Abstract
In heart failure (HF), the impaired left ventricular (LV) arterial coupling and diastolic dysfunction present at rest are exacerbated during exercise. We have previously shown that in HF at rest stimulation of β3-adrenergic receptors by endogenous catecholamine depresses LV contraction and relaxation. β3-Adrenergic receptors are activated at higher concentrations of catecholamine. Thus exercise may cause increased stimulation of cardiac β3-adrenergic receptors and contribute to this abnormal response. We assessed the effect of L-748,337 (50 μg/kg iv), a selective β3-adrenergic receptor antagonist (β3-ANT), on LV dynamics during exercise in 12 chronically instrumented dogs with pacing-induced HF. Compared with HF at rest, exercise increased LV end-systolic pressure (PES), minimum LV pressure (LVPmin), and the time constant of LV relaxation (τ) with an upward shift of early diastolic portion of LV pressure-volume loop. LV contractility decreased and arterial elastance (EA) increased. LV arterial coupling (EES/EA) (0.40 vs. 0.51) was impaired. Compared with exercise in HF preparation, exercise after β3-ANT caused similar increases in heart rate and PES but significantly decreased τ (34.9 vs. 38.3 ms) and LVPmin with a downward shift of the early diastolic portion of LV pressure-volume loop and further augmented dV/dtmax. Both EES and EES/EA (0.68 vs. 0.40) were increased. LV mechanical efficiency improved from 0.39 to 0.53. In conclusion, after HF, β3-ANT improves LV diastolic filling; increases LV contractility, LV arterial coupling, and mechanical efficiency; and improves exercise performance.
Keywords: β-adrenergic receptor blockers, pressure-volume relation, congestive heart failure
during exercise, the cardiac output increases to meet the elevated oxygen delivery to the muscles. Normally, this is achieved without an abnormal increase in left atrial (LA) pressure (P) (7, 10, 37, 57). Enhanced sympathetic stimulation during exercise increases heart rate and accelerates relaxation, resulting in smaller left ventricular (LV) end-systolic volume (VES), lower minimum LV pressure (LVPmin), and increased LA-LV pressure gradient. This produces a marked increase in the peak rate of LV filling (dV/dtmax) without an increase in LAP to abnormal levels. Exercise increases LV contractility (53), both through the adrenergic stimulation and a positive force-frequency relationship (51). These responses result in an increased stroke volume despite reduced duration of ventricular ejection and filling. Moreover, LV contractility, LV-arterial coupling (EES/EA), and LV mechanical efficiency [stroke work (SW)/P-V area (PVA)] are all increased during normal exercise.
In contrast, after the development of heart failure (HF), the slowed relaxation at rest is further impaired during exercise and LVPmin is elevated (11). Increased preload during exercise elevates LV end-diastolic pressure (PED) and volume (VED), and diastolic P-V relation goes upward throughout the filling phase. Thus mean LAP abnormally increases, which contributes to the development of exertional dyspnea and limited exercise tolerance. Furthermore, contractility during exercise is impaired due to blunted response to β-adrenergic stimulation (40, 60, 64) and an impaired force-frequency relationship (51, 60). Thus the abnormal LV arterial coupling and diastolic dysfunction present at rest are exacerbated during exercise (11, 37).
The β3-adrenergic receptor (β3-AR) was initially identified in fat and some vascular tissues and subsequently found in the myocardium. Unlike β1- and β2-ARs, β3-AR stimulation inhibits cardiac contraction and relaxation (14, 21, 22, 31, 46, 47, 58) through its link to inhibitory G proteins (Gi) and involves both nitric oxide (NO)-dependent (14, 22) and -independent effects (2, 14, 21, 22, 46, 67). Stimulation of cardiac β3-AR has a negative effect on cardiac contraction (i.e., negative inotropy states with the β3-AR) but has no direct chronotropic effects. In the failing heart, β3-ARs are upregulated (14, 46) and are relatively resistant to desensitization (58) due to a lack of βARK-1 phosphorylation sites (14, 22, 36, 46, 47), while cardiac β1-ARs are downregulated and β1- and β2-ARs are desensitized and uncoupled from the Gs protein in the failing heart (39). This altered balance of cardiac β3-, β1-, and β2-ARs in HF may have important effects.
Previously, we found that stimulation of β3-AR by endogenous catecholamine depresses LV contraction and relaxation at rest in conscious dogs with pacing-induced HF (47). In HF, during exercise, markedly elevated levels of catecholamine occur (18), which may cause enhanced activation of cardiac β3-ARs. Thus the adverse effects of β3-AR stimulation in HF may be accentuated during exercise. However, the role of β3-AR during exercise performance in HF is unknown. Whether the beneficial actions of β3-ANT we observed previously in HF at rest (47) can persist during exercise in HF remains to be determined. Investigations addressing this problem are timely and highly significant, because exercise intolerance is a hallmark of HF, but many existing HF therapies (including aldosterone antagonism, angiotensin-converting-enzyme-inhibitor therapy, and β-blockade) with clear salutary role on cardiac function at rest failed to improve exercise capacity in patients with HF reported by recent clinical trials (16, 28, 32–34, 48).
Accordingly, this study was undertaken to test the hypotheses that after HF there is an enhanced activation of the cardiac β3-AR system during exercise, which contributes to the abnormal responses of LV contraction, relaxation, and LV-arterial coupling to exercise. We assessed the acute effect of L-748,337, a selective β3-antagonist, on diastolic filling, LV arterial coupling, and mechanical efficiency at rest and during exercise in a conscious, chronically instrumented dog model with pacing-induced HF (34).
MATERIALS AND METHODS
Instrumentation
This investigation was approved by the Wake Forest School of Medicine Animal Care and Use Committee. Twelve healthy, adult, heartworm-negative mongrel dogs (body wt of 25–35 kg) were instrumented to measure three LV internal dimensions, LVP, and LAP. One myocardial lead (model 4312: Cardiac Pacemakers, Minneapolis, MN) was implanted within the myocardium of the right ventricle (RV), and the lead was attached to unipolar multiprogrammable pacemakers (model 8329: Medtronic, Minneapolis, MN) positioned under the skin in the chest. Hydraulic occluders were placed around the venae cavae by a technique described previously (12, 40, 47, 62).
