Abstract
Redox status has emerged as critical in modulating stemness and lineage commitment in several precursor cell types. However, a role for redox genes, specifically NADPH oxidases (Nox), in cardiac precursor cells (CPCs) has not been established. We tested whether CPCs marked by type III receptor tyrosine kinase c-kit (c-kit+) exhibit a unique NADPH oxidase signature that confers precursor status and whether alterations in this profile are functionally linked to changes in lineage specification. Dihydroethidium (DHE) microfluorography indicated reduced basal reactive oxygen species (ROS) formation within early postnatal c-kit+ CPCs. Real-time quantitative PCR revealed downregulation of ROS generator Nox2 and its subunit p67phox in c-kit+ CPCs under basal conditions but upregulation of Nox2 and Nox4 over the course of differentiation. Adenoviral silencing of Nox2 and Nox4 increased expression of CPC markers c-kit and Flk-1 and blunted smooth and cardiac muscle differentiation, respectively, while overexpression of Nox2 and Nox4 significantly reduced c-kit expression. These changes were accompanied by altered expression of transcription factors regulating cardiac lineage commitment, Gata6 and Gata4, and cytokine transforming growth factor (TGF)-β1. Similar to other precursor cell types, RT2Profiler PCR Arrays revealed that c-kit+ CPCs also exhibit enhanced antioxidant capacity at the mRNA level. In conclusion, we report that c-kit+ CPCs demonstrate reduced Nox2 expression and ROS levels and that increases in Nox2 and Nox4 influence their differentiation into mature cells. We speculate that ROS generators Nox2 and Nox4, along with the antioxidant genes identified by PCR Arrays, may be novel targets in CPCs that could prove useful in cell-based therapy of the heart.
Keywords: redox, reactive oxygen species, stem cells, cardiac differentiation
owing in large part to the low regenerative capacity of the adult heart (6), coronary heart disease (CHD) remains one of the leading causes of death in the developed world. In 2008, CHD accounted for one of every six deaths in the United States (43). Evidence from directed differentiation of embryonic stem cells (ESCs) and in neonatal mice indicates a significant population of cardiac precursor cells (CPCs) at this developmental stage capable of generating the three main types of cells that specify the heart: smooth muscle cells, cardiac muscle cells, and endothelial cells (22, 30, 52, 57). Unfortunately, the extent and capacity of adult CPCs (5) to cope with the loss of tissue following myocardial infarction or other damage is limited, suggesting that exogenous expansion and therapeutic delivery may be necessary to achieve substantial cardiac regeneration. It has been proposed that redox mechanisms play important roles both in maintaining stemness and in mediating lineage commitment of several precursor cell types (32, 36).
NADPH oxidase (Nox) enzymes are essential components of cardiac redox biology and generate reactive oxygen species (ROS) in a highly regulated manner (9, 16). Unlike the phagocytic NADPH oxidase, which releases a large “respiratory burst” of superoxide (O2−) to ward off pathogens, nonphagocytic NADPH oxidase enzymes generate lower levels of intracellular ROS that are necessary for cell signaling and survival (16). Of the NADPH oxidase homologs (Nox1, Nox2, and Nox4), Nox2 and Nox4 are most abundantly expressed in the myocardium (9). Although Nox2- and Nox4-knockout mice do not demonstrate defects in heart development (40, 60), likely because of compensatory mechanisms, recent evidence indicates that Nox2- and Nox4-derived ROS play critical roles in cardiovascular cell function. Nox2 has been linked to neointimal remodeling and neovascularization after hindlimb ischemia as well as left ventricular remodeling after myocardial infarction (10, 28, 55), while Nox4 has been linked to enhanced myocardial capillary density upon pressure overload as well as increased expression of smooth muscle-specific genes during vascular formation from ESCs (59, 60). The NADPH oxidase enzymes also are implicated in cardiac cell differentiation. For example, Rac, a critical NADPH oxidase subunit, was found to play an important role in early cardiomyogenesis by ESCs (41), while stimulation of peroxisome proliferator-activated receptor α (PPARα) is thought to enhance ESC-mediated cardiomyogenesis via pathways involving both ROS and NADPH oxidase activity (47). The majority of the studies implicating NADPH oxidase-derived ROS in smooth muscle generation and cardiac differentiation have been performed in ESCs (4, 8, 41, 47, 59). However, a recent study linking redox gene Ref-1 to the redox status of adult c-kit+ cardiac stem cells and their commitment to the cardiac fate suggests that NADPH oxidase regulation also might be a critical modulator of CPC function (17).
CPCs can be identified by their surface markers including Sca-1 (a member of the Ly-6 family) (34), Flk-1 [a receptor for vascular endothelial growth factor (VEGF)] (22), and c-kit (a type III receptor tyrosine kinase) (5, 52, 57). Research from our laboratory and others demonstrates that c-kit identifies a mesodermally derived cell population consisting of both precursors that coexpress Flk-1 and cardiac cells coexpressing α-actinin in the very early stages of lineage commitment and development (52, 57). When isolated from the early postnatal heart, c-kit+ cells expand and differentiate in vitro into all three cardiac lineages and display morphological characteristics of nodal, atrial, and ventricular action potentials (52). Discovering the mechanisms that regulate CPC function, including the potential interplay between redox state and CPC lineage commitment, is critical for optimizing CPC-based therapy aimed at repairing the adult heart.
As such, the goal of this study was to test the hypothesis that c-kit+ CPCs exhibit a unique NADPH oxidase molecular signature that confers precursor status and that alterations in this profile influence cardiac cell differentiation. Utilizing genetic tools to selectively manipulate the NADPH oxidase enzymes of early postnatal c-kit+ CPCs, our results suggest that Nox2 and Nox4 are involved in the balance between c-kit+ cell precursor and differentiation status. We found that silencing Nox2 and Nox4 blunts the differentiation of c-kit+ CPCs along the smooth muscle and cardiac lineages, while simultaneously increasing the expression of early precursor cell markers and altering the expression of transcription factors and a cytokine necessary for cardiac lineage specification. Nox2 and Nox4 overexpression studies confirmed a relationship between NADPH oxidase expression and c-kit+ CPC status. We also provide insight into the overall ROS balance in c-kit+ CPCs by examining their antioxidant capacity at the mRNA level. We speculate that the redox genes identified in this study will provide novel targets for improving the regenerative capacity of c-kit+ CPCs, which eventually may be integrated into cell-based therapy for myocardial infarction.
MATERIALS AND METHODS
Animals.
Bacterial artificial chromosome (BAC) transgenic mice in which enhanced green fluorescent protein (EGFP) is a transcriptional indicator of c-kit promoter activity (c-kitBAC-EGFP) and their wild-type (WT) C57BL/6 littermates [ages postnatal day (PN)0–4] were utilized for these studies (11, 52). c-kitBAC-EGFP heterozygote and homozygote transgenic mice were obtained from in-house colonies and bred. c-kitBAC-EGFP newborns (PN0–4) were screened for EGFP expression with a KL2500 cold light source (Leica) with safety glasses covered with Wratten filter no. 12 (Kodak). All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College and met the standards set forth by the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, US Department of Agriculture regulations, and the American Veterinary Medical Association Panel on Euthanasia.
Cell isolation and fluorescence-activated cell sorting.
Cardiac c-kit+ cells from PN0–4 heterozygote c-kitBAC-EGFP pups, hereafter referred to as “freshly isolated c-kit+ cells,” or total cardiomyocytes from age-matched WT littermates, hereafter referred to as “control cardiomyocytes,” were dispersed with a modified version of the Worthington Neonatal Cardiomyocyte Isolation System and isolated by flow cytometry (11, 52). Fluorescence-activated cell sorting (FACS) was undertaken with a Becton-Dickinson FACSVantage SE at the Flow Cytometry Facility at the Hospital for Special Surgery (New York, NY). For sorting, cells were resuspended in a DMEM medium (GIBCO) without phenol red containing Stasis Stem Cell Qualified U.S. Origin Fetal Bovine Serum (2%; Gemini Bio-Products) and sorted into an F-12K Nutrient Mixture, Kaighn's Modification medium (see Culturing conditions) with 20% FBS. Cells were sorted under 13 psi sheath pressure through a 70-μm nozzle. Cells were gated into a parent population with a side scatter area (SSC-A) vs. forward scatter (FSC)-A plot. This parent population was then gated to include only single cells with a SSC-width (W) vs. SSC-A plot. Finally, EGFP+ cells were identified with a 488-nm excitation laser and two band-pass filters [fluorescein isothiocyanate (FITC) 530/30 and phycoerythrin (PE) 575/26]. Total EGFP+ (tEGFP) cells were effectively separated from autofluorescing cells with a final plot, FITC-A vs. PE-A (11, 52). Total cardiomyocytes from age-matched WT littermates were sorted under identical conditions and served as control cells.
Culturing conditions.
Immediately after FACS, freshly isolated c-kit+ cells were seeded onto Lab-Tek II four-well chamber slides (Nunc) and cultured in a F-12K Nutrient Mixture, Kaighn's Modification medium (GIBCO) containing Stasis Stem Cell Qualified U.S. Origin Fetal Bovine Serum (5%; Gemini Bio-Products), basic (b)FGF (10 ng/ml; Invitrogen), leukemia inhibitory factor (LIF, 10 ng/ml; Millipore), and Pen Strep (1%; GIBCO) (52). For the NADPH oxidase differentiation time course, freshly isolated c-kit+ cells were cultured in the F12K medium for 3 days and then switched to a Dulbecco's modified Eagle medium:Nutrient Mixture F-12 (DMEM/F-12) medium (GIBCO) supplemented with B27 (2%; Invitrogen), bFGF (10 ng/ml), LIF (10 ng/ml), EGF (20 ng/ml; Invitrogen), and Pen Strep (1%) (52). Cells were incubated in a NAPCO series 8000 DH CO2 incubator (95% air-5% CO2) at 37°C, and medium was changed every 3–4 days.
ROS detection.
