microinjections of SHU9119 (0.25 mM) into the right IML (3 microinjections at T1, T2, and T3; 30 nl each) elicited no response (b). After 2 min, microinjection of NMDA (10 mM) into the right ARCN again elicited a smaller increase in HR (c). Fifty minutes later, tachycardic responses to microinjection of NMDA (10 mM) into the right ARCN showed recovery (d). B: group data for the experiment shown in A. HR responses to microinjections of NMDA (10 mM) into the right ARCN before, 2 min after blockade of MC4Rs into the right IML at T1–T3 by SHU9119 (0.25 mM) and 50 min after blockade of MC4Rs at the same site by SHU9119 (n = 5). Open bar = before, black bar = 2 min after SHU9119 microinjections into the right IML, and hatched bar = 50 min after SHU9119 microinjections into the IML. *P < 0.05.
Group data for this experiment are shown in Fig. 5B. Increases in HR to microinjections of NMDA (10 mM) into the right ARCN before, 2 min, and 50 min after the microinjections of SHU9119 (0.25 mM) into the ipsilateral right IML in T1–T3 were 53.4 ± 7.5, 40.6 ± 7.5, and 52 ± 7.7 beats/min, respectively (n = 5). Microinjections of SHU9119 (0.25 mM) into the right IML at T1–T3 significantly (P < 0.05) attenuated tachycardic responses elicited from the ipsilateral ARCN.
Identification of microinjection sites.
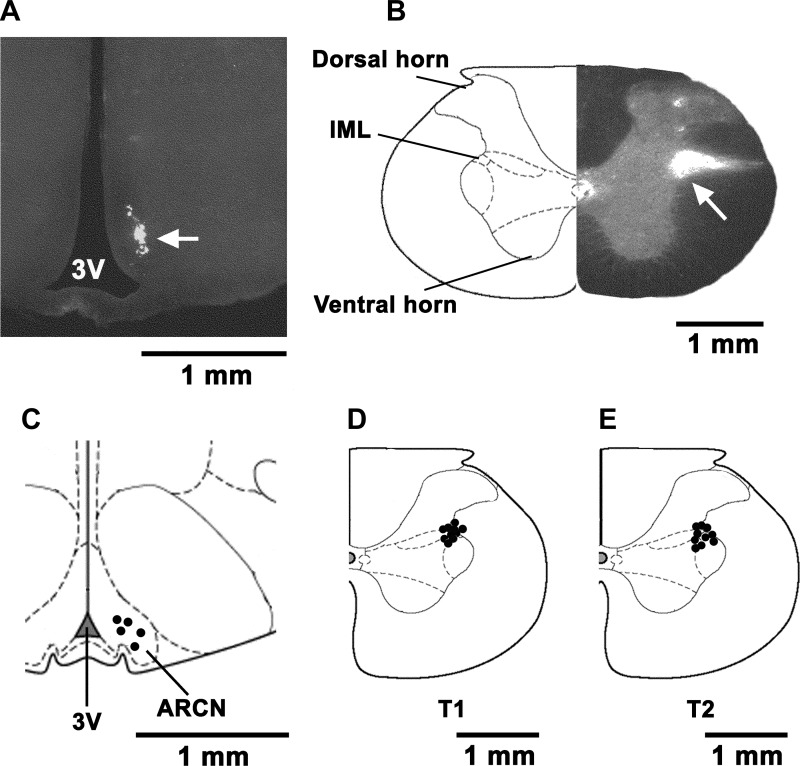
Typical microinjection sites in the ARCN and right IML (marked by diluted green retrobeads IX) are shown in Figs. 6, A and B, respectively. The volumes of green retrobeads IX microinjected into the ARCN and IML were 30 and 50 nl, respectively. Composite diagrams of the microinjection sites in the ARCN (n = 5) and IML at T1 and T2 (n = 10 each) are shown in Fig. 6, C, D, and E, respectively.
Fig. 6.