Data Collection
Studies were performed after full recovery from instrumentation (10 days after original surgery) with the dogs standing and then running on a motorized treadmill (model 1849C; Quinton, Seattle, WA) as previously described (11, 12).
Experimental Protocol
Induction of HF.
Studies were performed after the animals had fully recovered from instrumentation. After completion of the normal baseline studies as previously described (12, 47), rapid RV pacing (at 220–240 beats/min) was initiated using the pacing protocol to induce HF (13, 47). After 4–5 wk of rapid pacing, when the LVPED, during the nonpacing period, had increased by >15 mmHg over the prepacing control level, we obtained HF data.
Effect of exercise with HF without and with β3-ANT.
During the stable HF period, we examined the cardiac response to exercise before and after β3-ANT. Briefly, before each study, the pacer was turned off, and the dog was allowed to equilibrate for at least 40 min. Then, steady-state measurements were obtained and blood was collected from LA catheter at rest while the dogs stood on a motorized treadmill (model 1849C; Quinton). Variably loaded LV P-V loops were generated by transient occlusion of the venae cavae (VCO) as previously described (11, 47, 62). The first HF exercise was then performed with the dogs running on the treadmill.
The treadmill speed was gradually increased every 1–2 min from 2.5 mph up to the maximum tolerated level (4.5–6 mph). The animals exercised at this level until they could no longer keep up with the treadmill. At submaximal levels of exercise, blood was collected. Both steady-state and VCO data were obtained, and then the treadmill was suddenly stopped. Data were acquired during 12-s periods throughout the exercise protocol. We analyzed the data recorded during submaximal level of exercise to avoid marked fluctuation by respiration. The total exercise time ranged from 4 to 8 min.
After 40 min of recovery, a selective β3-ANT, L-748,337 (50 μg/kg), was administered intravenously. This dosage of L-748,337 (50 μg/kg iv) has been demonstrated to selectively block β3-ARs by our group and others (9, 44, 46, 47). We have also verified the adequacy of its effect on β3-blockade by abolishing BRL-37,344-induced responses.
Ten minutes after L-748,337 treatment, hemodynamic data and blood samples were collected at rest. Then treadmill exercise protocol was again performed (13) and at submaximal levels of exercise, blood was collected. We previously observed that there is no difference in the response to exercise repeated after a 40-min rest period (11, 13). The values of resting controls were also similar before initial exercise and with a 40-min resting period after exercise.
Plasma NO Determination
Plasma NO concentrations were determined by the Hypertension Center Core Laboratory of Wake Health as previously described (1).
Data Processing and Analysis
As previously described, LVV, LV PES-VES relation and its slope (EES), and SW-VED relation and its slope (MSW) were analyzed (12, 13). Relaxation was evaluated by determining the time constant of the isovolumic decrease of LVP (τ). LVP from the time of peak −dP/dt until mitral valve opening was fit to the exponential equation LVP = PA exp(−t/τ) + PB, where t is time and PA, PB, and τ are constants determined by the data. Although the decrease in isovolumic P is not exactly exponential (65), the time constant, which is derived from the exponential approximation, provides an index of the rate of LV relaxation (24, 42). In addition, τ was also calculated by the Weiss method (monoexponential decay model to zero asymptote). LV-arterial coupling was quantitated as the ratio of EES to EA, determined as PES/stroke volume (SV). The LV PVA, which represents the total mechanical energy, was determined as the area under the PES-VES relation and systolic P-V trajectory above the PED-VED curve. The efficiency of conversion of mechanical energy to external work of the heart was calculated as SW/PVA (50). Data acquisition and analysis were not blinded to group identity vis-a-vis β3-ANT treatment due to the study design.
Statistical Analysis
Statistical comparisons were made with repeated-measures ANOVA. If there was a significant overall effect, intergroup differences were assessed by using Fisher's protected least significant difference. Each dog also served as its own control. Significance was established as P < 0.05. Data for steady state are expressed as means ± SD; values for LV P-V relations are expressed as means ± SE.
Drugs
(S)-N-{4-[2-({3-[3-(acetamidomethyl) phenoxy]-2-hydroxypropyl}-amino)ethyl]phenyl} benzene sulfonamide (L-748,337) was a gift from Merck Research Laboratories (Rahway, NJ).
RESULTS
Abnormal Hemodynamic Responses in Pacing-Induced HF: Rest vs. Exercise
At rest, as summarized in Tables 1 and 2, consistent with our past reports (13, 47), chronic RV rapid pacing in a canine model produced progressive LV systolic and diastolic dysfunction. Compared with normal rest, LV PED, VED, and VES significantly increased. LV systolic dysfunction was shown by significant decreases in EES and Msw with reduced LV dp/dtmax. LV diastolic dysfunction was indicated by significantly elevated LVPmin, LVPED, and mean LAP, as well as prolonged LV relaxation with increased τ.
Table 1.