ROS production was assessed by using dihydroethidium (DHE) as an indicator. For in vitro ROS detection, c-kit+ cells were cultured at low density for 48 h. At this time, cell medium was aspirated and cells were rinsed twice with warmed Dulbecco's PBS (DPBS; GIBCO). Cells were then treated with 2.5 μM DHE (Invitrogen) dissolved in DMSO and diluted in DPBS for 30 min at 37°C in the dark. After DHE application, cells were rinsed for 5 min and coverslipped with Aqua-Mount (Fisher Scientific). Coverslipped cells were imaged the same day with an Orca-ER B/W camera (Hamamatsu Photonics) connected to a Zeiss Axioplan 2 microscope. DHE fluorescence intensity was quantified with NIH ImageJ software and expressed relative to c-kit+ cells (61). Background fluorescence was subtracted from the red and green channels. Next, the cell was identified in the green channel and traced with the elliptical function. This selection was then restored onto the corresponding red channel image, and pixel number was measured. c-kit+ cells were identified as those with bright EGFP fluorescence compared with non-c-kit-expressing cells within the same well (23). This process was repeated for each field. In total, ∼12 fields were analyzed (3 fields/well) for each of the three biological replicates.
For in vivo DHE microfluorography (14, 56), PN0–1 homozygote c-kitBAC-EGFP pups were anesthetized with hypothermia (38) for 3 min and injected with DHE (10 μl ip, 4 mg/ml; dissolved in DMSO and diluted in sterile saline) with a 30-gauge needle. After a 1.5- to 2-h incubation period on a warming pad, pups were euthanized and the heart tissue was harvested. Heart tissue was fixed for exactly 1 h in 4% paraformaldehyde (PFA) at 4°C and dehydrated overnight in 20% sucrose. Hearts were embedded in OCT and cryosectioned at 8 μm. Sections were coverslipped with Vectashield mounting medium for fluorescence (Vector) and imaged the same day on a LSM 5 Live confocal microscope (Zeiss). DHE fluorescence intensity was quantified as above except that three directly neighboring c-kit− cells were used for the comparison and areas of the tissue remote to the c-kit+ clusters were used to account for autofluorescence. Approximately 5–10 sections per neonatal heart were analyzed from a total of five biological replicates.
Real-time quantitative PCR.
RNA was harvested from freshly isolated c-kit+ cells and control cardiomyocytes directly after FACS or cultured c-kit+ cells with an optimized version of the TRIzol (Invitrogen) protocol to increase precipitation time in isopropanol. One or two randomly selected samples from each RNA batch were assigned RNA integrity number (RIN) values by an Agilent 2100 bioanalyzer to determine RNA integrity, and all samples were reverse transcribed into cDNA with a TaqMan Reverse Transcription Kit (Applied Biosystems) and the cycling parameters provided by the manufacturer. Quantitative PCR (qPCR) was performed with 8–20 ng of RNA template per well, PerfeCTa SYBR Green FastMix (Quanta), gene-specific primers (Table 1), and an iQ5 Cycler (Bio-Rad) as described previously (37). Cycling parameters were as follows: 10 min at 95°C, 40 × (15 s at 95°C, 1 min at 60°C). All primers (Table 1) were verified by standard and melt curve analyses using RNA isolated from ESCs, control cardiomyocytes, and/or PN4 WT hearts. Values were normalized to β-actin mRNA expression.
Table 1.
Primers used in this study
| Gene | GenBank | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|---|
| Acta2 (α-SMA) | NM007392 | ATTGTGCTGGACTCTGGAGATGGT | TGATGTCACGGACAATCTCACGCT |
| B-actin | NM007393 | CATCCTCTTCCTCCCTGGAGAAGA | ACAGGATTCCATACCCAAGAAGGAAGG |
| C-kit | NM021099 | TCATCGAGTGTGATGGGAAA | GGTGACTTGTTTCAGGCACA |
| Gata4 | NM008092 | GAAAACGGAAGCCCAAGAACC | TGCTGTGCCCATAGTGAGATGAC |
| Gata6 | NM010258 | TTGCTCCGGTAACAGCAGTG | GTGGTCGCTTGTGTAGAAGGA |
| Kdr (Flk-1) | NM010612 | TTTGGCAAATACAACCCTTCAGA | GCAGAAGATACTGTCACCACC |
| Ncf2 (p67phox) | NM010877 | AGCTTCTGCTCCTGTCCGAAGAAA | AATAAGACCTTGGTCACCCACCGT |
| Nox1 | NM172203 | CGTGATTACCAAGGTTGTCATGCAC | GATGCCACTCCAGGAAGGAAATGGA |
| Nox2 | NM007807 | TTCCAGTGCGTGTTGCTCGAC | GATGGCGGTGTGCAGTGCTAT |
| Nox4 | NM015760 | GGATCACAGAAGGTCCCTAGCAG | GCGGCTACATGCACACCTGAGAA |
| Tgf-β1 | NM011577 | GATCCTGTCCAAACTAAGGCTC | ACCTCTTTAGCATAGTAGTCCGC |
| Tnnt2 (cTnT) | NM011619 | CAGAGGAGGCCAACGTAGAAG | CTCCATCGGGGATCTTGGGT |
| Vegfa | NM009505 | CTTGTTCAGAGCGGAGAAAGC | CATCTGCAAGTACGTTCGTTT |
| Vwf | NM011708 | CTTCTGTACGCCTCAGCTATG | GCCGTTGTAATTCCCACACAAG |
Adenoviral vectors.
Recombinant E1-deleted adenoviral vectors (human Ad serotype 5) encoding small interference RNA (siRNA) targeted against Nox2 (AdsiNox2), Nox4 (AdsiNox4), and EGFP (AdsiCON) have been described in previous studies confirming the selective downregulation of Nox2 and Nox4 and a corresponding decrease in ROS levels both in vitro and in vivo (19, 20, 37, 58). For all adenoviral knockdown studies, c-kit+ cells were infected on day 7 of culture (100 pfu/cell) and RNA was isolated (see Real-time quantitative PCR), protein was harvested (see Western immunoblot), or cells were fixed for immunocytochemistry (see Immunocytochemistry) on day 14. Recombinant E1-deleted adenoviral vectors encoding murine Nox2 (AdNox2) and Nox4 (AdNox4) as well as EGFP (AdCON) also were utilized for these studies. For all adenoviral overexpression studies, c-kit+ cells were infected on day 7 of culture (100 pfu/cell) and RNA was harvested 3 days later. All adenoviral vectors were obtained from the Iowa Gene Transfer Vector Core.
Western immunoblot.
c-kit protein levels were assessed by Western immunoblot performed on c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdsiCON, AdsiNox2, AdsiNox4, or AdsiNox2/4 utilizing SDS-PAGE. Samples were incubated with polyclonal rabbit anti-c-kit antibody [sc-168, Santa Cruz Biotechnology; 1:100 in Tris-buffered saline (TBS) with 3% BSA and 0.1% Tween 20] followed by goat anti-rabbit HRP (sc-2030, Santa Cruz Biotechnology; 1:10,000) and subjected to chemiluminescence. Band intensity was quantified by densitometry using NIH ImageJ and normalized to GAPDH loading controls.
Immunocytochemistry.
c-kit+ cells cultured on Lab-Tek II four-well chamber slides (Nunc) and treated with adenovirus (AdsiCON, AdsiNox2, and/or AdsiNox4) were fixed in 4% PFA for 25 min at room temperature (RT) and washed three times with DPBS (GIBCO). Cells were stored at 4°C in DPBS until immunocytochemistry was performed.
For primary antibodies monoclonal mouse anti-α-SMA (1:15, Dako, M0851) (52) and monoclonal mouse anti-cTnT (1:150, Thermo Scientific, MS-295-P0) (52) the following protocol was followed. Cells were permeabilized for 15 min with 0.05% Triton X (Fisher Scientific) in TBS (Bio-Rad) and blocked for 1.5 h with Mouse Ig Blocking Reagent (M.O.M. Immunodetection Kit, Vector Laboratories) followed by 10% normal donkey serum (Millipore) for 30 min at RT. After a quick wash with TBS, primary antibodies were diluted in M.O.M. Diluent (M.O.M. Immunodetection Kit, Vector Laboratories) and applied overnight at 4°C in a humidified chamber. Cells were then washed three times with TBS and incubated with Alexa Fluor 594 donkey anti-mouse IgG (1:200, Invitrogen) diluted in M.O.M. Diluent for 1 h at RT. After secondary incubation, cells were washed four times with TBS. Stained cells were then mounted with Vectashield mounting medium with DAPI for fluorescence (Vector) and quantified. The percentage of positive cells in each condition was determined and expressed as fold AdsiCON. A “no primary antibody” control was utilized to determine specificity. Images were obtained with a Retiga 1300i camera (QImaging) connected to a Nikon Eclipse 80i microscope. Three biological samples were evaluated.
For primary antibody polyclonal rabbit anti-Ki67 (1:100; Abcam, ab15580) the following protocol was followed. Cells were permeabilized for 15 min with 0.2% Triton X in TBS and washed two times for 2 min each with TBS. Cells were then blocked in 10% normal donkey serum for 1 h and 15 min at RT. After a quick wash, the primary antibody was diluted in 0.05% Triton X-1% normal donkey serum-1% normal mouse serum (Jackson ImmunoResearch Laboratories) in TBS for 1 h at RT. Cells were then washed four times with TBS and incubated with Alexa Fluor 594 donkey anti-rabbit IgG (1:100; Invitrogen) diluted in 0.05% Triton X in TBS for 45 min at RT. After secondary incubation, cells were washed four times with TBS. Stained cells were then mounted with Vectashield mounting medium with DAPI for fluorescence (Vector) and imaged with a Retiga 1300i camera (QImaging) connected to a Nikon Eclipse 80i microscope. The percentage of positive cells in each condition was determined. A “no primary antibody” control was utilized to determine specificity. Three biological samples were evaluated.
RT2Profiler PCR arrays.
Directly after FACS, RNA was isolated from freshly isolated c-kit+ cells and control cardiomyocytes with an optimized version of the TRIzol (Invitrogen) protocol to increase precipitation time in isopropanol. RNA was pooled from two independent cell sorts to accumulate 1,000 ng of total RNA and assigned RIN values by an Agilent 2100 bioanalyzer. cDNA first was synthesized with the RT2 First Strand Kit and then added to the RT2qPCR Master Mix according to manufacturer's instructions (Qiagen-SABiosciences). The mixture was divided into aliquots across the PCR Array plates, and thermal cycling was performed (see Real-time quantitative PCR). Data were analyzed with the RT2Profiler PCR Array Data Analysis Template available on the manufacturer's website.