Histological identification of microinjection sites in the ARCN and IML. A: coronal section at a level 3.0 mm caudal to the bregma showing a microinjection site in the right ARCN which was marked by a microinjection of diluted (1:50) green retrobeads IX containing NMDA (10 mM) (30 nl; arrow). The center of the spot was 0.3 mm lateral to the midline and 9.6 mm deep from the dura. B: coronal section at T2 level of the spinal cord showing a microinjection site in the right IML which was marked by a microinjection of diluted (1:50) green retrobeads IX containing l-Glu (5 mM) (50 nl; arrow). The center of the spot was 1 mm lateral to the midline and 0.9 mm deep from the dura. C: drawing of coronal sections 3.0 mm caudal to the bregma showing the right ARCN microinjection sites as dark spots. Each spot represents a site in one animal (n = 5). The microinjection sites were located in the ARCN, 0.2–0.4 mm lateral to the midline and 9.4–9.8 mm deep from the dura. D and E: drawings of coronal sections at T1 and T2 levels of the spinal cord showing the microinjection sites in the right IML. Each spot represents a site in one animal (n = 5 each). The spots were located in the IML 0.8–1 mm lateral to the midline and 0.7–0.9 mm deep from the dura. 3V, 3rd ventricle.
Immunohistochemistry.
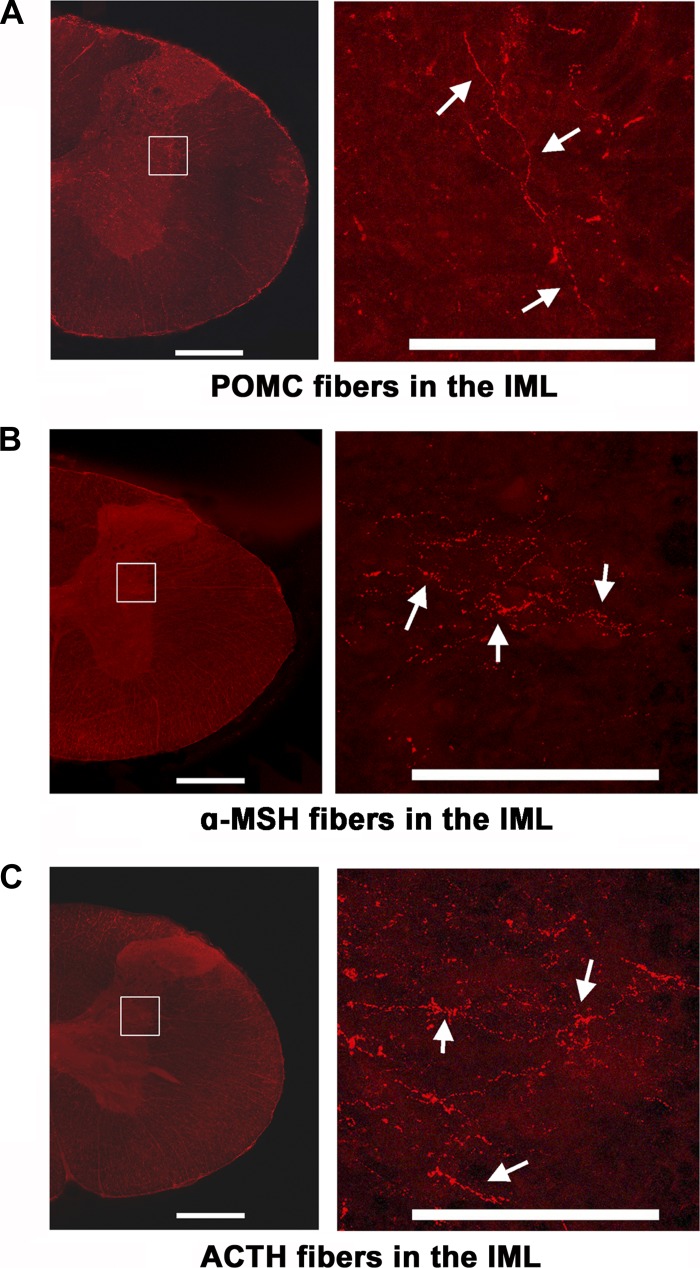
The presence of POMC, α-MSH, and ACTH immunoreactive fibers was demonstrated in the IML of spinal cord sections at T2 level (Fig. 7, A, B, and C, respectively) (n = 4). Microinjections of Fluoro-Gold (FG; 50 nl) into the right IML (Fig. 8A) resulted in retrograde labeling of neurons in the ipsilateral ARCN (Fig. 8, B–D) (n = 3). Numerous POMC, α-MSH, and ACTH immunoreactive neurons were present in the same sections (Fig. 8, E–G). Merging of corresponding sections showed that some neurons retrogradely labeled with FG in the ARCN contained POMC, α-MSH, and ACTH (Fig. 8, H–J, respectively). Sections in which antibodies for POMC, α-MSH, and ACTH were omitted from the protocol did not show any immunoreactivity for these peptides; omission controls for POMC and α-MSH are shown in Fig. 8, K and L, respectively. Sections in which the primary antibody for ACTH was preabsorbed with control peptide did not show immunoreactivity for this peptide (Fig. 8M).