Effects of β3-blockade on steady-state hemodynamic data at rest and during exercise after HF
| Control |
β3-Blockade |
|||
|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | |
| Heart rate, beats/min | 133 ± 16 | 170 ± 17* | 136 ± 21 | 166 ± 17* |
| Maximum dP/dt, mmHg/s | 1,482 ± 280 | 1,872 ± 404* | 1,565 ± 328 | 1,864 ± 348* |
| Minimum dP/dt, mmHg/s | −1,453 ± 158 | −1,761 ± 293* | −1,477 ± 188 | −1,746 ± 242* |
| Stroke volume, ml | 13.4 ± 5.0 | 14.5 ± 5.3* | 14.0 ± 5.4 | 15.2 ± 5.9* |
| LV end-diastolic pressure, mmHg | 33.2 ± 5.4 | 41.2 ± 7.1* | 31.7 ± 5.2† | 37.8 ± 6.5* |
| LV end-systolic pressure, mmHg | 94 ± 7 | 106 ± 11* | 93 ± 7 | 102 ± 10*‡ |
| Minimum LV pressure, mmHg | 16.9 ± 3.7 | 20.6 ± 4.0* | 15.6 ± 2.9† | 17.0 ± 3.7*‡ |
| Mean LA pressure, mmHg | 25.4 ± 4.9 | 33.0 ± 5.1* | 23.3 ± 3.8† | 28.2 ± 4.8*‡ |
| LV end-diastolic volume, ml | 52.7 ± 21.4 | 53.5 ± 21.8 | 52.1 ± 20.5 | 53.5 ± 21.8* |
| LV end-systolic volume, ml | 39.4 ± 16.1 | 38.8 ± 17.0 | 38.3 ± 16.9† | 38.0 ± 15.3‡ |
| Maximum dV/dt, ml/s | 172 ± 67 | 235 ± 86* | 191 ± 73 | 260 ± 97*‡ |
| Stroke work, mmHg·ml | 988 ± 452 | 1,117 ± 412* | 1,060 ± 521 | 1,210 ± 503*‡ |
| Cardiac output, ml/min | 1,740 ± 633§ | 2,383 ± 925* | 1,840 ± 744 | 2,448 ± 918* |
| Ea, mmHg/ml | 8.0 ± 2.6 | 8.2 ± 2.2 | 7.6 ± 3.0 | 7.5 ± 2.8‡ |
| Time constant of relaxation, ms | 36.3 ± 5.0 | 38.3 ± 4.8* | 35.2 ± 5.1† | 34.9 ± 4.9‡ |
Values are means ± SD (n =12). HF, heart failure; dP/dt, rate of rise of left ventricular pressure; LV, left ventricular; LA, left atrial; maximum dV/dt, peak rate of mitral flow; Ea, arterial elastance
P < 0.05, exercise vs. corresponding control rest.
P < 0.05, β3-blockade rest vs. control rest.
P < 0.05, HF β3-blockade exercise vs. HF control exercise.
Table 2.
Effects of β3-blockade on pressure-volume relations at rest and during exercise after HF
| Control |
β3-Blockade |
|||
|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | |
| EES, mmHg/ml | 3.5±0.7 | 3.0±0.7* | 4.1±0.7† | 4.6±0.7*‡ |
| EES/EA | 0.51±0.13 | 0.40±0.09 | 0.65±0.16† | 0.68±0.10‡ |
| MSW, mmHg | 61.1±1.0 | 63.3±6.7 | 65.5±2.9 | 75.1±3.2‡ |
| SW/PVA | 0.45±0.04 | 0.39±0.06 | 0.52±0.03 | 0.53±0.03‡ |
Values are means ± SE (n = 4). EES, slope of linear end-systolic pressure-volume (PES-VES) relation; SW, stroke work; PVA, LV pressure-volume area; MSW, slope of SW-end-diastolic volume (VED) relation
P < 0.05, exercise vs. control rest.
P < 0.05, β3-blockade rest vs. control rest.
P < 0.05, HF β3-blockade exercise vs. HF control exercise.
During exercise, as typically displayed in Figs. 1 and 2, such abnormalities at rest were exacerbated. During exercise after HF, heart rate, LVPES, τ (38.3 ± 4.8 vs. 36.3 ± 5.0 ms), LVPED, LVPmin, and mean LAP (33.0 ± 5.1 vs. 25.4 ± 4.9 mmHg) increased. These changes were accompanied by a consistent rightward and upward shift of the early diastolic portion of the LV P-V loop. During early diastole, at an equivalent LVV, the LVP was significantly higher during exercise than at rest after HF (62). In addition, the impaired LV arterial coupling present at rest were exacerbated during HF exercise. As summarized in Table 2, compared with HF at rest, HF exercise caused a significant decrease in EES, while EA was relatively unchanged, resulting in decreased EES/EA ratio with reduced SW/PVA (0.39 ± 0.06 vs. 0.45 ± 0.04).
Fig. 1.
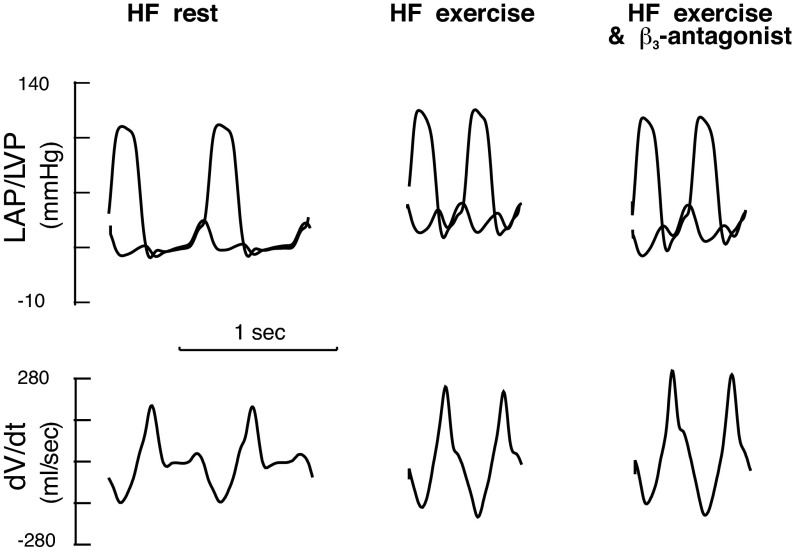
Examples of the effect of β3-antagonist on left ventricular (LV) diastolic filling during exercise after heart failure (HF). Analog recordings of LV pressure (LVP), left atrial pressure (LAP), and the peak rate of mitral flow (dV/dtmax) at rest, during exercise, and exercise with β3-antagonist obtained from one conscious chronically instrumented dog after pacing-induced HF. Compared with HF at rest, the maximum mitral peak filling rate (dV/dtmax) increased during exercise. Thus the increase in dV/dtmax during exercise after HF resulted entirely from an increase in LAP. Compared with HF exercise, after β3-antagonist, exercise caused similar increases in LV end-systolic pressure (PES) and heart rate. LAP was lower while τ and LVPmin decreased. There was an increase in the early diastolic mitral valve pressure gradient. dV/dtmax was further augmented with β3-antagonist during exercise.