Statistical analysis.
Results are expressed as means ± SE. Data were analyzed by Student's t-test for comparisons between two groups or ANOVA followed by the Tukey posttest for multiple comparisons. Statistical analyses were performed with Prism (GraphPad Software).
RESULTS
c-kit+ CPCs exhibit reduced overall ROS levels in vitro and in vivo.
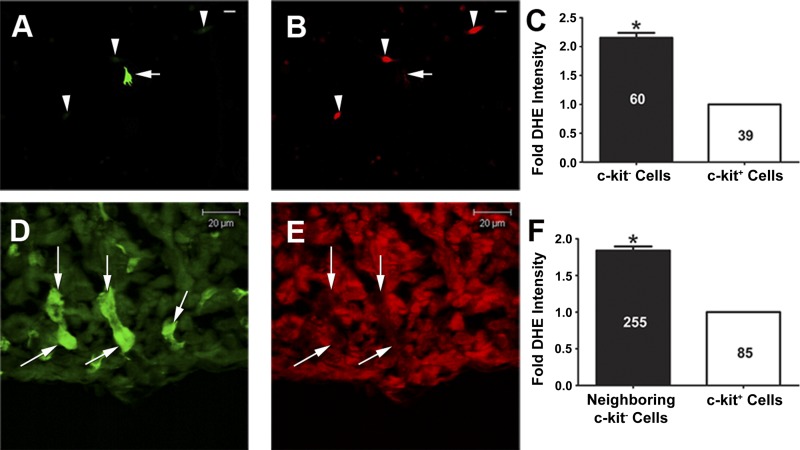
Emerging data indicate that some precursor cell types reside in relatively low-ROS niches that serve to maintain their stemness (12, 21, 35). To gain initial insight into basal overall intracellular ROS levels of c-kit+ CPCs in vitro and in vivo, we utilized DHE microfluorography. As shown in representative images (Fig. 1, A and B) and in the summary (Fig. 1C), there was a significant decrease in DHE fluorescence intensity in early postnatal c-kit+ CPCs compared with c-kit− cells. To rule out the possibility that cell isolation and culturing conditions altered the physiological redox state of c-kit+ CPCs, we also utilized in vivo DHE microfluorography to measure ROS levels of c-kit+ CPCs within the intact left ventricular myocardium of c-kitBAC-EGFP neonates. Similar to our observations from the in vitro study, representative DHE microfluorography confocal images (Fig. 1, D and E) and summary data (Fig. 1F) revealed a significant decrease in DHE fluorescence intensity in ventricular c-kit+ CPCs compared with neighboring c-kit− cells. Taken together, these results suggest reduced basal overall ROS levels within c-kit+ CPCs.
Fig. 1.
c-kit+ cardiac precursor cells (CPCs) exhibit reduced general reactive oxygen species (ROS) levels in vitro and in vivo. A: representative green fluorescent image of a c-kit+ cell (arrow) and 3 c-kit− cells (arrowheads) isolated from postnatal day (PN)0–4 heterozygote c-kitBAC-EGFP pup hearts and cultured for 48 h. Scale bar, 20 μm. B: red fluorescent image from same field demonstrating that the c-kit+ cell (arrow) shows lower dihydroethidium (DHE) fluorescence intensity than the c-kit− cells (arrowheads). Scale bar, 20 μm. C: summary of in vitro DHE fluorescence intensity in c-kit− and c-kit+ cells (3 separate experiments). D: green fluorescent image of c-kit+ cells (arrows) residing in the left ventricular myocardium of PN0 homozygote c-kitBAC-EGFP pup hearts. E: corresponding red fluorescent image from same field demonstrating that the c-kit+ cells (arrows) show lower DHE fluorescence intensity than neighboring c-kit− cells. F: summary of in vivo DHE fluorescence intensity in neighboring c-kit− cells and c-kit+ cells (n = 5 neonates). Numbers of cells analyzed in each experiment are depicted on the bars. *P < 0.05 vs. c-kit+ cells.
Nox2 is selectively downregulated in freshly isolated c-kit+ CPCs and is upregulated along with Nox4 over the course of differentiation.
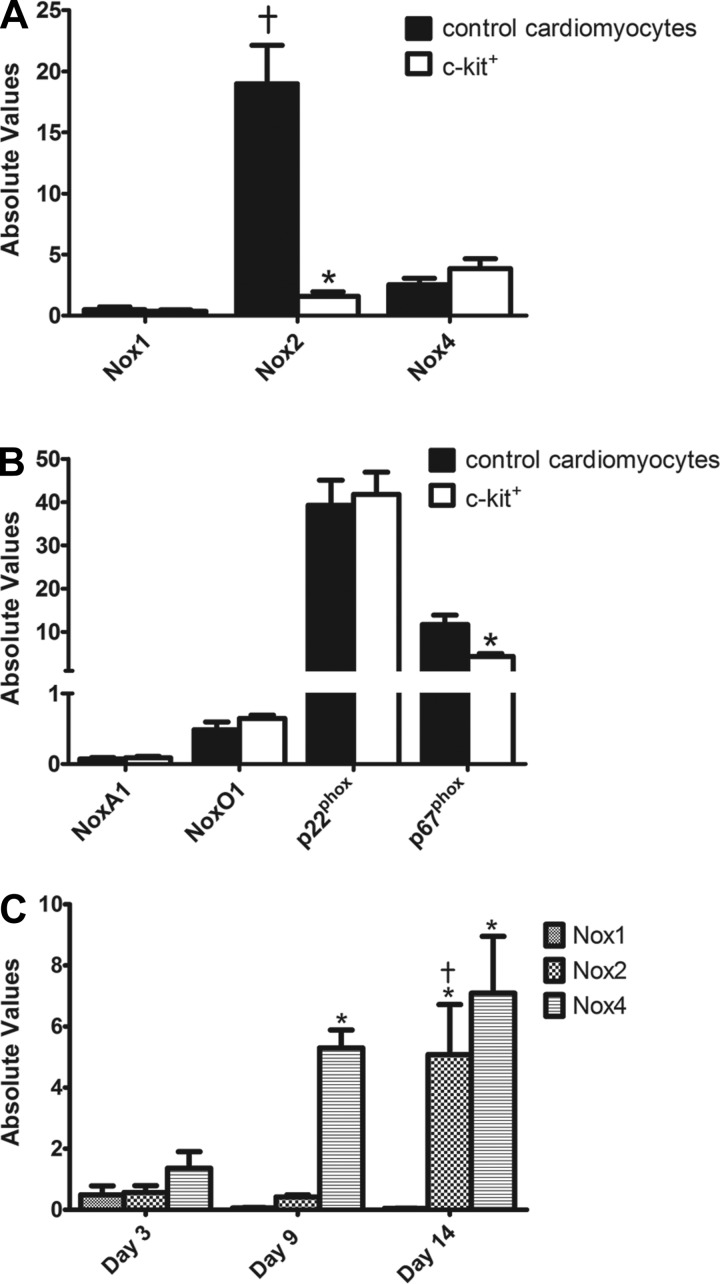
Given the importance of NADPH oxidase enzymes in cardiac ROS generation and signaling (9, 16), we utilized qPCR to profile basal transcript levels of Nox1, 2, and 4, along with a subset of their subunits, in freshly isolated early postnatal c-kit+ and control cells. As shown in Fig. 2A, Nox1 and Nox4 were expressed at low but detectable levels and showed no difference in expression between control cardiomyocytes and freshly isolated c-kit+ CPCs. Moreover, two subunits required for Nox1 activity, NoxA1 and NoxO1, displayed no difference in expression between the groups (Fig. 2B). Similarly, p22phox, a subunit utilized by all three of the cardiac NADPH oxidase enzymes and the only subunit necessary for Nox4 activity (9), was unchanged between c-kit+ and control cells (Fig. 2B). Interestingly, Nox2 was expressed at markedly higher levels than Nox1 and Nox4 in control cardiomyocytes, consistent with previous reports for primary rat neonatal cardiomyocytes (19). However, freshly isolated c-kit+ CPCs showed a dramatic reduction in basal Nox2 expression compared with control cardiomyocytes (12.0-fold, P < 0.05; Fig. 2A). Expression of p67phox, a critical player in Nox2 activity (9), also was reduced significantly in freshly isolated c-kit+ CPCs compared with controls (2.71-fold, P < 0.05; Fig. 2B). Together, these data reveal marked downregulation of the potent ROS generator Nox2 and its critical subunit p67phox in freshly isolated c-kit+ CPCs, which may contribute to the low ROS levels observed in these cells (see Fig. 1).
Fig. 2.
Nox2 is selectively downregulated in freshly isolated c-kit+ CPCs and is upregulated along with Nox4 over the course of differentiation. A and B: summary of basal NADPH oxidase homolog (A) and subgroup of NADPH oxidase subunit (B) mRNA levels analyzed by quantitative (q)PCR in control cardiomyocytes and freshly isolated c-kit+ cells. n = 6/group for NADPH oxidase homologs; n = 3/group for NADPH oxidase subunits. *P < 0.05 vs. control cardiomyocytes; †P < 0.05 vs. Nox1 and Nox4. C: transcript levels of Nox1, 2, and 4 in c-kit+ cells cultured for 3, 9, and 14 days. n = 4/group. *P < 0.05 vs. day 3; †P < 0.05 vs. day 9. Gene expression was normalized to β-actin.
Having identified a unique basal NADPH oxidase expression profile in freshly isolated c-kit+ CPCs, we next examined whether NADPH oxidase enzyme expression was induced upon differentiation as has been reported for ESCs (4, 8, 26) and adult cardiac stem cells (17). c-kit+ CPCs were cultured for 3, 9, or 14 days, and NADPH oxidase enzyme expression was profiled by qPCR as above. As seen in Fig. 2C, Nox1 transcript levels remained low, but detectable, and did not change significantly throughout the differentiation time course. Similar to basal expression data, Nox2 transcript levels were low and remained so at days 3 and 9; however, by day 14 there was a marked upregulation of this NADPH oxidase homolog. Nox4 exhibited a gradual increase in expression over time and by day 14 was similar to Nox2 expression. Together, these findings demonstrate that Nox2 and Nox4 are upregulated in a time-specific manner over the course of c-kit+ CPC differentiation, suggesting unique roles for these NADPH oxidase isozymes in ROS-mediated cardiac lineage commitment.