Fig. 7.
Immunoreactive melanocortin fibers in the IML. A–C: coronal sections of the spinal cord at T1 and T2 level are shown in the left panel. The area in the rectangle within each panel is magnified on the right side showing fibers in the IML immunoreactive for proopiomelanocortin (POMC) (A), α-MSH (B), and ACTH (C) (n = 4). Scale bars: left panels, 500 μm; right panels, 200 μm.
Fig. 8.
Retrograde labeling of ARCN neurons after microinjections of a tracer in the IML. Retrograde labeling and immunohistochemistry were done in 3 rats. A: Fluoro-Gold (FG) was microinjected into the right IML at T1–T2. B–D: neurons in the ipsilateral ARCN that were retrogradely labeled with FG. E–G: numerous neurons immunoreactive for POMC (E), α-MSH (F), and ACTH (G) are present in the sections shown in B–D; ARCN neurons retrogradely labeled with FG that were immunoreactive for melanocortin peptides are marked by arrows. H: merged images of B and E showing some retrogradely labeled neurons containing POMC (arrows). I: merged images of C and F showing some retrogradely labeled neurons containing α-MSH (arrows). J: merged images of D and G showing some retrogradely labeled neurons containing ACTH (arrows). K and L: controls in which primary antibodies for POMC (K) and α-MSH (L) were omitted from the immunohistochemical procedure showed no immunoreactivity. M: control in which primary antibody for ACTH was preabsorbed with control peptide showed no immunoreactivity.
DISCUSSION
The main observation in this study is that activation of MCRs in the right IML at T1–T2, by microinjections of α-MSH and ACTH, elicits cardioacceleratory responses. This is the first report of cardiac effects of melanocortins in the IML. We have previously reported that in the rat, SPGNs controlling HR are located in the right IML at T1–T3 (30–33). Melanocortins may excite these SPGNs and elicit tachycardic responses. Consistent with this notion is the report that some melanocortins (e.g., ACTH) depolarize neurons by enhancing voltage-dependent Ca2+ currents via L-type Ca2+ channels (37). Local distortion of IML tissue or any nonspecific effects were not responsible for these observations because microinjections of aCSF into the right IML did not elicit any response.
Cardioacceleration elicited by microinjections of l-Glu, α-MSH, and ACTH into the right IML did not result in increases in BP. This observation is in agreement with our previous reports in which other agents were used to stimulate the IML at T1–T3 (30–33). We have previously reported that increase in myocardial contractility is elicited by stimulation of the left IML, but not the right IML, at T1–T3 (30–33). Therefore, stimulation of the right IML at T1–T3 is not expected to increase stroke volume. The peak increase in HR elicited by microinjections of l-Glu, α-MSH, and ACTH into the IML at T1–T3 lasted for ∼2 min. Therefore, cardiac output may not increase due to lack of increase in stroke volume and short duration of peak increases in HR induced by l-Glu, α-MSH, and ACTH. Consequently, no changes in BP were observed in our present and previous studies (30–33).