Fig. 2.
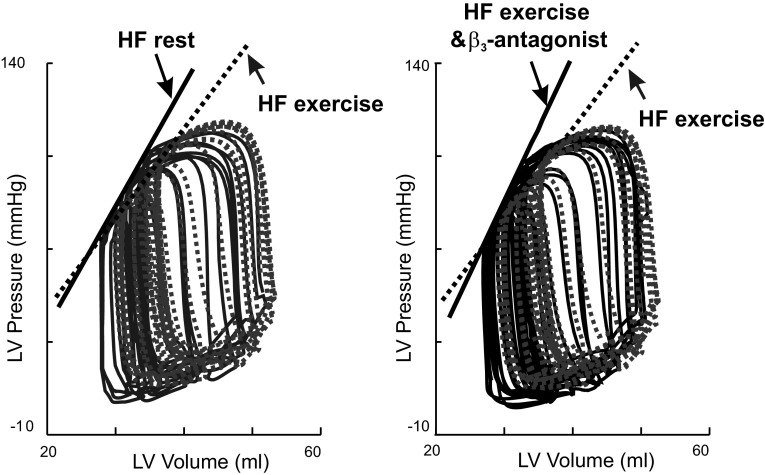
Examples of the effects of β3-antagonist on LVPES-LV end-systolic volume (VES) relations during exercise after HF. LV P-V loops recorded following transient caval occlusions in one conscious dog after HF at rest, during exercise, and during exercise after β3-antagonist treatment. After HF, compared with rest, exercise caused a decrease in the slope of the LV PES-VES relation, EES. Compared with HF exercise without treatment, HF exercise following treatment with β3-antagonist produced marked leftward shifts of the LV PES-VES relation with increase in the slope of EES, indicating that β3-antagonist increased LV contractility after HF during exercise.
β3-ANT Improves Hemodynamic Responses in Pacing-Induced HF: Rest vs. Exercise
At rest after HF, β3-ANT significantly improved LV systolic and diastolic function without significant changes of plasma levels of NO (43.0 ± 12.8 vs. 41.8 ± 13.2 μmol/l). As shown in Table 1 and Fig. 1, consistent with our past report (47), compared with HF at rest, β3-ANT produced no changes in heart rate and LVPES but significantly reduced EA, PED, and mean LAP. The dV/dtmax (191 ± 73 vs. 172 ± 67 mmHg/s) was increased due to decreased LVPmin (15.6 ± 2.9 vs. 16.9 ± 3.7 mmHg) and τ. SV and cardiac output were increased. After β3-ANT, EES was also increased. Both the EES/EA ratio and SW/PVA (0.52 ± 0.03 vs. 0.45 ± 0.04) were significantly improved (Table 2 and Fig. 2).
Importantly, during exercise after HF, treatment with β3-ANT prevented HF exercise-induced adverse effects on LV systolic and diastolic dysfunction (Fig. 2). As shown in Tables 1 and 2, compared with exercise in HF preparations, exercise after β3-ANT treatment caused similar increases in heart rate, PES, and NO (64.6 ± 14.8 vs. 60.2 ± 11.5 μmol/l) but significantly attenuated HF exercise-induced increase in LVPED and mean LAP; reversed exercise-induced abnormal increases in LVPmin, τ, and upward shift of the early diastolic portion of LV P-V loop; and further augmented dV/dtmax (Table 1 and Figs. 1 and 3). In addition, during HF exercise with β3-ANT treatment, there was a reversal of abnormal HF exercise response in ventricular-vascular coupling and cardiac mechanical efficiency. As demonstrated in Fig. 3 and summarized in Table 2, during HF exercise after treatment with β3-ANT, HF exercise-caused decreases in EES and EES/EA were converted to increased EES (5.3 vs. 3.7 mmHg/ml) and EES/EA (0.84 vs. 0.55). Thus treatment with β3-ANT further improved HF exercise SW/PVA from 0.39 to 0.53. The duration of exercise was also significantly increased (7.2 vs. 5.6 min).
Fig. 3.
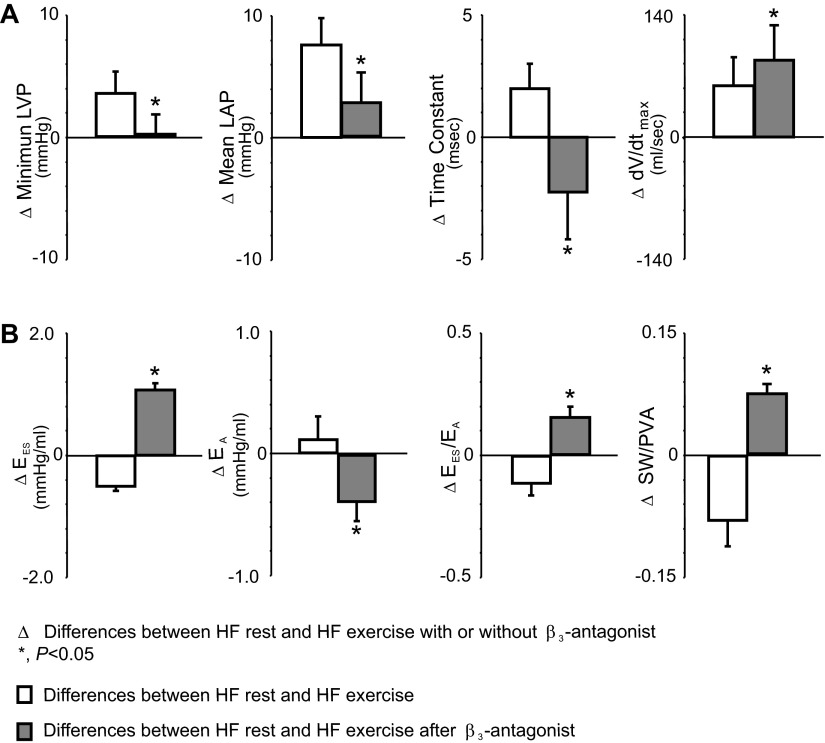
Group mean data on the differences between HF at rest and HF exercise with and without treatment of β3-antagonist on LV filling, LV arterial coupling, and stroke work (SW)/P-V area (PVA). Compared with HF at rest, HF exercise caused an increase in dV/dtmax due to significant increases in mean LAP, while τ and LVPmin were increased. In contrast, during HF exercise with treatment of β3-antagonist, there was a greater increase in dV/dtmax with decreased τ and smaller increase in mean LAP. During HF exercise, LV arterial coupling (EES/EA) decreased, resulting from a significantly increased EA, but decreased EES, which lead to a reduction in SW/PVA. In contrast, β3-antagonist reversed the HF exercise-induced abnormal responses and caused significant increases in EES, EES/EA, and SW/PVA during HF exercise.