Targeted silencing of Nox2 and Nox4 alters c-kit+ precursor and lineage commitment status.
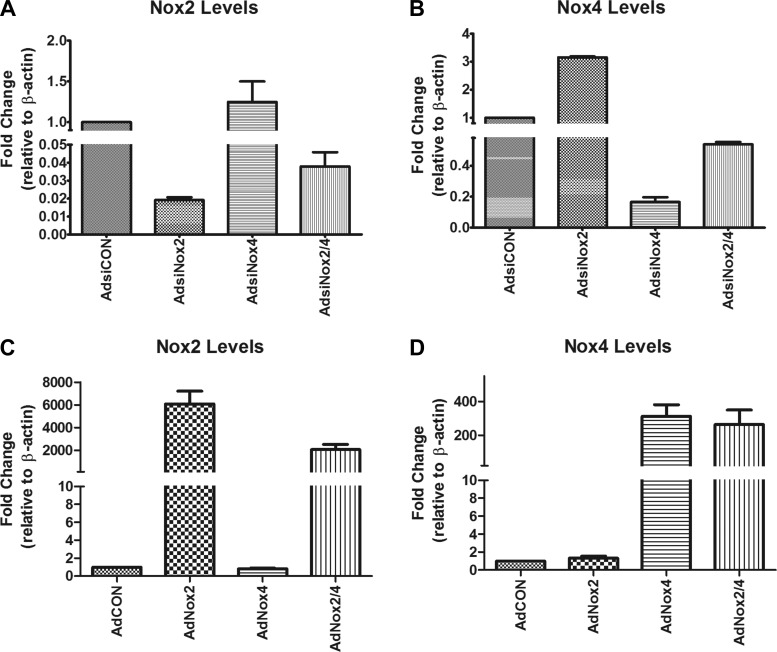
To determine whether Nox2 and Nox4 are functionally linked to c-kit+ precursor cell status and/or cardiac cell differentiation in vitro, we utilized adenoviruses encoding U6 promoter-driven siRNAs targeted to Nox2 (AdsiNox2) or Nox4 (AdsiNox4). These siRNAs were previously established and fully characterized in our laboratory (19, 20, 37) and have been used successfully by other researchers in multiple cell types and experimental settings (27, 31). Importantly, we have demonstrated that these viruses efficiently and selectively silence Nox2 and Nox4 at the mRNA and protein levels and significantly reduce ROS formation in cultured cardiomyocytes and in vivo (19, 20, 37). As shown in Fig. 3, A and B, transcript silencing also was demonstrated in neonatal c-kit+ CPCs.
Fig. 3.
Nox2 and Nox4 transcript levels by qPCR. A and B: Nox2 (A) and Nox4 (B) transcript levels analyzed by qPCR in c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdsiCON, AdsiNox2, AdsiNox4, or AdsiNox2/4 for 48 h. n = 3/group. C and D: Nox2 (C) and Nox4 (D) transcript levels analyzed by qPCR in c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdCON, AdNox2, AdNox4, or AdNox2/4 for 48 h. n = 5/group. Gene expression was normalized to β-actin.
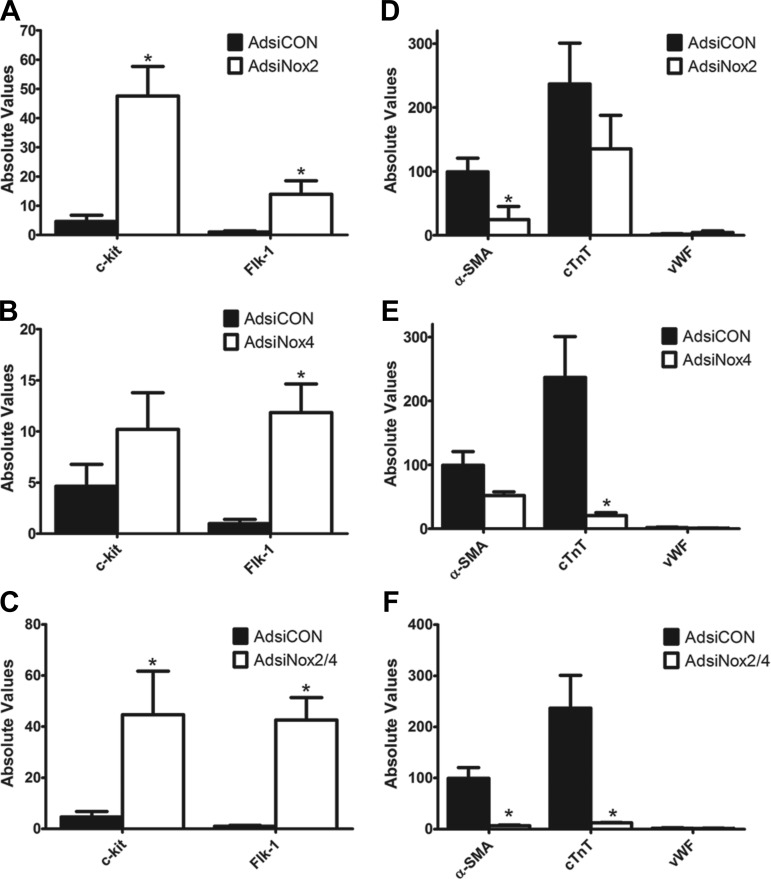
We next isolated c-kit+ CPCs from early postnatal c-kitBAC-EGFP hearts, infected them with one or both of these adenoviruses or a control virus (AdsiCON), and analyzed expression of several cardiac precursor (c-kit and Flk-1) (5, 22, 52) and differentiation genes [α-smooth muscle actin (α-SMA), cardiac troponin T (cTnT), endothelial von Willebrand factor (vWF)] (5, 52) by qPCR.
As shown in Fig. 4A, c-kit+ CPCs infected with AdsiNox2 exhibited a significant increase in precursor genes c-kit and Flk-1 compared with cells infected with a control siRNA (AdsiCON). This was accompanied by a marked downregulation of α-SMA but no effect on cTnT or vWF transcript levels (Fig. 4D). Interestingly, cells infected with AdsiNox4 also demonstrated a significant increase in one of the stemness markers (Flk-1), and in these cells there was a corresponding and selective decrease in cTnT, with no changes observed in the other differentiation markers compared with control treatment (Fig. 4, B and E). Finally, coinfection of c-kit+ CPCs with both AdsiNox2 and AdsiNox4 triggered a marked increase in both of the precursor genes c-kit and Flk-1 compared with AdsiCON (Fig. 4C). This was accompanied by marked decreases in both α-SMA and cTnT transcript levels but still no effect on the endothelial marker vWF (Fig. 4F).
Fig. 4.
Targeted silencing of Nox2 and Nox4 increases expression of cardiac precursor genes and decreases expression of specific differentiation genes. Transcript levels for stemness genes c-kit and Flk-1 (A–C) and differentiation genes α-smooth muscle actin (α-SMA), cardiac troponin T (cTnT), and endothelial von Willebrand factor (vWF) (D–F) analyzed by qPCR in c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdsiCON, AdsiNox2, AdsiNox4, or AdsiNox2/4. qPCR was performed 7 days after infection. n = 4–5/group. *P < 0.05 vs. AdsiCON. Gene expression was normalized to β-actin.
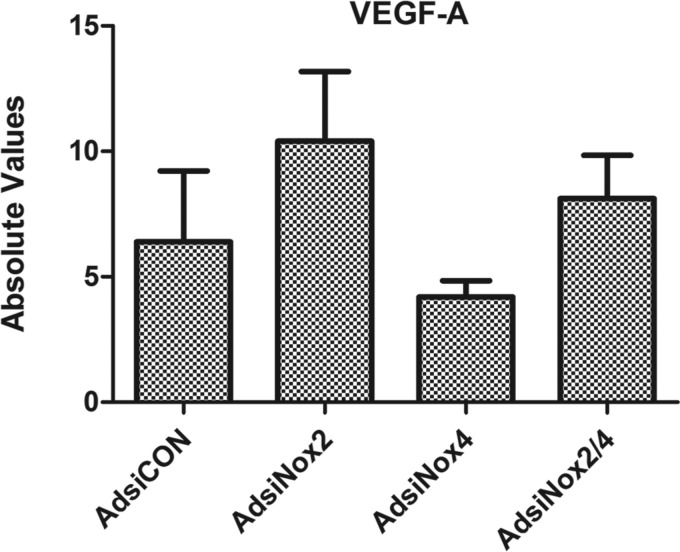
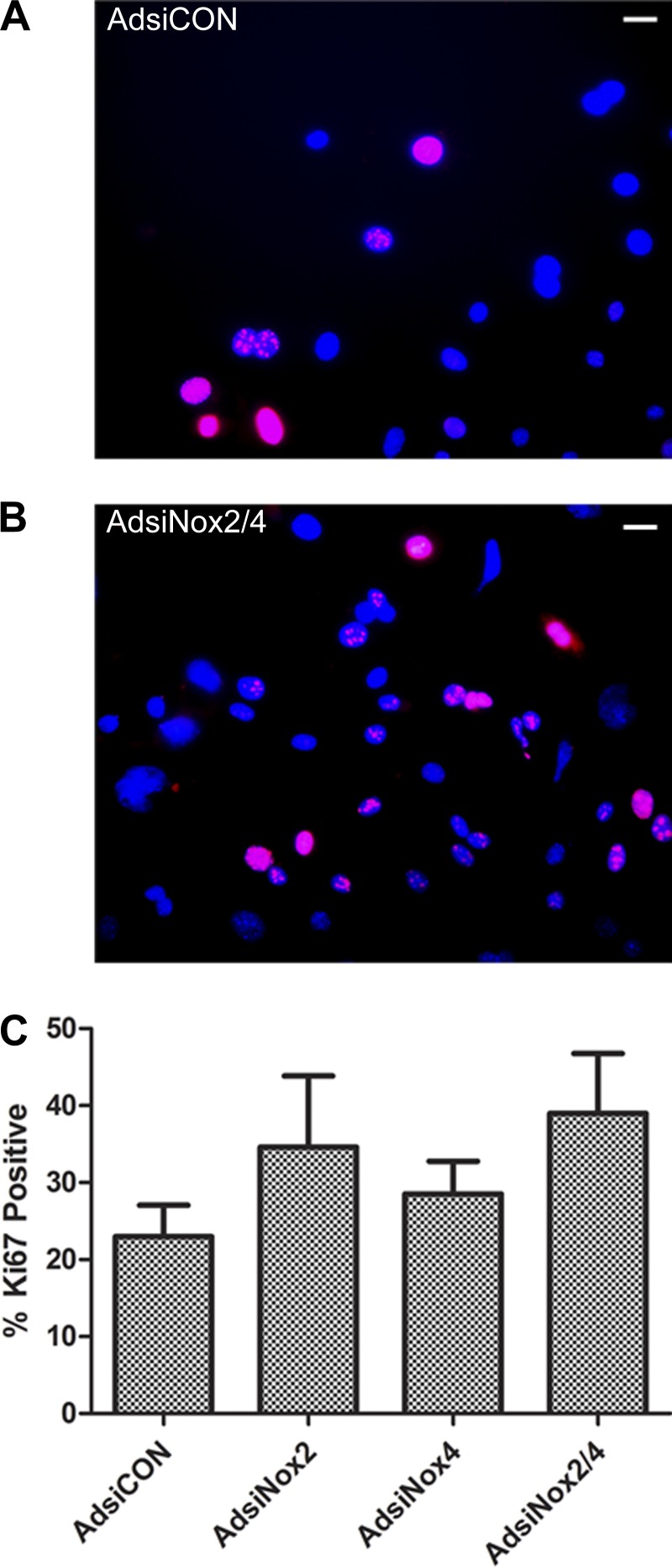
Because mRNA levels of vWF were low and unaltered in each of the experiments, we also investigated expression of VEGF-A, a growth factor critical for endothelial cell differentiation and proliferation (33, 53). We found that VEGF-A was expressed at detectable levels in c-kit+ CPCs; however, no changes in transcript levels were observed upon silencing of Nox2 and/or Nox4 (Fig. 5). In addition, we sought to verify that viral silencing of the NADPH oxidase enzymes, either separately or in combination, did not alter cell proliferation compared with cells transduced with the control vector. As seen in Fig. 6, there were no differences in expression of the cell proliferation marker Ki67 in any of the virus-treated groups compared with AdsiCON-treated c-kit+ CPCs.