Previously we have reported that very high concentrations (0.5–1 M) of l-Glu were needed to stimulate the ARCN in the rat (19). Other authors have also reported that high concentrations of l-Glu are needed to stimulate other hypothalamic areas (e.g., paraventricular nucleus; PVN) (8, 13). The reason for the necessity to use high concentrations of l-Glu to stimulate the ARCN and PVN may be that neurons in these nuclei are tonically inhibited by gamma aminobutyric acid (GABA) (27) or nitric oxide (29). Another possibility is that the injected material in these nuclei is dissipated relatively faster because they are highly vascularized regions of the brain (26). However, much lower concentration of NMDA (10 mM) was needed to stimulate the ARCN (15, 19). Therefore, NMDA was selected to stimulate the ARCN in this study. The concentrations of l-Glu needed to stimulate the IML are relatively small (5 mM); therefore, l-Glu was used to stimulate the IML (31).
The responses of microinjections of α-MSH and ACTH into the right IML at T1–T2 level were site specific. Microinjections of α-MSH and ACTH into an area outside the IML (0.5–0.6 mm lateral to the midline and 1.6–1.8 mm deep from the dorsal spinal surface) did not elicit any cardiovascular response.
The cardiac effects of α-MSH and ACTH elicited by microinjections of α-MSH and ACTH into the IML at T1–T2 were mediated via MC4Rs because prior microinjections of an antagonist for these receptors (SHU9119) at the same site blocked the responses to subsequent microinjections of these peptides at the same site. AGRP has been reported to be the endogenous antagonist of MC4Rs (25). This antagonist also blocked the effect of α-MSH and ACTH in the IML, supporting the conclusion that the responses elicited by these peptides were mediated via MC4Rs. Both SHU9119 and AGRP have been reported to be less potent antagonists at MC3Rs; therefore, the role of MC3Rs in mediating the responses elicited by α-MSH and ACTH cannot be excluded. Microinjections of the MCR antagonists (SHU9119 and AGRP) did not exert any deleterious effects at the site of injection because they did not alter responses to another unrelated agonist (l-Glu).
Microinjections of antagonists for MC3/4Rs (SHU9119 or AGRP) alone into the right IML at T1–T3 did not elicit a change in HR. Because microinjections of agonists for MC3/4Rs (α-MSH and ACTH) into the IML at T1–T3 increase HR, microinjections of the antagonists for these receptors would be expected to decrease HR if MCRs in the right IML were tonically active. Lack of any changes in HR in response to microinjections of MC3/4Rs into the IML at T1–T3 indicated that under normal physiological conditions MC3/4Rs in the right IML are not under tonic control of endogenous α-MSH and ACTH. It is possible that MCRs in the IML may play a role in mediating cardiac responses only during special situations such as stress.
Activation of ARCN neurons has been reported in response to stress (3). Our immunohistochemical experiments showed that some ARCN neurons were retrogradely labeled after microinjections of Fluoro-Gold into the IML at T1–T2. Some ARCN neurons retrogradely labeled with FG contained POMC or α-MSH or ACTH. α-MSH and ACTH are cleavage products of POMC (24). In these experiments, colchicine was used to raise the levels of peptides such as POMC, ACTH, and α-MSH in the neuronal cell body and facilitate their immunostaining. Colchicine is known to inhibit axonal transport in neurons (2). Although the presence of neurons immunoreactive for POMC, α-MSH, and ACTH has been reported previously (27), we have shown that these neurons send projections to the IML at T1–T2. In this study, we have demonstrated that fibers immunoreactive for POMC, α-MSH, and ACTH are present in the IML at T1–T2. It is possible that neurons containing POMC, α-MSH, and ACTH are activated in stressful situations and release one or more of these peptides in the IML. Release of α-MSH in the CSF of humans as well as rats has been documented (20). Activation of MCRs in the IML by these peptides elicits cardioacceleratory responses which are observed in stressful situations (4, 10). Indeed, in this paper we observed that stimulation of the ARCN by NMDA resulted in tachycardia which was attenuated by prior microinjections of MCR antagonists in the right IML at T1–T3, which is the region of the spinal cord involved in the regulation of cardioacceleratory responses in the rat (30–33). Although microinjections of MCR antagonists in the IML at T1–T3 attenuated the cardioacceleratory responses elicited by the stimulation of the ARCN, these responses were not completely abolished. This result is expected because other neurotransmitters have also been implicated in the tachycardic responses elicited from the stimulation of the ARCN. For example, we have previously reported that intrathecal injections of ionotropic glutamate receptor antagonists attenuated, but did not block, the tachycardic responses elicited by the stimulation of the ARCN, suggesting that glutamate is another neurotransmitter in the IML mediating cardioacceleratory responses elicited from the ARCN (19).