DISCUSSION
The major symptom and cause of disability in HF patients are exercise intolerance. Despite marked progress in the therapeutic approach of patients with HF, exercise intolerance remains to be a hallmark of the disease (16, 33, 39, 56). Current treatment of exercise intolerance is unsatisfactory in HF patients. The β-adrenergic agonist dobutamine and phosphodiesterase inhibitor milrinone were associated with worse survival and clinical outcomes and did not improve quality of life in severe HF patients (39, 52, 61). Aldosterone antagonism, angiotensin-converting-enzyme-inhibitor therapy, and β-blockade treatment improve survival in patients with HF but failed to improve exercise capacity in recent clinical trials (16, 28, 32–34, 48).
In the current investigation, we showed, for the first time, increased activation of cardiac β3-AR is an important contributor to exercise intolerance in HF and that administration of a β3-ANT in a canine model of HF enhances the response to exercise with improved LV diastolic filling, increases LV contractility, and decreases arterial elastance with an overall improvement in LV-arterial coupling and mechanic efficiency. This study is important in that it challenges the existing paradigm that general downregulation of adrenergic signaling in congestive HF is protective, unravels a new mechanism of exercise intolerance in HF, and points to a new molecular target for this serious and growing health problem.
How does β3-ANT administration prevent exercise-induced exacerbation of LV systolic and diastolic dysfunction and restore normal exercise response in HF? Previous observations from serial studies on the mechanism and treatment of exercise intolerance with HF performed by us and other groups have shown that abnormal exercise response in HF is attributed to several factors such as an enhanced sensitivity of LV relaxation to exercise-induced increased systolic load, altered force-frequency relation, chronotropic incompetence, impaired intrinsic contractility, reduced β-adrenergic reserve, abnormal ventricular-arterial interaction (5), and a blunted peripheral arterial vasodilator response to exercise (12, 13, 16, 27, 33, 37–39, 41, 51, 56). The current study indicates that excessive endogenous catecholamine stimulation during exercise in HF (18, 30) alters inotropic response to β-ARs due to enhanced β3-AR stimulation with higher concentrations of catecholamine (20) with less responsive β1- and β2-AR (14, 17, 45). Thus cardiac β-AR desensitization in HF at rest was further exacerbated due to enhanced activation of β3-AR.
It is noted that β3-AR stimulation has been reported to produce vasodilatation. However, blocking endogenous β3-AR with L-748,337 did not produce any evidence of vasodilatation. Compared with HF exercise without β3-ANT, similar increases in PES and heart rate were achieved during HF exercise after β3-ANT treatment. These effects are also different than that produced by existing HF therapies such as conventional β-blockers and ACE inhibitors. These observations are consistent with the findings of Pelat et al. (55) and suggest that endogenous β3-AR activation does not have a significant effect on HR or blood pressure in HF. Thus the improvement of exercise performance during HF following treatment with a β3-ANT should be due to the direct myocardial effects.
Our findings are comparable with some past reports on the effects of exogenous β3-AR agonists (31, 46). Exogenous administration of a β3-agonist induces marked vasodilation (63). Observations of the exogenous administration of β3-agonists showed that β3-AR-mediated signaling results in NO generation with elevated levels of NO (8, 14, 21). In rat thoracic aorta, SR59230A, a β3-ANT, antagonized the β3-dose-dependent relaxation evoked by SR58611 (23). Such findings raised concern that β3-ANT might be deleterious in the failing heart by increasing afterload (44). However, current results and our previous study (47) showed that blocking β3-AR with the current dosage of β3-ANT does not increase LV afterload. Instead, our studies showed that β3-ANT decreased EA both at rest and during exercise. In our study, the plasma levels of NO were not altered by β3-ANT during exercise, although we could not determine myocardial levels of NO. It has previously been known that activation of PDE2 via a β3/nitric oxide synthase/cGMP pathway may further reduce inotropy and aggravate systolic function, particularly in the presence of concomitant β1/β2-stimulation (43). Recently, we found that in HF, chronic β3-AR stimulation triggers upregulation of cardiac inducible nitric oxide synthase (iNOS). Oxidant stress from iNOS uncoupling aggravates cardiac dysfunction. These alterations were prevented in β3-AR knockout (β3KO) mice and also were reversed with the treatment of a β3-ANT. Thus it is possible that β3-ANT may inhibit β3-AR-iNOS/NO signaling and contribute to its beneficial actions in HF during exercise. However, whether and to what extent administration of β3-ANT affects the iNOS/NO pathway, thereby contributing to the beneficial action of L-748, 337, are unknown.
Our observation of the improvement of LV mechanical efficiency after acute treatment of β3-ANT during exercise is consistent with the reports that acute administration of SR59230A, a β3-ANT, attenuates the imbalance of systemic and myocardial oxygen transport induced by dopamine (25). β3-ANT by L-748,337 enhances cardiac positive inotropic and lusitropic responses to dobutamine in dogs with pacing-induced HF. Moreover, hypotension or poor ejection would not be anticipated in the use of β3-ANT, which is also different from the acute use of conventional HF drugs.