Fig. 5.
Vascular endothelial growth factor (VEGF)-A transcript levels by qPCR: VEGF-A transcript levels analyzed by qPCR in c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdsiCON, AdsiNox2, AdsiNox4, or AdsiNox2/4 for 7 days. n = 3–5/group. No significant differences were observed between the groups; P > 0.05 vs. AdsiCON. Gene expression was normalized to β-actin.
Fig. 6.
Proliferation in virally transduced cells. A and B: representative images of Ki67 immunocytochemistry in DAPI-stained c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and treated with AdsiCON (A) and AdsiNox2/4 (B) for 7 days. Scale bars, 10 μm. C: summary of % of total cells positive for Ki67 at day 7 after viral transduction. Three independent experiments were analyzed, comprising ≥200 cells/condition. No significant differences were observed between the groups; P > 0.05 vs. AdsiCON.
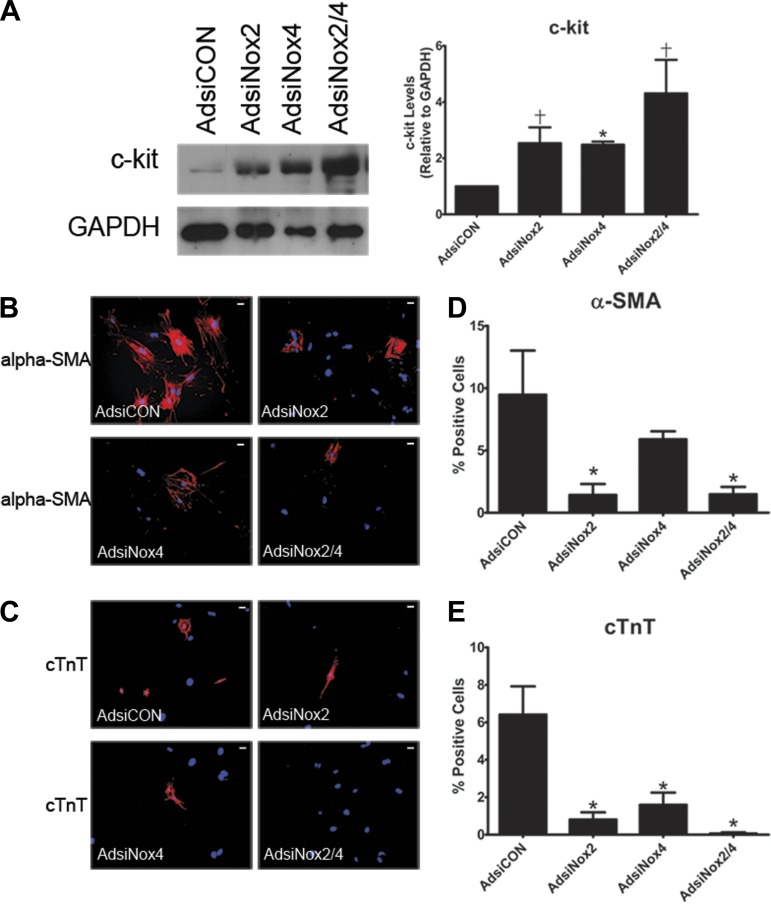
To further verify these findings and validate the mRNA data observed with NADPH oxidase isozyme silencing, we next performed a series of Western immunoblot and immunocytochemistry experiments. As shown in Fig. 7A, we observed significant increases in total c-kit protein after either AdsiNox2 or AdsiNox4 alone or in combination compared with AdsiCON. We also examined the percentage of cells expressing either α-SMA or cTnT by immunocytochemistry. Importantly, the proportions of control cells expressing differentiation markers α-SMA and cTnT (Fig. 7, D and E) were similar to our previous report (52). As shown in representative images (Fig. 7, B and C) and summary graphs (Fig. 7, D and E), and in line with our mRNA data, significant reductions in the percentage of α-SMA-expressing cells after treatment with AdsiNox2 or AdsiNox2/4 and cTnT-expressing cells after treatment with AdsiNox4 or AdsiNox2/4 were observed. Knockdown of Nox2 also led to a reduction in the percentage of cTnT-positive cells, even though the decrease in cTnT transcript level was not different from control (Fig. 4D). Collectively, these results suggest that low levels of Nox2 and Nox4 are critical for the maintenance of precursor status in c-kit+ CPCs and that increasing levels of Nox2 and Nox4 correlate with and may be important for expression of smooth muscle and cardiac cell markers.
Fig. 7.
Targeted silencing of Nox2 and Nox4 alters population of lineage-committed cells. A: representative immunoblot (left) and summary (right) of quantification of c-kit protein in cell lysates from c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdsiCON, AdsiNox2, AdsiNox4, or AdsiNox2/4 for 7 days. n = 3. *P < 0.05 vs. AdsiCON; †P = 0.05 vs. AdsiCON. B and C: immunocytochemistry for α-SMA (B) and cTnT (C) in virally transduced c-kit+ cells. Scale bars, 10 μm. D and E: summary of % of total cells positive for α-SMA (D) and cTnT (E) 7 days after viral transduction. Four independent experiments were analyzed comprising ≥550 cells/condition. *P < 0.05 vs. AdsiCON.
Overexpression of Nox2 and Nox4 alters c-kit+ precursor and lineage commitment status.
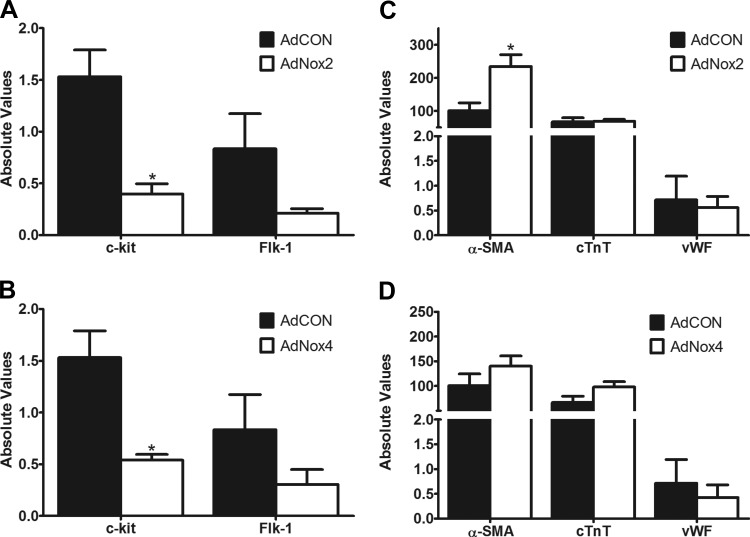
To further verify that Nox2 and Nox4 are functionally linked to c-kit+ precursor and cardiac cell differentiation status in vitro, we utilized adenoviruses encoding Nox2 (AdNox2) or Nox4 (AdNox4). First, we demonstrated that these viruses efficiently and selectively increase Nox2 and Nox4 expression, respectively, in neonatal c-kit+ CPCs (Fig. 3, C and D). Next, c-kit+ CPCs isolated from early postnatal c-kitBAC-EGFP hearts were infected with one or both of these adenoviruses or a control virus (AdCON) and expression of cardiac precursor (c-kit and Flk-1) (5, 22, 52) and differentiation genes (α-SMA, cTnT, vWF) (5, 52) were analyzed by qPCR. As shown in Fig. 8A, c-kit+ CPCs infected with AdNox2 exhibited a significant decrease in precursor cell marker c-kit compared with cells infected with control virus (AdCON). This was accompanied by a marked increase in α-SMA but no effect on cTnT or vWF transcript levels (Fig. 8C). Interestingly, cells infected with AdNox4 also demonstrated a significant decrease in stemness marker c-kit (Fig. 8B), but in these cells no changes were observed in the other differentiation markers compared with control treatment (Fig. 8D). Together, these data support a link between NADPH oxidase expression and c-kit+ CPC status.