Perspectives and significance.
The results presented suggest that MCRs in the IML are involved in the regulation of HR in some pathological or pathophysiological situations such as stress. Melanocortin levels are elevated in stressful situations such as cardiac arrest (36). ARCN neurons have been reported to be activated in stress (3). This information provides a basis for future studies on the role of MCRs in mediating cardiac responses in different models of stress.
GRANTS
This study was supported in part by National Institutes of Health Grants HL-024347 and HL-076248 awarded to H. N. Sapru.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.I. and H.N.S. conception and design of research; M.I. and K.K. performed experiments; M.I., K.K., and H.N.S. analyzed data; M.I., K.K., and H.N.S. interpreted results of experiments; M.I. and K.K. prepared figures; M.I. and H.N.S. drafted manuscript; M.I., K.K., and H.N.S. edited and revised manuscript; M.I., K.K., and H.N.S. approved final version of manuscript.
REFERENCES
- 1.Adan RA, Gispen WH. Melanocortins and the brain: from effects via receptors to targets. Eur J Pharmacol 405: 13–24, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Alonso G. Effects of colchicine on the intraneuronal transport of secretory material prior to the axon: a morphofunctional study in hypothalamic neurosecretory neurons of the rat. Brain Res 453: 191–203, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Baffi JS, Palkovits M. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinology 72: 102–113, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bedi M, Varshney VP, Babbar R. Role of cardiovascular reactivity to mental stress in predicting future hypertension. Clin Exp Hypertens 22: 1–22, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Brown S, Chitravanshi VC, Sapru HN. Cardiovascular action of adrenocorticotropin microinjections into the nucleus tractus solitarius of the rat. Neuroscience 143: 863–874, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chitravanshi VC, Sapru HN. Microinjections of alpha-melanocyte stimulating hormone into the nucleus ambiguus of the rat elicit vagally mediated bradycardia. Am J Physiol Regul Integr Comp Physiol 296: R1402–R1411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Darlington DN, Miyamoto M, Keil LC, Dallman MF. Paraventricular stimulation with glutamate elicits bradycardia and pituitary responses. Am J Physiol Regul Integr Comp Physiol 256: R112–R119, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Gee C, Chen CLC, Roberts J, Thompson R, Watson SJ. Identification of pro-opiomelanocortin neurons in rat hypothalamus by in situ cDNA-mRNA hybridization. Nature 306: 374–376, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 12: 1–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadley ME, Hruby VJ, Jiang J, Sharma SD, Fink JL, Haskell-Luevano C, Bentley DL, Al-Obeidi F, Sawyer TK. Melanocortin receptors: identification and characterization by melanotropic peptide agonists and antagonists. Pigment Cell Res 9: 213–234, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam alpha-melanotropin analogs of Ac-Nle4-cyclo[Asp5,d-Phe7-Lys10]alpha-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem 38: 3454–3461, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Katafuchi T, Oomura Y, Kurosawa M. Effects of chemical stimulation of paraventricular nucleus on adrenal and renal nerve activity in rats. Neurosci Lett 86: 195–200, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res 1102: 117–126, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kawabe T, Kawabe K, Sapru HN. Effect of barodenervation on cardiovascular responses elicited from the hypothalamic arcuate nucleus of the rat. PLoS ONE 7(12): e53111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khachaturian H, Lewis ME, Haber SN, Akil H, Watson SJ. Proopiomelanocortin peptide immunocytochemistry in rhesus monkey brain. Brain Res Bull 13: 785–800, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 Receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Mountjoy KG, Wild JM. Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res 107: 309–314, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Bhatt S, Sapru HN. Cardiovascular responses to hypothalamic arcuate nucleus stimulation in the rat: Role of sympathetic and vagal efferents. Hypertension 54: 1369–1375, 2009 [DOI] [PubMed] [Google Scholar]