The current observation is in agreement with our recent serial studies in HF models showing remarkable salutary effects of the β3-AR blockade on reversing β-AR desensitization and preventing HF progression. Our preliminary observations (abstract presented at American Heart Association Meeting) revealed that β3-AR-deficient mice were protected from isoproterenol-induced HF. Recently, we further found that aging-induced desensitization of cardiac β-adrenergic signaling was prevented in β3-AR knockout (β3KO) aged mice. β3KO reverses aging-induced downregulation of LV myocyte sarco(endo)plasmic reticulum Ca2+-ATPase and upregulation of iNOS. We also made observations on the restoration of normal cardiomyocyte basal and β-AR subtype modulation of L-type calcium current in HF by β3KO. Consistent with past reports that these β3KO animals had normal life spans with no cardiac pathological conditions detectable at any point (67). Our recent investigation is supported by evidence that the efficacy of some β blockers may result from the action of β3-ARs (3, 19, 26, 39, 59, 66). β3-AR antagonists improve clinical outcomes and limit HF progression and remodeling in experimental studies (19, 44, 47). Recent clinical studies have demonstrated that chronic treatment (6 mo) with carvedilol (a nonselective β-AR blocker with β3-AR antagonist) was associated with a positive inotropic effect with significant improvement in load-independent indexes of myocardial contractility beyond what can be attributed to changes in LV chamber size and load after 3 mo (6). However, long-term (6 mo) administration of nebivolol (a β3-AR agonist/β1-AR antagonist with associated NO-releasing properties) failed to improve exercise in HF patients (15). Similarly, 3 mo of treatment of nebivolol reduced exercise duration while that of carvedilol did not (P = 0.02 for the difference) in randomized controlled trial (54).
Study Limitations
Several methodological issues should be considered in the interpretation of our data: 1) Observations were obtained from a pacing-induced HF canine model. Although rapid pacing produces an animal model of HF that closely mimics that of clinical congestive cardiomyopathy, this experimental model demonstrated biventricular chamber dilatation with increased LV and RV filling pressures and striking abnormalities in systolic and diastolic function similar to that found in patients with dilated congestive cardiomyopathy (7, 12, 35). We cannot ascertain that these results are applicable to HF from other causes. 2) We did not examine the effects of β3-ANT on LV end-diastolic P-V relation during exercise in this investigation. Further studies are needed to focus on this point in HF. 3) Our study did not define the mechanisms of the action of β3-ANT during exercise of HF in addition to its ready known blocking β3-AR activation-induced enhanced inhibition on LV contraction and relaxation. It is possible that its beneficial actions are attributable to the alteration on β3-AR stimulation activated subtypes of NOS/NO pathways. We also did not measure oxidative stress. Indeed, it is emerging that cardioactive amines are also able to bypass the receptors, enter the myocytes, and trigger the activity of monoamine oxidases, leading to increased oxidative stress due to the monoamine oxidase-driven generation of H2O2. This has been shown both in the case of ischemia-reperfusion (4) and in transverse aortic constriction-induced HF (29). Clearly, more insight will be gained from ongoing work designed to assess the influence of β3-ANT on the interaction between β3-AR stimulation and the subtypes of NOS/NO pathways. It should be clarified in a future study whether β3-ANT may blunt an increased oxidative stress during exercise in HF preparations.
Our novel findings indicate that antagonizing detrimental effects of endogenous β3-adrenergic stimulation may help preserve β1- and β2-AR function, lead to resensitizing the activity of the β-adrenergic system, and provide inotropic support and thus improve exercise performance in HF. However, our study only elucidated the positive systolic and diastolic effects of acute β3-ANT administration after HF at rest and during exercise. Since acute gain of β3-ANT may not correlate with long-term benefits, whether these beneficial actions of acute β3-ANT administration during exercise in HF will persist after chronic long-term application is unclear. Thus the precise therapeutic role of chronic β3-ANT for exercise tolerance of HF remains to be determined.
In conclusion, we found that in dogs with pacing-induced HF, β3-AR activation contributes to exercise intolerance in HF. β3-ANT improves LV diastolic filling; increases LV contractility, LV arterial coupling, and mechanical efficiency; and improves response to Ex. The current study supports the hypothesis that upregulation of cardiac β3-ARs is the critical determinant of the dysfunctional β-AR regulation that occurs in HF at rest and during exercise and provides unique insight to the functional implications of β3-AR blockade in exercise intolerance of HF.
GRANTS
This study was supported, in part, by National Institutes of Health Grants AA-12335 and HL-074318) and American Heart Association Grant 0530079N (to C. P. Cheng) and National American Heart Association Scientist Development Grant 0530079N (to H.-J. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M., H.J.C., and C.P.C. conception and design of research; S.M., H.J.C., A.M., H.H., Q.-H.H., and C.P.C. performed experiments; S.M., H.J.C., A.M., H.H., Q.-H.H., and C.P.C. analyzed data; S.M., H.J.C., A.M., H.H., Q.-H.H., and C.P.C. interpreted results of experiments; S.M. and C.P.C. prepared figures; S.M. drafted manuscript; S.M., H.J.C., A.M., H.H., Q.-H.H., W.C.L., and C.P.C. approved final version of manuscript; W.C.L. and C.P.C. edited and revised manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the computer programming of Ping Tan, technical assistance of Michael Cross and Chun Xian Zhang, and administrative support of Amanda Burnette. We thank Merck Research Laboratories for providing L-748,337.
The abstract for this study was presented at the American Heart Association Meeting (November 2005, Dallas, TX).