Fig. 8.
Overexpression of Nox2 and Nox4 alters c-kit+ precursor and lineage commitment status: transcript levels for stemness genes c-kit and Flk-1 (A and B) and differentiation genes α-SMA, cTnT, and vWF (C and D) analyzed by qPCR in c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdCON, AdNox2, or AdNox4. qPCR was performed 3 days after infection. n = 4–5/group. *P < 0.05 vs. AdCON. Gene expression was normalized to β-actin.
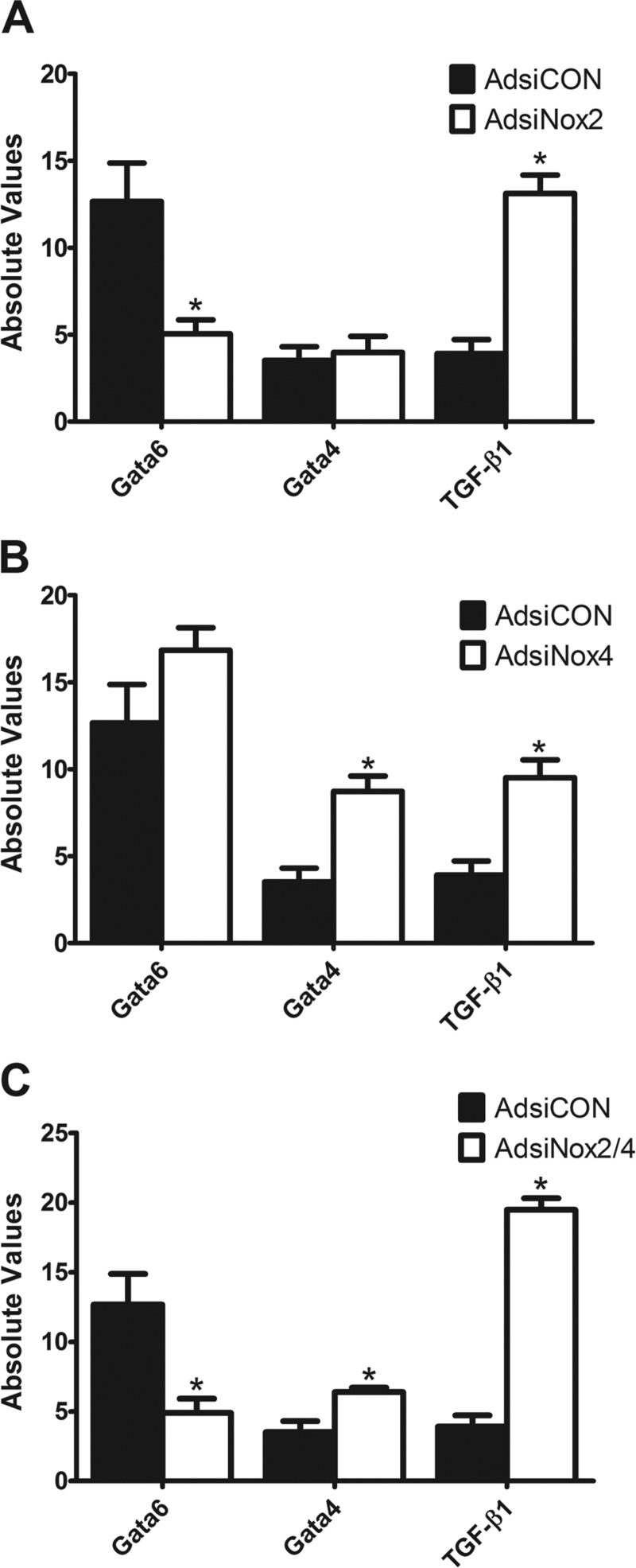
Targeted silencing of Nox2 and Nox4 alters expression of transcription factors and a cytokine.
To begin to tease apart the mechanisms by which NADPH oxidase isoforms act during c-kit+ CPC specification, we utilized qPCR to measure the expression levels of two transcription factors, Gata6 and Gata4, and the cytokine TGF-β1, each known to play important roles in cardiac lineage commitment (3). As shown in Fig. 9A, viral silencing of Nox2 reduced the expression of the smooth muscle-specific transcription factor Gata6 and significantly increased expression of TGF-β1, which is a critical modulator of ESC-mediated smooth muscle generation (48). AdsiNox4 significantly increased expression of the early cardiac transcription factor Gata4 and TGF-β1 but had no effect on Gata6 expression (Fig. 9B). In combination, knockdown of Nox2 and Nox4 led to a significant reduction in Gata6 mRNA expression along with a significant increase in both Gata4 and TGF-β1 mRNA levels (Fig. 9C). Thus it appears that Nox2- and Nox4-derived ROS target the early transcription pathways necessary for mesodermal differentiation.
Fig. 9.
Targeted silencing of Nox2 and Nox4 alters expression of transcription factors and a cytokine: Gata6, Gata4, and TGF-β1 transcript levels analyzed by qPCR in c-kit+ cells isolated from PN0–4 heterozygote c-kitBAC-EGFP pup hearts and infected with AdsiCON and AdsiNox2 (A), AdsiNox4 (B), or AdsiNox2/4 (C) for 7 days. n = 3–6/group. *P < 0.05 vs. AdsiCON. Gene expression was normalized to β-actin.
c-kit+ CPCs exhibit enhanced antioxidant capacity at the mRNA level.
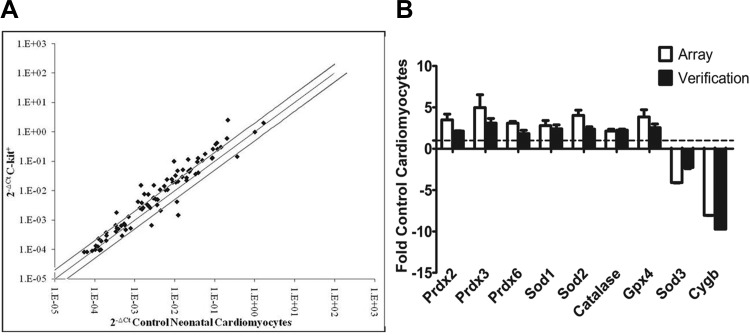
Our evidence suggests that the prooxidant generators Nox2 and Nox4 influence c-kit+ CPC function. However, as it has been established that overall redox balance is an important modulator of precursor cell state, a variety of antioxidants likely are involved in the pathways mediating CPC self-renewal and differentiation. Therefore, we utilized the RT2Profiler PCR Array system to simultaneously monitor mRNA expression of 84 different genes involved in Mouse Oxidative Stress and Antioxidant Defense systems in a population of freshly isolated c-kit+ CPCs. Utilizing qPCR, we found that 23 redox genes were upregulated and 4 genes were downregulated in freshly isolated c-kit+ CPCs compared with control cardiomyocytes (Fig. 10A, Table 2, and Table 3). Fold changes were considered significant at a twofold or greater change in either direction (P < 0.05). Of these 23 genes, many represented entire families of redox-related molecules. For example, upregulated genes included two of the three superoxide dismutase isoforms (Sod1, Sod2) and four members of the peroxiredoxin family (Prdx2, Prdx3, Prdx5, Prdx6), all of which are involved in ROS scavenging. The four downregulated genes included the third member of the Sod family (Sod3), apolipoprotein E, cytoglobin, as well as previously identified prooxidant p67phox. RT2Profiler PCR Array results were confirmed with independently designed qPCR primers for select target genes run on three distinct samples, and close alignment was found (Fig. 10B). These data reveal an intriguing genetic redox profile in freshly isolated c-kit+ CPCs that likely contributes to maintenance of a low-ROS state and preservation of a stemness phenotype in c-kit+ CPCs.
Fig. 10.
c-kit+ CPCs exhibit enhanced antioxidant capacity at the mRNA level. A: summary of transcript levels of 84 redox genes analyzed by RT2Profiler PCR Array in c-kit+ CPCs and control cardiomyocytes isolated from PN0–4 heterozygote c-kitBAC-EGFP and wild-type littermate pup hearts, respectively. Fold changes were considered significant at ≥2-fold change in either direction (solid lines). n = 3. P < 0.05. Ct, threshold cycle. B: summary of a subset of upregulated and downregulated genes analyzed by qPCR in c-kit+ CPCs and control cardiomyocytes expressed as fold control cardiomyocytes and compared with the Array data set. n = 3. P > 0.05. Gene expression was normalized to β-actin.
Table 2.
Twenty-three upregulated redox genes and their functions
| Upregulated Gene | Fold Change (≥2) | P Value (<0.05) | Gene Function |
|---|---|---|---|
| Mb | 12.09 | 0.0188 | Globin, storage/movement of O2 in muscle |
| Xirp1 | 10.8 | 0.0028 | Stabilizes actin filaments |
| Tmod1 | 10.56 | 0.0029 | Blocks depolarization of actin filaments |
| Prdx3 | 5.42 | 0.041 | Reduces H2O2 and alkyl hydroperoxides in mitochondria |
| Nqo1 | 5.13 | 0.0215 | Quinone reductase, cellular detoxification |
| Slc41a3 | 4.45 | 0.0108 | Magnesium transporter |
| Sod2 | 4.11 | 0.0077 | Dismutates superoxide in mitochondria |
| Gstk1 | 3.8 | 0.0211 | Glutathione transferase, cellular detoxification |
| Gpx4 | 3.78 | 0.0153 | Catalyzes reduction of H2O2 |
| Prdx2 | 3.67 | 0.0215 | Reduces H2O2 and alkyl hydroperoxides in cytosol |
| Txnrd2 | 3.53 | 0.0115 | Reduces thioredoxin |
| Slc38a1 | 3.47 | 0.0007 | Amino acid transporter |
| Prdx5 | 3.38 | 0.0028 | Reduces H2O2 and alkyl hydroperoxides in mitochondria/peroxisomes |
| Prdx6-rs1 | 3.27 | 0.0014 | Peroxiredoxin 6 related sequence-1 |
| Prdx6 | 3.17 | 0.0042 | Reduces H2O2 and alkyl hydroperoxides in cytosol |
| Psmb5 | 3.14 | 0.0163 | Proteasome subunit |
| Txnip | 3.02 | 0.0488 | Inhibits thioredoxin activity |
| Sod1 | 2.94 | 0.0486 | Dismutates superoxide in cytoplasmic compartment |
| Park7 | 2.63 | 0.0043 | Member of peptidase C56 family, sensor of oxidative stress |
| Nudt15 | 2.6 | 0.0205 | Helps prevent incorporation of 8-oxo-dGTP into DNA |
| Catalase | 2.22 | 0.0312 | Decomposes H2O2 |
| Dnm2 | 2.17 | 0.015 | GTP-binding protein, endocytosis |
| Ptgs1 | 2.13 | 0.0154 | Prostaglandin synthesis, cell proliferation |
Table 3.