- 20.O'Donohue T, Dorsa DM. The opiomelanotropinergic neuronal and endocrine system. Peptides 3: 353–395, 1982 [DOI] [PubMed] [Google Scholar]
- 21.Palkovits M. Stress-induced activation of neurons in the ventromedial arcuate nucleus: a blood-brain-CSF interface of the hypothalamus. Ann NY Acad Sci 1148: 57–63, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Palkovits M, Mezey E, Eskay RL. Pro-opiomelanocortin-derived peptides (ACTH/beta endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res 436: 323–328, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). London: Academic, 2007 [Google Scholar]
- 24.Pritchard LE, Turnbull AV, White A. Proopiomelanocortin processing in the hypothalamus: impact on melanocortin signaling and obesity. J Endocrinol 172: 411–421, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Quillan JM, Sadee W, Wei ET, Jimenez C, Ji L, Chang JK. A synthetic human Agouti-related protein-(83–132)-NH2 fragment is a potent inhibitor of melanocortin receptor function. FEBS Lett 428: 59–62, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Rivest S. Does circulating leptin have the ability to cross the blood-brain barrier and target neurons directly? Endocrinology 428: 3211–3213, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Seeley RJ, Drazen DL, Clegg DJ. The critical role of the melanocortin system in the control of energy balance. Annu Rev Nutr 24: 133–149, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Stern JE. Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Biol 84: 197–215, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Sundaram K, Murugaian J, Krieger AJ, Sapru HN. Microinjections of cholinergic agonists into the IML of the spinal cord at T1–T3 increase heart rate and contractility. Brain Res 503: 22–31, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Sundaram K, Murugaian J, Sapru HN. Cardiac responses to the microinjections of excitatory amino acids into the intermediolateral cell column of the rat spinal cord. Brain Res 482: 12–22, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Sundaram K, Murugaian J, Sapru HN. Microinjection of norepinephrine into the IML of the spinal cord exert excitatory as well as inhibitory effects on the cardiac function. Brain Res 544: 227–234, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Sundaram K, Sapru HN. NMDA receptors in the IML of the spinal cord mediate sympathoexcitatory responses elicited from the ventrolateral medullary pressor area. Brain Res 544: 33–41, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Van Der Kraan M, Tatro JB, Entwistle ML, Brakkee JH, Burbach JP, Adan RA, Gispen WH. Expression of melanocortin receptors and pro-opiomelanocortin in the rat spinal cord in relation to neurotrophic effects of melanocortins. Mol Brain Res 63: 276–286, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Wikberg JES, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, Skottner A. New aspects on the melanocortins and their receptors. Pharmacol Res 42: 393–420, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Wortsman J, Frank S, Wehrenberg WB, Petra PH, Murphy JE. Gamma-3-melanocyte-stimulating hormone immunoreactivity is a component of the neuroendocrine response to maximal stress (cardiac arrest). J Clin Endocrinol Metab 61: 355–360, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Young C, Huang YC, Lin CH, Shen YZ, Gean PW. Selective enhancement of L-type calcium currents by corticotropin in acutely isolated rat amygdala neurons. Mol Pharmacol 59: 604–611, 2001 [DOI] [PubMed] [Google Scholar]