REFERENCES
- 1.Alusik S, Jedickova V, Paluch Z, Zecova S. Plasma levels of nitrite/nitrate and inflammation markers in elderly individuals. Bratisl Lek Listy 109: 289–292, 2008 [PubMed] [Google Scholar]
- 2.Audigane L, Kerfant BG, El Harchi A, Lorenzen-Schmidt I, Toumaniantz G, Cantereau A, Potreau D, Charpentier F, Noireaud J, Gauthier C. Rabbit, a relevant model for the study of cardiac beta3-adrenoceptors. Exp Physiol 94: 400–411, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Beta-Blocker Evaluation of Survival Trial (B-BEoST) Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 344: 1659–1667, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112: 3297–3305, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Cardiol Clin 29: 447–459, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt B, Bolos M, Deswal A, Ather S, Chan W, Mann DL, Carabello B. New insights into mechanisms of action of carvedilol treatment in chronic heart failure patients–a matter of time for contractility. J Card Fail 18: 183–193, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR. [beta]-Adrenergic receptor blockade in chronic heart failure. Circulation 101: 558–569, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Brixius K, Bloch W, Pott C, Napp A, Krahwinkel A, Ziskoven C, Koriller M, Mehlhorn U, Hescheler J, Fleischmann B, Schwinger RH. Mechanisms of beta 3-adrenoceptor-induced eNOS activation in right atrial and left ventricular human myocardium. Br J Pharmacol 143: 1014–1022, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candelore MR, Deng L, Tota L, Guan XM, Amend A, Liu Y, Newbold R, Cascieri MA, Weber AE. Potent and selective human beta3-adrenergic receptor antagonists. J Pharmacol Exp Ther 290: 649–655, 1999 [PubMed] [Google Scholar]
- 10.Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res 70: 9–19, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Cheng CP, Noda T, Nozawa T, Little WC. Effect of heart failure on the mechanism of exercise-induced augmentation of mitral valve flow. Circ Res 72: 795–806, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Cheng CP, Suzuki M, Ohte N, Ohno M, Wang ZM, Little WC. Altered ventricular and myocyte response to angiotensin II in pacing-induced heart failure. Circ Res 78: 880–892, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Cheng CP, Ukai T, Onishi K, Ohte N, Suzuki M, Zhang ZS, Cheng HJ, Tachibana H, Igawa A, Little WC. The role of ANG II and endothelin-1 in exercise-induced diastolic dysfunction in heart failure. Am J Physiol Heart Circ Physiol 280: H1853–H1860, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Cheng HJ, Zhang ZS, Onishi K, Ukai T, Sane DC, Cheng CP. Upregulation of functional beta3-adrenergic receptor in the failing canine myocardium. Circ Res 89: 599–606, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail 14: 219–225, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Conraads VM, Van Craenenbroeck EM, De Maeyer C, Van Berendoncks AM, Beckers PJ, Vrints CJ. Unraveling new mechanisms of exercise intolerance in chronic heart failure. Role of exercise training. Heart Fail Rev 18: 65–77, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Dincer UD, Bidasee KR, Guner S, Tay A, Ozcelikay T, Altan VM. The effect of diabetes on expression of beta1-, beta2-, and beta3-adrenoreceptors in rat hearts. Diabetes 50: 455–461, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Ferrari R, Anand IS, Ceconi C, De Giuli F, Poole-Wilson PA, Harris P. Neuroendocrine response to standing and mild exercise in patients with untreated severe congestive heart failure and chronic constrictive pericarditis. Heart 76: 50–55, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan RT, Li WM, Xiu CH, Shen JX, Wang X, Wu S, Kong YH. Chronic blocking of beta3-adrenoceptor ameliorates cardiac function in rat model of heart failure. Chin Med J 120: 2250–2255, 2007 [PubMed] [Google Scholar]
- 20.Gauthier C, Langin D, Balligand JL. Beta3-adrenoceptors in the cardiovascular system. Trends Pharmacol Sci 21: 426–431, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, Marec HL. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102: 1377–1384, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauthier C, Leblais V, Moniotte S, Langin D. The negative inotropic action of catecholamines: role of beta3-adrenoceptors. Can J Physiol Pharmacol 78: 681–690, 2000 [PubMed] [Google Scholar]
- 23.Gauthier C, Tavernier G, Trochu JN, Leblais V, Laurent K, Langin D, Escande D, Le Marec H. Interspecies differences in the cardiac negative inotropic effects of beta3 adrenoceptor agonists. J Pharmacol Exp Ther 290: 687–693, 1999 [PubMed] [Google Scholar]
- 24.Gilbert JC, Glantz SA. Determinants of left ventricular filling and of the diastolic pressure-volume relations. Circ Res 64: 827–852, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Gill RS, Cheung PY, Yu X, Aklabi MA, Nagendran J, Quinonez LG, Li YQ, Miller J, Ross DB, Rebeyka IM, Li J. Beta3-adrenoceptor antagonist SR59230A attenuates the imbalance of systemic and myocardial oxygen transport induced by dopamine in newborn lambs. Clin Med Insights Cardiol 6: 45–51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology at human beta-adrenergic receptor subtypes – characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 369: 151–159, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Izawa H, Yokota M, Takeichi Y, Inagaki M, Nagata K, Iwase M, Sobue T. Adrenergic control of the force-frequency and relaxation-frequency relations in patients with hypertrophic cardiomyopathy. Circulation 96: 2959–2968, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Jorde UP, Vittorio TJ, Kasper ME, Arezzi E, Colombo PC, Goldsmith RL, Ahuja K, Tseng CH, Haas F, Hirsh DS. Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: time to pace? Eur J Heart Fail 10: 96–101, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106: 193–202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato M, Kinugawa T, Omodani H, Osaki S, Ahmmed GU, Ogino K, Hisatome I, Miyakoda H, Thames MD. Responses of plasma norepinephrine and renin-angiotensin system to dynamic exercise in patients with congestive heart failure. J Card Fail 2: 103–110, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Kitamura T, Onishi K, Dohi K, Okinaka T, Isaka N, Nakano T. The negative inotropic effect of beta3-adrenoceptor stimulation in the beating guinea pig heart. J Cardiovasc Pharmacol 35: 786–790, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 3: 659–667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kociol RD. Circulation: Heart Failure editor's picks: most important articles in heart failure and therapeutics. Circ Heart Fail 5: e73––e78., 2012 [DOI] [PubMed] [Google Scholar]