Four downregulated genes and their functions
| Downregulated Gene | Fold Change (≥2) | P Value (<.05) | Gene Function |
|---|---|---|---|
| Cygb | 0.12 | 0.0002 | O2 storage/transfer |
| Sod3 | 0.25 | 0.0188 | Dismutates superoxide in extracellular compartment |
| Ncf2 (p67phox) | 0.37 | 0.0196 | Subunit of NADPH oxidase complex in neutrophils |
| Apoe | 0.4 | 0.0062 | Lipoprotein metabolism |
DISCUSSION
With cell-based therapies emerging as potential strategies for a variety of disease states including heart failure (3), it is increasingly important to understand the mechanisms underlying precursor cell self-renewal and differentiation. Redox signaling is recognized to play key roles both in maintaining stemness and in mediating lineage commitment in multiple precursor cell types (32, 36). However, little is known about the redox status of c-kit+ CPCs and the interplay between ROS and CPC differentiation. Here we report that early postnatal c-kit+ CPCs exhibit reduced overall ROS levels under basal conditions and that Nox2 and Nox4 are involved in the balance between c-kit+ cell precursor and differentiation status, in part by altering the expression of key transcription factors Gata6 and Gata4 and the cytokine TGF-β1, all of which have been implicated in cardiac development.
Several precursor cell types, including endothelial progenitor cells (EPCs) and hematopoietic stem cells, have now been shown to exist in a ROS-protected state in which they exhibit lower levels of ROS than their differentiated counterparts (12, 21, 35). It is postulated that this may promote survival of progenitor cells by shielding them from DNA damage and limiting their exposure to molecules that trigger differentiation (12, 21, 35, 49, 50). Using DHE as a ROS indicator in freshly isolated c-kit+ CPCs in vitro and in the intact myocardium of early postnatal c-kitBAC-EGFP mice, we have provided the first evidence that early postnatal c-kit+ CPCs also exhibit reduced ROS levels. While we are aware that DHE fluorescence intensity is an indirect indicator of ROS, at this time there are no individual ROS indicators that when used alone can provide a comprehensive profile of cellular ROS (13). For the scope of this work, it was not our intention to discriminate between H2O2 and O2− as we were unable to obtain enough material from our severely limited cell population to utilize multiple ROS indicators. However, our DHE results were indicative of an altered general redox state, and our findings suggest that c-kit+ CPCs reside within well-protected niche environments, as has been observed in other precursor cell types (21, 35). Similar to other precursor cell populations (12), we also demonstrated that c-kit+ CPCs exhibit increased expression of antioxidant enzymes including catalase, SOD, and glutathione peroxidase. It is likely that upregulation of these antioxidants contributes to the low-ROS state of c-kit+ CPCs. Further studies are required to elucidate specific roles for these antioxidants on c-kit+ cell redox balance.
After demonstrating a decrease in overall ROS production in c-kit+ CPCs, we next turned to analyzing Nox1, Nox2, and Nox4 since they are the main ROS generators in cardiovascular tissues and are known to be involved in cardiac signaling and differentiation (9, 16). We examined Nox1, Nox2, and Nox4 at the mRNA level as this is currently the most suitable methodology given that our cell population is so limited. While we realize that mRNA levels do not always correlate with the amount of protein present because of posttranslational controls (18), a lack of high-quality antibodies coupled with our limited cell samples precluded us from performing immunocytochemistry studies. However, we are confident that the changes we observed in NADPH oxidase expression at the transcript level are biologically meaningful and provide novel insight into the redox status of CPCs. We found that Nox1, which has limited expression in the mature heart (9, 16), was expressed at low basal levels in both early postnatal c-kit+ CPCs and control cardiomyocytes, and, unlike the other two homologs, showed no induction upon c-kit+ CPC differentiation. In addition, the two required subunits for Nox1 activity, NoxA1 and NoxO1, also were not differentially expressed in c-kit+ CPCs. Therefore, Nox1 likely plays a very limited role in c-kit+ cell precursor status or lineage commitment. On the other hand, Nox2 and its functional subunit, p67phox, were significantly downregulated in early postnatal c-kit+ CPCs compared with control cells. Interestingly, this was not the case for Nox4, which leads us to speculate that the low-ROS state of early postnatal c-kit+ CPCs is due at least in part to selective downregulation of Nox2 expression and function in these cells.
Both Nox2 and Nox4 exhibited increased expression over the course of c-kit+ cell differentiation, with Nox4 showing upregulation somewhat earlier than Nox2. This is consistent with evidence that both of these homologs are expressed in multiple developing and mature cardiac cell types (4, 8, 9, 16, 26, 31, 59). Previously, we reported that c-kit+ cells differentiate into all three lineages of the heart in vitro (52), so to determine whether Nox2 and/or Nox4 are functionally linked to c-kit+ CPC differentiation into smooth muscle, cardiac muscle, and/or endothelial cells, we went on to utilize gene-silencing viruses targeted selectively to each of these two homologs (19, 20, 37). Upon knockdown of Nox2, there was a significant increase in both the CPC marker c-kit and the early mesodermal cell marker Flk-1, concomitant with a decrease in expression of α-SMA at the mRNA and protein levels. As expected, Nox2 overexpression led to a significant decrease in c-kit expression as well as an increase in α-SMA mRNA. Although it has been reported previously that Nox2 plays a key role in skeletal muscle differentiation (39) and in remodeling/revascularization after ischemic injury in other systems (10, 55), this is the first report that Nox2 is critical for the differentiation of CPCs along the smooth muscle lineage. In line with previous reports that Gata6 expression follows that of α-SMA (24), we also observed a significant downregulation of Gata6 mRNA in AdsiNox2-transduced c-kit+ CPCs, and this was not further altered by knockdown of Nox4. Nox2 knockdown also was accompanied by significant upregulation of TGF-β1, which is consistent with reports that Gata6 is critical for the maintenance of a differentiated phenotype in vascular smooth muscle cells and is a suppressor of TGF-β1 (2, 15). Because Gata6 and TGF-β1 have broad cellular implications, it is not surprising that downregulation of Nox2 also led to a significant reduction in the number of cTnT-expressing cells, even though this was not significant at the transcript level. A partial contribution of Nox2 to cardiomyogenesis by ESCs was recently reported; however, similar to our data, Nox4 was found to be the key NADPH oxidase determinant in cardiac commitment (4).
As mentioned above, a role for Nox4 in ESC-mediated cardiomyogenesis has emerged (8, 26), and our studies suggest that a similar mechanism contributes to the pathways that drive differentiation of c-kit+ CPCs along the cardiomyocyte lineage. Selective knockdown of Nox4 with AdsiNox4 reduced expression of cTnT mRNA as well as the percentage of cells expressing cTnT protein. As with AdsiNox2 treatment, this was accompanied by increased expression of stemness markers c-kit and Flk-1. We speculate that this change reflects a block in the molecular signaling necessary for the transition between a precursor cell and a mature cardiac cell rather than a shift from a mature cell to a more primitive state, as it is generally believed that cardiac cells are terminally and irreversibly differentiated. Overexpression of Nox4 led to the expected decrease in c-kit mRNA but showed no change in cTnT transcript expression. We hypothesize that the lack of change in cTnT transcript might be due to the fact that, in order to maintain viability, we were only able to culture AdNox-treated cells for 3 days. On the basis of our findings in this study, we expect that a longer time course likely is necessary to capture downstream changes in cTnT mRNA. Interestingly, although previous reports also link Nox4 to smooth muscle cell differentiation (59), our data do not support a similar role for Nox4 in c-kit+ CPCs, since AdsiNox4 did not significantly alter Gata6 expression, α-SMA transcript levels, or the percentage of α-SMA-positive cells. However, AdsiNox4 did increase expression of early cardiac transcription factor Gata4 along with cytokine TGF-β1, which is a known mediator of Gata4 expression (1, 29, 31). This is not completely unexpected given that Gata4 expression is sensitive to overall redox change rather than absolute ROS levels and can also be regulated by antioxidant-sensitive and ROS-independent mechanisms (8, 31, 46, 51). Similar to the findings of Schmelter et al. (46), the upregulation of Gata4 observed in our system was unmet by a subsequent rise in expression of mature cardiac marker cTnT. Together, these data highlight a delicate interplay between Nox4, Gata4, and TGF-β1 in cardiac commitment, and we speculate that a finely tuned balance between ROS-dependent and ROS-independent pathways orchestrates proper cardiogenic differentiation. Further studies will be required to elucidate the mechanisms by which these factors work together, as it is clear that upregulation of Gata4 and TGF-β1 alone is not sufficient to drive cardiac muscle specification and that Nox4 is critically linked to the expression of mature cardiac marker cTnT.
In line with our earlier report that a negligible number of c-kit+ CPCs analyzed 24 h after FACS express endothelial markers platelet endothelial cell adhesion molecule (PECAM)1 and vWF (52), we noted very low but detectable levels of vWF in all conditions and failed to observe differential effects of AdsiNox2 and/or AdsiNox4 on endothelial cell differentiation. This was verified further with VEGF-A, a growth factor critical for endothelial cell differentiation and proliferation (33, 53). It should be noted, however, that we cannot rule out the possibility that specific media conditions and growth factors vital for endothelial cells to differentiate from c-kit+ CPCs were lacking in our protocol and this may have contributed to our findings (52). While we believe it is unlikely that Nox2 or Nox4 plays a prominent role in the differentiation of c-kit+ CPCs into mature endothelial cells, it remains to be elucidated whether overall redox state and/or NADPH oxidase signaling via other NADPH oxidase homologs are critical for the differentiation of c-kit+ CPCs along the endothelial cell lineage in optimized media conditions.
Although it is clear that ROS derived from the different NADPH oxidase isoforms feed into divergent signaling cascades and lead to specific cellular outcomes (9), our data suggest that there also may be convergent signaling pathways for NADPH oxidase-derived ROS in c-kit+ CPCs. For example, when AdsiNox2 and AdsiNox4 were used concomitantly, we observed a significant and even more robust upregulation of cardiovascular precursor marker Flk-1 than that observed after the downregulation of either NADPH oxidase isoform alone. Interestingly, Flk-1 also has been reported as a marker of EPCs (54) and vascular stem cells (25). Because we did not observe an increase in endothelial cell markers after downregulation of Nox2 and Nox4, we do not believe that this increase in Flk-1 is indicative of a shift to an endothelial cell fate, but instead we hypothesize that it is a reversal to either a multipotent cardiovascular or a more restricted vascular precursor cell state. The idea of convergent signaling pathways for NADPH oxidase-derived ROS also was observed for TGF-β1 mRNA, where a more robust increase was seen after concurrent treatment with AdsiNox2 and AdsiNox4. The augmentation in induction observed here may reflect an attempt to overcompensate for the drastic loss of NADPH oxidase signaling, as TGF-β1 is a known agonist of both NADPH oxidase activity and expression in several systems including the kidney (7, 42).
In conclusion, our study reveals two novel prooxidant targets, Nox2 and Nox4, that are involved in the balance between neonatal c-kit+ cell precursor and differentiation status. With the use of microfluorography to measure ROS, along with genetic tools to profile and manipulate the redox status of early postnatal c-kit+ CPCs, our results demonstrate that c-kit+ CPCs exist in a low-ROS state and that Nox2 and Nox4 are functionally linked to their differentiation into smooth muscle and cardiac cell lineages. The downstream pathways modulated by NADPH oxidase-derived ROS production in CPCs will require further study but likely involve redox-sensitive signaling pathways such as JNK, ERK1/2, p38MAPK, and phosphatidylinositol 3-kinase (PI3K), which are key components in the process of cardiac commitment and differentiation of ESCs (31, 44–46). Here we focused mainly on prooxidant NADPH oxidase isozymes; however, we are cognizant that additional sources of ROS, most notably the mitochondria, as well as overall redox balance are likely important and that a variety of additional prooxidant and/or antioxidant molecules identified by the RT2PCR Arrays may be involved in the pathways mediating c-kit+ CPC self-renewal and differentiation. Although uncovering the precise redox pathways and mechanisms that drive these outcomes will require additional investigation, we believe that this study significantly advances the understanding of CPC biology by highlighting several key target genes that may be utilized in directed differentiation for cell-based therapy of the heart.
GRANTS
This work was supported by National Institutes of Health Grants HL-063887, DK-065992, and HL-045239. A. S. Nadworny was supported by an American Heart Association Founders Affiliate Predoctoral Fellowship (10PRE3450014) and is an American Heart Association Stanley Stahl Research Fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S.N., M.R.G., R.V.S., M.I.K., and R.L.D. conception and design of research; A.S.N., M.R.G., and D.P. performed experiments; A.S.N., M.R.G., R.M.D., R.V.S., and R.L.D. analyzed data; A.S.N., M.R.G., R.M.D., R.V.S., and R.L.D. interpreted results of experiments; A.S.N. prepared figures; A.S.N. drafted manuscript; A.S.N., M.I.K., and R.L.D. edited and revised manuscript; A.S.N., M.R.G., D.P., R.M.D., R.V.S., M.I.K., and R.L.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sergei Rudchenko and Stanka Semova for support of FACS, Dr. Gang Wang and Dr. Carmen Capone for experimental expertise, and Dr. Heidi Stuhlmann for helpful discussions. Special thanks to Dr. Jen Musa for her assistance with preparing the manuscript and insightful evaluation.
REFERENCES
- 1.Abdel-Latif A, Zuba-Surma EK, Case J, Tiwari S, Hunt G, Ranjan S, Vincent RJ, Srour EF, Bolli R, Dawn B. TGF-beta1 enhances cardiomyogenic differentiation of skeletal muscle-derived adult primitive cells. Basic Res Cardiol 103: 514–524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe M, Hasegawa K, Wada H, Morimoto T, Yanazume T, Kawamura T, Hirai M, Furukawa Y, Kita T. GATA-6 is involved in PPARgamma-mediated activation of differentiated phenotype in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23: 404–410, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation 113: 1451–1463, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bartsch C, Bekhite MM, Wolheim A, Richter M, Ruhe C, Wissuwa B, Marciniak A, Muller J, Heller R, Figulla HR, Sauer H, Wartenberg M. NADPH oxidase and eNOS control cardiomyogenesis in mouse embryonic stem cells on ascorbic acid treatment. Free Radic Biol Med 51: 432–443, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buggisch M, Ateghang B, Ruhe C, Strobel C, Lange S, Wartenberg M, Sauer H. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci 120: 885–894, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Keaney JF, Jr, Schulz E, Levison B, Shan L, Sakuma M, Zhang X, Shi C, Hazen SL, Simon DI. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc Natl Acad Sci USA 101: 13014–13019, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craven M, Kotlikoff MI, Nadworny AS. C-kit expression identifies cardiac precursor cells in neonatal mice. Methods Mol Biol 843: 177–189, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood 104: 3591–3597, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas RM, Ryu J, Kanaan A, Del Carmen Rivero M, Dugan LL, Haddad GG, Ali SS. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am J Physiol Cell Physiol 298: C1594–C1602, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froese N, Kattih B, Breitbart A, Grund A, Geffers R, Molkentin JD, Kispert A, Wollert KC, Drexler H, Heineke J. GATA6 promotes angiogenic function and survival in endothelial cells by suppression of autocrine transforming growth factor beta/activin receptor-like kinase 5 signaling. J Biol Chem 286: 5680–5690, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart 90: 491–493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurusamy N, Mukherjee S, Lekli I, Bearzi C, Bardelli S, Das DK. Inhibition of ref-1 stimulates the production of reactive oxygen species and induces differentiation in adult cardiac stem cells. Antioxid Redox Signal 11: 589–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19: 1720–1730, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics 26: 180–191, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106: 1763–1774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 11: 723–732, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kuhn LT, Liu Y, Advincula M, Wang YH, Maye P, Goldberg AJ. A nondestructive method for evaluating in vitro osteoblast differentiation on biomaterials using osteoblast-specific fluorescence. Tissue Eng Part C Methods 16: 1357–1366, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Lepparanta O, Pulkkinen V, Koli K, Vahatalo R, Salmenkivi K, Kinnula VL, Heikinheimo M, Myllarniemi M. Transcription factor GATA-6 is expressed in quiescent myofibroblasts in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 42: 626–632, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Leri A, Hosoda T, Kajstura J, Anversa P, Rota M. Identification of a coronary stem cell in the human heart. J Mol Med 89: 947–959, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17: 3978–3988, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Spencer NY, Pantazis NJ, Engelhardt JF. Alsin and SOD1G93A proteins regulate endosomal reactive oxygen species production by glial cells and proinflammatory pathways responsible for neurotoxicity. J Biol Chem 286: 40151–40162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Monzen K, Hiroi Y, Kudoh S, Akazawa H, Oka T, Takimoto E, Hayashi D, Hosoda T, Kawabata M, Miyazono K, Ishii S, Yazaki Y, Nagai R, Komuro I. Smads, TAK1, and their common target ATF-2 play a critical role in cardiomyocyte differentiation. J Cell Biol 153: 687–698, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127: 1151–1165, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Murray TV, Smyrnias I, Shah AM, Brewer AC. NADPH oxidase 4 regulates cardiomyocyte differentiation via redox activation of c-Jun protein and the cis-regulation of GATA-4 gene transcription. J Biol Chem 288: 15745–15759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal 7: 1456–1467, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, Torok-Storb B, Ratner BD, Pabon L, Murry CE. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol 30: 80–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 100: 12313–12318, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461: 537–541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal 11: 2777–2789, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiol Behav 38: 887–890, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ, Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J, Kim SS. Nox 2 stimulates muscle differentiation via NF-kappaB/iNOS pathway. Free Radic Biol Med 38: 989–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9: 202–209, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Puceat M, Travo P, Quinn MT, Fort P. A dual role of the GTPase Rac in cardiac differentiation of stem cells. Mol Biol Cell 14: 2781–2792, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocic P, Lucchesi PA. NAD(P)H oxidases and TGF-beta-induced cardiac fibroblast differentiation: Nox-4 gets Smad. Circ Res 97: 850–852, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125: e2–e220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauer H, Neukirchen W, Rahimi G, Grunheck F, Hescheler J, Wartenberg M. Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp Cell Res 294: 313–324, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Sauer H, Rahimi G, Hescheler J, Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett 476: 218–223, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J 20: 1182–1184, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Sharifpanah F, Wartenberg M, Hannig M, Piper HM, Sauer H. Peroxisome proliferator-activated receptor alpha agonists enhance cardiomyogenesis of mouse ES cells by utilization of a reactive oxygen species-dependent mechanism. Stem Cells 26: 64–71, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol 287: C1560–C1568, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA 97: 10032–10037, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinbeck MJ, Kim JK, Trudeau MJ, Hauschka PV, Karnovsky MJ. Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells. J Cell Physiol 176: 574–587, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki YJ. Cell signaling pathways for the regulation of GATA4 transcription factor: implications for cell growth and apoptosis. Cell Signal 23: 1094–1099, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA 106: 1808–1813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res 65: 550–563, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Thomas RA, Pietrzak DC, Scicchitano MS, Thomas HC, McFarland DC, Frazier KS. Detection and characterization of circulating endothelial progenitor cells in normal rat blood. J Pharmacol Toxicol Methods 60: 263–274, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci 28: 8138–8143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien C, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 127: 1137–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol 20: 1006–1010, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am J Physiol Cell Physiol 296: C711–C723, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004 [DOI] [PubMed] [Google Scholar]