- 34.Ladage D, Schwinger RH, Brixius K. Cardio-selective beta-blocker: pharmacological evidence and their influence on exercise capacity. Cardiovasc Ther 31: 76–83, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Lenfant C. Report of the Task Force on Research in Heart Failure. Circulation 90: 1118–1123, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Liggett SB, Freedman NJ, Schwinn DA, Lefkowitz RJ. Structural basis for receptor subtype-specific regulation revealed by a chimeric beta3-/beta2-adrenergic receptor. Proc Natl Acad Sci USA 90: 3665–3669, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Little WC, Kitzman DW, Cheng CP. Diastolic dysfunction as a cause of exercise intolerance. Heart Fail Rev 5: 301–306, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Little WC, Cheng CP. Effect of exercise on left ventricular-arterial coupling assessed in the pressure-volume plane. Am J Physiol Heart Circ Physiol 264: H1629–H1633, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res 93: 896–906, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Masutani S, Cheng HJ, Hyttila-Hopponen M, Levijoki J, Heikkila A, Vuorela A, Little WC, Cheng CP. Orally available levosimendan: dose-related positive inotropic and lusitropic effect in conscious, chronically-instrumented normal and heart failure dogs. J Pharmacol Exp Ther 325: 236–247, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Masutani S, Cheng HJ, Tachibana H, Little WC, Cheng CP. Levosimendan restores the positive force-frequency relation in heart failure. Am J Physiol Heart Circ Physiol 301: H488–H496, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyazaki S, Guth BD, Miura T, Indolfi C, Schulz R, Ross J., Jr Changes of left ventricular diastolic function in exercising dogs without and with ischemia. Circulation 81: 1058–1070, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98: 226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Moniotte S, Balligand JL. Potential use of beta3-adrenoceptor antagonists in heart failure therapy. Cardiovasc Drug Rev 20: 19–26, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Moniotte S, Belge C, Sekkali B, Massion PB, Rozec B, Dessy C, Balligand JL. Sepsis is associated with an upregulation of functional beta3 adrenoceptors in the myocardium. Eur J Heart Fail 9: 1163–1171, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL. Upregulation of beta3-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 103: 1649–1655, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Morimoto A, Hasegawa H, Cheng HJ, Little WC, Cheng CP. Endogenous beta3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am J Physiol Heart Circ Physiol 286: H2425–H2433, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Narang R, Swedberg K, Cleland JGF. What is the ideal study design for evaluation of treatment for heart failure? Eur Heart J 17: 120–134, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Nonogi H, Hess OM, Ritter M, Krayenbuehl HP. Diastolic properties of the normal left ventricle during supine exercise. Br Heart J 60: 30–38, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nozawa T, Cheng CP, Noda T, Little WC. Effect of exercise on left ventricular mechanical efficiency in conscious dogs. Circulation 90: 3047–3054, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Ohte N, Cheng CP, Little WC. Tachycardia exacerbates abnormal left ventricular-arterial coupling in heart failure. Heart Vessels 18: 136–141, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 325: 1468–1475, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Papanicolaou DA, Petrides JS, Tsigos C, Bina S, Kalogeras KT, Wilder R, Gold PW, Deuster PA, Chrousos GP. Exercise stimulates interleukin-6 secretion: inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol Endocrinol Metab 271: E601–E605, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Patrianakos AP, Parthenakis FI, Mavrakis HE, Diakakis GF, Chlouverakis GI, Vardas PE. Comparative efficacy of nebivolol versus carvedilol on left ventricular function and exercise capacity in patients with nonischemic dilated cardiomyopathy. A 12-month study. Am Heart J 150: 985. e9–985. e18, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Pelat M, Verwaerde P, Galitzky J, Lafontan M, Berlan M, Senard JM, Montastruc JL. High isoproterenol doses are required to activate beta3-adrenoceptor-mediated functions in dogs. J Pharmacol Exp Ther 304: 246–253, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: a statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention. Circulation 107: 1210–1225, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Ross J, Jr, Miura T, Kambayashi M, Eising GP, Ryu KH. Adrenergic control of force-frequency relation. Circulation 92: 2327–2332, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Rozec B, Gauthier C. Beta3-adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther 111: 652–673, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Rozec B, Noireaud J, Trochu JN, Gauthier C. Place of beta3-adrenoceptors among other beta-adrenoceptor subtypes in the regulation of the cardiovascular system. Arch Mal Coeur Vaiss 96: 905–913, 2003 [PubMed] [Google Scholar]
- 60.Senzaki H, Isoda T, Paolocci N, Ekelund U, Hare JM, Kass DA. Improved mechanoenergetics and cardiac rest and reserve function of in vivo failing heart by calcium sensitizer EMD-57033. Circulation 101: 1040–1048, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, Hutchins S, Leier CV, LeJemtel TH, Loh E, Nicklas J, Ogilby D, Singh BN, Smith W. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 102: 2222–2227, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Tachibana H, Cheng HJ, Ukai T, Igawa A, Zhang ZS, Little WC, Cheng CP. Levosimendan improves LV systolic and diastolic performance at rest and during exercise after heart failure. Am J Physiol Heart Circ Physiol 288: H914–H922, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Trochu JN, Leblais V, Rautureau Y, Beverelli F, Le Marec H, Berdeaux A, Gauthier C. Beta3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br J Pharmacol 128: 69–76, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ukai T, Cheng CP, Tachibana H, Igawa A, Zhang ZS, Cheng HJ, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation 103: 750–755, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Yellin EL, Hori M, Yoran C, Sonnenblick EH, Gabbay S, Frater RW. Left ventricular relaxation in the filling and nonfilling intact canine heart. Am J Physiol Heart Circ Physiol 250: H620–H629, 1986 [DOI] [PubMed] [Google Scholar]
- 66.Zhao Q, Wu TG, Jiang ZF, Cheng GW, Lin Y, Wang LX. Effect of beta blockers on beta3-adrenoceptor expression in chronic heart failure. Cardiovasc Drugs Ther 21: 85–90, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Ziskoven C, Grafweg S, Bolck B, Wiesner RJ, Jimenez M, Giacobino JP, Bloch W, Schwinger RH, Brixius K. Increased Ca2+ sensitivity and protein expression of SERCA 2a in situations of chronic beta3-adrenoceptor deficiency. Pflügers Arch 453: 443–453, 2007 [DOI] [PubMed] [Google Scholar]